©The Author(s) 2025.
World J Stem Cells. Dec 26, 2025; 17(12): 110564
Published online Dec 26, 2025. doi: 10.4252/wjsc.v17.i12.110564
Published online Dec 26, 2025. doi: 10.4252/wjsc.v17.i12.110564
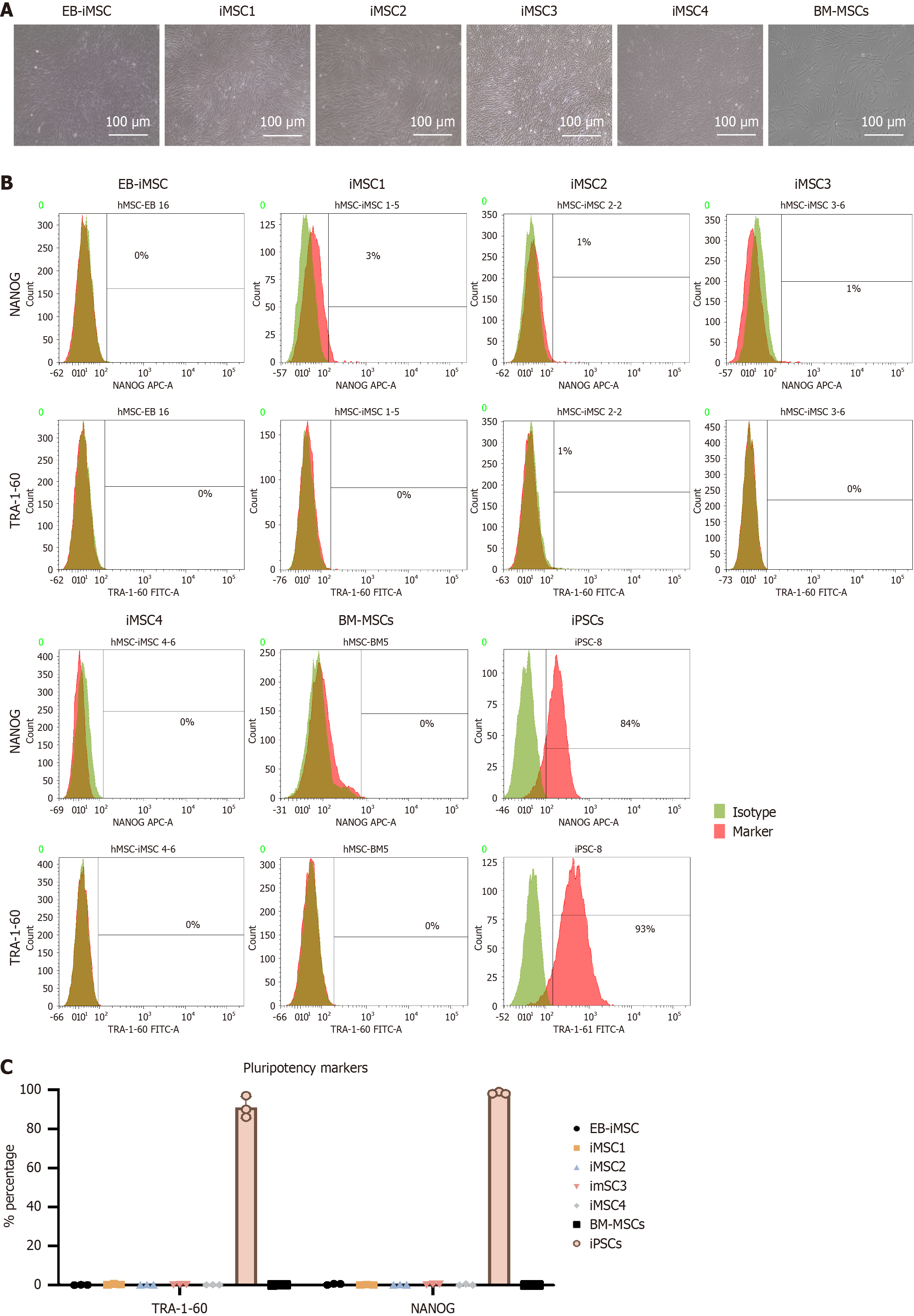
Figure 1 Flow cytometry analysis of pluripotency cell surface markers in induced pluripotent stem cells-derived mesenchymal stem cell lines generated using different protocols.
