Published online Dec 26, 2024. doi: 10.4252/wjsc.v16.i12.1002
Revised: October 17, 2024
Accepted: November 22, 2024
Published online: December 26, 2024
Processing time: 104 Days and 19.7 Hours
Extracellular vesicles (EVs) are cell-to-cell interaction tools that are attracting increasing interest in the literature in two opposing areas. In addition to their role in physiological development, there is growing evidence of their involvement in healing and protective processes. However, EVs also mediate pathological con
Core Tip: An increasing number of studies in the literature have focused on the role of extracellular vesicles (EVs) in the progression of several diseases, particularly neurodegenerative diseases, in which EVs are presumed to transfer pathological molecules to normal cells. Nevertheless, many therapeutic strategies focus on the use of EVs to deliver prosurvival factors; however, apparent discrepancies are noted. In this review, we focused on neurodegenerative diseases to shed light on the dual role that EVs play and explored, in particular, the potential therapeutic role of stem cell-derived EVs.
- Citation: Scuteri A, Donzelli E. Dual role of extracellular vesicles in neurodegenerative diseases. World J Stem Cells 2024; 16(12): 1002-1011
- URL: https://www.wjgnet.com/1948-0210/full/v16/i12/1002.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i12.1002
The ability of cells to interact and communicate with each other is essential for their survival and for the correct execution of their functions. Based on this premise, an increasing number of studies in the literature have focused on alterations in cellular interactions as the initiating mechanism of different types of diseases. Among the different communication mechanisms, such as gap junctions and tunneling nanotubes[1], extracellular vesicles (EVs) have attracted increasing interest in recent years, with considerable evidence of the involvement of these structures both in common physiological processes (cell maintenance and survival, myelin formation, neurite elongation, and cellular aging) and in the deve
EVs are vesicular structures delimited by a lipidic layer and are unable to replicate[6]. In a thorough review, Couch et al[7] described the discovery of these structures, which are now widely studied. Starting from the first report of their existence as a particulate fraction during blood clotting experiments, in approximately 1940[8], Couch et al[7] reported different hypotheses on these structures over the years, during which the scientific community gained awareness of their importance in an incremental fashion. From the first hypothesis on their possible role as simple cargo systems for molecules[9], until the current evidence of a dynamic communication system available to the cell was reached, changes in the extracellular environment and proper reactions were detected by adjusting the EV content[1,10,11]. Current gui
As putative carriers of this wide range of molecules, as stated before and according to several papers, EVs can play important roles in physiological processes in several tissues. In the cardiovascular system, they seem to be involved in blood pressure regulation via the transport of vasoactive molecules[16,17]. In the kidney, EVs can transport aquaporin-2, therefore regulating water balance. In the nervous system, they are involved in brain development[17,18]. The ability that makes these structures so interesting is their role in the development of pathological features[16], particularly for those diseases characterized by the propagation of a pathological protein, such as neurodegenerative diseases, which this review is focused.
