Published online Jun 26, 2023. doi: 10.4252/wjsc.v15.i6.576
Peer-review started: January 31, 2023
First decision: February 10, 2023
Revised: March 18, 2023
Accepted: May 8, 2023
Article in press: May 8, 2023
Published online: June 26, 2023
Processing time: 145 Days and 18.5 Hours
Lung cancer is the major cause of cancer-related deaths worldwide, it has one of the lowest 5-year survival rate, mainly because it is diagnosed in the late stage of the disease. Lung cancer is classified into two groups, small cell lung cancer (SCLC) and non-SCLC (NSCLC). In turn, NSCLC is categorized into three distinct cell subtypes: Adenocarcinoma, squamous cell carcinoma, and large cell carci
Core Tip: Lung cancer stem cells (CSCs) could have a functional role in primary tumor initiation, invasion and metastasis, resistance to chemotherapeutic drugs, and recurrence in lung cancer. To improve lung cancer treatments, it is necessary to identify and characterize CSCs populations in lung tissue and develop targeted therapies against these cell types. This review discusses the current knowledge on CSCs in lung tissue and future perspectives in lung cancer treatment.
- Citation: Romeo HE, Barreiro Arcos ML. Clinical relevance of stem cells in lung cancer. World J Stem Cells 2023; 15(6): 576-588
- URL: https://www.wjgnet.com/1948-0210/full/v15/i6/576.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i6.576
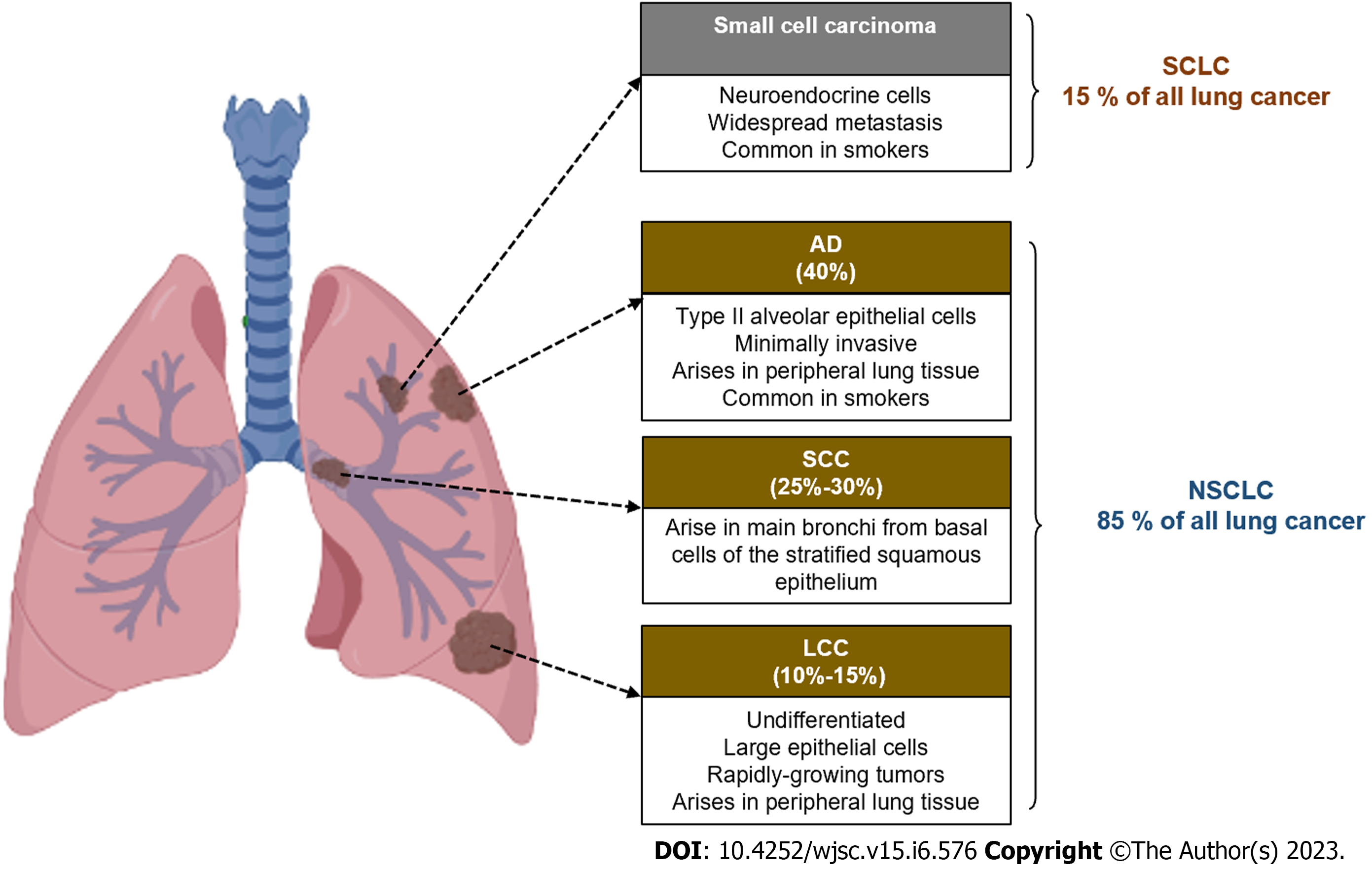
Lung cancer is the leading cause of cancer death worldwide, in both men and women. Lung cancer is classified into two histological types, small cell lung cancer (SCLC) derived from cells of the neuroendocrine lineage and non-SCLC (NSCLC) derived from epithelial cells. The latter is the most frequent type of lung cancer in the population, since represents approximately 85% of all lung tumors, while SCLC represents the remaining 15%[1].
Based on the morphology of the transformed cells, NSCLC is classified into three subtypes: Squamous cell carcinoma (SCC), adenocarcinoma (AD), and large cell carcinoma (LCC). AD arises in peripheral lung tissue from type II alveolar epithelial cells that line the small alveoli, while SCC arises in the central lung region from cells of the stratified squamous epithelium that line the airways from the trachea to the main bronchi. LCC is a heterogeneous group of tumors that lack the morphological characteristics of AD and SCC sub-types or SCLC and arise from epithelial cells that line the lungs[1,2].
SCLC arises from lung epithelial cells from the main bronchi to the terminal alveoli. Their histologic features correlate with the site of origin and reflect epithelial variations of the airways[2]. The classification and main characteristics of lung cancer are shown in Figure 1.
