Published online Dec 26, 2024. doi: 10.4252/wjsc.v16.i12.1047
Revised: October 9, 2024
Accepted: November 29, 2024
Published online: December 26, 2024
Processing time: 114 Days and 19.1 Hours
The gold standard of care for patients with severe peripheral nerve injury is autologous nerve grafting; however, autologous nerve grafts are usually limited for patients because of the limited number of autologous nerve sources and the loss of neurosensory sensation in the donor area, whereas allogeneic or xenografts are even more limited by immune rejection. Tissue-engineered peripheral nerve scaffolds, with the morphology and structure of natural nerves and complex bio
To prepare allogenic peripheral nerve scaffolds using a low-toxicity decellularization method, and use human umbilical cord mesenchymal stem cells (hUC-MSCs) as seed cells to cultivate scaffold-cell complexes for the repair of injured peripheral nerves.
After obtaining sciatic nerves from New Zealand rabbits, an optimal acellular scaffold preparation scheme was established by mechanical separation, varying lyophilization cycles, and trypsin and DNase digestion at different times. The scaffolds were evaluated by hematoxylin and eosin (HE) and luxol fast blue (LFB) staining. The maximum load, durability, and elastic modulus of the acellular scaffolds were assessed using a universal material testing machine. The acellular scaffolds were implanted into the dorsal erector spinae muscle of SD rats and the scaffold degradation and systemic inflammatory reactions were observed at 3 days, 1 week, 3 weeks, and 6 weeks following surgery to determine the histocompatibility between xenografts. The effect of acellular scaffold extracts on fibroblast proliferation was assessed using an MTT assay to measure the cyto
The experiments effectively decellularized the sciatic nerve of the New Zealand rabbits. After comparing the completed acellular scaffolds among the groups, the optimal decellularization preparation steps were established as follows: Mechanical separation of the epineurium, two cycles of lyophilization-rewarming, trypsin digestion for 5 hours, and DNase digestion for 10 hours. After HE staining, no residual nuclear components were evident on the scaffold, whereas the extracellular matrix remained intact. LFB staining showed a significant decrease in myelin sheath composition of the scaffold compared with that before preparation. Biomechanical testing revealed that the maximum tensile strength, elastic modulus, and durability of the acellular scaffold were reduced compared with normal peripheral nerves. Based on the histocompatibility test, the immune response of the recipient SD rats to the scaffold New Zealand rabbits began to decline3 weeks following surgery, and there was no significant rejection after 6 weeks. The MTT assay revealed that the acellular reagent extract had no obvious effects on cell proliferation. The cells were successfully isolated, cultured, and passaged from human umbilical cord WJ by MSC medium, and their ability to differentiate into Schwann-like cells was demonstrated by morphological and immunohistochemical identification. The differentiated cells could also myelinate in vitro.
The acellular peripheral nerve scaffold with complete cell removal and intact matrix may be prepared by com
Core Tip: The treatment of severe peripheral nerve injuries remains a clinical challenge, particularly in children. Autologous nerve grafts are the standard treatment for these severe neurologic deficits and the scarce number of autologous nerves and the loss of neurosensory function in the donor area are major obstacles, particularly in infants and young children. Al
- Citation: Qian C, Guo SY, Xu Z, Zhang ZQ, Li HD, Li H, Chen XS. Preliminary study on the preparation of lyophilized acellular nerve scaffold complexes from rabbit sciatic nerves with human umbilical cord mesenchymal stem cells. World J Stem Cells 2024; 16(12): 1047-1061
- URL: https://www.wjgnet.com/1948-0210/full/v16/i12/1047.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i12.1047
The treatment of severe peripheral nerve injury (PNI) remains a clinical challenge, even for children. PNI in the brachial plexus region caused by birth trauma is the most common PNI (obstetrical palsy) in newborns. Autologous nerve transplantation is the standard treatment for these severe neurological deficits[1]. The number of precious autologous nerves and the loss of sensory function of the donor nerve are the primary constraints, especially for the transplantation of autologous nerves in infants and young children; however, nerve allograft or nerve xenotransplantation is limited by immune rejection. Currently, there is an urgent need for peripheral nerve substitutes that can bridge the two broken ends of nerves, guide the axial growth of nerve axons, avoid the formation of neuromas, and guide the functional regeneration of peripheral nerves.
Tissue-engineered acellular nerve scaffolds are repair materials exhibiting natural nerve morphology, complex biological signals, and little immunogenicity obtained by the decellularization of homologous/heterogeneous peripheral nerves, which are the most promising nerve “substitutes”[2]. With the advent of tissue engineering, a variety of decellularization techniques have been applied to peripheral nerve scaffolds[3,4]; however, the use of detergents during the decellularization process increases the cytotoxicity of the scaffold itself and reduces the biomechanical properties of the material, which can affect the tissue reconstruction goals in vivo. Some researchers have completely abandoned detergents and simply used mechanical separation and enzymatic methods to obtain the ideal acellular scaffold[5,6].
Current studies suggest that Schwann cells (SCs) have important functions that other peripheral nerve-related cells do not in the process of peripheral nerve regeneration. A large number of experimental studies indicate that SC tran
All animal experiments were approved by the Association’s Animal Care and Use Committee and strictly adhered to animal protection guidelines. Twenty specific pathogen-free (SPF) 3-month-old New Zealand female rabbits and 30 SPF one-month-old SD rats were provided by the Animal Experimental Center of Fudan University. The animals were reared in an environment with an indoor temperature of 20 °C-25 °C and a relative humidity of 40%-50%. The experimental rabbits were fed by dedicated researchers with a quantitative diet and water intake each day. The animal experiment license number was SYXK (Shanghai) 2018.0003.
After an intraperitoneal injection of 30 mg/kg of pentobarbital sodium to anesthetize the New Zealand rabbits, an approximate 8 cm longitudinal incision was made in the middle of the bilateral posterior thigh. Blunt isolation of the posterior thigh muscles was then performed to find the deep sciatic nerve. The soft tissue around the sciatic nerve was freed, a nerve with a length of approximately 8 cm was selected and its far and near ends were tied together with silk thread. The nerve between the ligatures was cut with a sharp knife to remove it. After drying, the peripheral epineurium was mechanically separated. The 8 cm nerve was divided into two 4 cm long segments and stored at -80 °C. The nerve samples were transferred to a freeze-drying container pre-cooled to -56 °C, freeze-dried for 48 hours, rewarmed to 37 °C, and rinsed. After drying and removing the epineurium residue, the samples were stored at -80 °C.
The nerves were decellularized and the experimental samples were divided into groups 1, 2, 3, and 4, as well as groups A, B, C, and D based on their different lyophilization cycles (with 3 nerves in each group). Of these, groups 1-4 were lyophilized for one cycle and groups A-D were lyophilized for two cycles. Groups 1-4 and A-D were differentiated according to various enzyme treatment times. The specific steps were as follows: (1) Groups 1 and A were treated with serine protease inhibitors to stabilize the neural matrix components for 12 hours, pancreatic enzyme treatment for 5 hours to disrupts cell adhesion to the cell matrix, DNase treatment for 5 hours to destroy the nuclear components, and finally immersed in phosphate buffer saline (PBS) for 48 hours to remove the cellular components. Group 2 and group B were processed in the same manner, with the processing time being 12, 10, 5, and 48 hours, respectively. The treatment time for groups 3 and C were 12, 5, 10, and 48 hours, respectively, whereas that of groups 4 and D was 12, 10, 10, and 48 hours respectively. After drying the acellular scaffolds at a low temperature, 0.9% normal saline containing 5% penicillin (10000 U/mL)/streptomycin (10000 g/L) solution was added to prevent infection. After irradiation and disinfection with γ-rays (15K Gray), the samples were stored at -4 °C for later use.
