Published online Sep 26, 2020. doi: 10.4252/wjsc.v12.i9.952
Peer-review started: April 3, 2020
First decision: April 22, 2020
Revised: May 6, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: September 26, 2020
Processing time: 171 Days and 16.4 Hours
Tendon is a mechanosensitive tissue that transmits force from muscle to bone. Physiological loading contributes to maintaining the homeostasis and adaptation of tendon, but aberrant loading may lead to injury or failed repair. It is shown that stem cells respond to mechanical loading and play an essential role in both acute and chronic injuries, as well as in tendon repair. In the process of mechanotransduction, mechanical loading is detected by mechanosensors that regulate cell differentiation and proliferation via several signaling pathways. In order to better understand the stem-cell response to mechanical stimulation and the potential mechanism of the tendon repair process, in this review, we summarize the source and role of endogenous and exogenous stem cells active in tendon repair, describe the mechanical response of stem cells, and finally, highlight the mechanotransduction process and underlying signaling pathways.
Core Tip: Stem cells and mechanical loading are crucial to tendon injuries. In this review, we summarize the sources and roles of endogenous and exogenous stem cells for tendon repair, describe the mechanical response of stem cells, and finally highlight the mechanotransduction process and underlying signaling pathways. The deeper understanding of interactions between stem cells and mechanical loading offers great potential for the development of new therapeutic strategies for tendon repair.
- Citation: Wang HN, Huang YC, Ni GX. Mechanotransduction of stem cells for tendon repair. World J Stem Cells 2020; 12(9): 952-965
- URL: https://www.wjgnet.com/1948-0210/full/v12/i9/952.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i9.952
Tendon is a unique form of connective tissue that links muscle to bone. It is the anatomical structure that transmits muscle-contraction force to the skeleton in order to maintain posture or produce motion. Tendons are composed of triple-helical collagen I molecules assembled into fibrils that, in turn, form fibers, fascicles, and ultimately, tendon[1-3]. This mechanosensitive tissue has specific mechanical properties that enable it to respond and adapt to the loading transmitted by muscles; the collagen fibrils, considered to be the fundamental force-transmitting unit of the tendon, are densely arranged within the extracellular matrix (ECM), oriented parallel to the bone-muscle axis. A multitude of ECM molecules, including collagens, elastin, proteoglycans, and glycoproteins, are involved in tendon-specific collagen I. Tenocytes, the main type of cell located inside the collagen fibers, produce collagen I and ECM molecules[2]. Additionally, tendon stem/progenitor cells (TSPCs), also commonly termed as tendon-derived stem cells (TDSCs) or tendon stem cells (TSCs), located in the fascicular matrix, are responsible for replenishing tendon cells through differentiation and proliferation[4]. Under certain conditions, such as aberrant loading, TSPCs have multidifferentiation potential, namely, TSPCs can differentiate into tenocytes, chondrocytes, osteocytes, and adipocytes[4,5].
Tendon injuries range from chronic to acute, with partial or complete tendon rupture[2,6]. Chronic tendon injury mainly refers to tendinopathy, which is the most common tendon overuse injury and is characterized by pain and impaired function and performance[7]. The pathogenesis of tendinopathy is far from well understood and has been interchangeably defined as a degenerative condition or a failure of the healing process. Moreover, increased expression of inflammatory cytokines, such as COX-2 and interleukin (IL)-6, has been observed in overuse tendinopathy[8]; currently, the production of inflammatory cytokines is not considered to be a classic inflammatory response, but rather, the production expressed by resident tenocytes under overload[9,10]. Histologically, disorganization of collagen, increased noncollagenous ECM, hypercellularity, and neovascularization can be seen[11,12]. The exact relationship between tendinopathy and tendon rupture remains unknown, but it has been reported that tendinopathy increases the risk of tendon rupture[13]. Acute partial or complete tendon rupture interrupts tendon continuity, leading to bleeding, clotting, and the release of PDGF, TGF-β, ATP, and ADP from platelets and of epinephrine and norepinephrine from blood vessels at the wound site[9], resulting in a decrease or even loss of function and, potentially, in the loss of mobility. After injury, tendon undergoes a natural healing process involving successive steps—inflammation and formation of new cells, as well as ECM formation and remodeling[14]. Currently, the treatment for tendon injuries differs from that for other chronic and acute injuries. Conservative treatments include therapeutic exercise[15,16], shockwave[17], and injection therapy[18], which are helpful for reducing pain and improving function in tendinopathy, but pain during treatment, slow recovery, and risk of failure remain. A surgical procedure is usually the first choice for total tendon rupture, but patients may still face postoperative issues, such as proliferation of scar tissue, decreased mobility, and risk of second rupture.
To this end, stem-cell-based therapy has been introduced to clinical practice, and it may have broader future applications with the advantages of offering regeneration and repair. TSPCs, which tend to differentiate into tenocytes and are able to replace the loss of normal tenocytes, have been found to reside in tendon tissue. Thus, they have great potential to become the ideal cell source for stem-cell-based therapy[19,20].
Mechanical loading plays a crucial role in the biology of TSPCs[21]. In particular, proper loading aids in promoting the proliferation and tenogenic differentiation of TSPCs, which is beneficial for tendon repair, whereas aberrant loading might lead to nontenocyte differentiation, which could hinder tendon healing. In order to further understand the response and mechanism of stem cells to mechanical loading, related to tendon injury and repair, this paper summarizes the sources of stem cells and describes the mechanical response of TSPCs for tendon repair, and it further discusses the underlying signaling pathways of TSPCs responding to mechanical stimuli.
Several types of endogenous and exogenous stem cells have proven effective for tendon repair. TSPCs are fibroblast-like cells[22], which have been identified in mice, rabbits, and humans, with typical stem-cell makers[4,22,23]. Nevertheless, the exact source location of TSPCs remains unclear. TSPCs have been isolated and differentiated from tendon[4], peritenon[24], and perivascular sources[25,26]. A recent study reported that a PDGFRA+ cell population expressing tubulin polymerization-promoting protein family member 3 (TPPP3+), which is located in peritenon, has stem-cell characteristics, such that it may generate new tenocytes and self-renew upon injury[27]. TSPCs share some common markers with tenocytes, such as collagen I, collagen III, tenascin C, and tenomodulin (TNMD), but they express still more markers, like Oct-4, SSEA-1/4, and nucleostemin[4,5]. Both TSPCs isolated from the tendon and peritenon regions of mouse Achilles tendons have the Sca1, CD90, and CD44 markers[26], but progenitor cells from the tendon and peritenon regions can be distinguished with genes such as Scx, Mkx, Thbs4, and Wnt10a[24]. Moreover, perivascular stem cells isolated and cultured from human supraspinatus tendon biopsies express both tendon-like and stem/precursor-cell-like markers, including musashi-1, nestin, prominin-1/CD133, CD29, CD44, Scx, and Smad8[25]. TSPCs from the tendon proper lack CD133 markers, however, which may help distinguish TSPCs from tendon proper and perivascular sources. TSPCs have shown a high capacity for proliferation and multipotential differentiation into tenocytes, osteoblasts, chondrocytes, and adipocytes[4,28]. Although TSPCs show multipotential differentiation, they also show spontaneous tenogenic differentiation, which can be beneficial for tendon repair[20]. In a tendon-window-wound study, TSPCs participated in tendon repair by proliferation and activation of tenogenesis[29]. The therapeutic effect of TSPCs has also been confirmed by using animal models. It has been reported that TSPCs promote tendon repair by improving cell and collagen-fiber alignment, collagen birefringence, and Young’s modulus typical of tendon, as well as by increasing ultimate stress capacity[30]. Similarly, ultimate failure load and the expression of collagen I and collagen III in the ruptured Achilles tendon have been much improved by TSPC transplantation[31]. Nevertheless, the abnormal differentiation of TSPCs into nontendon cells has a negative effect on tendon development, homeostasis, and repair. For instance, tendon progenitor cells of injured tendon have strong chondrogenic potential, which may cause endochondral ossification as a result of ectopic mineralization[32]. To date, very scant clinical research has been performed using TSPCs for tendon-related diseases.
