Published online Nov 26, 2024. doi: 10.4252/wjsc.v16.i11.926
Revised: August 20, 2024
Accepted: September 6, 2024
Published online: November 26, 2024
Processing time: 266 Days and 19.9 Hours
Human periodontal ligament stem cells (PDLSCs) regenerate oral tissue. In vitro expansion causes replicative senescence in stem cells. This causes intracellular reactive oxygen species (ROS) accumulation, which can impair stem cell function. Tissue engineering efficiency is reduced by exogenous ROS stimulation, which causes premature senescence under oxidative stress. Melatonin (MT), a powerful free radical scavenger, can delay PDLSCs senescence but may not maintain stemness under oxidative stress. This experiment examined the effects of hyd
To determine if MT can reverse the above effects along with the underlying molecular mechanisms involved.
PDLSCs were isolated from human premolars and cultured in different con
MT increases Yes-associated protein expression and maintains cell stemness in an induced inflammatory microenvironment, which may explain its therapeutic effects. We examined how MT affects PDLSCs aging and stemness and its biological mechanisms.
Our study reveals MT’s role in regulating oxidative stress in PDLSCs and Yes-associated protein-mediated activity, providing insights into cellular functions and new therapeutic targets for tissue regeneration.
Core Tip: While the use of mesenchymal stem cells in preclinical and clinical studies for the treatment of various diseases has provided promising results, the microenvironment of oxidative stress inhibits the efficacy of mesenchymal stem cells-mediated tissue regeneration, which remains a major challenge limiting clinical applications. Our findings provide new insights into melatonin (MT) regulation in the biological functions of oxidative stress-induced periodontal ligament stem cells and elucidate the potential mechanism of Yes-associated protein-mediated MT action. These findings strengthen our comprehension of the role of MT during cellular oxidative stress and provide potential targets for the development of new therapeutic strategies to promote tissue regeneration.
- Citation: Gu K, Feng XM, Sun SQ, Hao XY, Wen Y. Yes-associated protein-mediated melatonin regulates the function of periodontal ligament stem cells under oxidative stress conditions. World J Stem Cells 2024; 16(11): 926-943
- URL: https://www.wjgnet.com/1948-0210/full/v16/i11/926.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i11.926
Human periodontal ligament stem cells (PDLSCs) are a source of oral tissue and the dominant seed cells in the oral tissue regeneration field[1]. With the extension of the human lifespan and the rise of an aging society, the aging of the body has become a major clinical factor that needs to be addressed through tissue regeneration. Aging microenvironments inhibit tissue regeneration mediated by mesenchymal stem cells (MSCs). Additionally, after multiple passages, the cells undergo mitotic arrest, replicative senescence, and stemness changes[2]. The changes over time include abnormal stem cell morphology, limited self-renewal ability, decreased differentiation potential, and a shortened lifespan.
Due to its simple operation and low cost, small- molecule drug therapy has become a recent research and development hotspot. Melatonin (MT), a pineal gland-secreted neurohormone peptide, is a powerful free radical scavenger and small-molecule drug detected at multiple sites in the body[3]. Prior studies have shown that MT regulates MSC proliferation, apoptosis, and stemness. Furthermore, MT effectively reverses the premature aging of bone marrow-MSCs caused by hydrogen peroxide (H2O2), in a dose-dependent manner[4] and can also restore the damaged osteogenic differentiation potential of senescent cells[4,5]. Under oxidative stress conditions, the self-renewal ability of MT-treated MSCs is enhanced, and apoptosis is reduced[6].
Yes-associated protein (YAP) is a downstream effector molecule of the Hippo signaling pathway[7]. As an evolu
The dental samples evaluated here consisted of complete premolars taken from 20 systemically healthy donors who required extraction for orthodontic treatment. The individuals were between 12 and 14 years of age and provided informed consent. All procedures were approved by the Research Ethics Committee of Shandong University (No. GR201902). From the middle third of the root surface, periodontal ligament tissue was scraped and inoculated into flasks and maintained in a complete medium, composed of α-MEM (HyClone) and 10% fetal bovine serum (Gibco), at 37 °C in 5% CO2. The culture medium was replaced every 2-3 days, and the PDLSCs were utilized between passages 3 and 4 (P3-4) to avoid passage-dependent changes in cell behavior.
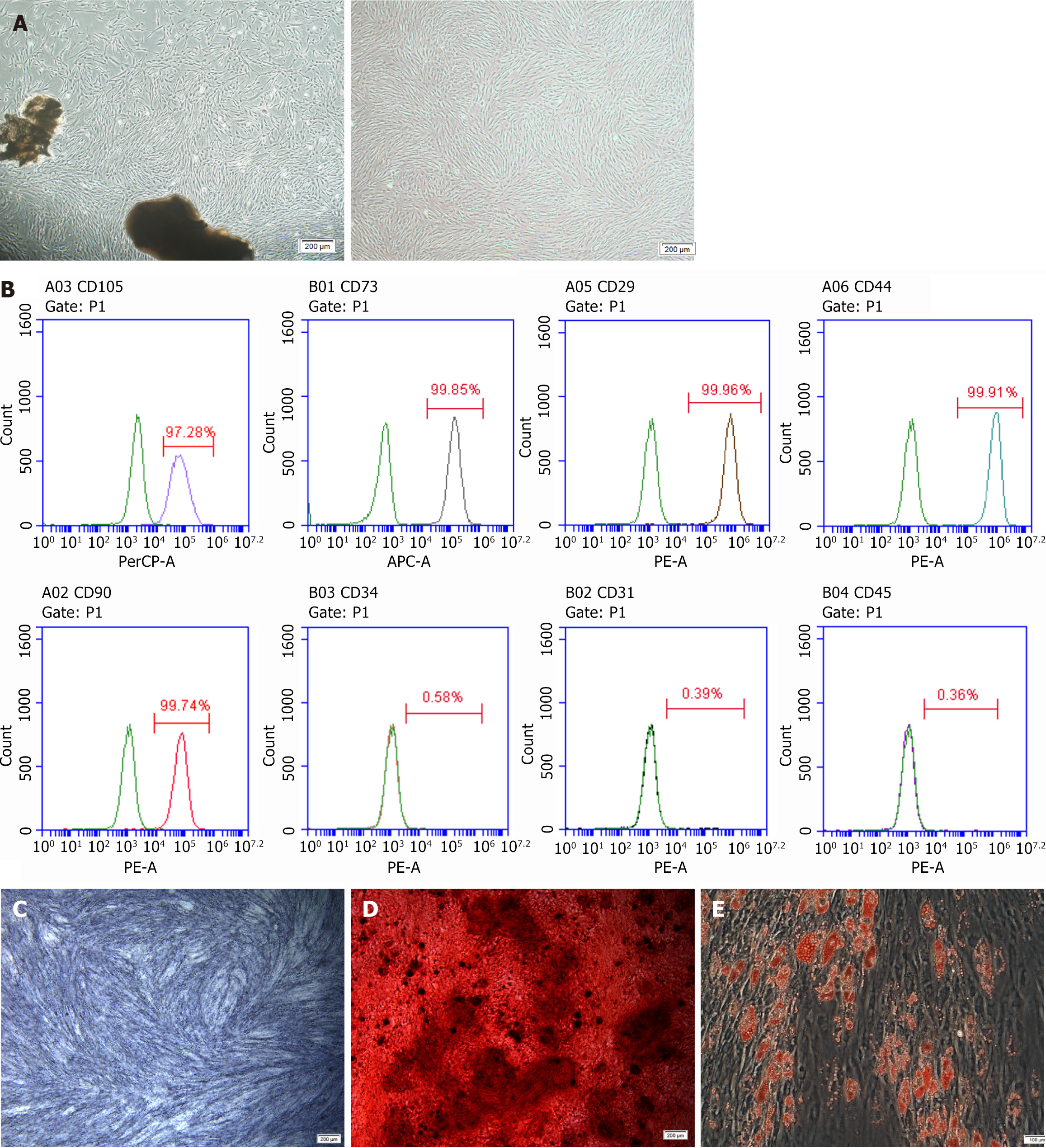
Flow cytometry (BD Biosciences) was used to analyze PDLSCs cell surface markers according to the manufacturer’s instructions. Briefly, after cleaning with phosphate buffered saline (PBS) containing 3% fetal bovine serum, cells were incubated with monoclonal antibodies against human CD90, CD105, CD29, CD44, CD73, CD31, CD34, and CD45 for 1 hours at 4 °C in the dark. Cells that were not antibody-pretreated served as blank controls. Subsequently, the cells were washed with PBS and analyzed by flow cytometry.