DNA quantitative detection: DNA was extracted from each group of acellular scaffolds using a TIA Namp Genomic DNA kit for sample DNA quantification. The DNA was quantitated at 280 nm using a spectrophotometer (Thermo Spectronic, Biomate3, Rochester, NY, United States). The DNA was then analyzed using a nucleic acid/protein analyzer (Du800 BECKMAN, United States).
Integrity test: A hydroxyproline test kit was used to measure the collagen content of the scaffold. After hydrolyzing the samples, 1 mL of each sample was dripped onto a 96-well plate, placed in a microplate reader, and the absorbance of each sample was measured at 550 nm. The collagen content was calculated as follows: Collagen content = hydroxyproline content/13.4%.
Hematoxylin and eosin (HE) and luxol fast blue (LFB) staining: Acellular scaffolds were immobilized with 10% paraformaldehyde, dehydrated, paraffin-embedded, and sliced, followed by histological observation. HE staining was performed and the structures, such as cells and myelin sheaths, were observed under a light microscope. The distribution of myelin sheaths was observed by LFB staining. Microscopically, the myelin sheaths of the peripheral nerve scaffolds were dark blue.
Rabbit sciatic nerve acellular scaffolds (7-10 cm) as well as 7-10 cm contralateral normal sciatic nerves (controls), were prepared and labeled individually according to the ptimal decellularization method (per 10 in each group). After in
Twenty-four SD rats were divided into two groups: The first group was a sham operation group and the second group was an operation group, with 12 rats per group. The acellular scaffold was implanted into the right erector spinae with minimal injury. Pentobarbital sodium (30 mg/kg) was injected intraperitoneally for anesthesia. A median longitudinal incision was made along the spinous process notch of the spine, the skin and fascia were cut in turn, and bluntly separated into the erector spinae muscle layer. The right erector spinae was used as the insertion point. The acellular scaffold was fixed with silk threads and embedded into the muscle with minimal damage through round needle gui
According to the time pattern for the post-transplantation rejection reactions in SD rats, three rats in each group were euthanized for local compatibility testing at 3, 7, 21, and 42 days following surgery. The rats in the operation group were dissected, the erector spinae muscle was removed, and fixed in a polyformaldehyde solution for preservation. Paraffin sectioning and HE staining were done to observe the degree of acellular scaffold degradation, inflammatory cell infiltration, and autologous cell migration. Systemic compatibility testing: After euthanizing the rats, three tubes (5 mL each) of blood were collected. After heparin anticoagulation, the samples were sent to the inspection center to test for three inflammatory indicators: Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and IL-6.
Fibroblasts from SD rats were used for cytotoxicity testing. After an intraperitoneal injection of 30 mg/kg of pentobarbital sodium, the rat tail was cut short and the tail skin was removed. The tail and coccyx were cut into a primary culture with a length of approximately 1 cm, placed onto the surface of a petri dish, and washed with DMEM three times. Subsequently, the tissue blocks were cut into 1 mm × 1 mm × 1 mm small pieces, with 2-3 mm spacing between the tissue blocks. After adding a high-sugar DMEM culture solution, the petri dish was incubated at 37 °C in a 5% CO2 incubator. After the tissue blocks were attached to the wall as much as possible (after 4 hours), culture medium was carefully added at a volume that prevented the tissue blocks from floating, with the media slightly over the tissue blocks. The tissue blocks were cultured for 24 hours and the same culture medium was added appropriately according to the decrease in absorption of the culture medium, without allowing the tissue blocks float. The medium was replaced every 3 days. The fibroblasts underwent routine culture, fluid exchange, digestion, and passage. The third generation of fibroblasts was used for the cytotoxicity assay.
The prepared acellular peripheral nerve scaffolds were immersed in a DMEM culture solution to prepare acellular scaffold extracts. The cultured third-generation SD rat fibroblasts were prepared at a concentration of 109/L, suspended, and inoculated into a 96-well plate. DMEM culture solution was added to the culture and incubated for 24 hours in a 5% CO2 cincubator at 37 °C. Once cell adhesion was observed, the remaining culture medium was discarded, and two different concentrations of lyophilized acellular scaffold extracts (50% and 100%) were added. DMEM (200 µL) was added to the negative control group. COne culture plate was removed from the incubator on day 0, 1, 3, 5, 7, and 9 followed by incubation with MTT solution (20 µL/well) for an additional 4 hours. After removing the culture solution, 150 µL of DMSO was added and shaken for 10 minutes. The absorbance value was measured with a microplate reader at 490 nm. The results were calculated by the six-level toxicity grading method for the relative proliferation and toxicity of fi
Healthy umbilical cords were obtained from full-term neonates born in the Obstetric Department of our hospital with the informed consent of their parents. Fresh umbilical cords of full-term neonates were obtained and sterilized. Blood was removed and placed in DMEM/F12 culture dishes to remove arteriovenous vessels. WJ was the remaining tissue after removal of the umbilical artery. The separated WJ tissue was sheared to a particle size of approximately 1 cm3, colla
Determination of the ability of hUC-MSCs to differentiate into osteoblasts: HUC-MSCs were subcultured for three generations and trypsinized (0.25%) to prepare a cell suspension. hUC-MSCs were re-inoculated at a density of 1 × 105/cm2 and cultured in a trypsin zed special MSC medium, which was changed every three days. The degree of cell fusion was observed using an inverted phase contrast microscope. When hUC-MSCs grew to near confluence, an osteogenic induction solution was added, and the cells were stained with alkaline phosphatase on the 7th day after continuous culture. On the 14th day, the mineralized crystals in the culture dish were stained with alizarin red and observed using an inverted phase contrast microscope. Alizarin red staining: After discarding the culture medium, the contents remaining in the petri dish were fixed with paraformaldehyde for 30 minutes, rinsed with PBS, stained with alizarin red for 8 minutes, and rinsed sequentially with distilled water and 0.5% acetic acid. Following dehydration with gradient ethanol, the samples were observed under a microscope.
Identification of the ability of hUC-MSCs to differentiate into adipocytes: The degree of cell fusion was the same as that of ‘Determination of the ability of hUC-MSCs to differentiate into osteoblasts’. When hUC-MSCs were nearly con
Induction into SCs: Third-generation hUC-MSCs were seeded into a 6-well plate at a density of 5 × 105/cm2 to prepare round coverslips. Special MSC culture medium containing 1 mmol/L β-mercaptoethanol was added. Once the cells were confluent, a pre-induction solution (DMEM medium, 10% FBS, and 35 ng/mL of all-trans retinoic acid) was added for 72 hours. After washing with 0.01 mol/L of PBS three times, an induction solution (MSC medium, 5 mmol/L Forskolin, 10 ng/mL bFGF, 5 ng/mL PDGF, and 200 ng/mL of heregulin) was added. The medium was replaced every three days and the cells were identified on day 7.