Many diseases of the nervous system, although characterized by different cellular targets and symptoms, share a common pathological feature, that is, the accumulation of altered proteins, which acquire a toxic function[19-22], as observed in Alzheimer’s disease (AD)[23] with β-amyloid and tau; α-synuclein in Parkinson’s disease (PD)[15] or huntingtin in Huntington’s disease (HD); and superoxide dismutase 1, TDP43 and Fus in amyotrophic lateral sclerosis[24,25]. This toxic content inside cells quickly spreads to other normal cells, leading to the progressive poisoning of the entire cell population, with the loss of a particular type of cell and overall loss of its function. This type of error propagation involves viral-like diffusion or, rather, prion-like spread, with misfolded proteins inducing alterations in normal proteins[21]. Given that cellular communication is the basis of this form of diffusion, many studies have focused on interaction structures, particularly on EVs, as a mechanism to propagate a “toxic” factor. Eitan et al[26] suggested that EV content could cause neuronal damage. Specifically, EVs derived from AD patients or animal disease models, which are therefore considered pathological, can increase and prolong the Ca2+ response to glutamate; moreover, they can reduce both basal and maximal mitochondrial respiration and ATP levels. Thus, these EVs can alter both Ca2+ homeostasis and mi
The main question arising from these studies revolves around the role of EVs: Are they merely passive mirrors reflecting what happens inside the cell and serving as cargo systems hijacked by pathogenic cells to spread their altered content? Alternatively, could they be considered the triggering factor of the disease? The majority of the literature supports the first hypothesis because of the presence of many peculiar neurotoxic proteins inside EVs. Poehler et al[31] reported that some mutations associated with early PD onset, namely, those in the SNCA gene, promote both α-synuclein aggregation and its accumulation in EVs, which are then secreted and transferred to other cells that start to show fibril aggregation[31]. Likewise, Zhang et al[30] reported the presence of expanded CAG triplets and HD-associated proteins in EVs, whereas Aβ-amyloid peptides have been found in AD patient-derived EVs[26]. In addition to the propagation of misfolded proteins, an unfavorable environment could also alter the EV content, thus further worsening cellular stress. Jeske et al[32] reported that the EV content could change in response to inflammatory stimuli, such as lipopolysaccharide or tumor necrosis factor-α, as well as stress stimuli, thus highlighting a detrimental role for EVs. Given that neuroinflammation is a hallmark of neurodegenerative diseases, this could also determine a pathological change in EV content.
EVs can be exchanged not only between neurons but also between neurons and glial cells[20]. The role of EVs derived from different types of glial cells has been identified as pivotal for neuroimmune communication, the regulation of neuron survival and excitability, and neurite elongation[33] in several experimental models of neurodegenerative di
In addition, other authors have suggested an alternative point of view. Starting from the evidence of the presence of altered/misfolded proteins inside EVs, Hill[14] considered the process as an ultimate attempt of the cell to clear the altered proteins. The pathological contents of EVs should not necessarily be transported but rather seized to eliminate undesired/dangerous proteins[38]. In this context, EVs should represent a protective tool used by the cell. Yuyama et al[38] observed the role of EVs in Aβ-amyloid clearance and reported that their downregulation could be related to disease development. This view apparently contradicts the other views previously cited; however, their role, which is initially protective, could change in particular circumstances, i.e., in aged individuals[39]. Upadhya et al[39] observed a dysregulation in EV production in aged animals, which could be related to a change in their role; however, the exact mechanisms of such a switch remain unsolved. Therefore, although EVs have not been confirmed to trigger neurodegenerative di
If it is certain that EVs play a role in neurodegenerative disease propagation, it is also clear that, because of their features, EVs could be exploited to import every type of molecule into the cell, making them suitable for fighting the same diseases. A possible strategy involves decreasing the number of EVs released, which is associated with disease pro
The idea of a positive role of EVs was raised first by the evidence that some cells actively contribute to correct nervous system development and maintenance through the release of EVs that modulate neuronal functions[41,42]. Oligoden
Despite these interesting observations, endogenous EVs, which may be released during physiological development and homeostasis, clearly fail to protect against damage for the reasons mentioned above. In contrast, the exogenous administration of EVs with a protective effect could better support a therapeutic effect. A limiting factor is, however, represented by the need for a sufficient number of EVs to achieve valid protection, which is not easy to obtain.