Human PDLCs at a density of 2 × 105 cells/well were inoculated in 6-well plates and incubated at 37 °C in 5% CO2 for 24 hours. When the cell confluence reached 60%, 50 μM, 100 μM, 200 μM, 300 μM, or 400 μM H2O2 (a gradient dilution in complete medium) was added, and the samples were allowed to incubate for 2 hours. Next, the cells were washed in PBS to remove residual H2O2 and then incubated in a fresh complete medium, which was replaced every 2-3 days. These samples were used for subsequent experiments. Different concentrations of MT (1, 10, or 100 μM) dissolved in anhydrous ethanol were added to the medium, along with different concentrations of verteporfin (25 or 200 nM) dissolved in dimethyl sulfoxide.
Cell cycle distribution was quantified using a DNA quantitative analysis kit (Solarbio), according to the manufacturer’s instructions. Cells at a density of 2 × 105 cells/well were seeded in 6-well plates, and 500 μL of pre-cooled 70% ethanol was added to allow the cells to fix overnight at 4 °C. Then, 100 μL of RNase A solution was added, and the samples were incubated in a water bath at 37 °C for 30 minutes. Subsequently, 400 μL of propidium iodide (PI) staining solution was added in a dark room, and the samples were incubated for 30 minutes at 4 °C, protected from light. The resulting fluorescence intensity was measured by flow cytometry using a PerCP laser.
A Cell Count Kit 8 (CCK-8) (Biosharp) assay was used to quantify cell proliferation according to the manufacturer’s instructions. Briefly, in the CCK-8 assay, PDLSCs were seeded in 96-well culture plates (2 × 103 cells/well) and cultured for 1, 2, or 3 days. At each specified time point, the medium was replaced with fresh MEM supplemented with 10% CCK-8, and the samples were incubated at 37 °C for 2 hours in the dark. The resulting absorbance was measured at 450 nm using a microplate reader (SPECTROstar Nano, BMG Labtech).
The percentage of apoptotic cells was determined using an annexin V-FITC/PI staining kit (Multisciences, Hangzhou, China). PDLSCs were seeded into six-well culture plates (1 × 105 cells/well), and after reaching 80%-90% confluence, the cells were washed with cold PBS and treated with binding buffer. Lastly, the cells were harvested and resuspended in 100 μL of binding buffer containing 5 μL annexin V-FITC and 10 μL PI for 15 minutes in the dark at room temperature. The percentage of apoptotic cells was determined using flow cytometry.
To assess osteogenic differentiation potential, 1 × 105 PDLSCs were inoculated into 6-well plates and cultured in the osteogenic induction medium (complete medium supplemented with 50 mg/L vitamin C, 10 mmol/L sodium-glycerophosphate, and 10 mmol/L dexamethasone). After 7 days, PDLSCs were stained with alkaline phosphatase (ALP), and according to the manufacturer’s instructions, an ALP activity assay kit (Nanjing Jiancheng Institute of Biological Engineering) was used to measure the ALP activity level, and a microplate reader was used to measure the absorbance at 520 nm. After 21 days, the mineralized nodules were stained with 2% alizarin red and measured at 560 nm using a microplate reader after solubilization in 10% acetyl pyridinium chloride (CPC, Sigma-Aldrich) for 30 minutes at room temperature.
To assess the adipogenic differentiation potential, 1 × 105 PDLSCs were seeded in 6-well plates and cultured in the adipogenic induction medium (complete medium supplemented with 1 mol/L dexamethasone, 200 mol/L indomethacin, 10 mg/L insulin, and 500 mol/L IBMX). After 14 days, the lipid droplets were stained with oil-red O for evaluation.
We analyzed cellular reactive oxygen species (ROS) levels in PDLSCs using a fluorescent intracellular ROS kit (Beyotime), according to the manufacturer’s instructions. Briefly, the cells were incubated with the ROS assay reagent stock solution for 30 minutes at 37 °C in 5% CO2 under light-protected conditions. After utilizing the FITC channel to excite green fluorescence, all subgroups were photographed in different fields of view under the same exposure conditions, and the single-channel fluorescence intensity was measured using ImageJ software. In addition, fluorescence intensity was measured using a flow cytometer with a FITC laser.
PDLCs (1 × 105 cells) were inoculated on 24-well plates at 37 °C in 5% CO2 overnight. In accordance with the manufacturer’s protocol, we processed the cells using the cell senescence-specific β-galactosidase assay kit (Beyotime), with incubation at 37 °C for 12 hours. Cell observation and counting under a microscope (Olympus) allowed us to identify cells expressing senescence-associated-galactosidase (SA-gal) by their blue color.