All forms of lung cancer have a poor prognosis, mainly SCLC and SCC, which are typically seen in smokers. SCLC is the most aggressive lung cancer because it grows quickly and spreads to other tissues, generating metastases in the liver, brain, bones, and adrenal glands[3]. LCC has a better prognosis, which depends on the cell subtype. The poor prognosis is generally associated with the ability of the cells to metastasize and the late diagnosis of the disease, since the symptoms and signs do not usually appear in the early stages[4]. People with lung cancer may have chest pain, frequent coughing, blood in the mucus, trouble breathing, swallowing or speaking, loss of appetite and weight, and tiredness[5].
The prevalence of lung cancer worldwide is 13%, with an incidence of 1.8 million new cases per year. Lung cancer is responsible for about 20% of all cancer-related deaths worldwide and has one of the lowest 5-year survival rates (7% in SCLC and 11-17% in NSCLC, depending on the subtype and stage of the cancer[6].
The main cause of lung cancer is long-term smoking, with a 25-fold increase in the risk of developing the disease. However, there are other risk factors such as genetic factors, long-term menopausal hormone replacement therapy, exposure to radon, asbestos, metals (arsenic, cadmium, and chromium), organic chemicals found in smoke coal, and a history of lung disease such as tuberculosis, emphysema, or chronic bronchitis[7]. Smoking can cause all types of lung cancer, but is most strongly linked to SCLC and SCC, while AD is the most common type of lung cancer in patients who have never smoked. In the first two, cancer development frequently occurs in people 60 to 70 years of age with a long-term history of tobacco smoking, while AD occurs in younger people[7].
In this review, we update knowledge about the properties of SCs and their participation in the initiation and progression of lung cancer, pointing out the biomarkers that may be useful in therapeutic strategies or as a prognosis of the disease. In addition, we discuss the role of SCs in the resistance to therapeutic treatments.
Lung carcinogenesis is a chronic process that involves multiple genetic, cellular, and tissue alterations as a consequence of mutations in genes that regulate growth, differentiation, and apoptosis. Cancers are caused by the accumulation of mutations in critical genes, specifically those that control cell growth and division or the repair of damaged DNA. These changes allow cells to grow and divide uncontrollably to form a tumor. In almost all cases of lung cancer, these genetic changes, which are present only in certain lung cells, are acquired during life as a result of exposure to carcinogens, long-term hormone therapy, or chronic lung disease[8].
The most frequently mutated genes in lung cancer are epidermal growth factor receptor (EGFR), Kirsten rat sarcoma virus (KRAS) and tumor protein 53 (TP53)[9,10]. The proteins encoded by these genes participate in signaling pathways that contribute to lung tumorigenesis. Mutations in the EGFR gene cause the expression of receptors on the cell membrane that are constitutively activated, triggering signaling pathways within cells involved in lung tumorigenesis. Binding of epidermal growth factor (EGF) to EGFR induces receptor phosphorylation at tyrosine residues and activates multiple downstream signaling pathways, such as the Ras-Raf-MAPK, PI3K, and STAT pathways, which induce proliferation and cell invasion, angiogenesis, inhibition of apoptosis and metastasis. The constitutive activity of EGFR has been observed in more than 60% of patients with NSCLC and is due to different mutations present in the receptor[11]. KRAS was first identified as a viral oncogene in the Kirsten RAt Sarcoma virus. The KRAS gene encodes the Ras proteins, low molecular weight enzymes with guanosine triphosphate hydrolase (GTPase) activity located on the inside of the plasma membrane. Ras GTPases are activated in response to the binding of ligands to several receptors such as EGF, platelet-derived growth factor, integrin, and cytokine receptors. Mutations in the KRAS gene cause loss of the ability of Ras to hydrolyze GTP to guanosine diphosphate leading to constant activation of Ras[12]. Similar to constitutive activation of EGFR, mutated Ras stimulates multiple signaling pathways relevant to tumor development. Mutations in the KRAS gene are of high frequency in NSCLC but are also present in other types of cancer such as colorectal and pancreatic cancer[13]. Another protein associated with the onset of lung cancer is the p53 tumor suppressor protein encoded by the TP53 gene. This tumor suppressor responds to several cellular stresses to regulate expression of target genes, thereby inducing DNA repair or cell cycle arrest and apoptosis[14]. Mutations in the TP53 gene synthesize a non-functional p53 protein, causing accumulation of damaged DNA in cells and dysregulation of the cell cycle. Mutations in the KRAS and TP53 genes have been found in up to 30% of patients with lung cancer and have been considered as predictors of poor prognosis[9]. Mutations in other genes such as
Lung tumor tissue is characterized by a heterogeneity of cell types with different phenotypic characteristics and properties as a result of the accumulation of gene mutations and differential cell signaling in the tumor microenvironment. However, it has been shown that only a small fraction of cells in tumor tissue have the ability to form tumor spheroids in vitro and develop tumors when they are transplanted into immunodeficient animals. These observations raise the need to study which are the cell populations in the tumor with tumorigenic potential.
Cancer stem cells (CSCs) are defined as a subpopulation of tumor cells residing in the tumor with SCs characteristics. CSCs have the ability to self-renew, generate identical daughter cells with stem cell properties, differentiate into multiple cancer cell lineages, facilitate tumor growth and survival, and metastasize to distal sites. In addition, they could be the cause of evasion of the immune system, resistance to chemotherapy and radiation therapy, and cancer relapse[15]. CSCs differ from normal tissue-specific SCs only in their uncontrolled growth and altered genotypes[16].
The seminal proposition that CSCs could be responsible for tumor initiation was introduced by the end of XIX century[17], however the first conclusive evidence for CSCs in acute myeloid leukemia was demonstrated in 1997 by Bonnet and Dick[18]. These authors isolated a subpopulation of CD34+/CD38- leukemic cells capable of initiating tumors in non-obese diabetic/severe combined immunodeficiency disease (NOD/SCID) mice histologically similar to the donor. In recent years, CSCs have been identified in several tumors, including breast, brain, colon, and lung cancer[19].
CSCs are a small proportion of the cell population of a tumor (less than 1%) that exhibit high tumorigenic potential. This is supported by experiments where thousands of tumor cells need to be inoculated into syngeneic or immunodeficient animals for a solid tumor to develop. Some years ago, this requirement was thought to be due to the loss of viability of tumor cells during transfer or the absence of a suitable niche for tumor growth. The new paradigm indicates that only CSCs (a small fraction of the transplanted cells) has the potential to generate a tumor[20]. This hypothesis can be reinforced by the fact that metastases are histologically heterogeneous and similar to the primary tumor, which implies that the cell that gave rise to it has the potential to differentiate into multiple cell types, such as a SCs[19].