As shown in Table 1, stem cells/progenitors derived from other tissues, such as bone marrow-derived mesenchymal stem cell (BMSCs) and adipose-derived stem cells (ASCs), are much easier to acquire than TSPCs[33,34], and they have been proven efficient for tendon repair[14,19]. BMSCs are spindle-shaped[35] and have the potential of tenogenic differentiation[36,37] and high proliferation[4,38]. Several mechanisms may contribute to tendon repair with exogenic BMSCs. First, BMSCs can differentiate into certain new cells (tenocytes) to replace lost normal cells[19,39]; second, BMSCs can secrete various cytokines and growth factors to promote the proliferation of cells in injured tissue[40]; and third, BMSCs can increase the deposition of collagenous proteins[41]. BMSC-based therapy has been found to improve histological and biomechanical properties and to increase the expression of collagen in animal injury[42,43]. But the application of BMSCs may also carry the risk of nontendon differentiation and of forming ectopic bone during tendon repair[44]. The clinical application of BMSCs was started very early, and four clinical trials (NCT03688308, NCT01788683, NCT02484950, and NCT01687777) using BMSCs for rotator-cuff repair are at the stage of recruiting, and the results have not yet been released. ASCs are spindle-shaped[45] with stem-cell marks[23,35,46]; these cells commonly being isolated from subcutaneous adipose tissue[34] and liposuction aspirates[47], have shown the multipotential ability of differentiation including tenogenic cells[48-50] and high proliferation[23,38,51]. ASC transplantation could enhance the secretion of collagen I and tenascin-C during healing and improve the mechanical strength of tendon[52,53], as well as improve the pathological changes of tendinopathy and the normalization of collagen ratios within the affected tendon[54]. Recently, a study indicated that ASCs improved tendon repair in tendinopathy by inhibiting inflammation and inducing neovascularization at the early stage of tendon healing, and ASCs are also effective for the inhibition of ectopic ossification in vivo[55]. Additionally, the clinical safety and efficacy of ASCs therapy have been reported. After allogeneic ASC treatment, patients with lateral elbow epicondylosis self-reported outcomes with reduced pain and improved function, without safety issues, as well as demonstrated decreased tendon defect areas in ultrasound images at 52 wk post-injection[56]. However, the application of ASCs may give rise to fibrotic tissue formation and scarring[57] as well as forming adipocytes[58] during tendon repair. In addition, induced pluripotent stem cells (iPSCs) can be reprogrammed from adult somatic cells. It has been found that human iPSC-derived neural crest stem cells (iPSC-NCSCs) can differentiate into mesenchymal-lineage tenocytes, which accelerate the process of tendon repair[59]. In a rat patellar-tendon window-defect trial, iPSC-NCSCs promoted healing by improving matrix synthesis and mechanical properties and by increasing fetal tendon-related matrix proteins, stem-cell recruitment factors, and the tenogenic differentiation factor[60].
| TSPCs | BM-MSCs | ASCs | |
| Morphology | Fibroblast-like shape[22] | Spindle-shaped[35] | Spindle-shaped[45] |
| Phenotypes | Positive: CD13, C29, CD44, CD54, CD73, CD90, CD105, CD146 and CD166 Negative: CD2, CD3, CD11b, CD14, CD15, CD16, CD18, CD19, CD31, CD34, CD45, CD56, CD71, CD106, CD117, CD123, and CD235a[4,22,23] | Positive: CD13, CD29, CD44, CD73, CD90, and CD105 Negative: CD14, CD19, CD34, CD45[35,37] | Positive: CD13, C29, CD44, CD49d, CD54, CD73, CD90, CD105, and CD166 Negative: CD14, CD19, CD31, CD34, CD45 and CD71[23,35,46] |
| Proliferation | TSPCs = BM-MSCs[4]; TSPCs ≤ ASCs[23] | BM-MSCs = TSPCs[4]; BM-MSCs < ASCs[38] | ASCs > BM-MSCs[38]; ASCs ≥ TSPCs[23] |
| Tenogenic differentiation | Spontaneous differentiation[20], or promoted by growth factors[120] and mechanical loading[75] | Induced by growth factors[36] and mechanical loading[39] | Induced by growth factor supplements[48,49] and extracorporeal shockwave[50] |
| Evidence for tendon repair in vitro | Tenogenic differentiation[20,75,120] and high proliferation potential[4,28] | Tenogenic differentiation and high proliferation potential[4]; enhanced secretion of bioactive factors[40] and the deposition of ECM[41] | Tenogenic differentiation and high proliferation rate[51] |
| Evidence for tendon repair in vivo | High proliferation and activation of tenogenesis[29]; improved collagen alignment and biomechanical properties[30,31] | Improved histological and biomechanical properties; increased expression of collagen[42,43] | Modulation of microenvironment[55]; enhancing the secretion of collagen and mechanical strength of tendon[52,53] |
| Evidence for tendon repair in clinics | None | Four registered trials, but the results are not available | Reduction of pain, tendon defect areas post intervention[56] |
| Advantages | Spontaneous tenogenic differentiation[20]; higher proliferation and therapeutic effectiveness[31] | Easier acquirement[33]; enhanced secretion of bioactive factors[40]; increased the deposition of collagenous proteins[41] | Easier acquirement[34]; inhibition of osteogenic differentiation[55]; confirmed clinical outcome[56] |
| Limitations | Limited number obtained from isolation[63] | High potential of osteogenic differentiation[44]; lower therapeutic effectiveness than TSPCs[31] | Risk of fibrotic tissue formation, scarring[57], and forming adipocytes[58] |
Compared with exogenic stem cells/progenitors, TSPCs possess higher regenerative potential for tendon repair. For instance, during treatment of rat Achilles tendon injury, TSPCs have a greater positive effect on morphological and histological alteration and biomechanical strength when compared to BMSC transplantation[31]. This distinction may be because TSPCs proliferate more rapidly and have a greater capacity for colony formation[41,61,62]; additionally, TSPCs undergo spontaneous tenogenic differentiation, whereas BMSCs do not[20]. It has been demonstrated that mouse TSPCs express higher levels of tenogenic markers, such as Scx, Comp, Sox9, and Runx2, than mouse BMSCs; similarly, human TSPCs express more TNMD than BMSCs[4]. Thus, TSPCs more rapidly differentiate to functional tenocytes. Moreover, the expression of collagen I and collagen III is higher in TSPCs, which results in greater biomechanical strength at the early stage of repair[31]. However, the limited number of resident TSPCs hinders the large-scale clinical application[63]. Hence, both endogenous and exogenous stem cells have therapeutic potential. According to current evidence, TSPCs possess some advantages for tendon repair, but the efficacy of endogenic and exogenic stem cells requires further investigation.
