Published online Feb 26, 2022. doi: 10.4252/wjsc.v14.i2.200
Peer-review started: February 23, 2021
First decision: April 20, 2021
Revised: May 1, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: February 26, 2022
Processing time: 367 Days and 1.8 Hours
Sustained injury, through radiotherapy, burns or surgical trauma, can result in fibrosis, displaying an excessive deposition of extracellular matrix (ECM), persisting inflammatory reaction, and reduced vascularization. The increasing recognition of fibrosis as a cause for disease and mortality, and increasing use of radiotherapy causing fibrosis, stresses the importance of a decent anti-fibrotic treatment.
To obtain an in-depth understanding of the complex mechanisms underlying fibrosis, and more specifically, the potential mechanisms-of-action of adipose-derived stomal cells (ADSCs) in realizing their anti-fibrotic effect.
A systematic review of the literature using PubMed, Embase and Web of Science was performed by two independent reviewers.
The injection of fat grafts into fibrotic tissue, releases ADSC into the environment. ADSCs’ capacity to directly differentiate into key cell types (e.g., ECs, fibroblasts), as well as to secrete multiple paracrine factors (e.g., hepatocyte growth factor, basis fibroblast growth factor, IL-10), allows them to alter different mechanisms underlying fibrosis in a combined approach. ADSCs favor ECM degradation by impacting the fibroblast-to-myofibroblast differentiation, favoring matrix metalloproteinases over tissue inhibitors of metalloproteinases, positively influencing collagen organization, and inhibiting the pro-fibrotic effects of transforming growth factor-β1. Furthermore, they impact elements of both the innate and adaptive immune response system, and stimulate angiogenesis on the site of injury (through secretion of pro-angiogenic cytokines like stromal cell-derived factor-1 and vascular endothelial growth factor).
This review shows that understanding the complex interactions of ECM accumulation, immune response and vascularization, is vital to fibrosis treatments’ effectiveness like fat grafting. It details how ADSCs intelligently steer this complex system in an anti-fibrotic or pro-angiogenic direction, without falling into extreme dilation or stimulation of a single aspect. Detailing this combined approach, has brought fat grafting one step closer to unlocking its full potential as a non-anecdotal treatment for fibrosis.
Core Tip: The goal of this review is to elucidate the potential mechanisms of action of fat grafting, and more specifically of adipose-derived stem cells (ADSCs), in hostile environment. Why can fat grafts turn the sclerotic environment after intense radiotherapy, burns or surgical trauma into a soft zone that can be further restored and reconstructed? In doing so, this review aims to complement existing literature by delivering an integrated approach to explain the positive effect of ADSCs on fibrosis, considering all 3 main fibrotic aspects, i.e., extracellular matrix accumulation, innate and adaptive immune response and vascularization. It aims at acknowledging the complexity and reciprocal impact these aspects have, both from a clinical as well as a molecular point of view. While available literature so far only focused on a single one of these aspects, the question remains whether an integrated approach and explanation on these combined levels could improve the effectiveness and application areas of this treatment.
- Citation: Vanderstichele S, Vranckx JJ. Anti-fibrotic effect of adipose-derived stem cells on fibrotic scars. World J Stem Cells 2022; 14(2): 200-213
- URL: https://www.wjgnet.com/1948-0210/full/v14/i2/200.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i2.200
Fibrosis, or scarring, is a potential consequence of a dysregulated wound-healing process. Tissue injury triggers wound-healing, a complex dynamic process characterized by four distinct but overlapping phases, all limited in time: homeostasis, inflammation, proliferation and remodeling. Interference of the immune system, deposition of the extracellular matrix (ECM) and alteration of the vascularization are indispensable and, typically, reversible elements of the wound-healing. However, sustained injury results in reduced vascularization, persisting inflammatory reaction and excessive deposition of ECM, inducing fibrosis[1]. Radiotherapy-induced skin fibrosis (RISF), a late cutaneous side effect of external radiation, is an example of chronic tissue injury leading to fibrosis. RISF is often characterized by pain, limited range of motion, tissue contraction and aesthetic deformation. All result in a significant loss of quality of life for patients.
Fibrosis can affect nearly every tissue in the body leading to common diseases such as idiopathic pulmonary fibrosis, cirrhosis, renal fibrosis, myocardial fibrotic remodeling, fibrotic stricture as a common complication of Crohn’s disease, and scar contractions after surgery, burns, trauma and radiotherapy. The increasing recognition of fibrosis as a cause for disease and mortality and the increasing use of radiotherapy, stresses the importance of a decent anti-fibrotic treatment[2].
In the early 20th century Hedrick, Zuk et al[3,4], reported the presence of adipose-derived stem cells (ADSCs), or adipose-derived stromal cells within adipose tissue obtained by lipoaspiration, the so-called stromal vascular fraction (SVF). Decades before, fat grafting was already used in reconstructive procedures as filler of defects, albeit without much confidence since this transfer of fat lobules occurred without intrinsic vascularization. Coleman et al[5] pioneered in making the fat graft aliquots soluble and performing the fat transfer as tiny liquid parcels of lipoaspirate, thus eliminating the need for vascularized transfer.
The discovery of ADSCs in ‘ordinary’ fat grafts was revolutionary in stem cell research, since it was assumed previously that stem cells resided mainly in embryonic tissues, placenta and bone marrow of the adult patient[3,4]. The option of obtaining large numbers of mesenchymal stem cells from lipoaspirates meant a significant paradigm change. Moreover, nowadays ADSCs can be reprogrammed[6,7]. Induced pluripotent stem cells, derived from skin or blood cells, that have been reprogrammed back into an embryonic-like pluripotent state, enable the development of an unlimited source of any type of human cell required for therapeutic strategies[8,9].
Considering the fact that the SVF from fat grafts is easily accessible by lipo-aspiration, multipotent ADSCs emerged as attractive alternatives for soft tissue restoration and regeneration by adding cells, growth factors and active molecules to the microenvironment of the wound[10,11].
Plastic surgeons observed that fat grafting had a smoothening effect on scars and even on radiation-induced fibrosis. The application of fat grafting extended on various types of fibrosis such as Parry-Romberg syndrome, sclerodermia, dupuytren, hypertrophic scars etc., A multitude of reports confirm these observations[12,13]. However, the mechanisms underlying these fibrosis-reducing effects of fat grafts remain unclear so far.
The goal of this review is to elucidate the potential mechanisms of action of fat grafting, and more specifically of ADSCs, in hostile environment. Why can fat grafts turn the sclerotic environment after intense radiotherapy, burns or surgical trauma into a soft zone that can be further restored and reconstructed? In doing so, this review aims to complement existing literature by delivering an integrated approach to explain the positive effect of ADSCs on fibrosis, considering all 3 main fibrotic aspects i.e., ECM accumulation, innate and adaptive immune response and vascularization. It aims at acknowledging the complexity and reciprocal impact these aspects have, both from a clinical as well as a molecular point of view. While available literature so far only focused on a single one of these aspects, the question remains whether an integrated approach and explanation on these combined levels could improve the effectiveness and application areas of this treatment.
