INTRODUCTION
The hair follicle is a skin appendage and dynamic mini-organ derived from tightly coordinated interactions between prototypic ectodermal–mesodermal cells early in embryogenesis. The hair follicle is easily accessible and contain stem cells from different developmental origins, such as epithelial stem cells, melanocyte stem cells, and mesenchymal stem cells (MSCs)[1]. These stem cells continuously self-renew, differentiate, regulate hair follicle development, and contribute to hair follicle cycles which consist of the growth phase (anagen), regression phase (catagen), and rest phase (telogen) throughout adult life[2]. During catagen and telogen, follicles prepare their stem cells for the next anagen. During anagen, bulge stem cells are activated by induction signals from the dermal papilla and migrate downward to the bulb region, where they proliferate and differentiate to regenerate the inner and outer root sheath, matrix, and hair shaft.
Many studies have focused on the epidermal stem cell lineage, which lies within the bulge region of the hair follicle, compared to MSCs derived from the dermal papilla or dermal sheath. In a pioneering study, Lako et al[3] first demonstrated that dermal papilla and dermal sheath cells from transgenically marked donor mice could produce multiple lineages of the hematopoietic system in lethally irradiated mice, indicating the presence of multipotent stem cells in the dermal papilla and dermal sheath. Subsequent studies showed that dermal papilla or sheath cells from rat follicles expressed the cell-surface markers CD44, CD73, and CD90 as bone marrow MSCs and resembled bone marrow MSCs in their ability to differentiate toward the adipogenic, osteogenic, and chondrogenic lineages[4,5]. In 2006, the International Society for Cellular Therapy issued the minimal criteria for characterizing human MSCs. Specifically, cultured MSCs should be adherent fibroblast-like cells, express the surface markers CD105, CD73, and CD90, and lack the expression of CD45, CD34, CD14 or CD11b, CD79α or CD19, and human leukocyte antigen-DR isotype. Furthermore, MSCs have osteogenic, adipogenic and chondrogenic differentiation potential in