Published online Sep 26, 2020. doi: 10.4252/wjsc.v12.i9.966
Peer-review started: May 27, 2020
First decision: June 15, 2020
Revised: June 23, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: September 26, 2020
Processing time: 117 Days and 22.4 Hours
Mesenchymal stem/stromal cells (MSCs) have various properties that make them promising candidates for stem cell-based therapies in clinical settings. These include self-renewal, multilineage differentiation, and immunoregulation. However, recent studies have confirmed that aging is a vital factor that limits their function and therapeutic properties as standardized clinical products. Understanding the features of senescence and exploration of cell rejuvenation methods are necessary to develop effective strategies that can overcome the shortage and instability of MSCs. This review will summarize the current knowledge on characteristics and functional changes of aged MSCs. Additionally, it will highlight cell rejuvenation strategies such as molecular regulation, non-coding RNA modifications, and microenvironment controls that may enhance the therapeutic potential of MSCs in clinical settings.
Core Tip: Mesenchymal stem cell (MSC) administration is a promising therapeutic strategy for various human diseases. However, cell aging limits MSC function and therapeutic properties via reducing their activities. We review the morphological changes, molecular expression alterations, and functional degeneration of aged MSCs, and the effects of aged MSCs on immune cells and other target cells. Additionally, we summarize the strategies to rejuvenate aged MSCs to enhance their clinical potential.
- Citation: Meng QS, Liu J, Wei L, Fan HM, Zhou XH, Liang XT. Senescent mesenchymal stem/stromal cells and restoring their cellular functions. World J Stem Cells 2020; 12(9): 966-985
- URL: https://www.wjgnet.com/1948-0210/full/v12/i9/966.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i9.966
Mesenchymal stem/stromal cells (MSCs) are multipotent progenitor cells that can retain postnatal capacity for both self-renewal and multilineage differentiation. The minimal criteria for MSCs as defined by the International Society for Cellular Therapy in 2006 are adherence to plastic under culture conditions; positivity for cell surface markers CD44, CD90, CD105, and CD73; negativity for hematopoietic markers CD45, CD34, CD14, CD11b, CD79α, CD19, and human leukocyte antigen-DR; and multi-differentiation potential of osteogenesis, chondrogenesis, and adipogenesis[1]. They are a heterogeneous population of cells isolated from a variety of mesodermal tissues. These cells are involved in a wide range of physiological and pathological processes, such as bone development, adipogenesis, fibrosis, and inflammatory regulation[2]. Over the past few decades, the amount of MSC-focused research has grown exponentially. These studies include both preclinical and clinical trials of either autologous or allogeneic MSCs. Infusion of MSCs has been performed to evaluate their safety and therapeutic efficacy in diseases of the immune[3], hematological[4], cardiovascular[5,6], nervous[7,8], respiratory[9], digestive[10], skeletal[11], endocrine[12], and reproductive[13] systems[14]. To date, more than 1000 MSC-based clinical trials have been registered in the United States National Institute of Health database[15,16]. It is well recognized that MSC administration is a safe and effective strategy in the treatment of a variety of diseases.
Emerging evidence has demonstrated that multiple factors, including cell species, tissue source, isolation method, culture conditions, and cellular status, may explain the inconsistency in the features and characteristics of MSCs in some preclinical and clinical trials. A recent study showed that aging is an important factor affecting MSC properties and functions[17]. Age-dependent decline in MSC number and function was found in old individuals[18]. Additionally, MSCs from young donors may also become senescent because of excessive cell passage, oxidative stress, and other injuries[19,20]. The senescent cells manifest a sequence of progressive changes in cellular morphology, biological function, and molecular expression[21,22], as well as weakened efficacy in cell-based therapies[23]. Therefore, appropriate quality controls or cellular rejuvenation processes are required to obtain clinical-grade MSCs. In this review, we will focus on investigations that have assessed the molecular features and functional changes of aged MSCs and highlight rejuvenation strategies that will enable more effective clinical translation.
Aging MSCs exhibit morphological changes and undergo a progressive decline in homeostasis, which contributes to the age-dependent deterioration of MSC function[24]. These changes in senescent MSCs include a general decrease in their regenerative capacity, a switch in differentiation potency, and weakened regulatory functions (such as immunosuppressive effects)[25]. A full understanding of these characteristics is fundamental for the development of strategies to delay or even prevent MSC senescence. In view of this, the phenotypes and functional characteristics of senescent MSCs will be summarized in this section.
The most noticeable changes in aged MSCs are morphological. In vitro imaging analysis demonstrated that MSCs from early passages (P1-P3) were remarkably uniform in size[24]. At P5, they exhibited a flattened and enlarged morphology compared with those at P1. Additionally, gradual telomere shortening is a typical characteristic of aging in MSCs[26]. Moreover, these changes in morphology represented the heterogeneous response to the cellular microenvironment in vitro and in vivo.
In aged MSCs, the balance of homeostasis is disrupted, the proliferative ability declines, and mitochondrial dysfunction increases. In addition, both the DNA repair and retrogression of anti-oxidative capacity are reduced[18,27]. Senescent MSCs display delayed self-cloning as well as restricted differentiation potency[28]. Additionally, they exhibit a shift in differentiation potency from osteoblasts to adipocytes[29-33]. Genetic stability, another biological index affecting MSCs activity, is involved in biosafety issues and therapeutic efficacy of these cells[34]. Mounting evidence suggested that long-term cultured MSCs acquired genetic alterations, with the promotion of cell senescence and potential increased risks of transformation. However, the relevance of increased genomic instability with culture passages is still being debated[35-38]. Roselli et al[39] reported that MSCs were genetically stable in long-term cultures at least up to passage 10, and abnormal MSC clones showed neither growth advantage nor senescence resistance. Some authors suggested that senescent cells are unlikely to undergo malignant transformation, even if the presence of few tumorigenic cells can not be excluded[40]. The inconsistencies may be caused by tissue sources, culture conditions, culture times, and cuture passages[41]. Nevertheless, it is of critical importance to evaluate MSC genetic stability before clinical application.
Several methods may be used to identify MSC senescence. The most widely used measures include increased senescence-associated (SA) beta-galactosidase activity (SA-beta-gal), cell cycle arrest, and persistent DNA damage response signaling[22]. Furthermore, specific markers for senescent MSCs have been discovered. Analysis of the MSC compartment revealed that MSC subpopulations differ between developmental and aged stages. CD271(-)CD146(+) cells only appeared in fetal bone marrow (BM)-containing colony-forming-unit-fibroblasts. The dominant MSC subset in pediatric and fetal samples was the CD271(bright)CD146(+) population, whereas the main cell type in adult samples was CD271(bright)CD146(-)[42]. The proportion of CD11b+, CD3+, Gr-1+, or F4/80+ cells is upregulated in BM from aged mice, while the percentage of B220+ cells was significantly decreased compared with those from young mice BM[43].
Recently, novel specific biomarkers were found to demonstrate the senescent state of MSCs[21]. MSC-derived microvesicles (MVs) are one such biomarker[44], and senescent late passage MSCs displayed a smaller MSC-MV size compared with early passage MSCs. Additionally, when comparing late and early passage MSC-MVs, there was a lower ratio of CD105+ cells and decreased osteogenesis in late passage MSC-MVs[45]. When the percentage of CD264+ cells was greater than 35%, CD264 expression was inversely correlated with the regenerative potential of MSCs. Above the 75% threshold, MSCs were enlarged and showed a decreased proliferation and differentiation potency.
CyBC9, a senescence-specific fluorescent probe, is a promising tool used to rapidly identify both early and late senescent phenotypes in clinically relevant MSCs[46], and it can be applied to live cells as a nontoxic probe. The mitochondrial Cox1 gene containing the differentially methylated CpG island 4 was upregulated in MSCs from human fetal heart tissues. This demonstrated that CpG hypo-methylation in mitochondria might serve as a biomarker for senescence of human fetal heart MSCs induced by chronic oxidative stress[20].
Recent studies have demonstrated significant changes in the expression profiles (including transcriptomic, proteomic, epigenetic, and non-coding RNAs) of senescent MSCs. Transcriptome drift even preceded replicative exhaustion and other aging metrics[47]. Utilizing a microarray assay, transcriptome analyses were performed using various types of aged MSCs (Table 1).
