Published online Oct 26, 2020. doi: 10.4252/wjsc.v12.i10.1196
Peer-review started: May 20, 2020
First decision: June 5, 2019
Revised: June 13, 2020
Accepted: August 16, 2020
Article in press: August 16, 2020
Published online: October 26, 2020
Processing time: 159 Days and 1.2 Hours
Nerve diseases and injuries, which are usually accompanied by motor or sensory dysfunction and disorder, impose a heavy burden upon patients and greatly reduce their quality of life. Dental pulp stem cells (DPSCs), derived from the neural crest, have many characteristics that are similar to those of neural cells, indicating that they can be an ideal source for neural repair.
To explore the potential roles and molecular mechanisms of DPSCs in crushed nerve recovery.
DPSCs were isolated, cultured, and identified by multilineage differentiation and flow cytometry. Western blot and immunofluorescent staining were applied to analyze the expression levels of neurotrophic proteins in DPSCs after neural induction. Then, we collected the secretions of DPSCs. We analyzed their effects on RSC96 cell proliferation and migration by CCK8 and transwell assays. Finally, we generated a sciatic nerve crush injury model in vivo and used the sciatic function index, walking track analysis, muscle weight, and hematoxylin & eosin (H&E) staining to further evaluate the nerve repair ability of DPSCs.
DPSCs highly expressed several specific neural markers, including GFAP, S100, Nestin, P75, and NF200, and were inclined toward neural differentiation. Furthermore, neural-induced DPSCs (N-DPSCs) could express neurotrophic factors, including NGF, BDNF, and GDNF. The secretions of N-DPSCs could enhance the proliferation and migration of Schwann cells. In vivo, both DPSC and N-DPSC implants alleviated gastrocnemius muscle atrophy. However, in terms of anatomy and motor function, as shown by H&E staining, immunofluorescent staining, and walking track analyses, the repair effects of N-DPSCs were more sustained, potent, and effective than those of DPSCs and the controls.
In summary, this study demonstrated that DPSCs are inclined to differentiate into neural cells. N-DPSCs express neurotrophic proteins that could enhance the proliferation and migration of SCs. Furthermore, our results suggested that N-DPSCs could help crushed nerves with functional recovery and anatomical repair in vivo. Thus, DPSCs or N-DPSCs could be a promising therapeutic cell source for peripheral nerve repair and regeneration.
Core Tip: Dental pulp stem cells (DPSCs) might be an ideal cell source for nerve repair and regeneration for having an inclination to neural differentiation, expressing neurotrophic proteins after induction, and enhancing stem cell proliferation after neural induction. Furthermore, neural induced-DPSCs provided an effective and long-term treatment for crushed nerve with functional recovery and anatomical repair. Thus, DPSCs could be a promising therapeutic cell source for peripheral nerve repair and regeneration. Collectively, our study provided a novel theoretical basis and a promising cell-related therapeutic strategy for peripheral nerve repair and regeneration.
- Citation: Wang DR, Wang YH, Pan J, Tian WD. Neurotrophic effects of dental pulp stem cells in repair of peripheral nerve after crush injury. World J Stem Cells 2020; 12(10): 1196-1213
- URL: https://www.wjgnet.com/1948-0210/full/v12/i10/1196.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i10.1196
Inferior alveolar nerve (IAN) and lingual nerve (LN) injuries are complications of mandibular third molar extraction, which usually lead to numbness of the lips and taste disorder and eventually impose an extremely large burden on the patient’s life[1]. The IAN and LN can be stretched or crushed during surgical operation[2], ultimately leading to functional disorder. The incidence of LN injury after third molar surgery varies from 0.4% to 1.5%, while the incidence of IAN injury involvement is approximately 1% to 5%[1]. The repair time following IAN and LN injury is a controversial topic and can range from 2 wk to 2 years or more[2]. Currently, there are no effective or satisfactory treatments that can help crushed IANs/LNs completely recover. Traditional treatments, including drugs, end-to-end sutures, or autologous nerve grafting, usually cannot meet the requirements of recovery[3,4]. A brand-new and effective treatment is urgently needed.
Currently, as advancements and achievements in mesenchymal stem cell (MSC) research are developing rapidly, the use of MSCs to alleviate peripheral nerve injury has drawn researchers’ attention. Studies have reported that both adipose tissue-derived stem cells and bone marrow-derived stem cells are able to differentiate into neural-like cells and finally demonstrate a great inclination to nerve repair and regeneration in vivo[5-7].
Among all kinds of MSCs, dental pulp stem cells (DPSCs), derived from the neural crest, have been shown to have several similarities to neural cells. DPSCs, isolated from dental pulp tissue, were first discovered by Gronthos et al[8] in 2000. DPSCs are positive for STRO-1, CD146, CD73, and CD90. Similar to other MSCs, DPSCs are capable of multilineage differentiation, including osteogenesis, chondrogenesis, adipogenesis, and myogenic lineages[9-15]. Regarding neural differentiation, DPSCs have a higher positive ratio for neural markers such as Nestin, GFAP, and s100-beta than other kinds of MSCs[16]. Moreover, some reports have shown that DPSCs can secrete neurotrophic factors that can help injured nerve recovery[17,18]. All these traits make DPSCs an ideal source for nerve repair and regeneration.
Research on the use of DPSCs in neural disease treatments has developed rapidly and demonstrated great achievements sparked by numerous reports of applications of DPSCs in nerve tissue repair and regeneration. Wang et al[19] found that DPSCs could promote neural repair in an Alzheimer's disease model by secreting growth factors, promoting neural stem cell proliferation, increasing cell viability, and reducing cell apoptosis. Moreover, DPSCs can help locomotor functional recovery in spinal cord injury rats by inhibiting cell apoptosis, inducing axon outgrowth, and differentiating into and replacing neural cells[20]. Regarding peripheral nerve repair, studies have reported that DPSCs can promote peripheral nerve regrowth and remyelination after injury. Furthermore, DPSCs, combined with collagen scaffolds, silicone tubes, or polylactic glycolic acid tubes, could help axonal outgrowth and myelination and promote recovery of injured facial nerves[7,21-23]. All such evidence indicates that DPSCs will become a promising stem cell source for neural disease treatments in the near future.
Based on previous studies, we hypothesized that DPSCs themselves have the ability to heal crushed nerves. In this study, we investigated the neural differentiation potential of DPSCs, compared the neural repair ability between DPSCs and neural-induced DPSCs (N-DPSCs), and further evaluated their neurotrophic effects. In vivo, we observed the therapeutic effects of DPSCs and N-DPSCs in a sciatic nerve crush injury model. Collectively, our study will provide a novel theoretical basis and a promising cell-related therapeutic strategy for peripheral nerve repair and regeneration.
All experiments involving humans followed the guidelines of the Ethics Committee of West China College of Stomatology. Human DPSCs were obtained and isolated from the extracted third molars or orthodontic teeth with no caries from 15- to 25-year-old healthy individuals at the Department of Oral and Maxillofacial Surgery, West China Hospital of Stomatology Sichuan University. Collected teeth were soaked in antibiotics with phosphate-buffered saline (PBS) after tooth extraction. Dental pulp tissues were aseptically dissected and placed into PBS with antibiotics and then cut into small tissue pieces. The pulp tissues were digested with collagenase type 1 (Gibco, United States) at 37 °C for 30 min and shaken to homogenize the mixture every 10 min. Then, the digestion was ended with 10% α-MEM (HyClone, United States) supplemented with 10% fetal bovine serum (FBS, Gibco, United States), 1% penicillin/streptomycin (HyClone), and 1 × glutamine. After neutralization and centrifugation, the supernatant was removed, and the sediment was resuspended in10% α-MEM medium. Then, the tissues were placed into T-25 cell culture flasks and incubated in a standard culture environment (37 °C, 5% CO2). The cell culture medium was changed every 2 d, and cells from passages 3 to 5 were used for experiments. Rat cell line RSC96 was purchased from CAS (Chinese Academy of Sciences, China) and cultured in DMEM (HyClone/2% FBS (2% DMEM).
The multi-differentiation potential of DPSCs was assessed by osteogenic and neurogenic differentiation. For osteogenic differentiation, DPSCs were seeded in a 6-well culture plate for osteogenic induction at a density of 1 × 105 cells/well. When reaching 70% confluence, the culture medium was removed and replaced with 2 mL of osteogenic inducing medium [100 nmol/L dexamethasone (Sigma, United States), 50 mg/mL ascorbic acid (Sigma), 10% FBS, 5 mmol/L l-glycerophosphate (Sigma)]. The inducing medium was changed every 3 d. After 2 wk of differentiation, cells were fixed with 4% paraformaldehyde for 30 min and stained with alizarin red S.
