©The Author(s) 2025.
World J Stem Cells. Sep 26, 2025; 17(9): 108657
Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.108657
Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.108657
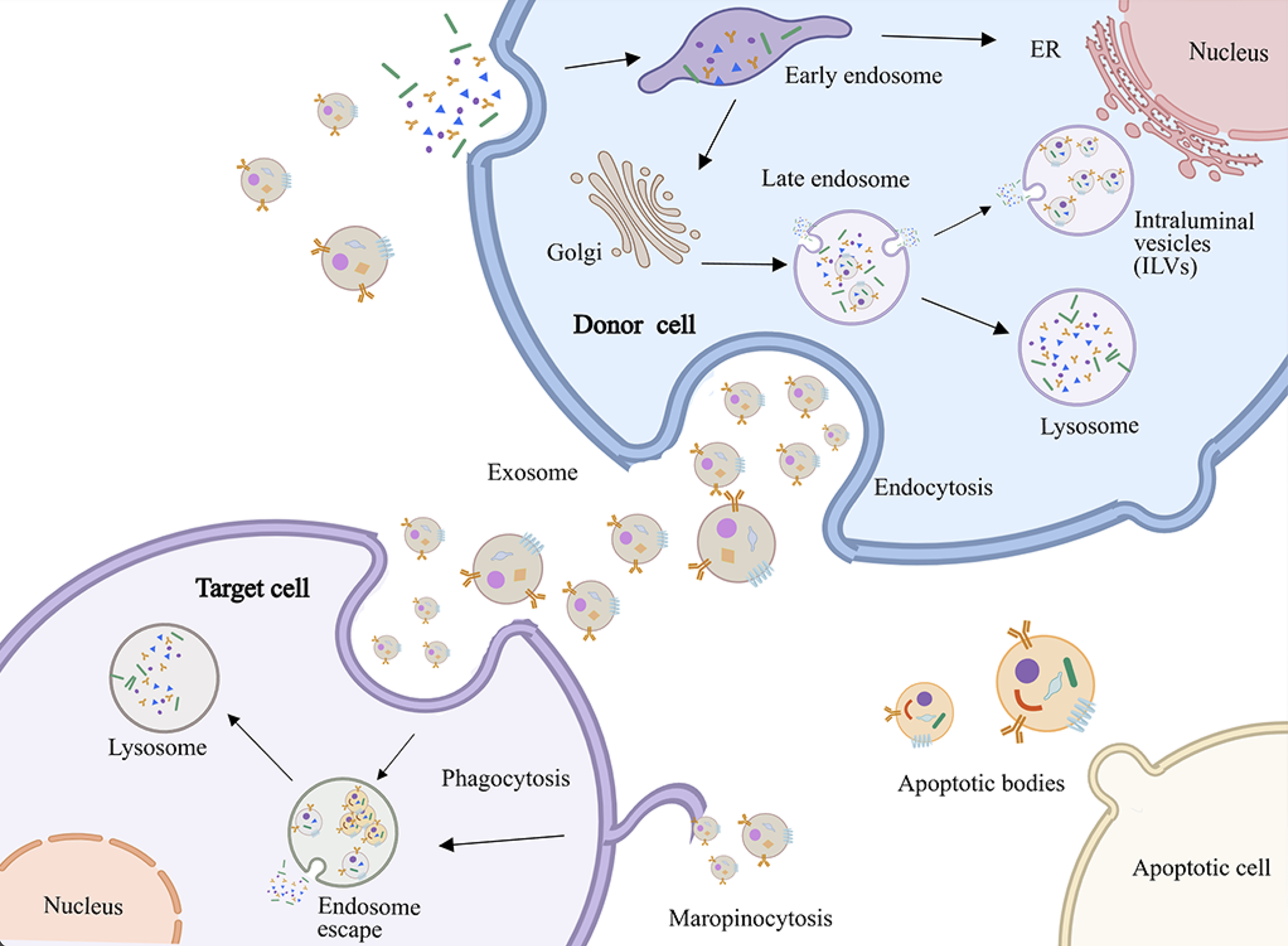
Figure 1 Biogenetic mechanism of extracellular vesicles.
The diagram depicts cellular processes including endocytosis, vesicle trafficking, and lysosomal degradation in donor cells. Recipient cells internalize exosomes via endocytosis or micropinocytosis, while phagocytosis mediates clearance of apoptotic bodies or apoptotic cells. ER: Endoplasmic reticulum.