A number of factors influence the homeostasis of tendon, in which mechanical loading plays a critical role[64]. Under normal or physiological loading, the magnitude of loading is much less than the ultimate tensile strength (UTS). Typically, tendon could return to its original length when the strain is less than 4% of elongation; but tendon will have macroscopic tearing and eventually rapture when the strain is beyond 8%-10% of elongation[65]. Researchers usually use 4% cyclic uniaxial stretching to mimic this loading condition in vitro, and to moderate treadmill running model of rats (13 m/min, 15 min/d, and 5 d/wk in the first week; 13 m/min, 50 min/d, and 5 d/wk for another 3 wk) in vivo[21]. In normal or physiological loading, tendon can maintain homeostasis and respond to loading through cellular anabolic adaptation[3,21]. By contrast, abnormal loading may be different from normal mechanical loading in magnitude, frequency, duration, and/or direction; typically, abnormal loading of tendon can be unload, overload, or high repetitive low load[66]. Compared with explants tensioned with constant 4% strain, nontensioned rabbit patellar tendon decreased linear stiffness, elongation to failure, and maximum failure force after 20 h[67]; undergoing cyclic loading at approximately 35% of the UTS led to tendon rapture in 15 min[68]; also, cyclic loading under 5% of UTS (around 1% strain) resulted in rupture within 15 h[69]. In vitro, researchers usually use 8% cyclic uniaxial stretching to mimic the overloading condition, as well as intensive treadmill running (13 m/min, 15 min/d, and 5 d/wk in the first week; 13 m/min, 3 h/d, 4 h/d, and 5 h/d in the second, third, and fourth weeks for 5 d)[21]. Abnormal loading can lead to failed repair or pathological changes by causing anabolic changes in tendon[14,21,70]. After high-intensity repetitive-exercise-induced injury, the expression level of IL-1β increases in mouse tendon[71]. Moreover, a greater production of inflammatory mediators induced by IL-1β, including COX-2, MMP-1, and PGE-2, has been reported for human tendon fibroblasts with excessive stretch loading than with moderate stretching or without stretch loading in vitro[72]. Similarly, the level of PGE-2 significantly increases in mouse patellar and Achilles tendons after rigorous treadmill running compared to caged control groups in vivo[73], indicating that overloading tendon may lead to a higher production of PGE-2. These inflammatory mediators may, in turn, promote the degradation of tendon, such as through neutrophil infiltration and decreased collagen production, thereby negatively impacting the repair of injured tendon.
TSPCs undergo similar mechanical loading as tenocytes. Mechanical loading, no matter what level, can increase TSC proliferation, which is indeed necessary for healing injured tendon[21]. Patellar and Achilles TSCs isolated from mice after moderate treadmill running have nearly double proliferation rates compared to the TSCs isolated from less active mice in vitro[21]; also, compared to inactive mice, cellular production of collagen increases by 70% and 200% for patellar and Achilles TSCs, respectively, for mice completing moderate treadmill running in vivo[74]. Currently, few studies have found that the magnitude of stretching could lead to different cell fate. In particular, a higher magnitude of stretching may cause aberrant differentiation compared to a lower magnitude of stretching in vitro. It was reported that 4% stretching promoted the differentiation of TSCs into tenocytes with increased gene expression of collagen I; 8% stretching, however, promoted the differentiation of TSCs into nontenocytes, including adipocytes, chondrocytes, and osteocytes, aside from differentiation into tenocytes, as evidenced by higher expression levels of genes such as PPARγ, collagen II, Sox-9, and Runx2 in vitro[75]. Similarly, increased differentiation into adipocytes, chondrocytes, osteocytes, and tenocytes with high gene expression of LPL, Sox-9, Runx2, Osterix, collagen I, and TNMD was found in mice after intensive treadmill running in vivo[76]. Also, mechanical loading can influence both TSC proliferation and differentiation due to an inflammatory mediator. In response to rigorous treadmill running, mouse patellar and Achilles tendons increase the production of PGE-2, which can decrease cell proliferation and induce both adipogenesis and osteogenesis of TSCs, as well as promote the production of fatty and calcified tissues within tendon[73]. These findings are consistent with the clinical understanding that complete rest without loading will decrease tendon strength and induce pathologic change in tendon, such that total rest is relatively contraindicated for tendinopathy[77,78]. In short, mechanical loading is necessary for TSC proliferation and collagen production, but excessive loading may cause abnormal differentiation of TSCs into nontenocytes, leading to tendon injury or failed tendon repair.
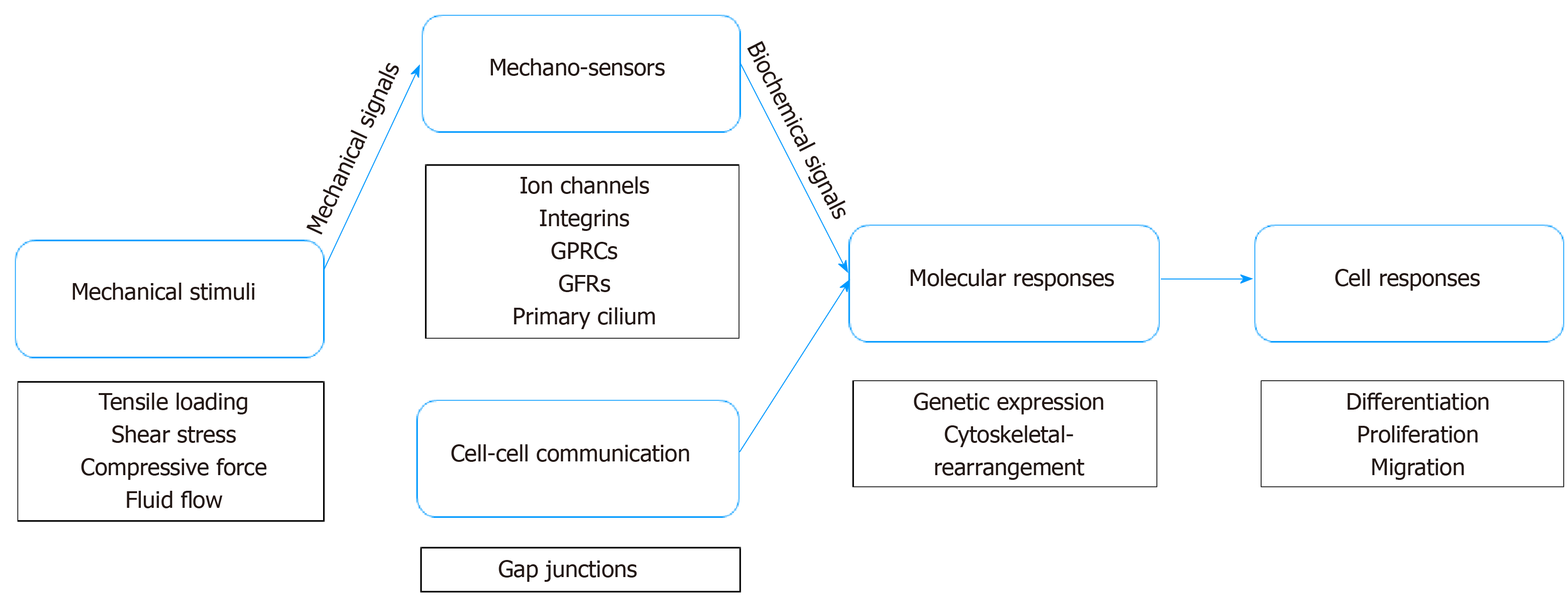
TSPCs would not be able to respond to mechanical loading without mechanotransduction, which converts mechanical signals from the environment into biochemical signals[79] (Figure 1). As a mechanical signal is transmitted to the microenvironment, it causes the physical perturbation of cells and deformation of the extracellular matrix[80,81]. Both TSPCs and tenocytes reside in the pericellular matrix, such that TSPCs experience a force similar to tenocytes. In tendon, tissue probably undergoes various types of force, including tensile loading, shear, and even compression force[81,82]. As tendon has the function of transmitting force from muscle to bone, tendon is exposed to high-tensile force, whereas the tendon-bone junction area commonly experiences compression force[83]. Further, the midportion of a tendon can potentially be exposed to both shear and compression forces due to the different forces to which the posterior and anterior areas of the tendon are exposed[84]. It has been reported that cyclic tensile loading on tendon may cause interstitial fluid flow, leading to shear force and perhaps hydrostatic force.
