Published online Jan 26, 2026. doi: 10.4252/wjsc.v18.i1.114751
Revised: November 2, 2025
Accepted: December 8, 2025
Published online: January 26, 2026
Processing time: 107 Days and 12.1 Hours
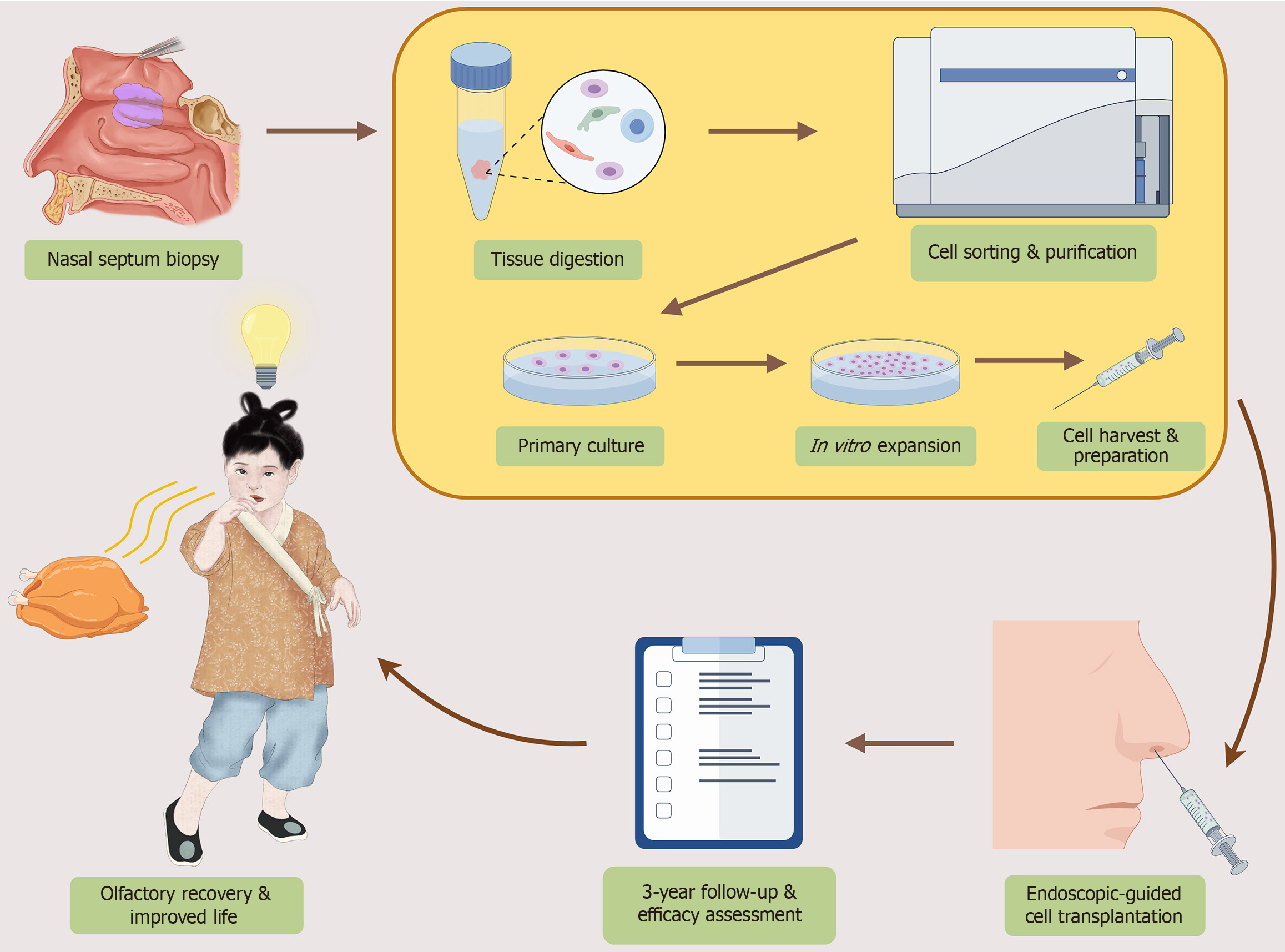
A recently published prospective study marks a breakthrough for congenital olfactory disorders in children. The study provides the first long-term, three-year follow-up data, robustly demonstrating the durable efficacy and safety of au
Core Tip: This letter highlights a landmark prospective study that provides the first three-year follow-up evidence of autologous nasal epithelial stem cell transplantation in children with congenital olfactory disorders. The findings confirm durable efficacy and safety, transforming what was once deemed untreatable into a feasible therapeutic pathway. However, to establish this pioneering approach as a global standard, multicenter, controlled clinical trials are urgently required to validate and extend these promising results.