vitro[6]. Therefore, dermal papilla or sheath cells from rat follicles may be a type of MSCs. A later study extended these findings to the human system and confirmed that dermal papilla or sheath cells from human hair follicles expressed the MSC immunophenotype and possessed multi-lineage differentiation potential; therefore, they were named human hair follicle-derived MSCs (hHF-MSCs)[7]. Based on these previous studies, the hair follicle may be a readily accessible source of autologous human MSCs that can be used for tissue engineering and regenerative medicine.
Here, methods for isolating and expanding hHF-MSCs are presented and important recent advances in understanding the multi-potential of hHF-MSCs are summarized.
ISOLATION OF hHF-MSCs
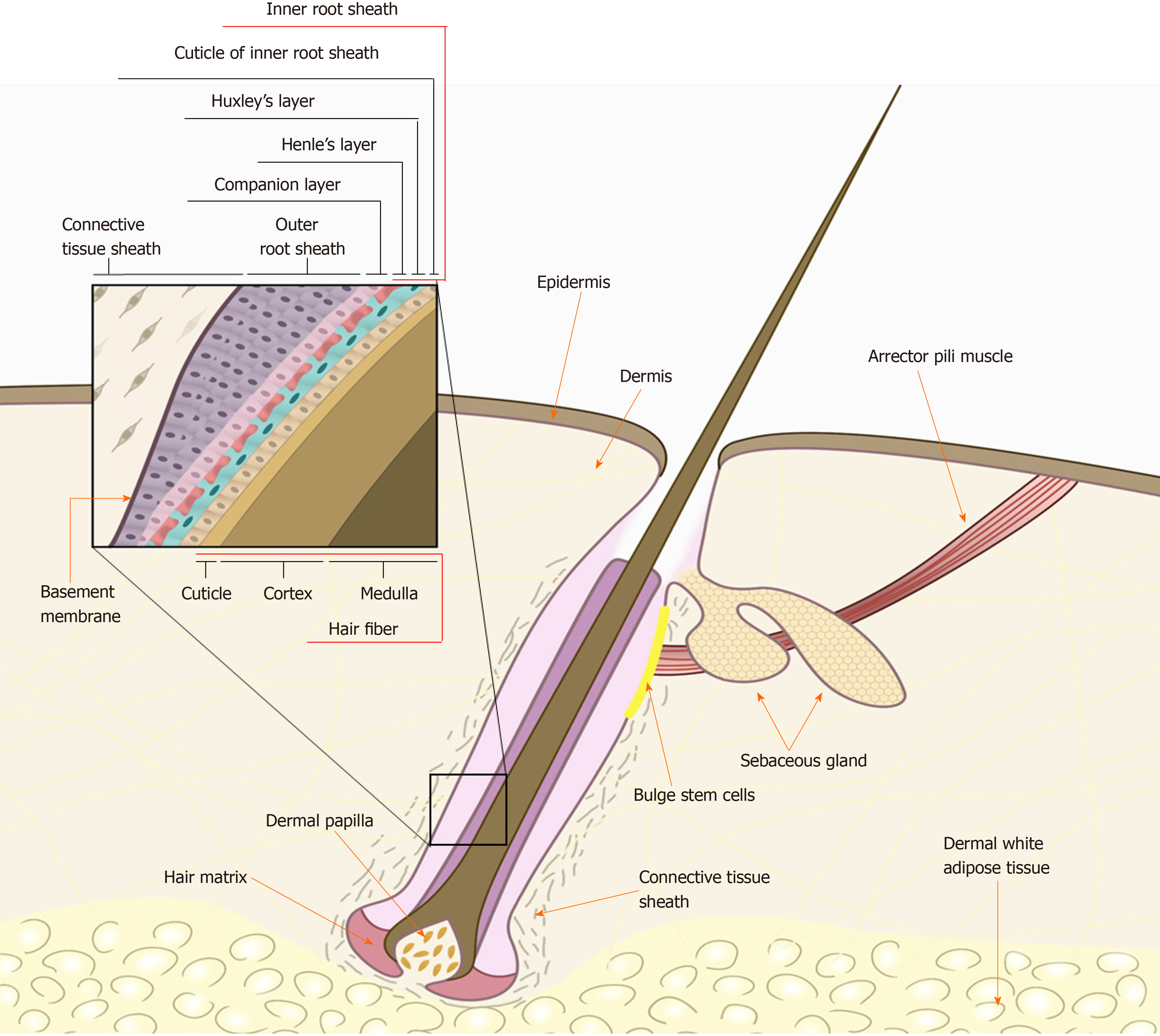
When isolating hHF-MSCs, the first step is to obtain a complete hair follicle. When obtaining human tissues, studies should be conducted in accordance with the guidelines of the Helsinki declaration and appropriate ethical approvals should be in place. A frequently used method is to use collagenase type I to separate the intact hair follicle from the human scalp skin[8]. An intact hair follicle usually includes inner root sheath, outer root sheath, and connective tissue sheath (dermal sheath) as shown in Figure 1. The human scalp skin is usually obtained by skin biopsy from the scalp of a donor under sterile conditions[7]. The obtained skin tissues are often full-thickness and therefore have an epidermis, dermis, and dermal white adipose tissue, as hair follicles are located in the adipose tissue. First, skin tissues are intensively rinsed with phosphate-buffered saline (PBS) containing 1% penicillin/streptomycin solution, trimmed to remove underlying adipose tissues, cut into 2-4-mm small pieces, and digested with 1 mg/mL collagenase type I at 37 °C with occasional agitation. After 4 h of enzymatic dissociation, the epidermis can be peeled off from the dermis, and single-hair follicles are released from the dermis, filtered through a 40-mm cell strainer, and washed thoroughly with PBS to prevent contaminating the epidermal or dermal cells.
Figure 1 Schematic of the human hair follicle.