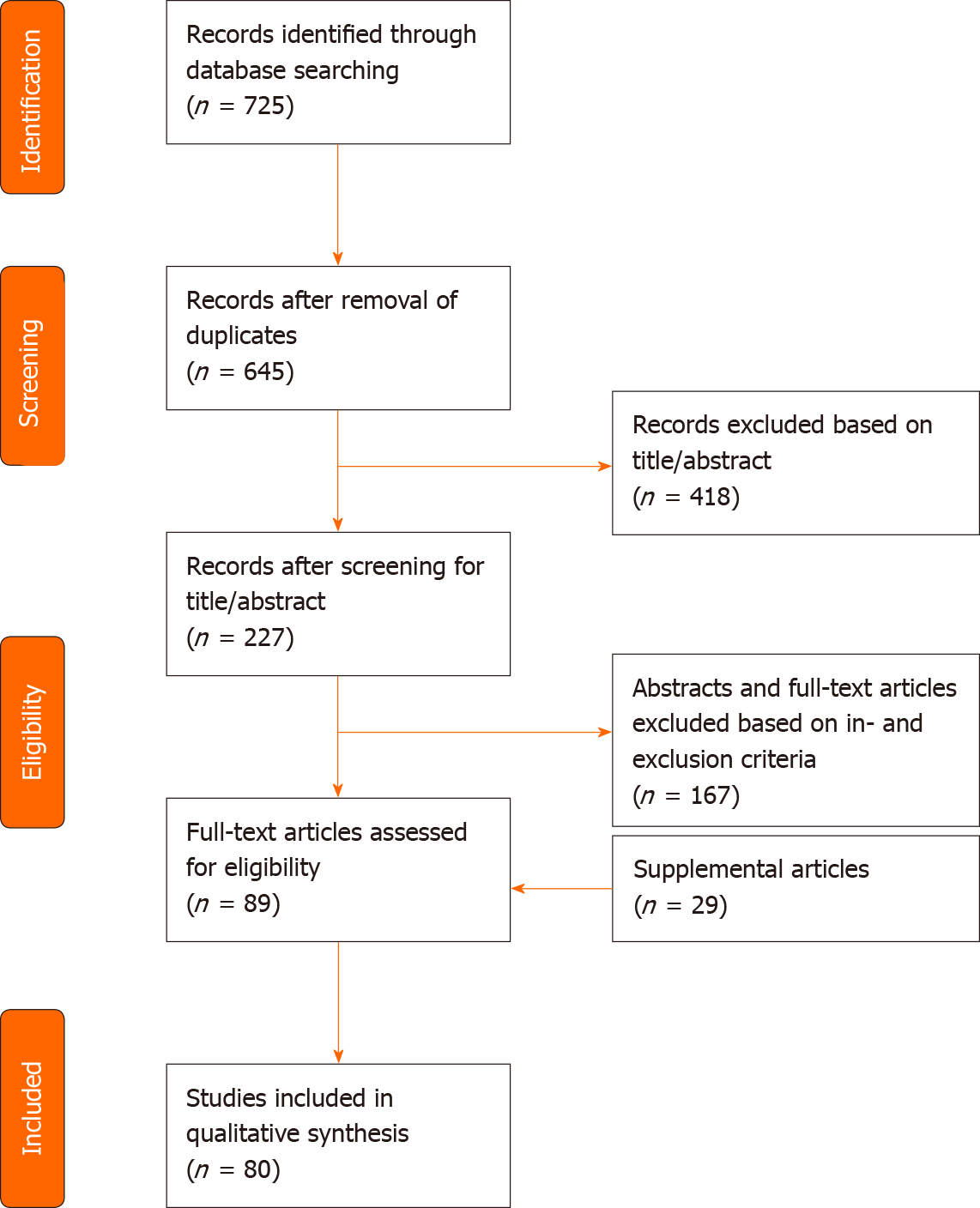
A systematic review of the literature using PubMed, Embase and Web of Science was performed by two independent reviewers. Terms applied to the search included fat grafting, lipofilling, adipose tissue transplantation/transfer, adipose-derived stem cell, adipose-derived stromal cell, fibrosis, scar, keloid, radiation-induced skin fibrosis, myofibroblast, fibroblast, collagen, regenerative medicine, tissue engineering, immunomodulation, neovascularization and angiogenesis modulating agents. Inclusion criteria included animal studies, randomized controlled trials, case-control studies and reviews that were relevant for elucidating the anti-fibrotic effect of ADSCs, applied to multiple features of fibrosis (e.g., dermal fibrosis after radiotherapy, hypertrophic scars, burns, sclerodermia) Exclusion criteria comprised of articles solely about clinical outcome measures, case reports, case series and literature focused on the technique of fat grafting. Language has been restricted to English. A structured summary of the review process is illustrated in Figure 1.
Introduction and histology of fibrosis: Fibrosis is caused by a dysregulation of regular wound-healing (mostly through sustained injury), leading to an excessive deposition of ECM, persisting inflammatory reaction and reduced vascularization.
ECM is a non-cellular three-dimensional molecular network composed of proteins (e.g., collagen, elastin, laminin, fibronectin) and ground substance (e.g., glycosaminoglycans, such as hyaluronan and proteoglycans)[14-16]. The ECM regulates tissue development and homeostasis and constantly undergoes remodeling. Excessive accumulation of ECM components, such as collagens, fibronectin, proteoglycans, glycosaminoglycans and laminin are the typical characteristics of fibrosis regardless of the etiology[17]. Although all the beforementioned ECM components participate in the overall pathogenic process, collagen type I, collagen type III and fibronectin are the most dominating proteins found in fibrotic tissue[1,2]. Despite the fact that there is an increase in the amount of ECM, some ECM components (such as decorin) are less abundant in scars[13]. Besides the increase of ECM accumulation, fibrosis is also defined by a high amount of alfa-smooth muscle actin (α-SMA).
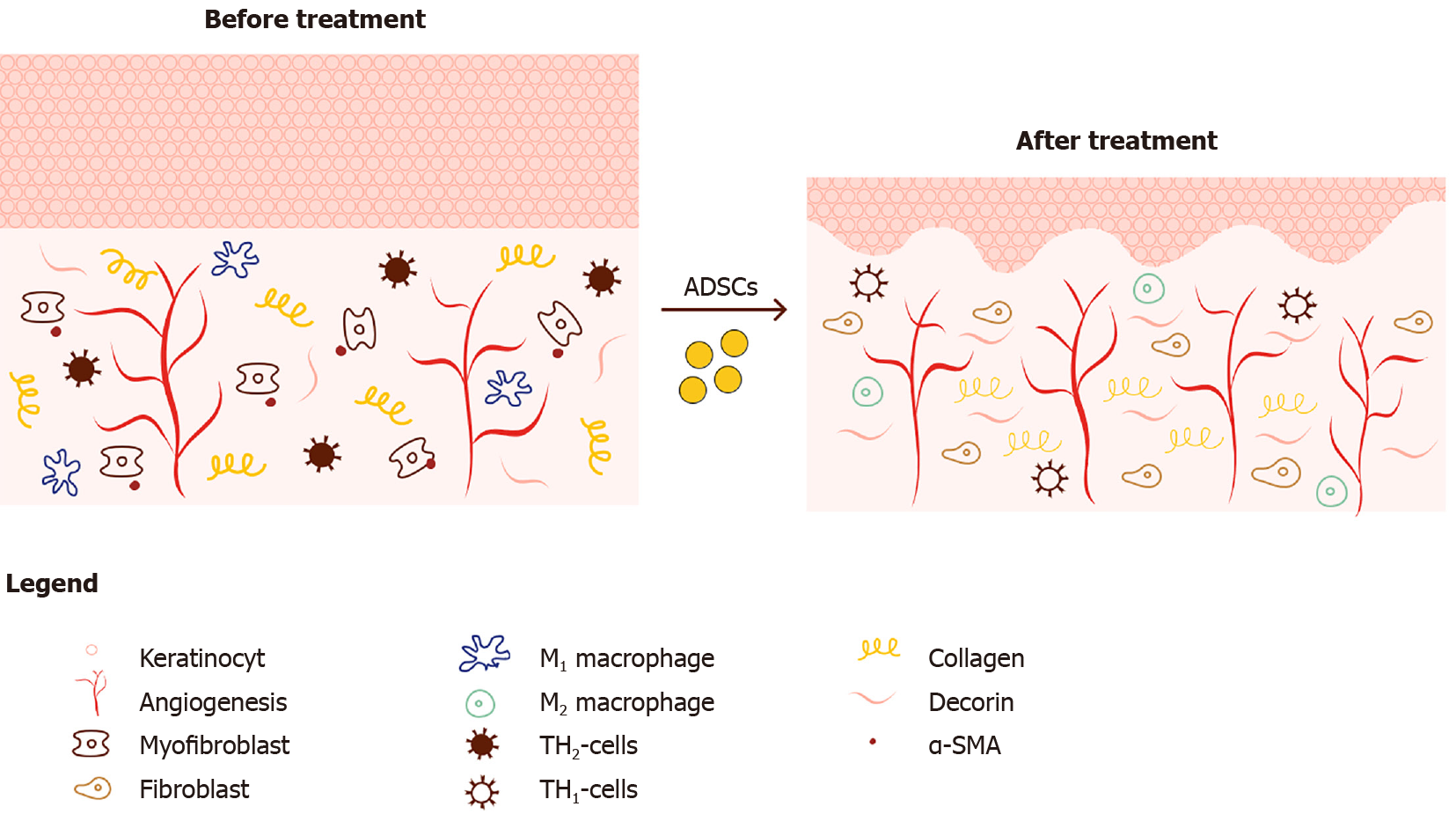
Microscopically, scar tissue (e.g., after radiation) is characterized by flattening of the rete ridges, a thickened epidermis and dermis, and an irregular collagen organization (see Figure 2). Excessive proliferation of keratinocytes causes the epidermal thickening, while it is the excessive ECM that causes the thickened dermis. The abnormal collagen behavior displays itself in an increase in the number of collagens, altered fiber thickness, more cross-linking as well as a decreased degree of collagen organization[18].