A: Representative microscopic images showing the morphology of induced pluripotent stem cells (iPSCs)-derived mesenchymal stem cell (iMSC) lines compared to bone marrow mesenchymal stem cells (BM-MSCs), scale bar = 100 μm; B: Flow cytometry histograms of pluripotency markers NANOG and TRA-1-60 in iMSC lines compared to iPSCs (positive control) and BM-MSCs (negative control); C: Bar graph displaying the percentages of pluripotency markers in iMSC lines relative to control cells (BM-MSCs and iPSCs). Data represent mean ± SE from at least three independent technical replicates. BM-MSCs: Bone marrow mesenchymal stem cells; EB-iMSCs: Embryoid body-induced pluripotent stem cells-derived mesenchymal stem cells; iMSC: Induced pluripotent stem cells-derived mesenchymal stem cell; iPSCs: Induced pluripotent stem cells.
Figure 2 Flow cytometric analysis of human mesenchymal stem cell surface markers.
A-D: Quantification of CD90 (A), CD105 (B), CD73 (C), and CD44 (D) expression levels in induced pluripotent stem cells-derived mesenchymal stem cell lines compared to bone marrow mesenchymal stem cells. Statistical analysis was performed using ordinary one-way ANOVA, with bone marrow mesenchymal stem cells as the control group. Data represent mean ± SE from at least three independent technical replicates. aP ≤ 0.05; bP ≤ 0.01; cP ≤ 0.001. BM-MSCs: Bone marrow mesenchymal stem cells; EB-iMSCs: Embryoid body-induced pluripotent stem cells-derived mesenchymal stem cells; iMSC: Induced pluripotent stem cells-derived mesenchymal stem cell.