Total RNA was isolated from PDLSCs using the RNAios Plus reagent (Takara) and reverse-transcribed to cDNA using the PrimeScript TM RT kit with gDNA Eraser (Takara). cDNA was amplified by polymerase chain reaction under the following thermocycler conditions: 95 °C for 2 minutes, followed by 95 °C for 15 seconds, and 60 °C for 1 minutes, for 40 cycles. The relative expression levels of the target gene were normalized to GAPDH levels and determined by the 2-∆∆Ct method. The primer sequences are shown in Table 1.
| Gene | Forward primer 5’-3’ | Reverse primer 5’-3’ |
| C-MYC | TTCGGGTAGTGGAAAACT | AGCAGCTCGAATTTCTTCC |
| OCT-4 | CCCCTGGTGCCGTGAAGG | GCAAATTGCTCGAGTTCT |
| NANOG | AAAGAATCTTCACCTATG | GAAGGAAGAGGAGAGAC |
| P21 | CGATGGAACTTCGACTTTGTCA | GCACAAGGGTACAAGACAGTG |
| GAPDH | AGAAGGCTGGGGCTGAGA | AGGGGCCATCCACAGTCT |
| RB | CTTCCTCATGCTGTTCAGGAG | TGCATGAAGACCGAGTTATAGAAT |
| ALP | TCCATCTGTAAAGGGCGG | AATACCAGCTACGCTGCA |
| COL1 | GCTGATGATGCCAATGTA | CCAGTCAGAGTGGCACAT |
| YAP | GCTACAGTGTCCCTCGAACC | CCGGTGCATGTGTCTCCTTA |
After washing PDLSCs three times with ice-cold PBS, the cells were lysed with RIPA reagent containing 1% PMSF and 1% phosphatase inhibitor cocktail. After centrifugation at 14000 × g for 15 minutes, the total protein concentration was measured using a BCA protein assay kit (Solarbio). Proteins were separated based on molecular weight using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were blocked with 5% milk for 1 hour and then incubated with primary antibodies overnight at 4 °C. The membranes were then washed three times with tris-buffered saline and tween-20 and then incubated with secondary antibodies for 1 hour at room temperature. Protein strips were chromogenized using Pierce ECL Western Blotting substrate (Thermo Fisher Scientific), and densitometry was performed on each strip using ImageJ software (National Institutes of Health). The following primary antibodies were used: P21 (#2947, CST), phospho-Rb (Ser807/811) (#8516, CST), NANOG (#4903, CST), OCT-4A (#2840, CST), C-MYC (#5605, CST), and GAPDH (#8884, CST).
In all of the experiments, which were performed at least three times independently, the data were expressed as mean ± SD. A one-way analysis of variance was performed to compare results between three or more groups, and a student’s t-test was used to compare results between two groups. Statistical analysis was performed using Prism (GraphPad Prism v7.02), and statistical significance was set at a threshold of P < 0.05.
PDLC evaluation using an inverted microscope revealed that the cells had long spindle shapes with abundant cytoplasm and well-defined cell margins (Figure 1A). Positive expression of the MSC-specific surface markers CD105, CD73, CD29, CD44, and CD90 was observed. Meanwhile, expression of the hematopoietic stem cell-specific molecule CD34, the platelet endothelial cell-specific molecule CD31, and the leukocyte-specific molecule CD45 was negative (Figure 1B). After 7 days of osteogenesis induction, the PDLSCs were uniformly and consistently stained dark blue upon ALP staining analysis (Figure 1C). After 21 days of osteogenic induction, PDLSCs were visible as a large number of reddish-brown mineralized nodules following alizarin red staining (Figure 1D). After 14 days of adipogenic induction, a large number of grape-vesicle-like lipid droplets were visible in the PDLSCs following oil-red staining (Figure 1E). Taken together, these results reveal that the cells used in this study possess multi-directional differentiation potential.
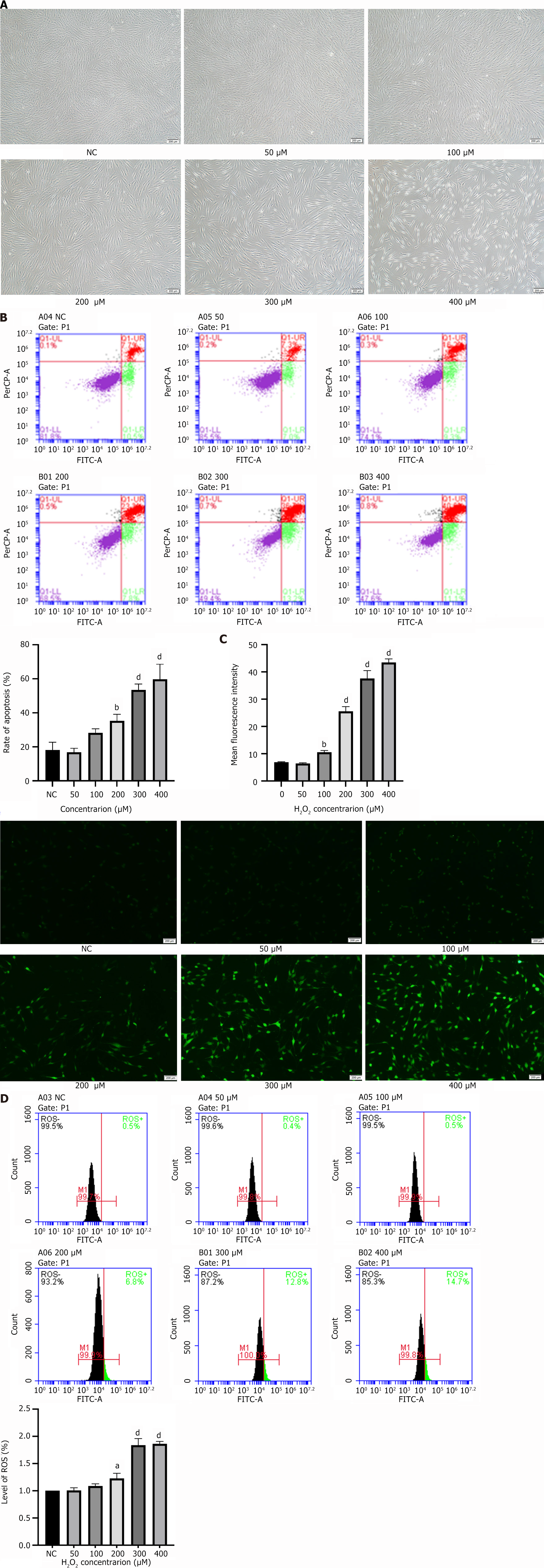
Microscopy observations revealed that the higher the concentration of H2O2, the lower the number of PDLSCs that adhered to the wall. Furthermore, the cell volume increased, and the morphology changed from the original short spindle shape to an elongated fibroblast-like appearance (Figure 2A). Notably, the apoptosis rate was proportional to the H2O2 concentration (Figure 2B). Furthermore, the ROS assay results showed that the number of ROS-positive PDLSCs increased with increasing H2O2 concentrations in a concentration-dependent manner (Figure 2C and D). In summary, we successfully established a model of H2O2-induced oxidative stress in PDLSCs.