Histological patterns do not only reflect the increased ECM deposition but also the enhanced inflammation and reduced vascularization, through decreased vessel density, microvascular obliteration and abnormal vascularization patterns[12].
The homeostasis of the ECM is a well-regulated process influenced by a variety of actors and is subject to four major delicate balances. In fibrosis these balances are dysregulated, favoring deposition over degradation which results in overproduction of ECM.
Imbalance between fibroblasts and myofibroblasts: Fibroblasts play a crucial role in tissue homeostasis by regulating the ECM, which is constantly being synthesized, degraded and remodeled[19]. However, when fibroblasts can no longer coordinate this meticulous cross talk and interplay of cells and factors in an environment of chronic inflammation or repeated tissue damage, they transform into professional repair cells i.e., myofibroblasts. Myofibroblasts do not typically appear in healthy connective tissue[20]. Myofibroblasts produce large amounts of ECM and are capable of contraction, due to the expression of α-SMA[21]. Juhl et al[19] found that cytokines and growth factors such as interleukin 6 (IL-6), platelet-derived growth factor, and transforming growth factor-beta 1 (TGF-β1) facilitate a prolonged myofibroblast activation, which results in excessive ECM production manifested as fibrosis.
Imbalance between TIMPs and MMPs: Fibrotic tissue can be characterized by a decreased matrix metalloproteinase (MMP)/tissue inhibitors of metalloproteinases (TIMPs) ratio resulting in ECM accumulation. ECM degradation enzymes called MMPs, vs their opposing tissue inhibitors known as TIMPs also play a role in ECM remodeling. MMPs are endopeptidases, mainly produced by macrophages (12), and are categorized by their substrates and structure, into collagenases, gelatinases (MMP-2 and MMP-9), stromelysins, membrane-type-MMPs and others.
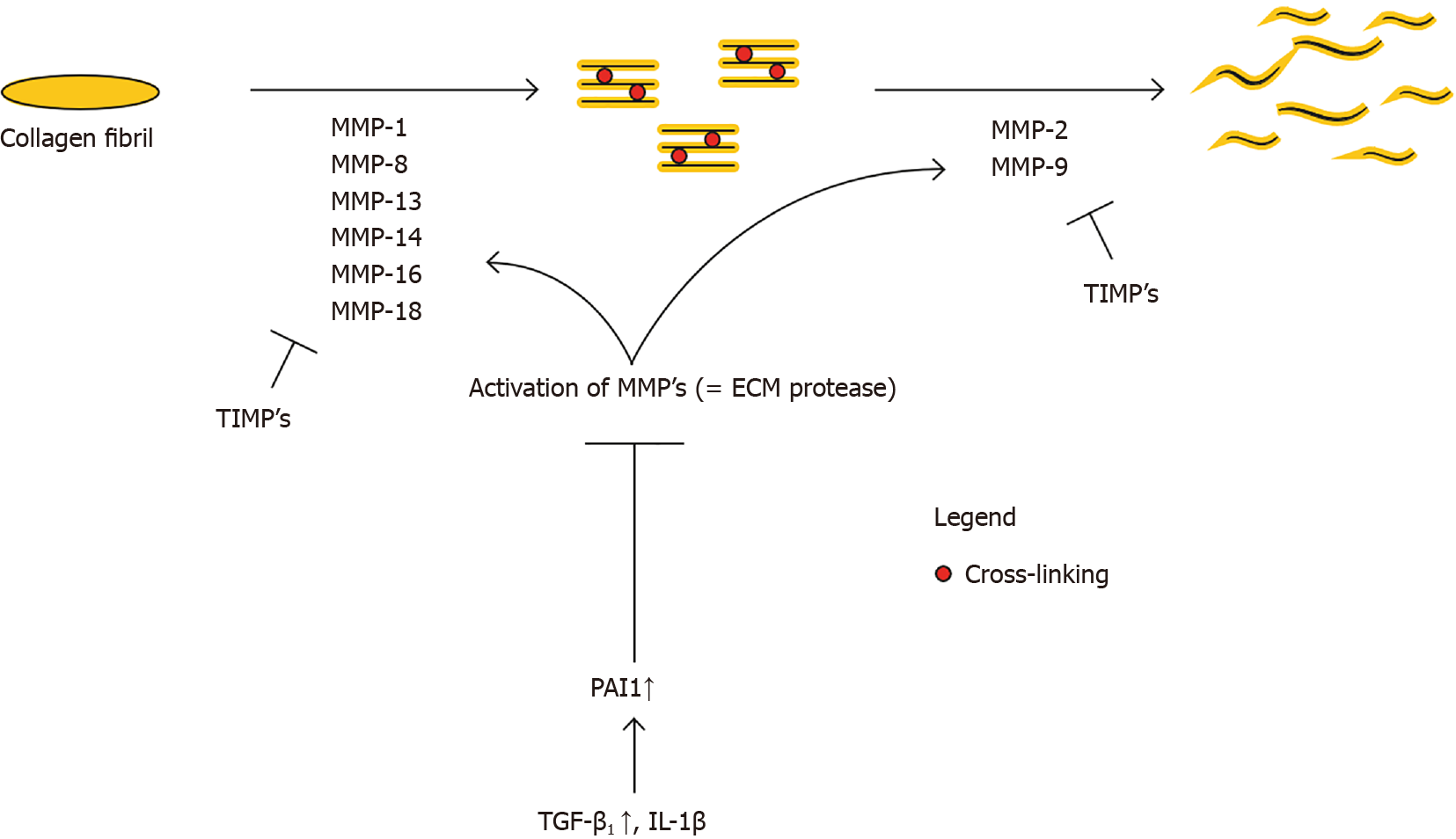
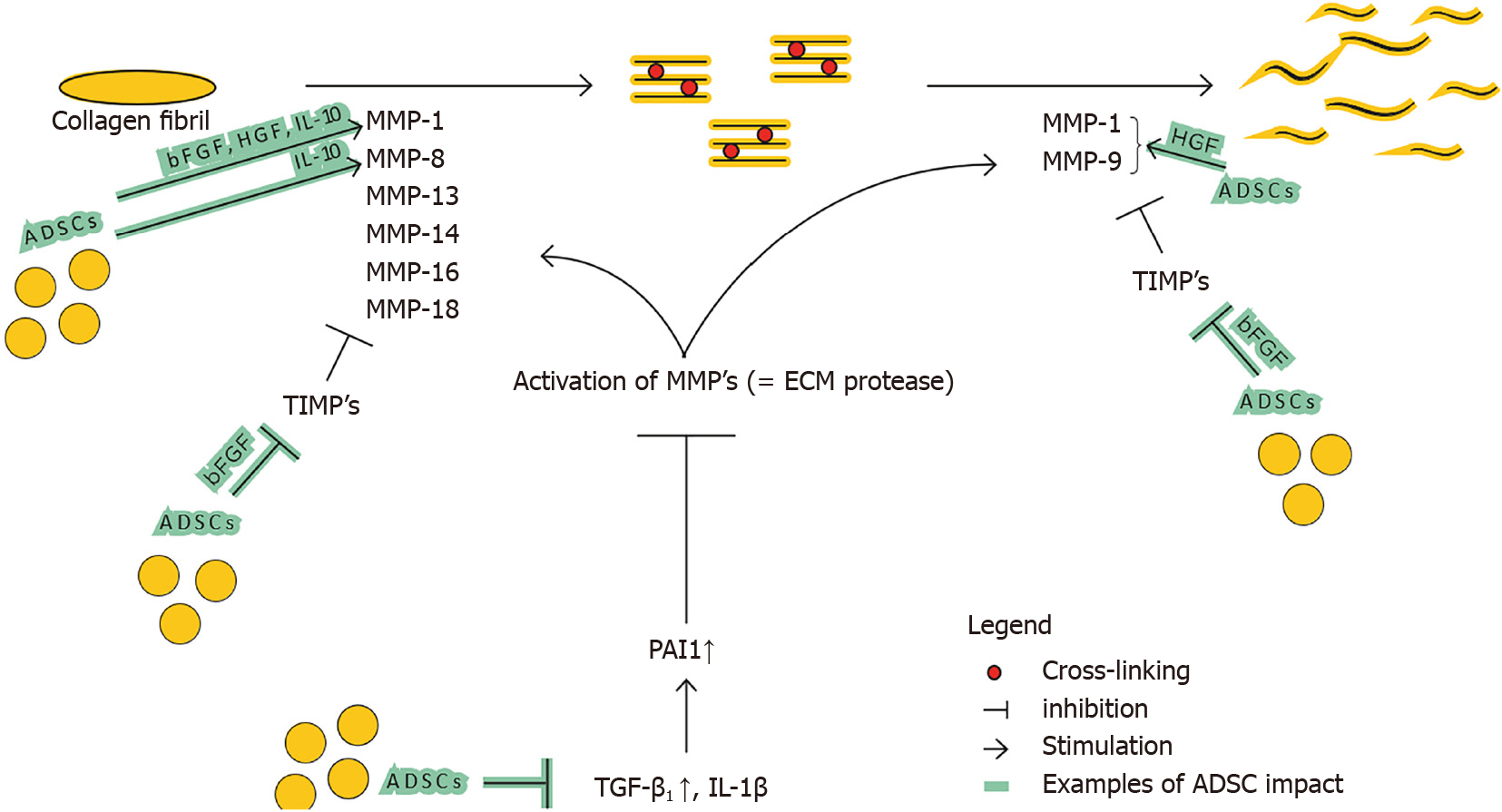
Zhao et al[2] show that fibrillar collagen, the pre-dominant structural protein in fibrotic tissue, is cleaved by MMP1, MMP8, MMP13, MMP14, MMP16, and MMP18, allowing other MMPs such as gelatinases (MMP2 and MMP9), to further degrade collagen (see Figure 3). Several mice studies confirm the relation of a certain MMP-deficiency or -stimulation with development of fibrosis or protection against fibrosis, respectively[12,22,23]. The opposite is applicable for TIMPs; TIMP upregulation has been associated with fibrosis[24]. This general classification however does not seem to be valid for all MMP and TIMP subtypes. The role of MMP-2 and MMP-9 is for example less clear, and MMP-9 has been associated with pro-fibrotic characteristics[25].