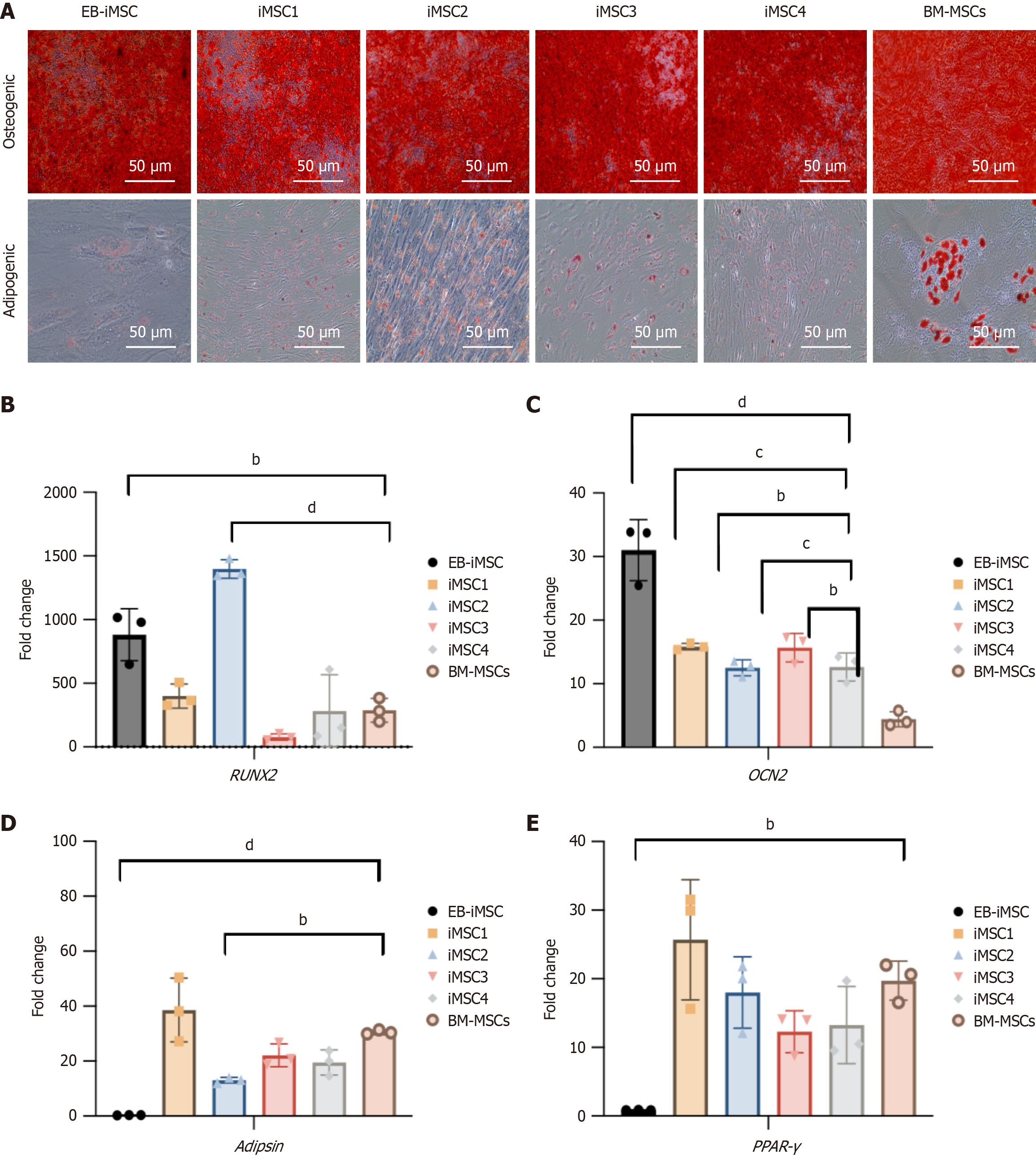
Figure 3 Analysis of osteogenic and adipogenic differentiation potential of induced pluripotent stem cells-derived mesenchymal stem cells.
A: Microscopy images showing red-stained calcium deposits in differentiated osteocytes (top row) and red-stained fat vacuoles in differentiated adipocytes (bottom row), scale bar = 50 μm; B and C: Quantitative reverse transcription polymerase chain reaction analysis of the two osteogenic-related genes runt-related transcription factor 2 (B) and OCN2 (C) in induced pluripotent stem cells-derived mesenchymal stem cells compared to bone marrow mesenchymal stem cells (control); D and E: Quantitative reverse transcription polymerase chain reaction analysis of the adipogenic-related genes Adipsin (D), and peroxisome proliferator-activated receptor gamma (E) in induced pluripotent stem cells-derived mesenchymal stem cells compared to bone marrow mesenchymal stem cells. Statistical analysis was performed using one-way ANOVA. All data are expressed as mean ± SE from at least three independent technical replicates. bP ≤ 0.01; cP ≤ 0.001; dP ≤ 0.0001. BM-MSCs: Bone marrow mesenchymal stem cells; EB-iMSCs: Embryoid body-induced pluripotent stem cells-derived mesenchymal stem cells; iMSC: Induced pluripotent stem cells-derived mesenchymal stem cell; RUNX2: Runt-related transcription factor 2; PPAR: Peroxisome proliferator-activated receptor.