For neurogenic differentiation, DPSCs were seeded in 6-well culture plates for neural induction at a density of 105 cells/well. After adherence, the culture medium was changed to neural inducing medium, which consisted of Neurobasal A Media (Gibco), 1 × B27 supplement (Gibco), 20 ng/mL epidermal growth factor (EGF, Peprotech, United States), and 40 ng/mL basic fibroblast growth factor (FGF, Peprotech)[12]. After 2 wk of differentiation, cells were fixed with 4% paraformaldehyde for 30 min, and qPCR was used to analyze the extent of neural differentiation.
Cells were digested with trypsin, and 1 × 106 cells were collected. After resuspending cells in PBS, the cells were divided into aliquots. The following conjugated antibodies (BD Biosciences) were used at the concentrations recommended by the manufacturer: CD73-PE, CD90-FITC, CD106-PE, CD146-PE, CD31-FITC, and CD34-FITC. After two washes in PBS, the cells were resuspended and analyzed using the C6 system. Blue represented the control, and red represented the indicated markers.
For further in vivo research, DPSCs were seeded in 6-well culture plates for neural induction at a density of 105 cells/well. After adherence, the culture medium was changed to neural inducing medium. The neural inducing medium was changed every 3 d, and cells were cultured for 2 wk for further use.
Immunofluorescent staining was performed to compare the neurotrophic effects between DPSCs and N-DPSCs. DPSCs and N-DPSCs were seeded in glass bottom cell culture dishes at a density of 4000 cells per dish. After adherence, the cells were fixed in 4% paraformaldehyde for 30 min. The cells were permeabilized with 0.3% Triton-100 for 15 min at room temperature. Then, the cells were rinsed 3 times with PBS and blocked with 1% BSA for 30 min. After that, the cells were incubated with primary antibodies overnight at 4 °C or for 2 h at 37 °C. Nuclei were stained with 100 ng/mL DAPI for 2 min.
Primary antibodies used were Nestin (mouse IgG, 1:200, Abcam, United Kingdom), GFAP (rabbit IgG, 1:200, Abcam), S100 (rabbit IgG, 1:200, Abcam), NF200 (mouse IgG, 1:200, Abcam), P75 (rabbit IgG, 1:200, Millipore, United States), brain-derived neurotrophic factor (BDNF, rabbit IgG, 1:200, ZENBIO, China), glial cell line-derived neurotrophic factor (GDNF, rabbit IgG, 1:200, ZENBIO), and nerve growth factor (NGF, rabbit IgG, 1:200, ZENBIO). The secondary antibodies used were Alexa FluoR 488 goat anti-mouse IgG (1:200, Invitrogen, United States) and Alexa FluoR 488 goat anti-rabbit IgG (1:200, Invitrogen).
The protein levels of BDNF, GDNF, and NGF in both DPSCs and N-DPSCs were analyzed by Western blot assay, with GAPDH as an internal control. DPSCs and N-DPSCs were cultured in 10% α-MEM medium and neural differentiation medium, respectively, for 2 wk in 6-well culture plates. Protein extraction was conducted using a total protein extraction kit (KEYGEN). Protein samples were treated with loading buffer, incubated at 98 °C for 10 min, added to 10% SDS-PAGE gels, and then transferred onto PVDF membranes. The membranes were blocked with 5% milk at room temperature for 1 h and incubated with the following primary antibodies at 4 °C overnight: BDNF (rabbit IgG, 1:1000, ZENBIO), GDNF (rabbit IgG, 1:1000, ZENBIO), and NGF (rabbit IgG, 1:1000, ZENBIO). Then, the secondary antibodies were added for another hour at room temperature before exposure.
To investigate whether DPSCs or N-DPSCs could improve the repair of injured peripheral nerves, we further analyzed the effects of conditioned media on RSC96 cells. After 2 wk of culture of DPSCs and N-DPSCs, the culture medium was changed to α-MEM without FBS. Two days later, the supernatants were collected for further research and named DPSCs-CM and N-DSPCS-CM. RSC96 cells were seeded on 96-well plates (2500 cells/well) and cultured with DMEM medium + 2% FBS (control), DPSCs-CM, and N-DSPCS-CM for the indicated time lengths. A Cell Counting Kit-8 (Sigma) was applied. The OD values were measured at 450 nm with a microplate reader (Thermo Scientific) every day for a week.
Transwell migration assays were performed according to the manufacturer’s instructions (Corning). RSC96 cells (4 × 104) were plated in the upper chamber. DMEM medium + 2% FBS (control), DPSCs-CM, and N-DSPCS-CM were separately added to the lower chamber. Cells were incubated for 24 h. Then, the cells in the upper chamber were cleared with cotton applicators. Cells that migrated across the membrane were then fixed with 4% paraformaldehyde for 5 min. Crystal violet was used to stain the cells. Pictures were taken with an Olympus IX71. Quantification was performed with ImageJ.
A total of 32 adult male SD rats (200-230 g) were used in this experiment. The rats were divided into four groups: Normal (rats without nerve crush treatment, n = 8), control (rats had nerve crush injury but without cure treatments, n = 8), DPSC (rats had nerve crush injury and were treated with DPSC cell sheet, n = 8), and N-DPSC (rats had nerve crush injury and were treated with N-DPSC cell sheet, n = 8). Prior to the start of the in vivo experiments, the rats were raised for 5 d to adapt to the laboratory environment. The rats were then anesthetized with pentobarbital. After incision of the skin and separation of the gluteal musculature, the right sciatic nerve was exposed. A vascular clip was placed on the nerve 6 mm from the sciatic notch, subjecting it to a 30 g crushing force for 2 min. The control group received no treatment after crushing. The DPSC and N-DPSC groups were implanted with DPSC cell sheets and N-DPSC cell sheets, respectively. Thirty days after treatment application, the rats were sacrificed. The crushed nerves and gastrocnemius muscles were harvested for assessment.
After undergoing the nerve crush procedure, the rats were tested on a limited walkway with a dark box at the end. The feet of the rats were soaked in red ink, after which the rats walked on a piece of white paper placed on the walkway to acquire the rats’ footprints. Three measurements were obtained in this experiment: PL (distance from the heel to the third toe), TS (distance from the first to the fifth toe), and IT (distance from the second to the fourth toe). The SFI (sciatic function index) was calculated by the following formula: SFI = -38.3 × (EPL - NPL)/NPL + 109.5 × (ETS - NTS)/NTS + 13.3 × (EIT - NIT)/NIT - 8.8. (N: Normal side; E: Experimental side). SFI values closer to -100 corresponded to increasingly worse impairment, whereas SFI values closer to 0 indicated increasingly normal function.
