©Author(s) (or their employer(s)) 2026.
World J Stem Cells. Feb 26, 2026; 18(2): 116184
Published online Feb 26, 2026. doi: 10.4252/wjsc.v18.i2.116184
Published online Feb 26, 2026. doi: 10.4252/wjsc.v18.i2.116184
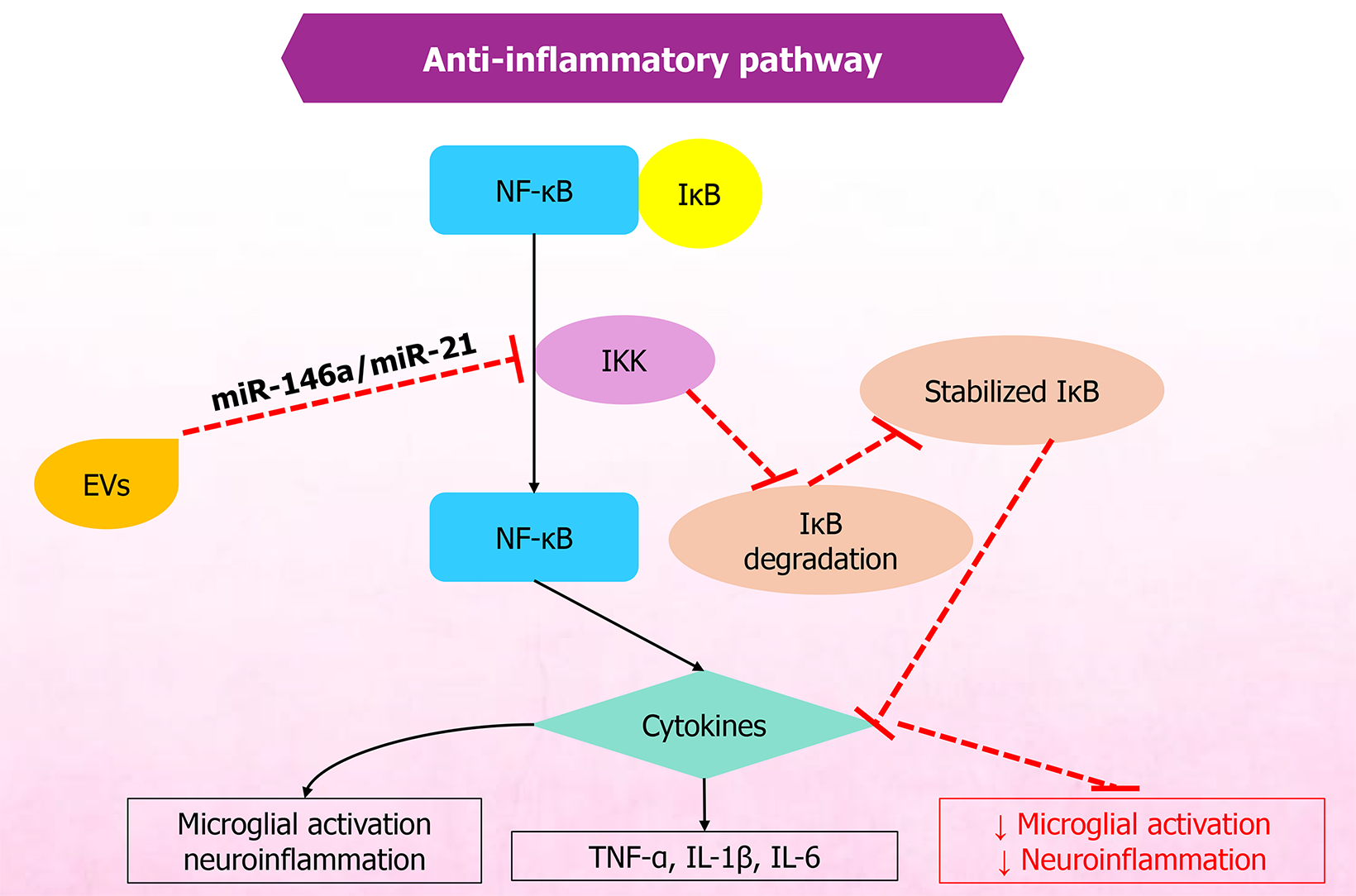
Figure 1 Schematic representation of extracellular vesicle-mediated modulation of nuclear factor kappa B-light chain enhancer of activated B cells signaling pathway.
Under inflammatory stimulation, receptor activation triggers the inhibitor of kappa B (IκB) kinase complex, leading to IκB phosphorylation and degradation, allowing nuclear factor kappa B to translocate into the nucleus and induce the expression of pro-inflammatory cytokines. Extracellular vesicle-derived microRNAs inhibit IκB kinase activity, thus stabilizing IκB and suppressing nuclear factor kappa B activation and downstream cytokine release. This mechanism helps reduce microglial activation and mitigate neuroinflammation. NF-кB: Nuclear factor kappa B; IκB: Inhibitor of kappa B; IKK: Inhibitor of kappa B kinase; EVs: Extracellular vesicles; TNF: Tumor necrosis factor; IL: Interleukin.
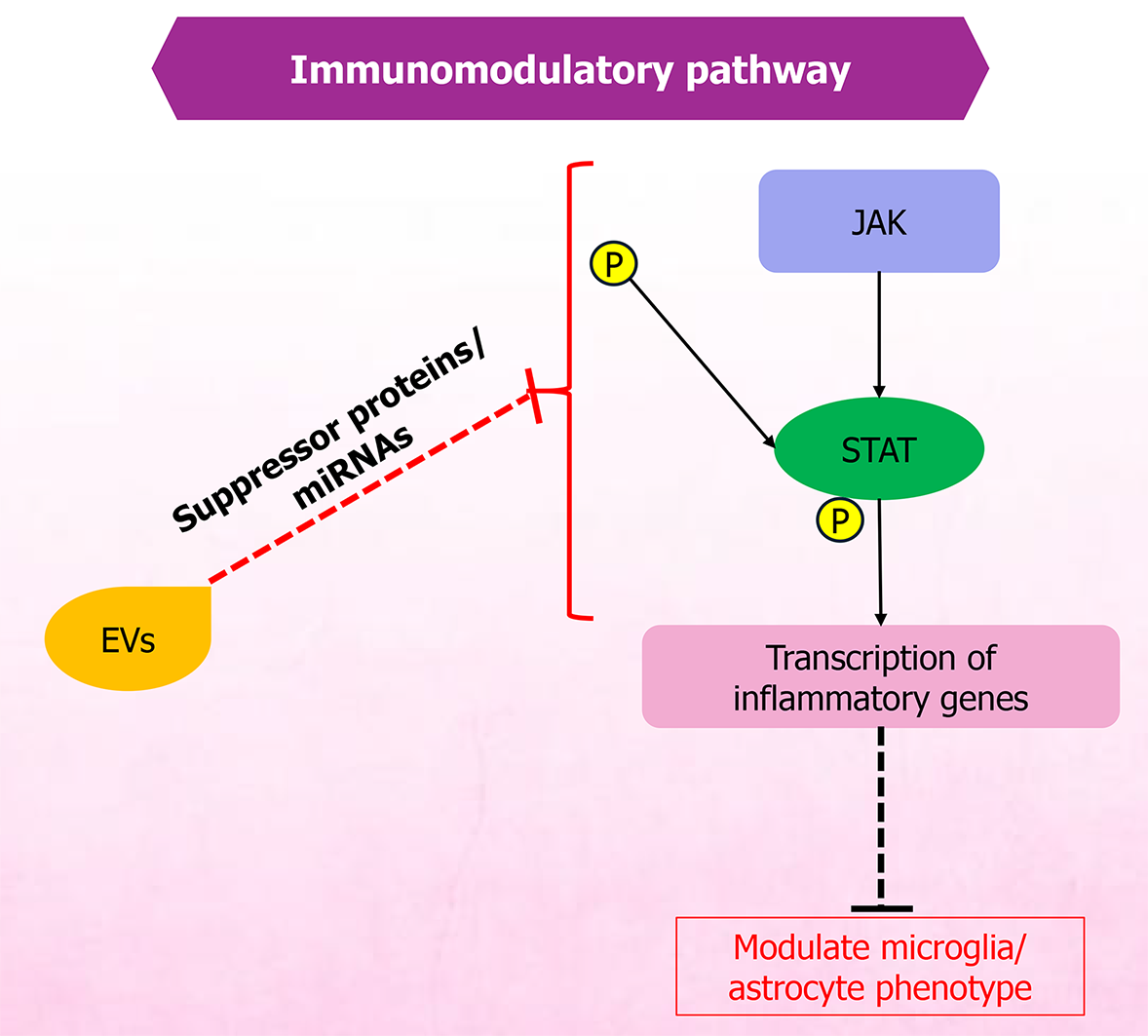
Figure 2 Extracellular vesicle-mediated regulation of Janus kinases/signal transducer and activator of transcription signaling pathway in glial immunomodulation.
Cytokine-receptor binding activates Janus kinases, leading to signal transducer and activator of transcription phosphorylation and nuclear translocation, which drives transcription of proinflammatory genes. Extracellular vesicle-derived cargo - including suppressor proteins and specific microRNAs - can inhibit Janus kinases/signal transducer and activator of transcription activation, modulating microglial and astrocytic phenotypes toward anti-inflammatory, neuroprotective states. EVs: Extracellular vesicles; miRNAs: MicroRNAs; JAK: Janus kinases; STAT: Signal transducer and activator of transcription.
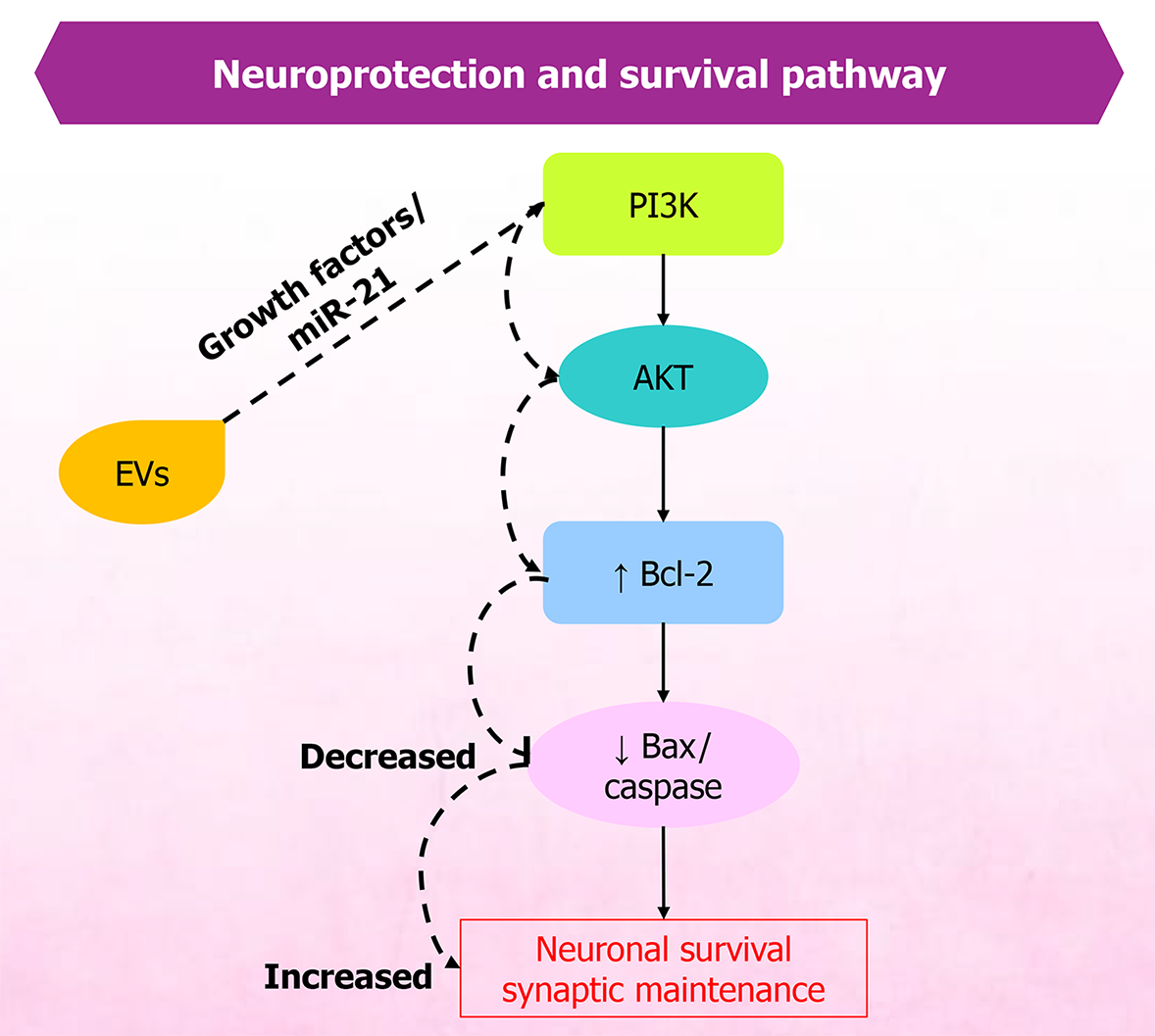
Figure 3 Extracellular vesicle-mediated activation of phosphoinositide 3-kinase/protein kinase B signaling pathway promotes neuronal survival and neuroprotection.
Ligand binding to surface receptors activates phosphoinositide 3-kinase, leading to protein kinase B phosphorylation, upregulation of antiapoptotic proteins, and downregulation of proapoptotic factors. Extracellular vesicle cargo enhances protein kinase B phosphorylation, supporting neuronal survival, synaptic maintenance, and resistance to neurodegenerative stress. EVs: Extracellular vesicles; PI3K: Phosphoinositide 3-kinase; AKT: Protein kinase B.
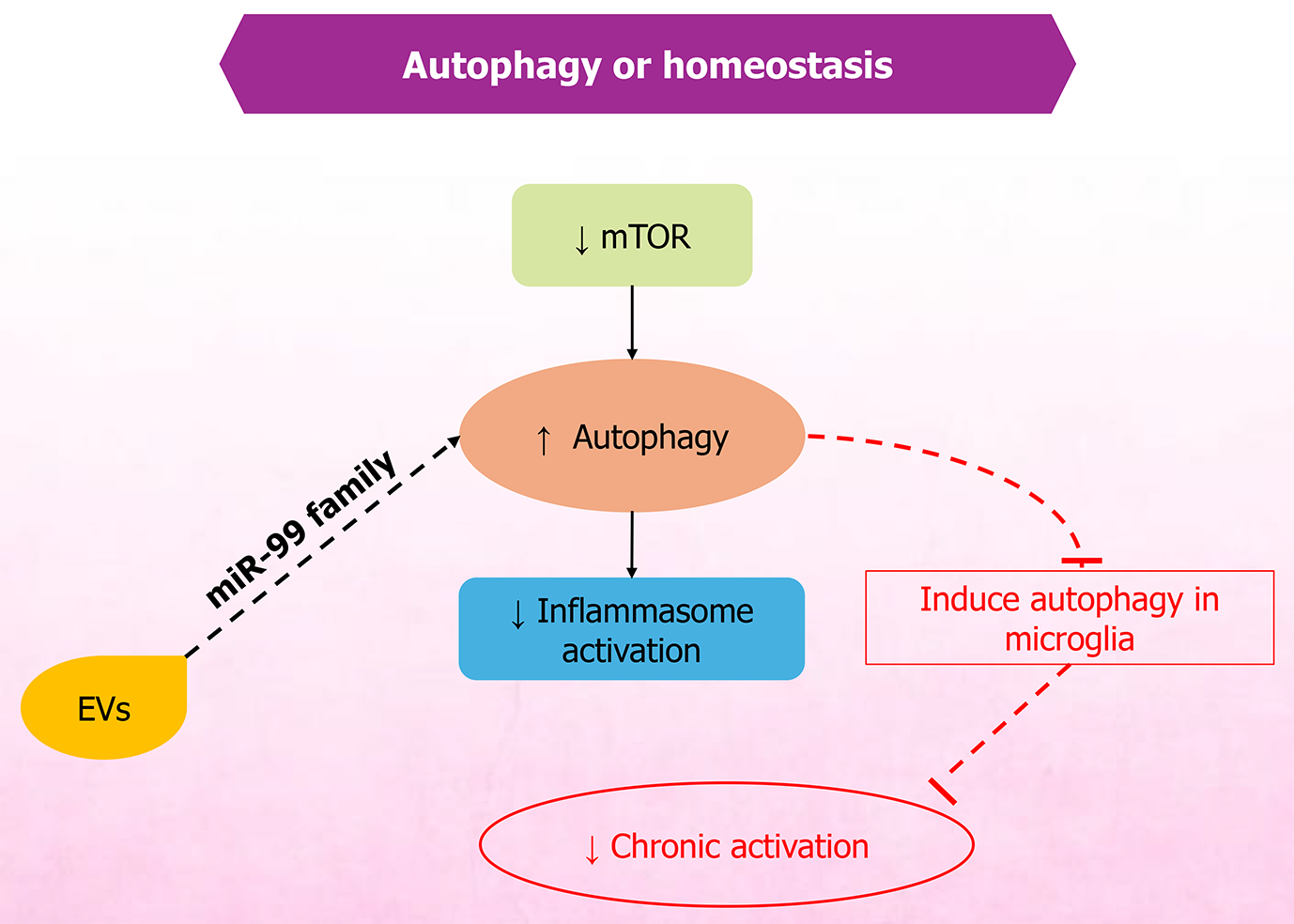
Figure 4 Extracellular vesicle-mediated modulation of mammalian target of rapamycin-autophagy pathway maintains cellular homeo
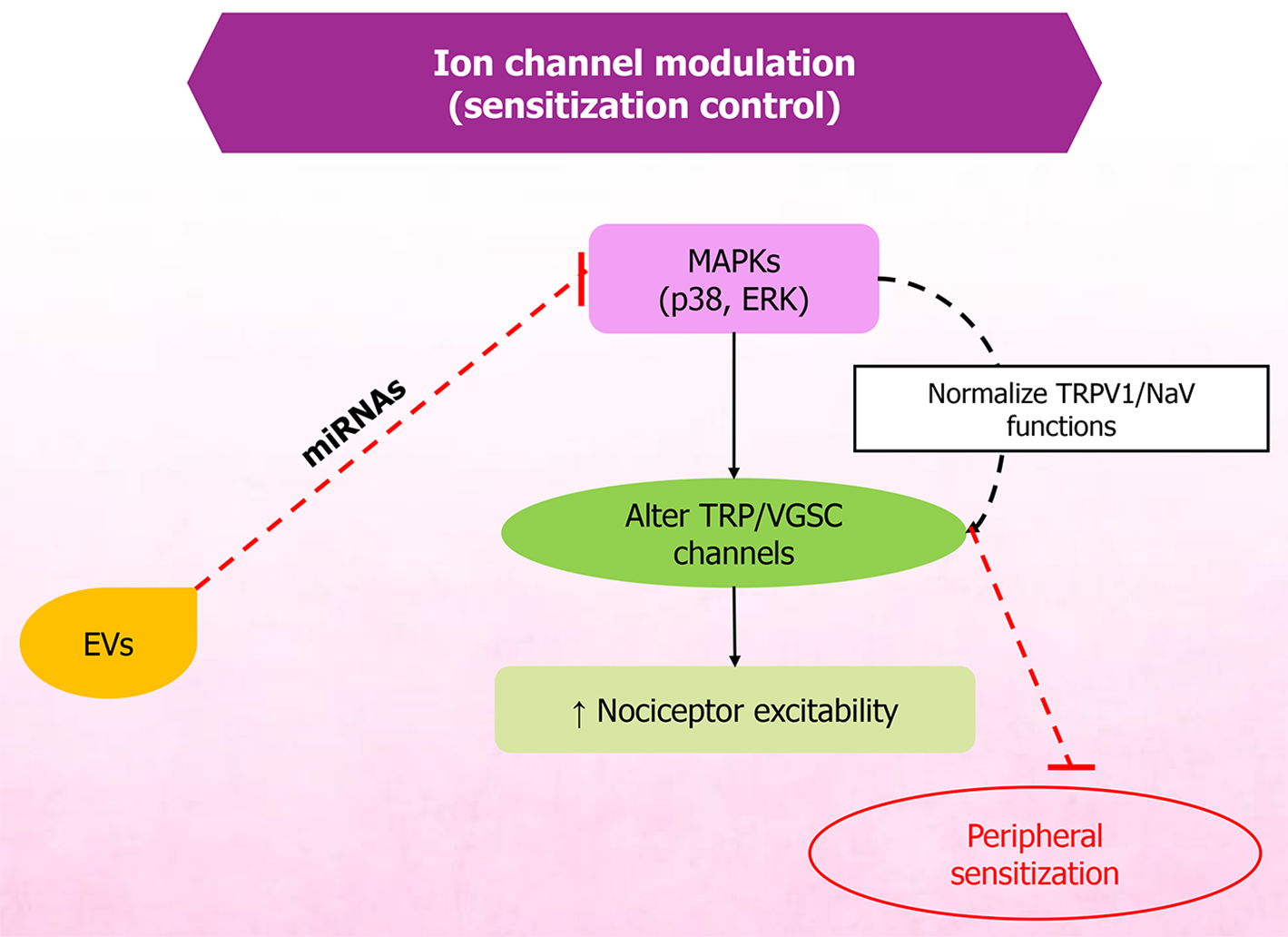
Figure 5 Extracellular vesicle-mediated modulation of mitogen-activated protein kinase and ion channel signaling in peripheral sen
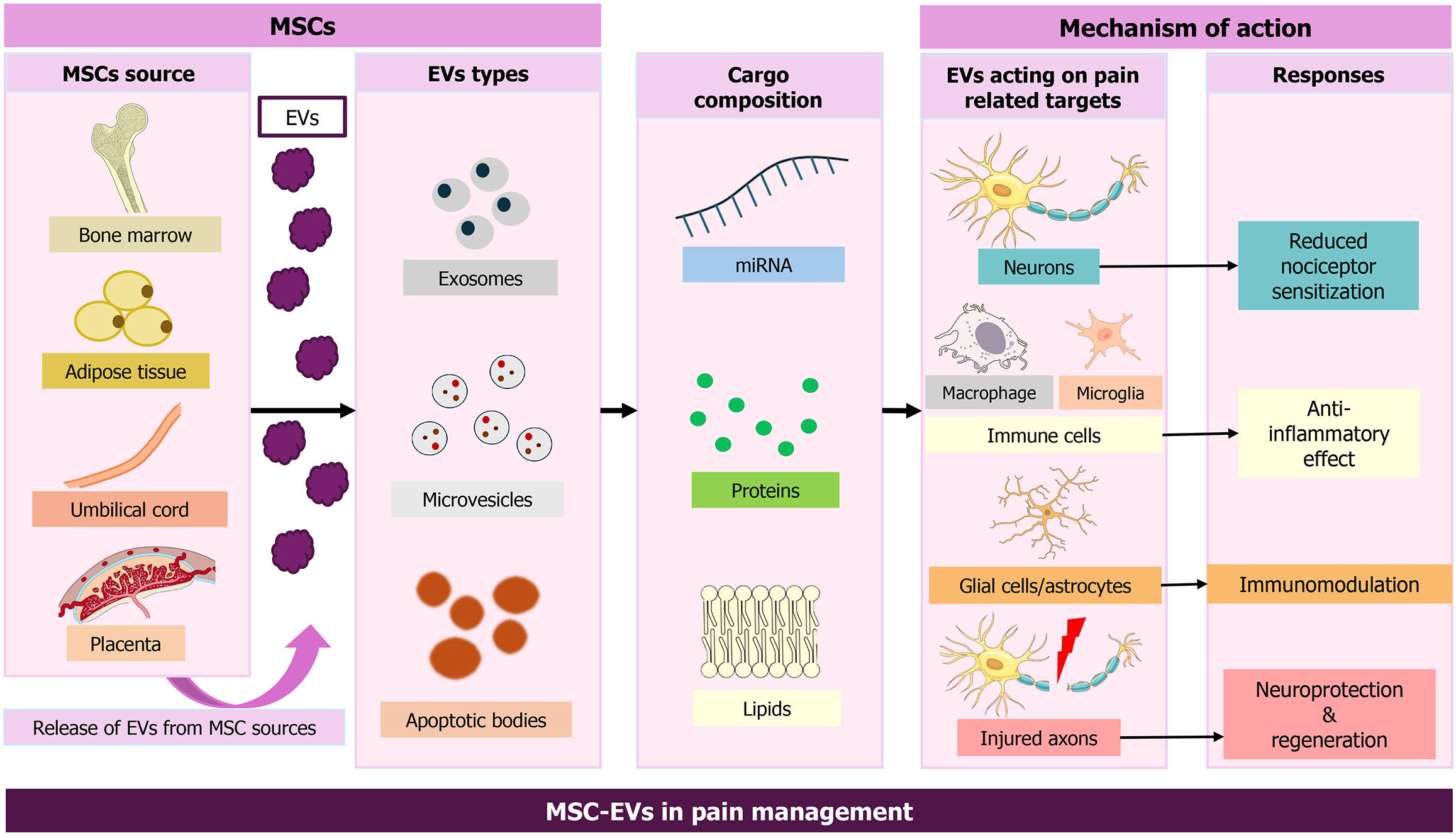
Figure 6 Summary of how mesenchymal stem cell-derived extracellular vesicles (from source to response) help in pain management.
Mesenchymal stem cells from various tissue sources secrete various types of extracellular vesicles, which contain bioactive cargo such as microRNAs, proteins, and lipids. These mesenchymal stem cell-derived extracellular vesicles target major pain-associated cells that result in therapeutic outcomes encompassing anti-inflammatory effects, neuroprotection, immunomodulation, and neuroprotection, thereby demonstrating their multifaceted mechanisms in reducing pain. MSCs: Mesenchymal stem cells; EVs: Extracellular vesicles; miRNAs: MicroRNAs; MSC-EVs: Mesenchymal stem cell-derived extracellular vesicles.
- Citation: Khan SA, Gangadaran P, Tiwari P, Rajendran RL, Jamal A, Hattiwale SH, Anand K, Jha SK, Hong CM, Ahn BC, Parvez S. Therapeutic applications of mesenchymal stem cell-derived extracellular vesicles in pain management: A narrative review of emerging evidence and future directions. World J Stem Cells 2026; 18(2): 116184
- URL: https://www.wjgnet.com/1948-0210/full/v18/i2/116184.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v18.i2.116184