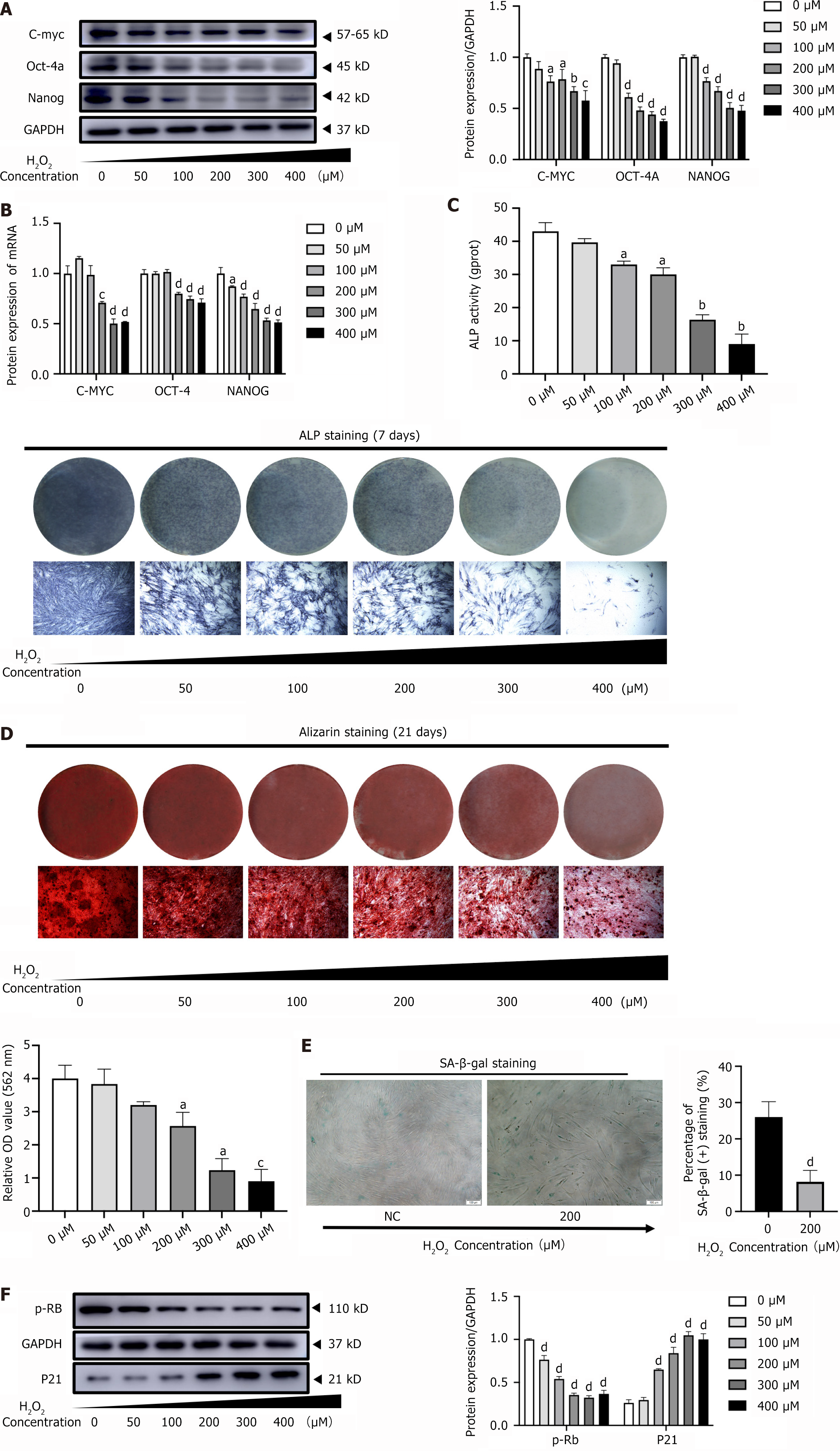
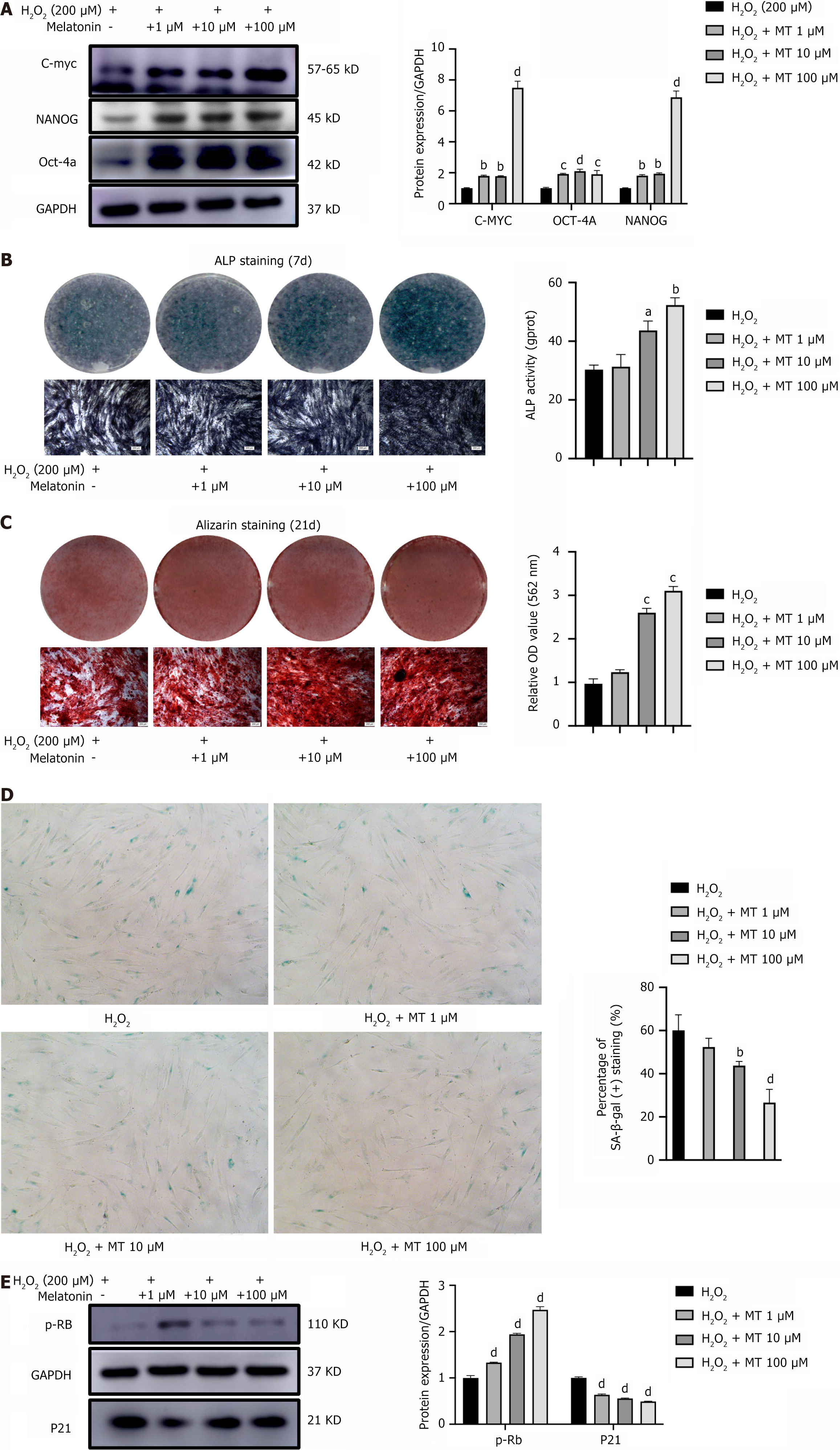
Compared with the control group, the protein expression levels of the stemness-related proteins C-MYC, OCT-4, and NANOG were significantly decreased in the H2O2 group in a concentration-dependent manner (Figure 3A). Furthermore, the mRNA expression levels of these genes were consistent with the protein expression results (Figure 3B). This indicates that H2O2 inhibits the ability of PDLSCs to maintain their stemness.