- Citation: Chen GY, Kang JB, Wang YK, Liu M. Dawn of a new era in olfactory regeneration: Pediatric stem cell therapy enters the era of long-term validation. World J Stem Cells 2026; 18(1): 114751
- URL: https://www.wjgnet.com/1948-0210/full/v18/i1/114751.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v18.i1.114751
Olfactory dysfunction is increasingly recognized as a significant public health issue, with more than 20% of the adult population affected when partial loss is included[1]. Beyond its sensory role, impaired olfaction compromises nutrition and safety awareness and contributes to psychosocial consequences, including depression and diminished quality of life[2]. While most cases are acquired, typically resulting from upper respiratory infections, sinonasal disease, trauma, or aging, congenital olfactory disorders (CODs) represent a rare but particularly impactful subset. Epidemiological estimates suggest that approximately 1 in 10000 individuals are born with congenital anosmia, encompassing both syndromic and non-syndromic forms[3]. Despite their low prevalence, CODs impose disproportionate burdens by affecting appetite regulation, developmental milestones, and emotional well-being, while no approved or standardized therapeutic interventions currently exist, leaving affected children and families without viable treatment options[4,5].
Preclinical research on olfactory regeneration has relied on several inducible hyposmia models, such as zinc sulfate (ZnSO4) intranasal instillation[6] and methimazole systemic administration[7], both of which selectively ablate olfactory receptor neurons and basal stem cells in rodents. Using these models, different cell types - including olfactory en
Against this backdrop, the recent prospective clinical study by Ni et al[14] constitutes a genuine milestone. Enrolling fifty pediatric patients with CODs, the investigators performed autologous NESC transplantation and conducted three-year longitudinal follow-up. Their findings demonstrate not only robust improvement in validated olfactory function tests (Sniffin’ Sticks and UPSIT-Children’s Version), but also significant enhancement in quality of life indices. Safety outcomes were particularly reassuring: No serious adverse events were observed, and only minor, self-limiting complications occurred in a small fraction of patients. Importantly, endoscopic and electro-olfactogram assessments corroborated functional recovery with morphological and electrophysiological evidence of olfactory epithelium regeneration. It is worth noting that stem cell-based approaches for olfactory regeneration have been extensively explored in preclinical and translational settings. Rodent and other experimental models of chemically or surgically induced anosmia have demonstrated that MSCs, olfactory ensheathing cells, and nasal epithelial progenitors can promote epithelial repair and behavioral recovery, providing a solid mechanistic and translational foundation for future clinical application[9,11,15-20] (Table 1). Taken together, this milestone study by Ni et al[14] provides the first robust, long-term pediatric clinical evidence supporting stem cell-based therapy for CODs, establishing both safety and sustained functional recovery over three years (Figure 1).
| Ref. | Cell type | Study model | Route of administration | Evaluation period | Main outcome |
| Kurtenbach et al[15], 2019 | c-KIT(+) globose basal cells (olfactory progenitors) | Mouse (hyposmia model) | Intranasal droplet delivery | 3-4 weeks (EOG/histology); 8-10 weeks (behavior) | Intranasally delivered c-KIT(+) progenitors generated olfactory neurons and restored function |
| Jiang et al[16], 2025 | Human olfactory neurospheres | Mouse (methimazole-induced hyposmia) | Intranasal delivery | 4 weeks (histology/behavior) | DNS-ONS showed superior regenerative capacity and function restoration compared to CRS-ONS |
| Ishikura et al[11], 2023 | Mouse ADSCs | Mouse (methimazole-induced OE damage) | Nasal administration | 14 days [histology (aversion test)] | ADSCs promote OE regeneration (OMP increase) and support the recovery of odor aversion behavior |
| Kim et al[17], 2009 | Rat ADSCs | Rat (olfactory nerve transection) | Intravenous injection | 30 days (histology) | Systemic ADSCs promote OE regeneration (OMP/PCNA increase) following nerve transection |
| Franceschini et al[18], 2014 | Human ADSCs | Mouse (dichlobenil-induced OE lesion) | Tail vein injection | 30 days and 60 days (EOG/histology) | Human ADSCs engraft, induce OE regeneration, and restore functional reactivity (EOG) |
| Hazir et al[19], 2025 | NSCs | Mouse (3-MI-induced anosmia) | Intranasal delivery | 2 weeks (early histology); 4 weeks [histology/behavior (FFT)/PCR] | NSCs transplantation promotes functional recovery (FFT) and OE epithelial repair |
| Lee et al[9], 2010 | NSCs | Mouse (3-MI-induced anosmia) | Intranasal delivery | 4 weeks [histology/behavior (FFT)/western blot] | NSCs transplantation promoted recovery of olfactory function and improved survival |
| Jo et al[20], 2015 | BMSCs | Rat (TX-100-induced OE damage) | Direct OE injection | 2 weeks and 4 weeks [histology/behavior (FFT)/IHC/qRT-PCR] | BMSCs transplantation promoted OE and olfactory function restoration, associated with NGF and BDNF regulation |
The study provides rare longitudinal evidence of recovery in CODs. The combination of functional gains, quality of life improvement, and reassuring safety profile underscores the therapeutic potential of NESCs. Importantly, the morphological and electrophysiological findings suggest not only symptomatic relief but also structural repair of the olfactory mucosa. To put these findings into context, the demonstration of three-year functional recovery in CODs contrasts with most prior attempts at sensory neural regeneration, such as in retinal or auditory systems, which have shown only partial or transient benefits[21,22]. By contrast, Ni et al[14] provide the first durable, longitudinal evidence in a pediatric sensory disorder.