An intact hair follicle usually includes the inner root sheath, outer root sheath, and connective tissue sheath (dermal sheath). The human hair follicle mesenchymal stem cells lie within the dermal papilla or dermal sheath (connective tissue sheath) of the hair follicle. Citation: Kiani MT, Higgins CA, Almquist BD. The Hair Follicle: An Underutilized Source of Cells and Materials for Regenerative Medicine. ACS Biomater Sci Eng 2018; 4: 1193-1207. Copyright© The Authors 2018. Published by American Chemical Society.
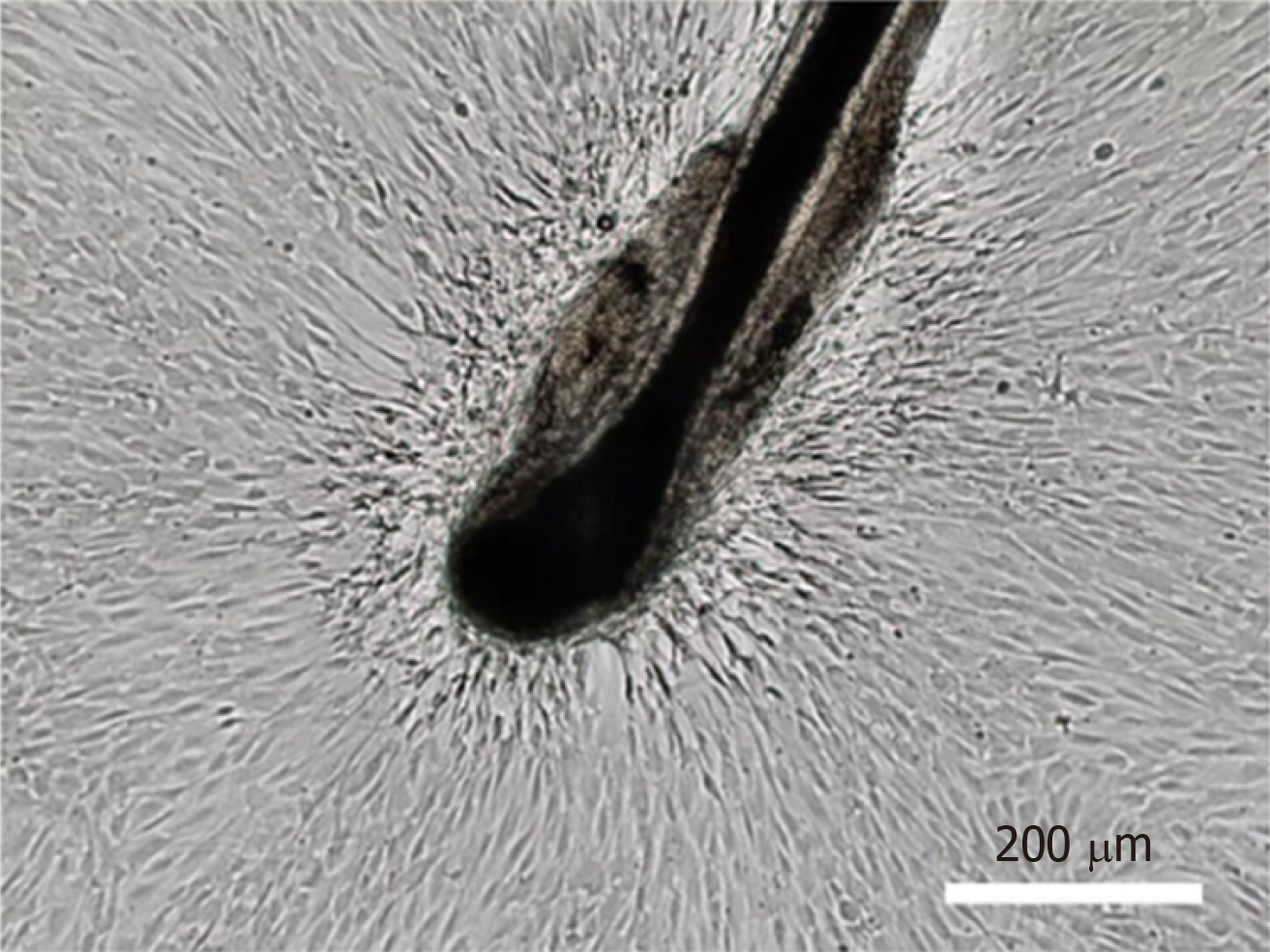
Another method is to obtain complete hair follicles by directly plucking them from the occipital region of the scalps, which eliminates invasive procedures associated with sampling[9,10]. Hairs with intact follicles should be extensively washed with PBS containing 1% penicillin/streptomycin solution. The hair shafts are cut off, and the remaining hair follicles are manually transferred to the bottom of a 96-well plate, with one follicle per well, and cultured in 100 µL of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 10 ng/mL basic fibroblast growth factor (bFGF) to allow for cell migration to the tissue culture plastic in a 37 °C/5% CO2 incubator. Cells originating from the bulge region can be visually identified as epidermal keratinocytes, whereas cells migrating out of the dermal sheath or papilla show the morphological appearance of mesenchymal cells (fibroblast-like cells) (Figure 2). The wells populated with cells migrating from the dermal sheath or papilla are digested, pooled, and expanded under the same culture conditions. It should be noted that the cells migrating from the dermal papilla may contain a small number of neural crest stem cell-like cells. They can form neurospheres under serum-free culture conditions containing N-2, B-27, bFGF, and epidermal growth factor (EGF)[11]. Li et al[12] found that the sphere-forming