Moreover, effector-cell mechanosensors detect mechanical signals and induce an intercellular response via various downstream signaling pathways to regulate stem-cell differentiation and proliferation[85,86]. Typically, effector cells can sense mechanical signaling from groups of transmembrane mechanosensitive proteins, namely, mechanosensors such as ion channels, integrins, G-protein coupled receptors (GPCRs), growth factor receptor (GFR), and primary cilium[85]. Ion channels play an essential role in cellular mechanotransduction. Cystic fibrosis transmembrane conductance regulator (CFTR) is a stretch-medicated activation channel that aids in passing chloride and water in and out of cells[87,88]. Recently, a study reported that CFTR regulates tenogenic differentiation through inhibiting the Wnt/β-catenin/ERK1/2 signaling pathway in TSPCs[89]. Also, Ca2+ is a greatly powerful second messenger regulating cell migration and influencing the differentiation of stem cells[90,91]. Several studies have shown that transient receptor potential melastatin 7 (TRPM7) works as a calcium channel conducting calcium influx[90,92], which, in turn, activates transcription factor NFATc1, which then induces osteogenesis in mesenchymal stem cells (MSCs)[93,94]. Interestingly, integrins become involved in the intracellular concentration of calcium by interaction with ion channels[95]. In addition to interaction with ion channels, accumulating evidence indicates that integrin systems can detect mechanical signals and then transduce them to nuclear signaling[96,97]. Once integrin receptors bind their ligands in the ECM, they move laterally in the plane of the membrane to form focal adhesions, which play a central role in cell motility and cytoskeletal dynamics, as well as in regulating cell proliferation, differentiation, and gene expression[98]. GPCRs have the ability to sense mechanical loading and regulate molecular mechanisms downstream via binding protein ligand. For instance, Wnt binds frizzled receptors, which span the plasma membrane and constitute a distinct family of GPCRs that regulate osteogenic differentiation in MSCs[99]. Studies have found that, when a stretching force is applied to TSPCs, Wnt5a activates a co-receptor, receptor tyrosine kinase-like orphan receptor 2 (ROR2), and regulates Wnt5a/JNK and Wnt5a/RhoA signaling pathways downstream for osteogenic differentiation in TSPCs[100,101]. Additionally, growth factors bind their GFRs and regulate signaling pathways downstream—in TSPCs, GFRs mediate bone morphogenetic protein (BMP) signaling pathways involved in biomechanical loading-induced differentiation[102,103]. Further, primary cilium, an extraordinary organelle that exists on nearly all somatic cells, also plays a role in the detection of mechanical signaling. Currently, research specifically focused on the primary cilium of TSPCs is rare, but it can be hypothesized that the principle might be similar to tenocytes and MSCs. In tenocytes, the length of primary cilium immediately and significantly increases in a stress-deprived environment and can be reversed by cyclic tensile loading[104]. In MSCs, frizzled receptors are thought to localize cilium membranes, such that primary cilium is involved in controlling differentiation by tuning Wnt signaling pathways in MSCs[105,106].
In addition, even if a cell is not to receive mechanical stimulation, the cell can still respond via a process known as cell-cell communication, whereby it can communicate with a distant cell receiving mechanical stimulation[107,108]. To date, limited research has investigated the mechanism of cell-cell communication in TSPCs. Because TSPCs are stem cells/progenitors of tendon, however, they may have similar communication characteristics as tenocytes and MSCs. Connexins form gap junctions between the cytoplasm of adjacent cells allow for the direct intercellular exchange of ions and molecules[109]. Gap junctions are immunohistochemically detected among tenocytes, and connexins 32 and 43 form a three-dimensional network to respond to mechanical stimulation together[110]—connexin 32 has a stimulatory function, whereas connexin 43 is inhibitory[109]. Additionally, cell-cell communication can be altered to some degree by stretching; specifically, communication increases under low-level stretching (4%) and decreases under high-level stretching (8%)[111]. Moreover, a recent study has shown that connexin 43 plays an impactful role in protecting MSCs from premature senescence, which results in the failure to properly differentiate in vitro[112]. Connexin 43 may contribute to early tenogenesis in MSCs, but the mechanism in mechanotransduction is still not clear[113].
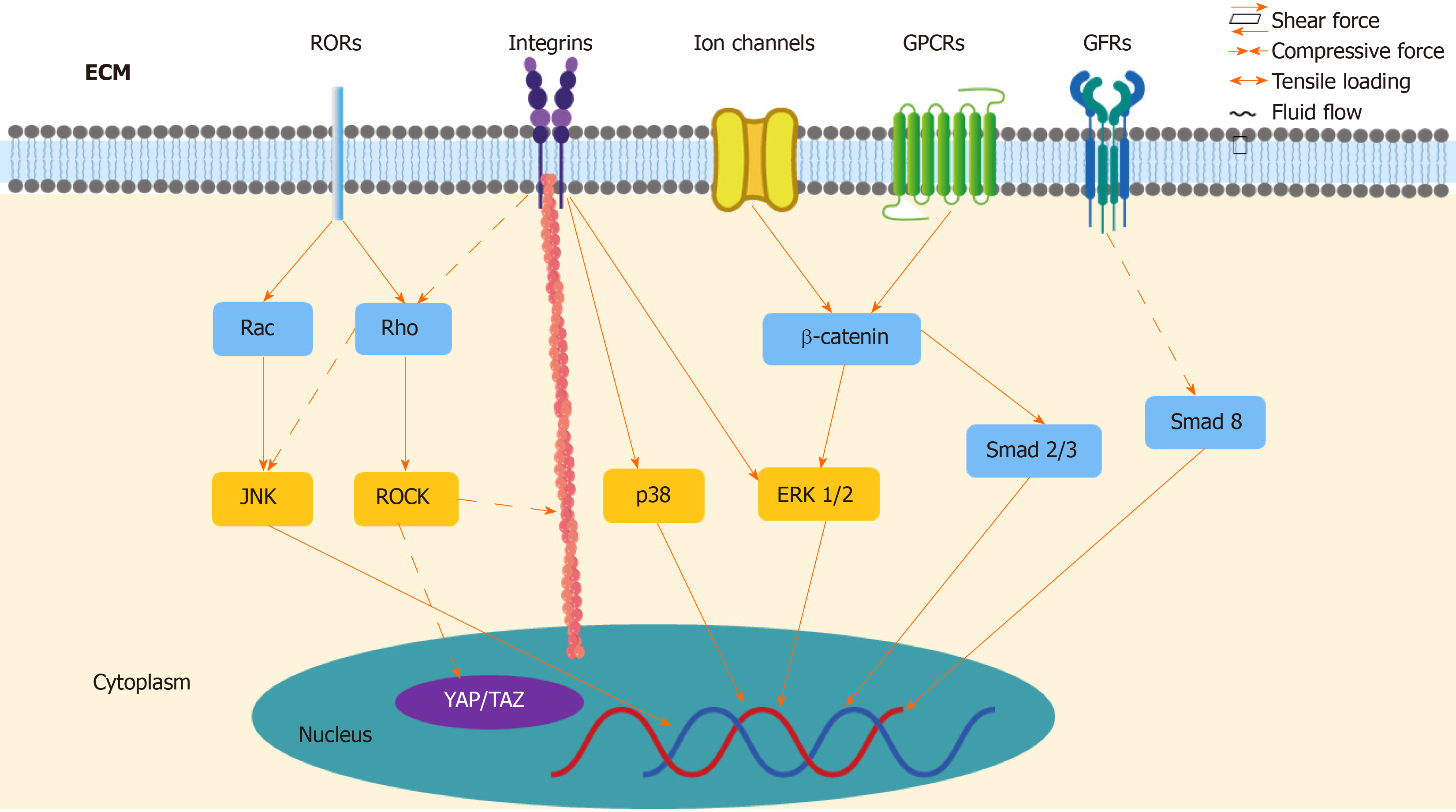
As the important role of mechanotransduction in TSPCs responding to mechanical loading has been realized, it has been demonstrated that various underlying signaling pathways transmit biomechanical signals to nuclei (Figure 2). With the noncanonical Wnts signaling pathway, Wnts binds co-receptor ROR2 and regulates signaling pathways downstream, in turn influencing osteogenic differentiation in TSPCs[114]. Mechanical tension promotes osteogenic differentiation of rat TSPCs via the Wnt5a/Wnt5b/JNK signaling pathway. Under 8% elongation uniaxial mechanical tension (UMT) stimulation, TSPCs exhibit increased protein levels of Wnt5a, Wnt5b, and P-JNK, as well as increased cytoskeletal rearrangement[100]. The mRNA expression of osteogenic genes, such as Runx2, Dlx5, Alpl, and collagen Ia1, also increases[100]. Additionally, UMT induces the appearance of osteogenic differentiation in rat TSCs through the Wnt5a/RhoA signaling pathway. RhoA and its effector protein, ROCK, play an important role in osteogenetic differentiation of MSCs[115], as they show increased mRNA expression of the osteogenic genes Runx2, Alpl, and collagen Ia1, along with ALP activity, as well as ALP cytochemical staining and Runx2 protein expression after 2% elongation mechanical tension[101]. Only the expression of Wnt5a increased under UMT, not the other noncanonical Wnts, such as Wnt5b, Wnt7a, and Wnt11, but it can be inferred that the difference in Wnt protein levels might be due to varying magnitude of loading[101]. To date, the interaction between Wnt5a/JNK and Wnt5a/RhoA remains to be further investigated, insofar as whether Wnt5a/JNK regulates cells independent of or depending on the RhoA pathway[116].
