Published online Jun 26, 2023. doi: 10.4252/wjsc.v15.i6.561
Peer-review started: January 7, 2023
First decision: April 10, 2023
Revised: April 22, 2023
Accepted: May 16, 2023
Article in press: May 16, 2023
Published online: June 26, 2023
Processing time: 170 Days and 11.8 Hours
The high incidence and disability rates of stroke pose a heavy burden on society. Inflammation is a significant pathological reaction that occurs after an ischemic stroke. Currently, therapeutic methods, except for intravenous thrombolysis and vascular thrombectomy, have limited time windows. Mesenchymal stem cells (MSCs) can migrate, differentiate, and inhibit inflammatory immune responses. Exosomes (Exos), which are secretory vesicles, have the characteristics of the cells from which they are derived, making them attractive targets for research in recent years. MSC-derived exosomes can attenuate the inflammatory response caused by cerebral stroke by modulating damage-associated molecular patterns. In this review, research on the inflammatory response mechanisms associated with Exos therapy after an ischemic injury is discussed to provide a new approach to clinical treatment.
Core Tip: Mesenchymal stem cell-derived exosome (MSC-Exos) transplantation is a novel treatment method for ischemic stroke that exhibits certain achievements in trials. Here, we review the strategies developed for MSC-Exos in the neuroinflammatory response of patients with stroke and provide potential therapeutic targets. These methods provide new insights for the future clinical application of MSC-Exos in the treatment of ischemic stroke.
- Citation: Chen N, Wang YL, Sun HF, Wang ZY, Zhang Q, Fan FY, Ma YC, Liu FX, Zhang YK. Potential regulatory effects of stem cell exosomes on inflammatory response in ischemic stroke treatment. World J Stem Cells 2023; 15(6): 561-575
- URL: https://www.wjgnet.com/1948-0210/full/v15/i6/561.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i6.561
Stroke is a common clinical condition that frequently occurs in middle-aged and elderly people and is a global public health problem with high disability and mortality rates; it ranks third in the list of diseases affecting human lifespan[1]. The main goals of stroke treatment are vascular recanalization and reduction of cerebral ischemic injury. At present, the main recanalization methods are intravenous thrombolysis and endovascular mechanical thrombectomy; however, owing to the restricted time window and various comorbidities, few patients can benefit from these procedures[2,3]. Increasing evidence has suggested that inflammatory cytokines promote the migration of immune cells to damaged tissues through the blood-brain barrier (BBB) after stroke, aggravating the inflammatory response and leading to nerve cell injury[4,5]. However, the exact molecular mechanisms underlying the inflammatory response after stroke remain unclear, hindering the development of effective and specific treatments.
The effectiveness of stem cell transplantation, which can regulate the immune-inflammatory response and the permeability of the BBB, in the treatment of ischemic stroke (IS) has been verified[6,7]. However, pluripotent stem cells are obstructed by the BBB and cannot effectively enter the central nervous system, leading to risks, such as tumorigenicity, thrombosis, and pulmonary embolism, limiting their clinical application. It has been suggested that the therapeutic mechanism of mesenchymal stem cells (MSCs) may involve secreted exosomes (Exos) rather than the direct replacement of brain cells[8]. MSC-derived extracellular vesicles (MSC-EVs) possess the biological characteristics of cells and can penetrate the BBB, reduce the risk of tumors and pulmonary embolism, considerably improve therapeutic efficiency, and reduce complications, thereby having broad therapeutic prospects. MSC-derived Exos (MSC-Exos) reduce inflammatory responses after stroke[9-12]. However, the specific mechanism by which Exos alleviate the inflammatory response after stroke has not yet been explored.
Therefore, in this review, we present the current progress in research on the unique biological characteristics of MSC-Exos and the specific mechanism of action of MSC-Exos in the neuroinflammatory response after stroke. This review aims to explore the role of Exos in the neuroinflammatory response in stroke and provide potential therapeutic targets, with the expectation of offering a reference for future clinical treatments.
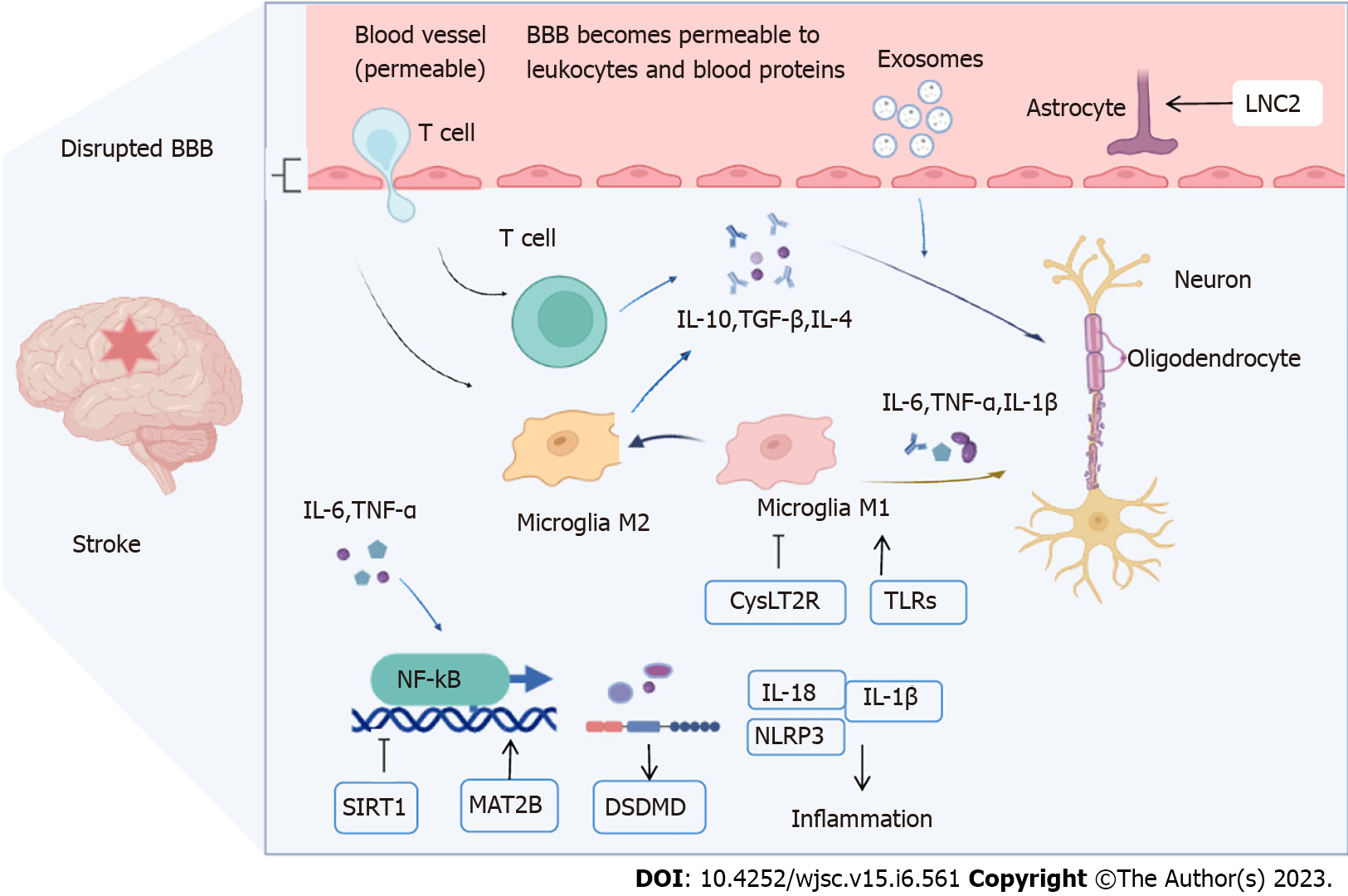
Brain cell death after stroke can lead to a series of pathological processes including cell energy failure, neuronal apoptosis, leukocyte infiltration, inflammatory immune responses, tight junction (TJ) protein breakage and degradation, BBB destruction, and increased permeability[13,14]. The main goals of IS treatment are to restore blood flow and improve functional outcomes as soon as possible[15]. In addition, methods of regulating immune inflammation and oxidative stress responses, anti-apoptosis, and promotion of angiogenesis and neurogenesis are of great significance for the treatment of cerebral apoplexy in ischemic and hypoxic injured brain tissues[16]. The BBB controls the inflow and outflow of biological substances necessary for metabolic activity and neuronal function in the brain; therefore, its structural and functional integrity is essential for maintaining the brain microenvironment. The BBB is mainly comprised of vascular endothelial cells, pericytes, the basement membrane, astrocytes, neurons, and microglia, which exchange substances that connect the central and peripheral nervous systems. The mechanisms of BBB injury after stroke include modification of TJ proteins, regulation of transporter expression, and inflammatory damage[17]. The intravascular inflammatory response marks the beginning of BBB disruption and leukocyte infiltration in ischemic brain tissue[18]. The inflammatory response after stroke is an important factor in BBB disruption and nerve cell edema, leading to damage to mental function and even death (Figure 1).