Several questions have been raised about the origin of CSCs in tumor tissue. The first hypothesis is that CSCs could originate from tissue-specific normal SCs that have dysregulated cell growth. In this sense, it has been shown that all tissues harbor SCs that play an essential role in tissue repair[16]. These normal SCs could be transformed into CSCs due to the accumulation of gene mutations or the action of chemical mediators released under conditions of tissue damage[21]. This hypothesis is accepted for tissues with a high degree of cell renewal, such as intestinal epithelium or skin, but it is controversial for tissues that are quiescent or that renew slowly, such as lung epithelium. In this sense, some authors have identified a fraction of cells in active division (less than 1.3%) located in specific niches of the tracheal and bronchiolar epithelium with characteristics of SCs that could become CSCs[22,23]. The second hypothesis is that CSCs could originate from more differentiated progenitor cells that have acquired the capacity for self-renewal or differentiated tumor cells (called CSCs-like cells) due to mutations in genes that regulate the cell cycle, hypoxia, or chemical mediators of the tumor microenvironment such as nitric oxide or certain interleukin[24].
Tumor tissue is heterogeneous, showing different cell populations that have their own signaling pathways, leading to more complex therapeutic strategies. Factors contributing to intratumoral heterogeneity include genetic mutations, epigenetic changes, interactions with the microenvironment and CSCs[25]. CSCs represent only a small group of cells within the heterogeneity of tumor tissue. SCs trigger specific signaling pathways, such as Notch, Hedgehog, and Wnt, that allow them to self-renew and proliferate in tissue repair processes. These pathways are tightly controlled, but their aberrant activation in CSCs can induce the expression of tumor molecular markers, leading to tumorigenicity and chemoresistance[26].
CSCs have been identified in several types of cancer through proteins that are differentially expressed in these cells. These proteins include cell membrane receptors, cell adhesion molecules, cell membrane transporters, enzymes involved in metabolism, and transcription factors. Its are used as biomarkers to predict the prognosis of the disease, evaluate the most appropriate chemotherapy treatments and the efficacy of the therapies in tumor remission[15].
Biomarkers that have been identified in lung cancer include cluster of differentiation-133 (CD133), cluster of differentiation-44 (CD44), epithelial cell adhesion molecule (EpCAM), aldehyde dehydrogenase 1A1 (ALDH1A1), ATP-binding cassette sub-family G member 2 (ABCG2) and the transcription factors octamer-binding transcription factor 4 (Oct-4), sex-determining region Y-box 2 (Sox2), and Nanog[15,26]. Although these biomarkers have different structures and functional roles, its are linked to the SCs properties and uncontrolled proliferation of tumor cells. The biomarkers identified in lung CSCs are shown in Figure 2.
Biomarkers are not specific for lung CSCs and may be expressed in other types of cancer, such as breast, brain, colon or liver cancer. Since a single marker is not sufficient to accurately identify CSCs, combinations of several markers are used to identify and isolate CSCs in tumor tissue[27].
CD133: CD133, also known as prominin-1, is a transmembrane glycoprotein involved in cell growth and differentiation through its involvement in multiple signaling pathways[27,28]. It has been identified as the main biomarker of SCs in normal tissue and of stemness in tumor tissue from patients with NSCLC or SCLC.CD133 expression is essential to maintain CSCs characteristics such as tumor cell proliferation, migration, and invasion, and the ability to resist chemotherapy[27].
In lung cancer, the high level of expression of CD133 has been correlated with epithelial to mesenchymal transitions and the formation of metastases in lymph nodes and other tissues, which reveals that CD133 is a biomarker of tumor aggressiveness and poor prognosis of the pathology[29-31]. The molecular mechanisms involved in CD133-mediated cell growth and resistance to chemotherapy are still under study, but appear to be associated with the Wnt/catenin, PI3K-AKT and SRC-FAK signaling pathways[32,33]. Furthermore, CD133 has been shown to directly interact with vascular endothelial growth factor (VEGF), stimulating angiogenesis and leading to tumor growth[34]. CD133 is also used as diagnostic, predictive, or therapeutic biomarkers in other types of cancer including breast, stomach, liver, prostate, colorectal, pancreatic, and renal[28].
CD44: CD44 is a transmembrane glycoprotein of high structural and functional diversity due to alternative splicing processes and post-translational modifications. It can bind to a wide variety of ligands on the cell surface, including hyaluronic acid (HA), and trigger multiple cellular processes such as cell-cell signaling, cell growth and differentiation, cell adhesion and migration, angiogenesis, or cell survival[26].
CD44 regulates several signaling pathways to promote cancer progression, including Notch, Hedgehog, and Wnt pathways[26]. In addition, it can act as a co-receptor and heterodimerizes with growth factor receptors [EGFR, fibroblast growth factor receptor (FGFR), hepatocyte growth factor receptor, VEGF receptor, transforming growth factorβ receptor] and leads to activation of the PI3K-AKT and MAPK pathways[15]. It is hypothesized that CD44 could play an important role in tumorigenesis mediated by the constitutive activation of EGFR, whose expression is frequently mutated in patients with NSCLC. In this last sense, CD44 could be an important marker to predict the efficacy of chemotherapy using EGFR-specific tyrosine kinase inhibitors in patients with lung cancer[15].
CD44 is expressed in almost all tumor types, but it has been identified as the main CSCs biomarker in lung ADs and in SCC. Its expression is associated with a more aggressive tumor phenotype, with the ability to metastasize and resist chemotherapy[35].
EpCAM: EpCAM is a transmembrane protein and acts as an adhesion molecule on the lung epithelium. It is a potential biomarker for lung tumors of epithelial origin[36]. EpCAM expression in lung tumor epithelium is upregulated by metastasis-associated protein 1 and leads to increased metastatic capacity of tumor cells[36]. The co-expression of EpCAM with CD44 and CD166 in NSCLC indicates a greater self-renewal capacity, clonal heterogeneity and stemness. These biomarkers in lung tumor tissue indicate a poor prognosis for the disease[37].
ABCG2: ABCG2 is a transporter of xenobiotic compounds into the extracellular space, which has been implicated in the development of multidrug resistance (MDR) in cancer. The wide variety of ABCG2 substrates includes several antitumoral drugs such as paclitaxel, doxorubicin, cisplatin, topotecan, mitoxantrone and irinotecan. ABCG2 also expels the fluorescent dye Hoechst 33342 out of the cell, a property that is unique to SCs and is used for identification of these cells[38]. The expression of ABCG2 in SCs has been conserved in many tissues, such as the pancreas, lung, heart, testis, liver, brain, prostate, and embryonic tissue, demonstrating the importance of this transporter for cell survival[39]. In lung cancer, increased expression of ABCG2 has been found in SCs, associated with the upregulation and activity of the transcription factors Sp1, Sp3, YAP1 and Nrf2[40-42]. Its expression in lung CSCs is associated with cancer relapse and poor prognosis[42].