In addition to noncanonical Wnts signaling pathways, the Wnt/β-catenin signaling pathway also contributes to differentiation in TSPCs. Wnt commonly binds frizzled receptors and downregulates β-catenin in the canonical Wnt/β-catenin signaling pathway[114]. In a rat tendinopathy model and a human tendon with tendinopathy, increased expression of Wnt3 and β-catenin has been observed. Wnt3a can increase ALP activity, calcium nodule formation, and the expression of osteogenic markers in TSPCs[117]. Similarly, strain loading promotes osteogenetic differentiation and inhibits adipogenesis via β-catenin[99]. A recent study reported that CFTR, a stretch-mediated activation channel, can regulate TSPCs during tenogenic differentiation under mechanical stretching. Mice with dysfunctional CFTR showed reduced levels of tendon markers, including Scx, TNMD, collagen Ia1 chain, and decorin, as well as abnormally active Wnt/β-catenin signaling, which, in turn, further activated the ERK1/2 signaling pathway[89]. Inhibiting ERK1/2 signaling can promote tenogenic differentiation in TSPCs, both in vitro and in vivo, however, and increase matrix formation and mechanical properties, which is helpful in the tendon healing process[89]. Moreover, activation of Wnt/β-catenin signaling suppressed the expression of tenogenic genes Scx, Mkx, and Tnmd in TSPCs by reducing the amount of Smad2 and Smad3, which are intracellular mediators for TGF-β signaling[118].
In rat TSPCs, both 4% and 8% stretching can increase the amount of BMP-2, and 4% stretching upregulates BMP-2 genetic expression, when compared to unstretching, which does not obviously promote the expression of BMP-2[119]. Studies have proven that BMP-2 causes aberrant proliferation and differentiation in TSPCs, in other words, the addition of BMP-2 to human TSPC cultures reduces the proliferation of cells and promotes osteogenic differentiation in vitro[120]. BMP-2 promotes osteogenic differentiation through ALP cytochemical staining, ALP activity, and calcium nodule formation. Additionally, BMP-2 inhibits tenogenic marker expression, but promotes osteogenic, adipogenic, and chondrogenic differentiation in TSPCs[103]. After BMP-2 stimulation, TSPCs show increased glycosaminoglycan (GAG) production and mRNA expression of aggrecan (Acan), along with decreased mRNA expression of decorin (Dcn), biglycan (Bgn), and fibromodulin (Fmod)[103]. To date, it remains to be determined whether the BMP-2 downstream molecular signaling pathways induce differentiation of TSPCs. Smad8 might play a role, as activated Smad8 promotes MSC tenogenic differentiation via inhibiting the BMP-2 induced osteogenic pathway[121].
ECM deformation initiates integrin signaling at focal adhesion sites where the ECM binds integrin, which activates downstream proteins, such as ERK1/2, p38, and JNK[98]. Higher matrix stiffness increases TDSC proliferation and forms more stress fibers, as well as inhibits the differentiation of TDSCs into tenogenic, chondrogenic, and osteogenic lineages via focal adhesion kinase (FAK) or ERK1/2 signaling pathways[122]. Similarly, another study found that ERK/MAPK signaling pathways increase the tenogenic expression level in mouse MSCs[123]. In addition to ERK, p38 kinases also affect integrin-induced signaling pathways in TSPCs, as it was reported that 8% mechanical stretching caused an upregulated response in ERK1/2 and p38 kinases, as well as altered expression of matrix proteins, integrins, and matrix metalloproteinases[124]. Interestingly, mechanical loading might precisely regulate ERK signaling, as the level of ERK1/2 phosphorylation induced by cyclic uniaxial mechanical stretching is related to stretching time in vitro[125]. Furthermore, RhoA/ROCK and the cytoskeleton may also contribute to integrin signaling. This demonstrates that FAK has the ability to regulate mechanical stretch-induced tenogenic differentiation by mediating RhoA/ROCK downstream and interacting with the cytoskeleton in human MSCs[126].
Recent studies have identified two important transcriptional coactivators—Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) in the Hippo signaling pathway[127]. Mechanical loading has the ability to influence YAP/TAZ activities through stretching, geometry, and substrate rigidity, which, in turn, regulates stem-cell fate and behavior[128]. In human MSCs, YAP/TAZ knockdown promotes adipogenic differentiation on rigid substrates, which commonly happens on soft substrates[127]. Also, shear stress can induce human MSC osteogenic and fibrochondrogenic differentiation and promote TAZ nuclear translocation via the RhoA/ROCK signaling pathway and YAP/TAZ[129,130]. In addition, another study suggests that YAP/TAZ may be a downstream effector of the noncanonical Wnts signaling pathway, which plays a crucial role in TSPC differentiation. Thus, YAP/TAZ might mediate gene expression, osteogenesis, and cell migration of TSPCs[131]. To date, limited research has been conducted into the deep mechanism of Hippo and YAP/TAZ signaling pathways in tenocytes and TSPCs, but the potential value of this area is certain. In short, several signaling pathways have been demonstrated to participate in the mechanotransduction of TSPCs, such as noncanonical Wnts, Wnt/β-catenin, BMP-2, and integrin, as well as YAP/TAZ.