Microglia are the resident immune cells of the brain that polarize into different phenotypes (M1 or M2)[19]. M1 inflamed microglia lead to BBB dysfunction and vascular ‘leakage,’ whereas M2 microglia have inflammation-inhibiting, immune-regulating, tissue-repair, and damage-eliminating functions; they also protect the BBB[20]. Activated M1 microglia release the pro-inflammatory factors tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6, which activate the nuclear factor kappa-B (NF-κB)-mediated inflammatory response of reactive astrocytes (A1s) and further amplify this effect[21]. Owing to inflammatory response stimulation, the structure of the neurovascular unit (NVU) changes, which inhibits central nervous system restoration. This change in the microenvironment stimulates M2 microglia to initiate phagocytosis and secrete transforming growth factor-β (TGFβ), IL-4, and IL-10, and the engulfment of immune cells, indirectly protecting against inflammation-induced BBB disruption[22]. M2 polarization promotes the release of anti-inflammatory cytokines and tissue repair, including neurogenesis, axonal remodeling, angiogenesis, and oligodendrogenesis[21,23]. Activated matrix metalloproteinase (MMP)-2 and MMP-9 by microglia after stroke degrade the basement membrane and TJ proteins, resulting in BBB disruption, leukocyte infiltration, and angioedema, thus aggravating brain injury[17,24,25]. Pericytes also release cytokines that play vital roles in maintaining the structural integrity of the BBB. Under pathological conditions, dysfunctional pericytes can cause basement membrane degradation or alter NVU coordination, leading to BBB instability[17]. In addition, BBB injury along with the activation of TGFβ signaling in astrocytes may be a mechanism to disrupt NVU structure, as TGFβ overproduction affects the function of pericytes and vascular smooth muscle cells[26,27]. Microglial polarization is closely related to stroke progression; M1 microglia promote astrocyte differentiation to the A1 phenotype through a variety of signaling pathways, including the immune inflammatory response, angioedema, BBB disruption, neuronal apoptosis, and glutamate excitotoxicity, thereby exacerbating brain injury caused by IS[28,29]. The inflammatory reaction of the nervous system is closely related to the polarization of microglia, pericyte-secreted factors, astrocyte differentiation, and leukocyte species. However, the underlying mechanism of action of Exos in the treatment of neuroinflammation in stroke remains unclear.
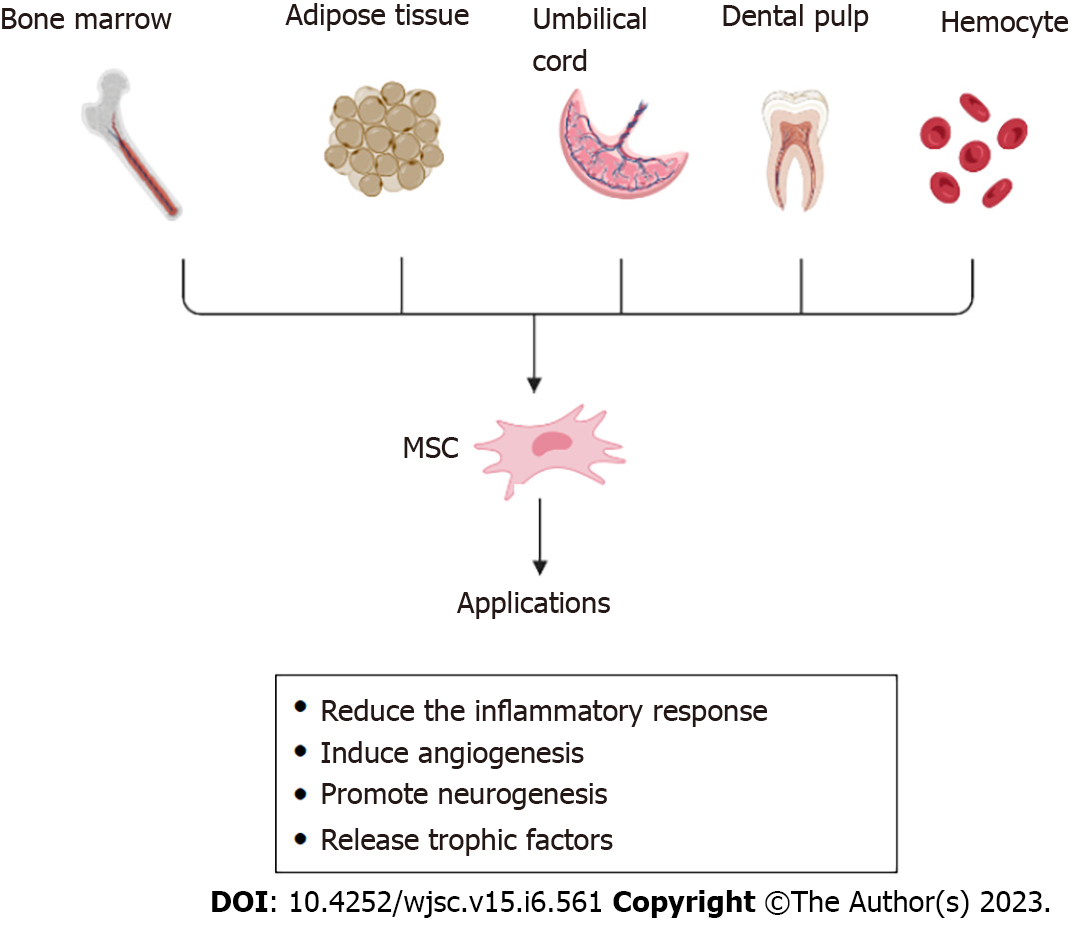
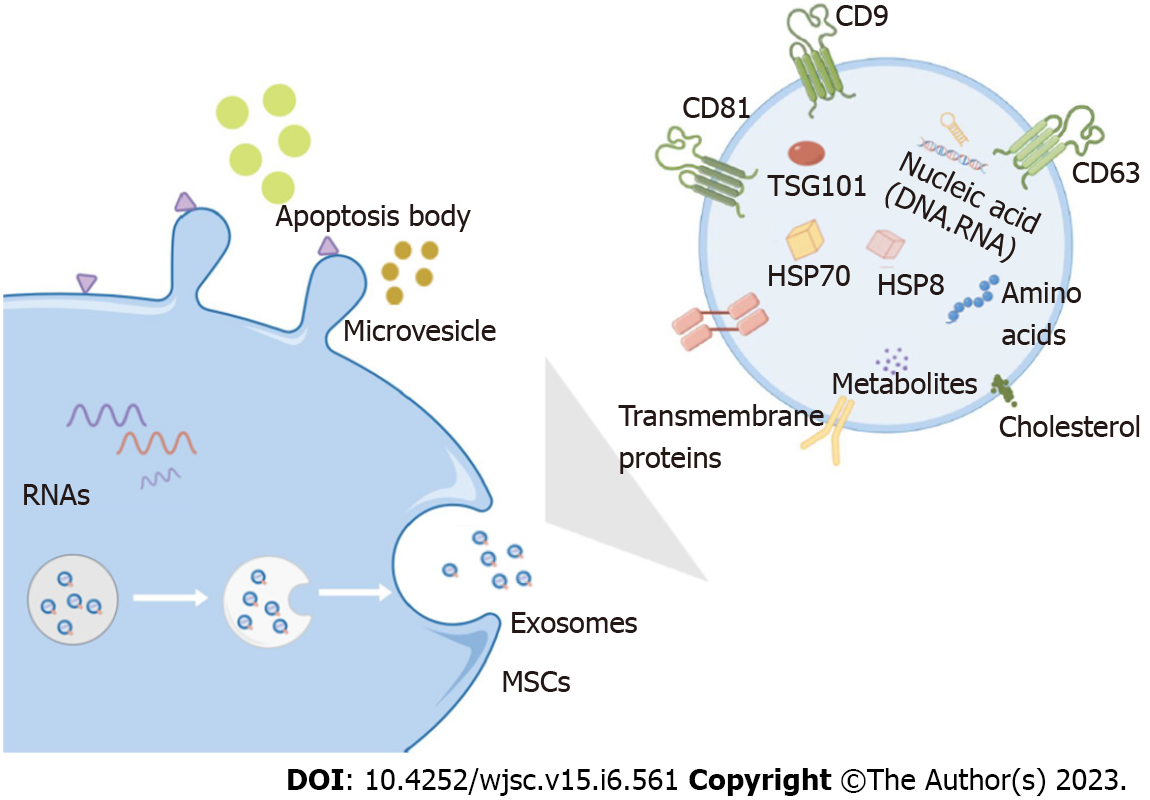
MSCs are pluripotent stem cells with self-renewal, differentiation, secretion, and homing properties. They were first discovered in the bone marrow, where they are abundant and easily extractable, and are also found in the dental pulp, umbilical cord, hemocytes, and adipose tissue (such as bone marrow MSCs, dental MSCs, umbilical cord-derived MSCs, adipose-derived MSCs, and hematopoietic stem cells)[30,31]. To overcome the problems with primary MSCs, human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and pluripotent stem cells (iPSCs), represent a promising solution[32]. MSCs can differentiate into lipogenic, chondrogenic, osteoblastic, endothelial, neural, and epithelial lineages, both in vivo and in vitro[33,34]. MSCs can reduce inflammatory responses, release trophic factors to promote therapeutic effects, induce angiogenesis, promote neurogenesis, reduce infarct volume, and replace damaged cells via immunomodulation[10,35,36]. Extracellular vesicles secreted by MSCs can be divided into three types based on their size and intracellular origin: Apoptotic bodies, microvesicles, and Exos. Apoptotic bodies are ≥ 1000 nm and microvesicles are 100–1000 nm in diameter. Exos (30–100 nm in diameter) originate from multivesicular bodies and are released by exocytosis, which is dependent on cytoskeletal reorganization, but independent of intracellular Ca2+ concentration[37,38]. When multivesicular bodies fuse with the cell membrane, Exos are released from the cells. Previously, these vesicles were considered waste products actively excreted by cells; however, studies have shown that Exos have key functions, such as transmitting information between cells, tissue regeneration, and immune regulation[39] (Figure 2).
