©The Author(s) 2025.
World J Stem Cells. Nov 26, 2025; 17(11): 111162
Published online Nov 26, 2025. doi: 10.4252/wjsc.v17.i11.111162
Published online Nov 26, 2025. doi: 10.4252/wjsc.v17.i11.111162
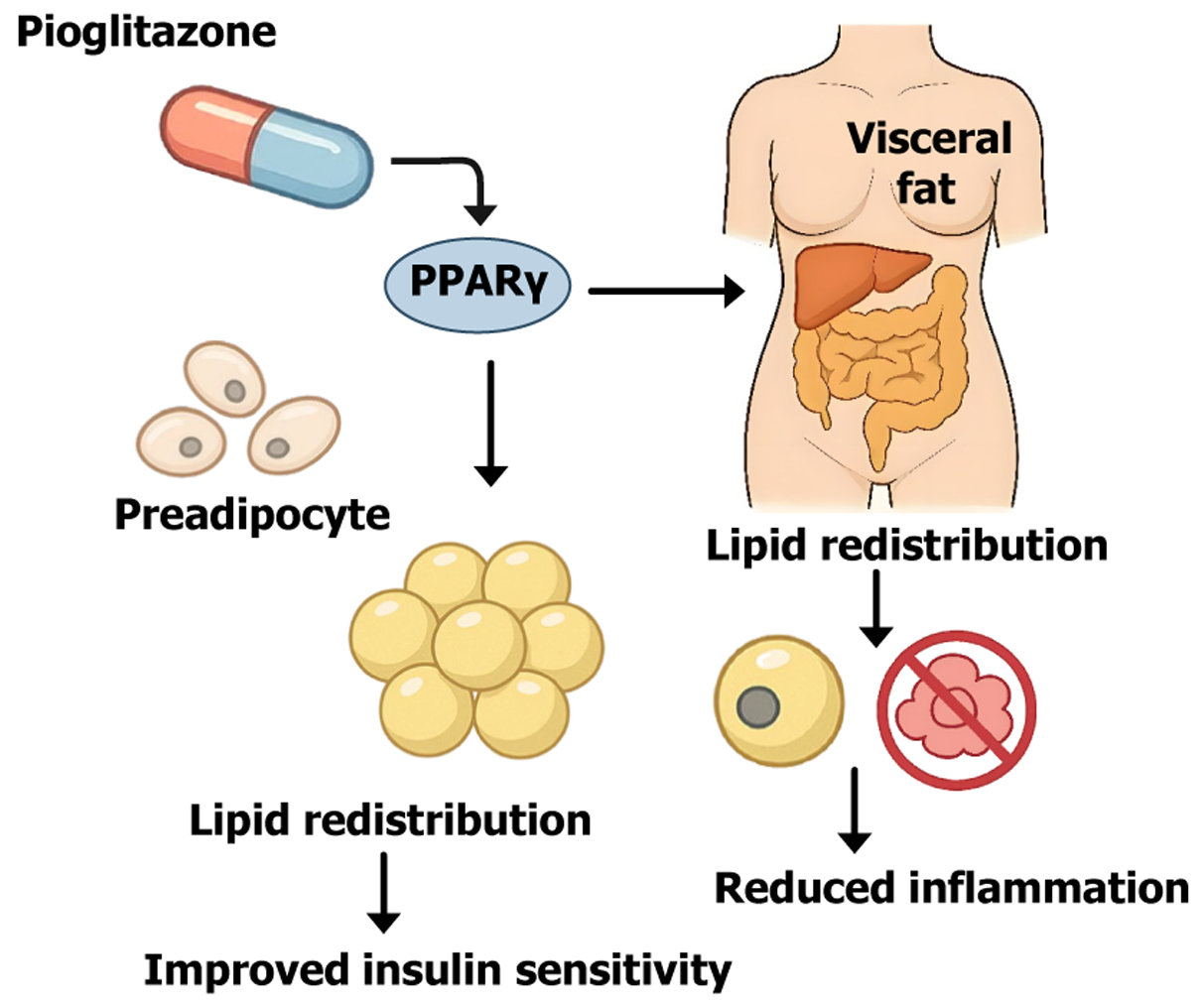
Figure 1 Mechanistic illustration of pioglitazone-induced adipose tissue remodeling via peroxisome proliferator-activated receptor gamma activation.