To investigate the effect of H2O2 on the osteogenic differentiation potential of PDLSCs, we examined the ALP activity and mineralized nodules. Compared to the control group, the ALP staining intensity in PDLSCs decreased with increasing H2O2 concentration, and ALP activity followed a similar trend (Figure 3C). After 21 days of osteogenic induction, alizarin red staining and quantitative analysis showed that the PDLSCs accumulated fewer mineralized nodules with increasing H2O2 concentrations than the control group (Figure 3D). These results suggest that H2O2 has an inhibitory effect on PDLSCs osteogenic differentiation. Senescence-associated β-galactosidase (SA β-gal) staining showed that the number of SA β-gal-positive cells was significantly higher in the 200 μM H2O2-treated PDLSCs group than in the control group (Figure 3E). Consistent with this phenotype, p-RB protein expression decreased and that of p21, a molecular marker of cellular senescence, increased significantly in PDLSCs with increasing H2O2 concentrations (Figure 3F). These results suggest that H2O2 accelerates senescence in PDLSCs. Based on the above results, we selected 200 μM as the most suitable H2O2 concentration for subsequent experiments.
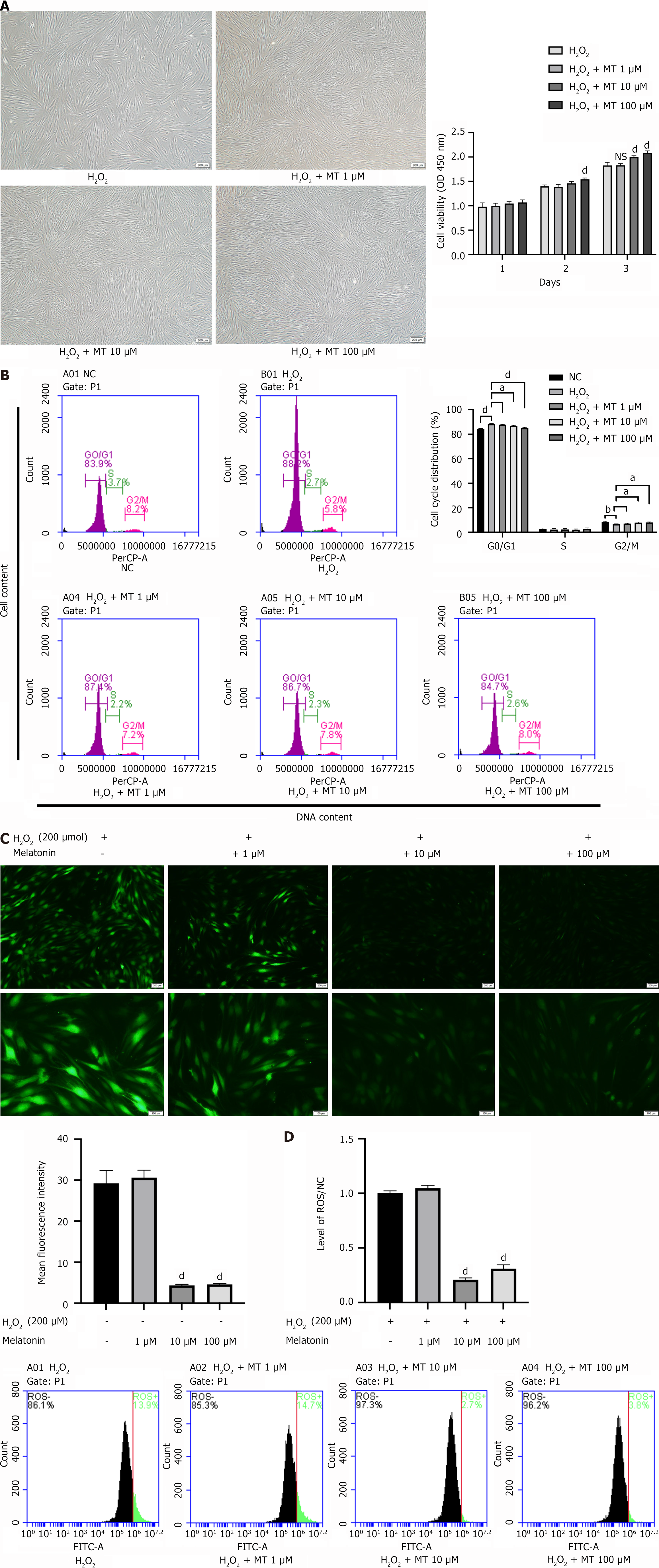
Microscopically, PDLSCs in the MT-treated group were in a good growth state, with a higher percentage of spindle-shaped cells compared to the H2O2 group (Figure 4A). The 3-day growth curves revealed that MT significantly enhanced the proliferation of H2O2-induced PDLSCs in a concentration-dependent manner (Figure 4A). Additionally, MT significantly reduced the proportion of H2O2-induced PDLSCs in the G0/G1 phase and increased the proportion of cells in the G2/M phase (Figure 4B). The ROS assay results showed that the number of H2O2-induced ROS-positive PDLSCs decreased significantly with increasing MT concentrations (Figure 4C and D). These findings suggest that MT effectively attenuates H2O2-induced oxidative stress in PDLSCs.