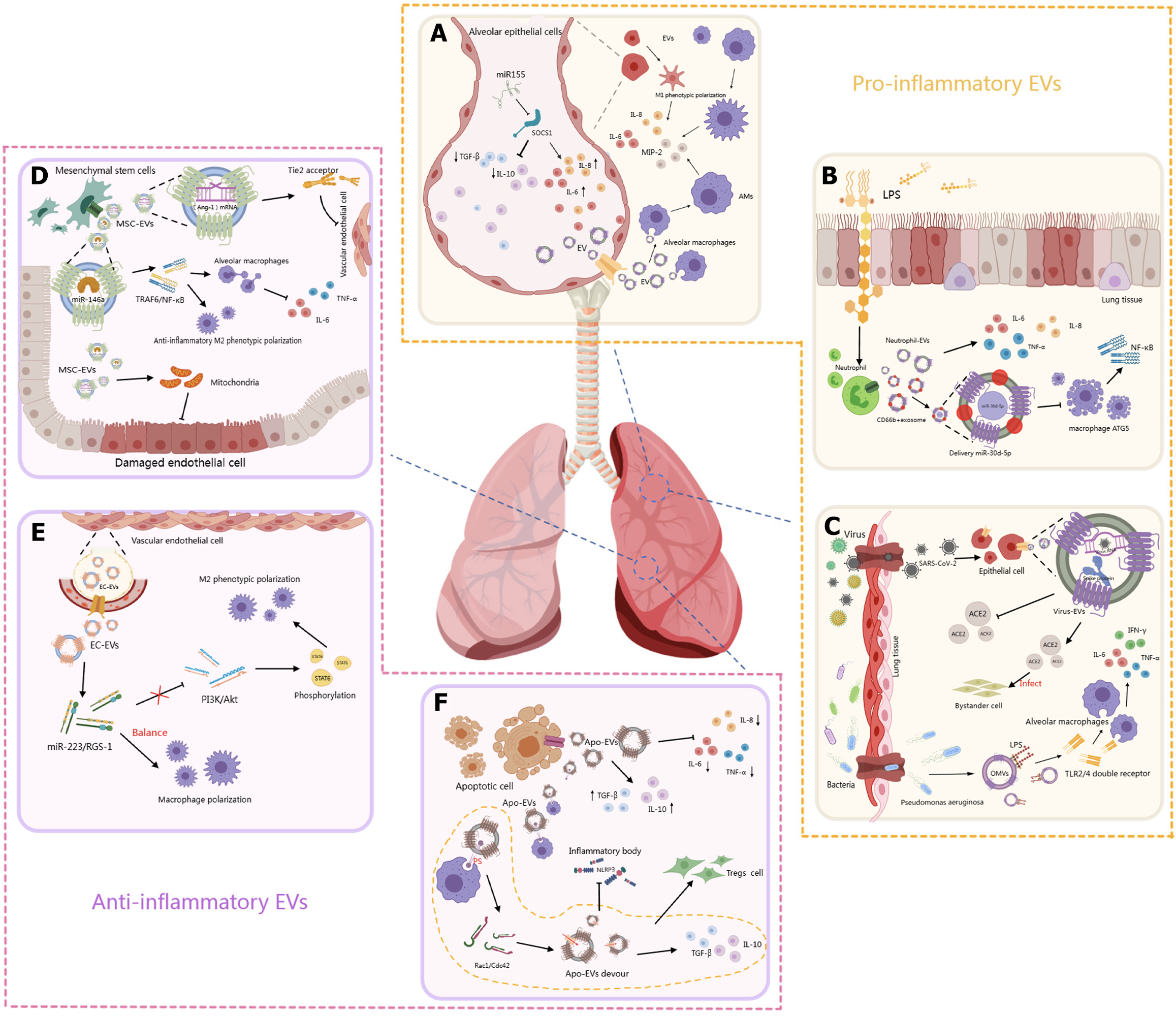
Figure 2 Pro-inflammatory and anti-inflammatory mechanisms of extracellular vesicles.
A: Alveolar epithelial cell-derived extracellular vesicles (EVs) contribute to lung inflammation by releasing pro-inflammatory factors such as miR-155, interleukin-1β, and interleukin-6. These EVs promote alveolar macrophage activation and cytokine release, exacerbating the inflammatory environment; B: In response to lipopolysaccharide and infection, lung epithelial cells release pro-inflammatory EVs carrying inflammatory mediators. These EVs stimulate macrophages and neutrophils, enhance nuclear factor-kappa B activation, and drive acute lung injury progression; C: Infections stimulate epithelial cells to release EVs containing viral RNA and cytokines, promoting alveolar macrophage M1 polarization and immune cell recruitment, thus amplifying pulmonary inflammation; D: Mesenchymal stem cell-derived extracellular vesicles attenuate lung injury by enhancing mitochondrial function, reducing endothelial damage, and promoting anti-inflammatory M2 macrophage polarization through tumor necrosis factor receptor-associated factor 1 inhibition and tumor necrosis factor-α downregulation; E: EVs from vascular endothelial cells promote M2 macrophage polarization via the phosphatidylinositol 3-kinase/protein kinase B pathway, mediated by miR-222 and miR-5. This maintains macrophage balance and contributes to resolution of inflammation in vascular-associated lung injury; F: Apoptotic bodies modulate immune responses by promoting regulatory T cell differentiation and reducing pro-inflammatory cytokines, thereby exerting anti-inflammatory effects and limiting tissue damage. EVs: Extracellular vesicles; SOCS1: Suppressing cytokine signaling suppressor 1; IL: Interleukin; TGF: Transforming growth factor; MIP-2: Macrophage inflammatory protein-2; AMs: Alveolar macrophages; PS: Phosphatidylserine; LPS: Lipopolysaccharide; TNF: Tumor necrosis factor; NF-κB: Nuclear factor-kappa B; ATG5: Autophagy related 5; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; ACE2: Angiotensin-converting enzyme 2; IFN: Interferon; TLR2/4: Toll-like receptor 2/4; OMVs: Outer membrane vesicles; Ang-1: Angiopoietin-1; MSC-EVs: Mesenchymal stem cell-derived extracellular vesicles; TRAF6: Tumor necrosis factor receptor-associated factor 6; EC-EVs: Endothelial cell-derived extracellular vesicles; PI3K: Phosphatidylinositol 3-kinase; Akt: Protein kinase B; STAT6: Signal transducer and activator of transcription 6; RGS-1: Regulator of G-protein signaling 1; Treg: Regulatory T cell; Apo-EVs: Apoptotic bodies; NLRP3: Nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain- containing receptor 3.
- Citation: Tie YF, Liu H, Zhang T, Meng TW, Liang Q. Dual role and clinical application of extracellular vesicles in acute respiratory distress syndrome: Mechanism analysis and translational challenges. World J Stem Cells 2025; 17(9): 108657
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/108657.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.108657