The first step to overcome such a problem was recently achieved based on research on EVs paired with that of stem cells. Over recent decades, the protective effect of different types of stem cells has been confirmed in many in vitro and in vivo models, suggesting that the release of rescue factors (such as neurotrophic factors) is a pivotal mechanism. In par
Different authors have highlighted the important role of endogenous EVs in the correct maintenance of neural fun
Several authors have reported that MSC-derived EVs can counteract several alterations responsible for cellular se
MSC-derived EVs have also been demonstrated to be useful in different in vivo models of neurological diseases, such as stroke[56], traumatic brain injury[57], and cisplatin-dependent cochlear damage[58,59]. They also counteract brain aging. For example, Zhang et al[60] administered EVs once a month for 3 months in a mouse model, reporting increased sirtuin 1 expression, as well as decreased apoptosis and ROS levels. In an in vivo model of cisplatin-induced neuropathy, in
Nevertheless, all these encouraging results obtained both in vitro and in vivo are limited by several important critical points. First, the large heterogeneity of the outcomes, mainly due to batch-to-batch variability in MSCs, highlights the current unreliability of this approach, as demonstrated by the small number of clinical trials that have reached phase 3[61]. In addition to variability, a further problem is represented by the large number of MSCs necessary to obtain an amount of EVs sufficient for therapeutic treatment[62]. To overcome these limitations, research has moved from the use of MSCs to the use of iPSCs or, even better, to the use of MSCs derived from iPSCs, so-called iPSC-derived MSCs (iMSCs). The switch to these cells could allow a more effective system to achieve a usable number of EVs, and currently, several protocols can be used to obtain iMSCs[62]. Some iMSCs were derived from embryonic stem cells, which may be cocu
| MSCs | iPSCs | iMSCs | |
| Differentiation potential | Differentiate into the 3 mesengenic lineages | Pluripotent | Do not differentiate into adipose cells |
| Proliferative potential | Low-medium | High | High |
| Immunomodulation | Immunomodulation properties | Immunomodulation properties | Immunomodulation properties |
| Gene signature | Age-related gene pathway | Rejuvenation-associated gene pathway | Age-related gene pathway |
| Variability | Donor and batch-dependent | Single-clone derivation | Single-clone derivation |
| Soluble factor release | Higher vascular development | Delayed effect | Neurological symptoms |
| Safety | No safety issues | Safety issues | Safety issues |
Billing et al[63] demonstrated that MSCs and iMSCs are very similar but not identical. Specifically, iMSCs are more useful for axon support and for reducing multiple sclerosis progression, whereas MSCs better support vascular de
From this starting point, different authors have attempted to modify the content of EVs, with the aim of improving the therapeutic strategy, by functionalizing them to transfer specific molecules (or drugs)[67-70]. Moreover, several tech