Identification after differentiation: After 7 days of induction and culture, the cells were placed under an inverted phase contrast microscope to evaluate their growth status and morphological changes. The induced MSCs were fixed with 4% paraformaldehyde for 30 minutes and washed three times with PBS. To eliminate endogenous peroxidase in cells, a peroxidase-blocking solution was added for 20 minutes followed by incubation with goat serum working solution. Three monoclonal antibodies (MBP, GFAP, and S100) were added separately and incubated overnight at -4 °C FITC and TRITC secondary antibodies were added, respectively, and incubated for 2 hours at 37 °C. The cells were observed by fluo
MPZ expression: When intracellular cAMP increases to a certain level, true SCs can differentiate into myelin-forming cells and express myelin protein 0 (MPZ, or p0). Therefore, based on the published method[12], the level of cAMP in the induced cells was measured by the FSK method, and the expression of MPZ was assessed by immunofluorescence. The treated cells were observed under a fluorescence microscope to determined whether there were any cells with positive MPZ expression.
Co-culture with PC12 cells: When PC12 cells were co-cultured with true SCs, which can induce and accelerate PC12 cell differentiation and form myelin sheath structures with PC12 neurites[13]. Therefore, we used PC12 to assess the myelination capacity of SC-like cells after induction and used transmission electron microscopy to observe and document the formation of myelin.
The data were analyzed using SPSS 26.0 software. A two-tailed Student’s t-test or one-way analysis followed by Tukey’s multiple comparisons post hoc test were used to determine statistical significance. All continuous variables for normality were tested and expressed as the mean ± SD. A P value of P < 0.05 (two-tailed) was considered statistically significant.
DNA content and collagen content were used to detect the degree of cell elution, and HE staining was used to observe the integrity of the scaffold. The decellularization method we tried remains a decellularization protocol that combines instrumental isolation, lyophilization and enzymatic methods and is detergent-free. After different cycles of lyophilization and application of different reagent times, we obtained eight scaffolds with different degrees of decellularization and different degrees of integrity. Figure 1 shows the residual DNA content in samples from the 8 groups following the various decellularization protocols (Group 1: 0.5560 ± 0.02, Group 2: 0.3552 ± 0.03, Group 3: 0.4913 ± 0.02, Group 4: 0.3406 ± 0.02, Group A: 0.2705 ± 0.02, Group B: 0.0525 ± 0.01, Group C: 0.0376 ± 0.01, Group D: 0.0358 ± 0.01), indicating a statistically decreased DNA content in each group compared with that in the control group (1.4294 ± 0.04). The decrease was the most significant in groups B-D, whereas no significant inter-group difference was observed in the DNA content between groups C and D (P = 0.783). Overall, the collagen content in the acellular scaffold decreased with extension trypsin and DNase digestion time (Group 1: 49.65 ± 1.62, Group 2: 52.62 ± 1.26, Group 3: 42.07 ± 2.73, Group 4: 49.06 ± 2.37, Group A: 50.24 ± 0.99, Group B: 42.42 ± 4.36, Group C: 49.20 ± 2.91, Group D: 45.25 ± 2.88). However, the collagen content of groups 1, 2, 4, A, and C showed no significant difference compared with the control group. Thus, for the preparation plans, we selected the method with the most complete degree of decellularization and preservation of the scaffold. Through this comparison, we proceeded with the decellularization method of group C.
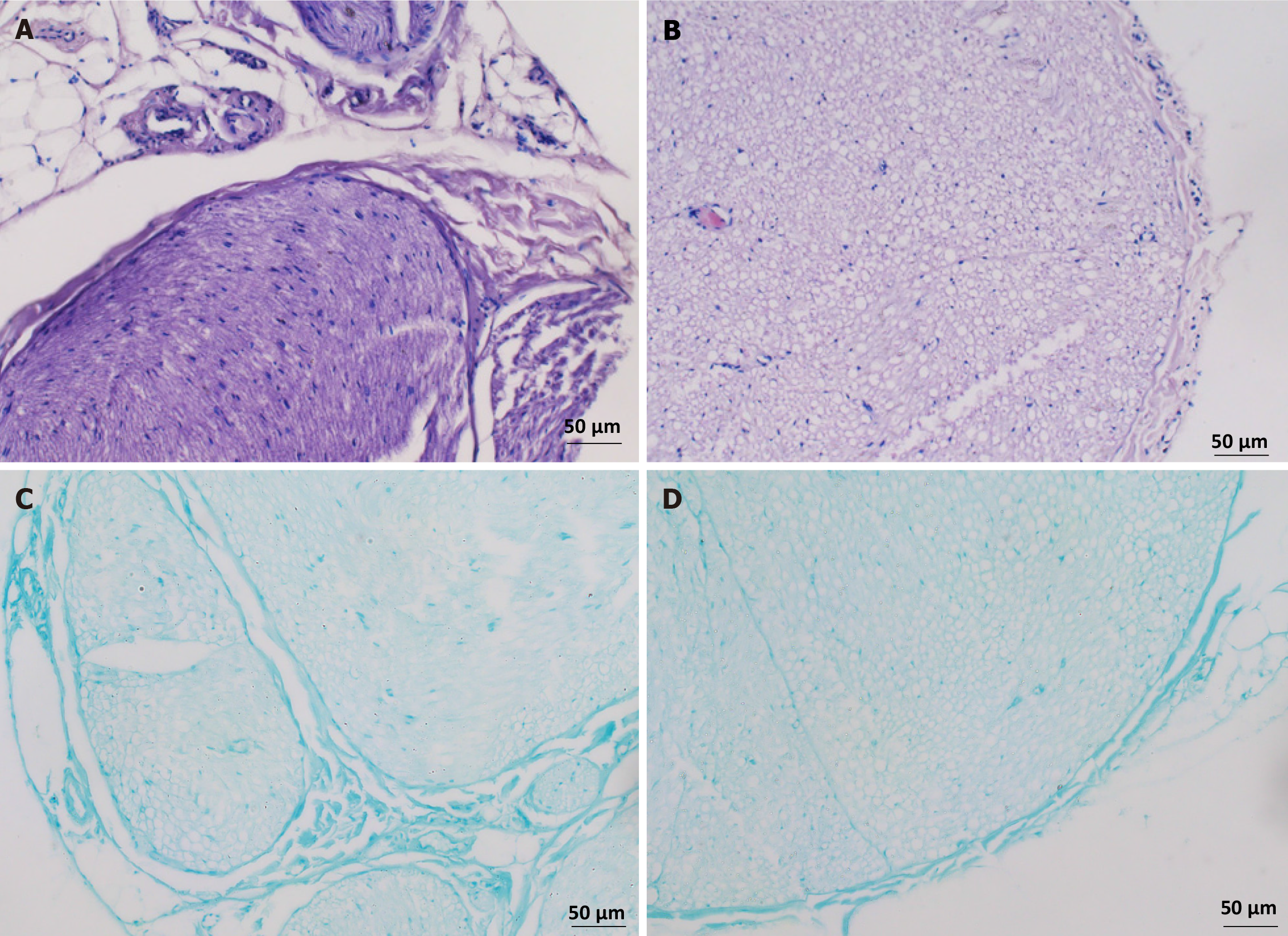
After HE staining, we found that the structure of the acellular peripheral nerve scaffold exhibited a regular tubular structural arrangement (Figure 2A). In the longitudinal section, the structure of the peripheral nerve was almost identical to that of normal peripheral nerves. After depleting the SCs and the cytoplasmic components of the neuron cells, the structure of the peripheral nerve neurolemma was nearly intact. At low or high magnification, little staining of the nuclei was observed (Figure 2B).
