©The Author(s) 2016.
World J Stem Cells. May 26, 2016; 8(5): 202-215
Published online May 26, 2016. doi: 10.4252/wjsc.v8.i5.202
Published online May 26, 2016. doi: 10.4252/wjsc.v8.i5.202
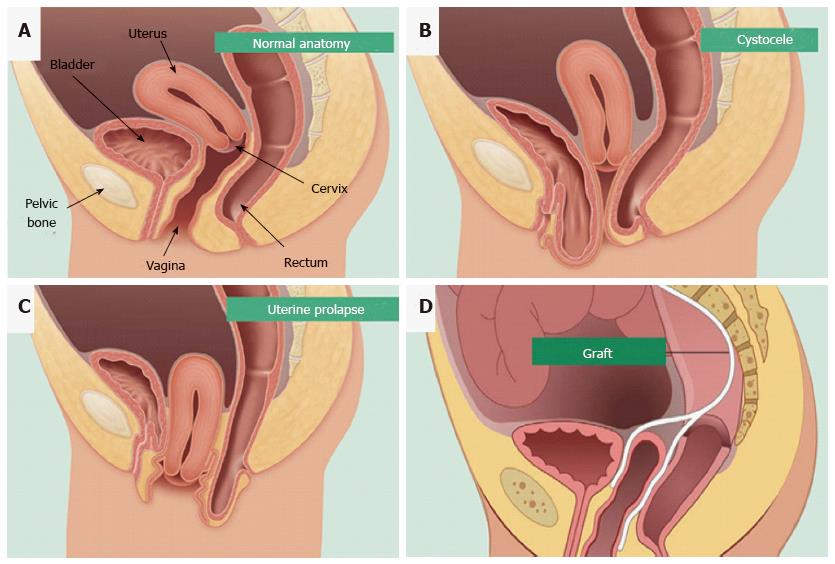
Figure 1 Pelvic organ prolapse mesh treatment.
Normal pelvic anatomy (A) and herniation of the bladder (B) and uterus into the vagina (C). Synthetic mesh augmentation of vaginal walls as a colporrhaphy treatment for pelvic organ prolapse (D). Hysterectomies are also used to treat uterine prolapse (reproduced with permission from BARD medical).
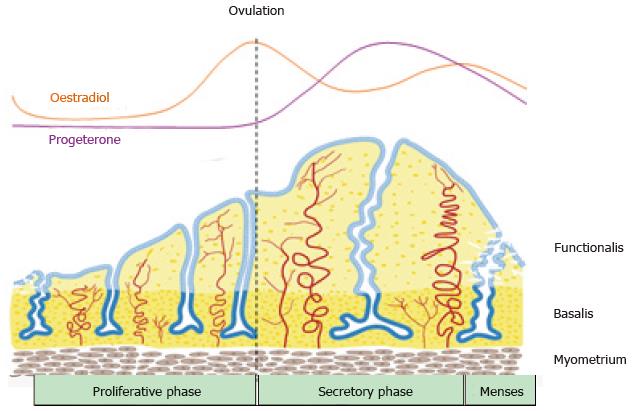
Figure 2 Schematic of changes in the human endometrium during the menstrual cycle, illustrating the growth, differentiation and shedding of the functionalis layer.
The functionalis layer regenerates 4-10 mm during the proliferative phase (10 d) as cells proliferate in response to rising circulating estrogen levels. During the secretory phase, progesterone induces differentiation of the epithelium and stroma to generate an endometrium receptive to implantation of an embryo. This entire process occurs over 400 times during a woman’s reproductive life indicating the regenerative potential of human endometrium (reproduced from ref.[63] with permission).