To date, some clinical trials have focused on EV use in the context of neurological diseases, although the majority have proposed EVs as biomarkers for disease detection (http://www.clinicaltrials.gov). In fact, regardless of whether their role is detrimental or defensive, some molecules are present inside EVs, and their presence is certainly attributed to the disease. This content paves the way for the use of these structures as “predictors”, which are suitable for the early detection of diseases using a minimally invasive method given that EVs are present in almost every body fluid, such as blood, urine, or saliva[76], and noticeable improvements in the current diagnostic techniques, particularly for neurological diseases. Furthermore, since the EV content may change during different stages of the disease, EVs could also be used as prognostic biomarkers or, at least, to classify the disease stage[67].
A alternative approach was offered by You et al[22], who identified some differences among EVs derived from di
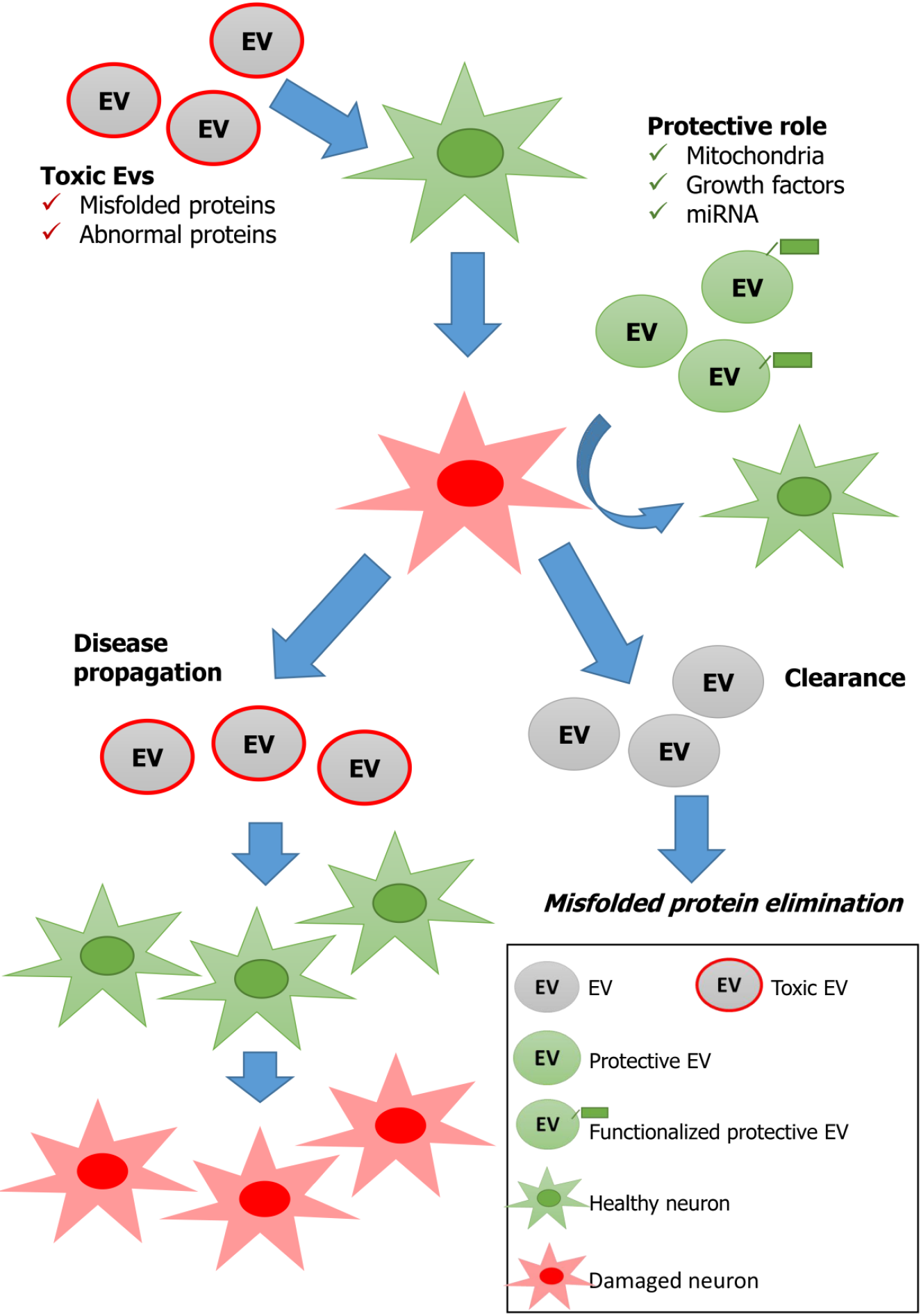
It is important to keep in mind the existence of a dual role of EVs in the context of current scientific research on neurodegenerative diseases, and understanding what could tip the scale toward a protective or detrimental role is fundamental for EV applications in the clinic. It is plausible and realistic to counteract a disease using EVs, which carry key factors, such as growth factors, regulatory miRNAs, antiapoptotic factors, anti-inflammatory factors and antioxidative factors, to support neuronal survival. In addition, EVs can be manipulated, and their content can be adjusted by changing the cellular microenvironment or the stimuli to which the cell is exposed. In any case, with respect to the clinical use of EVs, attention should be given to their putative disadvantages and our limited knowledge of many important parameters, such as the duration of their effects, how to adjust the EV content for different neurodegenerative diseases and, overall, their dual role. Neurodegenerative diseases, even more than other therapeutic areas, could benefit from treatments based on EVs because of their ability to cross the BBB, a peculiarity that could be further addressed through specific modifications of EVs[78,79]. A long list of challenges needs to be addressed in order to obtain robust data, starting from the choice of the best donor cell type for each therapeutic approach and moving toward the establishment of robust protocols for EV production and isolation[79,80]. Progress in EV research, particularly in in vivo studies, could help to improve the potential therapeutic role of EVs. Long-term studies should exclude the possibility that exogenous EVs administered with a therapeutic aim could also be hijacked by the body and turned into Trojan horses. Figure 1 recapitulates the different putative actions of EVs most frequently suggested by the papers considered in this review. The large amount of data presented in the literature should be critically analyzed to standardize culture conditions as well as characterization and extraction methods as much as possible, which may affect the results.
