Copyright
©The Author(s) 2023.
World J Stem Cells. May 26, 2023; 15(5): 438-452
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.438
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.438
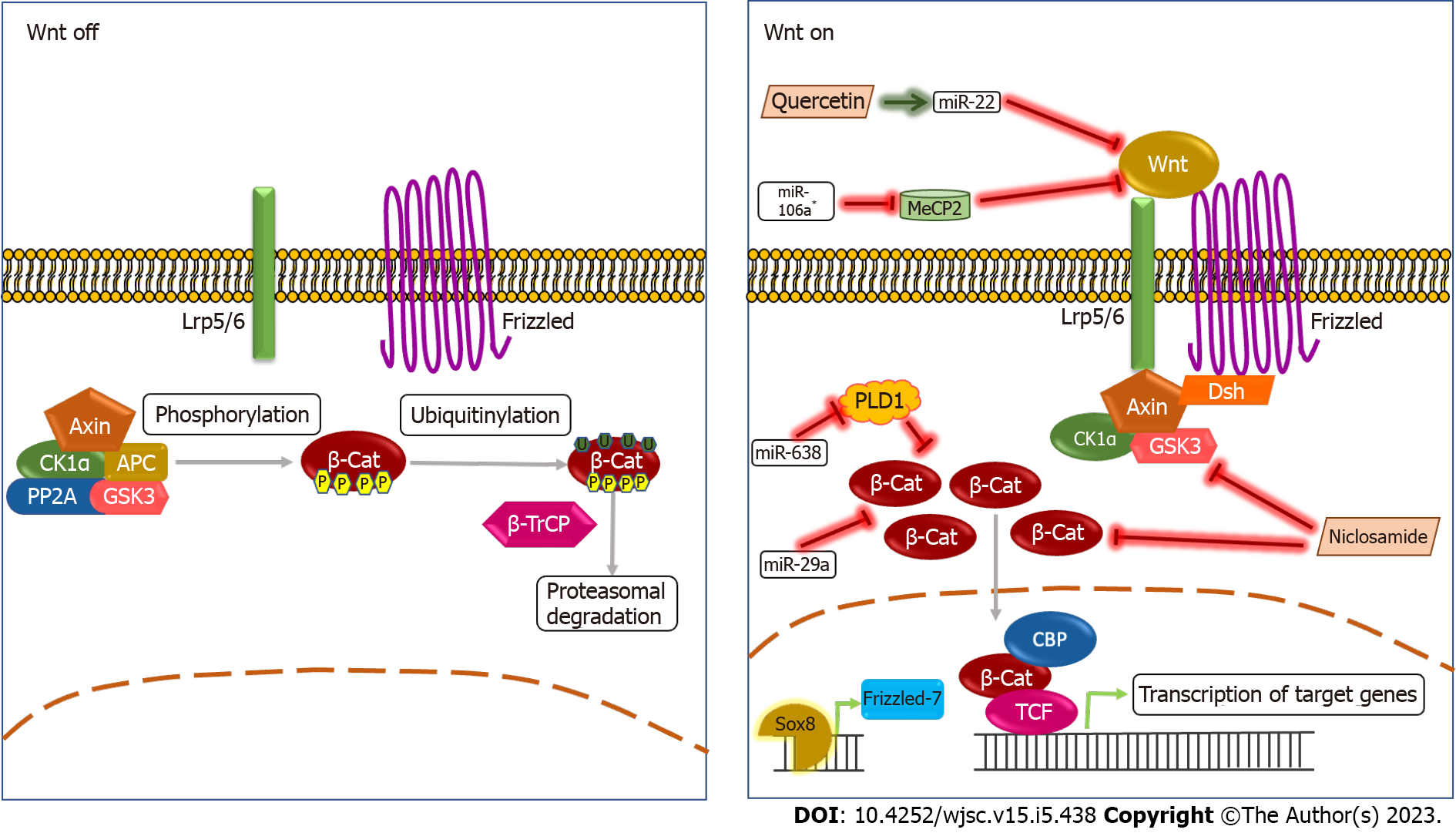
Figure 1 The Wnt signaling pathway and cancer stemness.