Exos are released upon fusion with the cell membrane and trigger the release of different cellular substances. Exos can carry the same bioactive substances as their source cells and are vital for information transmission between cells, such as immune regulation and promotion of cell migration, proliferation, differentiation, and matrix synthesis[40]. Moreover, the exogenous Exos of stem cells express specific surface markers (CD9, CD63, CD81, and CD92), express specific phenotypes of stem cells (CD29, CD44, CD73, and CD90)[41], and carry heat shock proteins (HSP) proteins (HSP8, HSP60, HSP70, and HSP9), signal transduction proteins, and multivesicular production-related proteins. More importantly, they can directly transfer bioactive molecules, including non-coding regulatory microRNAs (miRNAs), messenger RNAs (mRNAs), and proteins from donor cells to recipient cells. MiRNAs are short (approximately 22 nucleotides), single-stranded, non-coding RNAs transcribed in the nucleus by RNA polymerase II from one gene or between two different genes to regulate different cellular processes such as differentiation, proliferation, metabolism, inflammation, stress response, angiogenesis, and signaling transduction[42]. miRNAs mainly affect gene expression by degrading the corresponding miRNAs or suppressing translation[43]. Alexander et al[44] showed that exosomal miRNAs participate in regulating inflammatory responses; miR-146a-containing Exos can inhibit inflammation, whereas miR-155-containing Exos promote inflammation following exposure to the same inflammatory stimulus.
Exos contain a variety of active substances that form the basis for disease treatment. These bioactive substances carried by Exos can target specific cells for information transmission and enter the cytoplasm by fusion with receptor cell membranes or endocytosis, thereby changing the target cell function by transmitting proteins, lipids, and nucleic acids[45]. Exos can be effectively isolated from donor cells and protect their contents from the external environment, ensuring the complete transmission of effective information[46]. Exos act as mediators that facilitate intercellular communication and influence the recipient cell activity by delivering content. DiR-labeled MSC-Exos were injected into a rat model of stroke via the caudal vein, and the in vivo tracer showed that Exos could penetrate the BBB and reach the brain tissue. The fluorescence signal peaked on the third day and then gradually decreased[47]. Matsumoto et al[48] also demonstrated that Exos can increase long-term neuroprotective effects after stroke, modulate peripheral immune responses, and increase angiogenesis and axonal dendritic remodeling. Therefore, the use of Exos for the treatment of neurological diseases has great potential[49,50]. These results suggest that Exos is an important therapeutic target for the treatment of stroke (Figure 3).
The potential therapeutic mechanisms of stem cell Exos involve promoting dendritic and axonal growth, repairing nerves, and promoting angiogenesis through direct actions[51,52]. Through indirect action, it can promote the secretion of inflammatory factors by cells by exogenously producing Exos that appear to interact with recipient brain cells, thereby stimulating them to release their own Exos and playing a role in anti-inflammation and neurological repair. Transplantation of MSC-Exos improves inflammatory responses in IS, maintaining BBB function, decreasing brain edema, regulating energy metabolism, and promoting antioxidant, anti-inflammatory, and anti-apoptotic effects[53]. In IS, miRNAs are involved in a variety of cellular functions, such as injured tissue repair and remodeling, and different neuronal activities. Their target genes play a crucial regulatory role in the inflammatory process of post-ischemic reperfusion injury, which explains their potential use as therapeutic targets in IS and is the focus of Exos research[54,55]. According to current research, the main signaling pathways mediated by Exos after cerebral ischemia are as follows (Table 1)[12,41,56-63].