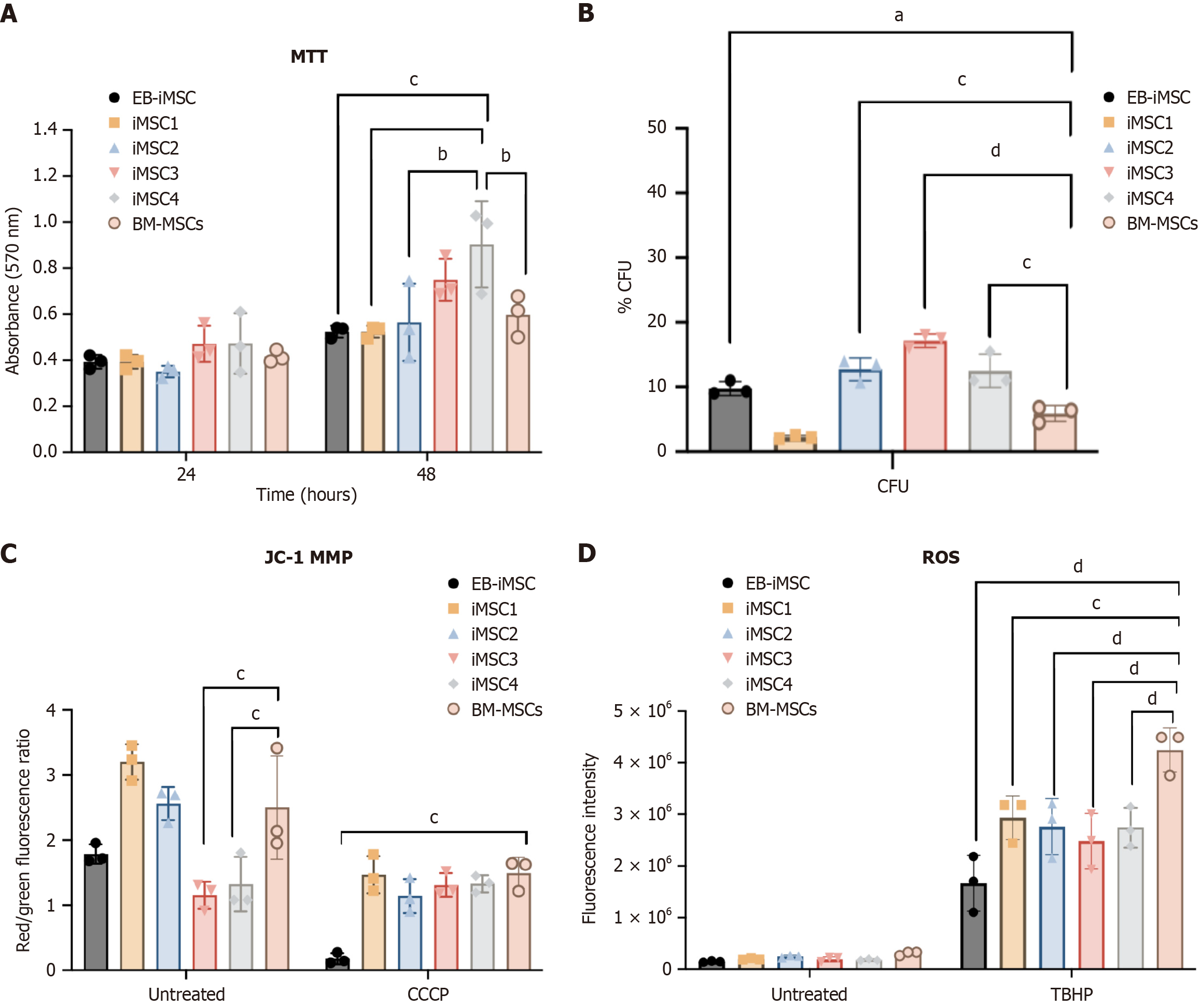
Figure 4 Analysis of cell viability, mitochondrial membrane potential, and intracellular reactive oxygen species cellular for induced pluripotent stem cells-derived mesenchymal stem cell lines.
A: Absorbance results of MTT assay showing viable cell numbers in induced pluripotent stem cells-derived mesenchymal stem cell (iMSC) lines compared to bone marrow mesenchymal stem cells at 24 hours and 48 hours after cell seeding; B: Percentage of colony-forming units among iMSC lines compared to bone marrow mesenchymal stem cells (control); C: Red/green fluorescence ratio indicating mitochondrial membrane potential in untreated and carbonyl cyanide 3-chlorophenylhydrazone-treated iMSC samples; D: Fluorescent intensities of total reactive oxygen species production in iMSC lines. Tert-butyl hydroperoxide was used as a positive control to induce reactive oxygen species production. bP ≤ 0.01; cP ≤ 0.001; dP ≤ 0.0001. BM-MSCs: Bone marrow mesenchymal stem cells; EB-iMSCs: Embryoid body-induced pluripotent stem cells-derived mesenchymal stem cells; iMSC: Induced pluripotent stem cells-derived mesenchymal stem cell; CFUs: Colony-forming units; MMP: Mitochondrial membrane potential; CCCP: Carbonyl cyanide 3-chlorophenylhydrazone; TBHP: Tert-butyl hydroperoxide; ROS: Reactive oxygen species.