The peripheral nerve myelin sheath was visible and enveloped around the central nerve fibers by LFB staining (Figure 2C). After decellularization, myelin staining of the peripheral nerves was attenuated, but the pore structure of the central nerve fibers was preserved (Figure 2D). These data indicate that after mechanical separation of the outer nerve membrane, lyophilization and reheating (2 cycles), five-hour pancreatic digestion, and ten-hour DNase digestion, a tissue-engineered peripheral nerve scaffold with complete acellular and extracellular matrix preservation was prepared.
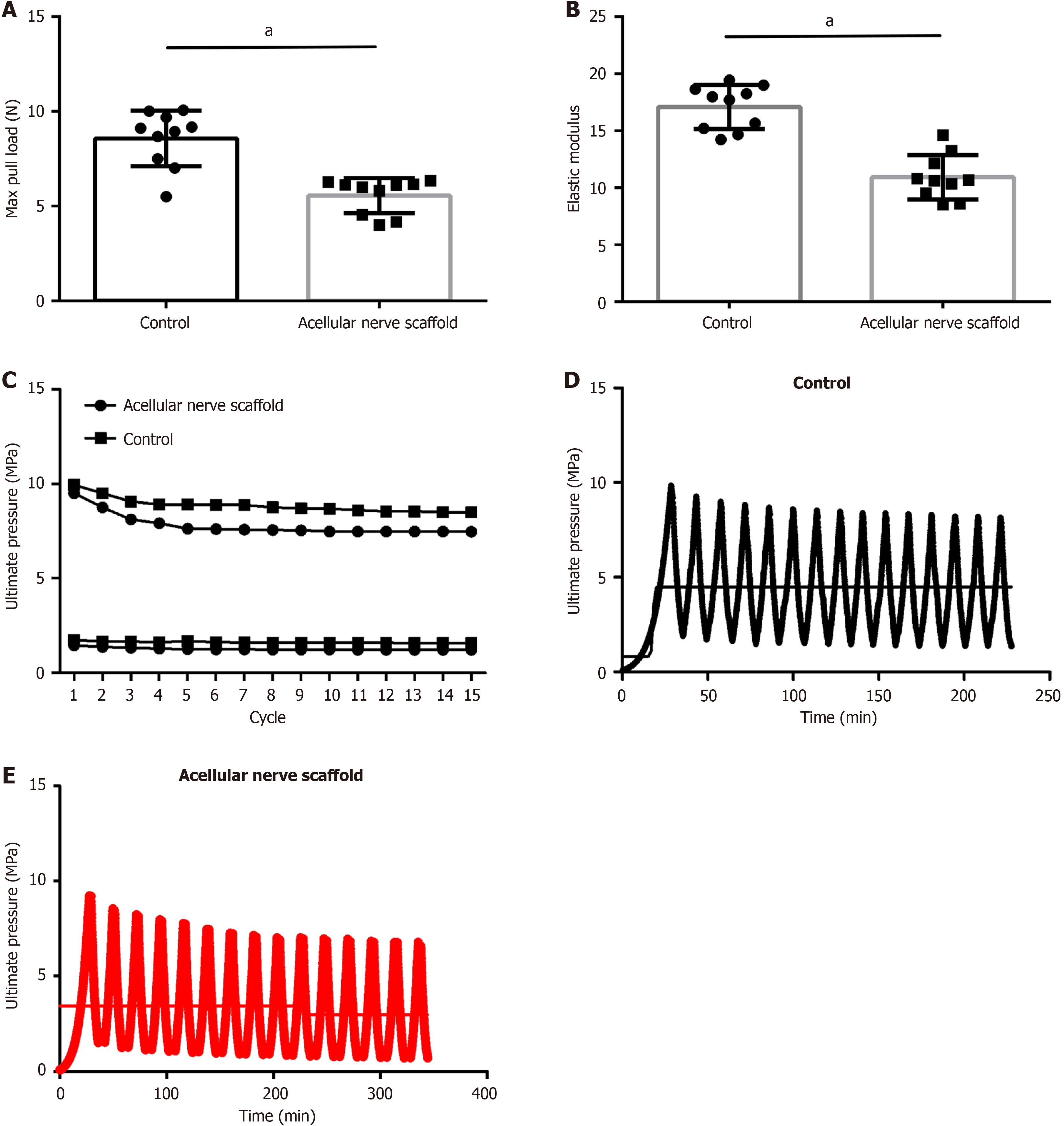
To examine the mechanical properties of acellular scaffolds, we performed measurements on durability, elastic modulus, and maximum tension of the acellular scaffolds. The results indicated that the maximum tensile strength of the sciatic nerve in normal rabbits reached an average of 9.007 ± 3.07 N, whereas that of the acellular rabbit nerve scaffold decreased significantly (current average maximum tensile strength: 6.005 ± 1.82 N, P < 0.05, Figure 3A). The elastic modulus of the control group was 16.75 ± 4.91 MPa compared with 10.91 ± 3.31 MPa for the acellular scaffold. Therefore, the tensile strength that the acellular scaffold can withstand was significantly smaller compared with that in the normal nerve tissue and its elastic modulus was also lower (Figure 3B).
A total of ten scaffolds and nerves were tested. The stress required for maintaining 2% of the sample length during stretching in the control group did not show a significant decrease after removing the viscoelastic effect in the first cycle, which indicated good durability of the sciatic nerve in the control group. However, two samples in the acellular scaffold group experienced breakage during the 7th and 11th cycles. The stress of the remaining eight samples continued to decrease for five cycles while maintaining a 2% tensile length before it began to plateau (Figure 3C). Figures 2E and 3D shows the test cycles for sample No. 3 in the control group and sample No. 2 in the acellular scaffold group, respectively.