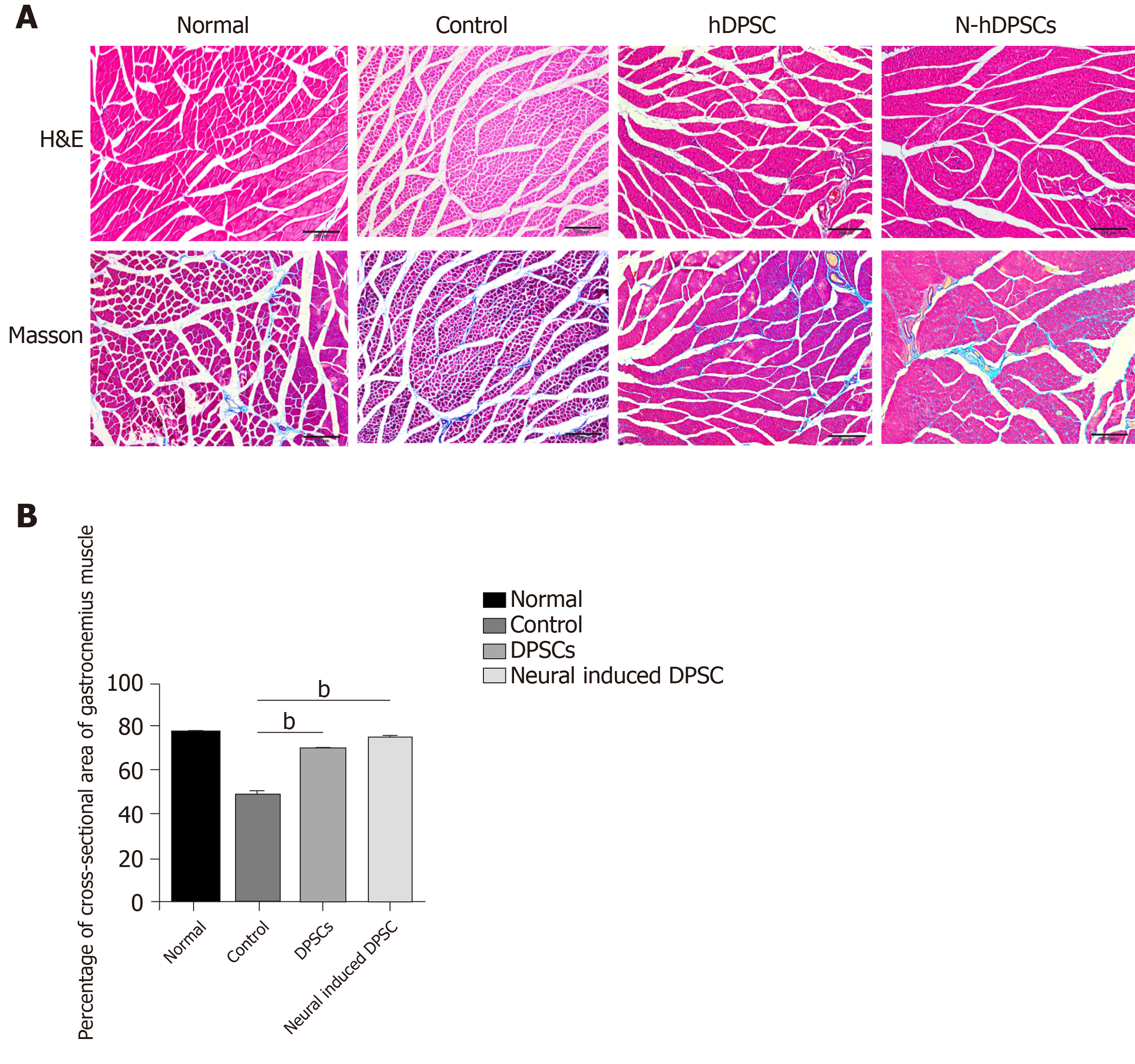
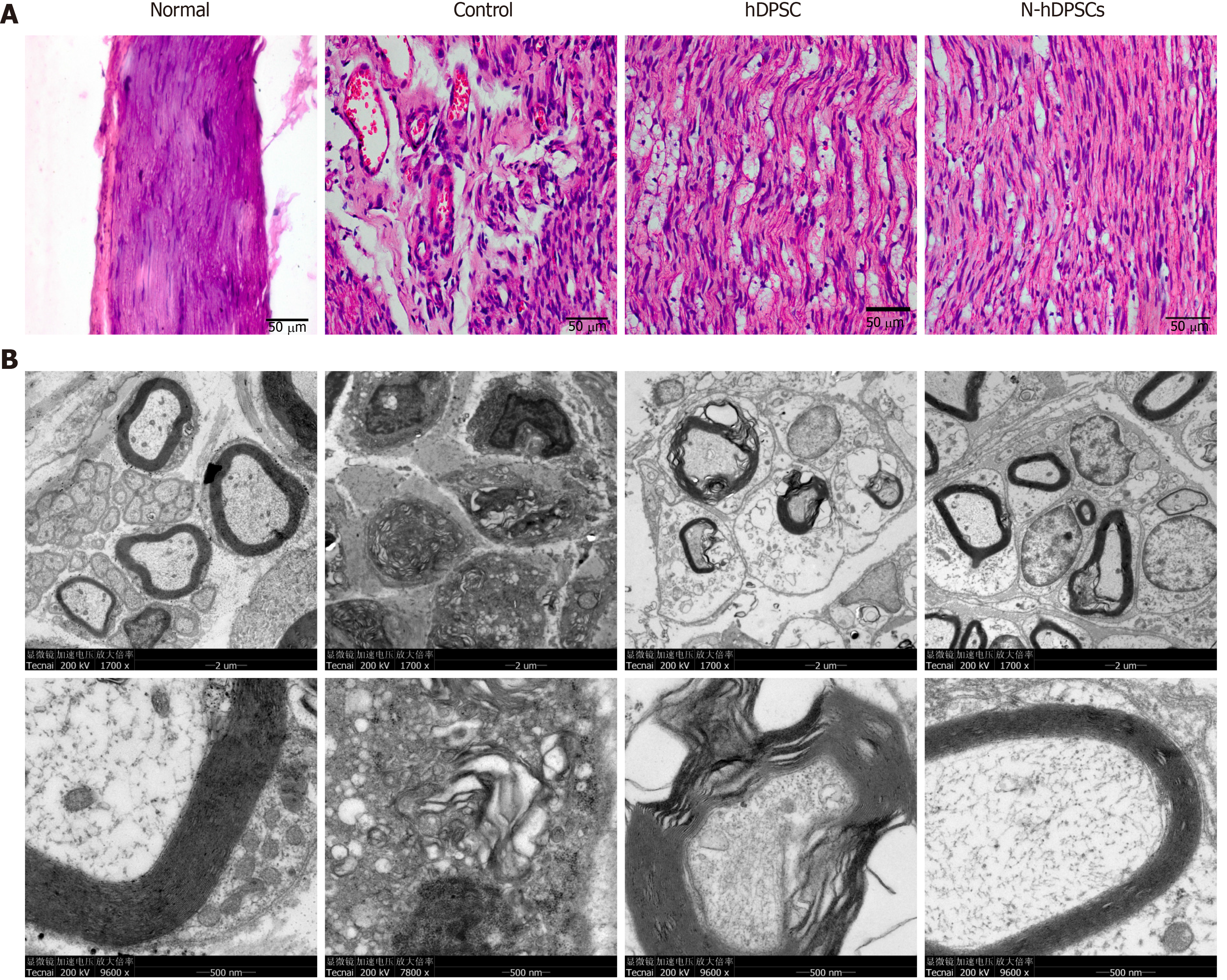
Bilateral gastrocnemius muscles were harvested, and their wet weights were measured. The wet weight ratio (%) was calculated as the wet weight of the injury side/the weight of the normal side × 100%. Muscle morphology was observed by hematoxylin & eosin (H&E) staining and Masson staining. The atrophy ratio of the muscle was calculated as (gastrocnemius muscle fiber area/total image area × 100%). The harvested nerve samples were stained with H&E. Recovery of the myelin sheath was assessed by transmission electron microscopy (TEM).