| MicroRNA | Source | Model(s) | Functional effects | Pathway(s) | Ref. |
| miR-21a-5p | MSCs | OGD microglia | Induces microglial M2 polarization by targeting STAT3 | STAT3 | Xin et al[12], 2022 |
| miR-138-5p | BMSCs | MCAO mouse, OGD astrocytes | Promotes astrocyte proliferation and inhibits inflammatory response | LCN2 | Deng et al[41], 2019 |
| miR-542-3p | MSCs | MCAO mice, OGD human glial cells | Suppresses inflammation and prevents cerebral infarction | TLR4 | Cai et al[56], 2021 |
| miR-146a-5p | HUMSCs | MCAO mouse, OGD microglia | Anti-inflammation | IRAK1/TRAF6 | Zhang et al[57], 2021 |
| miR-223-3p | MSCs | MCAO Rats, OGD microglia | Anti-inflammation | CysLT2R | Zhao et al[58,59], 2020 |
| miR-21-3p | MSCs | MCAO rats | BBB protection, anti-inflammation, anti-apoptosis | MAT2B | Li et al[60], 2019 |
| miR-26a-5p | HUMSCs | I/R mice, OGD microglia | Inhibits microglial M1 polarization | TLRs | Li et al[61], 2020 |
| miR-150-5p | BMSCs | MCAO rats | Decelerates neuronal apoptosis, reduces inflammation | TLR5 | Li et al[62], 2022 |
| miR-30d-5p | ADSCs | Patients, MCAO rats, OGD microglia | Promotes M2 microglial/macrophage polarization | Inflammatory mediators | Jiang et al[63], 2018 |
The transcription factor NF-κB regulates many aspects of innate and adaptive immunity and plays an important role in the inflammatory response. It is also involved in the migration of immune effector cells to the inflammatory system, thereby allowing the secretion of pro-inflammatory cytokines[64]. Han et al[65] showed that MSCs-Exos protect MCAO-injured rats, possibly by regulating the AMP-activated protein kinase (AMPK) and JAK2/STAT3/NF-κB signaling pathways. NF-κB is a central inflammatory mediator responding to many immune receptors. NF-κB mediates the induction of pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6, in monocytes/macrophages[66]. Fann et al[67] confirmed the involvement of NF-κB signaling in the activation of pyrin domain-containing inflammasomes following IS. Preconditioning MSCs with lithium modifies EV secretion patterns, enhancing the therapeutic potential of the derived EVs (Li-EVs) and significantly increasing the resistance of cultured astrocytes, microglia, and neurons to hypoxic injury compared with control and native EVs. Li-EVs reduce the abundance of post-hypoxic and post-ischemic TLR4 (leading to activation of the NF-κB signaling pathway) and decrease proteasomal activity, which together contribute to reduced levels of poststroke encephalitis[68]. The miRNAs carried by Exos play a significant physiological role. Cai et al[56] confirmed that MSC-derived exosomal microRNA-542-3p (miR-542-3p) prevented ischemia-induced glial cell inflammatory responses by inhibiting TLR4. Interleukin-1 receptor-associated kinase 1 (IRAK1) and tumor necrosis factor receptor-associated factor 6 (TRAF6) may be parts of an NF-κB-induced negative feedback loop[69]. Zhang et al[57] found that injected Exos produced by human umbilical cord MSCs (HUMSC-Exos) enter the site of ischemic injury and be internalized by cells, both in vivo and in vitro. In vitro, HUMSC-Exos treatment attenuates microglial inflammation induced by oxygen-glucose deprivation (OGD). In vivo, HUMSC-Exos treatment significantly reduced infarct volume, alleviated behavioral deficits, and improved microglial activation 3 d after transient cerebral ischemia. MiR-146a-5p from HUMSC-Exos can attenuate microglial-mediated neuroinflammation through the IRAK1/TRAF6 pathway and ensuing neurological deficits after IS. NF-κB signaling pathway activation is a ‘master regulator’ of inflammation and is associated with the generation of free radicals and the activation of proteolytic enzymes and pro-inflammatory cytokines, playing an important role in regulating apoptosis after stroke[70,71]. Taken together, these results show that NF-κB signaling is essential for the regulation of brain tissue inflammasomes under ischemic conditions. In addition, MSC-Exos treatment decreased the activation of the NF-κB signaling pathway, thereby attenuating inflammasome expression and activation under ischemic conditions. These findings suggest that therapeutic interventions targeting neuroinflammasome activation may provide new opportunities for the treatment of IS.
NOD-like receptor family pyrin domain-containing 3 (NLRP3) plays an important role in mediating the inflammatory responses during cerebral IS[72]. The NLRP3 inflammasome is a multiprotein complex comprising NLRP3 and pyroptosis-related factors (ASC and caspase 1)[73]. The NF-κB and mitogen-activated protein kinase (MAPK) pathways play a major role in the expression and activation of NLRP1 and NLRP3 inflammasomes in primary cortical neurons[67]. Bone marrow MSC-Exos (BMSC-Exos) can reduce brain infarct area and cerebral edema, thus improving neurological function. MSC-Exos can downregulate the expression of NLRP3 inflammasome and pyroptosis-related proteins on the surface of neurons[74]. Moreover, it improved the transition from M1 to M2 phenotype both in vivo and in vitro. BMSC-Exos relieve cerebral ischemia/reperfusion (I/R) injury by suppressing NLRP3 inflammasome-mediated inflammation and pyroptosis via modulation of microglial polarization[74]. Sarmah et al[75] came to similar conclusions by treating a rat MCAO model with intra-arterial injections of MSCs; the levels of NLRP-1 and NLRP-3 inflammasomes and their related components IL-1β, caspase-1, and ASC were significantly reduced. NLRP3 apoptotic bodies are involved in astrocyte and microglial polarization and are closely related to the development of the inflammatory cascade after stroke, and BMSC-Exos reduce the inflammatory response after stroke by inhibiting NLRP3 activation.
Sirtuins (SIRTs) are NAD+-dependent deacylases with multiple roles in energy metabolism regulation, cell survival, transcriptional regulation, inflammation, circadian regulation, and DNA repair[76]. SIRT-1 and SIRT-3 are both associated with the inflammatory response in stroke patients. Xin et al[12] used an in vivo neonatal male mouse model of hypoxic-ischemic (HI) injury and induced in vitro hypoxia-glucose deficiency, thus simulating microglial BV-2 cells to deliver miR-21a-5p (miR-21a-5p) as a therapeutic intervention through MSC-Exos. Treatment of BV-2 cells with MSC-EVs increased cell viability and miR-21a-5p levels, which were decreased after glucose-oxygen deprivation. In both in vitro and in vivo models of HI injury, the effects on microglial polarization and STAT3 phosphorylation decreased when miR-21a-5p levels were reduced in MSC-Exos. These results suggest that MSC-Exos attenuate HI brain injury in neonatal mice by delivering miR-21a-5p, which induces microglial M2 polarization by targeting STAT3. Adipose-derived MSC-derived miR-22-3p reduces infarct volume and apoptosis in a stroke model[77]. Sarmah et al[75] demonstrated that intraarterial MSCs increase SIRT-1 to inhibit the NF-κB pathway, reducing inflammasome signaling and apoptosis, thereby exerting a neuroprotective effect. SIRT1 may be an independent risk factor for cerebral infarction, and a high concentration of SIRT1 in cerebral infarction may be associated with disruption of the BBB[78].
Cysteinyl leukotrienes (CysLTs), including leukotriene C4 (LTC4), leukotriene D4 (LTD4), and leukotriene E4 (LTE4), are derived from 5-lipoxygenase metabolites of arachidonic acid after cell necrosis and are effective mediators of inflammation[79]. The effects of CysLTs are mainly mediated by the CysLT1 and CysLT2 receptors (CysLT1R and CysLT2R), which are active in various cell types during pathological brain injury. CysLT2 is expressed in the cerebral cortex, hippocampus, substantia nigra, and lateral ventricle[80]. Zhao et al[58,59] showed that the overexpression of miR-223-3p (miR-223-3p) in MSC-Exos can reduce MCAO-induced infarction, improve neurological deficits, and promote learning and memorization. MiR-223-3p inhibits the expression of pro-inflammatory factors and promotes the secretion of anti-inflammatory factors in the ischemic cortex and hippocampus. Western blot and quantitative real-time PCR analyses also showed that exosomal miR-223-3p reduced the mRNA and protein expression of CysLT2R in vitro and in vivo. Exosomal miR-223-3p from MSCs alleviated cerebral I/R injury by inhibiting the pro-inflammatory response mediated by M1 polarization of microglia, which may be related to the inhibition of CysLT2R by exosomal miR-223-3p.
Lipocalin-2 (LCN2), a 25 kDa protein, is a neutrophil gelatinase-associated protein that affects different cellular processes during stroke. The pro-inflammatory mediator LCN2 plays a key role in I/R injury[81]. Genetic or pharmacological inhibition of these pro-inflammatory mediators (iNOS, IL-6, CCL2, and CCL9) provides neuroprotection against stroke and reduces the expression of inflammatory factors by down-regulating LCN2[41]. Deng et al[41] used a mouse MCAO model to explore the effects of BMSC-derived exosomal miR-138-5p in IS in vivo. Overexpression of miR-138-5p promoted cell proliferation and inhibited apoptosis of OGD-damaged astrocytes, accompanied by decreased expression of inflammatory factors. This was achieved by downregulating LCN2, and the expression of LCN2 protein was subsequently detected by Western blot analysis. More importantly, BMSCs attenuated neuronal injury in IS mice by delivering miR-138-5p to astrocyte Exos. Therefore, the exogenous exosomal miR-138-5p from BMSCs promotes astrocyte proliferation and inhibits the inflammatory response after IS by targeting LCN2, thereby reducing neurological impairment, which may provide a new target for IS treatment.