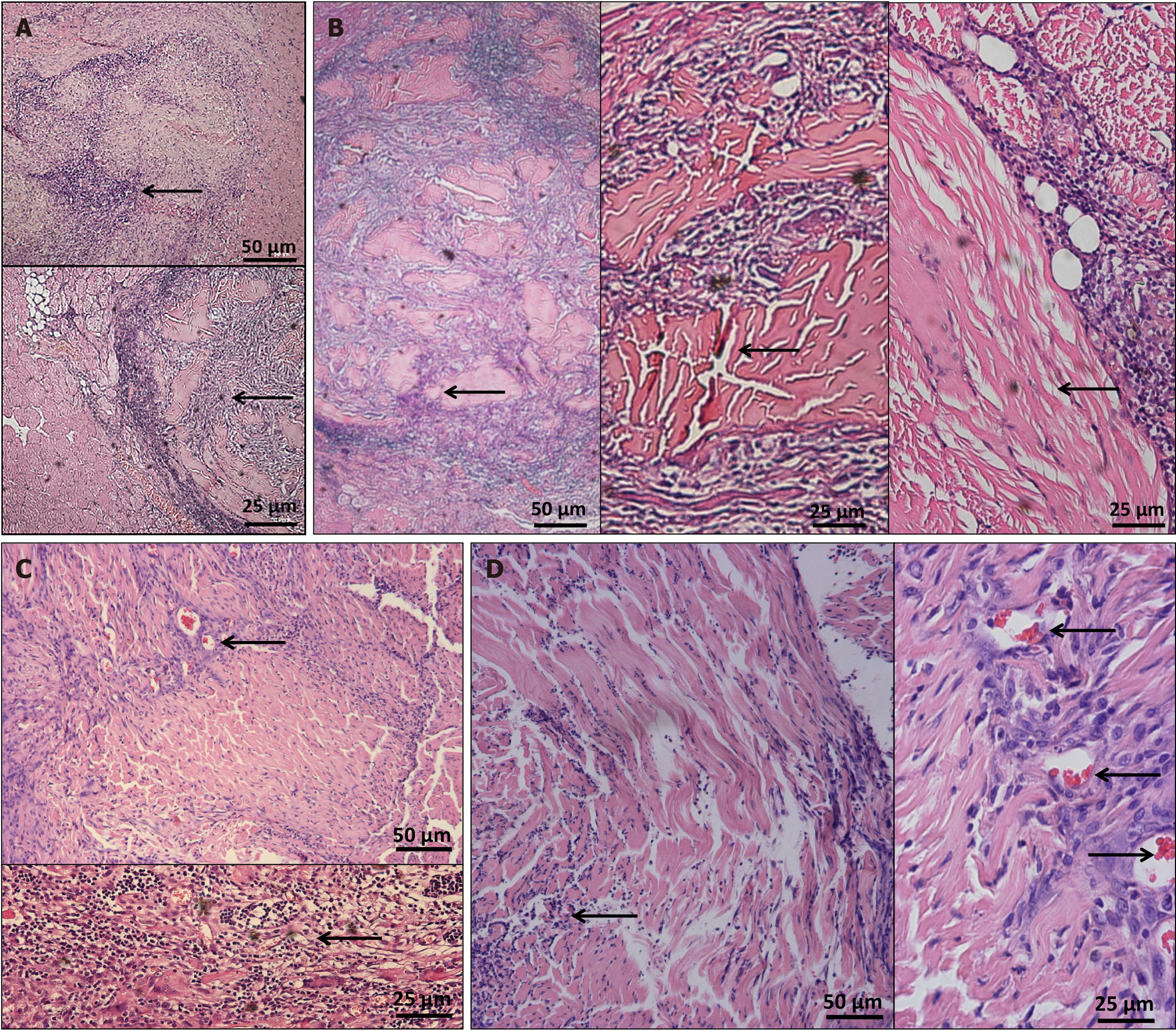
To test the histocompatibility of acellular scaffolds, we compatibilized the scaffolds with SD rat tissues and tested their compatibility on the 3rd postoperative day. A large number of inflammatory cells began to appear around the scaffold, including those surrounding the scaffold and spreading toward the middle. The overall shape of the scaffold could still be recognized; however, the pore structure associated with the original scaffold could not be identified (Figure 4A). On the 7th day following surgery, the number of inflammatory cells around the scaffold was further increased and the scaffold was further enveloped by the surrounding inflammatory cells, with some degradation compared with that on the 3rd day. In areas with relatively few inflammatory cells, the pore structure of the scaffold could be distinguished (Figure 4B). Three weeks after the operation, the inflammatory cells around the scaffold began to fade. Although the scaffold was degraded to a certain extent, its continuous morphology was maintained and the proliferation of connective scar tissue was observed around the scaffold (Figure 4C). Six weeks following the surgery, the inflammatory reaction further subsided and the shape of the scaffold was preserved. Neovascularization was observed at the scaffold–muscle junction (Figure 4D).
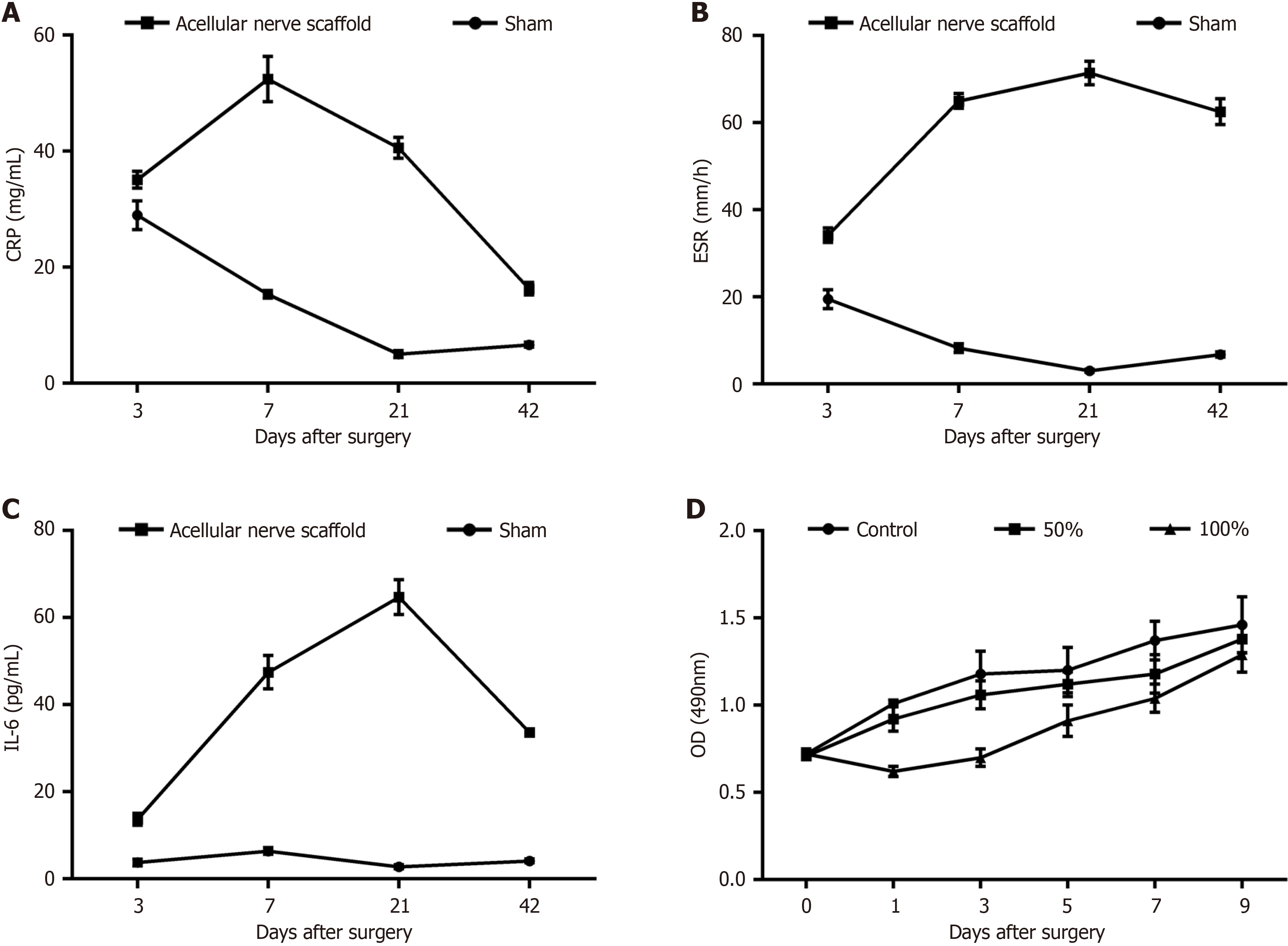
In this step of the study, we will compare the mechanical properties of this scaffold with those of nerve scaffolds synthesized from other materials to fully evaluate the advantages and disadvantages of lyophilized decellularized scaffolds. Autologous grafts of nerves do not undergo rejection, allogeneic grafts need to be transplanted either by comparing antigens or after treatment to remove antigens, whereas inter-allogeneic grafts are more likely to undergo rejection, and the grafts generally do not survive in the recipients without special treatment. Thus, we tested systemic compatibility. SD rats in the sham operation group and the acellular scaffold group showed increased CRP levels 3 days after surgery, whereas the levels in the sham operation group returned to normal 1 week following surgery. In contrast, CRP levels in the acellular scaffold group increased 7 days after surgery, but did not exhibit a downward trend until 3 weeks after surgery, and essentially returned to normal levels 6 weeks following surgery (Figure 5A).