cells contained 1.14% ± 0.03% of dermal papilla cells. However, these neural crest stem cell-like cells need to be supplemented with ITS supplement and EGF when cultured in vitro[13]. Therefore, they will gradually disappear with the increase of passage times under the hHF-MSCs culture conditions.
Figure 2 Isolation of human hair follicle mesenchymal stem cells.
Human hair follicle mesenchymal stem cells migrating out of the dermal sheath or papilla show the morphological appearance of fibroblast-like cells. Bar: 200 μm. Citation: Jiang Y, Liu F, Zou F, Zhang Y, Wang B, Zhang Y, Lian A, Han X, Liu Z, Liu X, Jin M, Wang D, Li G, Liu J. PBX homeobox 1 enhances hair follicle mesenchymal stem cell proliferation and reprogramming through activation of the AKT/glycogen synthase kinase signaling pathway and suppression of apoptosis. Stem Cell Res Ther 2019; 10: 268. Copyright© The Authors 2019. Published by Springer Nature.
MSCs can also be separated from the dermal papilla or dermal sheath alone. Briefly, each strand of the hair follicle can be gently separated by microdissection away from the scalp tissues, ensuring that the dermal sheath and dermal papilla are intact. To isolate dermal sheath MSCs, the hair follicle is digested with 0.1% collagenase and 0.25% Dispase II at 37 °C for 30 min and then the dermal sheath is carefully separated from the main shaft of the hair follicle with a 30G needle under a dissecting microscope[14]. After chopping the dermal sheath with scalpel blades, the samples are treated with 0.1% trypsin/0.02 M EDTA for 30 min at 37 °C. DMEM supplemented with 10% FBS and 10 ng/mL bFGF is used to quench the trypsinization process. After collecting the cell suspension from the dish into a centrifuge tube, the cell suspension is centrifuged at 200 × g for 5 min, aspirated to remove the supernatant, resuspended in an appropriate volume of the same medium, and expanded as hHF-MSCs.
