Published online Jun 26, 2020. doi: 10.4252/wjsc.v12.i6.471
Peer-review started: May 1, 2020
First decision: May 15, 2020
Revised: May 17, 2020
Accepted: May 21, 2020
Article in press: May 21, 2020
Published online: June 26, 2020
Processing time: 54 Days and 13.5 Hours
Respiratory diseases, including coronavirus disease 2019 and chronic obstructive pulmonary disease (COPD), are leading causes of global fatality. There are no effective and curative treatments, but supportive care only. Cell therapy is a promising therapeutic strategy for refractory and unmanageable pulmonary illnesses, as proved by accumulating preclinical studies. Stem cells consist of totipotent, pluripotent, multipotent, and unipotent cells with the potential to differentiate into cell types requested for repair. Mesenchymal stromal cells, endothelial progenitor cells, peripheral blood stem cells, and lung progenitor cells have been applied to clinical trials. To date, the safety and feasibility of stem cell and extracellular vesicles administration have been confirmed by numerous phase I/II trials in patients with COPD, acute respiratory distress syndrome, bronchial dysplasia, idiopathic pulmonary fibrosis, pulmonary artery hypertension, and silicosis. Five routes and a series of doses have been tested for tolerance and advantages of different regimes. In this review, we systematically summarize the global trends for the cell therapy of common airway and lung diseases registered for clinical trials. The future directions for both new clinical trials and preclinical studies are discussed.
Core tip: Preclinical studies demonstrate significant improvement of lung disorders by stem cells and extracellular vesicles. Completed clinical trials show cell-based therapies are safe and tolerant for acute and chronic respiratory diseases. Current challenges for cell therapy of pulmonary illnesses are long-term safety, efficacy, and personal medicines.
- Citation: Ji HL, Liu C, Zhao RZ. Stem cell therapy for COVID-19 and other respiratory diseases: Global trends of clinical trials. World J Stem Cells 2020; 12(6): 471-480
- URL: https://www.wjgnet.com/1948-0210/full/v12/i6/471.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i6.471
Respiratory diseases are a top-ranked cause of death toll worldwide[1]. Acute and chronic lung diseases, including coronavirus disease 2019 (COVID-19)[2], acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), bronchopulmonary dysplasia (BPD), pulmonary arterial hypertension (PAH), silicosis, sarcoidosis, extensively drug-resistant tuberculosis, chronic obstructive pulmonary diseases (COPD)[3], and idiopathic pulmonary fibrosis (IPF), have high morbidity and mortality. Amidst the list, COPD is the third leading cause of global fatality. Rapidly accumulating evidence in preclinical models suggests that cell-based therapy may be a promising therapeutic strategy for lung injury repair[4]. For example, mesenchymal stromal stem cells (MSCs) derived from the umbilical cord blood, bone marrow, adipose, placenta, and other tissues are tested by registered clinical trials. Stem cells are able to repair injured airways and lungs by modulating multiple biological processes of the immune response, alveolar fluid clearance, cell fate, and drug delivery through paracrine and autocrine mechanisms, predominantly via extracellular vesicles (i.e., exosomes)[5-7]. However, the safety and benefits of cell therapy for the airway and lung diseases are under investigation by increasingly registered clinical trials. To analyze the trend of the clinical trials globally for the cytotherapy of pulmonary diseases, we summarized completed and ongoing trials registered to four databases from 1997 to 2020. This review will provide a brief state-of-the-science overview of the clinical studies of the respiratory diseases using various stem cells and extracellular vesicles.