Postoperatively, the ESR in the sham operation group did not change, but fluctuated slightly from 3 days to 1 week after surgery. However, ESR in the acellular scaffold group began to rise continuously one week after surgery, but did not show a downward trend until the last test (Figure 5B).
There were no significant postoperative changes in IL-6 levels in the sham operation group. In the acellular scaffold group, IL-6 began to increase at 1 week after surgery, peaked at three weeks, and began to decline at 6 weeks following surgery (Figure 5C).
The A490 values measured on days 0-9 are shown in Figure 5D. Except for a decrease in the cell proliferation rate after 1 day of cultivation with 100% concentration of the extract, the toxicity of the extract in each group at other time points did not inhibit cell growth compared with the negative control group. On the 9th day of cytotoxicity testing, both groups of cells performed well at different concentrations of nerve scaffold extracts, with positive proliferation. The results indicated that there was no significant difference in cell proliferation following treatment with 50% extract compared with the control group (P > 0.05).
Above all, the lyophilized decellularized scaffolds in this experiment showed excellent anti-degradation properties and histocompatibility: the recipients were eventually able to tolerate the implantation of the scaffolds, the local reactions in the recipients after xenografting were not dramatic, the inflammatory reactions began to diminish at 6 weeks after the procedure, and the scaffolds ultimately retained a relatively intact continuity.
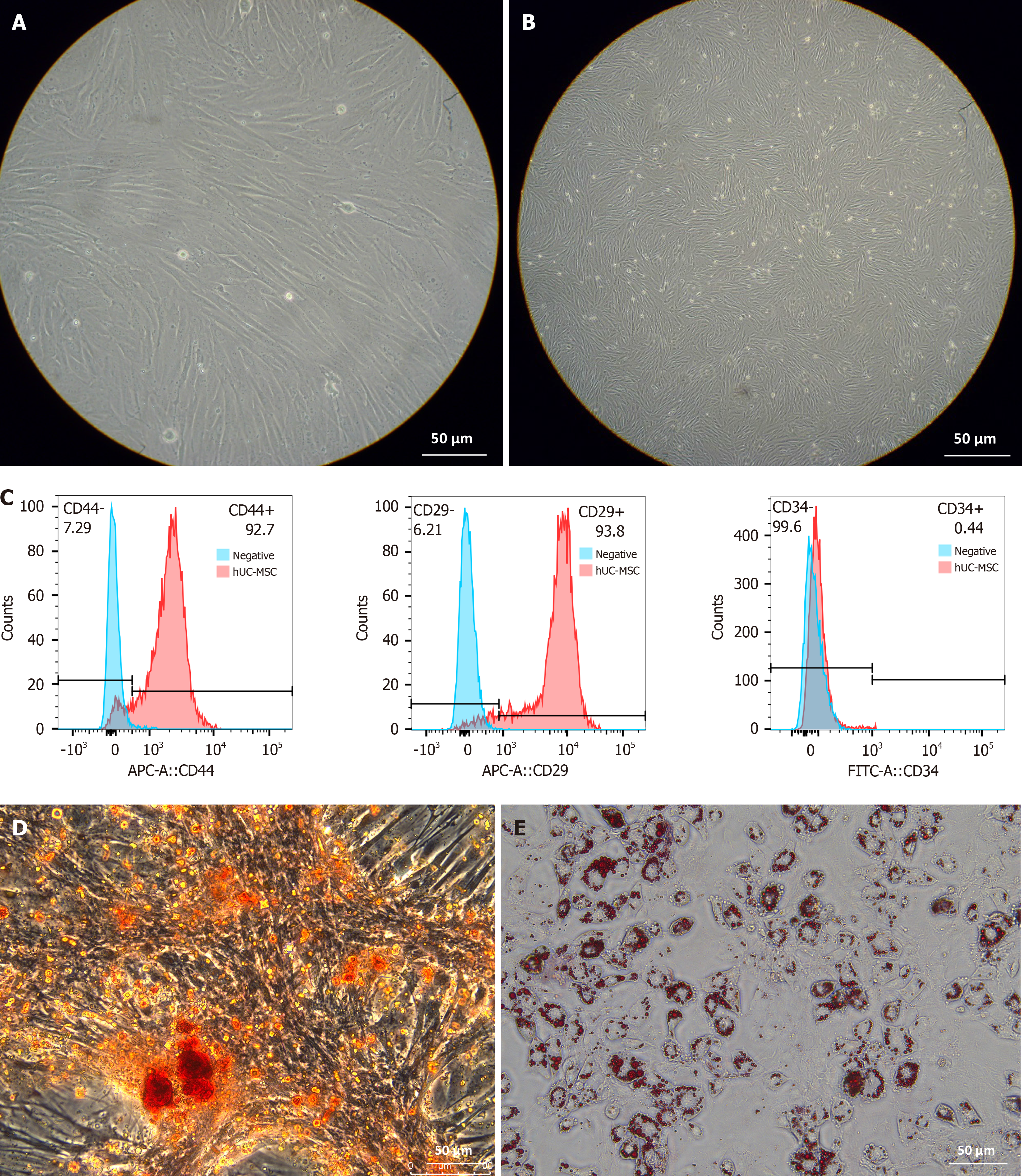
To further construct seed cells, we cultured hUC-MSCs. After six days of culture in the special MSC culture medium, the growth of round-like cells was observed under an inverted phase contrast microscope. After approximately one week of culture, the cells were adhering to the wall (Figure 6A). After passaging, no significant differences were observed in the cells microscopically. After 14 days of cultivation, the cells