A concluding remark should be made about the EV administration route. Many in vivo studies systemically provide EVs; however, several authors have demonstrated that systemic administration causes retention of EVs in the lungs, with a consequent reduction in their effectiveness[57]. Currently, local administration, particularly intranasal administration, should be employ standardize procedures that reduce EV clearance[81]. In conclusion, once the biological properties of EVs are completely elucidated, they have the potential for use as a disruptive and innovative therapeutic tool for several different diseases.
The authors are thankful to Dr. Silvia Fermi for language revision.
| 1. | Tarasiuk O, Ballarini E, Donzelli E, Rodriguez-Menendez V, Bossi M, Cavaletti G, Scuteri A. Making Connections: Mesenchymal Stem Cells Manifold Ways to Interact with Neurons. Int J Mol Sci. 2022;23:5791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Meldolesi J. Extracellular vesicles, news about their role in immune cells: physiology, pathology and diseases. Clin Exp Immunol. 2019;196:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1394] [Cited by in RCA: 2363] [Article Influence: 337.6] [Reference Citation Analysis (35)] |
| 4. | Yin Y, Chen H, Wang Y, Zhang L, Wang X. Roles of extracellular vesicles in the aging microenvironment and age-related diseases. J Extracell Vesicles. 2021;10:e12154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 5. | Wang J, Li L, Zhang Z, Zhang X, Zhu Y, Zhang C, Bi Y. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022;34:1264-1279.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 6. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 8300] [Article Influence: 1037.5] [Reference Citation Analysis (1)] |
| 7. | Couch Y, Buzàs EI, Di Vizio D, Gho YS, Harrison P, Hill AF, Lötvall J, Raposo G, Stahl PD, Théry C, Witwer KW, Carter DRF. A brief history of nearly EV-erything - The rise and rise of extracellular vesicles. J Extracell Vesicles. 2021;10:e12144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 353] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 8. | Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189-197. [PubMed] |
| 9. | Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2293] [Cited by in RCA: 2759] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 10. | Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4100] [Cited by in RCA: 4019] [Article Influence: 223.3] [Reference Citation Analysis (0)] |
| 11. | Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8246] [Cited by in RCA: 10084] [Article Influence: 530.7] [Reference Citation Analysis (0)] |
| 12. | Poupardin R, Wolf M, Strunk D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv Drug Deliv Rev. 2021;176:113872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Battistelli M, Falcieri E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology (Basel). 2020;9:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 14. | Hill AF. Extracellular Vesicles and Neurodegenerative Diseases. J Neurosci. 2019;39:9269-9273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 305] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 15. | Xia X, Wang Y, Zheng JC. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl Neurodegener. 2022;11:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 16. | Good ME, Musante L, La Salvia S, Howell NL, Carey RM, Le TH, Isakson BE, Erdbrügger U. Circulating Extracellular Vesicles in Normotension Restrain Vasodilation in Resistance Arteries. Hypertension. 2020;75:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Yates AG, Pink RC, Erdbrügger U, Siljander PR, Dellar ER, Pantazi P, Akbar N, Cooke WR, Vatish M, Dias-Neto E, Anthony DC, Couch Y. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology: Part I: Health and Normal Physiology. J Extracell Vesicles. 2022;11:e12151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 18. | Oosthuyzen W, Sime NE, Ivy JR, Turtle EJ, Street JM, Pound J, Bath LE, Webb DJ, Gregory CD, Bailey MA, Dear JW. Quantification of human urinary exosomes by nanoparticle tracking analysis. J Physiol. 2013;591:5833-5842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Yates AG, Pink RC, Erdbrügger U, Siljander PR, Dellar ER, Pantazi P, Akbar N, Cooke WR, Vatish M, Dias-Neto E, Anthony DC, Couch Y. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part II: Pathology: Part II: Pathology. J Extracell Vesicles. 2022;11:e12190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 20. | Li T, Tan X, Li S, Al-Nusaif M, Le W. Role of Glia-Derived Extracellular Vesicles in Neurodegenerative Diseases. Front Aging Neurosci. 