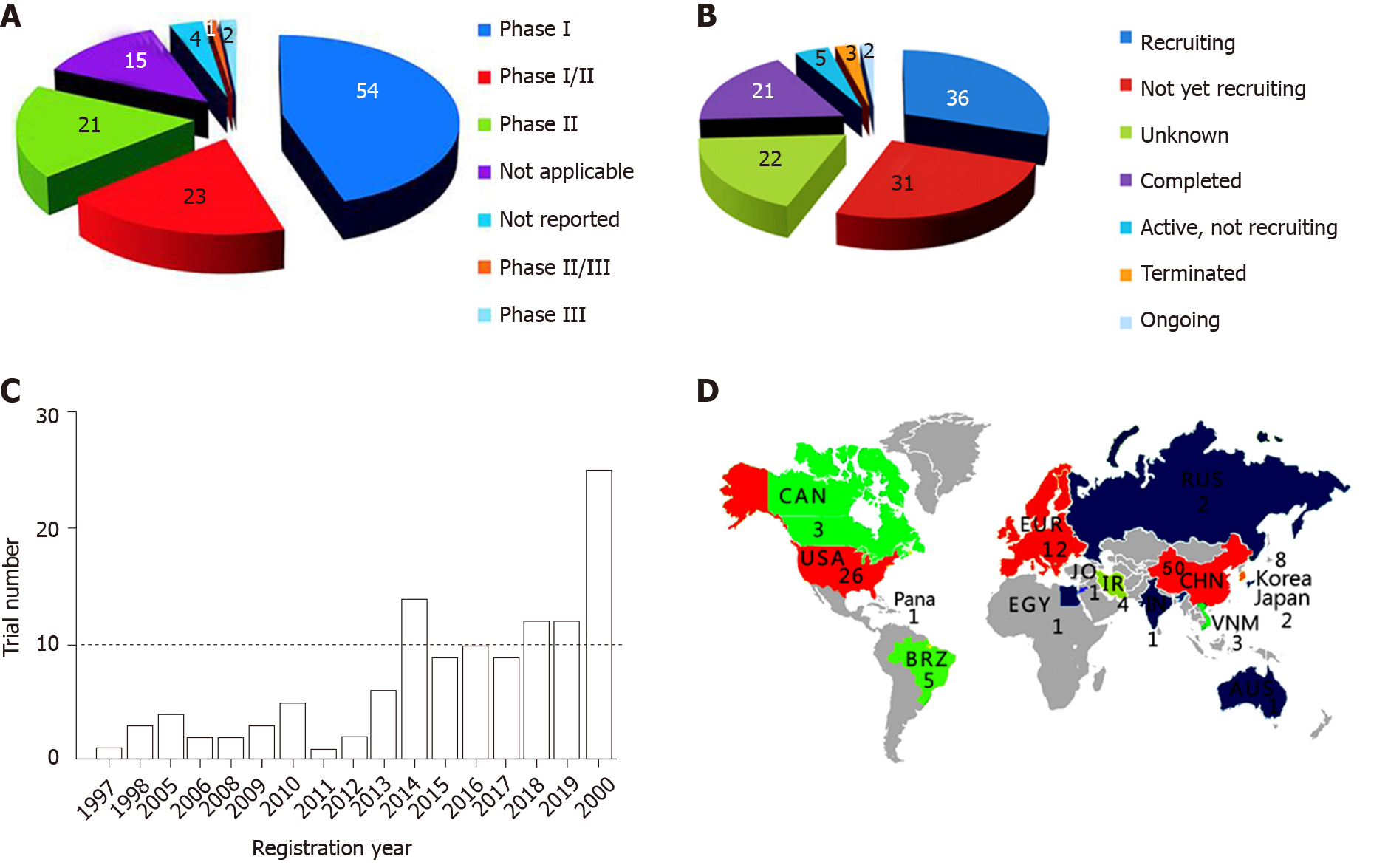
We searched four broadly recognized databases worldwide: (1) The Clinicaltrials.gov (CT, https://clinicaltrials.gov/); (2) The International Clinical Trials Registry Platform (ICTRP, http://apps.who.int/trialsearch/); (3) The European Union Clinical Trial Regulation (EUCTR, https://www.clinicaltrialsregister.eu/), and (4) The PubMed databases. The total hits were 329 trials composed of 96 (CT), 23 (ICTRP), 3 (EUCTR), and 21 (PubMed) in aforementioned four databases, respectively. Some trials are with a status of “withdrawn” or had a disease condition of “complications after transplantation of stem cells”. After combining the published and dual registered studies, there are 120 trials. The vast majority of these clinical trials (82%) are testing the safety of stem cells for feasibility, tolerance, and severe adverse events (54 trials for phase I, 23 for I/II, and 21 for II). Few trials (3%) are phase III (Figure 1A). The status of these trials shows (Figure 1B): “recruiting” (30%), “not yet recruiting” (26%), “unknown” (18%), “completed” (18%), and “others” (8%). The published trails (8 of 21) are listed in Table 1. The first trial was registered in 1997, and suddenly the number shoots up mainly due to COVID-19 (Figure 1C). Geologically, the trials are predominately registered by China (42%) and the USA (22%) (Figure 1D). Currently, the main purpose of the clinical trials is to test the safety of stem cell therapy except few moving to test effectiveness. A completed list and additional features of these clinical trials can be found in the Supplementary table.