ALDH1: ALDH1 is an enzyme that participates in cellular metabolism through the oxidation of aldehydes to carboxylic acids and is a marker of SCs of normal and tumor tissue[43]. It has three main isotypes, ALDH1A1, ALDH1A2 and ALDH1A3, but its activity is mainly attributed to the ALDH1A1 isotype[43].
Increased expression of ALDH has been found in NSCLC overexpressing a subpopulation of CD44+/EpCAM+ cells[44]. Overexpression of the ALDH1 and CD133 markers was found exclusively in SCC and AD[45]. ALDH1A1 overexpression is associated with an aggressive chemotherapy-resistant tumor phenotype. Despite this, ALDH1 could be a useful therapeutic target for tumors growing in tissues that do not normally express high levels of ALDH1A1, such as the lung, breast, colon, and stomach[43].
Otherwise, the transcription factors involved in the normal activity of SCs could be deregulated in cancer, and activate cellular pluripotency genes and suppress differentiation genes, triggering signaling pathways responsible for the characteristics of CSCs. Transcription factors that regulate the functions of lung CSCs, such as Oct-4, Sox2, and Nanog, have been identified.
Oct-4: Oct-4 also known as POU5F1, is a member of the POU transcription factor family that contains a binding domain to the ATGCAAAT sequence in target genes[15]. The Oct-4 gene encodes four protein isoforms called Oct4A, Oct4B-190, Oct4B-265, and Oct4B-164. Of these, Oct4A is known to transcribe a wide variety of genes that regulate SCs stemming. Several Oct-4A target genes have been identified in CSCs of lung cancer, including Fgf4, Utf1, Opn, Rex1/Zfp42, and Fbx15[15].
Oct-4A transcriptional activity is upregulated by post-translational modifications, through phosphorylation at residues Ser229, Ser236 and tyr327 or sumoylation at residue Lys118. Its expression is high in SCs, however Oct-4 is ubiquitinated and degraded by the proteasome in the cell differentiation process[15].
In lung cancer, Oct4 activity is associated with chemotherapy resistance, cancer relapse, and worse outcomes[15].
Sox2: Sox2 is a member of the high mobility group (HMG) box gene family encoded by the sex-determining region Y-box gene. HMG is a sequence of 80 amino acids that acts as a DNA-binding domain in several target genes related to the maintenance of pluripotency in embryonic SCs and CSCs[46]. Sox2 is closely associated with early embryonic development, neuronal differentiation, bronchial morphogenesis, and airway epithelial maturation[46].
In lung cancer, Sox2 can regulate the transcription of the c-MYC, Wnt and Notch oncogenes and increase metastatic capacity through the FGFR-ERK1/2-SOX2 signaling pathway[47]. In SCC, Sox2 can induce expression of tumor-related factors p63 and keratin 6 and lead to cancer differentiation, migration, and invasion. In SCLC, Sox2 is crucial in the PI3K-Akt-Sox2 signaling pathway and may mediate chemoresistance[47]. In addition, Sox2 together with Oct-4 increase its expression under hypoxia in tumor tissue, which induces the expression of the CD133 marker and the self-renewal and maintenance of lung CSCs[48].
Sox2 transcriptional activity can be increased by phosphorylation at amino acid residue Thr118 or methylation at Arg113, which inhibits its proteasome degradation or promotes its homodimerization, respectively[49].
Sox2 and Oct4 have been located in the cell nucleus of SCLC and lung AD, but not in their paracancerous tissues or benign tumor tissues, pointing to the importance of Sox2 and Oct4 as potential markers for cancer therapies[50].
Nanog: Nanog, a DNA-binding homeobox transcription factor, may promote cell proliferation, renewal, and stem properties. Nanog can regulate cell pluripotency through two mechanisms of action. Thus, Nanog can repress the transcription of genes essential for cell differentiation, such as Gata4 and Gata6, or it can activate the transcription of genes necessary for self-renewal, such as Rex1[15].
Nanog expression is upregulated by Nr5a2 promoting CSCs properties and tumorigenesis in NSCLC[51]. Nanog is highly expressed in pluripotent cells and its expression is downregulated during differentiation. Its transcriptional activity is increased by phosphorylation at amino acid residues Ser52, 65 and 71 and Thr287, which abolishes its ubiquitination and degradation by the proteasome[52].
Nanog is increased in many types of carcinomas, including lung cancers, and is associated with chemoresistance, cancer relapse, and poor prognosis[15,52].
Most clinical trials involve the use of drugs whose targets of action are protein tyrosine kinases, regulators of the cell cycle or cell signaling pathways. Several chemotherapy drugs are not effective in controlling tumor growth and metastases. Thus, novel therapeutic agents directed against CSCs are a hope for patients who do not respond to conventional therapies or who relapse after cancer treatment. These therapies include the use of synthetic or natural inhibitors, monoclonal or bispecific antibodies (BsAb), antibodies-drug conjugates, aptamer-drug conjugates, or chimeric antigen receptor T (CAR-T) therapies. Novel therapies using stem cell biomarkers as pharmacological targets are summarized in Table 1.