| Ref. | Species | Tissue sources | Classification | Cells | Groups | Database | Differentially expressed genes (DEGs) | Identification of targets |
| Benisch et al[48], 2012 | Human | Bone marrow of femoral heads | Affymetrix Gene Chip | Cultured in DMEM/Ham’s F-12 (1:1) medium supplemented with 10% fetal calf serum (FCS), 1 U/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL L-ascorbic acid 2-phosphate. Used after 1 to 2 passages | hMSC-C: Middle-aged donors (42-67 yr old); hMSC-old: The age-matched control group (79-89 yr old); hMSC-OP: Patients (79-94 yr old) who had primary osteoporosis; hMSC-senescent: Healthy donors of middle-age (42-64 yr old) until they entered senescence | GEO accession numbers: GSE35955; GSE35956; GSE35957; GSE35958; GSE35959 | One gene was upregulated and seven downregulated in all three groups, compared with the hMSC-C group; 38 genes with enhanced and 36 genes with reduced expression in hMSC-OP and hMSC-old groups, compared with hMSC-C; 2477 genes with higher and 1222 genes with lower expression in hMSC-OP, in comparison with hMSC-old | The reliable or promising candidates for osteoporosis, including susceptibility genes: Lrp5, Spp1 (Osteopontin), Col1a1, Sost, and Mab21l1 |
| Yoo et al[50], 2013 | Human | Bone marrow-derived MSCs | SSH analysis | Purchased from Cambrex Bio Science | Young human MSCs (Y-hMSCs): Approximately 10 population doubling levels (PDL); senescent MSCs (S-hMSCs): Until approximately 30 PDL, at least 80% of the cells were positive for SA-β-Gal staining | NA | Nineteen genes were down-regulated and 43 upregulated in S-hMSCs | Gradually downregulated mRNA in S-hMSCs: Pdia3, Wdr1, Fstl1, Copg1, Lman1, and Pdia6; significantly upregulated genes: Hsp90b1, Eid1, Atp2b4, Ddah1, Prnp, Rab1a, Psg5, Tm4sf1, and Ssr3 |
| Bustos et al[52], 2014 | Mouse | Bone marrow | Affymetrix Gene Chip | Sorted by fluorescence-activated cell sorting (FACS) | BM-MSCs from young (3-mo-old) and aged (24-mo-old) mice; young donor BM-MSCs vs aged ones | GEO accession number: GSE44403 | 927 genes were differentially expressed | Confirmed by qPCR: Cytokine receptors (15 genes), chemokines (Ccr7, Cxc3cr1, Cxcr5), markers of cell senescence (CDK, p21, p27, and p53), Marcks, Mmp9, and Timp2 |
| Duscher et al [54], 2014 | Mouse | Inguinal fat pads | Microfluidic-based single-cell gene expression platform | CD45-/CD31-/CD34+ cells were sorted | Adipose-derived mesenchymal stem cells (ADSCs) from young (3 mo) and aged (21 mo) mice | NA | Differences in transcriptional profiles of genes related to cell stemness, remodeling, and vasculogenesis: Adam10, Angpt1, Angpt2, Hif1a, Mef2c, and Sod2 | Age-related depletion of a subpopulation of MSCs characterized by a pro-vascular transcriptional profile, such as Angpt1, Vegfa, and Sod3 |
| Medeiros et al[19], 2017 | Human | Umbilical cord veins | The GeneChip Human Genome U133 Plus 2.0 array | The surface markers including CD105, CD73, CD90, CD14, CD34, and CD45 were analyzed by flow cytometry; differentiation capacity toward three lineages was assessed | hMSCs in the 9th (Y-hMSCs) and 18th passages (S-hMSCs) were used for assays, hMSCs/n from the donor with normal karyotype, and hMSCs/inv from the donor with a constitutional inversion of chromosome 3; the comparisons were as follows: (1) Y-hMSCs/n & S-hMSC/n; (2) Y-hMSCs/inv & S-hMSCs/inv; (3) Y-hMSCs/n & Y-hMSCs/inv; and (4) S-hMSCs/n & S-hMSCs/inv | GEO accession number: GSE56530 | 73 DEGs in S-hMSCs/n compared with Y-hMSCs/n and 279 DEGs in S-hMSCs/inv compared with Y-hMSCs/inv; 93 DEGs in Y-hMSCs/inv compared with Y-hMSCs/n; 425 DEGs in S-hMSCs/inv compared with S-hMSCs/n. The candidates for senescent markers: Dio2, Foxe1, Galnt5, Has3, Krt19, Krt34, Krtap1-55, Oc730755, Mrvi1, Plcb4, and Scube3 | Confirmed by qPCR: Ankrd1 and Mmp1 in S-hMSC/n vs Y-hMSC/n; Sfrp1, Ankrd1, G0s2, and Ndn in S-hMSC/inv vs Y-hMSC/inv; Adora2b, Sfrp1, Kynu, G0s2, Aldh1a1, and Mab21l1 in Y-hMSC/inv vs Y-hMSC/inv; and Adora2b, Ccl7, Sfrp1, Kynu, Ankrd1, Mmp1, Lamc2, G0s2, M ab21l1, and Ndn in S-hMSC/inv vs S-hMSC/n |
| Wu et al[49], 2019 | Human | Bone marrow of femoral heads | Affymetrix Gene Chip | Cultured in DMEM/Ham’s F-12 (1:1) medium supplemented with 10% FCS, 1 U/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL L-ascorbic acid 2-phosphate. used after 1 to 2 passages | Middle-aged group vs elderly group | GEO accession number: GSE35955 | 156 up-regulated and 343 down-regulated differentially expressed genes (DEGs) | Six hub genes identified by PPI network analysis: Ctnnb1, Ppp2r1a, Fyn, Mapk1, Pik3c2a and Ep300. 11 TFs identified by TFs screening: Creb1, Cux1, Egr1, Ep300, Foxc1, Hsf2, Mef2a, Plau, Sp1, Stat1 and Usf1 |
| Wiese et al[47], 2019 | Human | The perivascular region of Wharton’s jelly from umbilical cords | Affymetrix GeneChip U133A 2.0 arrays | Provided by Tissue Regeneration Therapeutics, Inc. Positive for the cell surface markers CD73, CD90, CD105, CD10, CD140b, CD146 (40%-60%), CD166, and MHC-I; negative for the cell surface markers CD45, CD31, CD34, and HLA-DR. Exhibit trilineage potential in directed differentiation assays | Human umbilical cord perivascular cells (HUCPVCs) from early passages (P2–P5), mid-passages (P6–P9), and pre-senescent passages (P10–P12) | GEO accession number: GSE119987 | The transcriptome of HUCPVCs was stable through P5. A single significantly DE gene was identified at P6 and P7 compared with P2, whereas 5 DE genes were detected at P8 and 27 at P9. The number of significantly DE probe sets increased from 27 (P9) to 301 (P10), then to 1094 (P12) | Significant transcriptome drift occurred only after P5 |
| Leveque et al[51], 2019 | Human | Bone marrow aspirates from the iliac crest of healthy donors (21 to 26 years old) | RNAseq Analysis | The surface markers including CD34, CD45, CD73, CD90, and CD105 were analyzed by flow cytometry; differentiation capacity toward three lineages was assessed | Four groups: Control MSCs (P3); 21 d pesticide mixture exposed MSCs (P4); long-term cultivated MSCs (P14); and MSCs from aging donor (72 yr old) | The SRA database under accession number PRJNA510912 | 394, 1073, and 2077 EST were significantly increased from pesticide exposed, P14 MSCs, and MSC from aged donor; 218, 1077, and1571 ESTs were down-regulated | Confirmed by QPCR: Igf-1, Prolactin, Leptin, and Cox-2 |
Transcriptomics: Assays for gene expression profiles have been performed on multiple types of aging MSCs. These MSCs were obtained from various species and tissues, and subjected to different treatments. Benisch et al[48] investigated the transcriptional profiles of human BM-MSCs from five elderly patients (79-94 years old) who had osteoporosis (hMSC-OP), the age-matched control group (hMSC-old; donor age 79-89 years), middle-aged donors (hMSC-C; donor age 42-67 years), and healthy middle-aged donors (42-64 years old) until they entered senescence (hMSC-senescent). By using hMSC-C as control cells, they found a small overlap of gene expression in the hMSC-old, hMSC-senescent, and hMSC-OP groups. By comparing the gene expression profiles of hMSC-OP, hMSC-old, and hMSC-senescent with hMSC-C, special transcriptomic features in each group were obtained. The differentially expressed genes in the three groups are mainly involved in proliferation, differentiation, osteoclastogenesis, and DNA repair[48]. Wu et al[49] identified six hub genes and eleven transcription factors related to adherens junctions, DNA damage induced by oxidative stress, attribution of telomeres, differentiation, and epigenetic modulation by comparing the gene alterations between hMSC-C and hMSC-old. Yoo et al[50] revealed that 19 genes were downregulated and 43 upregulated in senescent human BM-MSCs relative to young MSCs. And these genes were mainly involved in metabolic functions and cell adhesion. Additionally, 394, 1073, and 2077 genes were significantly up-regulated in BM-MSCs from pesticide exposed, P14 MSCs, and MSCs from aged donor, compared with control MSCs (P3), respectively[51]. And 218, 1077, and 1571 genes were down-regulated in BM-MSCs from pesticide exposed, P14 MSCs, and MSCs from aged donor, compared with P3 MSCs, respectively. Insulin-like growth factor-1 (Igf-1), prolactin, leptin, and Cox-2 were identified as key genes of the predicted protein–protein interactions[51]. In murine BM-MSCs that were freshly sorted by fluorescence-activated cell sorting, 927 differentially expressed genes were obtained in aged BM-MSCs. These genes contained cytokine receptors, chemokines, markers of cell senescence, and other groups, which were seen in the gene expression omnibus[52].
Human umbilical cord (hUC)-derived MSCs, cultured in chemically defined xeno- and serum-free medium, displayed comparable growth trajectories up to passage (P) 9 and variably approached senescence after P10. However, significant changes in the transcription profiles occurred earlier. Microarray analysis of 14500 human genes in aged hUC-MSCs revealed that a nonlinear evolution of aging MSCs appeared after P5 and accumulated rapidly after P9[47]. As for hUC vein-MSCs, young (P9) and senescent (P18) cells were used for transcriptome analysis assays. This study identified 73 differentially expressed genes in senescent cells, compared with young MSCs[19]. Among these, 18 upregulated genes were screened out as characteristic molecular signatures of senescence when comparing senescent and young hMSCs derived from donors with normal or constitutional chromosome inversion karyotype. Among them, 11 novel candidate markers for senescence were identified.
In response to IL-2 priming, human adipose-derived MSCs (ADSCs) showed increased expression of genes encoding potent growth factors, cytokines, angiogenic, and anti-apoptotic promoting factors, and they were defined as novel transcriptional signatures closely associated with senescence[53]. In CD45-CD31-CD34+ ADSCs from murine inguinal fat pads, aging has been shown to affect cellular signaling and function as well[54]. Single-cell transcriptional profiles of ADSCs isolated from both young and aged mice were analyzed by utilizing a microfluidic-based single-cell gene expression platform. About 70 gene targets related to stemness, vasculogenesis, and tissue remodeling were evaluated and used to define ADSC clusters in each group. Ingenuity Pathway Analysis of a subset of this heterogeneous cell collection was performed. This analysis suggested that deficiency of a putatively vasculogenic subpopulation of ADSCs was a potential risk for age-related impairments in ADSCs function (particularly with regard to wound healing)[54].
Proteomics: Proteomics is an efficient and accessible tool used to determine protein expression profiles. An SA secretome, also known as SA secretory phenotype (SASP), usually contains the expression of growth factors, cytokines, and extracellular proteases that modulate the microenvironmental phenotypes caused by senescent cells[55]. The SASP is useful for the development of biological markers and rejuvenation strategies in aged MSCs[22]. Secretome analyses for secretory protein profiles in senescent MSCs are summarized in Table 2. For example, elderly MSCs exhibited increased levels of pro‐inflammatory factors, including interleukin-6 (IL-6), IL-8 (IL-8/CXCL8), and monocyte chemoattractant protein-1 (MCP-1/CCL2). Neutralization of these factors improved their immunomodulatory function[56]. In the conditioned medium (CM) from senescent MSCs induced by the HIV protease inhibitor tipranavir, the soluble proteins were evaluated to find dysregulated secreted factors using antibody arrays and liquid chromatography-mass spectrometry (LC-MS)[57]. Semi-quantitative antibody arrays and LC-MS analysis identified altered secretion of 86 proteins related to the extracellular matrix, cell adhesion, angiogenesis, and wound healing. Among the identified secreted factors in the proteomic analysis, a series of TGF-β targets were significantly upregulated. Further investigation revealed that insulin-like growth factor-binding protein 7 (IGFBP7), one of the targets of TGF-β, is independent of any additional factors that induce osteogenesis in hMSCs. IGFBP7 is also essential for the viability of hMSCs during osteogenesis[57].
| Ref. | Species | Tissue sources | Classification | Cells | Groups | Differentially expressed proteins | Identification of targets |
| Kizilay et al[56], 2017 | Human | Subcutaneous and pericardial adipose tissue | R&D Systems Human Cytokine Array; multispot electrochemiluminescence immunoassay V-Plex Pro inflammatory Panel | CD44, CD73, CD105, and CD90 expression was more than 95%; CD45, CD34, CD19, CD14, and HLA-DR expression was less than 5%; differentiation capacity toward three lineages was assessed | E-MSCs: MSCs from elderly ATH patients (> 65 yr old); A-MSCs: MSCs from adult ATH patients (< 65 yr old) | The expression of IL-6, IL-8/CXCL8, MCP-1/CCL2, MIF, IFN-g, IL12p70, IL-13, IL-2, and IL-4 was elevated in E-MSCs relative to A-MSCs | Neutralization of IL-6, IL-8/CXCL8, and MCP-1/CCL2 significantly improved the E-MSCs’ immunomodulatory function |
| Infante et al[57], 2018 | Human | Bone marrow | Semi-quantitative antibody arrays; liquid chromatography-mass spectrometry (LC-MS): Version 4.0.4265.42984, Nonlinear Dynamics | Obtained from Lonza commercially; passages 3-4 | Ctrl-hMSCs: Incubated with dimethyl sulfoxide alone; PreA-hMSCs: Treated with the HIV protease inhibitor (tipranavir) every other day until passage 11 | A dysregulation in the secretion levels of 42 proteins was detected by antibody arrays; 44 were detected by LS-MS in preA-hMSCs; most of them were overexpressed in preA-hMSCs, in comparison with ctrl-hMSCs | IGFBP7 is essential for hMSCs viability during early osteogenic diferentiation |
| Madonna et al[58], 2019 | Mouse | Peri-epididymal visceral adipose tissue from 1-yr-old male C57BL/6 mice | Two-dimensional gel electrophoresis (2DE); matrix-assisted laser desorption/ionization time-of-fight mass spectrometry | The expression of CD45, CD34, CD133, ASMA, Desmin, CD105, CD73, CD90, CD79, and CD160 was analyzed by flow cytometry | Mock AT-MSCs: Mock-transduced AT-MSCs; rTMAT-MSCs: Rejuvenated by TERT and the anti-apoptotic transcription factor myocardin overexpression | 113 protein spots were picked up and identified from the whole CM and exosome-enriched fraction in rTMAT-MSCs | Two novel candidates supporting angiogenesis in the whole CM of rTMAT-MSCs: MMP2 and its inhibitor TIMP2 |
In the ADSCs from one-year-old male C57BL/6 mice, the anti-senescent protein, telomerase reverse transcriptase (TERT), and the anti-apoptotic transcription factor myocardin were overexpressed to restore their functions. The secretomes in CM and exosome-enriched fractions from the transgenic cells were analyzed using a proteomic approach. This approach involved combining two-dimensional gel electrophoresis with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry[58]. The comparative targeted proteomic analysis revealed that both matrix metalloproteinase-2 (MMP-2) and its inhibitor metalloproteinase inhibitor 2 (TIMP2) levels are increased by two-fold in the CM compared with those in mock-transduced cells.