| ID | Condition | Cell type | Case | Phase | Duration | Result |
| NCT00683722 | COPD | Progenitor cells | 62 | II | 2 yr | No infusional toxicities, deaths, and SAE |
| NCT01110252 | COPD/ Emphysema | BM-MSCs | 4 | NA | 1 yr | No SAE; significant improvement in the quality of life and clinical conditions |
| NCT01306513 | Emphysema | BM-MSCs | 10 | I | 8 wk | No SAE; increased CD31 expression |
| NCT01775774 | ARDS | BM-MSCs | 9 | I | 8 wk | No infusional toxicities, no SAE |
| NCT02097641 | ARDS | BM-MSCs | 60 | II | 8 wk | No SAE; improve MV and PEEP |
| NCT00257413 | PAH | EPCs | 31 | NA | 12 wk | No SAE; increased MWD, PAP, PVR, and cardiac output |
| NCT00469027 | PAH | EPCs | 7 | I | 1 yr | Well tolerated; increased MWD |
| NCT02181712 | BOS | MSC | 9 | I | 4 wk | No SAE |
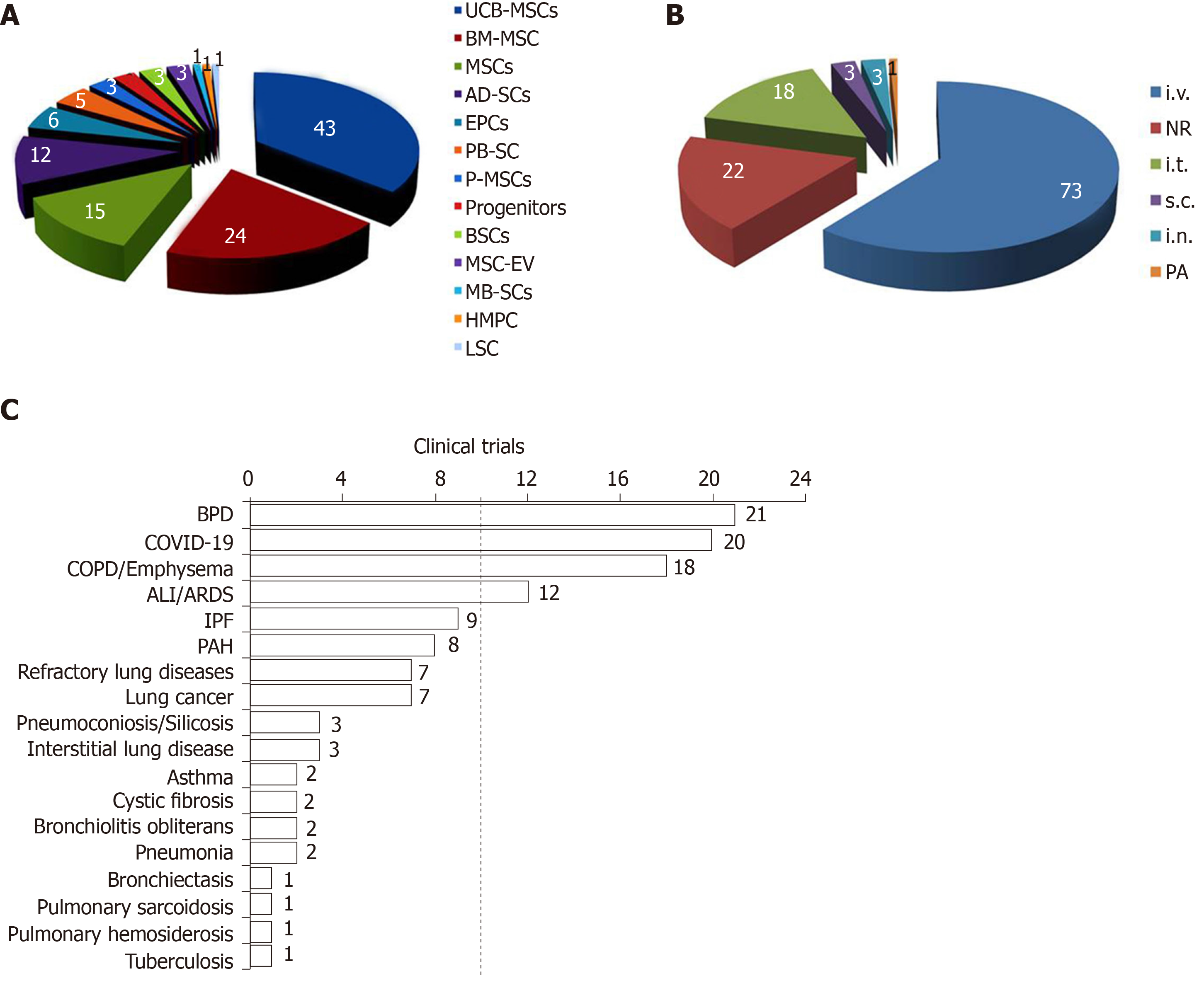
Stem cells include multipotent embryonic stem cells and progenitor cells. MSCs are used in most clinical trials, probably based on the fact of well-tolerated and free of serious adverse events[8]. Another consideration is availability. MSCs can be easily collected from the bone marrow, adipose tissue, muscle, peripheral blood, umbilical cord blood, and placenta[9]. Umbilical cord blood derived-MSCs (UCB-MSCs) were used in 43 trials, bone marrow derived-mesenchymal stromal cells (BM-MSCs) in 24 trials, mesenchymal stem cells in 15 trails, and adipose-derived stem cells (AD-SCs) for 12 trials (Figure 2A). Some trials are testing endothelial progenitor cells (EPCs), peripheral blood stem cells, placental mesenchymal stem cells, adult human stem cells, bronchi stem cells, menstrual blood-derived stem cells, bronchial basal cells, heart muscle progenitor cells, and lung stem cells. UCB-MSCs possess the highest proliferation rate, greatest anti-inflammatory ability, and lowest rate of senescence among all stem cells[10]. BM-MSCs and AD-SCs are the most popular autologous stem cells[11]. A combination of two or more types of stem cells is a standard regime for these clinical trials for lung diseases.