Despite these promising results, key limitations restrict generalizability. The absence of a randomized control arm and the modest sample size are consistent with challenges faced across early-stage neural cell therapy trials. Similar issues were observed in Parkinson’s disease stem-cell transplantation studies, where small, open-label cohorts initially suggested efficacy, but randomized trials tempered expectations[23,24]. These precedents highlight why multicenter randomized controlled trials are indispensable before this therapy can be considered standard of care[25]. Future controlled studies should further refine inclusion criteria, optimize transplantation protocols, and ensure extended follow-up for pediatric safety. These trials should refine inclusion criteria such as age and residual olfactory function, optimize dosing and transplantation protocols, and include rigorous safety endpoints. Extended long-term monitoring beyond three years will be critical, especially in children, to capture rare or delayed adverse effects.
Mechanistically, it remains unclear whether olfactory recovery results from neuronal replacement, paracrine signaling, or niche reactivation. Current evidence suggests that the beneficial effects of NESC transplantation may be largely driven by paracrine actions. Transplanted olfactory-supporting cells and MSCs have been shown to secrete neurotrophic and immunomodulatory factors (e.g., nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neu
Recent single-cell genomic and transcriptomic studies have shown the potential to delineate cellular plasticity and regenerative capacity[30], providing a framework for dissecting these mechanisms in future work. Elucidating these biological processes will be critical for optimizing therapeutic strategies and predicting long-term safety. Recent single-cell transcriptomic studies of human olfactory epithelium revealed profound aging-associated stem cell alterations, implicating niche dysfunction as a central barrier to regeneration[31,32]. In parallel, mechanistic studies using animal models, organoid systems, and advanced imaging are indispensable to clarify how regeneration is achieved and how grafted cells behave over time. For instance, in vivo two-photon microscopy provides high-resolution, non-invasive tracking of cell dynamics in living tissues, offering a powerful tool for monitoring graft behavior[33]. In addition, nasal and olfactory organoids have been successfully established to model epithelial differentiation and to test regenerative cues, thereby providing a complementary platform to de-risk future human trials[34]. Integrating such systems into preclinical pipelines would allow mechanistic validation - clarifying whether transplanted cells integrate, secrete trophic factors, or activate endogenous repair - before large-scale pediatric randomized controlled trials. In addition, leveraging recent advances in olfactory stem cell biology, including the roles of horizontal basal cells and globose basal cells in mucosal regeneration and aging-associated remodeling[31], inducible grafting of olfactory epithelial cell populations[35], and nasally delivered mesenchymal or adipose-derived stem cells secreting neurotrophic factors[11], may accelerate improvements in both outcome and feasibility.
Moving forward, translational progress will rely on practical optimization - improving graft survival and integration, engineering biomaterial scaffolds to guide axonal reconnection, and establishing scalable good manufacturing practice-compliant cell manufacturing. Advances in other regenerative fields suggest that paracrine modulation, immune engineering, and biomaterial design often yield synergistic benefits[11,36]. Finally, international collaborative networks will be essential to harmonize regulatory standards, share long-term safety data, and ensure equitable access. Ni et al’s work[14] offers compelling proof-of-concept for olfactory regeneration, marking a pivotal advance in the field. To translate this innovation into reproducible and globally accessible clinical care, future efforts must focus on mechanistic studies using organoid and animal models, alongside rigorous adherence to translational best practices.