Epigenetics: Epigenetic profiles of aged human BM-MSCs have been reported, and briefly reviewed by Cakouros and Gronthos[59]. Using the human methylation bead ChIP array, researchers identified a series of hypomethylated, hypermethylated, and hydroxymethylated CpG sites in MSCs from aged subjects[60-62]. However, differentially methylated CpG sites are a robust age-related DNA methylation signature, illustrating similar DNA changes independently of disease state, sex, tissue, and cell type[59,63]. Additionally, aged human BM-MSCs from long-term culture exhibited consistent epigenetic changes in vitro when the methylation profile of human BM-MSCs at early and late passages was assessed[64]. Following the application of enhanced reduced representation bisulfite sequencing, the global DNA methylation profiling demonstrated a greater breadth than previously reported. The genome-wide analyses using whole-genome bisulfite sequencing provided a better understanding of how the epigenetic modifications alter gene expression and regulate the biological characteristics[65].
Non-coding RNAs: It has been previously reported that some non-coding RNAs are associated with cellular senescence in different cell types[66-70]. MicroRNAs (miRNAs) are small non-coding RNAs that have highly conserved sequences and regulate target genes in cellular functions of metabolism, proliferation, apoptosis, and senescence[71,72]. In senescent MSCs, 43 miRNAs were identified and characterized using a miScript miRNA assay[73]. Among them, the expression of 24 miRNAs was closed related to cellular senescence as referred to previous studies. As for the rest, fourteen miRNAs (miR-10, miR-27b, miR-30b, miR-30d, miR-103a, miR-103a-2, miR-136, miR-140-5p, miR-323-3p, miR-330-5p, miR-361-5p, miR-409-3p, miR-424, and miR-455-3p) were upregulated, and five miRNAs (miR-16-2, miR-29b, miR-199b-5p, miR-454, and miR-618) were downregulated in response to cellular aging. Additionally, miR-29b and miR-199b-5p modulated cellular senescence via LAMC networks[73].
In MSCs cultured under hypoxic conditions, miRNA expression profiles of MSCs from young (≤ 30 years old) and aged (≥ 60 years old) donors were analyzed using an Agilent Technologies Bioanalyzer high sensitivity DNA chip[74]. Principal component analysis demonstrated differentially expressed miRNAs in normal and hypoxic groups. There was > 2-fold upregulation of nine miRNAs in young MSCs and two miRNAs in aged MSCs after culturing under hypoxic conditions. Also, the hypoxia induced downregulation of four miRNAs in young MSCs and thirty-one miRNAs in aged MSCs, respectively. MiR-543 and miR-590-3p were identified as regulators of cellular aging in hMSCs through direct binding to the aminoacyl tRNA synthetase-interacting multifunctional protein-3/p18 transcripts and decreasing the protein expression levels[75].
Long non-coding RNAs (lncRNAs) are non-coding transcripts, longer than 200 nucleotides, that play critical roles in the regulation of MSCs senescence. They are not only involved in age-related lineage fate switching but also in the reprogramming of old cells[76,77]. LncRNA microarray analysis of young and aged Sca-1+CD29+CD45-CD11b- murine BM-MSCs has demonstrated that 92 lncRNAs showed altered expression[76]. Among them, 83 lncRNAs were downregulated, and 9 were upregulated in cells isolated from aged mice. Further investigation demonstrated that the candidate BM stem cell-related lncRNA (BMNCR) was highly expressed in the BM-MSCs of young mice, and significantly decreased during aging. Moreover, the BMNCR levels in human BM-MSCs were negatively correlated with age. The effects of BMNCR were evidenced by Bmncr-KO and Bmncr-Tg mice simultaneously[76].
The senescent MSCs exhibit significant impairments in paracrine functions[78], resistance to oxidative stress, hypoxia, or serum deprivation-induced apoptosis[79-81]. The age-dependent decrease in cytokines, chemokines, and growth factors released by MSCs will impact cellular functions such as apoptosis, migration, osteogenesis, angiogenesis, cell adhesion, and immunomodulation[52,54,82]. Finally, the aged MSCs delay wound healing and exacerbate tissue injuries [54]. In summary, the functional regression of senescent MSCs limits their application in tissue engineering and regenerative medicine.
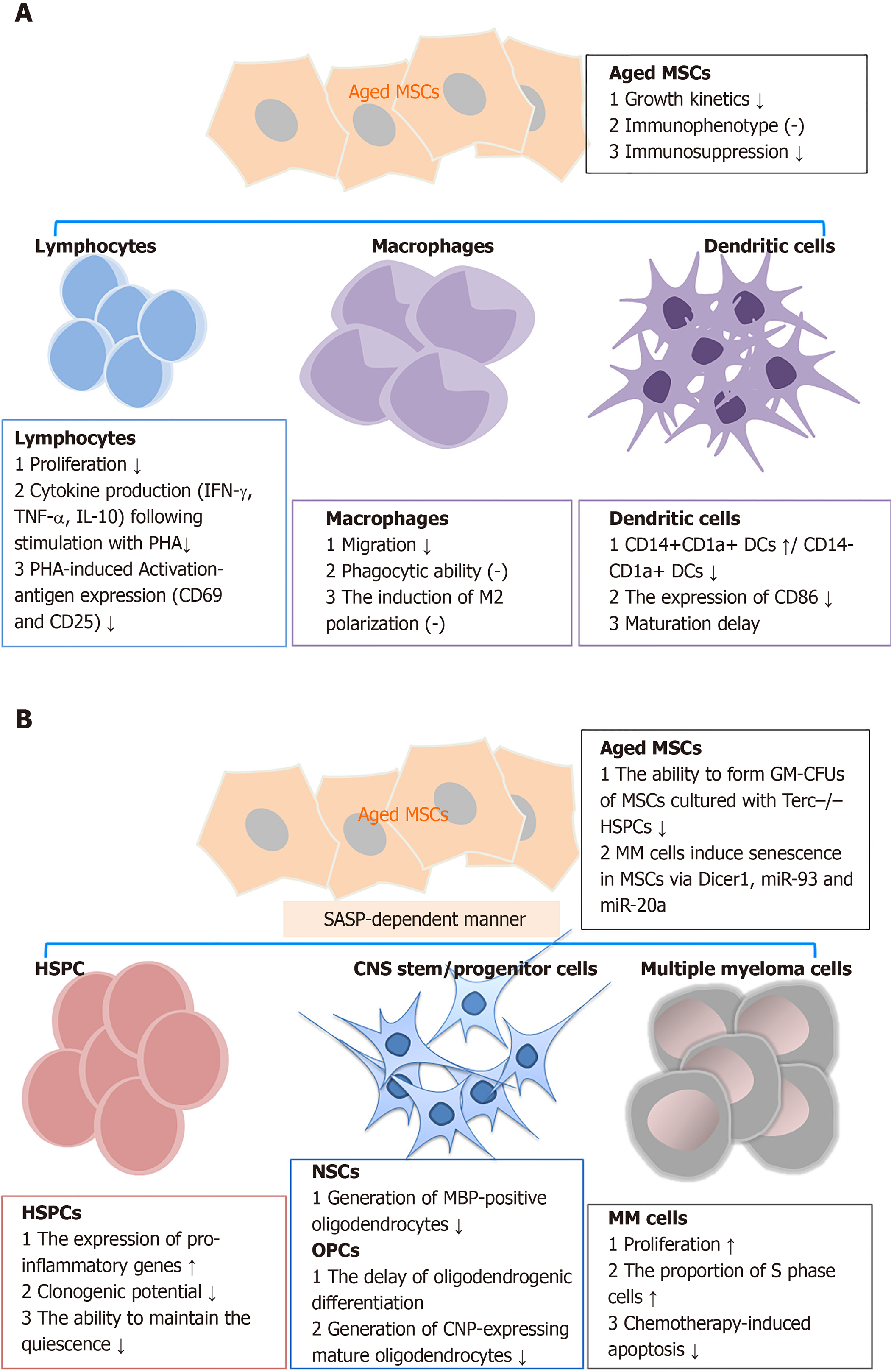
Bidirectional signaling exists between MSCs and their target cells[83,84]. The interaction between MSCs and target cells has been shown to occasionally follow a time-dependent model of regulation and feedback[85]. MSC senescence decreases the functions of a large variety of immune cells, hematopoietic stem and progenitor cells (HSPCs), oligodendrocytes, senescent chondrocytes, and other target cells through either direct or indirect cross-talk (Figure 1)[78,84,86].
Immune cells: Previous studies have demonstrated that MSC senescence retards immunosuppression in various types of immune cells. Replicative senescence of MSCs derived from BM or adipose tissue showed decreased ability to suppress T-cell, but not natural killer and B-cell proliferation[87,88]. Long-term expansion of MSCs reduced the capacity to inhibit CD4+ and CD8+ T cell proliferation. This phenomenon was observed by co-culturing MSCs with alphaCD3CD28-activated peripheral blood mononuclear cells. The inhibitory effect on T-cell proliferation significantly decreased along with increased passage number of hBM-MSCs, and the effect in hUC-MSCs was even more substantial[88]. ADSCs derived from elderly subjects also displayed a diminished capacity to suppress the proliferation of activated T cells. Similar results were observed in MSCs isolated in parallel from Lewis and Brown Norway rats of young (less than 4 wk old) and aged (older than 15 mo) groups[89]. Aside from proliferation, the senescent MSCs impair the suppressive effects of the activation-antigen expression and cytokine production in phytohemagglutinin-stimulated T cells[79]. Soluble factors and direct cell-cell contact partially mediate the decreased suppressive effect of aged MSCs on T cells[56].
In addition to lymphocytes, MSC senescence affects the phenotypes and functions of macrophages and dendritic cells[29,90]. When co-cultured with BM-MSCs from young mice, a macrophage cell line (RAW264.7 cells) exhibited higher migration rates, although they displayed similar phagocytic ability and induction of macrophage M2 polarization[29]. In dendritic cells, cellular maturation was inhibited when cultured with expanded marrow stromal cells relative to the parental MSCs[91].