TGF-β1 and plasminogen activator inhibitor 1 (PAI1) are major regulators of the MMP and TIMP expression[26,27]. The persistent activation and/or production of PAI1, resulting in MMP inhibition, directly stimulates fibrosis (see Figure 3). TGF-β1 also decreases the MMP/TIMP ratio, as will be further detailed in ‘Imbalance between multiple functions of TGF-b1 in regular vs hypertrophic scarring’. Fibroblasts derived from several fibrotic conditions such as keloid scars and scleroderma have demonstrated elevated levels of PAI1[27,28]. On the contrary, PAI1 deficiency protects mice from bleomycin induced lung fibrosis[27]. Taken together, upregulation of PAI1 substantially contributes to fibrosis. PAI1 is upregulated by TGF-β1, IL-1b, hypoxia and others. Ghosh et al[27] suggest that the increase of PAI1 Levels in fibroblasts from patients with scleroderma is linked to the activation of TGFβ1/ SMAD axis.
Imbalance between organized and unorganized collagen fibers: Collagen organization can also influence the ECM balance through the extent in which they are cross-linked. Multiple fibrotic organ types show that crosslinking is disproportionately present and contribute to decreased ECM degradation and increased tissue stiffness[2]. Thus, fibrosis is characterized by favoring unorganized and cross-linked collagen, over normal collagen organization.
Imbalance between multiple functions of TGF-β1 in regular vs hypertrophic scarring: Continuous activation of TGF-β1/Smad axis has been reported to mediate the excessive production of ECM without appropriate remodeling. In a study by Juhl et al[19], TGF-β-treated human dermal fibroblasts showed an increase in collagen I, fibronectin gene and protein levels. They also observed a gene-upregulation of α-SMA, type III, IV and V collagen, fibronectin and TGF-β1 genes, while type VI collagen was downregulated. In experimental animal studies, constitutive activation of TGF-β1 signaling leads to organ fibrosis, while inhibition of TGF-β1 reduces fibrosis[29].
The pro-fibrotic feature of TGF-β1 is multifactorial. TGF-β1 signaling stimulates directly the ECM accumulation by increasing the synthesis of ECM components such as collagen, fibronectin, elastin, proteoglycans, fibrillin and laminin[2]. TGF-β1 also enhances ECM production indirectly by inhibiting MMPs and stimulating TIMPs[26]. TGF-β1 induces fibroblast-to-myofibroblast differentiation, which results in the secretion of additional ECM components and which facilitates tissue contraction[2]. The entire TGF-β1 pathway promotes collagen cross-linking and leads to increased rigidity and decreased ECM degradation. The main sources of TGF-β1 production are fibroblasts and activated immune cells such as macrophages. Both of these are reciprocally upregulated by the presence of TGF-β1 creating a positive 2nd order TGF-β1 production loop.
The TGF-β1/Smad axis is also critical in regular wound healing, exerting influence on both the inflammation and revascularization processes. TGF-β1 increases inflammation by drawing neutrophils and monocytes to the injury environment and stimulates the differentiation of monocytes into activated macrophages[30]. However, TGF-β1 may also induce anti-inflammatory effects[31,32]. This duality may be explained by its double origin (from macrophages or T-cells)[31] or by the distinctive temporal expression patterns during the sequential phases in wound repair[30].
Aside from ECM accumulation, fibrosis is also driven by persistent low-grade inflammation, sustained by both the innate and adaptive immune response[31]. Multiple mechanisms lead to this increased or prolonged inflammation.
The first mechanism is governed by an increase of pro-inflammatory cytokines. Adipocytes, in a stress situation (e.g., radiotherapy), synthesize pro-inflammatory proteins such as tumor necrosis factor-α (TNF-α), IL-6 and IL-8[33]. IL-6 also has pro-fibrotic features by persistent stimulation of fibroblasts, which may lead to the differentiation into myofibroblasts[19]. Furthermore, various innate inflammatory cells (e.g., macrophages, neutrophils, mast cells and eosinophils) secrete a multitude of growth factors and cytokines, such as TNF-α, IL-1β. Studies by Miyazaki et al[34] and Kolb et al[35] found that mice who overexpress TNF-α or IL-1β in the lung, develop pulmonary fibrosis. In turn, TNF-α and IL-1β also increase expression of IL-6[31].
The second mechanism that leads to prolonged inflammation is via macrophages. Macrophages are vital in the wound healing process. However, redundant infiltration, overactivation, lack of differentiation and lack of clearance result in fibrotic tissue formation[18,36]. This pro-fibrotic activity manifests itself mostly in the early phases of the wound-healing process and results in the production of inflammatory mediators that can aggravate tissue injury, such as TNF-α, IL-1β, and a variety of toxic mediators, such as reactive oxygen and nitrogen species. Moreover, macrophages are known to produce the pro-fibrotic TGF-β1. Duffield et al[37] reported that early macrophage depletion effectively reduced the development of liver fibrosis in mice. Wynn et al[31] showed that limiting the pro-inflammatory activity of innate myeloid-lineage cells (incl. macrophages) may successfully treat fibrotic diseases. In later stages of the wound-healing process, a subset of macrophages convert into a suppressive phenotype and express multiple anti-inflammatory mediators (e.g., IL-10, Arg1, Relm-α,…) that direct the resolution of the inflammatory response[38].
Furthermore, one can distinguish anti-inflammatory/pro tissue repair macrophages (M2) from pro-inflammatory/pro-fibrotic (M1) macrophages[39]. Thus, the balance between both types also plays a role in the pathogenesis of fibrosis. Multiple studies[40,41] observed that M2 macrophages suppress fibrosis and reduce inflammation by competing with T-helper 2 (TH2) cells and fibroblasts for l-arginine. This competition reduces the TH2 response and suppresses collagen synthesis by myofibroblasts[31]. Furthermore, literature[38] indicates that M2 cells are important inducers of regulatory T-cells, that have an anti-fibrotic effect.
It seems useful to target the inflammatory cascade and the innate immune response, to treat fibrotic disorders. However, the extent of fibrosis is not per se linked with the severity of inflammation, which suggests that other immunological mechanisms, such as the adaptive immune response, play an important role in the fibrotic process. Wynn[42] described how the TH1/TH2 paradigm seems to play a key role in sustaining the immune response. There are 3 types of T helper cells; TH1 CD4+ T cells, TH2 CD4+ T cells and regulatory T cells. This categorization is based on the cytokines each of these cells secrete.