had fused into clusters were arranged in a spindle shape, with no significant differences in cell morphology (Figure 6B). Isolated and cultured hUC-MSCs were identified, and positive results of hUC-MSC surface markers CD29 and CD44 and a negative result of CD34 were obtained (Figure 6C), indicating successful isolation of hUC-MSCs. After continued culture of the hUC-MSCs with osteogenic induction solution, the morphology of the cells showed polygonal and oval changes. After 2 weeks of cultivation, obvious extracellular mineral crystals were observed following alizarin red staining (Figure 6D). This indicates that hUC-MSCs have the ability to differentiate into osteoblasts. After hUC-MSCs were continuously cultured with an adipogenic induction solution, the morphology of the cells was altered and lipid droplets with varying refractive indices were evident in the culture me
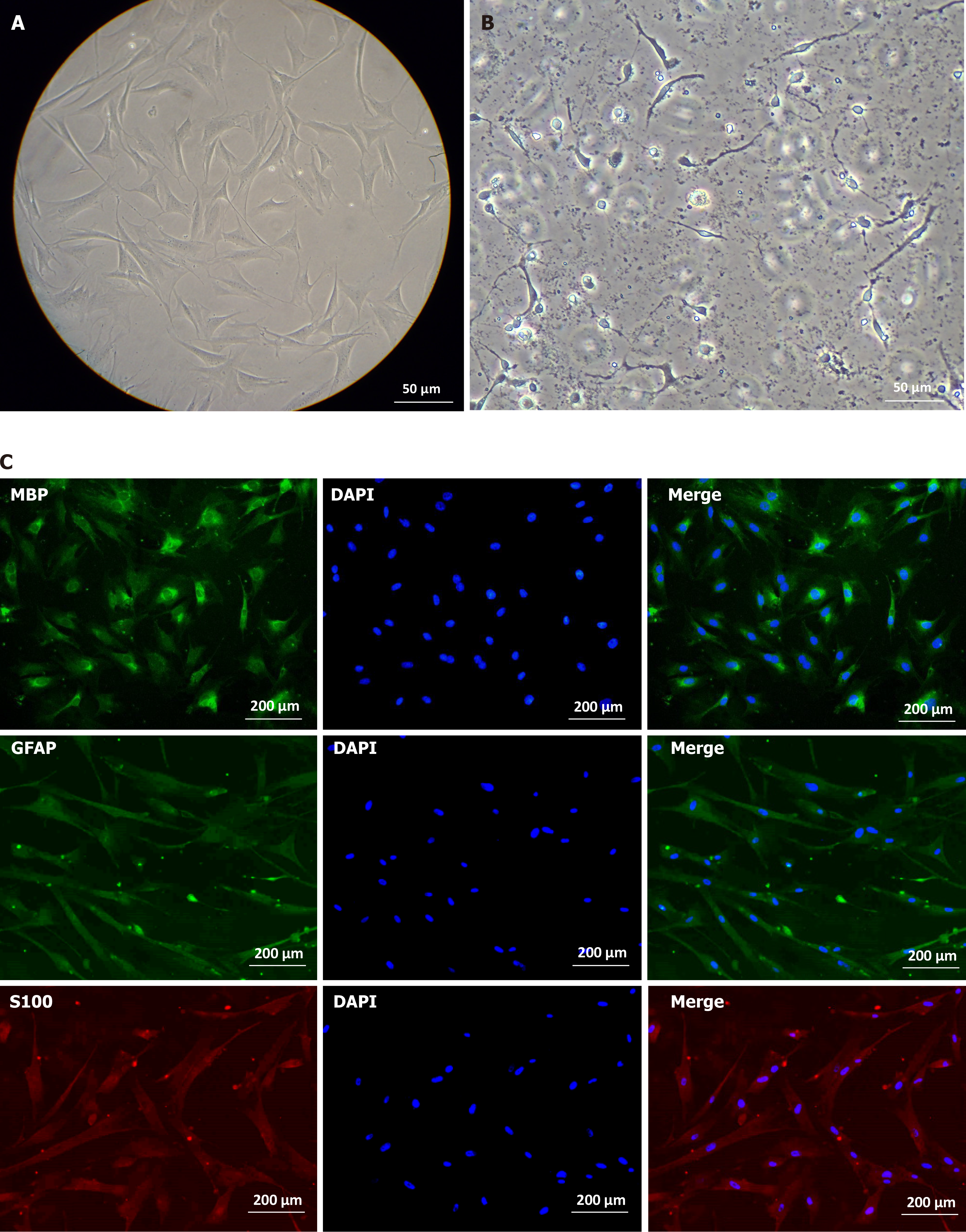
After induction, we observed that the cell morphology began to change approximately 24 hours after the addition of the inducer, and gradually became slender from the initial spindle shape (Figure 7A) to form a long-shuttle shape similar to SCs on the 7th day (Figure 7B). Immunofluorescence revealed positive expression of MBP, GFAP, and S100 (78.4%, 92.9%, and 77.6%, respectively) in differentiated SC-like cells (Figure 7C). These results indicate that hUC-MSCs differentiate into SCs after induction culture.
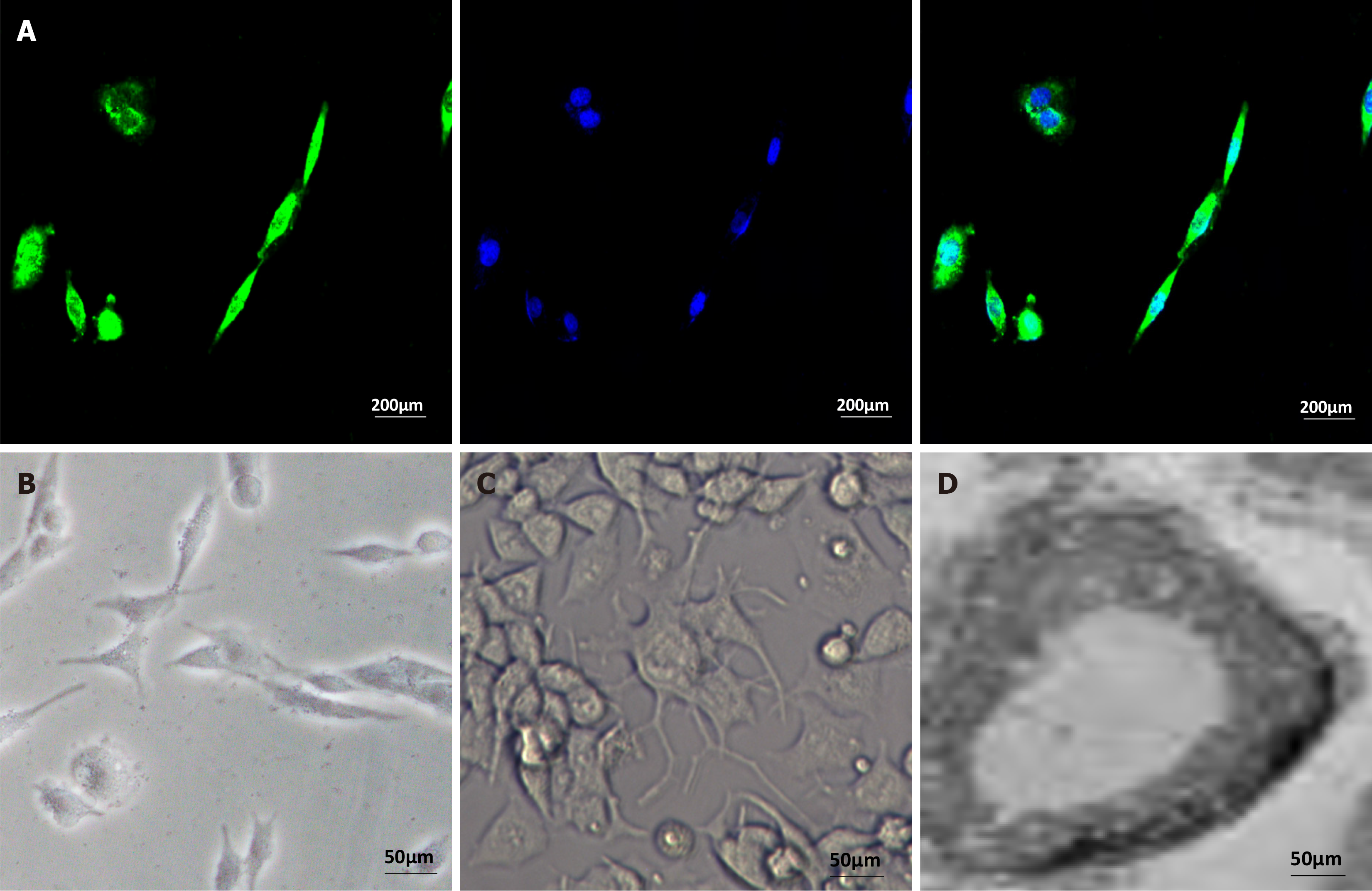
We confirmed whether hUC-MSC-differentiated SC-like cells have myelin-forming capacity. The medium for inducing differentiated cells was replaced with an MPZ induction medium to enhance intracellular cAMP levels. After seven days of culture, some SC-like cells exhibited a positive immune response to p0 (Figure 8A) and some MPZ-positive cells showed changes, such as irregular shape and swelling, which may be the result of cell membrane expansion caused by the secretion of myelin-like substances. These results suggest that SC-like cells differentiated from hUC-MSCs have a myelination ability (Schwann-like cells).