In the absence of a Wnt ligand, in the canonical pathway,, a complex of Axin, CK1α, APC, PP2A, and GSK3 (termed destruction complex) phosphorylates β-catenin targeting it for ubiquitinylation, that leads to its proteasomal degradation. When the Wnt ligand binds to the Frizzled receptor and LRP5/6 co-receptor, the destruction complex gets localized to the receptor, preventing the degradation of β-catenin that localizes to the nucleus that further activates transcription of the target genes. It has been reported that miRNAs such as miR-29a, miR-638, and miR-106a* reduce levels of β-catenin and Wnt ligand. The miR-638 targets PLD1, a generally accepted oncogene, leading to the reduction β-catenin levels[49]. The miR-29a also directly causes a reduction in β-catenin levels in TSCC cell lines[48]. The miRNA miR-106a* causes a reduction in MeCP2 (a gene expression regulator and oncogene) levels that inhibit the binding of Wnt ligand to the receptor that in turn causes downregulation of the Wnt pathway[50]. Chemical compounds such as Quercetin and Niclosamide downregulate the Wnt signaling pathway. Quercetin causes an increase in levels of miR-22 that in turn inhibits the Wnt1/β-catenin axis[51], while Niclosamide directly binds to DVL2, phosphorylated GSK3β, and Cyclin D1 that reduces levels of β-catenin[52]. Sox8 is shown to activate the Wnt pathway by inducing the expression of Frizzled-7[53].
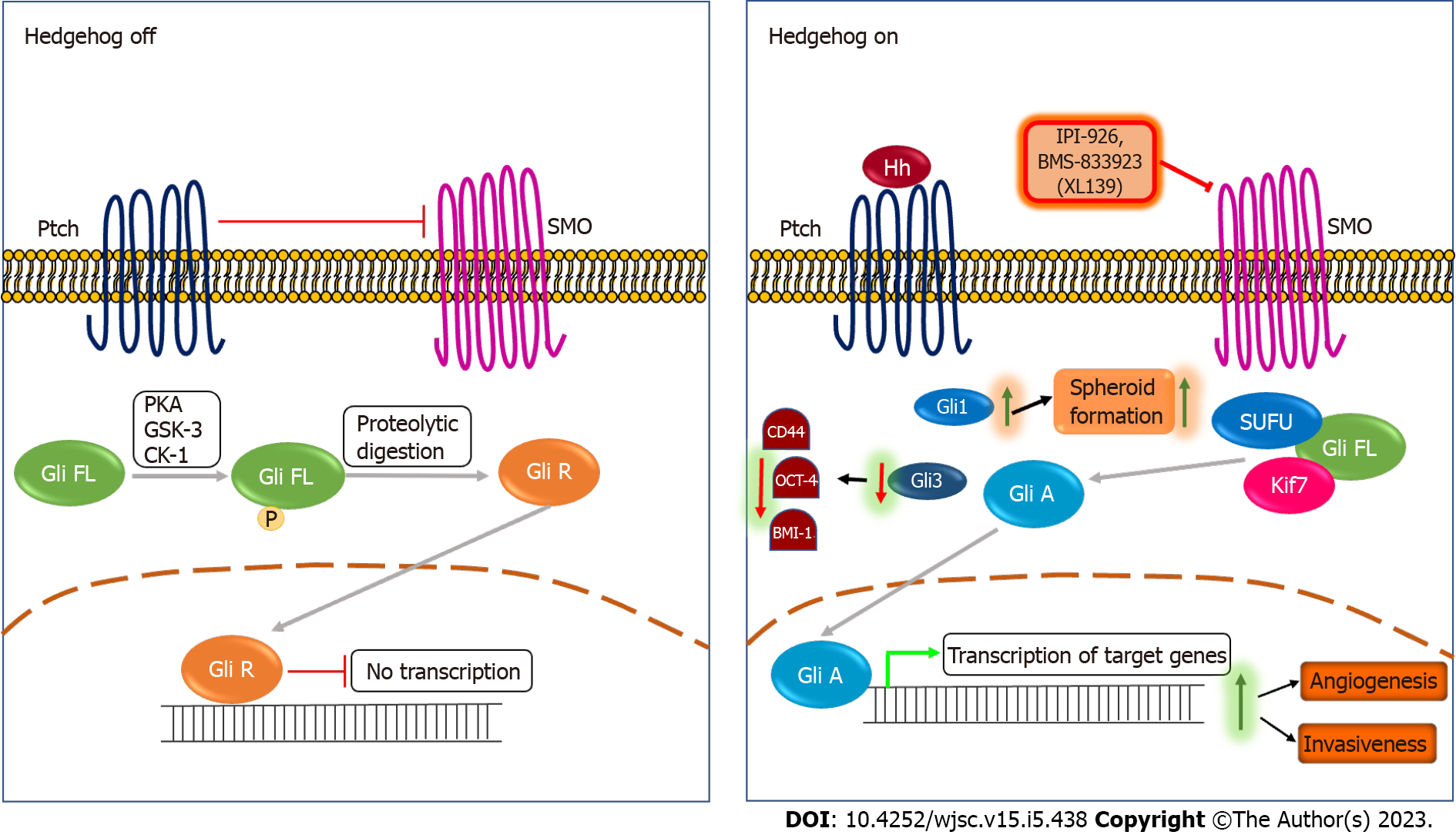
Figure 2 The Hedgehog signaling pathway and cancer stemness.