Methionine adenosyl transferase (MAT) is an enzyme involved in cell cycle regulation. Mammals have three major MAT genes: MAT1A, MAT2A, and MAT2B[60]. TNF-induced activation of MAT2B promotes tumor growth through the NF-κB pathway in hepatoma cells[81]. MiR-21-3p antagomir can control the inflammatory response by inhibiting NF-κB signaling; these functions of miR-21-3p are exerted by directly targeting MAT2B[82]. This interaction forms the basis of the function of miR-21-3p/MAT2B in regulating inflammation. Li et al[60] found that miR-21-3p expression was elevated in the MCAO model, and the inhibition of exogenous adipose-derived stem cell Exos miR-21-3p could inhibit the expression of MAT2B in neural cells, thereby improving the BBB status and inhibiting apoptosis and inflammatory responses. MiR-21-3p antagomir could inhibit the expression of pro-inflammatory cytokines (TNF-α, IL-1β, and IL6) and promote the expression of anti-inflammatory cytokines (IL-10). Thus, miR-21-3p can protect neural cells by inhibiting the expression of MAT2B and thus inhibiting apoptosis and inflammatory responses.
Evidence suggests that Toll-like receptors (TLRs) play important roles in the development of ischemic brain injury in adults[83]. TLRs, comprising 13 members (TLR-1 to TLR-13), are type 1 integral membrane proteins responsible for detecting invading pathogens and initiating immune responses[84,85]. The microglial TLR pathway is activated following cerebral ischemia and inhibition of TLR signaling by exosomal miR-26a-5p decreases cholesterol 25-hydroxylase protein expression, which in turn inhibits microglial M1 polarization and relieves nerve injury after brain injury[61]. The gene expression of many inflammatory mediators, such as TNF-α, IL-1, and IL-6, can reduce the development of nervous system inflammation by inhibiting TLR4 transduction pathway downregulation[86]. Upregulation of miR-326 attenuates IL-10, IL-1β, and TNF-α pro-inflammatory cytokine expression in response to lipopolysaccharide stimulation by targeting TLR4[87]. TLR5 activates NF-kB and MAPK pathways that regulate the transcription of genes encoding immune mediators[88]. Qiao et al[89] elucidated that TLR5 downregulation is accompanied by alleviated neurological deficits, reduced infarct volume, and reduced edema after IS[90]. Li et al[62] validated that BMSC-Exos can improve neurological function and pathological changes, decelerate neuronal apoptosis, and reduce inflammatory factors in MCAO rats. Exosomal miR-150-5p from BMSCs mitigates cerebral I/R injury by inhibiting TLR5 expression. These studies showed that TLRs and their related miRNAs are associated with inflammation after IS.
Dabrowska et al[91] transplanted human bone marrow stem cells and their secreted Exos into a rat model of local brain injury. The results showed that monocyte chemoattractant protein-1 (MCP-1) expression increased locally after brain injury, whereas MCP-1 expression decreased in the transplanted HUMSCs and Exos groups. In addition, they observed that the infusion of pro-inflammatory cytokines and chemokines with HUMSCs or EVs in rats with untreated focal brain injury was associated with reduced microglial/macrophage and astrocyte activation. MSC-Exos therapy can reduce the expression of the inflammatory cytokines TNF-α and IL-6, increase the expression of the cytokines IL-4 and IL-10, and reduce brain injury. Exos from stem cells can enhance the activation of CD4+ and CD8+ lymphocytes, decrease the number of dendritic cells, regulate peripheral immunosuppression caused by stroke[9], and pass antigens to the immune system through the BBB[92]. IL-4, CD206, and IL-10 are markers of M2 microglial secretion, whereas TNF-α, IL-6, and iNOS are markers of M1 microglial secretion[93]. Yang et al[94] found that the MCAO model also verified that Exos intervention reduced the infarct volume and promoted the polarization of microglia to M2 phenotype. These results demonstrate that adipose-derived stem cell Exos can prevent stroke by shifting microglia from an M1 to M2 phenotype in the hippocampus[94].
Microglia are macrophages of the central nervous system and are important components of innate and adaptive immune responses[95]. The microglial M1 type can secrete pro-inflammatory factors, whereas the M2 type can secrete anti-inflammatory factors; therefore, the fact that MSCs and Exos can promote the polarization of microglia to M2 is notable for the treatment of IS. The M2 type protects nerve cells mainly by engulfing debris and promoting the repair and regeneration of brain tissue. In contrast, inflammatory factors of the M1 phenotype aggravate post-stroke symptoms. Therefore, the microglial response after stroke is an important prognostic factor[96]. Different miRNAs transported by Exos contribute to the differentiation of microglia into distinct phenotypes. Increased levels of miR-124-3p in microglial Exos promote M2 microglial polarization, reduce brain damage, and improve stroke outcomes[97]. Adipose-derived MSCs (ADMSCs) participate in the repair process of tissues through paracrine effects after relieving nerve injury; ADMSCs have similar biological characteristics to MSCs. Stimulation of AMSC-derived Exos with inflammatory factors was found to convert M1 microglia into M2 microglia, suggesting that AMSC-derived Exos promote microglial polarization by activating pro-inflammatory microenvironment signals[98]. miRNAs are critical regulators of genes involved in various biological processes; miR-146a-5p-enriched BMSC-Exos directly target IRAK1 and nuclear factor-activated T cell 5 (NFAT5), which contributes to inflammatory responses and polarize M1 microglia/macrophages[99]. Exos containing miR-216a-5p, miR-124, miR-155, miR-182, miR-17-5p, miR-30d-5p, and miR-223-3p were found to promote microglial M2 polarization[63,100-103]. BMSC-Exos promote microglial polarization from M1 to M2, inhibit inflammation-related signaling pathways, and reduce endothelial cell injury and neurological impairment caused by IS[104-106]. Although astrocytes may play a role in brain inflammation, little is known about their role in stroke pathology[107].
Changes in regulatory T cell (Treg) numbers and function after stroke are accompanied by a decrease in immunosuppressive function, which affects stroke prognosis[108]. The immunosuppressive function of Tregs is largely impaired during stroke and Treg-derived anti-inflammatory factors, including transforming growth factor-beta (TGF-b) and IL-10, are reduced[109,110]. MiRNAs delivered by stem cell Exos can induce anti-inflammatory polarization as important regulators of Treg homeostasis and function[111,112]. MSC-Exos induce anti-inflammatory IL-10 and TGF-β transcription, attenuate pro-inflammatory factors IL-1β, IL-6, and TNF-β, and inhibit the differentiation and activation of Tregs[113,114]. Furthermore, MSC-Exos are absorbed by endothelial cells, impair T-cell function by inhibiting T-cell proliferation in vitro, and increase endothelial cell proliferation, migration, and capillary formation in a dose-dependent manner[115]. Wang et al[116] showed that the intravenous injection of MSC-EVs reduced neurological deficits, cerebral infarct volume, brain edema, and neuronal injury in both young and old mice. The neuroprotective and anti-inflammatory effects of MSC-EVs were demonstrated through a decrease in leukocyte infiltration and, specifically, polymorphonuclear neutrophil, monocyte, and macrophage infiltration, in the cerebral ischemic areas of aged mice. In addition, MSC-EVs significantly decreased the number of monocytes and activated Tregs. The expression and phos
Brain injury after stroke is a complex pathological process. This review summarizes the recent studies on the mechanism of action of MSC-Exos in regulating inflammatory responses during IS treatment. MSC-Exos regulate microglial polarization through various pathways such as NF-κB, NLRP3, and STATs, indicating that microglial M1 to M2 phenotype polarization is closely related to the inflammatory response after IS.
However, some essential questions remain unanswered. Stroke-induced brain injury involves multiple mechanisms that cannot be explained by a single one. Immune inflammation plays a crucial role in this process, especially the NF-κB, NLRP3, and other signaling pathways. After immune inflammation, microglia, leukocytes, and other inflammatory cells are activated and release many pro-inflammatory factors. Additionally, nerve cells are affected by varying degrees of damage caused by ischemia and hypoxia after stroke.