This schematic illustrates the core mechanisms by which pioglitazone exerts its insulin-sensitizing effects. Peroxisome proliferator-activated receptor gamma, a key transcription factor that promotes the differentiation of preadipocytes into mature adipocytes, particularly in subcutaneous fat depots. This expansion of subcutaneous fat enhances lipid storage capacity, facilitating the redistribution of lipids away from visceral and ectopic sites (e.g., liver, muscle). The resulting reduction in lipotoxicity and inflammation improves insulin sensitivity and metabolic homeostasis. Additionally, peroxisome proliferator-activated receptor gamma activation upregulates adiponectin and suppresses proinflammatory pathways, contributing to the anti-inflammatory effects of pioglitazone. PPARγ: Peroxisome proliferator-activated receptor gamma.
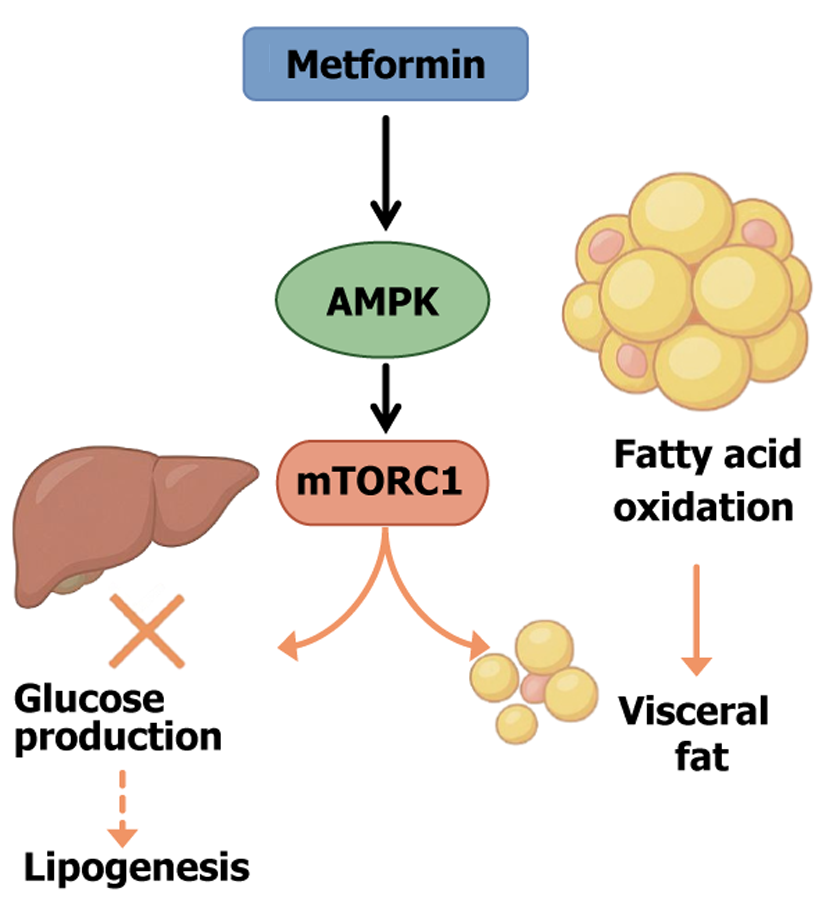
Figure 2 Metformin-mediated metabolic reprogramming via adenosine monophosphate-activated protein kinase/mechanistic target of rapamycin complex 1 pathway.
This diagram illustrates the core mechanisms by which metformin exerts its metabolic benefits in obesity and type 2 diabetes. Metformin activates adenosine monophosphate-activated protein kinase, a key energy sensor that downregulates anabolic processes and promotes catabolism. Activated adenosine monophosphate-activated protein kinase inhibits the mechanistic target of rapamycin complex 1, thereby suppressing hepatic gluconeogenesis and lipogenesis while enhancing fatty acid oxidation. These actions collectively contribute to reduced hepatic glucose output, decreased lipid accumulation, and diminished visceral fat mass. Moreover, through its impact on adipocyte metabolism and inflammation, metformin supports insulin sensitivity and improves adipose tissue function. AMPK: Adenosine monophosphate-activated protein kinase; mTORC1: Mechanistic target of rapamycin complex 1.