Extracellular vesicles (EVs) may serve as paracrine for MSCs to rescue damaged cells[12]. MSC-derived EVs replicate 70% of the beneficial effects of MSCs and carry a variety of bioactive factors, including signal molecules and growth factors, to recipient cells[13]. MSC-derived EVs are beneficial to the recovery of lung diseases in animal models[14]. Pre-clinical studies have demonstrated that MSC-derived EVs significantly reduce lung inflammation and restore the function of injured lungs. It could be partially attributable to the improvement in macrophage phagocytosis and bacterial killing[15-17]. Subsequently, the safety and efficacy of MSC-derived EVs are being tested for both BPD and COVID-19 pneumonia. It seems safer to deliver MSC-derived EVs rather than MSCs.
In general, the dosage of stem cells ranged from 1 × 106 to 1 × 109 cells/kg for a series of delivery, or a total dose of 2 × 106 to 1.2 × 109 cells. The dose of EVs is either 2.0 × 108 nanovesicles daily for 5 d or one dose of 20 pmol phospholipid/kg body weight. Stem cells and EVs were delivered via five routes: Intravenous perfusion for 73 trials, intratracheal administration for 18 trials, subcutaneous injection for 3 trials, intranasal instillation for 3 trials, and pulmonary artery injection for 1 trial (Figure 2B). Intravenous delivery (61% of analyzed trials) is a systemic route for cell therapy. Stem cells could be easily trapped in the pulmonary microcirculatory system and home to injured lobes[18]. In contrast, local delivery, including intratracheal, intranasal, and pulmonary artery administration is the second most used route. Local delivery possesses the advantages of prolonging the half-time of cells, increasing the utilization efficacy, and decreasing side effects to other organs (off-target effects). It could be better for local lung injury repair, particularly for epithelial injury. Systemic delivery of stem cells and EVs is applicable to systemic lung injury, including sepsis, multiple organ failure, or patients with severe pulmonary edema. A concern of local administration of stem cells and EVs is the distribution in both lungs. Based on the location of gastric acid aspiration injury and influenza animal models caused by the delivery of viruses intratracheally or intranasally, the impaired lobes are limited. In current trials, both local and systemic routes are used to BPD, pulmonary fibrosis, COPD, and silicosis. Local delivery of liposomal drugs and perfluorocarbon nanoparticles to the location of lung cancers is more effective than the systemic route[19,20]. Therefore, the types, routes, and dosages of stem cells should be justified based on the personal conditions (precision/individual medicine)[21].
Clinical trials registered are designed to test the safety and benefits of stem cells for BPD 21 (18%), COVID-19 20 (17%), COPD/Emphysema 18 (15%), ALI/ARDS 12 (10%), pulmonary fibrosis 9 (8%), PAH 8 (7%). Few trials are for lung cancer, pneumoconiosis, silicosis, asthma, cystic fibrosis (Figure 2C). There is a significant increase since 2014 (Figure 1C), particularly after the outbreak of COVID-19.