Other target cells: Apart from immune cells, senescent MSCs show impaired activity against multiple target cells. Senescent MSCs enhanced the expression of pro-inflammatory SASP factors in HSPCs and inhibited their clonogenic potentials[92]. These cells also have a reduced capacity to maintain CD34+CD38- HSPCs quiescence, as a result of increased IL-6 secretion[93]. In a study on telomere dysfunction in MSCs, using Terc-/- mice, Ju et al[94] found that aged Terc-/- BM-MSCs depressed the functions of HSPCs and early hematopoietic progenitors. Aging MSCs had a reduced ability to induce oligodendrogenic differentiation in neural stem cells. Additionally, the production of 2’,3’-cyclic-nucleotide 3’-phosphodiesterase-positive oligodendrocytes in oligodendrocyte progenitor cells was reduced. The impaired differentiation suppressed the generation of myelin-like sheaths during central nervous system remyelination[95]. Also, in the peri-infarct cortex of rats subjected to transient middle cerebral artery occlusion, aged MSC administration resulted in more microglia and reduced pericyte infiltration compared with that for young MSCs. The changes in cellular components probably correlated with reduced expression of brain-derived neurotrophic factor and MCP-1[96]. In a myocardial infarction (MI) model, the infusion of old MSCs resulted in a switch of the cellular profile in the infarct region. This was characterized by fewer endothelial cells, vascular smooth muscle cells, and macrophages, and more fibroblasts compared with young MSCs[97]. Similarly, senescent MSCs facilitated the growth of multiple myeloma (MM) cells, which aggravated disease progression[98]. In turn, MM cells (such as NCI-H929, OPM-2, KMS-12-BM, and primary CD138+ tumors cells) induced senescence in MSCs derived from healthy controls with decreased expression of Dicer1, miR-93, and miR-20a.
Simple isolation, standardized culture methods, and potential autologous application make MSCs a superior cell source for the treatment of various diseases and injuries[13]. Therefore, optimizing the viability and function of MSCs for infusion is of great significance. With an increased understanding of the characteristics of MSC senescence, further investigations are ongoing to resolve challenges linked to the clinical application of cellular therapies. To date, the rejuvenation strategies have demonstrated therapeutic potential including molecular regulation, non-coding RNA modification, and control of the microenvironment.
Multiple molecules have been confirmed to restore the proliferative ability and biological function of MSCs via gene modification, or the administration of recombinant proteins, agonists, or inhibitors.
Sirtuins: The sirtuins (SIRTs), which include seven sirtuin homologs, are wellknown for their ability to delay cellular senescence and extend the lifespan of organisms ranging from yeast to humans[99]. SIRT1 overexpression in aged MSCs ameliorated the senescence phenotype, recapitulated angiogenesis, and protected cells from oxidative stress. Infusion of SIRT1-modified aged MSCs promoted the expression of pro-angiogenic factors, such as angiopoietin 1, and basic fibroblast growth factor (bFGF). Additionally, SIRT1-modified aged MSCs increased Bcl-2/Bax ratio at the protein level, promoted cellular survival, inhibited fibrosis, upregulated vascular density, and improved heart function in an MI model, compared with vector-aged MSCs[100]. SIRT1 pathway activators, including nicotinamide mononucleotide[101], nicotinamide phosphoribosyl transferase[102], cell-deposited decellularized extracellular matrix[103], and SRT1720[104] have been applied in aged human MSCs. They improve cell viability and osteogenesis while inhibiting apoptosis and adipogenesis in aged MSCs. Pretreatment of aged MSCs with SRT1720 enhanced therapeutic efficacy by promoting angiogenesis and repressing fibrosis following rat MI[104].
Likewise, the overexpression of SIRT3 improves the antioxidant capacity and promotes the survival of old human MSCs through activating forkhead box O3a in the nucleus, manganese-superoxide dismutase, and catalase. In an MI model, the application of old human MSCs overexpressing SIRT3 enhanced cardiac function and decreased infarct size[105]. SIRT6 maintains hMSC homeostasis by co-activating the antioxidant nuclear factor erythroid 2-related factor 2 pathway, RNA polymerase II, and heme oxygenase 1[106].
Growth factors: Growth factors are a superfamily of proteins that promote cell survival, expansion, migration, and differentiation, as well as prevent disruption of homeostasis in vitro and in vivo[107]. Through stimulation of the FGFR1/2 pathway, LY294002 (a PI3K inhibitor) rescued MSCs from senescence[108]. Acadesine activates adenosine 5‘-monophosphate-activated protein kinase (AMPK), a downstream signal of FGF21, and abrogates the depletion of FGF21-induced senescence by inhibiting mitochondrial fusion[109]. Silencing mitofusin-2 has been found to inhibit MSC senescence induced by the abrogation of FGF21 as well. Knockdown of insulin-like growth factor binding protein 4 restored the osteogenic potency of aged MSCs via the activation of Erk and Smad signals[110]. Pretreatment of aged MSCs with macrophage migration inhibitory factor (MIF) enhanced the secretion of vascular endothelial growth factor (VEGF), bFGF, hepatocyte growth factor, and insulin-like growth factor, which promoted their growth, paracrine function, and survival[80]. MIF-rejuvenated MSCs release growth factors through interactions with CD74 and subsequent activation of AMPK-FOXO3a signaling, which protects cells from doxorubicin-induced senescence by modulating the PI3K-Akt signaling pathway[111].
Additional potential regulators: The AKT pathway plays an important role in the rejuvenation of features and functions of aged MSCs. This pathway can act via ERBB4/PI3K/AKT[112], lactoferrin/AKT[113], Vc/AKT/mTOR[114], and fucoidan/FAK-Akt-TWIST axes[115]. Administration of rapamycin, an inhibitor of the mTOR signaling pathway, raised the expression level of NANOG, postponed replicative arrest, and enhanced the lifespan increment of BM-MSCs[116]. NANOG, an embryonic transcription factor, is a pluripotency marker that facilitates myogenic differentiation and restores the contractile function of senescent MSCs[117]. Additionally, a high number of potential regulators involved in senescent MSC rejuvenation have been screened and investigated in vitro. For example, both L-carnitine[118] and curcumin[119] affect the methylation status of the TERT promoter, increase the telomerase activity, and consequently alleviate the aging-related features of ADSCs. In human BM-MSCs, the addition of L-carnitine during expansion also elevates cell production[120].
Many in vivo experiments using various disease models have been conducted to confirm the efficacy of the rescue strategies to rejuvenate aged MSCs. It is reported that melatonin can protect MSCs from senescence via prion protein (PrPc)-dependent enhancement of mitochondrial function[121]. Implantation of genetically-modified old human MSCs with tissue inhibitor of matrix metalloproteinase-3 or VEGF promotes angiogenesis, prevents adverse remodeling, and preserves cardiac function to a similar extent compared with young hMSCs[122]. Stem cell antigen 1 (Sca-1)+ MSCs resident in the heart increase angiogenesis, and activate cell proliferation in the infarcted heart, which improves cardiac function after MI[123,124]. Overexpression of neuron-derived neurotrophic factor rejuvenates human ADSCs and BM-MSCs from the elderly, reduces the ischemic area, and repairs cardiac function after MI by improving angiogenesis and decreasing apoptosis[125,126]. Ethyl pyruvate, a HMGB1 inhibitor, restores the senescent phenotype of BM-MSCs, alleviates clinical signs of lupus nephritis, and prolongs survival of MRL/Mp-lpr/lpr mice via TLR4-NF-kappaB signaling[127]. These candidates (both in vitro and in vivo) may be valuable for the identification of suitable targets with utility in the production of clinical-grade MSCs.
Non-coding RNAs are novel genetic regulators involved in regenerative medicine. With respect to aging, transfection of the miR-195 inhibitor restores the expression of anti-aging factors, including TERT and SIRT1, as well as phosphorylation of AKT and FOXO1. The miR-195 inhibitor reduced the expression of SA-beta-gal, which significantly induced telomere relengthening and restored the proliferative abilities of old MSCs. Administration of aged MSCs with miR-195 knockout alleviated infarction size and improved left ventricular function[128]. Additionally, miR-10a, miR-29c-3p, and miR-130b have been reported to rejuvenate MSC senescence by targeting different downstream pathways[128-131]. The overexpression of miR-10a in aged hBM-MSCs stimulates angiogenesis by inducing the expression of angiogenic factors via activated Akt. These cells then enhance implanted stem cell survival and improve cardiac function after MI[129]. The miR-29b-3p derived from BM-MSCs regulates aging-associated insulin resistance[132]. In multiple myeloma-MSCs, Dicer1 overexpression and upregulation of miR-93/miR-20a could reverse the effects on differentiation and reduce cellular senescence[98]. The lncRNA Bmncr regulates the age-related lineage switch between osteogenic and adipogenic differentiation in BM-MSCs. Overexpression of Bmncr (Bmncr-Tg) reduced bone loss and fat accumulation by maintaining extracellular matrix protein fibromodulin and activating the bone morphogenetic protein 2 pathway[76].
A conducive microenvironment is essential for maintaining MSC activity[133]. When BM-MSCs were treated with BM supernatant from systemic lupus erythernatosus (SLE) patients, they demonstrated characteristics of senescence. An inflammatory microenvironment is considered to play a primary role in the senescence of SLE BM-MSCs[127]. In unbalanced microenvironments caused by aging, the survival, proliferation, colony formation, migration, and appropriate differentiation of grafted BM-MSCs were significantly suppressed[43,84]. In addition, the BM pCO2 and HCO3 levels displayed a close correlation with MSC differentiation and proliferation[134]. Therefore, microenvironment regulation is a promising strategy to reverse the decline of aged MSCs and promote the clinical efficacy[135,136].
Rejuvenating the senescent MSCs is more effective in hypoxic conditions compared with that in normal conditions. The neuroprotective effects of CM from aged human BM-MSCs against cerebral ischemia were enhanced by hypoxia conditioning in vitro[137,138]. Stem cell-deposited decellularized extracellular matrix can rescue hUC-MSCs from oxidative stress-induced premature senescence and facilitate their clinical application in regenerative medicine[103]. The co-culture system is a convenient means of modulating the cellular microenvironment. BM-MSCs co-cultured with young (P3) human umbilical vein endothelial cells demonstrated a higher proliferative ability and decreased pro-inflammatory (cytokines and miRNA) phenotype, compared with the old cells (P13)[139]. In conclusion, the enhancement of the microenvironment has a significant effect on the prevention of MSC senescence.
Besides restoring the activities of aged autologous MSCs, some researchers have attempted to find an accessible source for the replenishment of autologous MSCs. In the past few decades, the differentiation of pluripotent stem cells into MSC-like cells has been explored to address the problems of viability and scarcity of autologous MSCs derived from old individuals[140-142]. Induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs), which acquire a rejuvenation gene signature, are the alternative sources of MSCs[141,143]. However, the production protocols used to derive MSCs from iPSCs and ESCs require optimization. The introduction of new technologies, such as 3D culture and gene engineering, might make them more valuable for further clinical application[142].