| Stem cell marker-target | Therapeutic strategies in clinical trials and preclinical studies |
| Differentiation cluster | |
| CD133 | Docetaxel-loaded liposomes conjugated to anti-CD133 aptamers |
| Gefitinib-loaded nanomicelles conjugated to anti-CD133 aptamers | |
| Salinomycin sodium-loaded nanoparticles conjugated to anti-CD133 antibody | |
| CD133-specific CAR-T cells plus anti-PD-1 antibody and a CD73 inhibitor | |
| CD44 | Salazosulfapyridine plus cisplatin and pemetrexed (Phase I clinical trial for the treatment of advanced non-squamous NSCLC) |
| mAb MEM-85 (monoclonal antibody) | |
| HA-Cisplatin conjugated | |
| HA-Irinotecan conjugated (Phase II study for treatment of SCLC) | |
| HA-Apoferritin conjugated | |
| HA-conjugated cisplatin-loaded nanoparticles | |
| Cell adhesion molecules | |
| EpCAM | Doxorubicin-loaded nanoparticles conjugated to EpCAM aptamer |
| Catumaxomab BsAb anti-human EpCAM/CD3 T-cell antigen (Phase I study for treatment of NSCLC) | |
| MT110 BsAb anti-EpCAM/CD3 T-cell antigen (Phase I clinical trial for treatment of lung adenocarcinoma) | |
| MuS110 BsAb anti-human EpCAM/CD3 T-cell antigen | |
| 2C11x4-7 BsAb anti-murine EpCAM/CD3 T-cell antigen | |
| Cell membrane transporters | |
| ABCG2 | Secalonic acid D |
| Axitinib in combination with topotecan or mitoxantrone | |
| FL118 (topoisomerase 1 inhibitor) in combination with irinotecan, topotecan or cisplatino | |
| A-803467 (tetrodotoxin-resistant sodium channel blocker) in combination with topotecan, doxorubicin or mitoxantrone | |
| Verteporfin (YAP1 inhibitor) | |
| Metabolic enzymes | |
| ALDH | Disulfiram in combination with cisplatin plus vinorelbine (Phase II clinical trial for the treatment of NSCLC) |
| Disulfiram alone or in combination with diethylaminobenzaldehyde and cisplatin | |
| FL118 in combination withirinotecan, topotecan or cisplatino | |
| Transcription factors and signaling pathway | |
| Oct-4 | FL118 in combination with irinotecan, topotecan or cisplatino |
| Notch-signaling pathway | BsAb-5 directed against c-MET and CTLA-4 in CD166+ NSCLC |
Salazosulfapyridine in combination with cisplatin and pemetrexed is under phase I study for advanced NSCLC (Trial registration-UMIN 000017854). This drug inhibits the intracellular uptake of cysteine by the cysteine-glutamate antiporter in CSCs that overexpress CD44 marker, preventing the synthesis of the antioxidant GSH, essential for cellular redox homeostasis, and leading to the inhibition of tumor growth[53].
Phase II trial using disulfiram in combination with cisplatin plus vinorelbine have shown to be effective for the treatment of metastatic NSCLC (Trial registration-NCT 00312819)[54]. In vitro studies in NSCLC cell lines treated with cisplatin have shown the growth of chemoresistant ALDH1+ cell subpopulations. These cells were sensitive to the cytotoxic effects of cisplatin after treatment with diethylaminobenzaldehyde and disulfiram, inhibitors of ALDH1 activity[55]. In xenograft models in NOD/SCID mice, it has been shown that disulfaran inhibits the activity of ALDH1A1 and the expression of Sox2, Oct-4 and Nanog, reducing the size of tumors derived from ALDH+ CSCs and cancer recurrence[56].
ABCG2 transporter is one of the main CSCs markers under study due to its role in MDR. It has been found that the secalonic acid D, a metabolite of marine-derived mangrove endophytic fungus, can down-regulate the expression of ABCG2 by activation of Calpaina 1, a protease that shortens the half-life of the transporter[57]. In addition, Verteporfin, an inhibitor of the YAP1 transcription factor, was found to down-regulate the expression of the ABCG2 transporter in lung CSCs[41].
A-803467, a tetrodotoxin-resistant sodium channel blocker, inhibits ABCG2 transporter activity, increasing the sensitivity to chemotherapeutic drugs such as topotecan, doxorubicin and mitoxantrone in multidrug resistant cells. A combination of A-803467 and ABCG2 substrates may potentially be a novel therapeutic treatment in tumors overexpressing the ABCG2 protein[58].
FL118, a topoisomerase 1 inhibitor similar to camptothecin analogues (such as irinotecan or topotecan) used in the clinic, selectively inhibits the expression of several members of the proapoptotic protein family such as Survivin, Xiap, CIAP2 and BCL-2. It has also been found that FL118 down-regulate the expression of ABCG2, ALDH1A1 and Oct-4. FL118 improves sensitivity to chemotherapy and inhibits the growth of CSCs and metastases. Moreover, FL118 is effective for human tumors that acquire irinotecan, topotecan and cisplatino resistance due to its ability to bypass the drug resistance induced by ABC transporters[59].
mAbMEM-85 is a monoclonal antibody of therapeutic interest since it inhibits the growth of lung cancer cells in murine models. It has been shown that mAbMEM-85 recognizes the hyaluronate binding site in the C-terminal region of CD44 in lung cancer SCs[60].
Axitinib is a monoclonal antibody that inhibits the activity of the ABCG2 transporter in CSCs, and reverses MDR. Studies in A549 human lung cancer cells and nude mice bearing S1-M1-80 xenografts that overexpress ABCG2 have shown that axitinib increases tumor cell apoptosis induced by chemotherapeutic drugs such as topotecan and mitoxantrone without causing additional toxicity[61].
BsAb target two different epitopes or antigens simultaneously. One of the Fab fragments recognizes epitopes on cytotoxic drugs or CD8+ T or NK immune cells, while the other Fab fragment can bind epitopes on CSCs. Then, BsAbs can selectively direct effector cells or chemotherapeutic drugs towards CSCs, promoting their destruction. BsAb have been extensively explored in translational and clinical studies in lung cancer.
Catumaxomab is a BsAb with binding sites directed to human EpCAM and CD3 T-cell antigen. A phase I study has shown that catumaxomab is safe and tolerable when administered intravenously in patients with NSCLC[62]. Other anti-CD3-EpCAM BsAbs have been studied in immunocompetent mice bearing lung tumors, showing potent inhibition of local and disseminated tumor growth[63]. MT110 BsAb targeting EpCAM/CD3 T-cell antigen has been tested in patients with colorectal, gastric, and lung cancer[64]. MuS110 BsAb was found to have similar in vitro characteristics and in vivo antitumor activity as MT110[65].
BsAb-5 target cellular mesenchymal-to-epithelial transition factor (c-MET) and cytotoxic T-lymphocyte-associated protein 4 in CD166+ lung CSCs with high affinity and specificity. BsAb-5 has been shown to reduce tumor size in mouse models by a mechanism that involves inhibition of the c-MET-Notch pathway in CSCs and the up-regulating effector T cells. BsAb could be a possible drug for the treatment of human NSCLC[66].
The extracellular domain of CD44 contains a HA binding site, a property that is used for the development of HA-conjugated antitumor drugs. Preclinical studies have shown that HA can be effectively used to deliver chemotherapy and selectively decrease CD44+ lung CSCs. Thus, a phase II clinical trial (ACTRN 12611000520932) using HA-irinotecan has shown to be effective in the treatment of SCLC[67]. Other studies show that cisplatin or apoferritin conjugated to HA are effective in eliminating lung CSCs[68,69].