As the dermal papilla is engulfed within the hair matrix, an inversion technique can be used to separate dermal papilla MSCs (DP-MSCs)[4,15]. First, using a pair of scissors, the follicle through the matrix just above the papilla is transected to isolate the end bulb. Next, a fine needle can be used to invert the collagen capsule structure of the end bulb and expose the hair matrix and the dermal papilla residing inside. After removing the matrix component and any epithelial tissue still present from the papilla, the samples are cultured in DMEM supplemented with 20% FBS to allow for cell migration to the culture plate in a 37 °C/5% CO2 incubator. Once the cells have proliferated to confluency, they can be passaged using standard cell culture techniques and the culture medium can be changed to DMEM supplemented with 10% FBS and 10 ng/mL bFGF.
EXPANSION OF hHF-MSCs
Currently, conventional cell culture techniques are still used to expand hHF-MSCs[16]. Briefly, the isolated hHF-MSCs are seeded into a 100-mm cell culture dish and cultured in DMEM supplemented with 10% FBS and 10 ng/mL bFGF in a 37 °C/5% CO2 incubator. The medium (10 mL for 100-mm plate) should be refreshed every 2 d. Once the cells are approximately 90% confluent, they should be sub-cultured using 0.1% trypsin/0.02 M EDTA in a 100-mm cell culture dish.
For cell therapy and tissue engineering, a large number of hHF-MSCs with highly proliferative and multipotent differentiation potential are required. However, application of hHF-MSCs is restricted because they show replicative cell senescence and loss of multipotency in long-term in vitro culture[17,18]. Bajpai et al[19] found that hHF-MSCs could be maintained in culture for 11-12 passages (approximately 36 population doublings) before they started to show signs of cellular senescence. In addition to the 8-10 population doublings that occurred during the initial isolation and expansion stage, they may also undergo a total of 44-46 population doublings. It was estimated that a hair follicle could yield approximately 1015 hHF-MSCs before senescence occurred[19]. It is important to develop effective strategies for maintaining hHF-MSCs in a highly proliferative but undifferentiated state after repeated in vitro passages. bFGF is a well-known growth factor that plays a critical role in the self-renewal, high proliferation, and multi-lineage differentiation potential of MSCs[20-22]. Similarly, bFGF has been widely used in large-scale expansion of hHF-MSCs, particularly to prevent myogenic differentiation[7]. Other growth factors, such as acidic FGF and EGF, have also been tested and shown to play a similar role in the expansion culture of hHF-MSCs[23]. In recent years, some transcription factors have also been found to play a role in maintaining the proliferative capacity and multipotency of hHF-MSCs. Studies showed that ectopic expression of NANOG promotes cell proliferation and delays hHF-MSC senescence by upregulating PBX1 and activating AKT signaling[16,24]. Lu et al[25] also found that overexpression of OCT4 promoted the transcriptional activation of DNMTs, leading to elevated methylation of the p21 promoter, which promoted the proliferation and suppression of senescence of hHF-MSCs.
Moreover, compared to conventional 2D cell culture techniques, 3D-cultured MSCs show a higher yield in the same culture volume and stronger multipotency in large-scale generation of MSCs[26,27]. Stirred-tank bioreactors with suspending microcarriers are the most widely used approach for the 3D culture of MSCs on a large scale. Stirred-tank bioreactors can make full use of the cultivation space and homogenized culture conditions, and enable process control, such as maintenance of pH and dissolved oxygen and medium supplementation[28]. In addition, microcarriers offer a high surface area to volume ratio for the immobilization and expansion of adherent cells, and avoid the potential risks of tumorigenesis due to mutations caused by consecutive passaging[29,30]. Our team utilized macroporous CultiSpher-G microbeads as microcarriers for the 3D culture of hHF-MSCs in stirred-tank bioreactors. The results revealed that hHF-MSCs quickly adhered to the microspheres and showed a 26-fold increase in the cumulative cell number after 12 d of expansion, with no significant difference in differentiation potential compared to 2D culture[31].