Biomaterials and various cellular components are potential carriers used for the modification of aged MSCs. MVs carrying mRNAs, miRNAs, non-coding RNAs, proteins, and DNA are a novel and promising tool used to reverse aging in cells by mediating intercellular communication[44,108]. For instance, exosomes containing miR-17 and miR-34a from young MSCs rejuvenate aged murine hematopoietic stem cells via AKT/autophagy-related mRNAs[144]. Extracellular vesicles from human iPSCs can reduce cellular reactive oxygen species levels and alleviate aged phenotypes of senescent MSCs by partially delivering intracellular peroxiredoxin antioxidant enzymes[145]. Media supplied with human platelet lysate from younger donors were able to facilitate MSC expansion and osteogenic differentiation[146]. Additionally, many bioactive hydrogels[147], biomimetic scaffolds[148], and other biomaterials[149,150] have been tested to assess whether they can modify aged MSCs. Removal of senescent cells in a high-throughput manner is another strategy that can be used to address the challenge of senescence[151]; this strategy, which has been explored in clinical trials, involves the isolation and enumeration of senescent MSCs from undiluted human whole blood.
Some chemical compounds and foods rejuvenate senescent MSCs. Zinc sulfate significantly reduced the doubling time and increased TERT gene expression of rat ADSCs under extremely low-frequency-electromagnetic field[152]. It also enhanced telomere length extension in human ADSCs by regulating telomerase and methylation of the TERT gene promoter CpG island[153]. Besides zinc sulfate, resveratrol mimics the effects of dietary restriction, improves osteogenic function, and promotes mitochondrial activities of senescent MSCs through the regulation of mitofilin[154]. NT-020, a dietary supplement containing blueberry, green tea, vitamin D3, and carnosine, rescued the reduced proliferation of MSCs in serum from aged rats[155]. Additionally, Undaria pinnatifida and its ethanol extracts improve replication ability and ameliorate functional decline in senescent hBM-MSCs (P17)[156].
The rejuvenation methods mentioned above have potentials to optimize the functional status of aged MSCs. However, most of them were in vitro or rodent model studies. Further research is needed to evaluate their long-term safety and efficacy before it can be clinically useful.
Senescence is an inevitable biological process for MSCs obtained from old individuals or long-term cultures. Although recent studies have revealed the characteristics and mechanisms of MSC senescence and attempted to rejuvenate aged MSCs, many issues remain unresolved. First, in studies of age-correlated phenotypic alterations, the expression of CD90 and CD73 in intervertebral disc cells was reduced in older individuals, while CD146 expression was increased[157]. However, the expression of these factors (MSC markers) is rarely compared between young and aged MSCs. The comparison of these two types of cells provides a better understanding of senescent MSCs. Second, the effects of cellular rejuvenation for aged MSCs need to be determined in vivo, especially in the context of the multidirectional functions of regulators[158]. For example, hypoxia not only promotes the expansion of MSCs[159,160], but also influences the activity of MSCs during osteogenic differentiation[161]. Future work in vivo can provide more information about clinical efficacy. Additionally, MSCs isolated from specific tissues usually maintain lineage differentiation towards a specific cell type, and this plays a crucial role in regenerative therapy[161]. Therefore, the directional differentiation capacities in aged MSCs must be clarified following the increase in available tissue sources. Finally, many newly developing technologies, such as MVs, three-dimensional spheroid culture, and nanobiotechnology, will aid in improving aged MSC function in clinical therapies. Additionally, the functional discrepancies in various rejuvenation factors reported in different studies should be evaluated. For example, although a decline of osteogenesis capacitiy in aged MSCs was reported, other studies suggested that bone formation capacity was not affected in aged MSCs[162]. The function of pigment epithelium-derived factor (PEDF) responding to the senescence is unanticipated to demonstrate the different results in different research teams[97,163]. Liang et al[97] showed that increased PEDF secretion resulted in the impaired therapeutic ability of aged MSCs. However, Cao et al[163] showed that PEDF delayed cellular senescence and allowed a greater expansion of MSCs by suppressing oxidative stress and preserving differentiation potentials, compared with that in the control group. The different PEDF functions are possibly attributable to MSC heterogeneity, varying research objectives, and the specific experimental models used.
The rejuvenation of aged MSCs holds great promise for the accelerated translation of cell-based approaches (especially autologous cell administration) into clinically relevant therapies.
| 1. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13033] [Article Influence: 685.9] [Reference Citation Analysis (12)] |
| 2. | Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76:3323-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 412] [Article Influence: 58.9] [Reference Citation Analysis (11)] |
| 3. | Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 432] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 4. | Kuci Z, Jordan C, Wehner S, Sörensen J, Jarisch A, Salzmann-Manrique E, Pfeffermann LM, Klingebiel T, Bader P, Kuҫi S. The Phenotype and Functional Activity of Mesenchymal Stromal Cells in Pediatric Patients with Non-Malignant Hematological Diseases. Cells. 2020;9:431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Kofoed KF, Haack-Sørensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail. 2020;22:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 6. | Hong Y, He H, Jiang G, Zhang H, Tao W, Ding Y, Yuan D, Liu J, Fan H, Lin F, Liang X, Li X, Zhang Y. miR-155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell. 2020;19:e13128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Staff NP, Jones DT, Singer W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin Proc. 2019;94:892-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Berry JD, Cudkowicz ME, Windebank AJ, Staff NP, Owegi M, Nicholson K, McKenna-Yasek D, Levy YS, Abramov N, Kaspi H, Mehra M, Aricha R, Gothelf Y, Brown RH. NurOwn, phase 2, randomized, clinical trial in patients with ALS: Safety, clinical, and biomarker results. Neurology. 2019;93:e2294-e2305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 9. | Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 469] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 10. | Carvello M, Lightner A, Yamamoto T, Kotze PG, Spinelli A. Mesenchymal Stem Cells for Perianal Crohn's Disease. Cells. 2019;8:764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Colombini A, Perucca Orfei C, Kouroupis D, Ragni E, De Luca P, ViganÒ M, Correa D, de Girolamo L. Mesenchymal stem cells in the treatment of articular cartilage degeneration: New biological insights for an old-timer cell. Cytotherapy. 2019;21:1179-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Yin Y, Hao H, Cheng Y, Zang L, Liu J, Gao J, Xue J, Xie Z, Zhang Q, Han W, Mu Y. Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice. Cell Death Dis. 2018;9:760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Fazeli Z, Abedindo A, Omrani MD, Ghaderian SMH. Mesenchymal Stem Cells (MSCs) Therapy for Recovery of Fertility: a Systematic Review. Stem Cell Rev Rep. 2018;14:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1298] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 15. | Nie WB, Zhang D, Wang LS. Growth Factor Gene-Modified Mesenchymal Stem Cells in Tissue Regeneration. Drug Des Devel Ther. 2020;14:1241-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Andrzejewska A, Lukomska B, Janowski M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 411] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 17. | Khademi-Shirvan M, Ghorbaninejad M, Hosseini S, Baghaban Eslaminejad M. The Importance of Stem Cell Senescence in Regenerative Medicine. Adv Exp Med Biol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Yu J, Shi J, Zhang Y, Zhang Y, Huang Y, Chen Z, Yang J. The replicative senescent mesenchymal stem / stromal cells defect in DNA damage response and anti-oxidative capacity. Int J Med Sci. 2018;15:771-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Medeiros Tavares Marques JC, Cornélio DA, Nogueira Silbiger V, Ducati Luchessi A, de Souza S, Batistuzzo de Medeiros SR. Identification of new genes associated to senescent and tumorigenic phenotypes in mesenchymal stem cells. Sci Rep. 2017;7:17837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Yu D, Du Z, Pian L, Li T, Wen X, Li W, Kim SJ, Xiao J, Cohen P, Cui J, Hoffman AR, Hu JF. Mitochondrial DNA Hypomethylation Is a Biomarker Associated with Induced Senescence in Human Fetal Heart Mesenchymal Stem Cells. Stem Cells Int. 2017;2017:1764549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Zhai W, Yong D, El-Jawhari JJ, Cuthbert R, McGonagle D, Win Naing M, Jones E. Identification of senescent cells in multipotent mesenchymal stromal cell cultures: Current methods and future directions. Cytotherapy. 2019;21:803-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Nadeau S, Cheng A, Colmegna I, Rodier F. Quantifying Senescence-Associated Phenotypes in Primary Multipotent Mesenchymal Stromal Cell Cultures. Methods Mol Biol. 2019;2045:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Turinetto V, Vitale E, Giachino C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int J Mol Sci. 2016;17:1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 378] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 24. | Oja S, Komulainen P, Penttilä A, Nystedt J, Korhonen M. Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res Ther. 2018;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Jung JS, Volk C, Marga C, Navarrete Santos A, Jung M, Rujescu D, Navarrete Santos A. Adipose-Derived Stem/Stromal Cells Recapitulate Aging Biomarkers and Show Reduced Stem Cell Plasticity Affecting Their Adipogenic Differentiation Capacity. Cell Reprogram. 2019;21:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Ganguly P, El-Jawhari JJ, Giannoudis PV, Burska AN, Ponchel F, Jones EA. Age-related Changes in Bone Marrow Mesenchymal Stromal Cells: A Potential Impact on Osteoporosis and Osteoarthritis Development. Cell Transplant. 2017;26:1520-1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 27. | Stab BR 2nd, Martinez L, Grismaldo A, Lerma A, Gutiérrez ML, Barrera LA, Sutachan JJ, Albarracín SL. Mitochondrial Functional Changes Characterization in Young and Senescent Human Adipose Derived MSCs. Front Aging Neurosci. 2016;8:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Li JJ, Ma FX, Wang YW, Chen F, Lu SH, Chi Y, Du WJ, Song BQ, Hu LD, Chen H, Han ZC. Knockdown of IL-8 Provoked Premature Senescence of Placenta-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2017;26:912-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Yin Y, Wu RX, He XT, Xu XY, Wang J, Chen FM. Influences of age-related changes in mesenchymal stem cells on macrophages during in-vitro culture. Stem Cell Res Ther. 2017;8:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 606] [Cited by in RCA: 961] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 31. | D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 639] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 32. | Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, Xia ZY, Zhou HD, Cao X, Xie H, Liao EY, Luo XH. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015;125:1509-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 33. | Li H, Liu P, Xu S, Li Y, Dekker JD, Li B, Fan Y, Zhang Z, Hong Y, Yang G, Tang T, Ren Y, Tucker HO, Yao Z, Guo X. FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. J Clin Invest. 2017;127:1241-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 34. | Neri S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int J Mol Sci. 2019;20:2406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 35. | Hladik D, Höfig I, Oestreicher U, Beckers J, Matjanovski M, Bao X, Scherthan H, Atkinson MJ, Rosemann M. Long-term culture of mesenchymal stem cells impairs ATM-dependent recognition of DNA breaks and increases genetic instability. Stem Cell Res Ther. 2019;10:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Wu H, Yang Z, Chi Y, Meng L, Mao A, Yan S, Hu S, Zhang J, Zhang Y, Yu W, Ma Y, Li T, Cheng Y, Wang Y, Wang S, Liu J, Han J, Li C, Liu L, Xu J, Han ZB, Han ZC. Human mesenchymal stem cells possess different biological characteristics but do not change their therapeutic potential when cultured in serum free medium. Stem Cell Res Ther. 2014;5:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Kundrotas G, Gasperskaja E, Slapsyte G, Gudleviciene Z, Krasko J, Stumbryte A, Liudkeviciene R. Identity, proliferation capacity, genomic stability and novel senescence markers of mesenchymal stem cells isolated from low volume of human bone marrow. Oncotarget. 2016;7:10788-10802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Sharma S, Bhonde R. Mesenchymal stromal cells are genetically stable under a hostile in vivo-like scenario as revealed by in vitro micronucleus test. Cytotherapy. 2015;17:1384-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Roselli EA, Lazzati S, Iseppon F, Manganini M, Marcato L, Gariboldi MB, Maggi F, Grati FR, Simoni G. Fetal mesenchymal stromal cells from cryopreserved human chorionic villi: cytogenetic and molecular analysis of genome stability in long-term cultures. Cytotherapy. 2013;15:1340-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Prockop DJ, Keating A. Relearning the lessons of genomic stability of human cells during expansion in culture: implications for clinical research. Stem Cells. 2012;30:1051-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Rebuzzini P, Zuccotti M, Redi CA, Garagna S. Chromosomal Abnormalities in Embryonic and Somatic Stem Cells. Cytogenet Genome Res. 2015;147:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Maijenburg MW, Kleijer M, Vermeul K, Mul EP, van Alphen FP, van der Schoot CE, Voermans C. The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica. 2012;97:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Yang YM, Li P, Cui DC, Dang RJ, Zhang L, Wen N, Jiang XX. Effect of aged bone marrow microenvironment on mesenchymal stem cell migration. Age (Dordr). 2015;37:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, Ren W, Guo H, Zhang L, Wang H, Chen Z, Guo AY, Li Q. Microvesicles as Potential Biomarkers for the Identification of Senescence in Human Mesenchymal Stem Cells. Theranostics. 2017;7:2673-2689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 45. | Madsen SD, Russell KC, Tucker HA, Glowacki J, Bunnell BA, O'Connor KC. Decoy TRAIL receptor CD264: a cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2017;8:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Ang J, Lee YA, Raghothaman D, Jayaraman P, Teo KL, Khan FJ, Reuveny S, Chang YT, Kang NY, Oh S. Rapid Detection of Senescent Mesenchymal Stromal Cells by a Fluorescent Probe. Biotechnol J. 2019;14:e1800691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Wiese DM, Ruttan CC, Wood CA, Ford BN, Braid LR. Accumulating Transcriptome Drift Precedes Cell Aging in Human Umbilical Cord-Derived Mesenchymal Stromal Cells Serially Cultured to Replicative Senescence. Stem Cells Transl Med. 2019;8:945-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Benisch P, Schilling T, Klein-Hitpass L, Frey SP, Seefried L, Raaijmakers N, Krug M, Regensburger M, Zeck S, Schinke T, Amling M, Ebert R, Jakob F. The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PLoS One. 2012;7:e45142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 49. | Wu Y, Yang J, Ai Z, Yu M, Li J, Li S. Identification of key genes and transcription factors in aging mesenchymal stem cells by DNA microarray data. Gene. 2019;692:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Yoo JK, Choi SJ, Kim JK. Expression profiles of subtracted mRNAs during cellular senescence in human mesenchymal stem cells derived from bone marrow. Exp Gerontol. 2013;48:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Leveque X, Hochane M, Geraldo F, Dumont S, Gratas C, Oliver L, Gaignier C, Trichet V, Layrolle P, Heymann D, Herault O, Vallette FM, Olivier C. Low-Dose Pesticide Mixture Induces Accelerated Mesenchymal Stem Cell Aging In Vitro. Stem Cells. 2019;37:1083-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, McVerry BJ, Richards TJ, Kaminski N, Cerdenes N, Mora AL, Rojas M. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 53. | Niu P, Smagul A, Wang L, Sadvakas A, Sha Y, Pérez LM, Nussupbekova A, Amirbekov A, Akanov AA, Gálvez BG, Jordan IK, Lunyak VV. Transcriptional profiling of interleukin-2-primed human adipose derived mesenchymal stem cells revealed dramatic changes in stem cells response imposed by replicative senescence. Oncotarget. 2015;6:17938-17957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Duscher D, Rennert RC, Januszyk M, Anghel E, Maan ZN, Whittam AJ, Perez MG, Kosaraju R, Hu MS, Walmsley GG, Atashroo D, Khong S, Butte AJ, Gurtner GC. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep. 2014;4:7144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 55. | Malaquin N, Martinez A, Rodier F. Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Exp Gerontol. 2016;82:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 56. | Kizilay Mancini Ö, Lora M, Shum-Tim D, Nadeau S, Rodier F, Colmegna I. A Proinflammatory Secretome Mediates the Impaired Immunopotency of Human Mesenchymal Stromal Cells in Elderly Patients with Atherosclerosis. Stem Cells Transl Med. 2017;6:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Infante A, Rodríguez CI. Secretome analysis of in vitro aged human mesenchymal stem cells reveals IGFBP7 as a putative factor for promoting osteogenesis. Sci Rep. 2018;8:4632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Madonna R, Angelucci S, Di Giuseppe F, Doria V, Giricz Z, Görbe A, Ferdinandy P, De Caterina R. Proteomic analysis of the secretome of adipose tissue-derived murine mesenchymal cells overexpressing telomerase and myocardin. J Mol Cell Cardiol. 2019;131:171-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Cakouros D, Gronthos S. Epigenetic Regulation of Bone Marrow Stem Cell Aging: Revealing Epigenetic Signatures associated with Hematopoietic and Mesenchymal Stem Cell Aging. Aging Dis. 2019;10:174-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 60. | Choi MR, In YH, Park J, Park T, Jung KH, Chai JC, Chung MK, Lee YS, Chai YG. Genome-scale DNA methylation pattern profiling of human bone marrow mesenchymal stem cells in long-term culture. Exp Mol Med. 2012;44:503-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Fernández AF, Bayón GF, Urdinguio RG, Toraño EG, García MG, Carella A, Petrus-Reurer S, Ferrero C, Martinez-Camblor P, Cubillo I, García-Castro J, Delgado-Calle J, Pérez-Campo FM, Riancho JA, Bueno C, Menéndez P, Mentink A, Mareschi K, Claire F, Fagnani C, Medda E, Toccaceli V, Brescianini S, Moran S, Esteller M, Stolzing A, de Boer J, Nisticò L, Stazi MA, Fraga MF. H3K4me1 marks DNA regions hypomethylated during aging in human stem and differentiated cells. Genome Res. 2015;25:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Toraño EG, Bayón GF, Del Real Á, Sierra MI, García MG, Carella A, Belmonte T, Urdinguio RG, Cubillo I, García-Castro J, Delgado-Calle J, Pérez-Campo FM, Riancho JA, Fraga MF, Fernández AF. Age-associated hydroxymethylation in human bone-marrow mesenchymal stem cells. J Transl Med. 2016;14:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Bork S, Pfister S, Witt H, Horn P, Korn B, Ho AD, Wagner W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 333] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 64. | Redaelli S, Bentivegna A, Foudah D, Miloso M, Redondo J, Riva G, Baronchelli S, Dalprà L, Tredici G. From cytogenomic to epigenomic profiles: monitoring the biologic behavior of in vitro cultured human bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2012;3:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Pasumarthy KK, Doni Jayavelu N, Kilpinen L, Andrus C, Battle SL, Korhonen M, Lehenkari P, Lund R, Laitinen S, Hawkins RD. Methylome Analysis of Human Bone Marrow MSCs Reveals Extensive Age- and Culture-Induced Changes at Distal Regulatory Elements. Stem Cell Reports. 2017;9:999-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Dellago H, Preschitz-Kammerhofer B, Terlecki-Zaniewicz L, Schreiner C, Fortschegger K, Chang MW, Hackl M, Monteforte R, Kühnel H, Schosserer M, Gruber F, Tschachler E, Scheideler M, Grillari-Voglauer R, Grillari J, Wieser M. High levels of oncomiR-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan. Aging Cell. 2013;12:446-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 67. | Bonifacio LN, Jarstfer MB. MiRNA profile associated with replicative senescence, extended cell culture, and ectopic telomerase expression in human foreskin fibroblasts. PLoS One. 2010;5:e12519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 68. | Tzatsos A, Paskaleva P, Lymperi S, Contino G, Stoykova S, Chen Z, Wong KK, Bardeesy N. Lysine-specific demethylase 2B (KDM2B)-let-7-enhancer of zester homolog 2 (EZH2) pathway regulates cell cycle progression and senescence in primary cells. J Biol Chem. 2011;286:33061-33069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 69. | Qiu W, Kassem M. miR-141-3p inhibits human stromal (mesenchymal) stem cell proliferation and differentiation. Biochim Biophys Acta. 2014;1843:2114-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Ukai T, Sato M, Akutsu H, Umezawa A, Mochida J. MicroRNA-199a-3p, microRNA-193b, and microRNA-320c are correlated to aging and regulate human cartilage metabolism. J Orthop Res. 2012;30:1915-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051-4060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2902] [Cited by in RCA: 3033] [Article Influence: 137.9] [Reference Citation Analysis (0)] |
| 72. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 28184] [Article Influence: 1281.1] [Reference Citation Analysis (0)] |