COVID-19 coronavirus started in Wuhan, China, in December 2019 and has been spreading rapidly worldwide[22]. There are no specific therapeutics yet for more than 1 million confirmed cases with 56-thousand deaths[23]. Twenty-four registered clinical trials are designed to investigate the therapeutic effects of MSCs on COVID-19 (Supplementary table). MSCs have anti-inflammatory, anti-apoptotic, antimicrobial, and anti-fibrotic properties[24,25]. COVID-19 has a higher risk of developing sepsis, multiple organ failure, including severe respiratory failure[26,27], MSCs are assumed to have a beneficial effect for COVID-19, as supported by the promising results of a pilot study[28]. A systemic review has recently summarized the ongoing trials regarding the cell therapy of COVID-19[29]. We will, thus, not duplicate here.
ALI is a common vital complication of systemic and pulmonary insults and developed as ARDS in the late stages[30,31]. The fatality of ARDS is approximately 30 to 40%, and even up to 49% in severe COVID-19 patients[27], brings a serious economic burden globally[32-34]. MSCs are promising, as shown in preclinical models of ARDS[33]. A phase I trial has demonstrated tolerance and short-term safety (up to 6 months) of MSCs for ARDS patients[35,36]. Further, a randomized phase IIa trial of ARDS treated by allogeneic mesenchymal stromal cells confirmed the safety of MSCs in 40 patients[36]. These two completed clinical trials were registered in the United States (NCT01775774 and NCT02097641) (Table 1). In addition, the safety of MSCs for ARDS is being examined in a new phase II trial for ARDS[37]. Besides MSCs, there are 12 clinical trials registered on the International Clinical Registration and five on the Chinese Clinical Trial Registrations for testing the MSCs from diverse resources. Although the benefits of cell therapy for ARDS are uncertain, the safety may not be a concern based on the results from completed trials.
COPD is characterized by tissue destruction, irreversible airflow limitation, caused by a combination of bronchitis and emphysema. COPD has high morbidity and mortality, which ranks the 3rd leading cause of deaths worldwide[38]. Common drugs for COPD are corticosteroids and bronchodilators[39]. MSCs are promising for COPD based on preclinical studies[40]. There are 18 clinical trials registered to evaluate cell therapy in COPD or emphysema totally (Figure 2). Of these, only 3 have been completed (Table 1), two phase I and one phase II demonstrated the safety of cell therapy for COPD[41-43]. Predominately, AD-MSCs and BM-MSCs are examined for COPD. Considering the limitations of small sample size and heterogeneity, a randomized, double-blind, placebo-controlled clinical trial is carried out in patients with COPD to follow up 2 years after MSCs infusion. Importantly, a phase I/II clinical study showed that four doses of UC-MSC treatment considerably alleviated the severity of symptoms of COPD[44]. Further studies are needed to confirm the effectiveness of MSCs, optimize the sources of MSCs, and select the best route to administer MSCs.
BPD is a chronic lung disease in premature infants, and usually causes various lifelong pulmonary complications (COPD and asthma)[45,46]. The current treatment strategies of the BPD are unsatisfactory. The safety and efficacy of MSCs for earlier preclinical and clinical studies have been evaluated for BPD[47,48]. Totally, 21 clinical trials are registered for cell therapy of BPD globally: China (8), Korea (7), United States (2), Spain (2), Canada (1), and Vietnam (1). Intratracheal infusion of allogeneic UCB-MSCs in preterm infants is safe and feasible[49]. Inflammatory markers and growth factors in tracheal aspirate samples decrease after MSC transplantation[50]. The same investigators have warranted a phase II clinical trial for intratracheal transplantation of UCB-MSCs to preterm infants with BPD (NCT01632475). The most of source of MSCs (90%) is UCB-MSCs in the 21 clinical trials, probably for UCB-MSCs are considered a better available source of MSCs than others[51]. Given the small sample size of these trials, the interpretations of the safety shall be cautious, and it may be too earlier to draw conclusions for the benefits of cell therapy for BPD.
PAH is a progressive chronic disorder with high mortality and increasing prevalence, characterized by the remodeling of the pulmonary arteries and increased pulmonary infiltrates[52]. Interventions with specific targets for PAH have been developed[53]; however, the fatality is not reduced. Animal studies show that cell therapy may be the most potent approach for PAH[54]. Therefore, 8 trials have been registered to date (Figure 2C). Amidst, 2 have been completed[55,56]. EPCs are used in 8 clinical trials. Intravenous administration of autologous EPCs with or without gene editing of endothelial NO-synthase (eNOS) seems to be feasible and safe[55,57]. A phase II trial of eNOS gene enhanced EPCs for PAH is ongoing (NCT03001414). Besides, the safety and effects of AD-MSCs on PAH is being tested. Due to the phase I/II trials are not a double-blind, placebo-controlled, the efficacy of EPCs for PAH is unknown. Taken together, the use of EPCs for formal clinical treatment requires a more rigorous review and experiment.