In recent years, nanoparticulate systems have been developed to encapsulate antitumor drugs. The efficacy of these systems in the treatment of cancer has been improved by the conjugation of the nanoparticles to specific ligands, antibodies or aptamers directed against SC markers. In this sense, it has been shown that the intratracheal administration of HA-conjugated cisplatin-loaded nanoparticles attenuated lung cancer growth in mice[70]. Other systems such as salinomycin sodium-loaded nanoparticles conjugated to anti-CD133 antibody, doxorubicin-loaded nanoparticles conjugated to EpCAM aptamer and gefitinib or docetaxel-loaded liposomes conjugated to anti-CD133 aptamers have been shown to be effective in inhibiting tumor growth[71-74].
CAR-T cell is a new therapeutic approach that involves the development of a receptor expressed in T cells that recognizes certain specific antigens on the membrane of cancer cells, triggering an antitumor immune response. The efficacy of specific CAR-T therapy against the AC133 epitope of CD133 has been studied in a mouse model with orthotopic xenograft. AC133-specific CAR-T cells reduced tumor size and prolonged survival in the humanized orthotopic SCLC model but were unable to eliminate tumors completely. However, the combination of AC133-specific CAR-T cells with an anti-PD-1 antibody and a CD73 inhibitor was able to eliminate chemoresistant CSCs[75].
Current chemotherapies for lung cancer involve the use of drugs whose targets of action are protein tyrosine kinases, regulators of the cell cycle or cell signaling pathways. Despite improvements in treatments, some patients do not respond to therapies or have cancer relapses months or years after treatment. It is hypothesized that one of the main causes of cancer relapse is the ineffectiveness of anticancer drugs to eliminate CSCs in tumor tissue. Chemotherapy and radiotherapy induce senescence of tumor cells. Factors released by senescent cells into the tumor microenvironment could activate signaling pathways that induce phenotypic and functional changes in SCs, increasing their plasticity and uncontrolled growth. In addition, CSCs could be chemoresistant due to the expression of transporters in cell membranes that expel xenobiotic compounds into the extracellular space.
In lung cancer, several biomarkers have been identified in CSCs associated with the maintenance of tumorigenicity, metastasis and chemoresistance. These biomarkers could be useful as targets for the effective treatment of lung cancer. Thus, new drugs directed against CSCs include the use of inhibitors, monoclonal and BsAb, antibody-drug conjugates or aptamer-drug conjugates, and CAR-T therapies. These therapies are a hope for patients who do not respond to conventional treatments or relapse in lung cancer.
| 1. | Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 475] [Cited by in RCA: 585] [Article Influence: 65.0] [Reference Citation Analysis (9)] |
| 2. | Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398:535-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 1723] [Article Influence: 344.6] [Reference Citation Analysis (0)] |
| 3. | Wang WZ, Shulman A, Amann JM, Carbone DP, Tsichlis PN. Small cell lung cancer: Subtypes and therapeutic implications. Semin Cancer Biol. 2022;86:543-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Nooreldeen R, Bach H. Current and Future Development in Lung Cancer Diagnosis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 526] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 5. | Beckles MA, Spiro SG, Colice GL, Rudd RM. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes. Chest. 2003;123:97S-104S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56720] [Article Influence: 7090.0] [Reference Citation Analysis (135)] |
| 7. | Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48:889-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 611] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 8. | Schwartz AG, Cote ML. Epidemiology of Lung Cancer. Adv Exp Med Biol. 2016;893:21-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Chen Y, Shi JX, Pan XF, Feng J, Zhao H. Identification of candidate genes for lung cancer somatic mutation test kits. Genet Mol Biol. 2013;36:455-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Wadowska K, Bil-Lula I, Trembecki Ł, Śliwińska-Mossoń M. Genetic Markers in Lung Cancer Diagnosis: A Review. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (32)] |
| 11. | Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020;61:167-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 447] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 12. | Ferrer I, Zugazagoitia J, Herbertz S, John W, Paz-Ares L, Schmid-Bindert G. KRAS-Mutant non-small cell lung cancer: From biology to therapy. Lung Cancer. 2018;124:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 13. | Timar J, Kashofer K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev. 2020;39:1029-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (12)] |
| 14. | Yang L, Zhou Y, Li Y, Zhou J, Wu Y, Cui Y, Yang G, Hong Y. Mutations of p53 and KRAS activate NF-κB to promote chemoresistance and tumorigenesis via dysregulation of cell cycle and suppression of apoptosis in lung cancer cells. Cancer Lett. 2015;357:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Maiuthed A, Chantarawong W, Chanvorachote P. Lung Cancer Stem Cells and Cancer Stem Cell-targeting Natural Compounds. Anticancer Res. 2018;38:3797-3809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Pathak S. Organ- and tissue-specific stem cells and carcinogenesis. Anticancer Res. 2002;22:1353-1356. [PubMed] |
| 17. | Cohnheim J. Congenitales, quergestreiftes Muskelsarkom der Nieren. Archiv f pathol Anat. 1875;65:64-69. [RCA] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 119] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4851] [Cited by in RCA: 4934] [Article Influence: 170.1] [Reference Citation Analysis (1)] |
| 19. | Rahman M, Deleyrolle L, Vedam-Mai V, Azari H, Abd-El-Barr M, Reynolds BA. The cancer stem cell hypothesis: failures and pitfalls. Neurosurgery. 2011;68:531-45; discussion 545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Naveen SV, Kalaivani K. Cancer stem cells and evolving novel therapies: a paradigm shift. Stem Cell Investig. 2018;5:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Chen Y, Xue Y, Jin Y, Ji H. Lung stem cells in regeneration and tumorigenesis. J Genet Genomics. 2021;48:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 292] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1623] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 24. | Sasai K, Takao-Rikitsu E, Sukezane T, Yanagita E, Nakagawa H, Itoh-Yagi M, Izumi Y, Itoh T, Akagi T. Engineering cancer stem-like cells from normal human lung epithelial cells. PLoS One. 2017;12:e0175147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park). 2014;28:1101-1107, 1110. [PubMed] |