In the absence of Hedgehog ligand, Patched inhibits smoothened (SMO), leading to the full-length Gli protein that gets phosphorylated by PKA, GSK-3 and CK-1 and converted into Gli repressor through proteolytic digestion. The Gli repressor further inhibits the Hedgehog pathway. When the Hedgehog ligand binds to the Patched receptor, the inhibition on SMO is released, leading to the dissociation of Gli from SUFU and Kif7 that lead to the activation of the Gli protein (Gli A). Further, Gli A translocates to the nucleus that further activates transcription of target genes. Activation of the Hedgehog pathway promotes angiogenesis and invasiveness in vivo[57,58]. Downregulation of Gli3 reduces expression of stemness markers such as CD44, OCT-4, and BMI-1 in tongue carcinoma (TSCC) cells[59], while upregulation of Gli1 increases the spheroid formation ability of TSCC cells[60]. IPI-926 and BMS-833923 (XL139) are SMO inhibitors used to therapeutically target the Hedgehog pathway. IPI-926 is a semi-synthetic derivative of cyclopamine, while BMS-833923 (XL139) is a small molecule inhibitor of SMO. Ptch: Patched; SMO: Smoothened; Hh: Hedgehog ligand; Gli FL: Full length glioma associated oncogene; Gli A: Gli activator; Gli R: Gli repressor; IPI-926, BMS-833923 (XL139): Smoothened inhibitors.