| 73. | Yoo JK, Kim CH, Jung HY, Lee DR, Kim JK. Discovery and characterization of miRNA during cellular senescence in bone marrow-derived human mesenchymal stem cells. Exp Gerontol. 2014;58:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Mohd Ali N, Boo L, Yeap SK, Ky H, Satharasinghe DA, Liew WC, Ong HK, Cheong SK, Kamarul T. Probable impact of age and hypoxia on proliferation and microRNA expression profile of bone marrow-derived human mesenchymal stem cells. PeerJ. 2016;4:e1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Lee S, Yu KR, Ryu YS, Oh YS, Hong IS, Kim HS, Lee JY, Kim S, Seo KW, Kang KS. miR-543 and miR-590-3p regulate human mesenchymal stem cell aging via direct targeting of AIMP3/p18. Age (Dordr). 2014;36:9724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Li CJ, Xiao Y, Yang M, Su T, Sun X, Guo Q, Huang Y, Luo XH. Long noncoding RNA Bmncr regulates mesenchymal stem cell fate during skeletal aging. J Clin Invest. 2018;128:5251-5266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 77. | Bernardes de Jesus B, Marinho SP, Barros S, Sousa-Franco A, Alves-Vale C, Carvalho T, Carmo-Fonseca M. Silencing of the lncRNA Zeb2-NAT facilitates reprogramming of aged fibroblasts and safeguards stem cell pluripotency. Nat Commun. 2018;9:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Cárdenes N, Álvarez D, Sellarés J, Peng Y, Corey C, Wecht S, Nouraie SM, Shanker S, Sembrat J, Bueno M, Shiva S, Mora AL, Rojas M. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res Ther. 2018;9:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 79. | Li XY, Ding J, Zheng ZH, Li XY, Wu ZB, Zhu P. Long-term culture in vitro impairs the immunosuppressive activity of mesenchymal stem cells on T cells. Mol Med Rep. 2012;6:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Xia W, Zhang F, Xie C, Jiang M, Hou M. Macrophage migration inhibitory factor confers resistance to senescence through CD74-dependent AMPK-FOXO3a signaling in mesenchymal stem cells. Stem Cell Res Ther. 2015;6:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Malaise O, Tachikart Y, Constantinides M, Mumme M, Ferreira-Lopez R, Noack S, Krettek C, Noël D, Wang J, Jorgensen C, Brondello JM. Mesenchymal stem cell senescence alleviates their intrinsic and seno-suppressive paracrine properties contributing to osteoarthritis development. Aging (Albany NY). 2019;11:9128-9146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 82. | Abolhasani M, Rezaee MA, Mohammadi M, Ghadimi T, Mohammadi M, Rahmani MR. Immunomodulatory properties of umbilical cord vein mesenchymal stromal cells influenced by gestational age and in vitro expansion. Immunol Lett. 2018;194:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 83. | Ding W, Nowakowski GS, Knox TR, Boysen JC, Maas ML, Schwager SM, Wu W, Wellik LE, Dietz AB, Ghosh AK, Secreto CR, Medina KL, Shanafelt TD, Zent CS, Call TG, Kay NE. Bi-directional activation between mesenchymal stem cells and CLL B-cells: implication for CLL disease progression. Br J Haematol. 2009;147:471-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 84. | Cao X, Luo P, Huang J, Liang C, He J, Wang Z, Shan D, Peng C, Wu S. Intraarticular senescent chondrocytes impair the cartilage regeneration capacity of mesenchymal stem cells. Stem Cell Res Ther. 2019;10:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Petri RM, Hackel A, Hahnel K, Dumitru CA, Bruderek K, Flohe SB, Paschen A, Lang S, Brandau S. Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function. Stem Cell Reports. 2017;9:985-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 86. | Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol. 2011;23:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 87. | Loisel S, Dulong J, Ménard C, Renoud ML, Meziere N, Isabelle B, Latour M, Bescher N, Pedeux R, Bertheuil N, Flecher E, Sensebé L, Tarte K. Brief Report: Proteasomal Indoleamine 2,3-Dioxygenase Degradation Reduces the Immunosuppressive Potential of Clinical Grade-Mesenchymal Stromal Cells Undergoing Replicative Senescence. Stem Cells. 2017;35:1431-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | de Witte SFH, Lambert EE, Merino A, Strini T, Douben HJCW, O'Flynn L, Elliman SJ, de Klein AJEMM, Newsome PN, Baan CC, Hoogduijn MJ. Aging of bone marrow- and umbilical cord-derived mesenchymal stromal cells during expansion. Cytotherapy. 2017;19:798-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 89. | Wu LW, Wang YL, Christensen JM, Khalifian S, Schneeberger S, Raimondi G, Cooney DS, Lee WP, Brandacher G. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl Immunol. 2014;30:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 90. | Xu LL, Fu HX, Zhang JM, Feng FE, Wang QM, Zhu XL, Xue J, Wang CC, Chen Q, Liu X, Wang YZ, Qin YZ, Kong Y, Chang YJ, Xu LP, Liu KY, Huang XJ, Zhang XH. Impaired Function of Bone Marrow Mesenchymal Stem Cells from Immune Thrombocytopenia Patients in Inducing Regulatory Dendritic Cell Differentiation Through the Notch-1/Jagged-1 Signaling Pathway. Stem Cells Dev. 2017;26:1648-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Dao MA, Tate CC, Aizman I, McGrogan M, Case CC. Comparing the immunosuppressive potency of naïve marrow stromal cells and Notch-transfected marrow stromal cells. J Neuroinflammation. 2011;8:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Gnani D, Crippa S, Della Volpe L, Rossella V, Conti A, Lettera E, Rivis S, Ometti M, Fraschini G, Bernardo ME, Di Micco R. An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell. 2019;18:e12933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 93. | O'Hagan-Wong K, Nadeau S, Carrier-Leclerc A, Apablaza F, Hamdy R, Shum-Tim D, Rodier F, Colmegna I. Increased IL-6 secretion by aged human mesenchymal stromal cells disrupts hematopoietic stem and progenitor cells' homeostasis. Oncotarget. 2016;7:13285-13296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 94. | Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 95. | Rivera FJ, de la Fuente AG, Zhao C, Silva ME, Gonzalez GA, Wodnar R, Feichtner M, Lange S, Errea O, Priglinger E, O'Sullivan A, Romanelli P, Jadasz JJ, Brachtl G, Greil R, Tempfer H, Traweger A, Bátiz LF, Küry P, Couillard-Despres S, Franklin RJM, Aigner L. Aging restricts the ability of mesenchymal stem cells to promote the generation of oligodendrocytes during remyelination. Glia. 2019;67:1510-1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Yamaguchi S, Horie N, Satoh K, Ishikawa T, Mori T, Maeda H, Fukuda Y, Ishizaka S, Hiu T, Morofuji Y, Izumo T, Nishida N, Matsuo T. Age of donor of human mesenchymal stem cells affects structural and functional recovery after cell therapy following ischaemic stroke. J Cereb Blood Flow Metab. 2018;38:1199-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 97. | Liang H, Hou H, Yi W, Yang G, Gu C, Lau WB, Gao E, Ma X, Lu Z, Wei X, Pei J, Yi D. Increased expression of pigment epithelium-derived factor in aged mesenchymal stem cells impairs their therapeutic efficacy for attenuating myocardial infarction injury. Eur Heart J. 2013;34:1681-1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Guo J, Zhao Y, Fei C, Zhao S, Zheng Q, Su J, Wu D, Li X, Chang C. Dicer1 downregulation by multiple myeloma cells promotes the senescence and tumor-supporting capacity and decreases the differentiation potential of mesenchymal stem cells. Cell Death Dis. 2018;9:512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 99. | Lee SH, Lee JH, Lee HY, Min KJ. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019;52:24-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 100. | Liu X, Chen H, Zhu W, Chen H, Hu X, Jiang Z, Xu Y, Zhou Y, Wang K, Wang L, Chen P, Hu H, Wang C, Zhang N, Ma Q, Huang M, Hu D, Zhang L, Wu R, Wang Y, Xu Q, Yu H, Wang J. Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. J Heart Lung Transplant. 2014;33:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Song J, Li J, Yang F, Ning G, Zhen L, Wu L, Zheng Y, Zhang Q, Lin D, Xie C, Peng L. Nicotinamide mononucleotide promotes osteogenesis and reduces adipogenesis by regulating mesenchymal stromal cells via the SIRT1 pathway in aged bone marrow. Cell Death Dis. 2019;10:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 102. | Ma C, Pi C, Yang Y, Lin L, Shi Y, Li Y, Li Y, He X. Nampt Expression Decreases Age-Related Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Sirt1. PLoS One. 2017;12:e0170930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 103. | Zhou L, Chen X, Liu T, Zhu C, Si M, Jargstorf J, Li M, Pan G, Gong Y, Luo ZP, Yang H, Pei M, He F. SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells. J Tissue Eng Regen Med. 2018;12:e1008-e1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 104. | Liu X, Hu D, Zeng Z, Zhu W, Zhang N, Yu H, Chen H, Wang K, Wang Y, Wang L, Zhao J, Zhang L, Wu R, Hu X, Wang J. SRT1720 promotes survival of aged human mesenchymal stem cells via FAIM: a pharmacological strategy to improve stem cell-based therapy for rat myocardial infarction. Cell Death Dis. 2017;8:e2731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 105. | Zhang DY, Zhang CF, Fu BC, Sun L, Wang XQ, Chen W, Liu W, Liu KY, Du GQ, Ma CY, Jiang SL, Li RK, Tian H. Sirtuin3 protects aged human mesenchymal stem cells against oxidative stress and enhances efficacy of cell therapy for ischaemic heart diseases. J Cell Mol Med. 2018;22:5504-5517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 106. | Pan H, Guan D, Liu X, Li J, Wang L, Wu J, Zhou J, Zhang W, Ren R, Zhang W, Li Y, Yang J, Hao Y, Yuan T, Yuan G, Wang H, Ju Z, Mao Z, Li J, Qu J, Tang F, Liu GH. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 2016;26:190-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 107. | Mitchell AC, Briquez PS, Hubbell JA, Cochran JR. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 108. | Kulkarni RS, Bajaj MS, Kale VP. Induction and Detection of Autophagy in Aged Hematopoietic Stem Cells by Exposing Them to Microvesicles Secreted by HSC-Supportive Mesenchymal Stromal Cells. Methods Mol Biol. 2019;1854:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Li X, Hong Y, He H, Jiang G, You W, Liang X, Fu Q, Han S, Lian Q, Zhang Y. FGF21 Mediates Mesenchymal Stem Cell Senescence via Regulation of Mitochondrial Dynamics. Oxid Med Cell Longev. 2019;2019:4915149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 110. | Wu J, Wang C, Miao X, Wu Y, Yuan J, Ding M, Li J, Shi Z. Age-Related Insulin-Like Growth Factor Binding Protein-4 Overexpression Inhibits Osteogenic Differentiation of Rat Mesenchymal Stem Cells. Cell Physiol Biochem. 2017;42:640-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Xia W, Hou M. Macrophage migration inhibitory factor rescues mesenchymal stem cells from doxorubicin-induced senescence though the PI3K-Akt signaling pathway. Int J Mol Med. 2018;41:1127-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Liang X, Ding Y, Lin F, Zhang Y, Zhou X, Meng Q, Lu X, Jiang G, Zhu H, Chen Y, Lian Q, Fan H, Liu Z. Overexpression of ERBB4 rejuvenates aged mesenchymal stem cells and enhances angiogenesis via PI3K/AKT and MAPK/ERK pathways. FASEB J. 2019;33:4559-4570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 113. | Park SY, Jeong AJ, Kim GY, Jo A, Lee JE, Leem SH, Yoon JH, Ye SK, Chung JW. Lactoferrin Protects Human Mesenchymal Stem Cells from Oxidative Stress-Induced Senescence and Apoptosis. J Microbiol Biotechnol. 2017;27:1877-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 114. | Yang M, Teng S, Ma C, Yu Y, Wang P, Yi C. Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology. 2018;70:1301-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 115. | Lee JH, Yun CW, Hur J, Lee SH. Fucoidan Rescues p-Cresol-Induced Cellular Senescence in Mesenchymal Stem Cells via FAK-Akt-TWIST Axis. Mar Drugs. 2018;16:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 116. | Antonioli E, Torres N, Ferretti M, Piccinato CA, Sertie AL. Individual response to mTOR inhibition in delaying replicative senescence of mesenchymal stromal cells. PLoS One. 2019;14:e0204784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 117. | Shahini A, Mistriotis P, Asmani M, Zhao R, Andreadis ST. NANOG Restores Contractility of Mesenchymal Stem Cell-Based Senescent Microtissues. Tissue Eng Part A. 2017;23:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 118. | Farahzadi R, Fathi E, Mesbah-Namin SA, Zarghami N. Anti-aging protective effect of L-carnitine as clinical agent in regenerative medicine through increasing telomerase activity and change in the hTERT promoter CpG island methylation status of adipose tissue-derived mesenchymal stem cells. Tissue Cell. 2018;54:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 119. | Pirmoradi S, Fathi E, Farahzadi R, Pilehvar-Soltanahmadi Y, Zarghami N. Curcumin Affects Adipose Tissue-Derived Mesenchymal Stem Cell Aging Through TERT Gene Expression. Drug Res (Stuttg). 