TH1 cells are predominant in the initial response of the adaptive immune system to chronic injury. TH1 cells mainly secrete interferon-γ (IFN-γ) and IL-12, as well as associated pro-inflammatory cytokines. The TH1 responses are pro-inflammatory yet anti-fibrotic. They directly inhibit collagen production by fibroblasts, stimulate ECM degradation by favoring MMPs over TIMP and inhibit TGF-b1 production[43]. Moreover, they indirectly inhibit fibrosis by reducing pro-fibrotic cytokine expression by TH2 cells (e.g., IL-13)[44].
However, when the stimulus persists, TH2 cells and regulatory T cells collaborate to suppress the TH1 response to prevent the immune system from causing additional damage. In their response, TH2 cells mainly secrete IL-4, IL-5 and IL-13[44]. These factors stimulate fibrosis by enhancing collagen deposition through various mechanisms. On top of that, IL-13 specifically strengthens the pro-fibrotic TGF-β1-SMAD mechanism, while also independently stimulating collagen production by fibroblasts[45-48]. Therefore, despite TH2’s facilitating function in wound healing, their response contributes to fibrosis. Specifically applied to post-irradiation fibrosis, Buttner et al[49] showed the increased concentrations of IL-4.
At last, the regulatory T-cells mainly secrete IL-10, which is anti-fibrotic by directly suppressing collagen synthesis by fibroblasts, and cooperates with TH1 cytokines to suppress collagen deposition[50-52].
Radiation injury on the skin is characterized by microvascular obliteration and poor revascularization. The reduced vascularization results in diminished transport of oxygen, nutrients and essential factors of the immune system to the damage tissues. In the end, this may lead to the deterioration of the hypoxia, resulting in fibrosis.
Histological changes mediated by ADSCs on scar tissue: When injecting ADSCs into a fibrotic injury environment through fat grafting, multiple histological changes occur (see Figure 2). A restoration of the normal skin ridge pattern is observed. Several animal models[23,53] and in vitro models[12,54] show a decrease in ECM components’ deposition such as collagen I, collagen III, fibronectin and elastin. The expression of α-SMA, SMAD-3 proteins and TGF-β1 seems to be decreased while the amount of decorin and MMP-1/TIMP-1 ratio is increased[12]. Furthermore, Zonari et al[55] and Zhang et al[53] show improved collagen fiber alignment, organization and less cross-linking. One can also distinguish a decrease in pro-inflammatory mediators/profibrotic factors (e.g., IL-6, IL-8, connective tissue growth factor)[12]. Finally, an increase in vascularization and a normalization of the microvascular architecture is observed[13].
This histological overview is however not exhaustive. Multiple other factors and substances are impacted by the insertion of a fat graft, which will be further described through the impact they have on the key molecular balances of fibrosis.
The antifibrotic potential of ADSC can be exerted in a direct or indirect fashion.
ADSCs as multipotent progenitor cells: ADSCs are multipotent progenitor cells that have the intrinsic possibility to differentiate into various sorts of cells that play a role in the wound-healing process (e.g., fibroblasts, keratinocytes, osteocytes, neural cells, endothelial cells, etc.). These ADSC cell products can in turn cause tissue regeneration and cell restoration[7]. ADSCs can also differentiate into mature adipocytes and serve as building blocks for a subcutaneous adipose layer that adds elasticity and pliability to the skin and volume required for thermoregulation. Adipogenesis is key for the effectiveness of ADSCs/fat grafts as it preserves the necessary fat tissue[33].
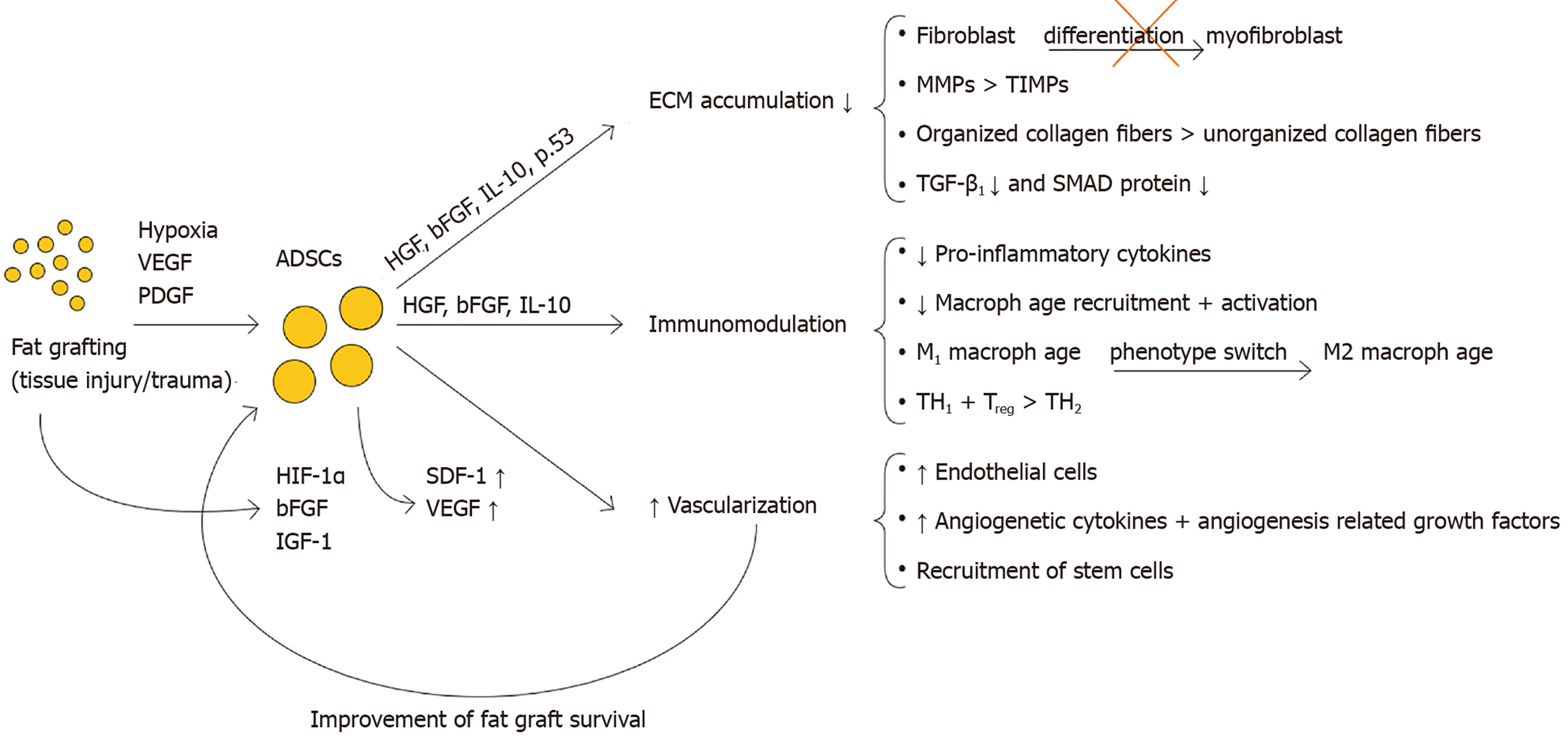
Paracrine: Moreover, ADSCs produce a myriad of trophic factors, which influence the formation and modulation of the ECM, and interact with the immune response and angiogenesis[7,13,30,32] (see Figure 4).
Influence on fibroblast to myofibroblast differentiation: Studies show that gene expression of the pro-fibrotic marker α-SMA, produced specifically by myofibroblasts, is decreased upon administration of ADSCs[23,53,55,56]. Borovikova et al[30] describe multiple mechanisms: ADSCs inhibit the fibroblast-to-myofibroblast differentiation by secreting hepatocyte growth factor (HGF), IL-10 and p53. ADSCs also stimulate myofibroblast apoptosis by the secretion of basis fibroblast growth factor (bFGF) via the Rho/Rho kinase pathway and HGF via the FAK-extracellular signal-regulated kinase (ERK)-MMP signaling pathway. FGF is also reported to reverse the myofibroblast phenotype through its activation of ERK/MAP kinase pathway.
MMP vs TIMP balance: The administration of ADSCs by lipofilling has an antifibrotic effect by modulating MMP/TIMP ratio by various pathways (see Figure 5).