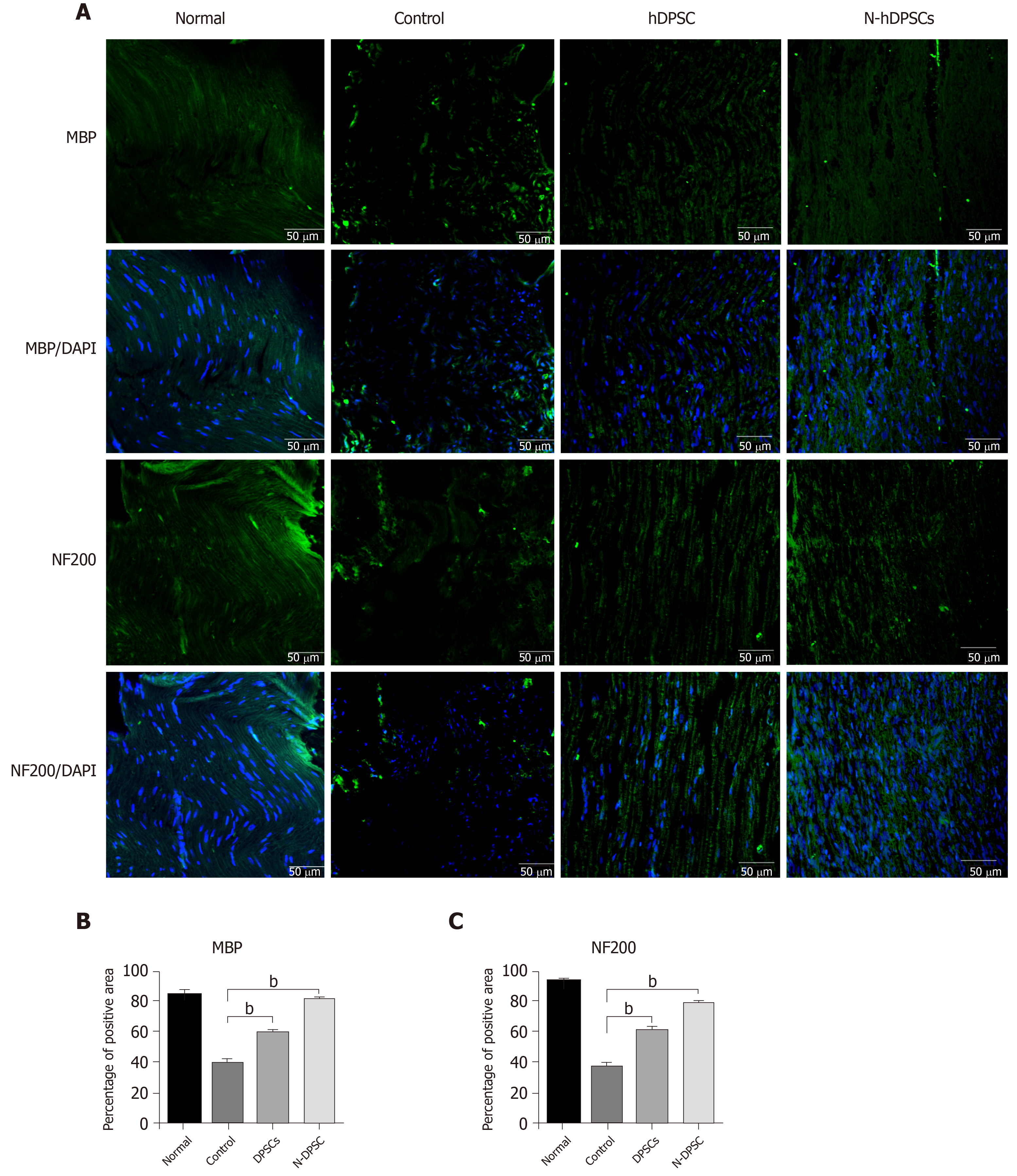
Immunofluorescence was applied to evaluate the levels of recovery and regeneration of the crushed nerve. The immunohistochemistry protocol was described previously; the primary antibodies used in nerve sample sections were MBP (myelin marker, mouse IgG, 1:200, Abcam) and NF200 (nerve fiber axons, mouse IgG, 1:200, Abcam).
All numerical data are presented as the mean ± SD. Student’s t test was used for comparisons between two groups, and one-way ANOVA was used for comparisons among three or more groups. P < 0.05 was considered statistically significant.
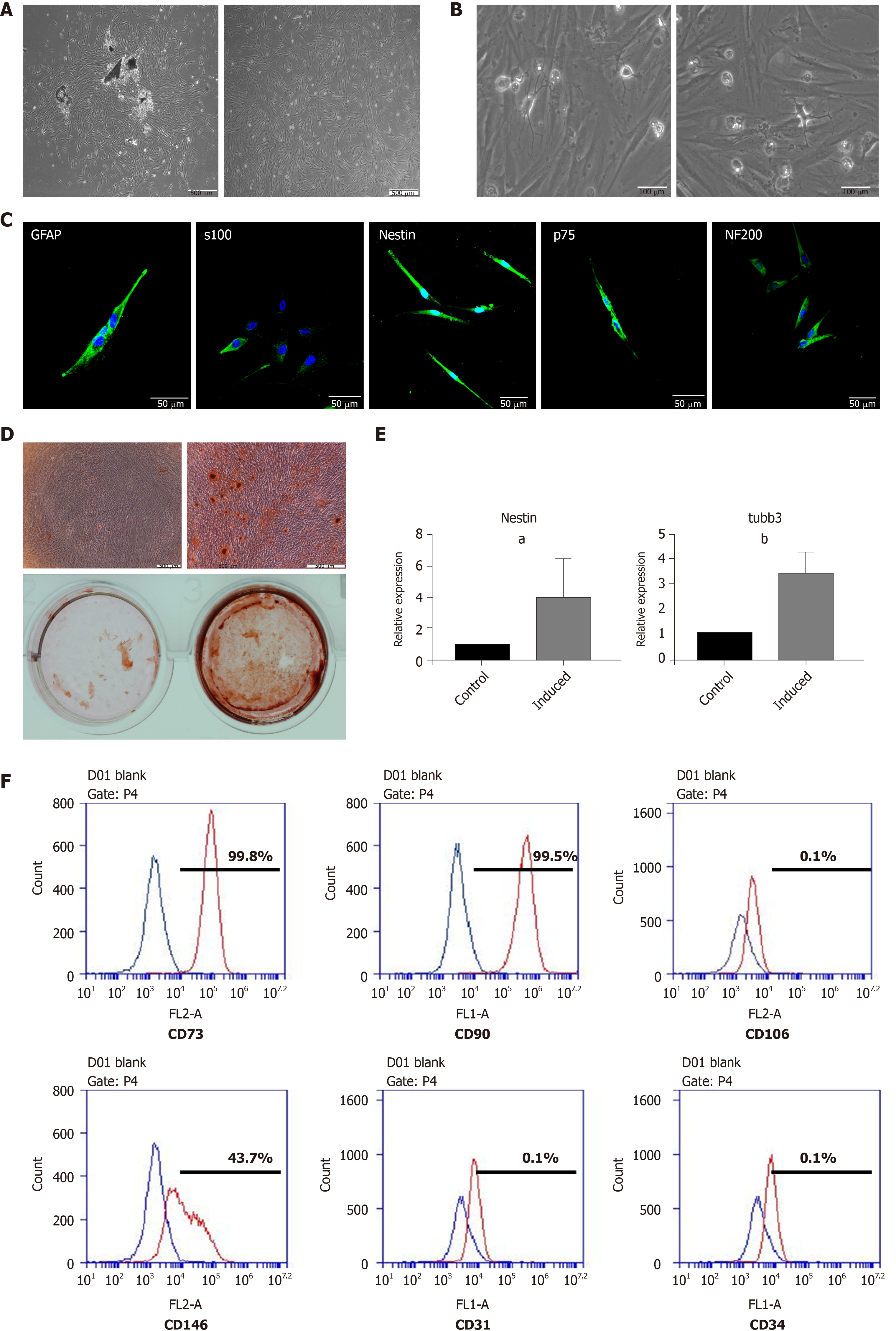
DPSCs emerged from the dental pulp tissue pieces approximately 5 to 7 d after isolation. The DPSCs presented a spindle-like shape, which is the typical morphology of MSCs (Figure 1A). The DPSCs possessed the ability to differentiate into osteoblasts after 14 d of osteogenic induction (Figure 1D). Calcium nodules could be observed under a microscope and positively stained for alizarin red S. Meanwhile, we detected the expression of neural-related genes after neural induction. The results showed that, at the mRNA level, the expression of Nestin and Tubulin-3 was significantly enhanced after neural induction (Figure 1E). Moreover, the results of flow cytometry indicated that the DPSCs expressed canonical MSC surface markers, including CD73, CD90, and CD146, but negatively expressed CD106, CD31, and CD34 (hematopoietic cell surface markers) (Figure 1F). These results implied that the cells that emerged from the dental pulp tissues had a similar morphology to MSCs, possessed the potential of multilineage differentiation, and were positive for the typical surface markers of MSCs. Thus, the cells that we isolated and cultured from dental pulp tissue could be defined as DPSCs.
We found some neural-like cells with multipolar and stellate appearances consistent with those of sensory and motor neurons under the microscope when the DPSCs had been cultured for 3-5 passages. The presence of neural-like cells implied that DPSCs could differentiate into neural cells without neural induction (Figure 1B). The results of immunofluorescent staining also showed that the DPSCs were strongly positive for Nestin, S100, GFAP, p75, and NF200, which are typical markers for neural cells (Figure 1C).