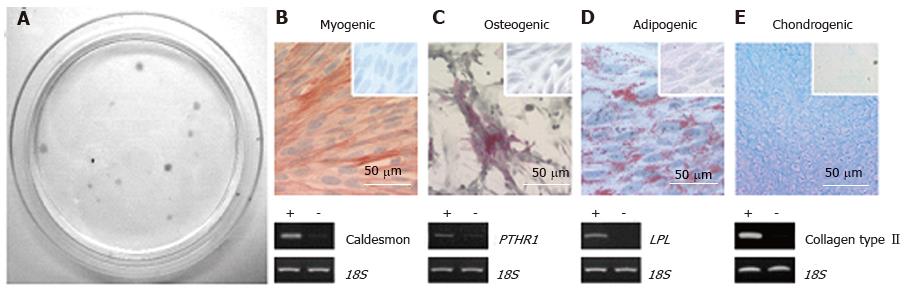
Figure 3 Endometrial mesenchymal stem cells.
Clonogenic (A); and differentiate into 4 mesodermal lineages from a single clonogenic cell (B-E); myocytes (B); osteocytes (C); adipocytes (D); chondrocytes (reproduced from ref. [44] with permission) (E). PTHR1: Parathyroid hormone 1 receptor; LPL: Lipoprotein lipase.
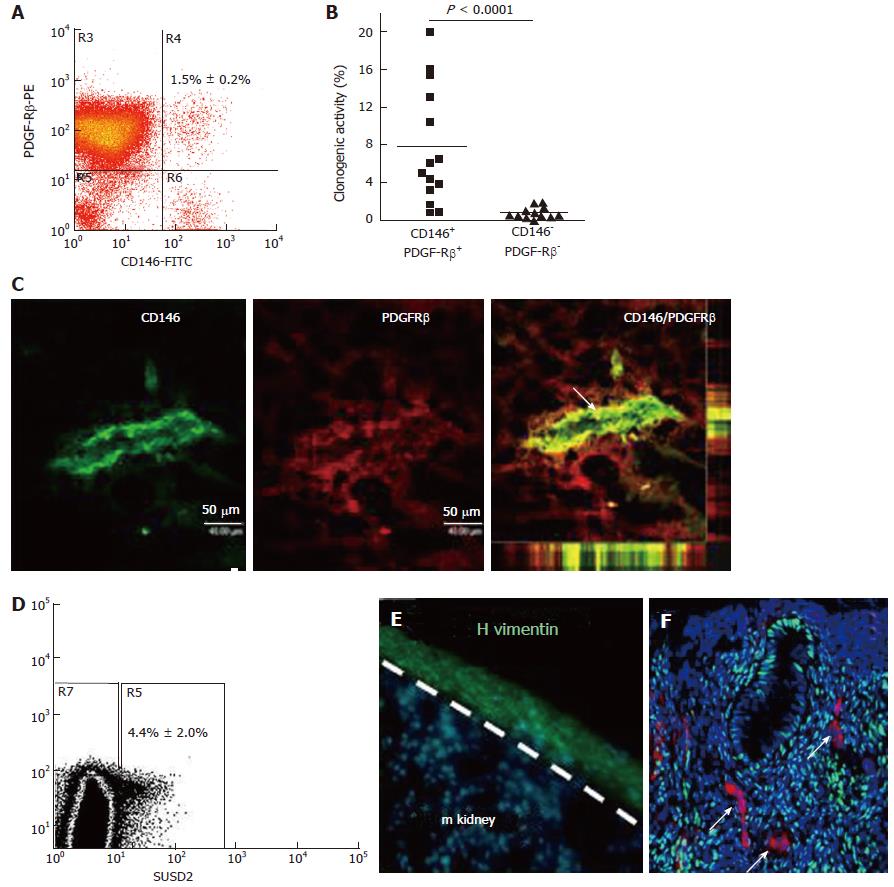
Figure 4 Specific enriching for endometrial mesenchymal stem cells.
Flow cytometry plot of CD146+PDGFRB+ fraction (A) which contains most of the clonogenic stromal cells (B) and reveals their pericyte identity in vivo (C); SUSD2+ cells in endometrial cell suspensions (D) which E reconstitute human vimentin+ stromal tissue when transplanted under the kidney capsule of NSG mice, and F have a perivascular location in human endometrium. SUSD2+ cells (red) do not express estrogen receptor-α (green), but endometrial stromal cells do (DNA blue). The white arrow indicates perivascular SUSD2+ cells (reproduced ref. [70,72,78] with permission).
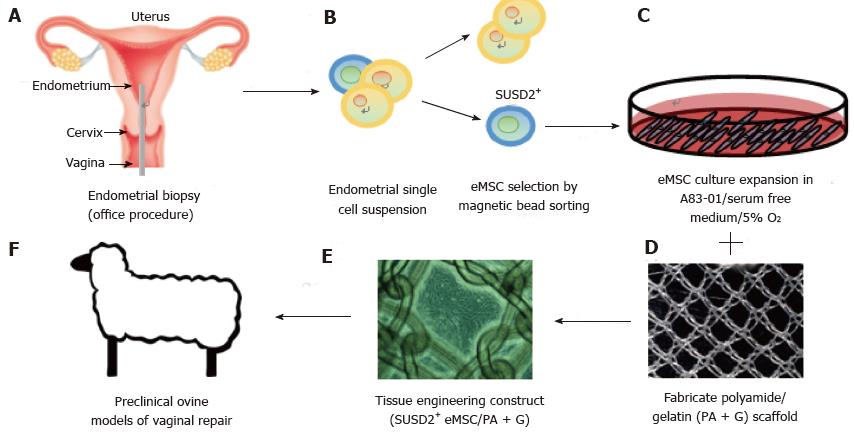
Figure 5 Isolation and application of e-mesenchymal stem cells in pelvic organ prolapse vaginal repair.
(A) simple office based endometrial biopsies can be used to obtain patients’ tissues, which are dissociated, then (B) eMSC selected using SUSD2 magnetic bead sorting, followed by (C) culture expansion in A83-01/serum free medium in 5% O2 to generate large numbers of undifferentiated SUSD2+ eMSC (90%-95%) for (D) seeding onto fabricated scaffolds which will create an (E) eMSC/PA-G tissue engineering construct for implantation into (F) a large animal preclinical model to assess their efficacy in vaginal repair of parous ewes with evidence of POP (reproduced with permission from ref.[57,103] with permission). POP: Pelvic organ prolapse; MSC: Mesenchymal stem cells.
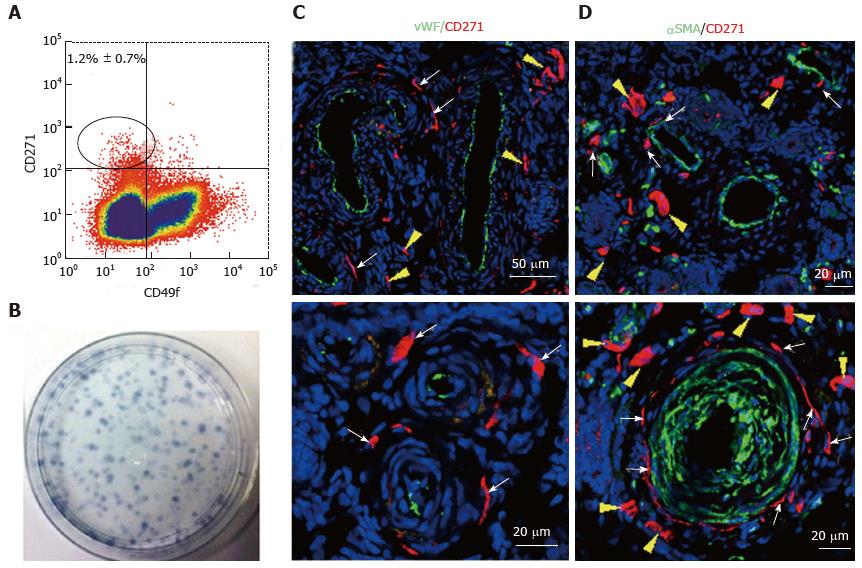
Figure 6 Specific markers for ovine e-mesenchymal stem cells.
Flow cytometry plot of ovine endometrial cells immunolabelled with CD271 and CD49f antibodies (A). The CD271+CD49f- population enriches; Clonogenic stromal cells (B); Immunofluorescence images of ovine endometrium stained with CD271 (red) and vascular markers reveals their in vivo perivascular location in the adventitia of veins and arteries (C, D); vWF an endothelial marker (green), showing CD271+ cells are perivascular but not pericytes (C); αSMA, a perivascular marker (green) showing CD271+ cells located adjacent to αSMA+ cells in the adventitia of vessels rather than expressing αSMA themselves (D). White arrows: perivascular CD271+ cells; yellow arrows: CD271+ cells not associated with vessels (reproduced from ref.[81] with permission). vWF: Von Willebrand factor; αSMA: Alpha smooth muscle actin.
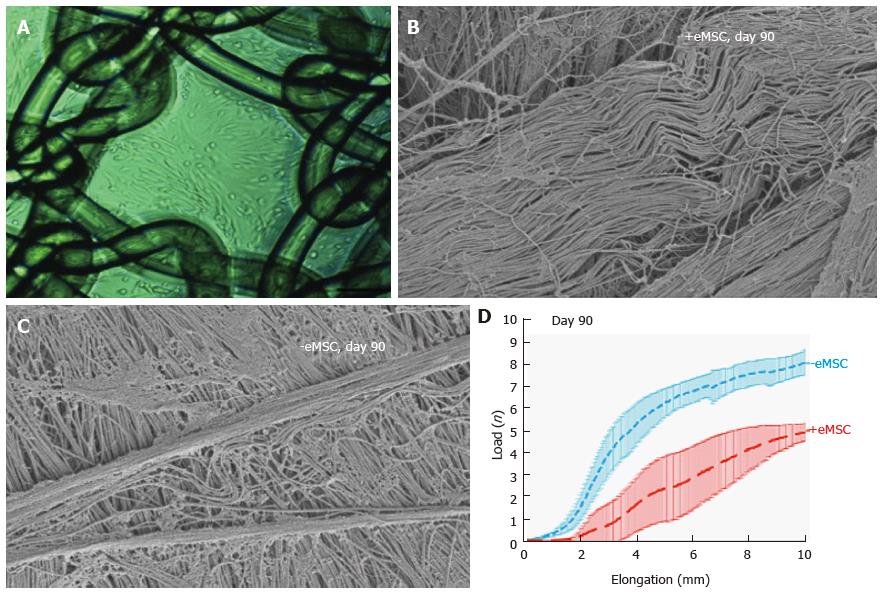
Figure 7 Human e-mesenchymal stem cells improves the biocompatibility of polyamide/gelatin (PA + G) mesh in a fascial wound defect in nude rats.
PA + G mesh seeded with 100000 eMSC/cm2 and cultured for 48 h, prior to implantation (A); Physiological crimped collagen deposited around eMSC+/PA + G mesh (B); Scar-like collagen in PA + G mesh alone as observed by SEM (C); Load-elongation curves of explanted meshes with (red) and without (blue) eMSC showing less stiffness (slope) and longer toe region for mesh seeded with eMSC, indicating improved biomechanical properties (reproduced from ref.[57,86,89] with permission) (D). MSC: Mesenchymal stem cells; PA : Polyamide; G: Gelatin.
- Citation: Emmerson SJ, Gargett CE. Endometrial mesenchymal stem cells as a cell based therapy for pelvic organ prolapse. World J Stem Cells 2016; 8(5): 202-215
- URL: https://www.wjgnet.com/1948-0210/full/v8/i5/202.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i5.202