However, these studies have some limitations. MSC-Exos can mediate different signaling pathways to reduce inflammatory responses after stroke in animal models. However, these results have not been translated into clinical practice. Most studies have focused on exosomal miRNAs, indicating that they play an important role in regulating cellular functions. However, research on other bioactive molecules contained in Exos, such as miRNAs, mRNAs, and proteins, is limited. This does not mean that this mechanism of action of miRNAs can explain how Exos attenuate post-stroke inflammation. The dosage, mode of administration, and duration of action of the Exos should be elucidated. Exos are considered ideal biomarkers and drug delivery vehicles, with great potential for overcoming the limitations of stem cell therapy[119]. The use of Exos as drug-loaded systems will facilitate breakthroughs in the research and development of targeted drugs for clinical treatment. Moreover, new directions and methods will be provided for stroke treatment.
| 1. | Yang L, Qian J, Yang B, He Q, Wang J, Weng Q. Challenges and Improvements of Novel Therapies for Ischemic Stroke. Front Pharmacol. 2021;12:721156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of Vascular Aging. Circ Res. 2018;123:849-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 660] [Article Influence: 94.3] [Reference Citation Analysis (14)] |
| 3. | Yang P, Zhang Y, Zhang L, Treurniet KM, Chen W, Peng Y, Han H, Wang J, Wang S, Yin C, Liu S, Wang P, Fang Q, Shi H, Yang J, Wen C, Li C, Jiang C, Sun J, Yue X, Lou M, Zhang M, Shu H, Sun D, Liang H, Li T, Guo F, Ke K, Yuan H, Wang G, Yang W, Li Z, Xing P, Zhang P, Zhou Y, Wang H, Xu Y, Huang Q, Wu T, Zhao R, Li Q, Fang Y, Wang L, Lu J, Li Y, Fu J, Zhong X, Wang Y, Goyal M, Dippel DWJ, Hong B, Deng B, Roos YBWEM, Majoie CBLM, Liu J; DIRECT-MT Investigators. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N Engl J Med. 2020;382:1981-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 625] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 4. | Tariq MB, Lee J, McCullough LD. Sex differences in the inflammatory response to stroke. Semin Immunopathol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | Zou L, Han R. Inflammatory Response and Immune Regulation in Brain-Heart Interaction after Stroke. Cardiovasc Ther. 2022;2022:2406122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 6. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1360] [Article Influence: 194.3] [Reference Citation Analysis (0)] |
| 7. | Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158:94-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | Li Y, Cheng Q, Hu G, Deng T, Wang Q, Zhou J, Su X. Extracellular vesicles in mesenchymal stromal cells: A novel therapeutic strategy for stroke. Exp Ther Med. 2018;15:4067-4079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 2015;4:1131-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 620] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 10. | Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 230] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 11. | Yu H, Xu Z, Qu G, Wang H, Lin L, Li X, Xie X, Lei Y, He X, Chen Y, Li Y. Hypoxic Preconditioning Enhances the Efficacy of Mesenchymal Stem Cells-Derived Conditioned Medium in Switching Microglia toward Anti-inflammatory Polarization in Ischemia/Reperfusion. Cell Mol Neurobiol. 2021;41:505-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Xin DQ, Zhao YJ, Li TT, Ke HF, Gai CC, Guo XF, Chen WQ, Liu DX, Wang Z. The delivery of miR-21a-5p by extracellular vesicles induces microglial polarization via the STAT3 pathway following hypoxia-ischemia in neonatal mice. Neural Regen Res. 2022;17:2238-2246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | An H, Zhou B, Ji X. Mitochondrial quality control in acute ischemic stroke. J Cereb Blood Flow Metab. 2021;41:3157-3170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 14. | Datta A, Sarmah D, Mounica L, Kaur H, Kesharwani R, Verma G, Veeresh P, Kotian V, Kalia K, Borah A, Wang X, Dave KR, Yavagal DR, Bhattacharya P. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl Stroke Res. 2020;11:1185-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 15. | Mendelson SJ, Prabhakaran S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA. 2021;325:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 527] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 16. | Onose G, Anghelescu A, Blendea D, Ciobanu V, Daia C, Firan FC, Oprea M, Spinu A, Popescu C, Ionescu A, Busnatu Ș, Munteanu C. Cellular and Molecular Targets for Non-Invasive, Non-Pharmacological Therapeutic/Rehabilitative Interventions in Acute Ischemic Stroke. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 2018;315:C343-C356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 453] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 18. | Jurcau A, Simion A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 324] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 19. | Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 547] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 20. | Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 21. | Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 1162] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 22. | Qiu YM, Zhang CL, Chen AQ, Wang HL, Zhou YF, Li YN, Hu B. Immune Cells in the BBB Disruption After Acute Ischemic Stroke: Targets for Immune Therapy? Front Immunol. 2021;12:678744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 282] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 23. | Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1401] [Cited by in RCA: 1570] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 24. | Shu ZM, Shu XD, Li HQ, Sun Y, Shan H, Sun XY, Du RH, Lu M, Xiao M, Ding JH, Hu G. Ginkgolide B Protects Against Ischemic Stroke Via Modulating Microglia Polarization in Mice. CNS Neurosci Ther. 2016;22:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Zhu J, Cao D, Guo C, Liu M, Tao Y, Zhou J, Wang F, Zhao Y, Wei J, Zhang Y, Fang W, Li Y. Berberine Facilitates Angiogenesis Against Ischemic Stroke Through Modulating Microglial Polarization via AMPK Signaling. Cell Mol Neurobiol. 2019;39:751-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 26. | Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 855] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 27. | Kato T, Sekine Y, Nozaki H, Uemura M, Ando S, Hirokawa S, Onodera O. Excessive Production of Transforming Growth Factor β1 Causes Mural Cell Depletion From Cerebral Small Vessels. Front Aging Neurosci. 2020;12:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Wan T, Huang Y, Gao X, Wu W, Guo W. Microglia Polarization: A Novel Target of Exosome for Stroke Treatment. Front Cell Dev Biol. 2022;10:842320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Jolivel V, Bicker F, Binamé F, Ploen R, Keller S, Gollan R, Jurek B, Birkenstock J, Poisa-Beiro L, Bruttger J, Opitz V, Thal SC, Waisman A, Bäuerle T, Schäfer MK, Zipp F, Schmidt MHH. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015;129:279-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 30. | Sherman LS, Romagano MP, Williams SF, Rameshwar P. Mesenchymal stem cell therapies in brain disease. Semin Cell Dev Biol. 2019;95:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Srithanyarat SS, Choosiri M, Sa-Ard-Iam N, Petcharat P, Osathanon T. Characteristics of mesenchymal stem cells from supracrestal gingival connective tissue. J Periodontol. 2023;94:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 32. | Liu TM. Application of mesenchymal stem cells derived from human pluripotent stem cells in regenerative medicine. World J Stem Cells. 2021;13:1826-1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Chen YH, Pruett-Miller SM. Improving single-cell cloning workflow for gene editing in human pluripotent stem cells. Stem Cell Res. 2018;31:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Maqsood M, Kang M, Wu X, Chen J, Teng L, Qiu L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020;256:118002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Dong N, Hong H, Qi J, Zhang S, Wang J. Mesenchymal Stem Cells: Therapeutic Mechanisms for Stroke. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Gu Y, Zhang Y, Bi Y, Liu J, Tan B, Gong M, Li T, Chen J. Mesenchymal stem cells suppress neuronal apoptosis and decrease IL-10 release via the TLR2/NFκB pathway in rats with hypoxic-ischemic brain damage. Mol Brain. 2015;8:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KE, Sadik M, Alaarg A, Smith CI, Lehtiö J, El Andaloussi S, Wood MJ, Vader P. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 755] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 38. | Yang Y, Ye Y, Su X, He J, Bai W, He X. MSCs-Derived Exosomes and Neuroinflammation, Neurogenesis and Therapy of Traumatic Brain Injury. Front Cell Neurosci. 2017;11:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 39. | Jafari D, Shajari S, Jafari R, Mardi N, Gomari H, Ganji F, Forouzandeh Moghadam M, Samadikuchaksaraei A. Designer Exosomes: A New Platform for Biotechnology Therapeutics. BioDrugs. 