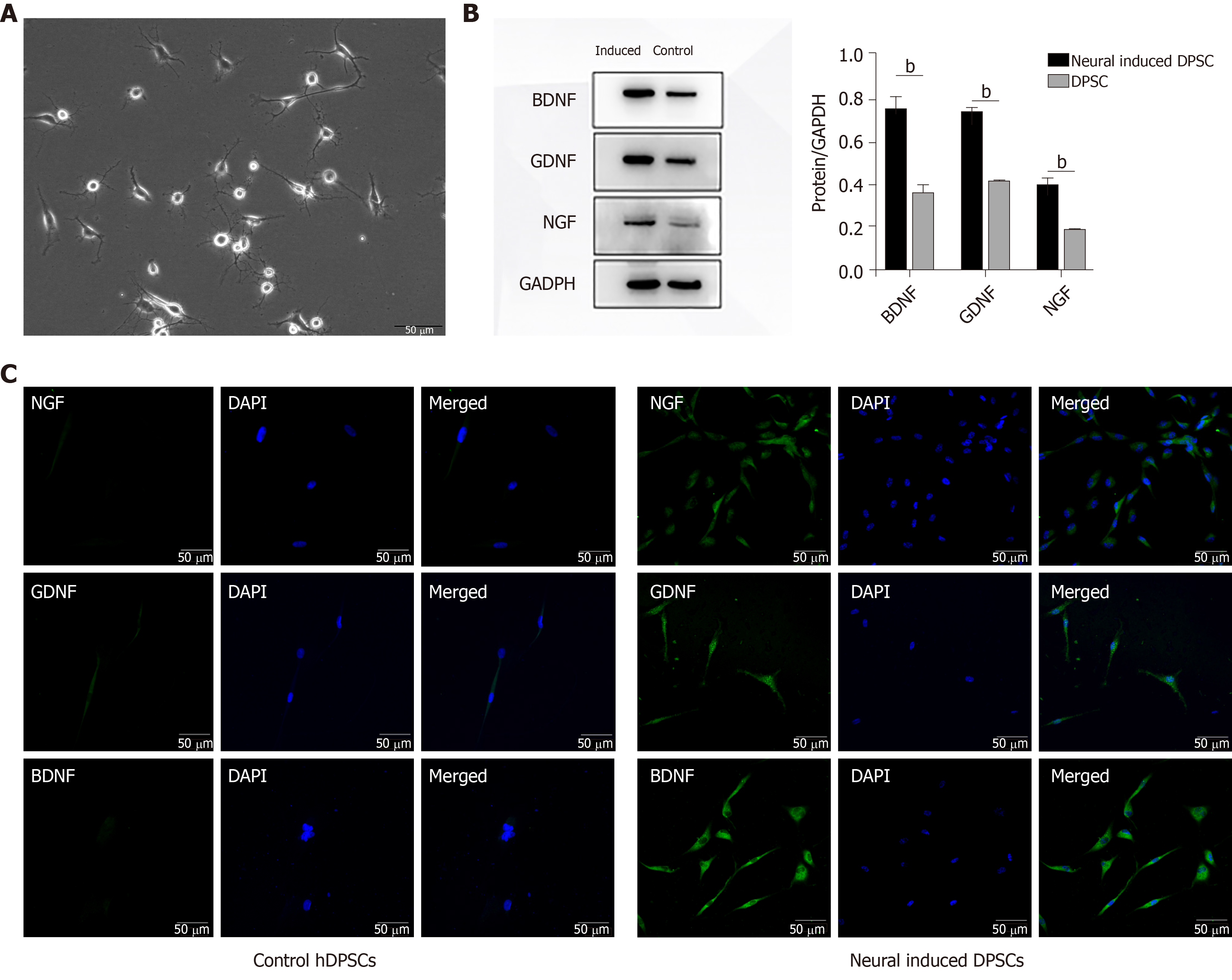
After induction for 2 wk, the morphology of the DPSCs began to change into a neuron-like shape that was similar to that of a motor or sensory neuron (Figure 2A). We then detected the expression of neurotrophic proteins in DPSCs after 2 wk of neural induction. The results showed that after being induced for 2 wk, the transcription and expression levels of BDNF, GDNF, and NGF in DPSCs were enhanced (Figure 2B). The results of immunofluorescent staining were consistent with those of the Western blot, which implied that the expression of neurotrophic proteins was quite enhanced after neural induction (Figure 2C). Collectively, these results suggested that the DPSCs had acquired a phenotype similar to that of neurons and had begun to express neurotrophic factors after neural induction. DPSCs could be a potential and ideal cell source for neural disease treatment.
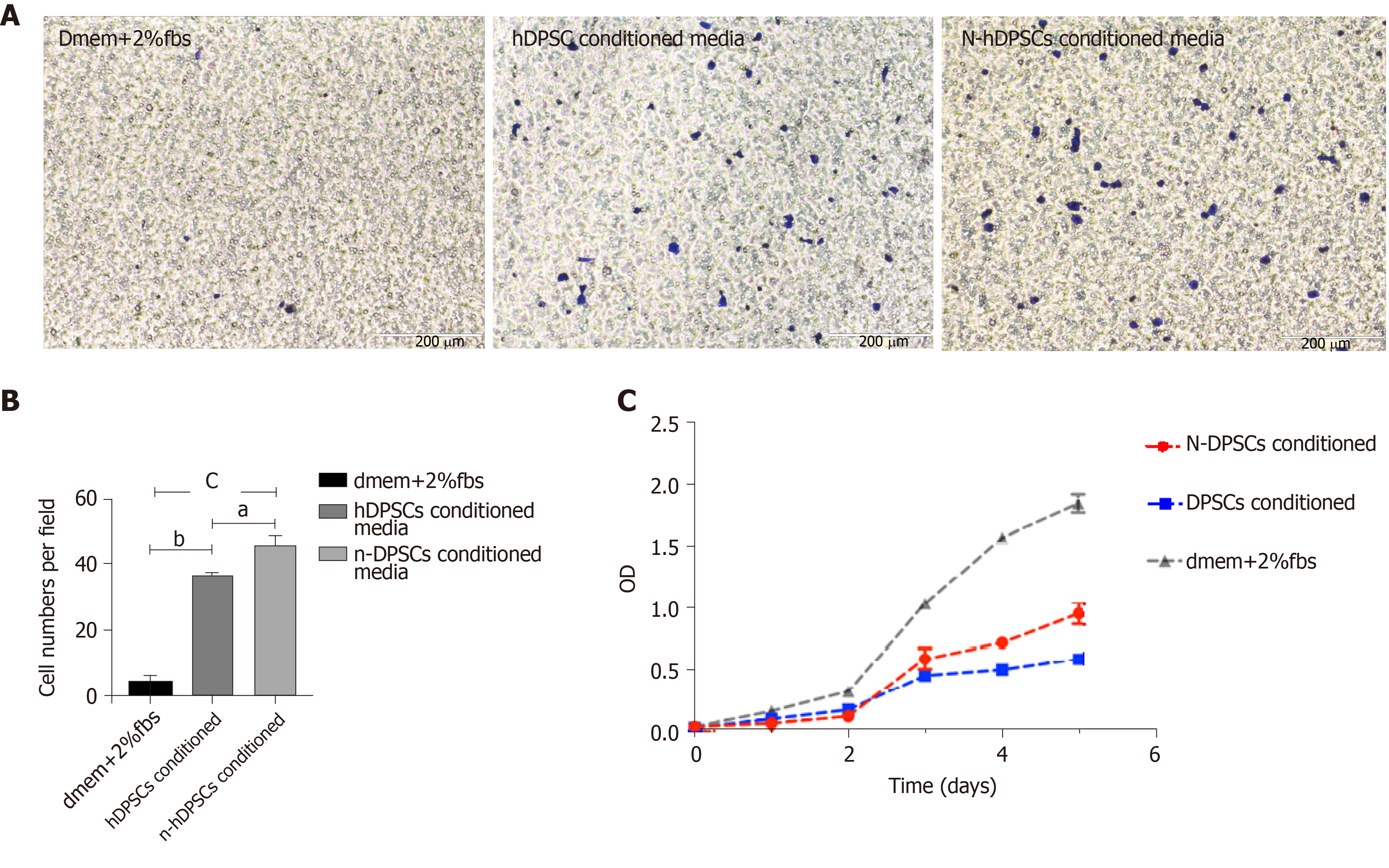
To investigate whether the secretions of DPSCs or N-DPSCs could enhance the proliferation of neural cells and finally induce nerve repair, we collected the conditioned media of both the DPSCs and N-DPSCs. We treated RSC96 cells with DPSCs-CM, N-DPSCs-CM, and basic media and compared their effects. The results of the transwell assays implied that the conditioned media of both the DPSCs and N-DPSCs could improve RSC96 cell migration compared with basic culture media (Figure 3A and B). Furthermore, according to the results of the CCK-8 assay, we found that RSC96 cells could proliferate under the cultures of both the DPSCs-CM and N-DPSCs-CM. However, N-DPSCs-CM-treated RSC96 cells possessed a higher proliferation rate than DPSCs-CM-treated RSC96 cells, which may be due to the stronger neurotrophic effects of N-DPSC secretions (Figure 3C). Collectively, the secretion of DPSCs could maintain the growth of neural cells. However, the secretions of N-DPSCs, especially the neurotrophic factors, better promoted the proliferation and migration of neural cells. These facts imply that N-DPSCs could help nerve repair due to their neurotrophic effects.