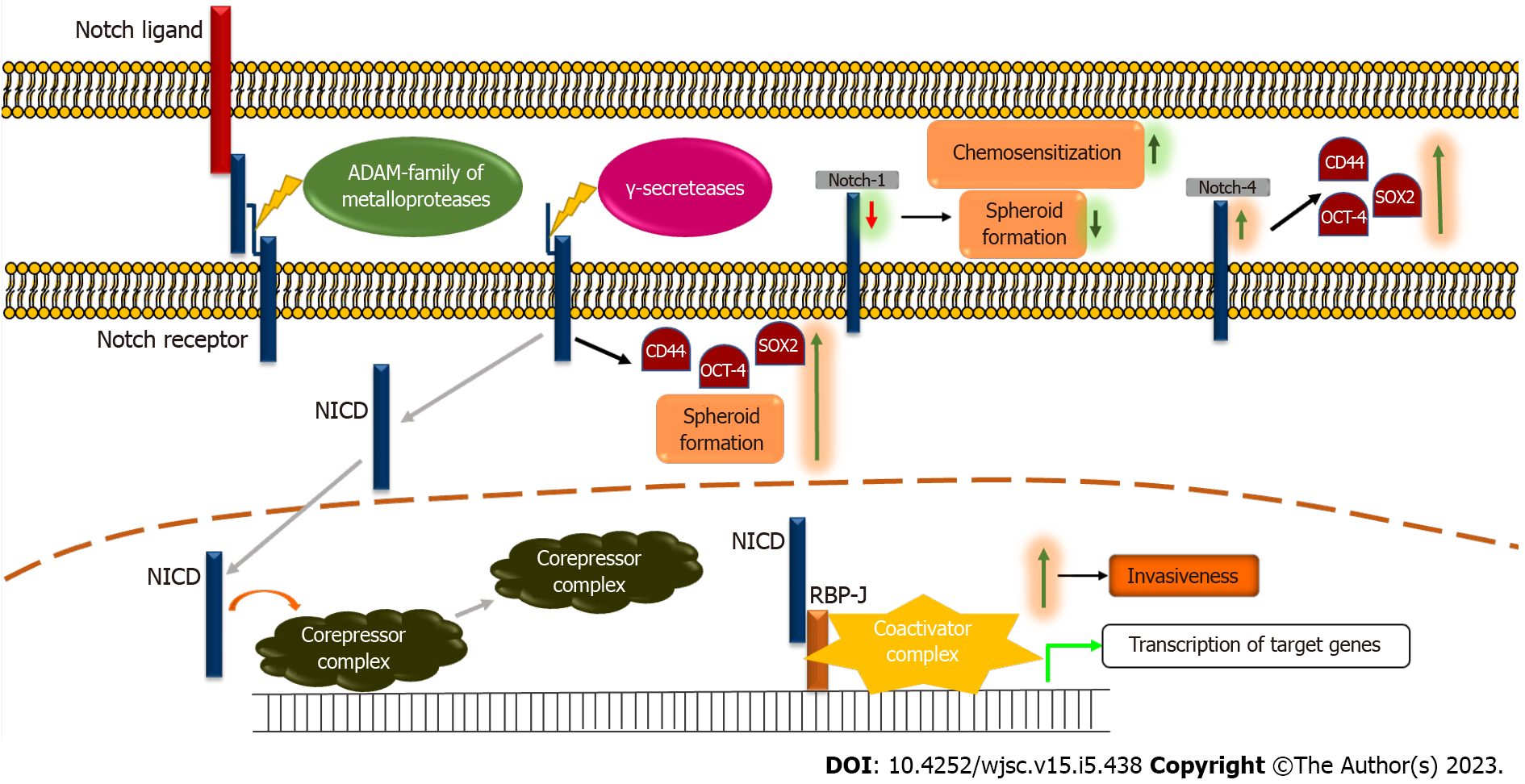
Figure 3 The Notch signaling pathway and cancer stemness.
In absence of the Notch ligand, the Notch pathway is in the inactivate state. The binding of ligand to the Notch receptor leads to cleavage by ADAM-family metalloproteases releasing the extracellular domain of the receptor. Further, the receptor is cleaved by γ-secretase leading to the formation of the Notch intracellular domain (NICD), thereby activating it. The NICD then translocates to the nucleus and releases inhibition on the target genes by dissociating the corepressor complex and forms a complex with RBP-J and co-activator complex thereby activating the transcription of target genes. Further, knockdown of Notch-1 expression has been shown to increase chemo-sensitization and decrease the spheroid formation ability of tongue squamous cell carcinoma (TSCC) cells[64]. An increase in Notch-4 levels increases stemness markers such as CD44, SOX2, and OCT-4 in TSCC cells[66]. NICD activation has been shown to increase levels of CD44, OCT-4, and SOX2 in TSCC cells[65]. Activation of the Notch pathway increases the invasiveness in TSCC cells[62,63,66,67]. NICD: Notch intracellular domain.