In summary, Ni et al’s study[14] provides the first durable clinical evidence for stem cell therapy in CODs, redefining a condition long regarded as untreatable. While replication in multicenter trials and deeper mechanistic insights are essential, this work establishes a foundation upon which true therapeutic innovation in sensory regeneration can be built.
| 1. | Whitcroft KL, Altundag A, Balungwe P, Boscolo-Rizzo P, Douglas R, Enecilla MLB, Fjaeldstad AW, Fornazieri MA, Frasnelli J, Gane S, Gudziol H, Gupta N, Haehner A, Hernandez AK, Holbrook EH, Hopkins C, Hsieh JW, Huart C, Husain S, Kamel R, Kim JK, Kobayashi M, Konstantinidis I, Landis BN, Lechner M, Macchi A, Mazal PP, Miri I, Miwa T, Mori E, Mullol J, Mueller CA, Ottaviano G, Patel ZM, Philpott C, Pinto JM, Ramakrishnan VR, Roth Y, Schlosser RJ, Stjärne P, Van Gerven L, Vodicka J, Welge-Luessen A, Wormald PJ, Hummel T. Position paper on olfactory dysfunction: 2023. Rhinology. 2023;61:1-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 2. | Lipp C, Laamari L, Bertsch A, Podlesek D, Bensafi M, Hummel T, Brugger J. Devices for the electrical stimulation of the olfactory system: A review. Biosens Bioelectron. 2025;271:117063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Kamarck ML, Trimmer C, Murphy NR, Gregory KM, Manoel D, Logan DW, Saraiva LR, Mainland JD. Identifying candidate genes underlying isolated congenital anosmia. Clin Genet. 2024;105:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Boesveldt S, Parma V. The importance of the olfactory system in human well-being, through nutrition and social behavior. Cell Tissue Res. 2021;383:559-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 5. | Jaime-Lara RB, Brooks BE, Vizioli C, Chiles M, Nawal N, Ortiz-Figueroa RSE, Livinski AA, Agarwal K, Colina-Prisco C, Iannarino N, Hilmi A, Tejeda HA, Joseph PV. A systematic review of the biological mediators of fat taste and smell. Physiol Rev. 2023;103:855-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 6. | McBride K, Slotnick B, Margolis FL. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem Senses. 2003;28:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Ren W, Ma Z, Wang L, Feng X, Yu H, Yu Y. Lgr5(+) cells are required and dynamically participate in olfactory epithelium regeneration: a revisiting shows Lgr5 expression in multiple cell lineages. Theranostics. 2022;12:5631-5644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Johansson S, Lee IH, Olson L, Spenger C. Olfactory ensheathing glial co-grafts improve functional recovery in rats with 6-OHDA lesions. Brain. 2005;128:2961-2976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Lee CH, Jeon SW, Seo BS, Mo JH, Jeon EH, Choi AR, Kim JW. Transplantation of neural stem cells in anosmic mice. Clin Exp Otorhinolaryngol. 2010;3:84-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 11. | Ishikura T, Shiga H, Nakamura Y, Kanitani T, Ishigaki Y, Miwa T. Olfactory Regeneration with Nasally Administered Murine Adipose-Derived Stem Cells in Olfactory Epithelium Damaged Mice. Cells. 2023;12:765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Jaloux C, Bonnet M, Vogtensperger M, Witters M, Veran J, Giraudo L, Sabatier F, Michel J, Legré R, Guiraudie-Capraz G, Féron F. Human nasal olfactory stem cells, purified as advanced therapy medicinal products, improve neuronal differentiation. Front Neurosci. 2022;16:1042276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Kim DH, Wang M, Kim S, Jang DW, Ko T, Goldstein BJ. Strategies to Develop Regenerative Medicine Approaches for Olfactory Disorders. Clin Exp Otorhinolaryngol. 2025;18:204-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Ni X, Shi J, Ning J, Tian XL. Long-term follow-up of autologous nasal epithelial stem cell transplantation for congenital olfactory disorders in children. World J Stem Cells. 2025;17:109942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Kurtenbach S, Goss GM, Goncalves S, Choi R, Hare JM, Chaudhari N, Goldstein BJ. Cell-Based Therapy Restores Olfactory Function in an Inducible Model of Hyposmia. Stem Cell Reports. 2019;12:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Jiang RS, Lee CW, Lin YH, Wang JJ, Liao JB, Peng KT, Chiang YC, Chi PL. Differential efficacy of olfactory neurospheres from deviated nasal septum and chronic rhinosinusitis patients in regenerating olfactory epithelium. Stem Cell Res Ther. 2025;16:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Kim YM, Choi YS, Choi JW, Park YH, Koo BS, Roh HJ, Rha KS. Effects of systemic transplantation of adipose tissue-derived stem cells on olfactory epithelium regeneration. Laryngoscope. 