Deng et al[12] show that ADSCs enhance ECM degradation by increasing MMP-1/TIMP-1 ratio. On top of that, Spiekman et al[54] show that ADSCs promote the expression of MMP-1, MMP-2 and MMP-14. Presumably ADSCs enhance the ratio in favor of degradation by decreasing the major regulator of MMP and TIMP expression, TGF-β1. Decreased SMAD signaling also decreases the other crucial director, PAI1 which in turn results in MMP activation. Furthermore, ADSCs secrete HGF, which activates MMP-1, MMP-2 and MMP-9[57,58]. ADSCs also produce bFGF and IL-10 that influence the ratio significantly. IL-10 does this by increasing MMP-1 and MMP-8, and bFGF presumably by increasing MMP-1 and decreasing TIMP-1 expression[59-61]. bFGF also stimulates HGF production, strengthening the said HGF effect.
Finally, not all MMPs and TIMPs will necessarily have a singular effect (e.g., increase of MMP-9 can in certain circumstances be pro-fibrotic vs the anti-fibrotic effect of other MMPs).
Improvement of collagen linking: Zonari et al[55] and Zhang et al[53] show improved collagen fiber alignment, organization and less cross-linking when ADSCs are inserted into the fibrotic injury environment. Given that cross-linking contributes to decreased ECM degradation[2], this decreased crosslinking has an anti-fibrotic effect.
TGF-b1/Smad axis: Section ‘Imbalance between multiple functions of TGF-β1 in regular vs hypertrophic scarring’ described the pro-fibrotic feature of the TGF-β1/Smad axis, through its multifactorial influence on the key mentioned balances leading to ECM accumulation, and thus fibrosis. Studies by Zonari et al[55], Uysal et al[56] and Spiekman et al[54], report that ADSCs decrease the presence of TGF-β1 as well as SMAD 2 and SMAD 3 proteins. An effect possibly contributing to this is given by Ejaz et al[62], who in their study describe a downregulation of TGF-β1 as a result of increased concentrations of HGF (also secreted by ADSCs). In turn, decreased TGF-β1 reduces ECM accumulation both through its direct as well as indirect effects (by reducing myofibroblast activation, favoring MMPs over TIMPs and decreasing collagen cross-linking), all mitigating fibrosis.
For completeness on ADSC impact, one can mention the impact of another member of the TGF-β cell group, i.e., TGF-β3. Impacting the TGF-β1/TGF-β3 balance towards increasing TGF-β3, is linked to reduced scar formation in adult wound healing[30]. Multiple studies[23,55,63] have shown such an increase of TGF-β3 in fibrotic tissue after injection of ADSCs, resulting in decreased tissue stiffness.
Immunomodulation: The anti-fibrotic effect of ADSCs through their impact on elements of both the innate and adaptive immune response, has multiple facets.
ADSCs downregulate key pro-inflammatory cytokines. Carceller et al[64] indicates that, in an in vivo mouse model, ADSCs effectively suppress the inflammatory response through the downregulation of selected inflammatory mediators (e.g., IL-1b, TNF-α, IL-6, leukotriene B4). A similar effect of ADSCs could be seen through their secretion of HGF, which is documented to lead to a decrease of pro-inflammatory cytokines (TNF-α, IL-12, monocyte chemoattractant protein 1, IFN-g) in a fibrosis model[30]. Also, ADSCs have the potency to modulate macrophage recruitment and activation, mostly by secreting bFGF, HGF and IL-10[30]. Kotani et al[32] show that ADSCs induce apoptosis of activated macrophages, and reduce infiltrations of macrophages, neutrophils and T lymphocytes. Therefore, through their immunomodulatory ability, ADSCs have an inhibitory effect on active macrophages. Moreover, Xie et al[65] showed that ADSCs promote a macrophage phenotype switch, favoring the anti-inflammatory M2 phenotype over the pro-inflammatory M1 in a mouse model.
Finally, ADSCs exhibit a suppressive effect on lymphocyte responses and induce a phenotypic conversion of T-cells. Kotani et al[32] describes, in the context of pulmonary fibrosis, that ADSCs inhibit the differentiation and proliferation of Th2-type mCD4+ T cells while promoting this for regulatory T cells. Given the pro-fibrotic effect of TH2-cells (see section ‘Role of the innate and adaptive immune response in fibrosis’), this could suggest the phenotypic conversion of T cells as an important mechanism underlying the anti-inflammatory effect of ADSCs. Cho et al[66] in turn suggest that ADSCs have an inhibitory effect on inflammatory diseases either directly or by inducing T-Regulatory cells (through PGE2 and TGF-β1) and inhibiting TH2 cytokines.
Stimulating angiogenesis and revascularization has a positive effect on fibrosis. Rebuilding natural blood flow improves the delivery of supplemental oxygen and other key substances to the injury site. On top of that, angiogenesis allows for better survival of administered ADSC/fat grafts in general, enhancing beforementioned treatment effects. Evans et al[67] describe how ADSCs, like bone marrow-derived mesenchymal stem cells, have the capacity to differentiate into endothelial cells (ECs), that provide the required cellular building blocks for angiogenesis.
Additionally ADSCs secrete an array of pro-angiogenetic cytokines and growth factors such as HGF, vascular endothelial growth factor (VEGF), bFGF, G-CSF, GM-CSF, IL-7, M-CSF, stromal cell-derived factor-1 (SDF-1), etc.[33,68]. These factors may promote the angiogenic sprouting process based on endothelial cell migration, proliferation and tube formation[69].
In response to entering a hypoxic environment, ADSCs activate hypoxia-inducing factor-1α (HIF-1α), and release bFGF and insulin-like growth factor-1 (IGF-1), that in turn promote neovascularization by establishing high levels of VEGF at the graft site. This VEGF promotes EC proliferation and migration to the graft, as well as inhibits EC apoptosis[70]. Studies also show[71-73] that high quantities of VEGF found at the grafting site, promote monocyte differentiation into M2 type macrophages, reducing fibrosis. On the paracrine side, increasingly more literature details the importance of SDF-1 in angiogenesis. Murohara et al[68] states that SDF-1 Likely plays a key role in the ADSC-mediated angiogenesis. Other studies mention that SDF-1, like VEGF, improves revascularization and angiogenesis by recruiting stem cells such as endothelial progenitor cells and hematopoietic stem cells to the engrafted/ischemic site. It is reported that SDF-1/CXCR4 axis exerts one of the strongest chemotactic effects on BMSCs[74,75].
Macrophages play an important role in angiogenesis. Cai et al[76] reported that early macrophage infiltration in the graft environment appears to be key for angiogenesis and revascularization. However, when present for an extended period of time they can stimulate fibrosis. By initially releasing angiogenetic cytokines (e.g., VEGF, bFGF), macrophages stimulate vessel growth in a VEGF-dependent manner, while also generating recruitment signals for stem cells such as ECs. On top of that, M2 macrophages are a major source of SDF-1. Through all this, the macrophages role in angiogenesis is regulated in an important, though delicate and time-sensitive balance. ADSCs influences this balance, by stimulating a phenotype switch to M2 macrophages and by inhibiting a prolonged infiltration. Further research needs to clarify the impact of ADSCs on early macrophage infiltration, possibly through M-CSF.
Finally, it must be stated that in select cases of fibrosis (e.g., liver fibrosis), angiogenesis can have a fibrosis stimulating effect[77]. This paradox indicates that the anti-fibrotic effect of ADSCs through angiogenetic stimulation, is case-dependent and remains particularly complex.
When injected into fibrotic tissue by using fat grafts, ADSCs exert anti-fibrotic and pro-angiogenic effects by impacting multiple distinctive mechanisms. Through their capacity to directly differentiate into key cell types that influence the wound healing process, as well as secrete multiple paracrine factors (e.g., HGF, bFGF, IL-10), they carefully alter different mechanisms underlying fibrosis. ADSCs favor ECM degradation by modifying the fibroblast-to-myofibroblast differentiation, by favoring MMPs over TIMPs, by positively influencing collagen organization, and by inhibiting the pro-fibrotic effects of TGF-β1. In addition ADSCs influence both the innate and adaptive immune response system. The pro-angiogenic effect of ADSCs can be categorized into direct (direct differentiation into ECs) as well as indirect effects (secreting pro-angiogenic cytokines such as stromal cell-derived factor-1 and VEGF).
Increasingly more in vitro and in vivo studies tend to focus on the development of anti-fibrotic drugs on a single aspect of fibrosis, not considering the complex interactions nor the time dependency of these mechanisms. This review has shown that the understanding of the complex interaction between ECM accumulation, immune response and vascularization, is vital for the effectiveness of treatments against fibrosis, like fat grafting.