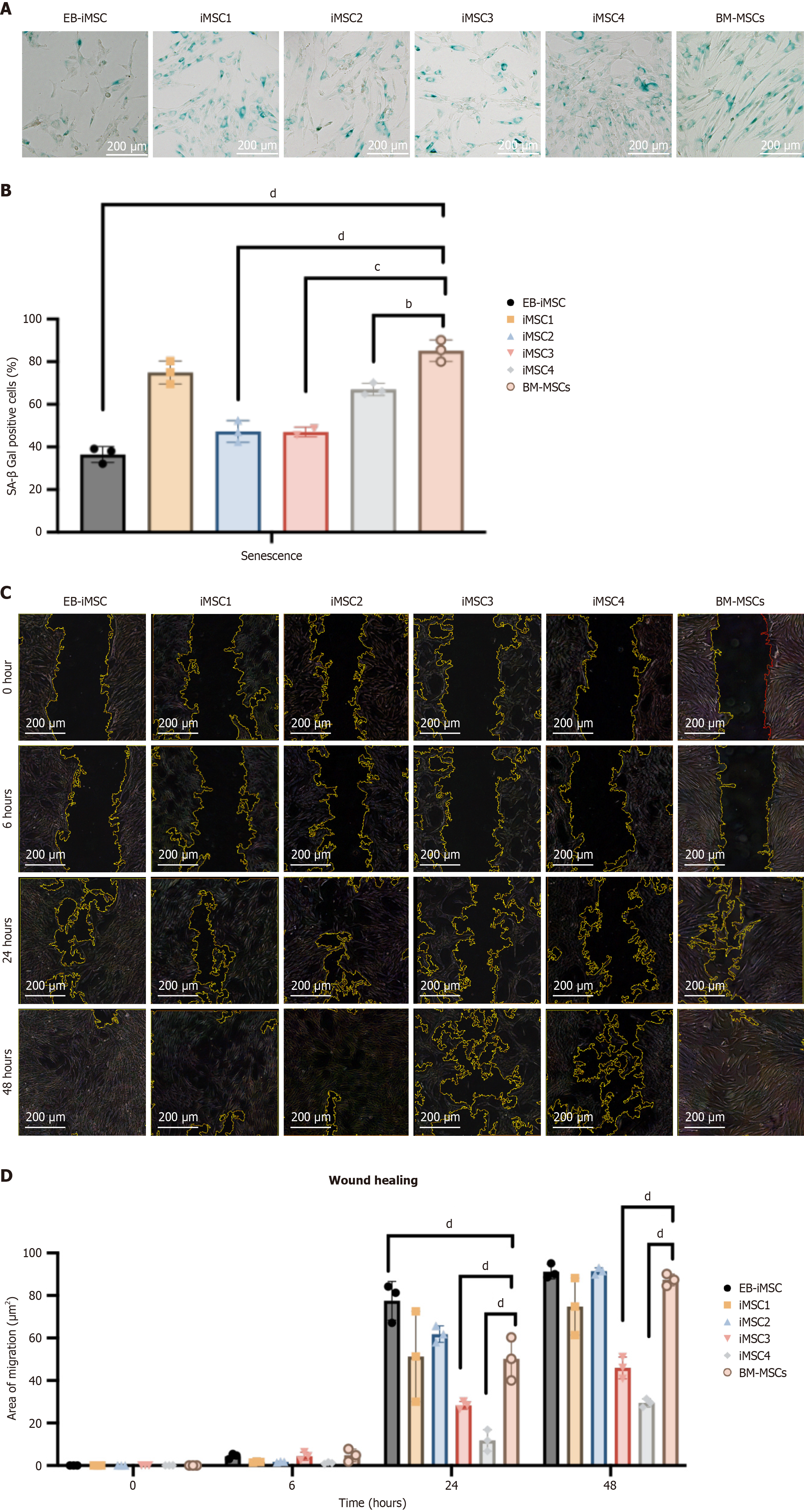
Figure 5 Assessment of senescence and migration in induced pluripotent stem cells-derived mesenchymal stem cell lines.
A: Repre
- Citation: Ababneh NA, Alwohoush E, AlDiqs R, Ismail MA, Al-Kurdi B, Barham R, Al-Atoom R, Nairat F, Al Hadidi S, Whaibi S, Gharandouq MH, Zalloum S, Al Shboul S, Al-Qaisi T, Abuhammad A, Saleh T, Awidi A. Impact of differentiation protocols on the functionality of mesenchymal stem cells derived from induced pluripotent stem cells. World J Stem Cells 2025; 17(12): 110564
- URL: https://www.wjgnet.com/1948-0210/full/v17/i12/110564.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i12.110564