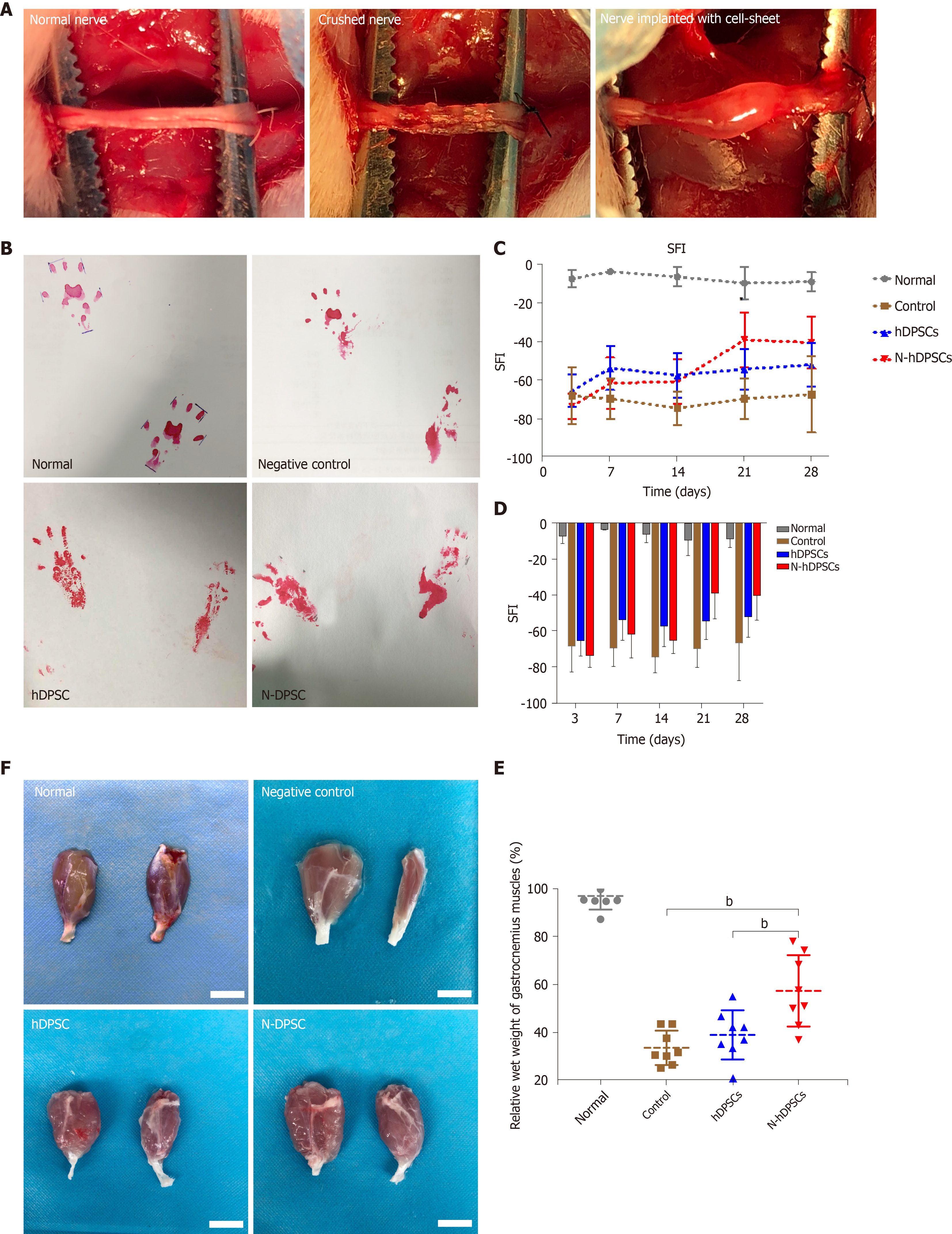
To evaluate whether the implanted DPSCs or N-DPSCs have the ability to help recover the locomotor activity of rats after nerve injury, the walking tracks of rats were collected and evaluated at the indicated time points. The footprint results showed that the hind footprint at the injury side became thinner and longer than that at the contralateral side. Four weeks post-operation, the footprints of the DPSC group and the N-DPSC group were normal compared to those of the control group (Figure 4). The SFI values displayed no difference among all three groups 3 d after the crush injury. Two weeks after the crush injury, both the DPSC group and the N-DPSC group showed signs of recovery. However, the SFI of the control group had worsened. Three weeks after the operation, the SFI indicated that the motor function of the rats in the N-DPSC group had a continuous recovery compared with that of the rats in the DPSC group and the control group, which tended to be stable 2 wk after surgery but showed no inclination toward recovery (Figure 4C; control group: -68.53 ± 11.61 vs N-hDPSC group: -38.73 ± 14.22, P = 0.0012). The differences between these three groups were obvious 4 wk after operation (Figure 4C; control group: -67.23 ± 19.91 vs N-hDPSC group: -40.08 ± 13.73, P = 0.0085). Taken together, the SFIs of these three groups implied that the implanted DPSCs and N-DPSCs cell sheet could help locomotor recovery after nerve crush injury. The repair effects of the implanted DPSCs were only obvious in the first week and showed almost no effects in the later period. However, the implanted N-DPSCs presented sustained and stable effects of motor function recovery until the fourth week. The secretion of neurotrophic factors may contribute to recovery. Among all groups, the N-DPSC rats showed the greatest recovery effect, which indicates that N-DPSCs possess great potential for nerve repair.
Four weeks after the nerve crush surgery, the rats were sacrificed for investigation. We separated the gastrocnemius muscles of the rats from all four groups and measured their wet weights. Moreover, the atrophy level of the muscles was also analyzed. In terms of appearance, the gastrocnemius muscles in both hind legs of the normal rats were almost the same size, while those of the operative side in the control group showed a greater degree of atrophy and a significant reduction in muscle volume (Figure 4F). The degree of gastrocnemius atrophy on the operative side was smaller and the volume was significantly greater in the DPSC group than in the control group. The degree of gastrocnemius atrophy on the operative side of the rats in the N-DPSC group was significantly alleviated compared with that of the control group. Furthermore, compared with the normal side gastrocnemius muscle, there was only slight atrophy, and the volume was almost the same. In general, N-DPSCs have good potential for nerve function repair, thus promoting the recovery of motor function and preventing muscle atrophy. Furthermore, the results of H&E and Masson staining showed that the total area of the isolated muscle fibers on the injured side was obviously smaller than that on the contralateral side in the control, DPSC, and N-DPSC groups. The results implied that the crushed nerve could lead to atrophy of the muscle that it controlled. However, among all the groups, the muscle atrophy degree in the N-DPSC group was significantly alleviated, as shown by the larger area ratio of muscle tissue and well-organized muscle fibers compared to those of the control and DPSC groups (Figure 5A; control group: 48.94% ± 5.357% vs DPSC group: 68.19% ± 0.7635%, P = 0.002; control group: 48.94% ± 5.357% vs N-DPSC group: 73.03% ± 2.596%, P < 0.0001; DPSC group: 68.19% ± 0.7635% vs N-DPSC group: 73.03% ± 2.596%, P = 0.2741). Meanwhile, the wet weight ratio of the gastrocnemius muscles in the N-DPSC group was obviously higher than those of the control and DPSC groups, which was consistent with previous results (Figure 4F and E; control group: 33.47% ± 7.217% vs DPSC group: 38.88% ± 10.34%, P = 0.7174; control group: 33.47% ± 7.217% vs N-DPSC group: 57.45% ± 15.01%, P = 0.0004; DPSC group: 38.88% ± 10.34% vs N-DPSC group: 57.45% ± 15.01%, P = 0.0057). In conclusion, these statistics suggested that N-DPSCs can prevent muscle atrophy after sciatic nerve crush injury, which means that N-DPSCs rather than DPSCs showed an advantage in nerve repair (Table 1).
| Control | Normal | DPSC | n-DPSC | ||
| Percentage of area of muscle fiber (%) | 48.94 ± 5.357 | 77.85 ± 0.5146 | 68.19 ± 0.7635 | 73.03 ± 2.596 | |
| Wet weight ratio of the gastrocnemius muscles (%) | 33.47 ± 7.217 | 97.33 ± 5.781 | 38.88 ± 10.34 | 57.45 ± 15.01 |
H&E staining was used to evaluate the levels of impairment and regeneration in the crushed nerves after 4 wk of recovery. The results showed that the arrangement of nerve fibers in the normal group was dense and regulated. However, the nerve fibers in the control group were disorganized, discontinuous, and scarce. In contrast, the number of nerve fibers in the DPSC and N-DPSC groups was larger than that in the control group. Moreover, the nerve fibers of these two groups presented clearer outlines and were well organized (Figure 6A).