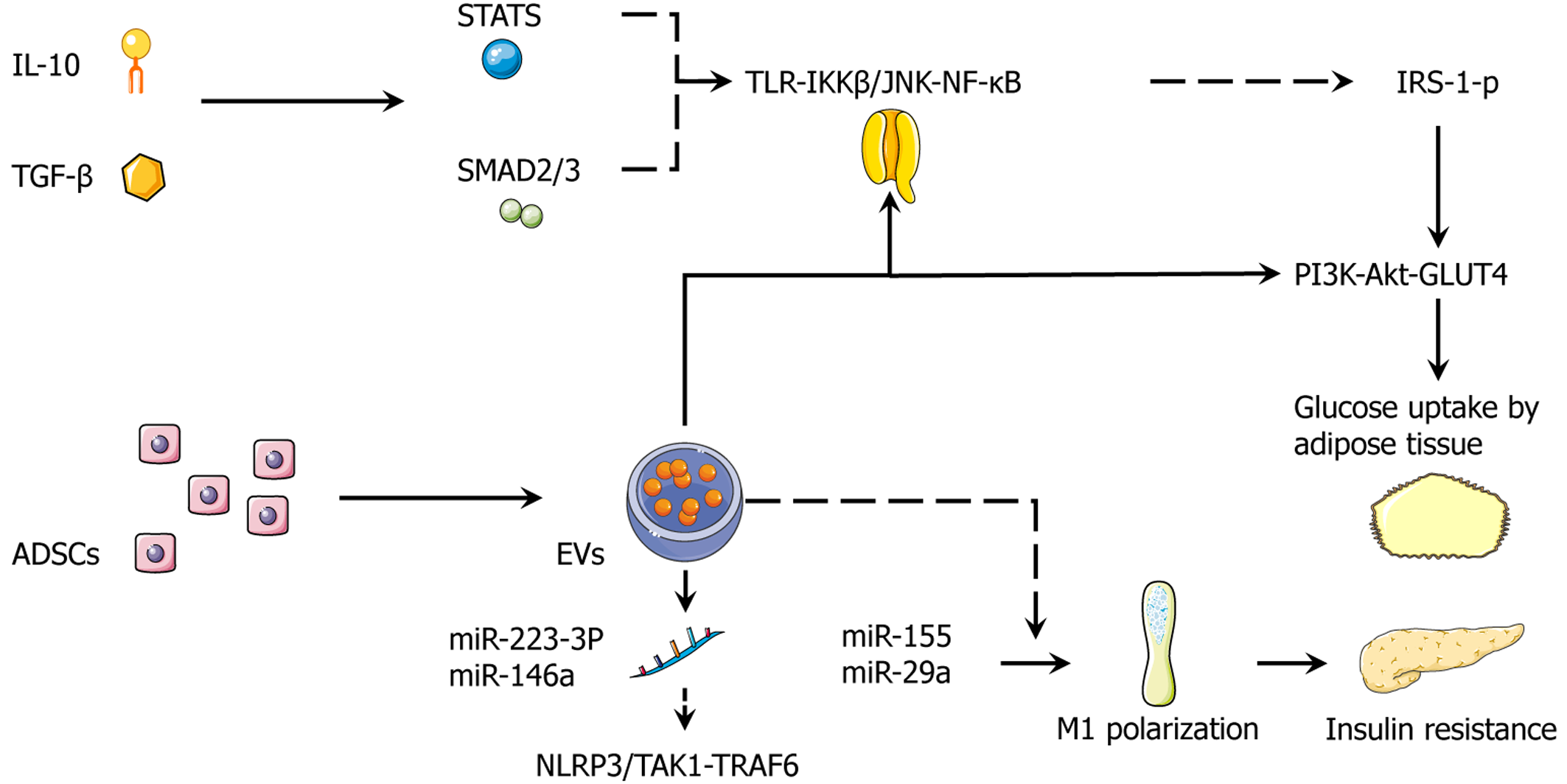
Figure 3 Stem cell secretome.