IPF is a chronic and irreversible interstitial lung disease characterized by diffuse alveolar inflammation and extracellular matrix remodeling[58]. There are no effective regimes yet, but the administration of MSCs is evaluated as a new therapy[59]. MSCs can prevent the progression of IPF in animal models[60]. In this way, there are 9 registered clinical trials based on the benefits of cell therapy in preclinical studies. In a completed trail, a single dose of 2, 10, or 20 × 107 cells/kg allogeneic BM-MSCs was intravenously delivered into 9 patients, whereas AD-MSCs were used. All three trails show the safety and well-tolerated of cell therapy and improved quality-of-life of IPF patients by MSCs[61-63]. Of note, a standardized protocol is available for clinicians[64]. Additional types of MSCs are tested in China, Australia, and Greece, including placental-derived MSCs and bronchial stem cells to compare the efficacy of them[64,65]. Given that no severe adverse effects were observed during a period of 6-month follow-up, the safety and efficacy of intravenous infusion of autologous lung spheroid stem cells are recruiting patients.
In addition to the aforementioned pulmonary diseases, clinical trials are registered for testing the safety and efficacy of stem cells for other refractory lung diseases, including lung cancer, silicosis, asthma, bronchiolitis obliterans, and tuberculosis (Figure 2C). Two clinical trials are recruiting bronchiolitis obliterans patients to evaluate the safety and feasibility of MSCs infusions. One phase I trial is evaluating the safety of allogeneic BM-MSCs (2-10 × 107 cells/kg, i.v.) for asthma. The safety of intranasal delivery of MSC-trophic factor for asthma (NCT02192736) has initiated too. Autologous BM-MCs is further tested for silicosis (NCT01239862) based on a previous study[66]. Radiation-induced lung injury is a new target of MSCs in the near future[67].
In conclusion, there is a rapid pace of clinical trials on stem cell therapy for lung diseases in the last 5 years. Because of the heterogeneity of pulmonary diseases, a broad spectrum of stem/progenitor cells has chosen by registered trials. Meanwhile, diverse routes for delivering and doses have applied based on both preclinical and clinical studies. It is a long-lasting debate if MSCs result in aggregating or clumping in the injured microcirculation and carry the risk of mutagenicity and oncogenicity. EVs could at least partially resolve these concerns. Mechanistically, the restoration of stem cell niches could be an innovative mechanism for cell therapy[68,69]. To date, most of these trials are at an early stage for evaluating safety, feasibility, tolerance, and potential efficacy. Few clinical studies have described clinical improvements. Therefore, further optimization for cell therapy on respiratory diseases needs to be explored by more phase III and IV clinical trials. Cell therapy has significant challenges for gene editing stem cells, optimized route and dose, intervention regimes and applications for individual case, nevertheless, cell-therapy offers a most innovative strategy for unmanageable respiratory diseases.
We are grateful of Yana Ma for her assistance in searching databases. The authors thank Dr. Michael A. Matthay (UCSF) for his kind comments.
| 1. | Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, Rock J, Snitow M, Krummel M, Stripp BR, Vu T, White ES, Whitsett JA, Morrisey EE. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 696] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 2. | Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, Pastore Y Piontti A, Mu K, Rossi L, Sun K, Viboud C, Xiong X, Yu H, Halloran ME, Longini IM, Vespignani A. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368:395-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2443] [Cited by in RCA: 1847] [Article Influence: 307.8] [Reference Citation Analysis (0)] |
| 3. | Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, Nair H, Gasevic D, Sridhar D, Campbell H, Chan KY, Sheikh A, Rudan I; Global Health Epidemiology Reference Group (GHERG). Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015;5:020415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 389] [Reference Citation Analysis (0)] |
| 4. | Weiss DJ, Chambers D, Giangreco A, Keating A, Kotton D, Lelkes PI, Wagner DE, Prockop DJ; ATS Subcommittee on Stem Cells and Cell Therapies. An official American Thoracic Society workshop report: stem cells and cell therapies in lung biology and diseases. Ann Am Thorac Soc. 2015;12:S79-S97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1196] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 7. | Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 661] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 8. | Zhao R, Su Z, Wu J, Ji HL. Serious adverse events of cell therapy for respiratory diseases: a systematic review and meta-analysis. Oncotarget. 2017;8:30511-30523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 10. | Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986-18001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 496] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 11. | Hoogduijn MJ, Dor FJ. Mesenchymal stem cells: are we ready for clinical application in transplantation and tissue regeneration? Front Immunol. 