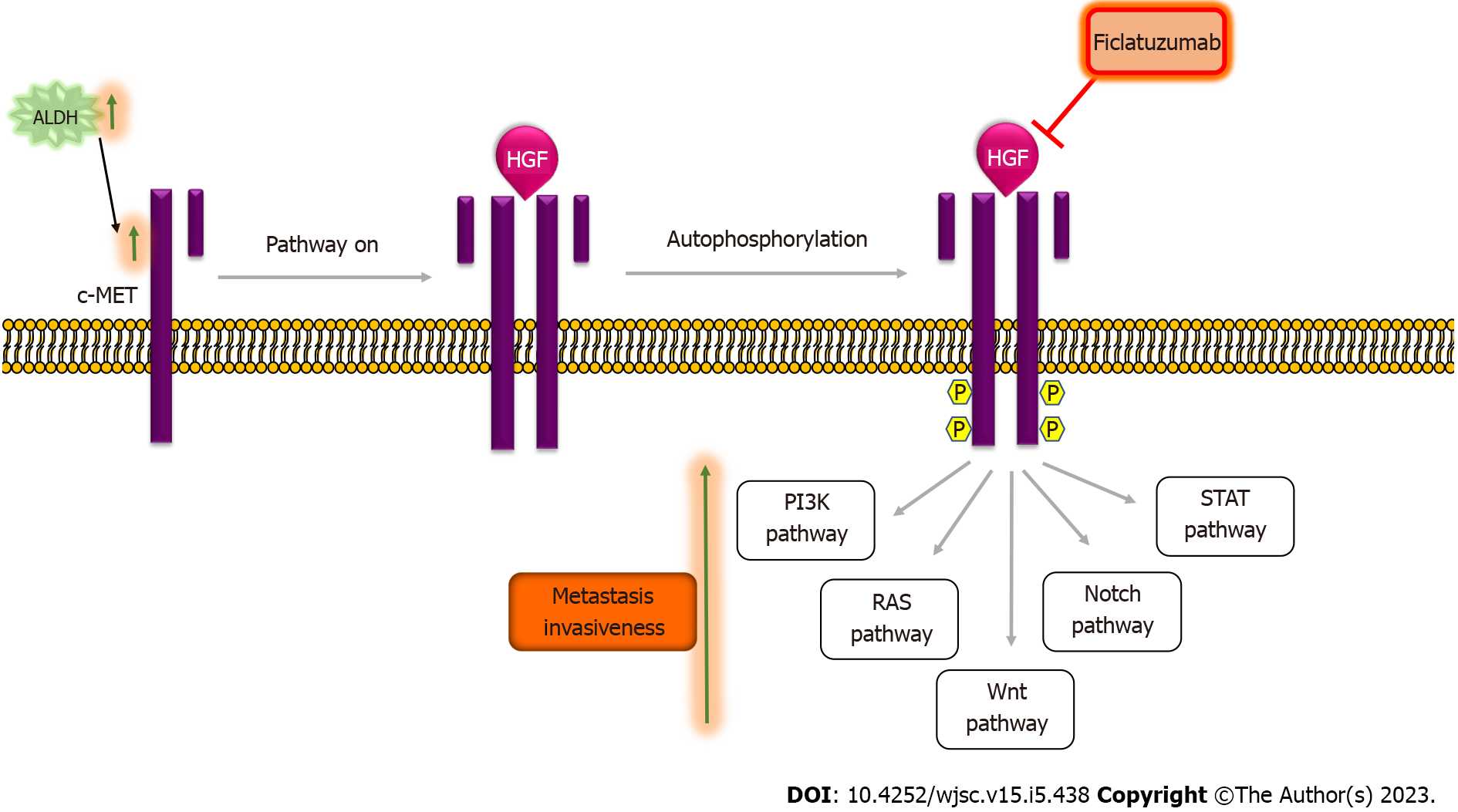
Figure 4 The HGF/c-MET signaling pathway and cancer stemness.
In the absence of the HGF ligand, the HGF/c-MET pathway remains inactivated. The binding of the HGF ligand to the c-MET kinase receptor results in the dimerization of two subunits that further leads to auto-phosphorylation of the tyrosine residues in the cytoplasmic domain of the receptor creating a docking site for adaptor proteins, which regulate pathways, such as PI3K, RAS, Wnt, Notch and STAT pathway. Transcriptional levels of c-MET were high in ALDHhigh cells[70]. Activation of c-MET results in an increase in invasiveness and metastasis in vitro as well as in vivo[73,74]. Ficlatuzumab is a humanized IgG1 monoclonal antibody targeting HGF used to therapeutically target the HGF/c-met pathway. ALDH: Aldehyde dehydrogenase; Ficlatuzumab: HGF/c-MET pathway inhibitor.
- Citation: Joshi P, Waghmare S. Molecular signaling in cancer stem cells of tongue squamous cell carcinoma: Therapeutic implications and challenges. World J Stem Cells 2023; 15(5): 438-452
- URL: https://www.wjgnet.com/1948-0210/full/v15/i5/438.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i5.438