Integrated adipose-derived stem cell secretome suppresses inflammatory signaling and restores insulin action in adipose tissue with interleukin-10, transforming growth factor-β activate signal transducer and activator of transcription 3, and mothers against decapentaplegic homolog 2/3, which repress the toll-like receptor-IκB kinase β/c-Jun N-terminal kinase-nuclear factor kappa B axis, lowering serine-phosphorylated insulin receptor substrate-1 and re-establishing phosphoinositide 3-kinase-protein kinase B-glucose transporter 4-dependent glucose uptake in adipocytes. Adipose-derived stem cell-derived extracellular vesicles deliver miR-223-3p and miR-146a to inhibit NOD-like receptor family pyrin domain containing 3 and transforming growth factor-β-activated kinase 1/tumor necrosis factor receptor-associated factor 6 signaling, further dampening innate immune activation. By contrast, obesogenic miR-155 and miR-29a (dashed) promote M1 polarization and exacerbate insulin resistance. The net effect is a shift of the adipose immune milieu toward resolution with improved insulin signaling and glucose uptake. Solid arrows indicate activation; dashed lines denote context-dependent or deleterious routes. IL-10: Interleukin-10; TGF-β: Transforming growth factor-β; STAT3: Signal transducer and activator of transcription 3; SMAD2/3: Mothers against decapentaplegic homolog 2/3; TLR: Toll-like receptor; IKKβ: IκB kinase β; JNK: C-Jun N-terminal kinase; NF-κB: Nuclear factor kappa B; IRS-1: Insulin receptor substrate-1; PI3K: Phosphoinositide 3-kinase; Akt: Protein kinase B; GLUT4: Glucose transporter 4; ADSCs: Adipose-derived stem cells; EVs: Extracellular vesicles; NLRP3: NOD-like receptor family pyrin domain containing 3; TAK1: Transforming growth factor-β-activated kinase 1; TRAF6: Tumor necrosis factor receptor-associated factor 6; miR: MicroRNA.
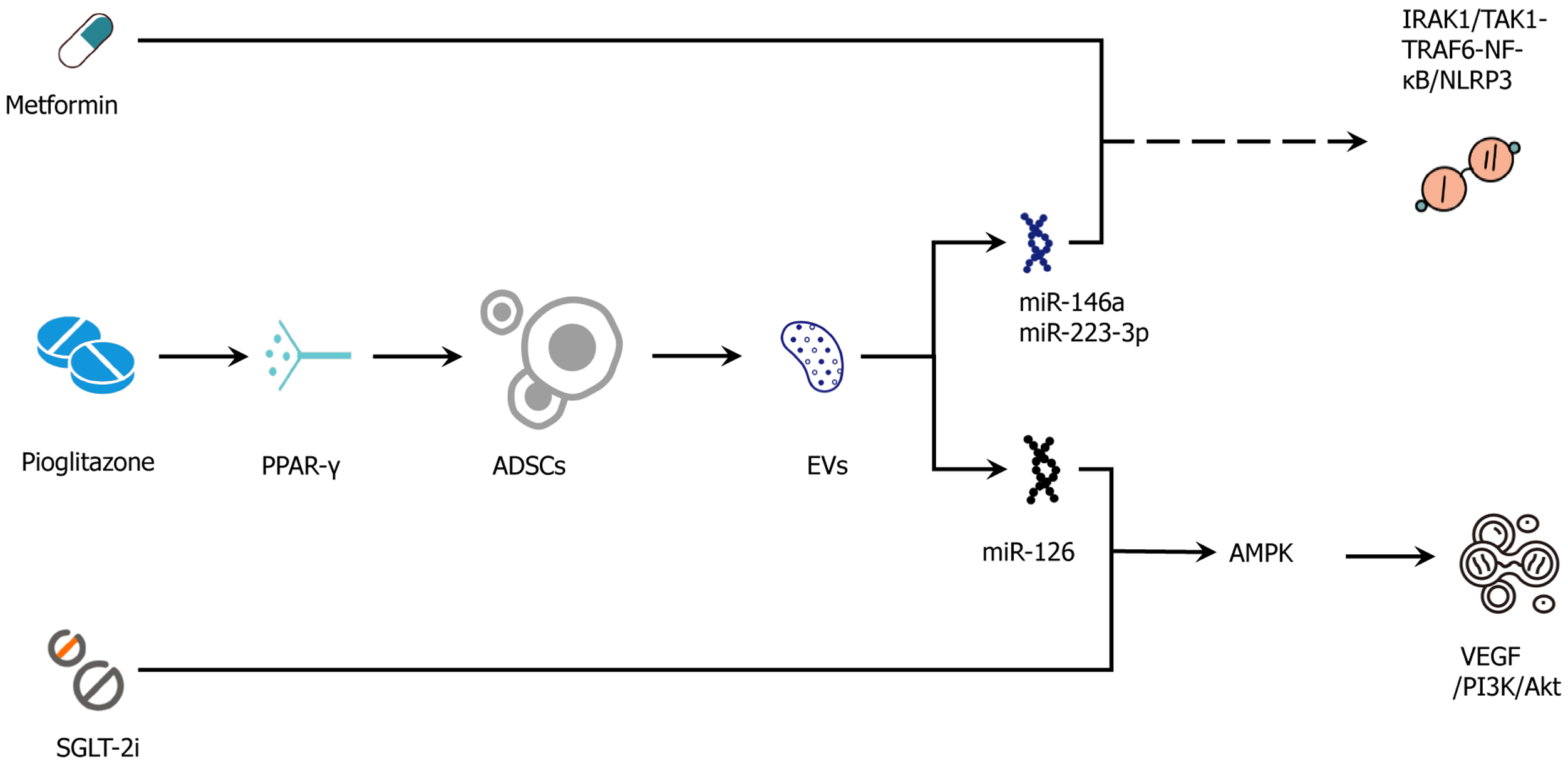
Figure 4 Adipose-derived stem cell-drug synergy map.