This review summarizes the sources and roles of the endogenous and exogenous stem cells that can be used for tendon repair, describes the mechanical response of stem cells in tendon repair, and finally, highlights the mechanotransduction process and its underlying signaling pathways. Mechanical loading plays a crucial role in both tendon injury and repair. When mechanical loading is applied, the stimulation is detected by mechanosensors, such as ion channels, integrin, GPCRs, GFR, and primary cilium, which then transmute the mechanical signal into a biological signal, and in turn, regulate various downstream signaling pathways. Suitable mechanical loading is helpful for promoting both the proliferation and differentiation of TSPCs, which are crucial for tendon repair. These findings promise a bright future with new therapeutic strategies for tendon repair. Further studies are necessary to identify mechanosensors and deeply understand the signaling pathways of stem cells.
| 1. | Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 686] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 2. | Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. 2015;11:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 340] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 3. | Galloway MT, Lalley AL, Shearn JT. The role of mechanical loading in tendon development, maintenance, injury, and repair. J Bone Joint Surg Am. 2013;95:1620-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1079] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 5. | Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 6. | Flint JH, Wade AM, Giuliani J, Rue JP. Defining the terms acute and chronic in orthopaedic sports injuries: a systematic review. Am J Sports Med. 2014;42:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Scott A, Squier K, Alfredson H, Bahr R, Cook JL, Coombes B, de Vos RJ, Fu SN, Grimaldi A, Lewis JS, Maffulli N, Magnusson SP, Malliaras P, Mc Auliffe S, Oei EHG, Purdam CR, Rees JD, Rio EK, Gravare Silbernagel K, Speed C, Weir A, Wolf JM, Akker-Scheek IVD, Vicenzino BT, Zwerver J. ICON 2019: International Scientific Tendinopathy Symposium Consensus: Clinical Terminology. Br J Sports Med. 2020;54:260-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 8. | Legerlotz K, Jones ER, Screen HR, Riley GP. Increased expression of IL-6 family members in tendon pathology. Rheumatology (Oxford). 2012;51:1161-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Scott A, Khan KM, Cook JL, Duronio V. What is "inflammation"? Are we ready to move beyond Celsus? Br J Sports Med. 2004;38:248-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford). 2004;43:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 343] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 531] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 12. | Lipman K, Wang C, Ting K, Soo C, Zheng Z. Tendinopathy: injury, repair, and current exploration. Drug Des Devel Ther. 2018;12:591-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Yasui Y, Tonogai I, Rosenbaum AJ, Shimozono Y, Kawano H, Kennedy JG. The Risk of Achilles Tendon Rupture in the Patients with Achilles Tendinopathy: Healthcare Database Analysis in the United States. Biomed Res Int. 2017;2017:7021862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Docheva D, Müller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv Drug Deliv Rev. 2015;84:222-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 474] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 15. | Malliaras P, Barton CJ, Reeves ND, Langberg H. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 16. | Beyer R, Kongsgaard M, Hougs Kjær B, Øhlenschlæger T, Kjær M, Magnusson SP. Heavy Slow Resistance Versus Eccentric Training as Treatment for Achilles Tendinopathy: A Randomized Controlled Trial. Am J Sports Med. 2015;43:1704-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 17. | Korakakis V, Whiteley R, Tzavara A, Malliaropoulos N. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: a systematic review including quantification of patient-rated pain reduction. Br J Sports Med. 2018;52:387-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Lin MT, Chiang CF, Wu CH, Huang YT, Tu YK, Wang TG. Comparative Effectiveness of Injection Therapies in Rotator Cuff Tendinopathy: A Systematic Review, Pairwise and Network Meta-analysis of Randomized Controlled Trials. Arch Phys Med Rehabil. 2019;100:336-349.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Liu L, Hindieh J, Leong DJ, Sun HB. Advances of stem cell based-therapeutic approaches for tendon repair. J Orthop Translat. 2017;9:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Guo J, Chan KM, Zhang JF, Li G. Tendon-derived stem cells undergo spontaneous tenogenic differentiation. Exp Cell Res. 2016;341:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Zhang J, Wang JH. The effects of mechanical loading on tendons--an in vivo and in vitro model study. PLoS One. 2013;8:e71740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Randelli P, Conforti E, Piccoli M, Ragone V, Creo P, Cirillo F, Masuzzo P, Tringali C, Cabitza P, Tettamanti G, Gagliano N, Anastasia L. Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am J Sports Med. 2013;41:1653-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Stanco D, Viganò M, Perucca Orfei C, Di Giancamillo A, Peretti GM, Lanfranchi L, de Girolamo L. Multidifferentiation potential of human mesenchymal stem cells from adipose tissue and hamstring tendons for musculoskeletal cell-based therapy. Regen Med. 2015;10:729-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Mienaltowski MJ, Cánovas A, Fates VA, Hampton AR, Pechanec MY, Islas-Trejo A, Medrano JF. Transcriptome profiles of isolated murine Achilles tendon proper- and peritenon-derived progenitor cells. J Orthop Res. 2019;37:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Tempfer H, Wagner A, Gehwolf R, Lehner C, Tauber M, Resch H, Bauer HC. Perivascular cells of the supraspinatus tendon express both tendon- and stem cell-related markers. Histochem Cell Biol. 2009;131:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Mienaltowski MJ, Adams SM, Birk DE. Regional differences in stem cell/progenitor cell populations from the mouse achilles tendon. Tissue Eng Part A. 2013;19:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Harvey T, Flamenco S, Fan CM. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat Cell Biol. 2019;21:1490-1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 28. | Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 29. | Tan Q, Lui PP, Lee YW. In vivo identity of tendon stem cells and the roles of stem cells in tendon healing. Stem Cells Dev. 2013;22:3128-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Ni M, Lui PP, Rui YF, Lee YW, Lee YW, Tan Q, Wong YM, Kong SK, Lau PM, Li G, Chan KM. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2012;30:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 31. | Al-Ani MKh, Xu K, Sun Y, Pan L, Xu Z, Yang L. Study of Bone Marrow Mesenchymal and Tendon-Derived Stem Cells Transplantation on the Regenerating Effect of Achilles Tendon Ruptures in Rats. Stem Cells Int. 2015;2015:984146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Asai S, Otsuru S, Candela ME, Cantley L, Uchibe K, Hofmann TJ, Zhang K, Wapner KL, Soslowsky LJ, Horwitz EM, Enomoto-Iwamoto M. Tendon progenitor cells in injured tendons have strong chondrogenic potential: the CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells. 2014;32:3266-3277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Zhang B, Luo Q, Halim A, Ju Y, Morita Y, Song G. Directed Differentiation and Paracrine Mechanisms of Mesenchymal Stem Cells: Potential Implications for Tendon Repair and Regeneration. Curr Stem Cell Res Ther. 2017;12:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 804] [Cited by in RCA: 761] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 35. | Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 306] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 36. | Perucca Orfei C, Viganò M, Pearson JR, Colombini A, De Luca P, Ragni E, Santos-Ruiz L, de Girolamo L. In Vitro Induction of Tendon-Specific Markers in Tendon Cells, Adipose- and Bone Marrow-Derived Stem Cells is Dependent on TGFβ3, BMP-12 and Ascorbic Acid Stimulation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13072] [Article Influence: 688.0] [Reference Citation Analysis (12)] |