TEM was used to determine the level of regeneration of the myelin sheath. The results were consistent with those of H&E staining. In the control group, the structure of the myelin sheath had nearly disappeared; the thickness of the sheath was far too thin to be observed. In the DPSC group, the sheath was thicker than that of the control group, but it also had vacuolar-like defects, which were indicative of demyelination. In the N-DPSC group, however, little to no demyelination could be observed. The thickness of the sheath was greater than that of the control and DPSC groups. The structure of the myelin sheath was nearly identical to that of the normal group. However, there were still some demyelinated regions in the N-DPSC group (Figure 6B).
The potential ability of implanted DPSCs and N-DPSCs to regenerate nerves was further investigated. We used immunofluorescence to detect the level of nerve regeneration by staining with antibodies against Myelin basic protein (MBP) (a marker of remyelination) and Neurofilament 200 (NF200, a neurofilament marker). The staining results showed that the MBP- and NF200-positive areas were dense and regularly arranged in the normal group. In the control group, however, the MBP- and NF200-positive regions were scarce and irregularly arranged. Compared to the control group, the distribution of MBP- and NF200-positive nerve fibers was dense and better regulated in both the DPSC and N-DPSC groups. Moreover, the expression of DAPI, a marker of the cell nucleus, was much higher in the N-DPSC group than in the other three groups, which indicated high cell proliferation and increased active nerve repair in the N-DPSC group, thus leading to nerve regeneration (Figure 7). In conclusion, both DPSCs and N-DPSCs could help injured nerves reduce the degree of demyelination and improve anatomical recovery to a certain extent. N-DPSCs had more obvious effects on nerve remyelination, repair, and regeneration.
Nerve diseases and injuries, which are usually accompanied by motor or sensory dysfunction and disorder, impose a heavy burden upon patients and greatly reduce their quality of life. Trigeminal nerve injury is one of the most severe complications of dental surgery[1]. Common stomatological operations, such as local anesthetic block injection and third molar, tumor, facial trauma, and tooth implant surgeries, have a risk of trigeminal nerve injury, especially IAN/LN injury[2]. However, because of the particular anatomical position of the IAN/LN[24], traditional surgical methods, such as end-end sutures to fix nerve injury, are too difficult to perform and carry a risk of secondary injury. The common treatments are physiotherapeutics and medical treatments, for which the recovery effects and duration are unstable and unpredictable[25-27].
DPSCs, originating from the neural crest, have many similarities to neural cells, including a high positive ratio for neural markers, high expression of neural proteins and neurotrophic factors, and inclination to differentiate into neural-like cells without induction[12,20,28,29]. Furthermore, reports have revealed that DPSCs have vascularization and immunomodulatory properties that can speed up blood flow and improve neural repair[30,31]. Thus, the use of DPSCs to repair damaged nerves is a promising research direction.
In our work, DPSCs emerged from dental pulp tissue pieces after approximately 5 d of isolation. DPSCs presented as fibroblastic and spindle-like shapes, which are typical morphologies of MSCs. After osteogenic induction for 14 d, DPSCs positively stained for alizarin red and formed calcium nodules, which could be observed under a microscope. After neural induction for 2 wk, the relative expression of Nestin and tubulin-3 was higher than that prior to induction. Furthermore, flow cytometry indicated that the hDPSCs expressed canonical MSC surface markers, including CD73, CD90, and CD146. The above results indicated that DPSCs possessed properties of MSCs and were capable of multilineage differentiation. Moreover, we not only found that DPSCs were strongly positive for Nestin, S100, GFAP, p75, and NF200 (typical markers for neural cells) when cultured without neural induction but also observed some neural-like cells among the hDPSCs under a microscope when the DPSCs were simply cultured in basic media. This phenomenon indicated that DPSCs have a strong inclination toward neural differentiation. After neural induction for 2 wk, the DPSC morphology began to change into a neuron-like shape, which was bipolar and stellate and consistent with that of motor or sensory neurons. This evidence supports the fact that DPSCs have great potential to differentiate into neural cells, which makes them an ideal source for nerve disease treatments and nerve defect repair and regeneration.
Neurotrophic factors, including BDNF, GDNF and NGF, play significant roles in promoting axon regeneration. As the expression of neurotrophic factors declines, the capacities of neurons to regenerate and of Schwann cells to support regenerating neurons also decline[32]. These facts prove that neurotrophic factors are indispensable for nerve repair and regeneration. Moreover, the intimate relationship between Schwann cells and axons makes Schwann cells a focus in nerve regeneration research. Schwann cells promote peripheral nerve regeneration by elaborating the basement membrane and producing neurotrophic factors[33]. As SCs proliferate, they contact regenerative axons and form myelin. Therefore, promoting SC proliferation is a critical point for nerve repair[34,35]. In our research, the results of the Western blot and immunofluorescent staining showed that the expression of BDNF, GDNF, and NGF was higher in N-DPSCs than in DPSCs. Moreover, N-DPSC-CM promoted RSC96 proliferation and migration better than DPSC-CM. These results imply that N-DPSCs may have a stronger neural repair and regeneration ability than DPSCs owing to their neurotrophic effects. N-DPSCs can be a potent candidate cell source for nerve regeneration.
To investigate whether DPSCs and N-DPSCs were indeed capable of nerve functional and anatomical recovery, we designed four experimental rat groups to be observed for 4 wk. Our results showed that both N-DPSCs and DPSCs could improve motor functional recovery in rats after a nerve crush injury. However, the recovery effect of DPSCs on motor function only lasted for the first week, while N-DPSCs demonstrated long-term recovery effects. Furthermore, N-DPSCs and DPSCs alleviated gastrocnemius muscle atrophy levels after crush injury. Moreover, the results of H&E, immunofluorescence, and TEM showed that N-DPSCs and DPSCs improved nerve repair and remyelination. The results of our in vivo experiments provide evidence that DPSCs and N-DPSCs possess the potential for nerve repair, while N-DPSCs possess a stronger repair effect owing to their secretion of neurotrophic factors.
Although our research provided basic evidence for the use of N-DPSCs in nerve tissue repair and regeneration, this study was accompanied by several limitations. Cell death after implantation, the low activity status of implanted cells, and degradation of proteins might limit the effectiveness of nerve repair in vivo. Moreover, photobiomodulation therapy (PBMT) has an intimate relationship with tissue recovery especially for peripheral nerve damage[36]. The combination of N-DPSCs and PBMT might have more effective effects on nerve repair. What’s more, the mechanism that underlying the neurotrophic effects of DPSC and N-DPSC is unknown. Our next aim is to investigate the underlying mechanism.
The application of DPSCs has several advantages, including ready accessibility, damage-free extraction from the donor’s body, few ethics issues, and low injection rates. The immune rejection problem and the source problem are two major obstacles to stem cell transplantation in disease treatment. The use of autologous dental pulp cells for repairing nerve injury after tooth extraction can greatly reduce immune rejection and solve the cell source problem. We are able to isolate and culture dental pulp cells from extracted teeth and store them as seed cells for nerve repair, which can turn discarded clinical waste into a potential therapeutic resource. Our research has provided DPSCs as another possibility for nerve repair and regeneration.
In summary, DPSCs might be an ideal cell source for nerve repair and regeneration, as they are inclined to undergo neural differentiation, express neurotrophic proteins after induction, and enhance SC proliferation after neural induction. Furthermore, N-DPSCs provide an effective and long-term treatment for crushed nerves with functional recovery and anatomical repair. Thus, DPSCs could be a promising therapeutic cell source for peripheral nerve repair and regeneration.
Inferior alveolar nerve (IAN) and lingual nerve (LN) injuries are complications of mandibular third molar extraction, which usually lead to numbness of the lips and taste disorder and eventually impose an extremely large burden on the patient’s life. Among all kinds of mesenchymal stem cells, dental pulp stem cells (DPSCs), derived from the neural crest, have been shown to have several similarities to neural cells. DPSCs may have a great potential for nerve repair.
A brand-new and effective stem cell based treatment for nerve repair and regeneration is urgently needed.
Nerve diseases and injuries, which are usually accompanied by motor or sensory dysfunction and disorder, impose a heavy burden upon patients and greatly reduce their quality of life. DPSCs, derived from the neural crest, have many characteristics that are similar to those of neural cells, indicating that they can be an ideal source for neural repair. In this study, we aimed to explore the potential roles and molecular mechanisms of DPSCs in crushed nerve recovery.
DPSCs were isolated, cultured, and identified by multilineage differentiation and flow cytometry. Western blot and immunofluorescent staining were applied to analyze the expression levels of neurotrophic proteins in DPSCs after neural induction. Then, we collected the secretions of DPSCs. We analyzed their effects on RSC96 cell proliferation and migration by CCK8 and transwell assays. Finally, we generated a sciatic nerve crush injury model in vivo and used the sciatic function index, walking track analysis, muscle weight, and hematoxylin & eosin (H&E) staining to further evaluate the nerve repair ability of DPSCs.
DPSCs highly expressed several specific neural markers, including GFAP, S100, Nestin, P75, and NF200, and were inclined toward neural differentiation. Furthermore, we also found that neural-induced DPSCs (N-DPSCs) could express neurotrophic factors, including NGF, BDNF, and GDNF. The secretions of N-DPSCs could enhance the proliferation and migration of Schwann cells. In vivo, both DPSC and N-DPSC implants alleviated gastrocnemius muscle atrophy. However, in terms of anatomy and motor function, as shown by H&E staining, immunofluorescent staining, and walking track analyses, the repair effects of N-DPSCs were more sustained, potent, and effective than those of DPSCs and the controls.
In summary, DPSCs might be an ideal cell source for nerve repair and regeneration, as they are inclined to undergo neural differentiation, express neurotrophic proteins after induction, and enhance SC proliferation after neural induction. Furthermore, N-DPSCs provide an effective and long-term treatment for crushed nerves with functional recovery and anatomical repair. Thus, DPSCs could be a promising therapeutic cell source for peripheral nerve repair and regeneration.
The application of DPSCs has several advantages, including ready accessibility, damage-free extraction from the donor’s body, few ethics issues, and low injection rates. We are able to isolate and culture dental pulp cells from extracted teeth and store them as seed cells for nerve repair, which can turn discarded clinical waste into a potential therapeutic resource. Our research has provided DPSCs as another possibility for nerve repair and regeneration.
The authors thank the healthy donors from the Department of Oral and Maxillofacial Surgery, West China Hospital of Stomatology Sichuan University. We also extend special thanks to State Key Laboratory of Oral Disease, National Clinical Research Center for Oral Diseases & the Department of Oral and Maxillofacial Surgery, West China Hospital of Stomatology, Sichuan University, and National Engineering Laboratory for Oral Regenerative Medicine.
| 1. | Patel N, Ali S, Yates JM. Quality of life following injury to the inferior dental or lingual nerve - a cross-sectional mixed-methods study. Oral Surg. 2018;11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Kushnerev E, Yates JM. Evidence-based outcomes following inferior alveolar and lingual nerve injury and repair: a systematic review. J Oral Rehabil. 2015;42:786-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | López-Cebral R, Silva-Correia J, Reis RL, Silva TH, Oliveira JM. Peripheral Nerve Injury: Current Challenges, Conventional Treatment Approaches, and New Trends in Biomaterials-Based Regenerative Strategies. ACS Biomater Sci Eng. 2017;3:3098-3122. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 455] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Yamada Y, Ohazama A, Maeda T, Seo K. The Sonic Hedgehog signaling pathway regulates inferior alveolar nerve regeneration. Neurosci Lett. 2018;671:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Geuna S. The sciatic nerve injury model in pre-clinical research. J Neurosci Methods. 2015;243:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Sanen K, Martens W, Georgiou M, Ameloot M, Lambrichts I, Phillips J. Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: potential for peripheral nerve repair? J Tissue Eng Regen Med. 2017;11:3362-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3444] [Article Influence: 132.5] [Reference Citation Analysis (1)] |
| 9. | Wang D, Wang Y, Tian W, Pan J. Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Prolif. 2019;52:e12572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 10. | Liu L, Ling J, Wei X, Wu L, Xiao Y. Stem cell regulatory gene expression in human adult dental pulp and periodontal ligament cells undergoing odontogenic/osteogenic differentiation. J Endod. 2009;35:1368-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 436] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 13. | Wang YX, Ma ZF, Huo N, Tang L, Han C, Duan YZ, Jin Y. Porcine tooth germ cell conditioned medium can induce odontogenic differentiation of human dental pulp stem cells. J Tissue Eng Regen Med. 2011;5:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 276] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Luo L, He Y, Wang X, Key B, Lee BH, Li H, Ye Q. Potential Roles of Dental Pulp Stem Cells in Neural Regeneration and Repair. Stem Cells Int. 2018;2018:1731289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 16. | Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014;78:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Yamamoto T, Osako Y, Ito M, Murakami M, Hayashi Y, Horibe H, Iohara K, Takeuchi N, Okui N, Hirata H, Nakayama H, Kurita K, Nakashima M. Trophic Effects of Dental Pulp Stem Cells on Schwann Cells in Peripheral Nerve Regeneration. Cell Transplant. 2016;25:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Kolar MK, Itte VN, Kingham PJ, Novikov LN, Wiberg M, Kelk P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep. 2017;7:12605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Wang F, Jia Y, Liu J, Zhai J, Cao N, Yue W, He H, Pei X. Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer's disease. Cell Biol Int. 2017;41:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 21. | Carnevale G, Pisciotta A, Riccio M, Bertoni L, De Biasi S, Gibellini L, Zordani A, Cavallini GM, La Sala GB, Bruzzesi G, Ferrari A, Cossarizza A, de Pol A. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J Tissue Eng Regen Med. 2018;12:e774-e785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Sasaki R, Matsumine H, Watanabe Y, Takeuchi Y, Yamato M, Okano T, Miyata M, Ando T. Electrophysiologic and functional evaluations of regenerated facial nerve defects with a tube containing dental pulp cells in rats. Plast Reconstr Surg. 2014;134:970-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Ogiuchi H, Okano T, Ando T. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med. 2011;5:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Dias GJ, de Silva RK, Shah T, Sim E, Song N, Colombage S, Cornwall J. Multivariate assessment of site of lingual nerve. Br J Oral Maxillofac Surg. 2015;53:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Matsuyama T, Mackay M, Midha R. Peripheral nerve repair and grafting techniques: a review. Neurol Med Chir (Tokyo). 2000;40:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Evans GR. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec. 2001;263:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 274] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 28. | Karaöz E, Demircan PC, Sağlam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol. 2011;136:455-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e109305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 30. | Tran-Hung L, Laurent P, Camps J, About I. Quantification of angiogenic growth factors released by human dental cells after injury. Arch Oral Biol. 2008;53:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Luo L, Albashari AA, Wang X, Jin L, Zhang Y, Zheng L, Xia J, Xu H, Zhao Y, Xiao J, He Y, Ye Q. Effects of Transplanted Heparin-Poloxamer Hydrogel Combining Dental Pulp Stem Cells and bFGF on Spinal Cord Injury Repair. Stem Cells Int. 2018;2018:2398521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 33. | Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 782] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 35. | Li R, Li Y, Wu Y, Zhao Y, Chen H, Yuan Y, Xu K, Zhang H, Lu Y, Wang J, Li X, Jia X, Xiao J. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials. 2018;168:24-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 36. | Rosso MPO, Buchaim DV, Kawano N, Furlanette G, Pomini KT, Buchaim RL. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering (Basel). 2018;5:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Buchaim RL, Khan A S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Xing YX