Adipose-derived stem cell secretome/extracellular vesicles target drug-action pathways to improve vascular, metabolic, and anti-inflammatory outcomes. Mechanistic intersections whereby adipose-derived stem cell (ADSC) secretome/extracellular vesicles (EVs) augment and complement conventional agents. Metformin (adenosine monophosphate-activated protein kinase) cooperates with ADSC-EV-delivered miR-126 to enhance vascular endothelial growth factor → phosphoinositide 3-kinase/protein kinase B → endothelial nitric oxide synthase signaling and improve endothelial function. Glucagon-like peptide-1 receptor agonist (cyclic adenosine monophosphate/protein kinase A → cyclic adenosine monophosphate response element-binding protein) aligns with ADSC-driven adenosine monophosphate-activated protein kinase/sirtuin-1 → peroxisome proliferator-activated receptor γ coactivator-1α → PR domain zinc finger protein 16/uncoupling protein-1 programs to promote beiging, mitochondrial repair, and fatty-acid oxidation, reducing ceramide/diacylglycerol lipotoxicity. The hemodynamic/anti-inflammatory effects of sodium-glucose cotransporter-2 inhibitors combine with EV miR-146a and miR-223-3p to suppress interleukin-1 receptor-associated kinase-1/transforming growth factor-β-activated kinase-1-tumor necrosis factor receptor-associated factor-6-nuclear factor kappa B → NOD-like receptor protein-3 signaling and reinforce anti-fibrotic responses (mothers against decapentaplegic homolog 2/3 modulation). Peroxisome proliferator-activated receptor γ activation by pioglitazone increases ADSC survival, migration, and vascular endothelial growth factor secretion, providing microenvironmental optimization for subsequent cell/EV therapy. At the insulin-signaling node ADSC effects (suppressor of cytokine signaling-3 ↓ and reduced serine-phosphorylated insulin receptor substrate-1) synergize with drug actions to restore phosphoinositide 3-kinase/protein kinase B/glucose transporter-4 translocation and adipose glucose uptake. These interactions are testable by paired adipose biopsies and serial EV-cargo profiling (e.g., miR-126/146a/223), together with functional readouts (flow-mediated dilation, estimated glomerular filtration rate slope, adipose peroxisome proliferator-activated receptor γ coactivator-1α/uncoupling protein-1, phosphorylated insulin receptor substrate-1). Solid arrows indicate activation/flow; T-bars or dashed lines indicate inhibition or context-dependent routes. IRAK1: Interleukin-1 receptor-associated kinase-1; TAK1: Transforming growth factor-β-activated kinase-1; TRAF6: Tumor necrosis factor receptor-associated factor-6; NF-κB: Nuclear factor kappa B; NLRP3: NOD-like receptor protein-3; PPARγ: Peroxisome proliferator-activated receptor γ; ADSC: Adipose-derived stem cell; EVs: Extracellular vesicles; AMPK: Adenosine monophosphate-activated protein kinase; SGLT-2i: Sodium-glucose cotransporter-2 inhibitor; VEGF: Vascular endothelial growth factor; PI3K: Phosphoinositide 3-kinase; Akt: Protein kinase B; miR: MicroRNA.
- Citation: Luo C, Yu XM, Hua LY, Zeng MQ, Xu H, Duan CZ, Xu SY, Sun D, Ye LY, He DJ. Targeting adipose remodeling: Synergistic mechanisms of drugs and adipose-derived stem cells in obese type 2 diabetes mellitus. World J Stem Cells 2025; 17(11): 111162
- URL: https://www.wjgnet.com/1948-0210/full/v17/i11/111162.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i11.111162