| 38. | Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 39. | Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 40. | Baberg F, Geyh S, Waldera-Lupa D, Stefanski A, Zilkens C, Haas R, Schroeder T, Stühler K. Secretome analysis of human bone marrow derived mesenchymal stromal cells. Biochim Biophys Acta Proteins Proteom. 2019;1867:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Wu T, Liu Y, Wang B, Sun Y, Xu J, Yuk-Wai LW, Xu L, Zhang J, Li G. The Use of Cocultured Mesenchymal Stem Cells with Tendon-Derived Stem Cells as a Better Cell Source for Tendon Repair. Tissue Eng Part A. 2016;22:1229-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH, Lim BH. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg Am. 2007;89:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 43. | He M, Gan AW, Lim AY, Goh JC, Hui JH, Chong AK. Bone Marrow Derived Mesenchymal Stem Cell Augmentation of Rabbit Flexor Tendon Healing. Hand Surg. 2015;20:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 45. | Arnhold S, Elashry MI, Klymiuk MC, Geburek F. Investigation of stemness and multipotency of equine adipose-derived mesenchymal stem cells (ASCs) from different fat sources in comparison with lipoma. Stem Cell Res Ther. 2019;10:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, Idris SB. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (1)] |
| 47. | De Francesco F, Ricci G, D'Andrea F, Nicoletti GF, Ferraro GA. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng Part B Rev. 2015;21:572-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 48. | Li X, Pongkitwitoon S, Lu H, Lee C, Gelberman R, Thomopoulos S. CTGF induces tenogenic differentiation and proliferation of adipose-derived stromal cells. J Orthop Res. 2019;37:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Dai L, Hu X, Zhang X, Zhu J, Zhang J, Fu X, Duan X, Ao Y, Zhou C. Different tenogenic differentiation capacities of different mesenchymal stem cells in the presence of BMP-12. J Transl Med. 2015;13:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Rinella L, Marano F, Paletto L, Fraccalvieri M, Annaratone L, Castellano I, Fortunati N, Bargoni A, Berta L, Frairia R, Catalano MG. Extracorporeal shock waves trigger tenogenic differentiation of human adipose-derived stem cells. Connect Tissue Res. 2018;59:561-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Cheng X, Tsao C, Sylvia VL, Cornet D, Nicolella DP, Bredbenner TL, Christy RJ. Platelet-derived growth-factor-releasing aligned collagen-nanoparticle fibers promote the proliferation and tenogenic differentiation of adipose-derived stem cells. Acta Biomater. 2014;10:1360-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Lee SY, Kwon B, Lee K, Son YH, Chung SG. Therapeutic Mechanisms of Human Adipose-Derived Mesenchymal Stem Cells in a Rat Tendon Injury Model. Am J Sports Med. 2017;45:1429-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 53. | Norelli JB, Plaza DP, Stal DN, Varghese AM, Liang H, Grande DA. Tenogenically differentiated adipose-derived stem cells are effective in Achilles tendon repair in vivo. J Tissue Eng. 2018;9:2041731418811183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Oshita T, Tobita M, Tajima S, Mizuno H. Adipose-Derived Stem Cells Improve Collagenase-Induced Tendinopathy in a Rat Model. Am J Sports Med. 2016;44:1983-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Kokubu S, Inaki R, Hoshi K, Hikita A. Adipose-derived stem cells improve tendon repair and prevent ectopic ossification in tendinopathy by inhibiting inflammation and inducing neovascularization in the early stage of tendon healing. Regen Ther. 2020;14:103-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Lee SY, Kim W, Lim C, Chung SG. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells. 2015;33:2995-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 57. | Costa-Almeida R, Calejo I, Reis RL, Gomes ME. Crosstalk between adipose stem cells and tendon cells reveals a temporal regulation of tenogenesis by matrix deposition and remodeling. J Cell Physiol. 2018;233:5383-5395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Uysal AC, Mizuno H. Tendon regeneration and repair with adipose derived stem cells. Curr Stem Cell Res Ther. 2010;5:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Bavin EP, Smith O, Baird AE, Smith LC, Guest DJ. Equine Induced Pluripotent Stem Cells have a Reduced Tendon Differentiation Capacity Compared to Embryonic Stem Cells. Front Vet Sci. 2015;2:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Xu W, Wang Y, Liu E, Sun Y, Luo Z, Xu Z, Liu W, Zhong L, Lv Y, Wang A, Tang Z, Li S, Yang L. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng Part A. 2013;19:2439-2451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Lui PP, Chan KM. Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev Rep. 2011;7:883-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 62. | Thaker H, Sharma AK. Engaging stem cells for customized tendon regeneration. Stem Cells Int. 2012;2012:309187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Costa-Almeida R, Calejo I, Gomes ME. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 64. | Gracey E, Burssens A, Cambré I, Schett G, Lories R, McInnes IB, Asahara H, Elewaut D. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat Rev Rheumatol. 2020;16:193-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 65. | Wang JH, Guo Q, Li B. Tendon biomechanics and mechanobiology--a minireview of basic concepts and recent advancements. J Hand Ther. 2012;25:133-40; quiz 141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 66. | Thornton GM, Hart DA. The interface of mechanical loading and biological variables as they pertain to the development of tendinosis. J Musculoskelet Neuronal Interact. 2011;11:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Nabeshima Y, Grood ES, Sakurai A, Herman JH. Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J Orthop Res. 1996;14:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Fung DT, Wang VM, Laudier DM, Shine JH, Basta-Pljakic J, Jepsen KJ, Schaffler MB, Flatow EL. Subrupture tendon fatigue damage. J Orthop Res. 2009;27:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 69. | Parent G, Huppé N, Langelier E. Low stress tendon fatigue is a relatively rapid process in the context of overuse injuries. Ann Biomed Eng. 2011;39:1535-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Snedeker JG, Foolen J. Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017;63:18-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 71. | Fedorczyk JM, Barr AE, Rani S, Gao HG, Amin M, Amin S, Litvin J, Barbe MF. Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J Orthop Res. 2010;28:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 72. | Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 73. | Zhang J, Wang JH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res. 2010;28:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Zhang J, Pan T, Liu Y, Wang JH. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res. 2010;28:1178-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 75. | Zhang J, Wang JH. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010;28:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 76. | Zhang J, Li B, Wang JH. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011;32:6972-6981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 77. | Kubo K, Akima H, Ushiyama J, Tabata I, Fukuoka H, Kanehisa H, Fukunaga T. Effects of 20 days of bed rest on the viscoelastic properties of tendon structures in lower limb muscles. Br J Sports Med. 2004;38:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Uchida H, Tohyama H, Nagashima K, Ohba Y, Matsumoto H, Toyama Y, Yasuda K. Stress deprivation simultaneously induces over-expression of interleukin-1beta, tumor necrosis factor-alpha, and transforming growth factor-beta in fibroblasts and mechanical deterioration of the tissue in the patellar tendon. J Biomech. 2005;38:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Elosegui-Artola A, Trepat X, Roca-Cusachs P. Control of Mechanotransduction by Molecular Clutch Dynamics. Trends Cell Biol. 2018;28:356-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 80. | Warden SJ, Thompson WR. Become one with the force: optimising mechanotherapy through an understanding of mechanobiology. Br J Sports Med. 2017;51:989-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Khan KM, Scott A. Mechanotherapy: how physical therapists' prescription of exercise promotes tissue repair. Br J Sports Med. 2009;43:247-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 82. | Docking S, Samiric T, Scase E, Purdam C, Cook J. Relationship between compressive loading and ECM changes in tendons. Muscles Ligaments Tendons J. 2013;3:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Cook JL, Purdam C. Is compressive load a factor in the development of tendinopathy? Br J Sports Med. 2012;46:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 84. | Almekinders LC, Weinhold PS, Maffulli N. Compression etiology in tendinopathy. Clin Sports Med. 2003;22:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 85. | Wall M, Butler D, El Haj A, Bodle JC, Loboa EG, Banes AJ. Key developments that impacted the field of mechanobiology and mechanotransduction. J Orthop Res. 2018;36:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Sun Z, Guo SS, Fässler R. Integrin-mediated mechanotransduction. J Cell Biol. 2016;215:445-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 778] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 87. | Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 705] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 88. | Zhang WK, Wang D, Duan Y, Loy MM, Chan HC, Huang P. Mechanosensitive gating of CFTR. Nat Cell Biol. 2010;12:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 89. | Liu Y, Xu J, Xu L, Wu T, Sun Y, Lee YW, Wang B, Chan HC, Jiang X, Zhang J, Li G. Cystic fibrosis transmembrane conductance regulator mediates tenogenic differentiation of tendon-derived stem cells and tendon repair: accelerating tendon injury healing by intervening in its downstream signaling. FASEB J. 2017;31:3800-3815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Xiao E, Yang HQ, Gan YH, Duan DH, He LH, Guo Y, Wang SQ, Zhang Y. Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells. 2015;33:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 91. | Tonelli FM, Santos AK, Gomes DA, da Silva SL, Gomes KN, Ladeira LO, Resende RR. Stem cells and calcium signaling. Adv Exp Med Biol. 2012;740:891-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 92. | Liu YS, Liu YA, Huang CJ, Yen MH, Tseng CT, Chien S, Lee OK. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci Rep. 2015;5:16522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Winslow MM, Pan M, Starbuck M, Gallo EM, Deng L, Karsenty G, Crabtree GR. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell. 2006;10:771-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 274] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 94. | Fromigué O, Haÿ E, Barbara A, Marie PJ. Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. J Biol Chem. 2010;285:25251-25258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 95. | Xiao E, Chen C, Zhang Y. The mechanosensor of mesenchymal stem cells: mechanosensitive channel or cytoskeleton? Stem Cell Res Ther. 2016;7:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Ingber DE, Wang N, Stamenovic D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep Prog Phys. 2014;77:046603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 97. | Lavagnino M, Wall ME, Little D, Banes AJ, Guilak F, Arnoczky SP. Tendon mechanobiology: Current knowledge and future research opportunities. J Orthop Res. 2015;33:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 98. | Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 99. | Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065-6075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 100. | Liu X, Chen W, Zhou Y, Tang K, Zhang J. Mechanical Tension Promotes the Osteogenic Differentiation of Rat Tendon-derived Stem Cells Through the Wnt5a/Wnt5b/JNK Signaling Pathway. Cell Physiol Biochem. 2015;36:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 101. | Shi Y, Fu Y, Tong W, Geng Y, Lui PP, Tang T, Zhang X, Dai K. Uniaxial mechanical tension promoted osteogenic differentiation of rat tendon-derived stem cells (rTDSCs) via the Wnt5a-RhoA pathway. J Cell Biochem. 2012;113:3133-3142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 102. | Brown JP, Finley VG, Kuo CK. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J Biomech. 2014;47:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 103. | Rui YF, Lui PP, Wong YM, Tan Q, Chan KM. BMP-2 stimulated non-tenogenic differentiation and promoted proteoglycan deposition of tendon-derived stem cells (TDSCs) in vitro. J Orthop Res. 2013;31:746-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 104. | Gardner K, Arnoczky SP, Lavagnino M. Effect of in vitro stress-deprivation and cyclic loading on the length of tendon cell cilia in situ. J Orthop Res. 2011;29:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 400] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 106. | Bodle JC, Loboa EG. Concise Review: Primary Cilia: Control Centers for Stem Cell Lineage Specification and Potential Targets for Cell-Based Therapies. Stem Cells. 2016;34:1445-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 107. | Banes AJ, Weinhold P, Yang X, Tsuzaki M, Bynum D, Bottlang M, Brown T. Gap junctions regulate responses of tendon cells ex vivo to mechanical loading. Clin Orthop Relat Res. 1999;S356-S370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 108. | Wall ME, Banes AJ. Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J Musculoskelet Neuronal Interact. 2005;5:70-84. [PubMed] |
| 109. | Waggett AD, Benjamin M, Ralphs JR. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol. 2006;85:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 110. | McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Maeda E, Ohashi T. Mechano-regulation of gap junction communications between tendon cells is dependent on the magnitude of tensile strain. Biochem Biophys Res Commun. 2015;465:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Shao Q, Esseltine JL, Huang T, Novielli-Kuntz N, Ching JE, Sampson J, Laird DW. Connexin43 is Dispensable for Early Stage Human Mesenchymal Stem Cell Adipogenic Differentiation But is Protective against Cell Senescence. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Theodossiou SK, Tokle J, Schiele NR. TGFβ2-induced tenogenesis impacts cadherin and connexin cell-cell junction proteins in mesenchymal stem cells. Biochem Biophys Res Commun. 2019;508:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 114. | van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 115. | Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 116. | Wang C, Zhao Y, Su Y, Li R, Lin Y, Zhou X, Ye L. C-Jun N-terminal kinase (JNK) mediates Wnt5a-induced cell motility dependent or independent of RhoA pathway in human dental papilla cells. PLoS One. 2013;8:e69440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Lui PP, Lee YW, Wong YM, Zhang X, Dai K, Rolf CG. Expression of Wnt pathway mediators in metaplasic tissue in animal model and clinical samples of tendinopathy. Rheumatology (Oxford). 2013;52:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 118. | Kishimoto Y, Ohkawara B, Sakai T, Ito M, Masuda A, Ishiguro N, Shukunami C, Docheva D, Ohno K. Wnt/β-catenin signaling suppresses expressions of Scx, Mkx, and Tnmd in tendon-derived cells. PLoS One. 2017;12:e0182051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 119. | Rui YF, Lui PP, Ni M, Chan LS, Lee YW, Chan KM. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res. 2011;29:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 120. | Zhang J, Wang JH. BMP-2 mediates PGE(2) -induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J Orthop Res. 2012;30:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 121. | Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G, Gross G, Gazit D. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 122. | Liu C, Luo JW, Liang T, Lin LX, Luo ZP, Zhuang YQ, Sun YL. Matrix stiffness regulates the differentiation of tendon-derived stem cells through FAK-ERK1/2 activation. Exp Cell Res. 2018;373:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 123. | Havis E, Bonnin MA, Olivera-Martinez I, Nazaret N, Ruggiu M, Weibel J, Durand C, Guerquin MJ, Bonod-Bidaud C, Ruggiero F, Schweitzer R, Duprez D. Transcriptomic analysis of mouse limb tendon cells during development. Development. 2014;141:3683-3696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 124. | Popov C, Burggraf M, Kreja L, Ignatius A, Schieker M, Docheva D. Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol Biol. 2015;16:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 125. | Paxton JZ, Hagerty P, Andrick JJ, Baar K. Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A. 2012;18:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 126. | Xu B, Song G, Ju Y, Li X, Song Y, Watanabe S. RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2012;227:2722-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 127. | Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4707] [Cited by in RCA: 4453] [Article Influence: 296.9] [Reference Citation Analysis (5)] |
| 128. | Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 129. | Kim KM, Choi YJ, Hwang JH, Kim AR, Cho HJ, Hwang ES, Park JY, Lee SH, Hong JH. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PLoS One. 2014;9:e92427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 130. | Zhong W, Zhang W, Wang S, Qin J. Regulation of fibrochondrogenesis of mesenchymal stem cells in an integrated microfluidic platform embedded with biomimetic nanofibrous scaffolds. PLoS One. 2013;8:e61283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 131. | Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 589] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Park YS S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Xing YX