2018;68:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 120. | Fujisawa K, Takami T, Fukui Y, Quintanilha LF, Matsumoto T, Yamamoto N, Sakaida I. Evaluating effects of L-carnitine on human bone-marrow-derived mesenchymal stem cells. Cell Tissue Res. 2017;368:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 121. | Han YS, Kim SM, Lee JH, Jung SK, Noh H, Lee SH. Melatonin protects chronic kidney disease mesenchymal stem cells against senescence via PrPC -dependent enhancement of the mitochondrial function. J Pineal Res. 2019;66:e12535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 122. | Yao J, Jiang SL, Liu W, Liu C, Chen W, Sun L, Liu KY, Jia ZB, Li RK, Tian H. Tissue inhibitor of matrix metalloproteinase-3 or vascular endothelial growth factor transfection of aged human mesenchymal stem cells enhances cell therapy after myocardial infarction. Rejuvenation Res. 2012;15:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 123. | Li SH, Sun L, Yang L, Li J, Shao Z, Du GQ, Wu J, Weisel RD, Li RK. Young Bone-Marrow Sca-1+ Stem Cells Rejuvenate the Aged Heart and Improve Function after Injury through PDGFRβ-Akt pathway. Sci Rep. 2017;7:41756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 124. | Li J, Li SH, Dong J, Alibhai FJ, Zhang C, Shao ZB, Song HF, He S, Yin WJ, Wu J, Weisel RD, Liu SM, Li RK. Long-term repopulation of aged bone marrow stem cells using young Sca-1 cells promotes aged heart rejuvenation. Aging Cell. 2019;18:e13026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Yang K, Song HF, He S, Yin WJ, Fan XM, Ru F, Gong H, Zhai XY, Zhang J, Peng ZX, Xi GX, Xie J, Li RK. Effect of neuron-derived neurotrophic factor on rejuvenation of human adipose-derived stem cells for cardiac repair after myocardial infarction. J Cell Mol Med. 2019;23:5981-5993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 126. | Song HF, He S, Li SH, Yin WJ, Wu J, Guo J, Shao ZB, Zhai XY, Gong H, Lu L, Wei F, Weisel RD, Xie J, Li RK. Aged Human Multipotent Mesenchymal Stromal Cells Can Be Rejuvenated by Neuron-Derived Neurotrophic Factor and Improve Heart Function After Injury. JACC Basic Transl Sci. 2017;2:702-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 127. | Ji J, Fu T, Dong C, Zhu W, Yang J, Kong X, Zhang Z, Bao Y, Zhao R, Ge X, Sha X, Lu Z, Li J, Gu Z. Targeting HMGB1 by ethyl pyruvate ameliorates systemic lupus erythematosus and reverses the senescent phenotype of bone marrow-mesenchymal stem cells. Aging (Albany NY). 2019;11:4338-4353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 128. | Okada M, Kim HW, Matsu-ura K, Wang YG, Xu M, Ashraf M. Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells. 2016;34:148-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 129. | Dong J, Zhang Z, Huang H, Mo P, Cheng C, Liu J, Huang W, Tian C, Zhang C, Li J. miR-10a rejuvenates aged human mesenchymal stem cells and improves heart function after myocardial infarction through KLF4. Stem Cell Res Ther. 2018;9:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 130. | Shang J, Yao Y, Fan X, Shangguan L, Li J, Liu H, Zhou Y. miR-29c-3p promotes senescence of human mesenchymal stem cells by targeting CNOT6 through p53-p21 and p16-pRB pathways. Biochim Biophys Acta. 2016;1863:520-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 131. | Xu J, Huang Z, Lin L, Fu M, Song Y, Shen Y, Ren D, Gao Y, Su Y, Zou Y, Chen Y, Zhang D, Hu W, Qian J, Ge J. miRNA-130b is required for the ERK/FOXM1 pathway activation-mediated protective effects of isosorbide dinitrate against mesenchymal stem cell senescence induced by high glucose. Int J Mol Med. 2015;35:59-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 132. | Su T, Xiao Y, Xiao Y, Guo Q, Li C, Huang Y, Deng Q, Wen J, Zhou F, Luo XH. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MiR-29b-3p Regulates Aging-Associated Insulin Resistance. ACS Nano. 2019;13:2450-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 133. | Redondo J, Sarkar P, Kemp K, Virgo PF, Pawade J, Norton A, Emery DC, Guttridge MG, Marks DI, Wilkins A, Scolding NJ, Rice CM. Reduced cellularity of bone marrow in multiple sclerosis with decreased MSC expansion potential and premature ageing in vitro. Mult Scler. 2018;24:919-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 134. | Miettinen JA, Salonen RJ, Ylitalo K, Niemelä M, Kervinen K, Säily M, Koistinen P, Savolainen ER, Mäkikallio TH, Huikuri HV, Lehenkari P. The effect of bone marrow microenvironment on the functional properties of the therapeutic bone marrow-derived cells in patients with acute myocardial infarction. J Transl Med. 2012;10:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 135. | Oses C, Olivares B, Ezquer M, Acosta C, Bosch P, Donoso M, Léniz P, Ezquer F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS One. 2017;12:e0178011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 136. | Di Maggio N, Mehrkens A, Papadimitropoulos A, Schaeren S, Heberer M, Banfi A, Martin I. Fibroblast growth factor-2 maintains a niche-dependent population of self-renewing highly potent non-adherent mesenchymal progenitors through FGFR2c. Stem Cells. 2012;30:1455-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 137. | Zhang Y, Ma L, Su Y, Su L, Lan X, Wu D, Han S, Li J, Kvederis L, Corey S, Borlongan CV, Ji X. Hypoxia conditioning enhances neuroprotective effects of aged human bone marrow mesenchymal stem cell-derived conditioned medium against cerebral ischemia in vitro. Brain Res. 2019;1725:146432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 138. | Ratushnyy AY, Rudimova YV, Buravkova LB. Alteration of Hypoxia-Associated Gene Expression in Replicatively Senescent Mesenchymal Stromal Cells under Physiological Oxygen Level. Biochemistry (Mosc). 2019;84:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 139. | Lazzarini R, Caffarini M, Tang H, Cerqueni G, Pellegrino P, Monsurrò V, Di Primio R, Orciani M. The senescent status of endothelial cells affects proliferation, inflammatory profile and SOX2 expression in bone marrow-derived mesenchymal stem cells. Exp Gerontol. 2019;120:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 140. | Umezaki Y, Hashimoto Y, Nishishita N, Kawamata S, Baba S. Human Gingival Integration-Free iPSCs; a Source for MSC-Like Cells. Int J Mol Sci. 2015;16:13633-13648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 141. | Spitzhorn LS, Megges M, Wruck W, Rahman MS, Otte J, Degistirici Ö, Meisel R, Sorg RV, Oreffo ROC, Adjaye J. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res Ther. 2019;10:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 142. | Zhao C, Ikeya M. Generation and Applications of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells. Stem Cells Int. 2018;2018:9601623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 143. | Wang LT, Jiang SS, Ting CH, Hsu PJ, Chang CC, Sytwu HK, Liu KJ, Yen BL. Differentiation of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells Results in Downregulation of c-Myc and DNA Replication Pathways with Immunomodulation Toward CD4 and CD8 Cells. Stem Cells. 2018;36:903-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 144. | Kulkarni R, Bajaj M, Ghode S, Jalnapurkar S, Limaye L, Kale VP. Intercellular Transfer of Microvesicles from Young Mesenchymal Stromal Cells Rejuvenates Aged Murine Hematopoietic Stem Cells. Stem Cells. 2018;36:420-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 145. | Liu S, Mahairaki V, Bai H, Ding Z, Li J, Witwer KW, Cheng L. Highly Purified Human Extracellular Vesicles Produced by Stem Cells Alleviate Aging Cellular Phenotypes of Senescent Human Cells. Stem Cells. 2019;37:779-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 146. | Lohmann M, Walenda G, Hemeda H, Joussen S, Drescher W, Jockenhoevel S, Hutschenreuter G, Zenke M, Wagner W. Donor age of human platelet lysate affects proliferation and differentiation of mesenchymal stem cells. PLoS One. 2012;7:e37839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 147. | Pescador D, Ibáñez-Fonseca A, Sánchez-Guijo F, Briñón JG, Arias FJ, Muntión S, Hernández C, Girotti A, Alonso M, Del Cañizo MC, Rodríguez-Cabello JC, Blanco JF. Regeneration of hyaline cartilage promoted by xenogeneic mesenchymal stromal cells embedded within elastin-like recombinamer-based bioactive hydrogels. J Mater Sci Mater Med. 2017;28:115. [PubMed] [DOI] [Full Text] |
| 148. | Manferdini C, Guarino V, Zini N, Raucci MG, Ferrari A, Grassi F, Gabusi E, Squarzoni S, Facchini A, Ambrosio L, Lisignoli G. Mineralization behavior with mesenchymal stromal cells in a biomimetic hyaluronic acid-based scaffold. Biomaterials. 2010;31:3986-3996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 149. | Šponer P, Kučera T, Brtková J, Urban K, Kočí Z, Měřička P, Bezrouk A, Konrádová Š, Filipová A, Filip S. Comparative Study on the Application of Mesenchymal Stromal Cells Combined with Tricalcium Phosphate Scaffold into Femoral Bone Defects. Cell Transplant. 2018;27:1459-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 150. | Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 622] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 151. | Chen Y, Mao P, Snijders AM, Wang D. Senescence chips for ultrahigh-throughput isolation and removal of senescent cells. Aging Cell. 2018;17:e12722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 152. | Fathi E, Farahzadi R. Zinc Sulphate Mediates the Stimulation of Cell Proliferation of Rat Adipose Tissue-Derived Mesenchymal Stem Cells Under High Intensity of EMF Exposure. Biol Trace Elem Res. 2018;184:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 153. | Farahzadi R, Fathi E, Mesbah-Namin SA, Zarghami N. Zinc sulfate contributes to promote telomere length extension via increasing telomerase gene expression, telomerase activity and change in the TERT gene promoter CpG island methylation status of human adipose-derived mesenchymal stem cells. PLoS One. 2017;12:e0188052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 154. | Lv YJ, Yang Y, Sui BD, Hu CH, Zhao P, Liao L, Chen J, Zhang LQ, Yang TT, Zhang SF, Jin Y. Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice. Theranostics. 2018;8:2387-2406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 155. | Bickford PC, Kaneko Y, Grimmig B, Pappas C, Small B, Sanberg CD, Sanberg PR, Tan J, Douglas Shytle R. Nutraceutical intervention reverses the negative effects of blood from aged rats on stem cells. Age (Dordr). 2015;37:103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 156. | Jeong SG, Oh YS, Joe IS, Jeong SY, Cho HM, Lee JS, Oh WK, Cho TO, Cho GW. Functional restoration of replicative senescent mesenchymal stem cells by the brown alga Undaria pinnatifida. Anim Cells Syst (Seoul). 2017;21:108-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 157. | Molinos M, Cunha C, Almeida CR, Gonçalves RM, Pereira P, Silva PS, Vaz R, Barbosa MA. Age-Correlated Phenotypic Alterations in Cells Isolated From Human Degenerated Intervertebral Discs With Contained Hernias. Spine (Phila Pa 1976). 2018;43:E274-E284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 158. | Čamernik K, Zupan J. Complete Assessment of Multilineage Differentiation Potential of Human Skeletal Muscle-Derived Mesenchymal Stem/Stromal Cells. Methods Mol Biol. 2019;2045:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 159. | Gao S, Xiang C, Qin K, Sun C. Mathematical Modeling Reveals the Role of Hypoxia in the Promotion of Human Mesenchymal Stem Cell Long-Term Expansion. Stem Cells Int. 2018;2018:9283432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 160. | Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 161. | Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y, Liu J, Geng Z, Wang Y. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp Mol Pathol. 2013;94:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 162. | Carbonneau CL, Despars G, Beaudry GM, Benabdallah B, Bouhanik S, Dépôt J, Moreau A, Beauséjour CM. Heterotopic bone formation derived from multipotent stromal cells is not inhibited in aged mice. Cytotherapy. 2014;16:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 163. | Cao Y, Yang T, Gu C, Yi D. Pigment epithelium-derived factor delays cellular senescence of human mesenchymal stem cells in vitro by reducing oxidative stress. Cell Biol Int. 2013;37:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccocioppo R S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Xing YX