When subculturing PC12 cells separately, only some stretching processes in PC12 cells were observed by microscopy (Figure 8B). Next, we co-cultured PC12 and SC-like cells in the SC medium for 14 days. PC12 rapidly differentiated into neuron-like cells after the addition of SC-like cells, whereas PC12 with obvious neurites were observed (Figure 8C). After 14 days of co-culture, transmission electron microscopy revealed that the SC-like cells formed a layered myelin structure with PC12 neurites (Figure 8D). The results also indicated that SC-like cells differentiated from hUC-MSCs have a myelination ability. Therefore, we found that when differentiated hUC-MSCs (Schwann-like cells) were co-cultured with PC12 cells, the Schwann-like cells could induce PC12 cells to rapidly differentiate and form myelin structures with PC12 cell neural protrusions.
Thus far, various methods of decellularization have been developed to make acellular scaffolds for peripheral nerves in the field of tissue engineering. Sondell et al[3] used allogenic acellular peripheral nerves as transplants for the first time. They used Triton X-100 and SDC to decellularize the sciatic nerves of SD rats. Compared with Sondell’s method, the detergent Triton-200 can better preserve the extracellular matrix of the scaffold[4]. Considering that residual detergents are cytotoxic and can penetrate cells, researchers have tried to reduce or remove such detergents using hyperosmotic methods for cell washing[14,15] and apoptosis for cell removal[16,17].
In the present study, we used a decellularization scheme that combines instrument separation, lyophilization, and an enzyme method without detergents. After various cycles of lyophilization and application of reagents for different times, we obtained eight scaffolds with varying degrees of decellularization and integrity. After the identification of DNA and collagen on the scaffold, we found the most suitable peripheral nerve decellularization method among the eight protocols. Lyophilization-rewarming of peripheral nerves can effectively destroy the cell membrane structure of myelin and neuronal cells, whereas DNase and pancreatic enzymes can effectively act following cell membrane destruction and has a similar role as detergents. In addition, the successful preparation of peripheral nerve scaffolds demonstrated the convenience of lyophilization and decellularization for organ preparation. For identification after scaffold preparation, we were satisfied to find no staining of the myelin sheath, neurons, and other cell nuclei in the acellular scaffold by HE staining. In addition, a porous structure remained between the perineurium and the endoneurium, which is conducive to nerve repair, neurotrophic factor transmission, and cell excreta transport. LFB staining revealed that after various steps of decellularization, the myelin tissue that was originally filled with nerves and the SCs that comprised the myelin sheath were significantly reduced. This indicates that the myelin tissue of the acellular scaffold was completely washed, which meets the criteria for clinical use of peripheral neural scaffolds.
The performance evaluation of acellular scaffolds includes many aspects, including their biomechanical properties, pore size, degradation performance, histocompatibility, and cell tropism[18]. A good tissue substitute must meet most of the above requirements to achieve better substitution effects. We tested the mechanical properties of the acellular sca
Following the successful fabrication of the acellular scaffold, we considered the repair of the nerve. SCs are constituent cells of the myelinated nerve fiber myelin sheath, which are widely present in the peripheral nervous system of ver
In summary, by combining lyophilization and enzymatic digestion, we successfully prepared acellular peripheral nerve scaffolds with complete cell depletion, intact matrix preservation, good histocompatibility between different species, and extremely low cytotoxicity. In addition, hUC-MSCs can differentiate into Schwann-like cells with myelinogenic ability after in vitro induction. However, there were some limitations to this study. It was the first attempt to prepare peripheral nerve scaffolds. Although seed cells more suitable for neonates were found, neonatal animal models or models with neonate-specific PNI were not used. In follow-up experiments, it will be necessary to prioritize the production of neonatal and animal models of obstetrical palsy before further research on treatment.
| 1. | Hale HB, Bae DS, Waters PM. Current concepts in the management of brachial plexus birth palsy. J Hand Surg Am. 2010;35:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Wang Q, Zhang C, Zhang L, Guo W, Feng G, Zhou S, Zhang Y, Tian T, Li Z, Huang F. The preparation and comparison of decellularized nerve scaffold of tissue engineering. J Biomed Mater Res A. 2014;102:4301-4308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 208] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 277] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Mahdian M, Tabatabai TS, Abpeikar Z, Rezakhani L, Khazaei M. Nerve regeneration using decellularized tissues: challenges and opportunities. Front Neurosci. 2023;17:1295563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Buckenmeyer MJ, Meder TJ, Prest TA, Brown BN. Decellularization techniques and their applications for the repair and regeneration of the nervous system. Methods. 2020;171:41-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. Mechanisms and roles of axon-Schwann cell interactions. J Neurosci. 2004;24:9250-9260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Jessen KR, Mirsky R. Why do Schwann cells survive in the absence of axons? Ann N Y Acad Sci. 1999;883:109-115. [PubMed] |
| 9. | Kangari P, Talaei-Khozani T, Razeghian-Jahromi I, Razmkhah M. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther. 2020;11:492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 10. | Wang H, Jia Y, Li J, Liu Q. Schwann cell‑derived exosomes induce bone marrow‑derived mesenchymal stem cells to express Schwann cell markers in vitro. Mol Med Rep. 2020;21:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Bojanic C, To K, Zhang B, Mak C, Khan WS. Human umbilical cord derived mesenchymal stem cells in peripheral nerve regeneration. World J Stem Cells. 2020;12:288-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991;112:457-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 290] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Keilhoff G, Stang F, Goihl A, Wolf G, Fansa H. Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol Neurobiol. 2006;26:1235-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Porzionato A, Stocco E, Barbon S, Grandi F, Macchi V, De Caro R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 15. | Kim JK, Koh YD, Kim JO, Seo DH. Development of a decellularization method to produce nerve allografts using less invasive detergents and hyper/hypotonic solutions. J Plast Reconstr Aesthet Surg. 2016;69:1690-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Cornelison RC, Wellman SM, Park JH, Porvasnik SL, Song YH, Wachs RA, Schmidt CE. Development of an apoptosis-assisted decellularization method for maximal preservation of nerve tissue structure. Acta Biomater. 2018;77:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Vasudevan S, Huang J, Botterman B, Matloub HS, Keefer E, Cheng J. Detergent-free Decellularized Nerve Grafts for Long-gap Peripheral Nerve Reconstruction. Plast Reconstr Surg Glob Open. 2014;2:e201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Echeverria Molina MI, Malollari KG, Komvopoulos K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front Bioeng Biotechnol. 2021;9:617141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 19. | Siemionow M, Sonmez E. Nerve allograft transplantation: a review. J Reconstr Microsurg. 2007;23:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Bosch-Queralt M, Fledrich R, Stassart RM. Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis. 2023;176:105952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 132] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 21. | Monje PV. Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/