MT significantly increased the protein expression levels of the stemness-related proteins C-MYC, OCT-4, and NANOG in H2O2-induced PDLSCs in a concentration-dependent manner, compared to the corresponding levels in the H2O2 group (Figure 5A). This observation suggests that MT enhances the ability of PDLSCs to maintain their stemness. To investigate the effect of MT on the osteogenic differentiation potential of H2O2-induced PDLSCs, we measured ALP activity in mineralized nodules. Compared to the H2O2 group, the intensity of ALP staining in PDLSCs became darker with increasing MT concentrations, and ALP activity followed a similar trend (Figure 5B). After 21 days of osteogenic induction, alizarin red staining and quantitative analysis showed that the PDLSCs accumulated more mineralized nodules with increasing MT concentrations (Figure 5C). These results suggest that MT enhances the osteogenic differentiation potential of H2O2-induced PDLSCs. Furthermore, SA β-gal staining of H2O2-induced PDLSCs showed that MT significantly decreased the number of SA β-gal-positive cells (Figure 5D). Consistent with this phenotype, the protein expression level of p-RB increased and that of p21 decreased significantly with increasing MT concentrations (Figure 5E). These results suggest that MT delays the senescence of PDLSCs.
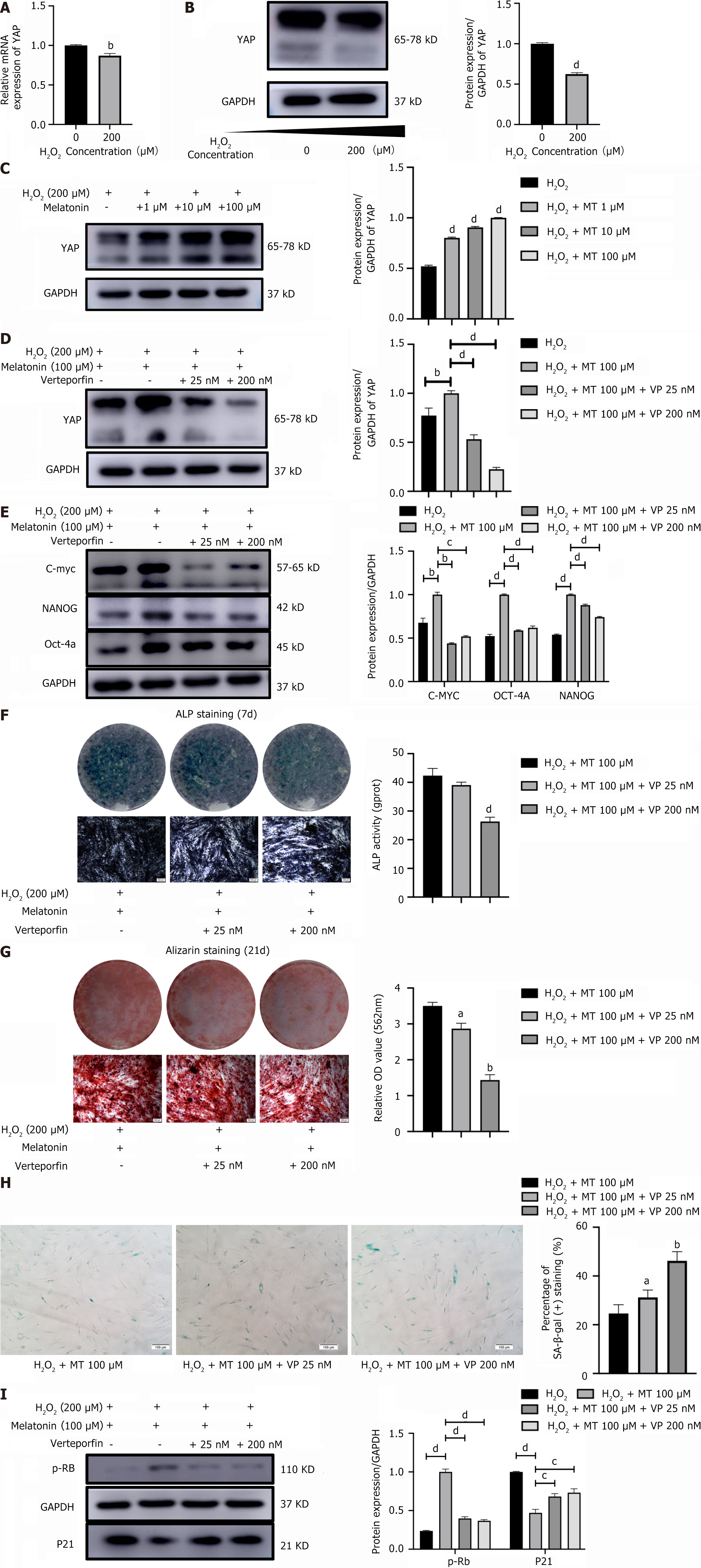
Compared with the control group, YAP mRNA expression and protein levels were decreased in the PDLSCs of the H2O2 group (Figure 6A and B), which indicates that oxidative stress inhibits YAP expression in PDLSCs. Furthermore, MT significantly elevated YAP protein levels in H2O2-induced PDLSCs in a concentration-dependent manner (Figure 6C). This finding suggests that YAP mediates the role of MT in enhancing stemness and reducing senescence in H2O2-induced PDLSCs. To further elucidate the underlying mechanism, verteporfin was used to antagonize YAP expression in MT-upregulated PDLSCs (Figure 6D). We also found that verteporfin significantly reduced the protein expression levels of C-MYC, OCT-4, and NANOG in MT-upregulated PDLSCs (Figure 6E). This observation suggests that YAP-mediated MT enhances the ability of H2O2-induced PDLSCs to maintain stemness.
ALP activity and mineralized nodules were examined to determine if YAP mediates MT’s osteogenic differentiation potential of H2O2-induced PDLSCs. The results showed that verteporfin decreased the intensity of ALP staining in MT-treated PDLSCs, with ALP activity following a similar trend (Figure 6F). After 21 days of osteogenic induction, alizarin red staining showed that verteporfin decreased the MT-induced accumulation of mineralized nodules in PDLSCs (Figure 6G). These results suggest that through a YAP-mediated pathway, MT enhances the osteogenic differentiation potential of H2O2-induced PDLSCs. Furthermore, SA-gal staining revealed that verteporfin significantly increased the number of SA β-gal-positive, MT-downregulated PDLSCs (Figure 6H). Consistent with this phenotype, verteporfin decreased the protein expression level of p-RB in MT-upregulated PDLSCs and elevated the expression level of p21 in MT-downregulated PDLSCs (Figure 6I). These results also suggest that MT delays the senescence of H2O2-induced PDLSCs through a YAP-mediated mechanism.