In addition to microcarrier culture, 3D aggregate or spheroid culture without carrier and substrate provides enhanced cell–cell interactions and more accurately mimics the in vivo niche of MSC, which has been developed to expand MSCs[32]. Previous studies demonstrated that 3D aggregates of MSCs exhibited higher proliferation efficiency, increased stemness and differentiative capacity, enhanced anti-inflammatory and angiogenic properties, and increased survival of transplanted cells compared to conventional 2D cell culture techniques[33-35]. Recently, Topouzi et al[15] and Higgins et al[36] reported that 3D aggregates of DP-MSCs created by hanging drop cultures can restore the intact dermal papilla transcriptional signature and induce de novo hair follicles in non-hair-bearing human skin. However, few studies have evaluated 3D aggregates of hHF-MSCs, which remains a promising research direction.
DIFFERENTIATION OF hHF-MSCs
Similar to bone marrow MSCs, hHF-MSCs show adipogenic, osteogenic, and chondrogenic differentiation in the appropriate induction medium[23,37]. Apart from their trilineage differentiation potential, accumulating evidence has demonstrated the potential therapeutic value of these cells in regenerative medicine by differentiating into smooth muscle cells (SMCs) and cell types of multiple different lineages. Here, we describe the differentiation potential of hHF-MSCs in detail.
Myogenic differentiation of hHF-MSCs
SMCs play a critical role in the occurrence and development of prevalent cardiovascular and respiratory diseases, such as atherosclerosis[38] and asthma[39], because of their contractile dysfunction. Emerging tissue engineering techniques offer the possibility of reconstructing functional vessel walls by SMCs[40]. Andrique et al[41] used SMCs and endothelial cells to produce functional blood vessels with the correct configuration of lumen, which could also react to vasoconstrictor agents. hHF-MSCs have great potential to differentiate into SMCs. Using a tissue-specific promoter, a previous study showed that the smooth muscle alpha-actin promoter (P-αSMA) and fluorescence-activated cell sorting method can be used to isolate P-αSMA cells from hHF-MSCs[7]. P-αSMA cells expressed specific markers of SMCs, including αSMA, calponin, and smooth muscle myosin heavy chain, and generated strong contractility in response to vasoactive agonists[7]. The defining property of SMCs is their ability to generate contraction; thus, P-αSMA cells are considered as SMCs[7]. Although this technique can achieve separation of a pure population of SMCs, it has the potential risk of foreign virus integration into the chromosome. Xu et al[42] induced hHF-MSCs into contractile SMCs by stimulation with transforming growth factor‑β1 and platelet‑derived growth factor BB, which avoided the risk of effects from the lentiviral vector. In addition, Gao et al[43] constructed tissue-engineered blood vessels using filled acellular umbilical arteries with hHF-MSCs under the regulation of transforming growth factor‑β1, and the arterial grafts showed considerable vasoreactivity in response to humoral constrictors.
Application of hHF-MSCs in hair regeneration
hHF-MSCs are mainly located in the dermal papilla and dermal sheath of hair follicles and play an important role in regulating repeated hair follicle morphogenesis in adult life. A decrease in the number of hHF-MSCs per follicle can cause hair thinning and loss[44]. Recently, Gentile et al[45,46] used the medical device Rigeneracons to develop autologous micro-grafts enriched in hHF-MSCs to treat androgenetic alopecia. The micro-grafts were obtained by centrifugation of a 2-mm punch biopsy of the scalp with the selection of a cell population with a diameter of 50 µm. The mean hair density was increased significantly over baseline value after treatment with micro-grafts enriched of hHF-MSCs[45]. Hair follicle morphogenesis is induced by tightly coordinated epithelial–mesenchymal interactions in the developing embryo. Similarly, bioengineered hair follicles can be prepared by the self-organization of epithelial and mesenchymal cells[47]. In previous studies, murine hair follicle regeneration was achieved by intracutaneous transplantation of the bioengineered hair-follicle germ, which is generated by multicellular organization of follicle-derived epithelial stem cells and HF-MSCs in 3D stem cell culture. The bioengineered hair follicle exhibited similar tissue structures to the murine natural vibrissa follicle and grow pelage[48]. These results suggest that hHF-MSCs are an important source of seed cells for human hair tissue engineering.