2020;34:567-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 40. | Kawabori M, Shichinohe H, Kuroda S, Houkin K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 41. | Deng Y, Chen D, Gao F, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Zhang J, Miao Z. Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng. 2019;13:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 42. | Rahmani A, Saleki K, Javanmehr N, Khodaparast J, Saadat P, Nouri HR. Mesenchymal stem cell-derived extracellular vesicle-based therapies protect against coupled degeneration of the central nervous and vascular systems in stroke. Ageing Res Rev. 2020;62:101106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 43. | Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 521] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 44. | Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O'Connell RM. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 629] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 45. | Chen J, Chopp M. Exosome Therapy for Stroke. Stroke. 2018;49:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 46. | Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom Rev. 2015;34:474-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 47. | Xu R, Bai Y, Min S, Xu X, Tang T, Ju S. In vivo Monitoring and Assessment of Exogenous Mesenchymal Stem Cell-Derived Exosomes in Mice with Ischemic Stroke by Molecular Imaging. Int J Nanomedicine. 2020;15:9011-9023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 48. | Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N, Shi M, Banks WA, Zhang J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol Commun. 2017;5:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 49. | Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res. 2017;12:19-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 50. | Liu W, Bai X, Zhang A, Huang J, Xu S, Zhang J. Role of Exosomes in Central Nervous System Diseases. Front Mol Neurosci. 2019;12:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 51. | Xu Y, Hu Y, Xu S, Liu F, Gao Y. Exosomal microRNAs as Potential Biomarkers and Therapeutic Agents for Acute Ischemic Stroke: New Expectations. Front Neurol. 2021;12:747380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 52. | Otero-Ortega L, Laso-García F, Gómez-de Frutos MD, Rodríguez-Frutos B, Pascual-Guerra J, Fuentes B, Díez-Tejedor E, Gutiérrez-Fernández M. White Matter Repair After Extracellular Vesicles Administration in an Experimental Animal Model of Subcortical Stroke. Sci Rep. 2017;7:44433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 53. | Wang J, Chen S, Zhang W, Chen Y, Bihl JC. Exosomes from miRNA-126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neurosci Ther. 2020;26:1255-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 54. | Vasudeva K, Munshi A. miRNA dysregulation in ischaemic stroke: Focus on diagnosis, prognosis, therapeutic and protective biomarkers. Eur J Neurosci. 2020;52:3610-3627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Ghoreishy A, Khosravi A, Ghaemmaghami A. Exosomal microRNA and stroke: A review. J Cell Biochem. 2019;120:16352-16361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Cai G, Cai G, Zhou H, Zhuang Z, Liu K, Pei S, Wang Y, Wang H, Wang X, Xu S, Cui C, Sun M, Guo S, Jia K, Zhang D. Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Res Ther. 2021;12:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 57. | Zhang Z, Zou X, Zhang R, Xie Y, Feng Z, Li F, Han J, Sun H, Ouyang Q, Hua S, Lv B, Hua T, Liu Z, Cai Y, Zou Y, Tang Y, Jiang X. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging (Albany NY). 2021;13:3060-3079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 58. | Zhao Y, Gan Y, Xu G, Hua K, Liu D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. 2020;260:118403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 59. | Zhao Y, Gan Y, Xu G, Yin G, Liu D. MSCs-Derived Exosomes Attenuate Acute Brain Injury and Inhibit Microglial Inflammation by Reversing CysLT2R-ERK1/2 Mediated Microglia M1 Polarization. Neurochem Res. 2020;45:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 60. | Li C, Fei K, Tian F, Gao C, Yang S. Adipose-derived mesenchymal stem cells attenuate ischemic brain injuries in rats by modulating miR-21-3p/MAT2B signaling transduction. Croat Med J. 2019;60:439-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Li G, Xiao L, Qin H, Zhuang Q, Zhang W, Liu L, Di C, Zhang Y. Exosomes-carried microRNA-26b-5p regulates microglia M1 polarization after cerebral ischemia/reperfusion. Cell Cycle. 2020;19:1022-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 62. | Li X, Bi T, Yang S. Exosomal microRNA-150-5p from bone marrow mesenchymal stromal cells mitigates cerebral ischemia/reperfusion injury via targeting toll-like receptor 5. Bioengineered. 2022;13:3030-3043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell Physiol Biochem. 2018;47:864-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 256] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 64. | Barnabei L, Laplantine E, Mbongo W, Rieux-Laucat F, Weil R. NF-κB: At the Borders of Autoimmunity and Inflammation. Front Immunol. 2021;12:716469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 442] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 65. | Han M, Cao Y, Xue H, Chu X, Li T, Xin D, Yuan L, Ke H, Li G, Wang Z. Neuroprotective Effect of Mesenchymal Stromal Cell-Derived Extracellular Vesicles Against Cerebral Ischemia-Reperfusion-Induced Neural Functional Injury: A Pivotal Role for AMPK and JAK2/STAT3/NF-κB Signaling Pathway Modulation. Drug Des Devel Ther. 2020;14:2865-2876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023-17023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2830] [Cited by in RCA: 6128] [Article Influence: 680.9] [Reference Citation Analysis (0)] |
| 67. | Fann DY, Lim YA, Cheng YL, Lok KZ, Chunduri P, Baik SH, Drummond GR, Dheen ST, Sobey CG, Jo DG, Chen CL, Arumugam TV. Evidence that NF-κB and MAPK Signaling Promotes NLRP Inflammasome Activation in Neurons Following Ischemic Stroke. Mol Neurobiol. 2018;55:1082-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 265] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 68. | Haupt M, Zheng X, Kuang Y, Lieschke S, Janssen L, Bosche B, Jin F, Hein K, Kilic E, Venkataramani V, Hermann DM, Bähr M, Doeppner TR. Lithium modulates miR-1906 levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation. Stem Cells Transl Med. 2021;10:357-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Zilahi E, Tarr T, Papp G, Griger Z, Sipka S, Zeher M. Increased microRNA-146a/b, TRAF6 gene and decreased IRAK1 gene expressions in the peripheral mononuclear cells of patients with Sjögren's syndrome. Immunol Lett. 2012;141:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 70. | Harari OA, Liao JK. NF-κB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010;1207:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 71. | Vidale S, Consoli A, Arnaboldi M, Consoli D. Postischemic Inflammation in Acute Stroke. J Clin Neurol. 2017;13:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 72. | Gong Z, Pan J, Shen Q, Li M, Peng Y. Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury. J Neuroinflammation. 2018;15:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 73. | Alfonso-Loeches S, Ureña-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci. 2014;8:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 74. | Liu X, Zhang M, Liu H, Zhu R, He H, Zhou Y, Zhang Y, Li C, Liang D, Zeng Q, Huang G. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol. 2021;341:113700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 75. | Sarmah D, Datta A, Kaur H, Kalia K, Borah A, Rodriguez AM, Yavagal DR, Bhattacharya P. Sirtuin-1 - Mediated NF-κB Pathway Modulation to Mitigate Inflammasome Signaling and Cellular Apoptosis is One of the Neuroprotective Effects of Intra-arterial Mesenchymal Stem Cell Therapy Following Ischemic Stroke. Stem Cell Rev Rep. 2022;18:821-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 76. | Kane AE, Sinclair DA. Sirtuins and NAD(+) in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ Res. 2018;123:868-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 371] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 77. | Li B, Wang Z, Yu M, Wang X, Chen C, Zhang Z, Zhang M, Sun C, Zhao C, Li Q, Wang W, Wang T, Zhang L, Ning G, Feng S. miR-22-3p enhances the intrinsic regenerative abilities of primary sensory neurons via the CBL/p-EGFR/p-STAT3/GAP43/p-GAP43 axis. J Cell Physiol. 2020;235:4605-4617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Liang X, Liu Y, Jia S, Xu X, Dong M, Wei Y. SIRT1: The Value of Functional Outcome, Stroke-Related Dementia, Anxiety, and Depression in Patients with Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2019;28:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Sasaki F, Yokomizo T. The leukotriene receptors as therapeutic targets of inflammatory diseases. Int Immunol. 2019;31:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 80. | Wang Y, Yang Y, Zhang S, Li C, Zhang L. Modulation of neuroinflammation by cysteinyl leukotriene 1 and 2 receptors: implications for cerebral ischemia and neurodegenerative diseases. Neurobiol Aging. 