ADSCs have the ability to interact intensively via multiple mechanisms-of action. They intelligently steer multiple molecular balances in an anti-fibrotic or pro-angiogenic direction in a delicate manner. It is by these synergistic actions that ADSCs injected through fat grafts successfully soften fibrotic scars.
The successful anti-fibrotic effect of clinical fat grafting has been described extensively in literature. However, the mechanisms leading to fibrosis and how adipose-derived stomal cells (ADSCs) can interact with these mechanisms to reduce fibrosis, are far from clarified today.
Fibrosis is increasingly recognized as an important cause for morbidity and mortality. Moreover, the increasing clinical use of radiotherapy results in an enhanced incidence of severe tissue damage by fibrosis. Therefore, an efficient anti-fibrotic treatment and a thorough understanding of its mechanism-of-action is mandatory.
The objective of this systematic review was to obtain an in-depth understanding of the complex mechanisms underlying fibrosis, and more specifically, the potential mechanisms-of-action of ADSCs in realizing their anti-fibrotic effect.
This systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) methodology. We clearly defined a set of objectives with pre-defined eligibility criteria. We performed a thorough and disciplined literature search to obtain all relevant studies that met the eligibility criteria.Each citation is associated with a set of Mesh terms that describe the content.
Systematic assessment of available literature was performed by two reviewers and one control to avoid bias. This process resulted in 80 references cited as reported in the PRISMA flow diagram. These references served as basic scientific platform to investigate the previously mentioned research objectives. Due to some contradictory findings in the molecular balances, we performed a supplementary literature search trying to elucidate some of the more specific mechanisms-of-action. This review has shown that the understanding of the complex interaction between extracellular matrix (ECM) accumulation, immune response and vascularization, is vital for the effectiveness of treatments against fibrosis, like fat grafting.
ADSCs have the ability to interact intensively with the healing environment via multiple mechanisms of action. Through their capacity to directly differentiate into key cell types that influence the wound healing process, as well as secrete multiple paracrine factors, ADSCs meticulously alter distinctive mechanisms underlying fibrosis. ADSCs stimulate ECM degradation by modifying the fibroblast-to-myofibroblast differentiation, by favoring matrix metalloproteinase over tissue inhibitors of metalloproteinases, by positively influencing collagen organization, and by inhibiting the pro-fibrotic effects of transforming growth factor-beta 1. In addition, ADSCs influence both the innate and adaptive immune response system. The pro-angiogenic effect of ADSCs exerted by direct differentiation into ECs, as well as by the secretion of pro-angiogenic cytokines such as stromal cell-derived factor-1 and vascular endothelial growth factor. ADSCs intelligently steer these molecular balances in a delicate manner. It is by these synergistic actions that ADSCs injected through fat grafts successfully soften fibrotic scars.
This thorough systematic review describes the intensive and cross talk of ADSCs with surrounding cells and active molecules all having a significant effect on the outcome of fibrosis. This manuscript invites to further research to unravel the complex interactions of ADSCs and the seemingly contradictory effects depending on time and place of occurrence.
We would like to thank Prof. Opdenakker G, Professor of Microbiology and Immunology, Chairman BoD, Rega Institute, KU Leuven, for his expert opinion and guidance.
| 1. | Milenkovic U, Albersen M, Castiglione F. The mechanisms and potential of stem cell therapy for penile fibrosis. Nat Rev Urol. 2019;16:79-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Zhao X, Kwan JYY, Yip K, Liu PP, Liu FF. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020;19:57-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 396] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 3. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5837] [Article Influence: 233.5] [Reference Citation Analysis (1)] |
| 4. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5057] [Article Influence: 210.7] [Reference Citation Analysis (0)] |
| 5. | Coleman SR. Long-Term Survival of Fat Transplants: Controlled Demonstrations. Aesthetic Plast Surg. 2020;44:1268-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Naderi N, Combellack EJ, Griffin M, Sedaghati T, Javed M, Findlay MW, Wallace CG, Mosahebi A, Butler PE, Seifalian AM, Whitaker IS. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J. 2017;14:112-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Shukla L, Yuan Y, Shayan R, Greening DW, Karnezis T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front Pharmacol. 2020;11:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Deinsberger J, Reisinger D, Weber B. Global trends in clinical trials involving pluripotent stem cells: a systematic multi-database analysis. NPJ Regen Med. 2020;5:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Mora C, Serzanti M, Consiglio A, Memo M, Dell'Era P. Clinical potentials of human pluripotent stem cells. Cell Biol Toxicol. 2017;33:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Opdenakker G, Van Damme J, Vranckx JJ. Immunomodulation as Rescue for Chronic Atonic Skin Wounds. Trends Immunol. 2018;39:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Hendrickx B, Verdonck K, Van den Berge S, Dickens S, Eriksson E, Vranckx JJ, Luttun A. Integration of blood outgrowth endothelial cells in dermal fibroblast sheets promotes full thickness wound healing. Stem Cells. 2010;28:1165-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Deng J, Shi Y, Gao Z, Zhang W, Wu X, Cao W, Liu W. Inhibition of Pathological Phenotype of Hypertrophic Scar Fibroblasts Via Coculture with Adipose-Derived Stem Cells. Tissue Eng Part A. 2018;24:382-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Spiekman M, van Dongen JA, Willemsen JC, Hoppe DL, van der Lei B, Harmsen MC. The power of fat and its adipose-derived stromal cells: emerging concepts for fibrotic scar treatment. J Tissue Eng Regen Med. 2017;11:3220-3235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 807] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 15. | Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1731] [Article Influence: 173.1] [Reference Citation Analysis (0)] |
| 16. | Vermeulen P, Dickens S, Degezelle K, Van den Berge S, Hendrickx B, Vranckx JJ. A plasma-based biomatrix mixed with endothelial progenitor cells and keratinocytes promotes matrix formation, angiogenesis, and reepithelialization in full-thickness wounds. Tissue Eng Part A. 2009;15:1533-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Vranckx JJ, Yao F, Eriksson E. Gene transfer of growth factors for wound repair. The epidermis in wound healing. Boca Raton, FL USA: CRC Press, 2004: 265-283. |
| 18. | El Ayadi A, Jay JW, Prasai A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 19. | Juhl P, Bondesen S, Hawkins CL, Karsdal MA, Bay-Jensen AC, Davies MJ, Siebuhr AS. Dermal fibroblasts have different extracellular matrix profiles induced by TGF-β, PDGF and IL-6 in a model for skin fibrosis. Sci Rep. 2020;10:17300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 20. | Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16:11-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 449] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 21. | Stempien-Otero A, Kim DH, Davis J. Molecular networks underlying myofibroblast fate and fibrosis. J Mol Cell Cardiol. 2016;97:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 976] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 23. | Yun IS, Jeon YR, Lee WJ, Lee JW, Rah DK, Tark KC, Lew DH. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012;38:1678-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Aoki M, Miyake K, Ogawa R, Dohi T, Akaishi S, Hyakusoku H, Shimada T. siRNA knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type I. J Invest Dermatol. 2014;134:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Lim DH, Cho JY, Miller M, McElwain K, McElwain S, Broide DH. Reduced peribronchial fibrosis in allergen-challenged MMP-9-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2006;291:L265-L271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Finnson KW, McLean S, Di Guglielmo GM, Philip A. Dynamics of Transforming Growth Factor Beta Signaling in Wound Healing and Scarring. Adv Wound Care (New Rochelle). 2013;2:195-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 27. | Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227:493-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 550] [Article Influence: 39.3] [Reference Citation Analysis (10)] |
| 28. | Tuan TL, Wu H, Huang EY, Chong SS, Laug W, Messadi D, Kelly P, Le A. Increased plasminogen activator inhibitor-1 in keloid fibroblasts may account for their elevated collagen accumulation in fibrin gel cultures. Am J Pathol. 2003;162:1579-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Derrett-Smith EC, Denton CP, Sonnylal S. Animal models of scleroderma: lessons from transgenic and knockout mice. Curr Opin Rheumatol. 2009;21:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Borovikova AA, Ziegler ME, Banyard DA, Wirth GA, Paydar KZ, Evans GRD, Widgerow AD. Adipose-Derived Tissue in the Treatment of Dermal Fibrosis: Antifibrotic Effects of Adipose-Derived Stem Cells. Ann Plast Surg. 2018;80:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2470] [Cited by in RCA: 2789] [Article Influence: 199.2] [Reference Citation Analysis (2)] |