2021;13:765395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Shippey LE, Campbell SG, Hill AF, Smith DP. Propagation of Parkinson's disease by extracellular vesicle production and secretion. Biochem Soc Trans. 2022;50:1303-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | You Y, Muraoka S, Jedrychowski MP, Hu J, McQuade AK, Young-Pearse T, Aslebagh R, Shaffer SA, Gygi SP, Blurton-Jones M, Poon WW, Ikezu T. Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer's disease brain. J Extracell Vesicles. 2022;11:e12183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 23. | Yuan Q, Li XD, Zhang SM, Wang HW, Wang YL. Extracellular vesicles in neurodegenerative diseases: Insights and new perspectives. Genes Dis. 2021;8:124-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Bonafede R, Mariotti R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Front Cell Neurosci. 2017;11:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Bonafede R, Turano E, Scambi I, Busato A, Bontempi P, Virla F, Schiaffino L, Marzola P, Bonetti B, Mariotti R. ASC-Exosomes Ameliorate the Disease Progression in SOD1(G93A) Murine Model Underlining Their Potential Therapeutic Use in Human ALS. Int J Mol Sci. 2020;21:3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 26. | Eitan E, Hutchison ER, Marosi K, Comotto J, Mustapic M, Nigam SM, Suire C, Maharana C, Jicha GA, Liu D, Machairaki V, Witwer KW, Kapogiannis D, Mattson MP. Extracellular Vesicle-Associated Aβ Mediates Trans-Neuronal Bioenergetic and Ca(2+)-Handling Deficits in Alzheimer's Disease Models. NPJ Aging Mech Dis. 2016;2:16019-16019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Tofaris GK. A Critical Assessment of Exosomes in the Pathogenesis and Stratification of Parkinson's Disease. J Parkinsons Dis. 2017;7:569-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Chang C, Lang H, Geng N, Wang J, Li N, Wang X. Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett. 2013;548:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 29. | Silverman JM, Christy D, Shyu CC, Moon KM, Fernando S, Gidden Z, Cowan CM, Ban Y, Stacey RG, Grad LI, McAlary L, Mackenzie IR, Foster LJ, Cashman NR. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem. 2019;294:3744-3759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 30. | Zhang X, Abels ER, Redzic JS, Margulis J, Finkbeiner S, Breakefield XO. Potential Transfer of Polyglutamine and CAG-Repeat RNA in Extracellular Vesicles in Huntington's Disease: Background and Evaluation in Cell Culture. Cell Mol Neurobiol. 2016;36:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Poehler AM, Xiang W, Spitzer P, May VE, Meixner H, Rockenstein E, Chutna O, Outeiro TF, Winkler J, Masliah E, Klucken J. Autophagy modulates SNCA/α-synuclein release, thereby generating a hostile microenvironment. Autophagy. 2014;10:2171-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 32. | Jeske R, Bejoy J, Marzano M, Li Y. Human Pluripotent Stem Cell-Derived Extracellular Vesicles: Characteristics and Applications. Tissue Eng Part B Rev. 2020;26:129-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Delpech JC, Herron S, Botros MB, Ikezu T. Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease. Trends Neurosci. 2019;42:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 34. | Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, Cui M, Tieu K. Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease. Brain. 2020;143:1476-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 392] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 35. | Clayton K, Delpech JC, Herron S, Iwahara N, Ericsson M, Saito T, Saido TC, Ikezu S, Ikezu T. Plaque associated microglia hyper-secrete extracellular vesicles and accelerate tau propagation in a humanized APP mouse model. Mol Neurodegener. 2021;16:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 36. | Shakespear N, Ogura M, Yamaki J, Homma Y. Astrocyte-Derived Exosomal microRNA miR-200a-3p Prevents MPP(+)-Induced Apoptotic Cell Death Through Down-Regulation of MKK4. Neurochem Res. 2020;45:1020-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 37. | de Rus Jacquet A, Tancredi JL, Lemire AL, DeSantis MC, Li WP, O'Shea EK. The LRRK2 G2019S mutation alters astrocyte-to-neuron communication via extracellular vesicles and induces neuron atrophy in a human iPSC-derived model of Parkinson's disease. Elife. 2021;10:e73062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, Kimura N, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y. A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide. FEBS Lett. 2015;589:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Upadhya R, Zingg W, Shetty S, Shetty AK. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J Control Release. 2020;323:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 40. | Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer's disease. Neurobiol Aging. 2014;35:1792-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 388] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 41. | Feliciano DM, Zhang S, Nasrallah CM, Lisgo SN, Bordey A. Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification. PLoS One. 2014;9:e88810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Schiera G, Di Liegro CM, Di Liegro I. Extracellular Membrane Vesicles as Vehicles for Brain Cell-to-Cell Interactions in Physiological as well as Pathological Conditions. Biomed Res Int. 2015;2015:152926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 337] [Article Influence: 37.4] [Reference Citation Analysis (1)] |
| 44. | Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1117] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 45. | Milutinovic B, Mahalingam R, Mendt M, Arroyo L, Seua A, Dharmaraj S, Shpall E, Heijnen CJ. Intranasally Administered MSC-Derived Extracellular Vesicles Reverse Cisplatin-Induced Cognitive Impairment. Int J Mol Sci. 2023;24:11862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 46. | Xhima K, Aubert I. The therapeutic potential of nerve growth factor combined with blood-brain barrier modulation by focused ultrasound for neurodegenerative disorders. Neural Regen Res. 2021;16:1783-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Barreca MM, Cancemi P, Geraci F. Mesenchymal and Induced Pluripotent Stem Cells-Derived Extracellular Vesicles: The New Frontier for Regenerative Medicine? Cells. 2020;9:1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Pusic AD, Kraig RP. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia. 2014;62:284-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (2)] |
| 49. | Rudnitsky E, Braiman A, Wolfson M, Muradian KK, Gorbunova V, Turgeman G, Fraifeld VE. Stem cell-derived extracellular vesicles as senotherapeutics. Ageing Res Rev. 2024;99:102391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 50. | Zuo R, Liu M, Wang Y, Li J, Wang W, Wu J, Sun C, Li B, Wang Z, Lan W, Zhang C, Shi C, Zhou Y. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res Ther. 2019;10:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 51. | Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev Rep. 2015;11:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 52. | Li Q, Niu X, Yi Y, Chen Y, Yuan J, Zhang J, Li H, Xia Y, Wang Y, Deng Z. Inducible Pluripotent Stem Cell-Derived Small Extracellular Vesicles Rejuvenate Senescent Blood-Brain Barrier to Protect against Ischemic Stroke in Aged Mice. ACS Nano. 2023;17:775-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 53. | Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 54. | Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y, Wang D, Xu L, Bi J, Yang H. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer's Disease. Neurochem Res. 2018;43:2165-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 55. | Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019;8:1605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 590] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 56. | Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 838] [Cited by in RCA: 781] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 57. | Hering C, Shetty AK. Extracellular Vesicles Derived From Neural Stem Cells, Astrocytes, and Microglia as Therapeutics for Easing TBI-Induced Brain Dysfunction. Stem Cells Transl Med. 2023;12:140-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 58. | Tsai SC, Yang KD, Chang KH, Lin FC, Chou RH, Li MC, Cheng CC, Kao CY, Chen CP, Lin HC, Hsu YC. Umbilical Cord Mesenchymal Stromal Cell-Derived Exosomes Rescue the Loss of Outer Hair Cells and Repair Cochlear Damage in Cisplatin-Injected Mice. Int J Mol Sci. 2021;22:6664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Oliva J. Role of Extracellular Vesicles Produced by Stem Cells in Tissue Repair. Int J Mol Sci. 2023;24:4798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Zhang X, Liu T, Hou X, Zhou Z, Zhang F, Ma H, Wu X, Jiang J. Exosomes secreted by mesenchymal stem cells delay brain aging by upregulating SIRT1 expression. Sci Rep. 2023;13:13213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 61. | Bertolino GM, Maumus M, Jorgensen C, Noël D. Recent Advances in Extracellular Vesicle-Based Therapies Using Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells. Biomedicines. 2022;10:2281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 62. | Kim S, Kim TM. Generation of mesenchymal stem-like cells for producing extracellular vesicles. World J Stem Cells. 2019;11:270-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Billing AM, Ben Hamidane H, Dib SS, Cotton RJ, Bhagwat AM, Kumar P, Hayat S, Yousri NA, Goswami N, Suhre K, Rafii A, Graumann J. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci Rep. 2016;6:21507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 64. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13051] [Article Influence: 686.9] [Reference Citation Analysis (12)] |