Hematopoietic differentiation potential of hHF-MSCs
Shortage of red blood cells caused by a lack of voluntary donations can threaten the lives of patients who require transfusion. hHF-MSCs may alleviate this dilemma because of their ability to differentiate into blood cells. A previous study showed that dermal papilla and dermal sheath cells generate hematopoietic colonies in vitro, and can contribute to multi-lineage hematopoietic reconstitution in vivo after transplantation into lethally irradiated recipient mice[3]. Recently, Liu et al[49] induced mature erythrocytes from hHF-MSCs by overexpressing OCT4 and hematopoietic cytokine exposure. This mature erythrocyte contained no nuclei, and expressed mainly the adult β-globin chain and rarely the fetal γ-globin chain. Numerous studies have shown that red blood cells produced from induced pluripotent stem cells (iPSCs) are generally incompletely enucleated and rarely express the adult β-globin chain, although iPSCs have been widely used to investigate treatments for diseases of the blood system[50,51]. Therefore, hHF-MSCs may provide an alternative source of erythrocytes for potential autologous transfusion.
iPSCs from hHF-MSCs
iPSCs are a suitable seed cell source for regenerative medicine because their broad differentiation potential is similar to that of embryonic stem cells[52]. iPSCs can be induced from somatic cells by ectopic expression of defined transcription factors[53]. In a groundbreaking study, Wang et al[10] successfully reprogrammed hHF-MSCs into iPSCs by lentiviral transduction with Yamanaka factors (OCT4, SOX2, C-MYC, and KLF4). These HF-MSC-derived iPSCs (HF-iPSCs) showed similar characteristics to embryonic stem cells in colony morphology, expression of alkaline phosphatase, and expression of specific human embryonic stem cells (hESCs) surface markers and endogenous pluripotent genes; additionally, HF-iPSCs formed teratomas containing representatives of all three germ layers after intramuscular injection into immunocompromised mice[10]. HF-iPSCs have further expanded the application of hHF-MSCs in regenerative medicine. Bajpai et al[54] derived contractile SMCs using HF-iPSCs. Shi et al[55] reprogrammed HF-iPSCs into functional hepatocytes expressing hepatic markers and drug metabolism-related genes. However, reprogramming of hHF-MSCs into iPSCs by lentiviral transduction with Yamanaka factors, although highly reproducible, is an inefficient method; therefore, identifying important transcription factors that can improve the efficiency of programming has become a research focus. Recently, our team found that co-transduction of PBX1 and Yamanaka factors into hHF-MSCs significantly improved the reprogramming efficiency by activating the AKT/GSK3β signaling pathway[56]. These results contribute to mass production of HF-iPSCs and further support the potential of hHF-MSCs in regenerative medicine.
CONCLUSION
hHF-MSCs have high proliferation ability and broad differentiation potential, and can be easily accessed by direct plucking of human hairs, showing numerous advantages over other cell sources in various clinical applications. We have described the tremendous capacities of hHF-MSCs in obtaining induced SMCs and tissue-engineered blood vessels, regenerated hair follicle, and induced red blood cells, as well as produced iPSCs in this review. However, hHF-MSC multipotency remains relatively unexplored as compared to the epidermal stem cell lineage in hair follicles or other human MSCs. The capacity for differentiation into other cell lineages as well as epigenetic modification during differentiation require further analysis. In addition, the role of hHF-MSCs in cell therapy has been investigated. hHF-MSCs transduced with the human hepatocyte growth factor (hHGF) gene can continuously secrete transgenic hHGF to promote liver cell regeneration and alleviate hepatic fibrosis[57]. Engineered hHF-MSCs transduced with the release-controlled insulin gene can release human insulin in response to rapamycin and exhibited dramatic functionality in reversing hyperglycemia with no formation of detectable tumors after engraftment into mice[58]. In summary, hHF-MSCs are a promising source of cells for the rapidly emerging field of cell therapy and tissue engineering.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Labusca L S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Xing YX