| 32. | Kotani T, Masutani R, Suzuka T, Oda K, Makino S, Ii M. Anti-inflammatory and anti-fibrotic effects of intravenous adipose-derived stem cell transplantation in a mouse model of bleomycin-induced interstitial pneumonia. Sci Rep. 2017;7:14608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Doornaert M, Colle J, De Maere E, Declercq H, Blondeel P. Autologous fat grafting: Latest insights. Ann Med Surg (Lond). 2019;37:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, Piguet PF, Vassalli P. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 270] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107:1529-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 620] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 36. | Cai J, Li B, Liu K, Li G, Lu F. Macrophage infiltration regulates the adipose ECM reconstruction and the fibrosis process after fat grafting. Biochem Biophys Res Commun. 2017;490:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 740] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 38. | Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4053] [Cited by in RCA: 4040] [Article Influence: 269.3] [Reference Citation Analysis (0)] |
| 39. | Perciani CT, MacParland SA. Lifting the veil on macrophage diversity in tissue regeneration and fibrosis. Sci Immunol. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Herbert DR, Hölscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Förster I, Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 600] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 41. | Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 625] [Cited by in RCA: 675] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 42. | Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1343] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 43. | Marth T, Strober W, Seder RA, Kelsall BL. Regulation of transforming growth factor-beta production by interleukin-12. Eur J Immunol. 1997;27:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, Sher A. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 313] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med. 2001;194:809-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 749] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 46. | Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol. 2003;163:1261-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173:4020-4029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 302] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 48. | Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 690] [Article Influence: 25.6] [Reference Citation Analysis (10)] |
| 49. | Büttner C, Skupin A, Reimann T, Rieber EP, Unteregger G, Geyer P, Frank KH. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol. 1997;17:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406-6416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 350] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 51. | Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, Wynn TA. Regulation of hepatic fibrosis and extracellular matrix genes by the th response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol. 2001;167:7017-7026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Wangoo A, Laban C, Cook HT, Glenville B, Shaw RJ. Interleukin-10- and corticosteroid-induced reduction in type I procollagen in a human ex vivo scar culture. Int J Exp Pathol. 1997;78:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther. 2015;6:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 54. | Spiekman M, Przybyt E, Plantinga JA, Gibbs S, van der Lei B, Harmsen MC. Adipose tissue-derived stromal cells inhibit TGF-β1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg. 2014;134:699-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Zonari A, Martins TM, Paula AC, Boeloni JN, Novikoff S, Marques AP, Correlo VM, Reis RL, Goes AM. Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomater. 2015;17:170-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Uysal CA, Tobita M, Hyakusoku H, Mizuno H. The Effect of Bone-Marrow-Derived Stem Cells and Adipose-Derived Stem Cells on Wound Contraction and Epithelization. Adv Wound Care (New Rochelle). 2014;3:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Iekushi K, Taniyama Y, Azuma J, Sanada F, Kusunoki H, Yokoi T, Koibuchi N, Okayama K, Rakugi H, Morishita R. Hepatocyte growth factor attenuates renal fibrosis through TGF-β1 suppression by apoptosis of myofibroblasts. J Hypertens. 2010;28:2454-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Sherriff-Tadano R, Ohta A, Morito F, Mitamura M, Haruta Y, Koarada S, Tada Y, Nagasawa K, Ozaki I. Antifibrotic effects of hepatocyte growth factor on scleroderma fibroblasts and analysis of its mechanism. Mod Rheumatol. 2006;16:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Eto H, Suga H, Aoi N, Kato H, Doi K, Kuno S, Tabata Y, Yoshimura K. Therapeutic potential of fibroblast growth factor-2 for hypertrophic scars: upregulation of MMP-1 and HGF expression. Lab Invest. 2012;92:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Shi HX, Lin C, Lin BB, Wang ZG, Zhang HY, Wu FZ, Cheng Y, Xiang LJ, Guo DJ, Luo X, Zhang GY, Fu XB, Bellusci S, Li XK, Xiao J. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS One. 2013;8:e59966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 61. | Shi JH, Guan H, Shi S, Cai WX, Bai XZ, Hu XL, Fang XB, Liu JQ, Tao K, Zhu XX, Tang CW, Hu DH. Protection against TGF-β1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res. 2013;305:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 62. | Ejaz A, Epperly MW, Hou W, Greenberger JS, Rubin JP. Adipose-Derived Stem Cell Therapy Ameliorates Ionizing Irradiation Fibrosis via Hepatocyte Growth Factor-Mediated Transforming Growth Factor-β Downregulation and Recruitment of Bone Marrow Cells. Stem Cells. 2019;37:791-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Borrelli MR, Patel RA, Adem S, Diaz Deleon NM, Shen AH, Sokol J, Yen S, Chang EY, Nazerali R, Nguyen D, Momeni A, Wang KC, Longaker MT, Wan DC. The antifibrotic adipose-derived stromal cell: Grafted fat enriched with cd74+ adipose-derived stromal cells reduces chronic radiation-induced skin fibrosis. Stem Cells Transl Med. 2020;9:1401-1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Carceller MC, Guillén MI, Ferrándiz ML, Alcaraz MJ. Paracrine in vivo inhibitory effects of adipose tissue-derived mesenchymal stromal cells in the early stages of the acute inflammatory response. Cytotherapy. 2015;17:1230-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Xie J, Jones TJ, Feng D, Cook TG, Jester AA, Yi R, Jawed YT, Babbey C, March KL, Murphy MP. Human Adipose-Derived Stem Cells Suppress Elastase-Induced Murine Abdominal Aortic Inflammation and Aneurysm Expansion Through Paracrine Factors. Cell Transplant. 2017;26:173-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Cho KS, Park MK, Mun SJ, Park HY, Yu HS, Roh HJ. Indoleamine 2,3-dioxygenase is not a pivotal regulator responsible for suppressing allergic airway inflammation through adipose-derived stem cells. PLoS One. 2016;11:e0165661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Evans BGA, Gronet EM, Saint-Cyr MH. How Fat Grafting Works. Plast Reconstr Surg Glob Open. 2020;8:e2705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Murohara T, Shintani S, Kondo K. Autologous adipose-derived regenerative cells for therapeutic angiogenesis. Curr Pharm Des. 2009;15:2784-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004;117:3-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 70. | Dickens S, Vermeulen P, Hendrickx B, Van den Berge S, Vranckx JJ. Regulable vascular endothelial growth factor165 overexpression by ex vivo expanded keratinocyte cultures promotes matrix formation, angiogenesis, and healing in porcine full-thickness wounds. Tissue Eng Part A. 2008;14:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Phipps KD, Gebremeskel S, Gillis J, Hong P, Johnston B, Bezuhly M. Alternatively activated M2 macrophages improve autologous Fat Graft survival in a mouse model through induction of angiogenesis. Plast Reconstr Surg. 2015;135:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Dong Z, Peng Z, Chang Q, Lu F. The survival condition and immunoregulatory function of adipose stromal vascular fraction (SVF) in the early stage of nonvascularized adipose transplantation. PLoS One. 2013;8:e80364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Seaman SA, Cao Y, Campbell CA, Peirce SM. Macrophage Recruitment and Polarization During Collateral Vessel Remodeling in Murine Adipose Tissue. Microcirculation. 2016;23:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Hutchings G, Janowicz K, Moncrieff L, Dompe C, Strauss E, Kocherova I, Nawrocki MJ, Kruszyna Ł, Wąsiatycz G, Antosik P, Shibli JA, Mozdziak P, Perek B, Krasiński Z, Kempisty B, Nowicki M. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 75. | Xu FT, Li HM, Yin QS, Liu DL, Nan H, Zhao PR, Liang SW. Human breast adipose-derived stem cells transfected with the stromal cell-derived factor-1 receptor CXCR4 exhibit enhanced viability in human autologous free fat grafts. Cell Physiol Biochem. 2014;34:2091-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Cai J, Feng J, Liu K, Zhou S, Lu F. Early Macrophage Infiltration Improves Fat Graft Survival by Inducing Angiogenesis and Hematopoietic Stem Cell Recruitment. Plast Reconstr Surg. 2018;141:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 77. | Zadorozhna M, Di Gioia S, Conese M, Mangieri D. Neovascularization is a key feature of liver fibrosis progression: anti-angiogenesis as an innovative way of liver fibrosis treatment. Mol Biol Rep. 2020;47:2279-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casado-Diaz A, Mehanna RA, Salvadori M S-Editor: Wu YXJ L-Editor: A P-Editor: Li X