| 26. | Zheng Y, Wang L, Yin L, Yao Z, Tong R, Xue J, Lu Y. Lung Cancer Stem Cell Markers as Therapeutic Targets: An Update on Signaling Pathways and Therapies. Front Oncol. 2022;12:873994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 27. | Prabavathy D, Swarnalatha Y, Ramadoss N. Lung cancer stem cells-origin, characteristics and therapy. Stem Cell Investig. 2018;5:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Walcher L, Kistenmacher AK, Suo H, Kitte R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S, Kossatz-Boehlert U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front Immunol. 2020;11:1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 672] [Article Influence: 112.0] [Reference Citation Analysis (2)] |
| 29. | Koren A, Rijavec M, Kern I, Sodja E, Korosec P, Cufer T. BMI1, ALDH1A1, and CD133 Transcripts Connect Epithelial-Mesenchymal Transition to Cancer Stem Cells in Lung Carcinoma. Stem Cells Int. 2016;2016:9714315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Wu H, Qi XW, Yan GN, Zhang QB, Xu C, Bian XW. Is CD133 expression a prognostic biomarker of non-small-cell lung cancer? A systematic review and meta-analysis. PLoS One. 2014;9:e100168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Yamashita N, Oyama T, So T, Miyata T, Yoshimatsu T, Nakano R, Matsunaga W, Gotoh A. Association Between CD133 Expression and Prognosis in Human Lung Adenocarcinoma. Anticancer Res. 2021;41:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Jang JW, Song Y, Kim SH, Kim J, Seo HR. Potential mechanisms of CD133 in cancer stem cells. Life Sci. 2017;184:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Aghajani M, Mansoori B, Mohammadi A, Asadzadeh Z, Baradaran B. New emerging roles of CD133 in cancer stem cell: Signaling pathway and miRNA regulation. J Cell Physiol. 2019;234:21642-21661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Adini A, Adini I, Ghosh K, Benny O, Pravda E, Hu R, Luyindula D, D'Amato RJ. The stem cell marker prominin-1/CD133 interacts with vascular endothelial growth factor and potentiates its action. Angiogenesis. 2013;16:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD, Fink LM, Ma Y, Wong MP. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One. 2010;5:e14062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 36. | Zhou N, Wang H, Liu H, Xue H, Lin F, Meng X, Liang A, Zhao Z, Liu Y, Qian H. MTA1-upregulated EpCAM is associated with metastatic behaviors and poor prognosis in lung cancer. J Exp Clin Cancer Res. 2015;34:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Satar NA, Fakiruddin KS, Lim MN, Mok PL, Zakaria N, Fakharuzi NA, Abd Rahman AZ, Zakaria Z, Yahaya BH, Baharuddin P. Novel triplepositive markers identified in human nonsmall cell lung cancer cell line with chemotherapy-resistant and putative cancer stem cell characteristics. Oncol Rep. 2018;40:669-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Smith PJ, Furon E, Wiltshire M, Campbell L, Feeney GP, Snyder RD, Errington RJ. ABCG2-associated resistance to Hoechst 33342 and topotecan in a murine cell model with constitutive expression of side population characteristics. Cytometry A. 2009;75:924-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Gorczyca L, Aleksunes LM. Transcription factor-mediated regulation of the BCRP/ABCG2 efflux transporter: a review across tissues and species. Expert Opin Drug Metab Toxicol. 2020;16:239-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Yang WJ, Song MJ, Park EY, Lee JJ, Park JH, Park K, Kim HP. Transcription factors Sp1 and Sp3 regulate expression of human ABCG2 gene and chemoresistance phenotype. Mol Cells. 2013;36:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Dai Y, Liu S, Zhang WQ, Yang YL, Hang P, Wang H, Cheng L, Hsu PC, Wang YC, Xu Z, Jablons DM, You L. YAP1 regulates ABCG2 and cancer cell side population in human lung cancer cells. Oncotarget. 2017;8:4096-4109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Yang B, Ma YF, Liu Y. Elevated Expression of Nrf-2 and ABCG2 Involved in Multi-drug Resistance of Lung Cancer SP Cells. Drug Res (Stuttg). 2015;65:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Tomita H, Tanaka K, Tanaka T, Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7:11018-11032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 454] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 44. | Masciale V, Grisendi G, Banchelli F, D'Amico R, Maiorana A, Sighinolfi P, Stefani A, Morandi U, Dominici M, Aramini B. CD44+/EPCAM+ cells detect a subpopulation of ALDH(high) cells in human non-small cell lung cancer: A chance for targeting cancer stem cells? Oncotarget. 2020;11:1545-1555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Roudi R, Korourian A, Shariftabrizi A, Madjd Z. Differential Expression of Cancer Stem Cell Markers ALDH1 and CD133 in Various Lung Cancer Subtypes. Cancer Invest. 2015;33:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Wang J, Zeng H, Li H, Zhang J, Wang S. Roles of sex-determining region Y-box 2 in cell pluripotency and tumor-related signaling pathways. Mol Clin Oncol. 2015;3:1203-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Grimm D, Bauer J, Wise P, Krüger M, Simonsen U, Wehland M, Infanger M, Corydon TJ. The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol. 2020;67:122-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 48. | Wang P, Wan WW, Xiong SL, Feng H, Wu N. Cancer stem-like cells can be induced through dedifferentiation under hypoxic conditions in glioma, hepatoma and lung cancer. Cell Death Discov. 2017;3:16105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Cai N, Li M, Qu J, Liu GH, Izpisua Belmonte JC. Post-translational modulation of pluripotency. J Mol Cell Biol. 2012;4:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Li X, Wang J, Xu Z, Ahmad A, Li E, Wang Y, Qin S, Wang Q. Expression of Sox2 and Oct4 and their clinical significance in human non-small-cell lung cancer. Int J Mol Sci. 2012;13:7663-7675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Ye T, Li J, Sun Z, Liu Y, Kong L, Zhou S, Tang J, Wang J, Xing HR. Nr5a2 promotes cancer stem cell properties and tumorigenesis in nonsmall cell lung cancer by regulating Nanog. Cancer Med. 2019;8:1232-1245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Gong S, Li Q, Jeter CR, Fan Q, Tang DG, Liu B. Regulation of NANOG in cancer cells. Mol Carcinog. 