2020;87:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2029] [Cited by in RCA: 1976] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 82. | Yang H, Ara AI, Magilnick N, Xia M, Ramani K, Chen H, Lee TD, Mato JM, Lu SC. Expression pattern, regulation, and functions of methionine adenosyltransferase 2beta splicing variants in hepatoma cells. Gastroenterology. 2008;134:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Ge X, Li W, Huang S, Yin Z, Yang M, Han Z, Chen F, Wang H, Lei P, Zhang J. Increased miR-21-3p in Injured Brain Microvascular Endothelial Cells after Traumatic Brain Injury Aggravates Blood-Brain Barrier Damage by Promoting Cellular Apoptosis and Inflammation through Targeting MAT2B. J Neurotrauma. 2019;36:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 84. | Paschon V, Takada SH, Ikebara JM, Sousa E, Raeisossadati R, Ulrich H, Kihara AH. Interplay Between Exosomes, microRNAs and Toll-Like Receptors in Brain Disorders. Mol Neurobiol. 2016;53:2016-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 85. | Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 629] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 86. | Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol. 2014;426:1246-1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 570] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 87. | Zhang XS, Li W, Wu Q, Wu LY, Ye ZN, Liu JP, Zhuang Z, Zhou ML, Zhang X, Hang CH. Resveratrol Attenuates Acute Inflammatory Injury in Experimental Subarachnoid Hemorrhage in Rats via Inhibition of TLR4 Pathway. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 88. | Chaurasiya V, Kumari S, Onteru SK, Singh D. Up-regulation of miR-326 regulates pro-inflammatory cytokines targeting TLR-4 in buffalo granulosa cells. Mol Immunol. 2020;119:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Qiao H, Zhang X, Zhu C, Dong L, Wang L, Xing Y, Wang C, Ji Y, Cao X. Luteolin downregulates TLR4, TLR5, NF-κB and p-p38MAPK expression, upregulates the p-ERK expression, and protects rat brains against focal ischemia. Brain Res. 2012;1448:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 90. | Gu L, Huang J, Tan J, Wei Q, Jiang H, Shen T, Liang B, Tang N. Impact of TLR5 rs5744174 on stroke risk, gene expression and on inflammatory cytokines, and lipid levels in stroke patients. Neurol Sci. 2016;37:1537-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Dabrowska S, Andrzejewska A, Strzemecki D, Muraca M, Janowski M, Lukomska B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation. 2019;16:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 92. | Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871:455-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 735] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 93. | Jiang CT, Wu WF, Deng YH, Ge JW. Modulators of microglia activation and polarization in ischemic stroke (Review). Mol Med Rep. 2020;21:2006-2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 94. | Yang H, Tu Z, Yang D, Hu M, Zhou L, Li Q, Yu B, Hou S. Exosomes from hypoxic pre-treated ADSCs attenuate acute ischemic stroke-induced brain injury via delivery of circ-Rps5 and promote M2 microglia/macrophage polarization. Neurosci Lett. 2022;769:136389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 95. | Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother. 2018;105:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 96. | Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063-3070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 1251] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 97. | Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, Chen F, Wang H, Zhang J, Lei P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018;32:512-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 98. | Domenis R, Cifù A, Quaglia S, Pistis C, Moretti M, Vicario A, Parodi PC, Fabris M, Niazi KR, Soon-Shiong P, Curcio F. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep. 2018;8:13325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 99. | Duan S, Wang F, Cao J, Wang C. Exosomes Derived from MicroRNA-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization. Drug Des Devel Ther. 2020;14:3143-3158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 100. | Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, Zhao S, Kong F, Gu C, Fan J, Cai W. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 435] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 101. | Zha Z, Gao YF, Ji J, Sun YQ, Li JL, Qi F, Zhang N, Jin LY, Xue B, Yang T, Fan YP, Zhao H, Wang L. Bu Shen Yi Sui Capsule Alleviates Neuroinflammation and Demyelination by Promoting Microglia toward M2 Polarization, Which Correlates with Changes in miR-124 and miR-155 in Experimental Autoimmune Encephalomyelitis. Oxid Med Cell Longev. 2021;2021:5521503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 102. | Yang J, Chen Y, Jiang K, Zhao G, Guo S, Liu J, Yang Y, Deng G. MicroRNA-182 supplies negative feedback regulation to ameliorate lipopolysaccharide-induced ALI in mice by targeting TLR4. J Cell Physiol. 2020;235:5925-5937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 103. | Li Y, Guo W, Cai Y. NEAT1 Promotes LPS-induced Inflammatory Injury in Macrophages by Regulating MiR-17-5p/TLR4. Open Med (Wars). 2020;15:38-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 104. | Zhang Q, Bian G, Chen P, Liu L, Yu C, Liu F, Xue Q, Chung SK, Song B, Ju G, Wang J. Aldose Reductase Regulates Microglia/Macrophages Polarization Through the cAMP Response Element-Binding Protein After Spinal Cord Injury in Mice. Mol Neurobiol. 2016;53:662-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 105. | Lin L, Yihao T, Zhou F, Yin N, Qiang T, Haowen Z, Qianwei C, Jun T, Yuan Z, Gang Z, Hua F, Yunfeng Y, Zhi C. Inflammatory Regulation by Driving Microglial M2 Polarization: Neuroprotective Effects of Cannabinoid Receptor-2 Activation in Intracerebral Hemorrhage. Front Immunol. 2017;8:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 106. | Zhang Y, Xu N, Ding Y, Zhang Y, Li Q, Flores J, Haghighiabyaneh M, Doycheva D, Tang J, Zhang JH. Chemerin suppresses neuroinflammation and improves neurological recovery via CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage in neonatal rats. Brain Behav Immun. 2018;70:179-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 107. | Coulibaly AP, Provencio JJ. Aneurysmal Subarachnoid Hemorrhage: an Overview of Inflammation-Induced Cellular Changes. Neurotherapeutics. 2020;17:436-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 108. | Wang M, Thomson AW, Yu F, Hazra R, Junagade A, Hu X. Regulatory T lymphocytes as a therapy for ischemic stroke. Semin Immunopathol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 109. | Ruhnau J, Schulze J, von Sarnowski B, Heinrich M, Langner S, Pötschke C, Wilden A, Kessler C, Bröker BM, Vogelgesang A, Dressel A. Reduced Numbers and Impaired Function of Regulatory T Cells in Peripheral Blood of Ischemic Stroke Patients. Mediators Inflamm. 2016;2016:2974605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Hu Y, Zheng Y, Wu Y, Ni B, Shi S. Imbalance between IL-17A-producing cells and regulatory T cells during ischemic stroke. Mediators Inflamm. 2014;2014:813045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 111. | Zhu D, Johnson TK, Wang Y, Thomas M, Huynh K, Yang Q, Bond VC, Chen YE, Liu D. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res Ther. 2020;11:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 112. | Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 450] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 113. | Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front Immunol. 2014;5:556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 114. | Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 531] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 115. | Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 554] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 116. | Wang C, Börger V, Mohamud Yusuf A, Tertel T, Stambouli O, Murke F, Freund N, Kleinschnitz C, Herz J, Gunzer M, Popa-Wagner A, Doeppner TR, Giebel B, Hermann DM. Postischemic Neuroprotection Associated With Anti-Inflammatory Effects by Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles in Aged Mice. Stroke. 2022;53:e14-e18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 117. | Bellon M, Lepelletier Y, Hermine O, Nicot C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood. 2009;113:4914-4917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 118. | Zhang M, Johnson-Stephenson TK, Wang W, Wang Y, Li J, Li L, Zen K, Chen X, Zhu D. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17(+) regulatory T cell. Stem Cell Res Ther. 2022;13:484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 109] [Reference Citation Analysis (0)] |
| 119. | Chen J, Jin J, Li K, Shi L, Wen X, Fang F. Progresses and Prospects of Neuroprotective Agents-Loaded Nanoparticles and Biomimetic Material in Ischemic Stroke. Front Cell Neurosci. 2022;16:868323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Henan Stroke Association.
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen X, United States; Song BW, South Korea; Liu W, China S-Editor: Li L L-Editor: Wang TQ P-Editor: Li L