2013;4:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 439] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 13. | Ding Y, Zhao R, Zhao X, Matthay MA, Nie HG, Ji HL. ENaCs as Both Effectors and Regulators of MiRNAs in Lung Epithelial Development and Regeneration. Cell Physiol Biochem. 2017;44:1120-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Worthington EN, Hagood JS. Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int J Mol Sci. 2020;21:2318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1745] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 16. | Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 641] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 17. | Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 525] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 18. | Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 505] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 19. | Rocha FA, Saito CA, Silveira LC, de Souza JM, Ventura DF. Twelve chromatically opponent ganglion cell types in turtle retina. Vis Neurosci. 2008;25:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Wu L, Wen X, Wang X, Wang C, Sun X, Wang K, Zhang H, Williams T, Stacy AJ, Chen J, Schmieder AH, Lanza GM, Shen B. Local Intratracheal Delivery of Perfluorocarbon Nanoparticles to Lung Cancer Demonstrated with Magnetic Resonance Multimodal Imaging. Theranostics. 2018;8:563-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Dow SW, Schwarze J, Heath TD, Potter TA, Gelfand EW. Systemic and local interferon gamma gene delivery to the lungs for treatment of allergen-induced airway hyperresponsiveness in mice. Hum Gene Ther. 1999;10:1905-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A Novel Coronavirus Emerging in China - Key Questions for Impact Assessment. N Engl J Med. 2020;382:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 839] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 23. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3646] [Article Influence: 607.7] [Reference Citation Analysis (7)] |
| 24. | Silva LHA, Antunes MA, Dos Santos CC, Weiss DJ, Cruz FF, Rocco PRM. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res Ther. 2018;9:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 448] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 26. | Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 27. | Ji HL, Zhao R, Matalon S, Matthay MA. Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility. Physiol Rev. 2020;100:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 28. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 862] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 29. | Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev Rep. 2020;1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (2)] |
| 30. | Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 913] [Cited by in RCA: 1748] [Article Influence: 249.7] [Reference Citation Analysis (0)] |
| 31. | Joslyn LR, Pienaar E, DiFazio RM, Suliman S, Kagina BM, Flynn JL, Scriba TJ, Linderman JJ, Kirschner DE. Integrating Non-human Primate, Human, and Mathematical Studies to Determine the Influence of BCG Timing on H56 Vaccine Outcomes. Front Microbiol. 2018;9:1734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3976] [Cited by in RCA: 3903] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 33. | Laffey JG, Matthay MA. Fifty Years of Research in ARDS. Cell-based Therapy for Acute Respiratory Distress Syndrome. Biology and Potential Therapeutic Value. Am J Respir Crit Care Med. 2017;196:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 34. | Rubenfeld GD, Shankar-Hari M. Lessons From ARDS for Non-ARDS Research: Remembrance of Trials Past. JAMA. 2018;320:1863-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 36. | Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 473] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 37. | Antebi B, Mohammadipoor A, Batchinsky AI, Cancio LC. The promise of mesenchymal stem cell therapy for acute respiratory distress syndrome. J Trauma Acute Care Surg. 2018;84:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389:1931-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 719] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 39. | Weiss DJ. Cell-based Therapy for Chronic Obstructive Pulmonary Disease. Rebuilding the Lung. Ann Am Thorac Soc. 2018;15:S253-S259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Balkissoon R. Stem Cell Therapy for COPD: Where are we? Chronic Obstr Pulm Dis. 2018;5:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Ribeiro-Paes JT, Bilaqui A, Greco OT, Ruiz MA, Marcelino MY, Stessuk T, de Faria CA, Lago MR. Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int J Chron Obstruct Pulmon Dis. 2011;6:63-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 43. | Stolk J, Broekman W, Mauad T, Zwaginga JJ, Roelofs H, Fibbe WE, Oostendorp J, Bajema I, Versteegh MI, Taube C, Hiemstra PS. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM. 2016;109:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Le Thi Bich P, Nguyen Thi H, Dang Ngo Chau H, Phan Van T, Do Q, Dong Khac H, Le Van D, Nguyen Huy L, Mai Cong K, Ta Ba T, Do Minh T, Vu Bich N, Truong Chau N, Van Pham P. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res Ther. 2020;11:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Davidson LM, Berkelhamer SK. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J Clin Med. 2017;6:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 290] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 46. | Namba F. Mesenchymal stem cells for the prevention of bronchopulmonary dysplasia. Pediatr Int. 2019;61:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Simones AA, Beisang DJ, Panoskaltsis-Mortari A, Roberts KD. Mesenchymal stem cells in the pathogenesis and treatment of bronchopulmonary dysplasia: a clinical review. Pediatr Res. 2018;83:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Pierro M, Thébaud B, Soll R. Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;11:CD011932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Ahn SY, Chang YS, Kim JH, Sung SI, Park WS. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J Pediatr. 2017;185:49-54.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 50. | Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, Park WS. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014;164:966-972.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 324] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 51. | Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 397] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 52. | Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 694] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 53. | Humbert M, Ghofrani HA. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax. 2016;71:73-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 54. | Fukumitsu M, Suzuki K. Mesenchymal stem/stromal cell therapy for pulmonary arterial hypertension: Comprehensive review of preclinical studies. J Cardiol. 2019;74:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Zhu JH, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 224] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 56. | Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, Chen JZ. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant. 2008;12:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Granton J, Langleben D, Kutryk MB, Camack N, Galipeau J, Courtman DW, Stewart DJ. Endothelial NO-Synthase Gene-Enhanced Progenitor Cell Therapy for Pulmonary Arterial Hypertension: The PHACeT Trial. Circ Res. 2015;117:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 58. | Lederer DJ, Martinez FJ. Idiopathic Pulmonary Fibrosis. N Engl J Med. 2018;378:1811-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 1366] [Article Influence: 170.8] [Reference Citation Analysis (4)] |
| 59. | Antoniou KM, Karagiannis K, Tsitoura E, Tzanakis N. Mesenchymal stem cell treatment for IPF-time for phase 2 trials? Lancet Respir Med. 2017;5:472-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Srour N, Thébaud B. Mesenchymal Stromal Cells in Animal Bleomycin Pulmonary Fibrosis Models: A Systematic Review. Stem Cells Transl Med. 2015;4:1500-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 61. | Glassberg MK, Minkiewicz J, Toonkel RL, Simonet ES, Rubio GA, DiFede D, Shafazand S, Khan A, Pujol MV, LaRussa VF, Lancaster LH, Rosen GD, Fishman J, Mageto YN, Mendizabal A, Hare JM. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest. 2017;151:971-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 62. | Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A, Zissimopoulos A, Boussios N, Dardzinski B, Gritzalis D, Antoniadis A, Froudarakis M, Kolios G, Bouros D. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med. 2013;11:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 63. | Ntolios P, Manoloudi E, Tzouvelekis A, Bouros E, Steiropoulos P, Anevlavis S, Bouros D, Froudarakis ME. Longitudinal outcomes of patients enrolled in a phase Ib clinical trial of the adipose-derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Clin Respir J. 2018;12:2084-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 64. | Chuang HM, Shih TE, Lu KY, Tsai SF, Harn HJ, Ho LI. Mesenchymal Stem Cell Therapy of Pulmonary Fibrosis: Improvement with Target Combination. Cell Transplant. 2018;963689718787501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Chambers DC, Enever D, Ilic N, Sparks L, Whitelaw K, Ayres J, Yerkovich ST, Khalil D, Atkinson KM, Hopkins PM. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 66. | Morales MM, Souza SA, Loivos LP, Lima MA, Szklo A, Vairo L, Brunswick TH, Gutfilen B, Lopes-Pacheco M, Araújo AJ, Cardoso AP, Goldenberg RC, Rocco PR, Fonseca LM, Lapa e Silva JR. Pilot safety study of intrabronchial instillation of bone marrow-derived mononuclear cells in patients with silicosis. BMC Pulm Med. 2015;15:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Xu S, Liu C, Ji HL. Concise Review: Therapeutic Potential of the Mesenchymal Stem Cell Derived Secretome and Extracellular Vesicles for Radiation-Induced Lung Injury: Progress and Hypotheses. Stem Cells Transl Med. 2019;8:344-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 68. | Savukinas UB, Enes SR, Sjöland AA, Westergren-Thorsson G. Concise Review: The Bystander Effect: Mesenchymal Stem Cell-Mediated Lung Repair. Stem Cells. 2016;34:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Gibran A, Zhao R, Zhang M, Jain KG, Chang J, Komatsu S, Fang X, Zhou B, Liang J, Jiang D, Ikebe M, Matthay MA, Ji HL. Fibrinolytic niche is requested for alveolar type 2 cell-mediated alveologenesis and injury repair. bioRxiv. 2020; Available from: https://www.biorxiv.org/content/10.1101/2020.03.24.006270v1. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Specialty type: Cell and tissue engineering
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohammadi M S-Editor: Wang JL L-Editor: A E-Editor: Ma YJ