Previous studies have shown that PDLSCs have self-renewal, multi-directional differentiation, and immune regulation abilities, while oxidative stress-induced impaired regenerative capacity can lead to tissue dysfunction and organismal aging[1-3]. Therefore, it is necessary to identify ways to modulate PDLSC dysfunction and promote tissue regeneration. Small-molecule drugs have gradually become a research hotspot for delaying cellular senescence because of their simple production, low cost, and relatively mature understanding[4]. MT, a small-molecule drug, is a powerful free radical scavenger that regulates MSC proliferation and apoptosis[5]. However, its effects and mechanisms on PDLSCs remain unclear. Therefore, the present study investigated the role and mechanisms of MT in regulating the biological characteristics of PDLSCs under oxidative stress.
The incomplete intracellular oxidation of MSCs during in vitro expansion generates high levels of ROS, including H2O2 and hydroxyl radicals. Although it has been shown that H2O2 is a component of cellular signaling pathways and is necessary for the growth, development, and adaptation of the organism[6], long-term exogenous exposure to H2O2 induces cellular oxidative stress damage and impairs biological functions, thus leading to senescence[7-9]. In this study, we established an oxidative stress model by observing a gradual increase in PDLSCs-generated ROS with increasing H2O2 concentration. Our results showed that under oxidative stress conditions, PDLSCs’ morphology gradually changed from a short spindle-like appearance to a slender fibroblast-like appearance. This may be due to the downregulated expression of dynamic actin cytoskeleton gene clusters, which would slow actin renewal[10], along with changes in the number and size of nuclei and organelles, including the mitochondria, endoplasmic reticulum, and lysosomes under oxidative stress conditions[11], resulting in morphological changes in PDLSCs.
Furthermore, the proliferative capacity of PDLSCs was reduced under oxidative stress, whereas apoptosis significantly increased, in agreement with previous findings[8]. Notably, it has been shown that MSCs are highly resistant to injury-induced apoptosis and preferentially activate premature cellular senescence in response to injury[12]. Cellular senescence is followed by increased lysosomal content and enhanced activity of SA β-gal, which is the current gold standard for cellular senescence assays. Notably, our results show a significant increase in the number of SA β-gal-positive PDLSCs under oxidative stress conditions. Additionally, the RB protein is a tumor suppressor protein that regulates cell proliferation, apoptosis, and differentiation and modulates cell biological functions[13]. Cell cycle-dependent protein kinases can phosphorylate RB[14], thereby advancing cell cycle progression[15]. P21 is a negative regulatory protein of the cell cycle that is closely associated with cellular senescence and is involved in regulating the G1/S phase transition[16]. Our results showed that p-RB expression was significantly downregulated and p21 expression was significantly upregulated in PDLSCs under oxidative stress, suggesting that oxidative stress induces senescence. Our results also showed that the stemness and osteogenic differentiation potential of PDLSCs were reduced under oxidative stress, consistent with the results of previous studies[17]. In the future, it will be crucial to identify small-molecule drugs that can counteract the effects of oxidative stress to delay cellular senescence and enhance cell stemness.
There are various types of antioxidants, including glutathione, MT, metformin, and resveratrol[3,8,18,19]. Among these, MT, a powerful endogenous free radical scavenger, has promising anti-aging applications. Our results showed that MT reduced oxidative stress-induced ROS in PDLSCs, indicating that it can scavenge free radicals and reduce intracellular oxidative stress. Moreover, MT restored the normal morphology of PDLSCs, enhanced their proliferation, and relieved the negative cell cycle regulatory effects of oxidative stress. Notably, previous studies have shown that MT enhances the proliferation and migration capacity of adipose-derived MSCs that have been passaged for a long time, thus delaying cellular senescence and maintaining cell stemness[18]. MT has also been shown to delay the senescence of MSCs in rats with chronic kidney disease and promote cell proliferation and adhesion by maintaining mitochondrial I and IV complex activity[20]. MT has also been found to prevent senescence and restore the impaired osteogenic differentiation potential in bone marrow MSCs in ovariectomized rats by activating the AMP-activated protein kinase-sirtuin 1 signaling pathway through the MT receptor[21]. In addition, MT can also promote osteogenic differentiation of dental pulp stem cells in vivo[22]. Our results indicate that MT significantly delays the oxidative stress-induced senescence of PDLSCs and enhances their stemness and osteogenic differentiation potential. This finding is generally consistent with those of prior studies; however, the molecular mechanisms by which MT regulates the biological functions of PDLSCs under oxidative stress remain unclear.
A growing number of studies have shown that the Hippo-YAP signaling pathway is associated with cellular oxidative stress. YAP1 protects cardiomyocytes from ROS-induced cell death and mediates cellular defense against oxidative stress[23]. Furthermore, in neurofibromatosis type 2 tumor cells, interference with YAP/TAZ expression has been found to enhance mitochondrial respiratory function and lead to ROS accumulation, which causes oxidative stress and cell death[24]. In the present study, we found that YAP expression in PDLSCs was significantly downregulated under oxidative stress conditions and significantly upregulated by MT. This finding is consistent with the results of previous studies and suggests that YAP signaling regulates oxidative stress-induced changes in PDLSCs. Verteporfin, an inhibitor of TEAD-YAP association[25], mimics a YAP knockdown phenotype and has been used to inhibit YAP in related studies[26,27]. Our results showed that MT-upregulated YAP levels were suppressed after YAP antagonism by verteporfin. Fur
Although the use of MSCs in preclinical and clinical studies for the treatment of various diseases has provided promising results, the oxidative stress microenvironment inhibits the efficacy of MSC-mediated tissue regeneration by suppressing endogenous and exogenous MSC physiological functions. This is currently a major challenge that limits the clinical application of MSCs. Our findings provide new insights into the biological functions of MT-regulated oxidative stress-induced PDLSCs and elucidate the mechanisms of YAP-mediated MT activity. These findings not only strengthen our understanding of the role of MT in processes related to cellular oxidative stress but also present potential targets for developing new therapeutic strategies for promoting tissue regeneration.