2009;119:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Franceschini V, Bettini S, Pifferi S, Menini A, Siciliano G, Ognio E, Brini AT, Di Oto E, Revoltella RP. Transplanted human adipose tissue-derived stem cells engraft and induce regeneration in mice olfactory neuroepithelium in response to dichlobenil subministration. Chem Senses. 2014;39:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Hazir B, Ceylan A, Bagriacik EÜ, Dayanir D, Araz M, Ceylan BT, Oruklu N, Sahin MM. Publisher Correction: Effects of intranasal neural stem cells transplantation on olfactory epithelium regeneration in an anosmia-induced mouse model. Sci Rep. 2025;15:29721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Jo H, Jung M, Seo DJ, Park DJ. The effect of rat bone marrow derived mesenchymal stem cells transplantation for restoration of olfactory disorder. Biochem Biophys Res Commun. 2015;467:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010;16:193-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77:58-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 23. | Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, Jeon J, Cha Y, Kim K, Li Q, Henchcliffe C, Kaplitt M, Neff C, Rapalino O, Seo H, Lee IH, Kim J, Kim T, Petsko GA, Ritz J, Cohen BM, Kong SW, Leblanc P, Carter BS, Kim KS. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson's Disease. N Engl J Med. 2020;382:1926-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 388] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 24. | Liu Z, Cheung HH. Stem Cell-Based Therapies for Parkinson Disease. Int J Mol Sci. 2020;21:8060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 1049] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 26. | Yi KI, Park JH, Kim SD, Mun SJ, Cho KS. Stem Cells and Cell-Free Therapies for Olfactory Epithelium Regeneration: Insights from Experimental Models. Int J Mol Sci. 2025;26:9024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Chen DY, Zhang WJ, Zuo C, Xu YS, Fu LX. Immune characteristics of olfactory ensheathing cells and repair of nerve injury. Front Immunol. 2025;16:1571573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Rui K, Hong Y, Zhu Q, Shi X, Xiao F, Fu H, Yin Q, Xing Y, Wu X, Kong X, Xu H, Tian J, Wang S, Lu L. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate murine Sjögren's syndrome by modulating the function of myeloid-derived suppressor cells. Cell Mol Immunol. 2021;18:440-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 29. | Ge L, Xun C, Li W, Jin S, Liu Z, Zhuo Y, Duan D, Hu Z, Chen P, Lu M. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J Nanobiotechnology. 2021;19:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 30. | Salcher S, Sturm G, Horvath L, Untergasser G, Kuempers C, Fotakis G, Panizzolo E, Martowicz A, Trebo M, Pall G, Gamerith G, Sykora M, Augustin F, Schmitz K, Finotello F, Rieder D, Perner S, Sopper S, Wolf D, Pircher A, Trajanoski Z. High-resolution single-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Cancer Cell. 2022;40:1503-1520.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 313] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 31. | Li W, Wu T, Zhu K, Ba G, Liu J, Zhou P, Li S, Wang L, Liu H, Ren W, Yu H, Yu Y. A single-cell transcriptomic census of mammalian olfactory epithelium aging. Dev Cell. 2024;59:3043-3058.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Oliva AD, Gupta R, Issa K, Abi Hachem R, Jang DW, Wellford SA, Moseman EA, Matsunami H, Goldstein BJ. Aging-related olfactory loss is associated with olfactory stem cell transcriptional alterations in humans. J Clin Invest. 2022;132:e155506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Malide D. In Vivo Cell Tracking Using Two-Photon Microscopy. Methods Mol Biol. 2016;1444:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Liu J, Zhang Y, Yu Y. Establishment of nasal and olfactory epithelium organoids for unveiling mechanism of tissue regeneration and pathogenesis of nasal diseases. Cell Mol Life Sci. 2025;82:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 35. | Peterson J, Lin B, Barrios-Camacho CM, Herrick DB, Holbrook EH, Jang W, Coleman JH, Schwob JE. Activating a Reserve Neural Stem Cell Population In Vitro Enables Engraftment and Multipotency after Transplantation. Stem Cell Reports. 2019;12:680-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR, Heke M, Nguyen LT. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 630] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/