| 65. | Branscome H, Paul S, Khatkar P, Kim Y, Barclay RA, Pinto DO, Yin D, Zhou W, Liotta LA, El-Hage N, Kashanchi F. Stem Cell Extracellular Vesicles and their Potential to Contribute to the Repair of Damaged CNS Cells. J Neuroimmune Pharmacol. 2020;15:520-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Ma Y, Xu X, Li C, Wang Y, Zhu J, Xia X, Zheng JC. Induced neural progenitor cell-derived extracellular vesicles promote neural progenitor cell survival via extracellular signal-regulated kinase pathway. CNS Neurosci Ther. 2021;27:1605-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Armstrong JPK, Stevens MM. Strategic design of extracellular vesicle drug delivery systems. Adv Drug Deliv Rev. 2018;130:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 68. | Yang Y, Hong Y, Nam GH, Chung JH, Koh E, Kim IS. Virus-Mimetic Fusogenic Exosomes for Direct Delivery of Integral Membrane Proteins to Target Cell Membranes. Adv Mater. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 69. | Haney MJ, Zhao Y, Jin YS, Batrakova EV. Extracellular Vesicles as Drug Carriers for Enzyme Replacement Therapy to Treat CLN2 Batten Disease: Optimization of Drug Administration Routes. Cells. 2020;9:1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 70. | Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL, Kabanov AV, Batrakova EV. Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. J Neuroimmune Pharmacol. 2020;15:487-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 71. | Lu M, DiBernardo E, Parks E, Fox H, Zheng SY, Wayne E. The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Autoimmune Disorders. Front Immunol. 2021;12:566299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 72. | Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;18:100522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 255] [Article Influence: 85.0] [Reference Citation Analysis (37)] |
| 73. | Cheng J, Chen Z, Liu C, Zhong M, Wang S, Sun Y, Wen H, Shu T. Bone mesenchymal stem cell-derived exosome-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. Nanomedicine (Lond). 2021;16:1567-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 74. | Liu Z, Tong H, Li J, Wang L, Fan X, Song H, Yang M, Wang H, Jiang X, Zhou X, Yuan H, Wang Y. Low-Stiffness Hydrogels Promote Peripheral Nerve Regeneration Through the Rapid Release of Exosomes. Front Bioeng Biotechnol. 2022;10:922570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 75. | Li L, Zhang Y, Mu J, Chen J, Zhang C, Cao H, Gao J. Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury. Nano Lett. 2020;20:4298-4305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 290] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 76. | Ali Moussa HY, Manaph N, Ali G, Maacha S, Shin KC, Ltaief SM, Gupta V, Tong Y, Ponraj J, Salloum-Asfar S, Mansour S, Al-Shaban FA, Kim HG, Stanton LW, Grivel JC, Abdulla SA, Al-Shammari AR, Park Y. Single Extracellular Vesicle Analysis Using Flow Cytometry for Neurological Disorder Biomarkers. Front Integr Neurosci. 2022;16:879832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 77. | Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015;11:600-7.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 740] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 78. | Li C, Qin S, Wen Y, Zhao W, Huang Y, Liu J. Overcoming the blood-brain barrier: Exosomes as theranostic nanocarriers for precision neuroimaging. J Control Release. 2022;349:902-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 79. | Rahnama M, Heidari M, Poursalehi Z, Golchin A. Global Trends of Exosomes Application in Clinical Trials: A Scoping Review. Stem Cell Rev Rep. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 80. | Malaguarnera M, Cabrera-Pastor A. Emerging Role of Extracellular Vesicles as Biomarkers in Neurodegenerative Diseases and Their Clinical and Therapeutic Potential in Central Nervous System Pathologies. Int J Mol Sci. 2024;25:10068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 81. | Imafuku A, Sjoqvist S. Extracellular Vesicle Therapeutics in Regenerative Medicine. Adv Exp Med Biol. 2021;1312:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/