While the use of MSCs in preclinical and clinical studies for the treatment of various diseases has provided promising results, the microenvironment of oxidative stress inhibits the efficacy of MSC-mediated tissue regeneration by suppressing endogenous and exogenous MSC physiological functions and transmigration, which currently remains a major challenge that limits the clinical application of MSCs. Our findings provide new insights into the biological functions of MT-regulated oxidative stress-induced PDLSCs and elucidate the potential mechanisms by which YAP-mediated MT acts. These findings not only strengthen our comprehension of the role of MT in the process of cellular oxidative stress, but also present potential targets to develop new therapeutic strategies for promoting tissue re
| 1. | Kim J, Kang JW, Park JH, Choi Y, Choi KS, Park KD, Baek DH, Seong SK, Min HK, Kim HS. Biological characterization of long-term cultured human mesenchymal stem cells. Arch Pharm Res. 2009;32:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Fickert S, Schröter-Bobsin U, Gross AF, Hempel U, Wojciechowski C, Rentsch C, Corbeil D, Günther KP. Human mesenchymal stem cell proliferation and osteogenic differentiation during long-term ex vivo cultivation is not age dependent. J Bone Miner Metab. 2011;29:224-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Shuai Y, Liao L, Su X, Yu Y, Shao B, Jing H, Zhang X, Deng Z, Jin Y. Melatonin Treatment Improves Mesenchymal Stem Cells Therapy by Preserving Stemness during Long-term In Vitro Expansion. Theranostics. 2016;6:1899-1917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Spehar K, Pan A, Beerman I. Restoring aged stem cell functionality: Current progress and future directions. Stem Cells. 2020;38:1060-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Chen LY, Tiong C, Tsai CH, Liao WC, Yang SF, Youn SC, Mai FD, Chang HM. Early-life sleep deprivation persistently depresses melatonin production and bio-energetics of the pineal gland: potential implications for the development of metabolic deficiency. Brain Struct Funct. 2015;220:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Van de Bittner GC, Dubikovskaya EA, Bertozzi CR, Chang CJ. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc Natl Acad Sci U S A. 2010;107:21316-21321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 7. | Ksiazek K. A comprehensive review on mesenchymal stem cell growth and senescence. Rejuvenation Res. 2009;12:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Kuang Y, Hu B, Feng G, Xiang M, Deng Y, Tan M, Li J, Song J. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology. 2020;21:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Wei H, Li Z, Hu S, Chen X, Cong X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem. 2010;111:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Saidova AA, Vorobjev IA. Lineage Commitment, Signaling Pathways, and the Cytoskeleton Systems in Mesenchymal Stem Cells. Tissue Eng Part B Rev. 2020;26:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Ogrodnik M, Salmonowicz H, Jurk D, Passos JF. Expansion and Cell-Cycle Arrest: Common Denominators of Cellular Senescence. Trends Biochem Sci. 2019;44:996-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Turinetto V, Vitale E, Giachino C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 378] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 13. | Manning AL, Dyson NJ. RB: mitotic implications of a tumour suppressor. Nat Rev Cancer. 2012;12:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Witkiewicz AK, Knudsen ES. Retinoblastoma tumor suppressor pathway in breast cancer: prognosis, precision medicine, and therapeutic interventions. Breast Cancer Res. 2014;16:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. Cellular Senescence: Defining a Path Forward. Cell. 2019;179:813-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2147] [Cited by in RCA: 2192] [Article Influence: 313.1] [Reference Citation Analysis (0)] |
| 16. | Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, Donato AJ. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol. 2013;305:H251-H258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 2011;10:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Liao N, Shi Y, Zhang C, Zheng Y, Wang Y, Zhao B, Zeng Y, Liu X, Liu J. Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res Ther. 2019;10:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Zhou T, Yan Y, Zhao C, Xu Y, Wang Q, Xu N. Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production via AMPK activation. Redox Rep. 2019;24:62-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Han YS, Kim SM, Lee JH, Jung SK, Noh H, Lee SH. Melatonin protects chronic kidney disease mesenchymal stem cells against senescence via PrP(C) -dependent enhancement of the mitochondrial function. J Pineal Res. 2019;66:e12535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Chen W, Lv N, Liu H, Gu C, Zhou X, Qin W, Chen AC, Chen L, Yang H, Chen X, Liu T, He F. Melatonin Improves the Resistance of Oxidative Stress-Induced Cellular Senescence in Osteoporotic Bone Marrow Mesenchymal Stem Cells. Oxid Med Cell Longev. 2022;2022:7420726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Chan YH, Ho KN, Lee YC, Chou MJ, Lew WZ, Huang HM, Lai PC, Feng SW. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects. Stem Cell Res Ther. 2022;13:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 23. | Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, Lim DS, Pan D, Sadoshima J. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2014;5:3315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 24. | White SM, Avantaggiati ML, Nemazanyy I, Di Poto C, Yang Y, Pende M, Gibney GT, Ressom HW, Field J, Atkins MB, Yi C. YAP/TAZ Inhibition Induces Metabolic and Signaling Rewiring Resulting in Targetable Vulnerabilities in NF2-Deficient Tumor Cells. Dev Cell. 2019;49:425-443.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 25. | Wang B, Shao W, Shi Y, Liao J, Chen X, Wang C. Verteporfin induced SUMOylation of YAP1 in endometrial cancer. Am J Cancer Res. 2020;10:1207-1217. [PubMed] |
| 26. | Liang J, Wang L, Wang C, Shen J, Su B, Marisetty AL, Fang D, Kassab C, Jeong KJ, Zhao W, Lu Y, Jain AK, Zhou Z, Liang H, Sun SC, Lu C, Xu ZX, Yu Q, Shao S, Chen X, Gao M, Claret FX, Ding Z, Chen J, Chen P, Barton MC, Peng G, Mills GB, Heimberger AB. Verteporfin Inhibits PD-L1 through Autophagy and the STAT1-IRF1-TRIM28 Signaling Axis, Exerting Antitumor Efficacy. Cancer Immunol Res. 2020;8:952-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Zhang Y, Feng X, Tian H, Fu X, Gu W, Wen Y. Metformin inhibits mTOR and c-Myc by decreasing YAP protein expression in OSCC cells. Oncol Rep. 2021;45:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Wen Y, Ji Y, Zhang Y, Jiang B, Tang C, Wang Q, Chen X, Jia L, Gu W, Xu X. Knockdown of Yes-Associated Protein Induces the Apoptosis While Inhibits the Proliferation of Human Periodontal Ligament Stem Cells through Crosstalk between Erk and Bcl-2 Signaling Pathways. Int J Med Sci. 2017;14:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Jia L, Gu W, Zhang Y, Jiang B, Qiao X, Wen Y. Activated Yes-Associated Protein Accelerates Cell Cycle, Inhibits Apoptosis, and Delays Senescence in Human Periodontal Ligament Stem Cells. Int J Med Sci. 2018;15:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Chen X, Wang Q, Gu K, Li A, Fu X, Wang Y, Gu W, Wen Y. Effect of YAP on an Immortalized Periodontal Ligament Stem Cell Line. Stem Cells Int. 2019;2019:6804036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |