©The Author(s) 2026.
World J Stem Cells. Jan 26, 2026; 18(1): 114119
Published online Jan 26, 2026. doi: 10.4252/wjsc.v18.i1.114119
Published online Jan 26, 2026. doi: 10.4252/wjsc.v18.i1.114119
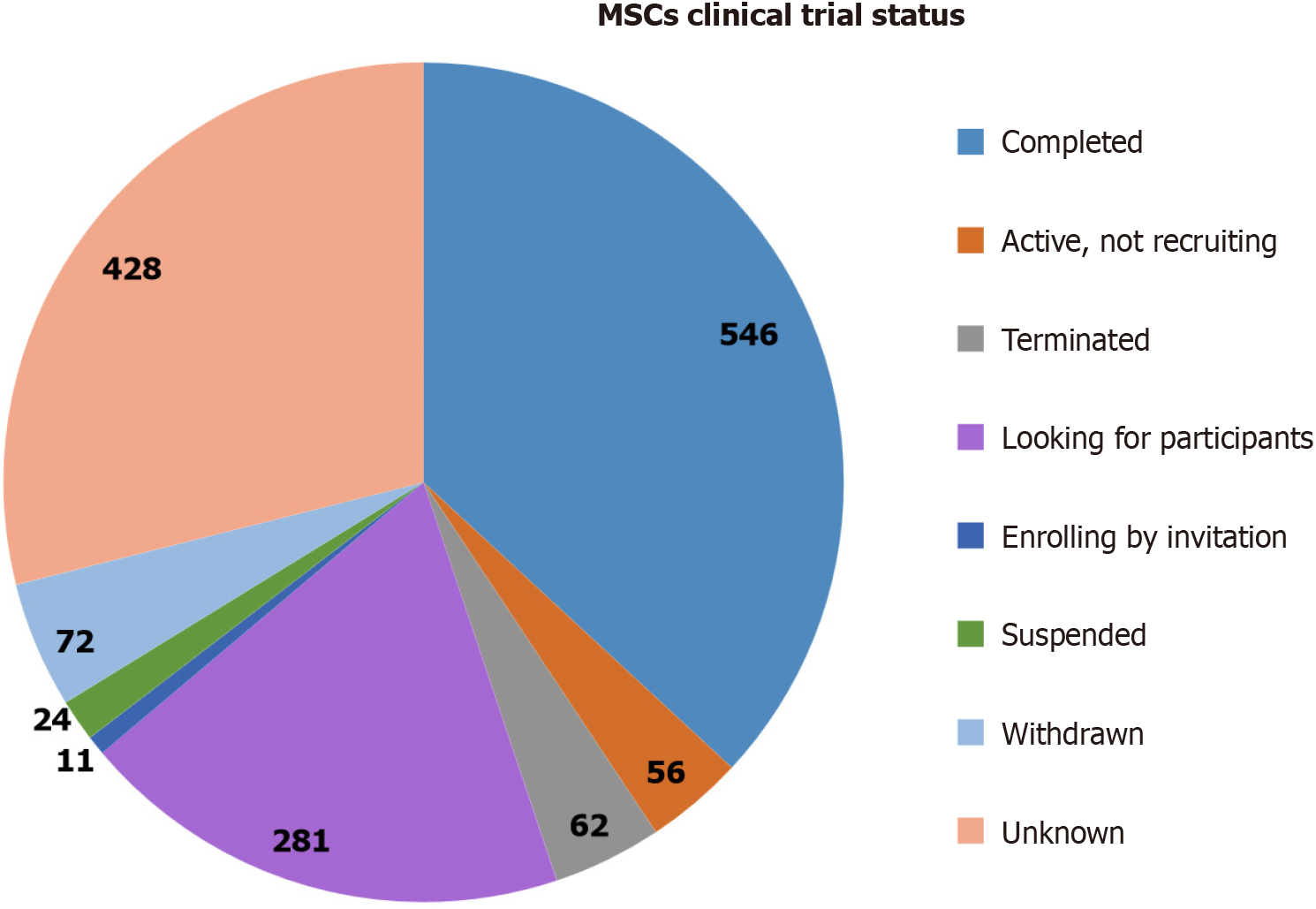
Figure 1 Clinical trials status of mesenchymal stem cell-based therapies.
This pie chart displays the distribution of clinical trials related to mesenchymal stem cells, showing various statuses of these trials. The majority of trials are either completed (546 trials) or active but not recruiting (428 trials). A smaller number of trials are enrolled by invitation (281 trials) and seeking participants (62 trials). There are also trials that are suspended (24 trials), terminated (72 trials), withdrawn (11 trials), or have an unknown status (36 trials). Each section of the chart is color-coded to indicate the respective trial status. MSCs: Mesenchymal stem cells.
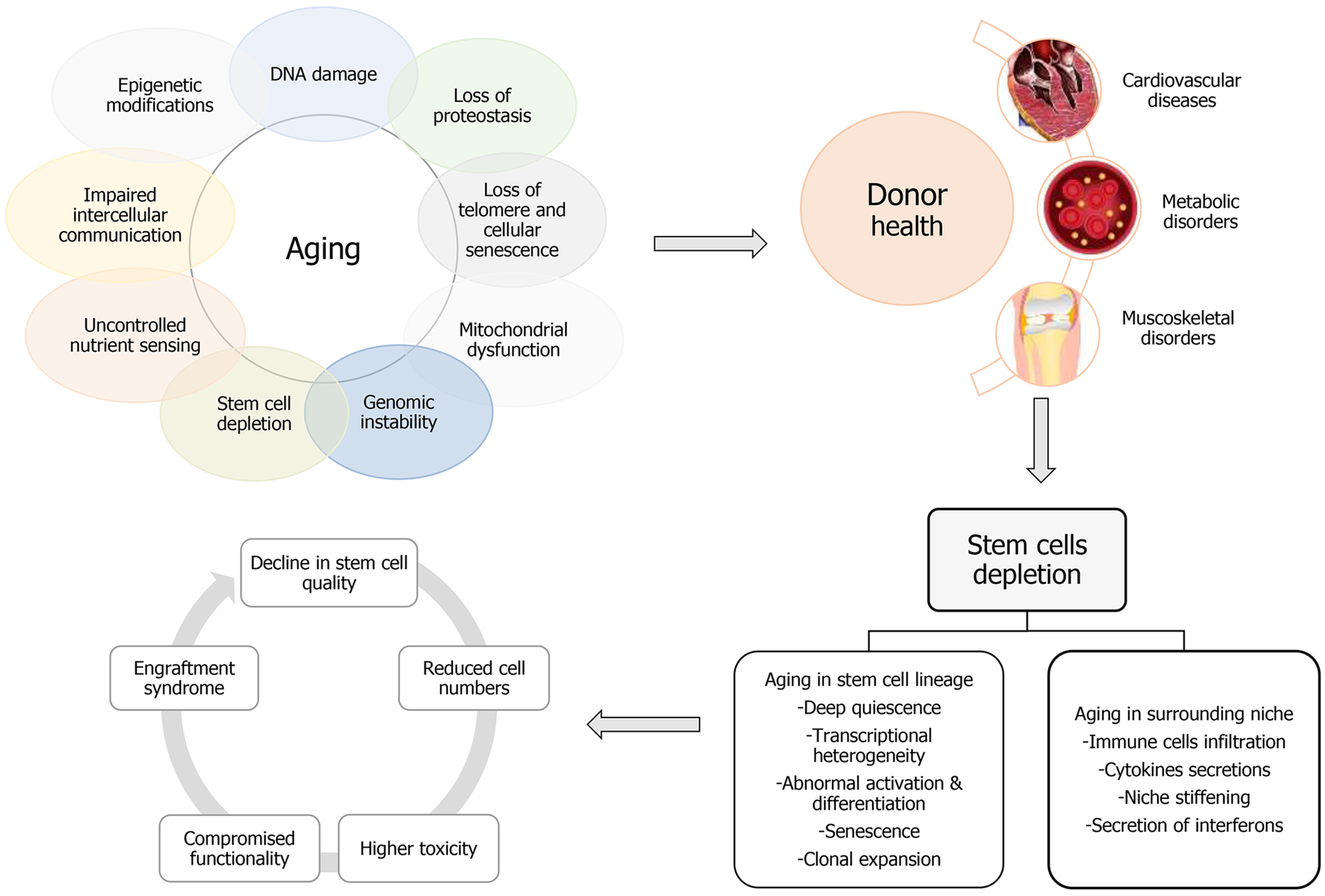
Figure 2 The aging puzzle in autologous stem cell therapy.
Schematic representation illustrating the complex interplay between aging, health status, and the regenerative potential of autologous stem cells. As the donor’s age increases and health status decreases, the regenerative capabilities of stem cells are compromised. This figure emphasizes the challenges associated with utilizing autologous stem cell therapy in the elderly, prompting the exploration of alternative strategies to enhance regenerative potential.
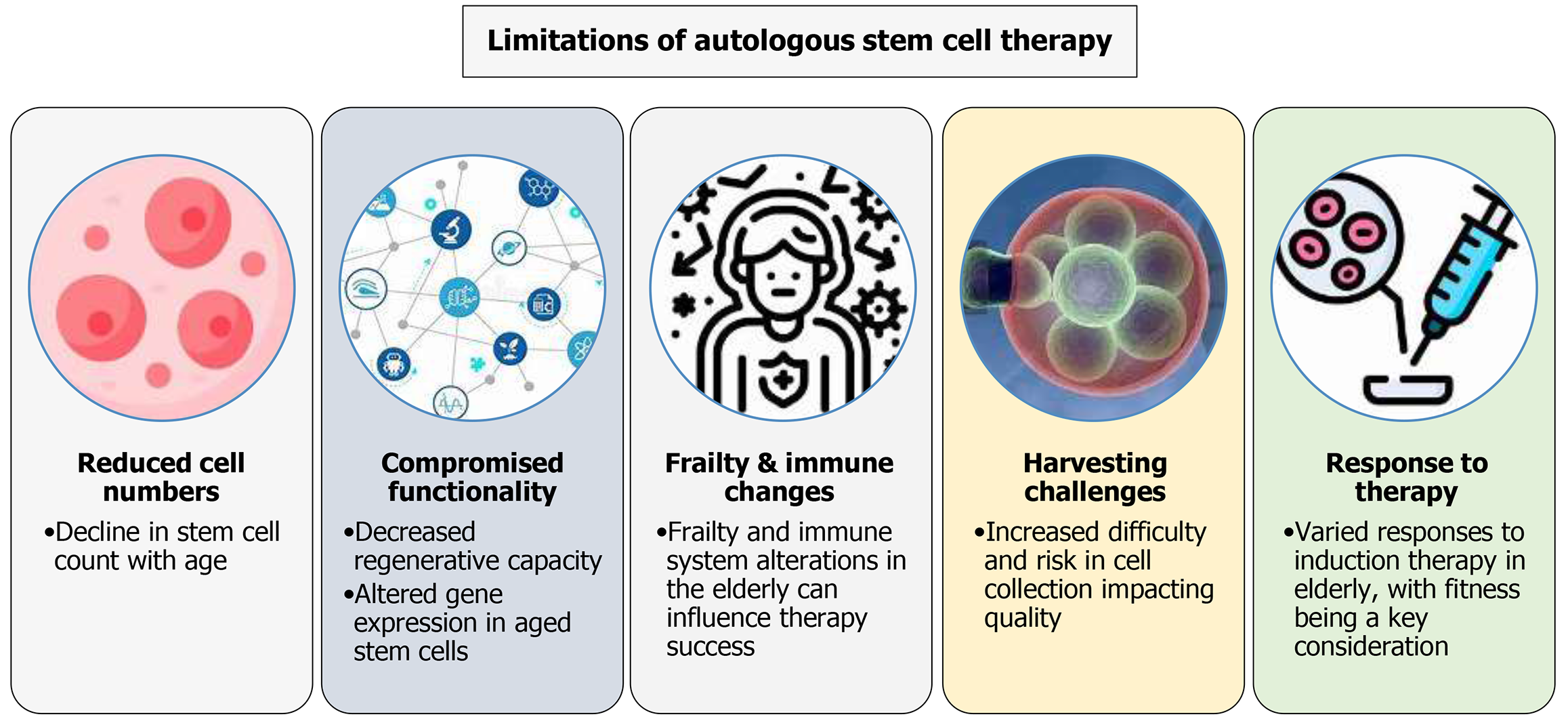
Figure 3 Limitations of autologous stem cell therapy in the elderly.
This figure outlines the key limitations of autologous stem cell therapy in elderly pa
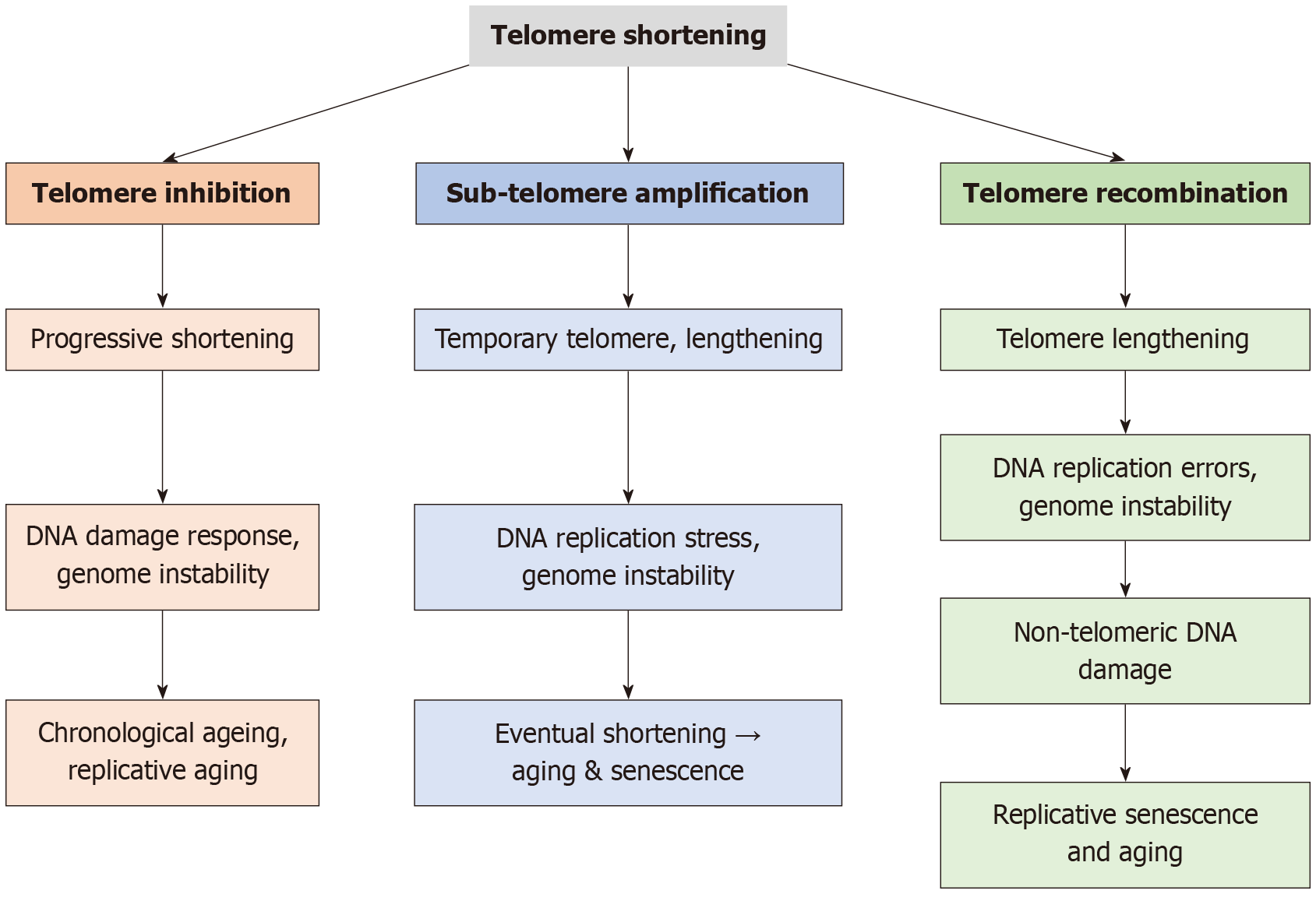
Figure 4 The cellular consequences of telomere shortening through three distinct pathways: Telomere inhibition, sub-telomere amp
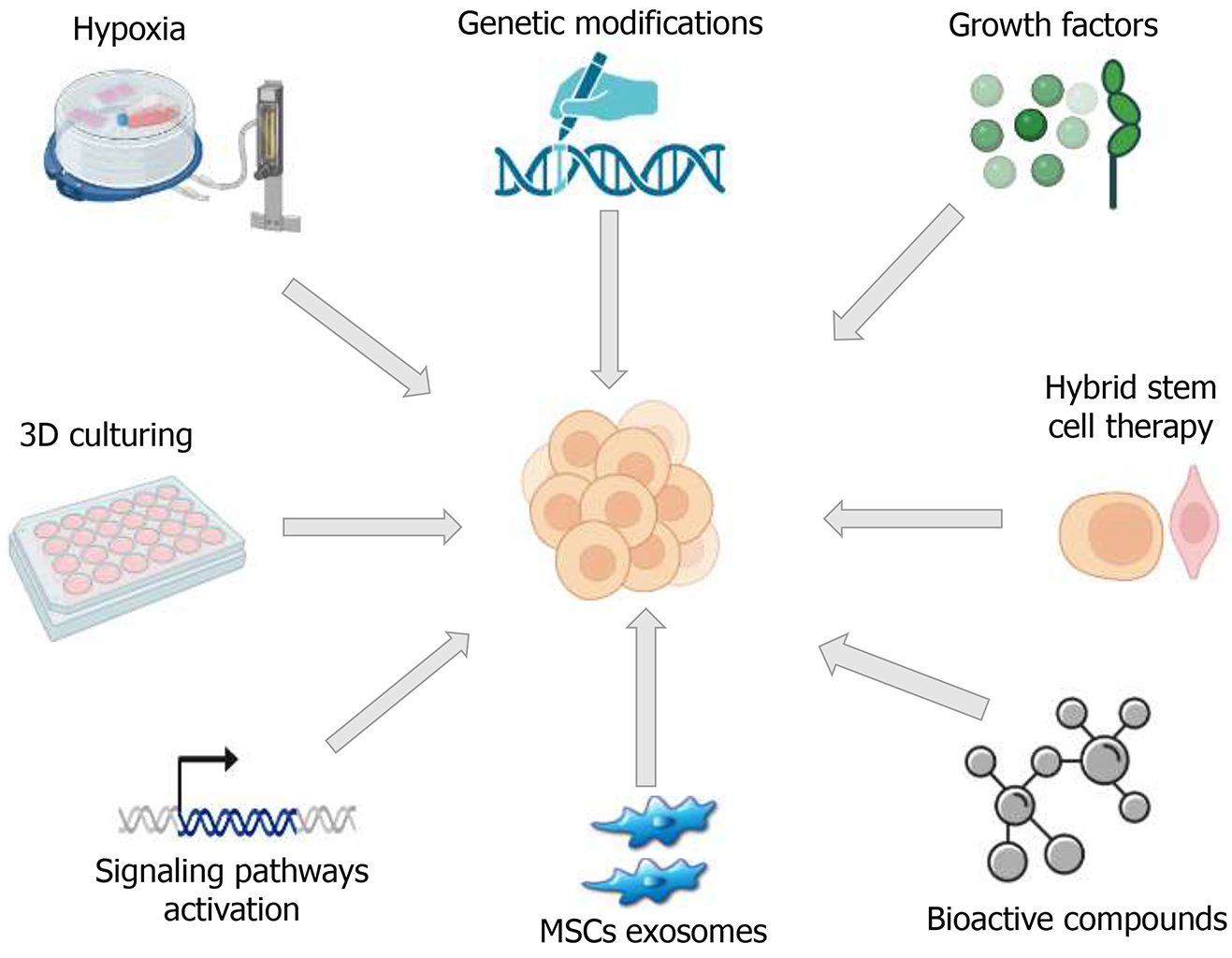
Figure 5 Overview of emerging approaches to restore the regenerative capacity of stem cells compromised by age and health status.
These include hypoxic conditioning, genetic modifications, activation of signaling pathways, growth factor supplementation, bioactive compounds, exosome therapy, hybrid cell strategies, and advanced three-dimensional culture systems. Applying these methods to aged stem cells may rejuvenate their function and open new avenues for therapeutic interventions in elderly patients. 3D: Three-dimensional; MSCs: Mesenchymal stem cells.
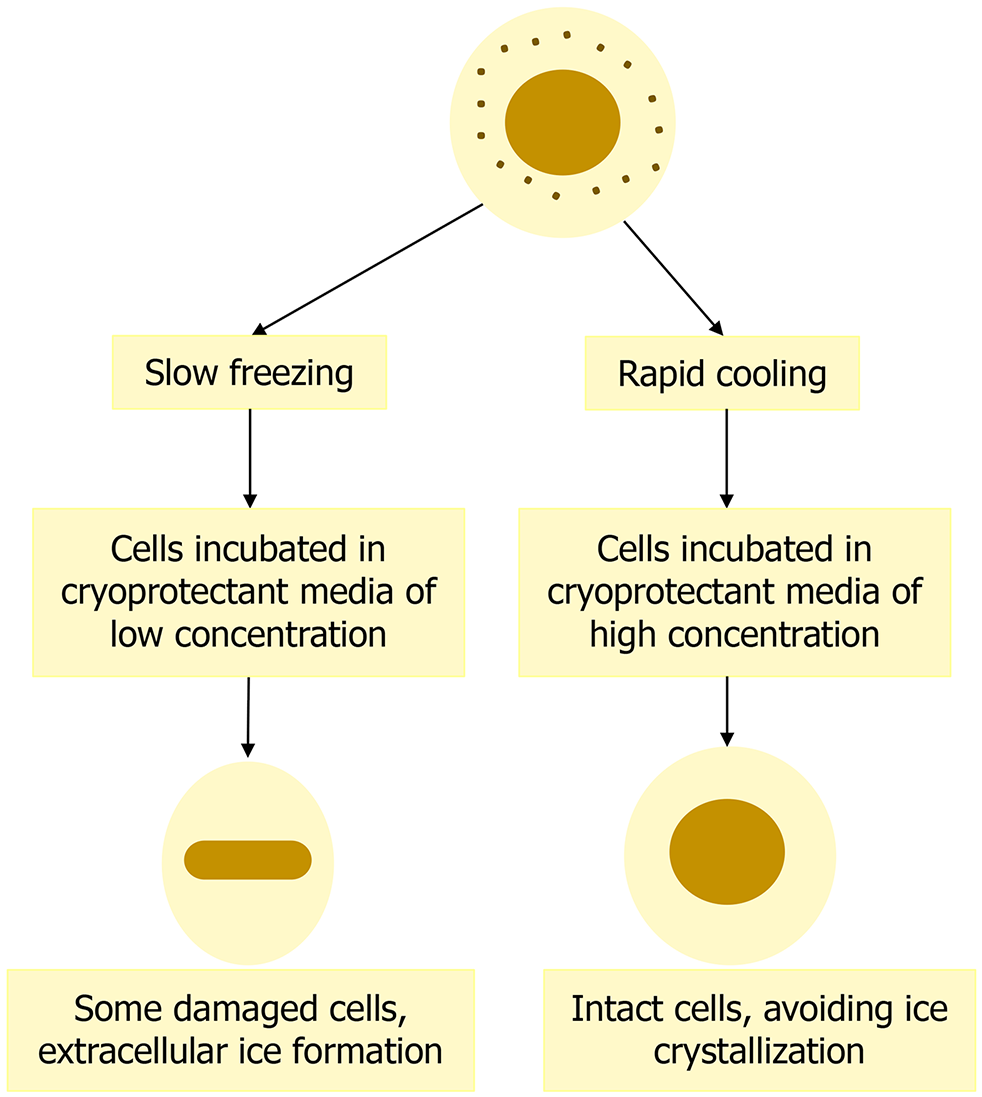
Figure 6 Cryopreservation of cells at slow freezing and rapid cooling.
This figure illustrates the two main cryopreservation techniques, slow freezing and rapid cooling. In the slow freezing method, cells are incubated in a cryoprotectant medium with low concentration, leading to some cell damage and extracellular ice formation. In contrast, rapid cooling involves the use of a cryoprotectant medium with high concentration, preserving cell integrity and preventing the formation of ice crystals within the cells.
- Citation: Choudhery MS, Arif T, Mahmood R. Aging puzzle: A closer look on the complex dilemma of autologous stem cell therapy. World J Stem Cells 2026; 18(1): 114119
- URL: https://www.wjgnet.com/1948-0210/full/v18/i1/114119.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v18.i1.114119