2015;54:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 53. | Otsubo K, Nosaki K, Imamura CK, Ogata H, Fujita A, Sakata S, Hirai F, Toyokawa G, Iwama E, Harada T, Seto T, Takenoyama M, Ozeki T, Mushiroda T, Inada M, Kishimoto J, Tsuchihashi K, Suina K, Nagano O, Saya H, Nakanishi Y, Okamoto I. Phase I study of salazosulfapyridine in combination with cisplatin and pemetrexed for advanced non-small-cell lung cancer. Cancer Sci. 2017;108:1843-1849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Nechushtan H, Hamamreh Y, Nidal S, Gotfried M, Baron A, Shalev YI, Nisman B, Peretz T, Peylan-Ramu N. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist. 2015;20:366-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 55. | MacDonagh L, Gallagher MF, Ffrench B, Gasch C, Breen E, Gray SG, Nicholson S, Leonard N, Ryan R, Young V, O'Leary JJ, Cuffe S, Finn SP, O'Byrne KJ, Barr MP. Targeting the cancer stem cell marker, aldehyde dehydrogenase 1, to circumvent cisplatin resistance in NSCLC. Oncotarget. 2017;8:72544-72563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Liu X, Wang L, Cui W, Yuan X, Lin L, Cao Q, Wang N, Li Y, Guo W, Zhang X, Wu C, Yang J. Targeting ALDH1A1 by disulfiram/copper complex inhibits non-small cell lung cancer recurrence driven by ALDH-positive cancer stem cells. Oncotarget. 2016;7:58516-58530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Hu YP, Tao LY, Wang F, Zhang JY, Liang YJ, Fu LW. Secalonic acid D reduced the percentage of side populations by down-regulating the expression of ABCG2. Biochem Pharmacol. 2013;85:1619-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Anreddy N, Patel A, Zhang YK, Wang YJ, Shukla S, Kathawala RJ, Kumar P, Gupta P, Ambudkar SV, Wurpel JN, Chen ZS, Guo H. A-803467, a tetrodotoxin-resistant sodium channel blocker, modulates ABCG2-mediated MDR in vitro and in vivo. Oncotarget. 2015;6:39276-39291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Wang J, Liu Z, Zhang D, Liu R, Lin Q, Liu J, Yang Z, Ma Q, Sun D, Zhou X, Jiang G. FL118, a novel survivin inhibitor, wins the battle against drug-resistant and metastatic lung cancers through inhibition of cancer stem cell-like properties. Am J Transl Res. 2017;9:3676-3686. [PubMed] |
| 60. | Škerlová J, Král V, Kachala M, Fábry M, Bumba L, Svergun DI, Tošner Z, Veverka V, Řezáčová P. Molecular mechanism for the action of the anti-CD44 monoclonal antibody MEM-85. J Struct Biol. 2015;191:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Wang F, Mi YJ, Chen XG, Wu XP, Liu Z, Chen SP, Liang YJ, Cheng C, To KK, Fu LW. Axitinib targeted cancer stemlike cells to enhance efficacy of chemotherapeutic drugs via inhibiting the drug transport function of ABCG2. Mol Med. 2012;18:887-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Sebastian M, Passlick B, Friccius-Quecke H, Jäger M, Lindhofer H, Kanniess F, Wiewrodt R, Thiel E, Buhl R, Schmittel A. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM x anti-CD3): a phase I study. Cancer Immunol Immunother. 2007;56:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Schlereth B, Kleindienst P, Fichtner I, Lorenczewski G, Brischwein K, Lippold S, da Silva A, Locher M, Kischel R, Lutterbüse R, Kufer P, Baeuerle PA. Potent inhibition of local and disseminated tumor growth in immunocompetent mouse models by a bispecific antibody construct specific for Murine CD3. Cancer Immunol Immunother. 2006;55:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Fiedler WM, Ritter B, Seggewiss R, Bokemeyer C, Fettes P, Klinger M, Vieser E, Ruettinger D, Kaubitzsch S, Wolf M. Phase I safety and pharmacology study of the EpCAM/CD3-bispecific BiTE antibody MT110 in patients with metastatic colorectal, gastric, or lung cancer. J Clin Oncol. 2010;28:2573-2573. |
| 65. | Amann M, Brischwein K, Lutterbuese P, Parr L, Petersen L, Lorenczewski G, Krinner E, Bruckmeier S, Lippold S, Kischel R, Lutterbuese R, Kufer P, Baeuerle PA, Schlereth B. Therapeutic window of MuS110, a single-chain antibody construct bispecific for murine EpCAM and murine CD3. Cancer Res. 2008;68:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Li JF, Niu YY, Xing YL, Liu F. A novel bispecific c-MET/CTLA-4 antibody targetting lung cancer stem cell-like cells with therapeutic potential in human non-small-cell lung cancer. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Alamgeer M, Neil Watkins D, Banakh I, Kumar B, Gough DJ, Markman B, Ganju V. A phase IIa study of HA-irinotecan, formulation of hyaluronic acid and irinotecan targeting CD44 in extensive-stage small cell lung cancer. Invest New Drugs. 2018;36:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Quan YH, Kim B, Park JH, Choi Y, Choi YH, Kim HK. Highly sensitive and selective anticancer effect by conjugated HA-cisplatin in non-small cell lung cancer overexpressed with CD44. Exp Lung Res. 2014;40:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 69. | Luo Y, Wang X, Du D, Lin Y. Hyaluronic acid-conjugated apoferritin nanocages for lung cancer targeted drug delivery. Biomater Sci. 2015;3:1386-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Ishiguro S, Cai S, Uppalapati D, Turner K, Zhang T, Forrest WC, Forrest ML, Tamura M. Intratracheal Administration of Hyaluronan-Cisplatin Conjugate Nanoparticles Significantly Attenuates Lung Cancer Growth in Mice. Pharm Res. 2016;33:2517-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Zhou J, Sun J, Chen H, Peng Q. Promoted delivery of salinomycin sodium to lung cancer cells by dual targeting PLGA hybrid nanoparticles. Int J Oncol. 2018;53:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Alibolandi M, Ramezani M, Abnous K, Sadeghi F, Atyabi F, Asouri M, Ahmadi AA, Hadizadeh F. In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J Control Release. 2015;209:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 73. | Huang X, Huang J, Leng D, Yang S, Yao Q, Sun J, Hu J. Gefitinib-loaded DSPE-PEG2000 nanomicelles with CD133 aptamers target lung cancer stem cells. World J Surg Oncol. 2017;15:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Ma J, Zhuang H, Zhuang Z, Lu Y, Xia R, Gan L, Wu Y. Development of docetaxel liposome surface modified with CD133 aptamers for lung cancer targeting. Artif Cells Nanomed Biotechnol. 2018;46:1864-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Taromi S, Firat E, Simonis A, Braun LM, Apostolova P, Elze M, Passlick B, Schumacher A, Lagies S, Frey A, Schmitt-Graeff A, Burger M, Schmittlutz K, Follo M, von Elverfeldt D, Zhu X, Kammerer B, Diederichs S, Duyster J, Manz MG, Niedermann G, Zeiser R. Enhanced AC133-specific CAR T cell therapy induces durable remissions in mice with metastatic small cell lung cancer. Cancer Lett. 2022;538:215697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei X, China; Zhao W, China S-Editor: Fan JR L-Editor: A P-Editor: Cai YX