©The Author(s) 2020.
World J Stem Cells. Oct 26, 2020; 12(10): 1133-1151
Published online Oct 26, 2020. doi: 10.4252/wjsc.v12.i10.1133
Published online Oct 26, 2020. doi: 10.4252/wjsc.v12.i10.1133
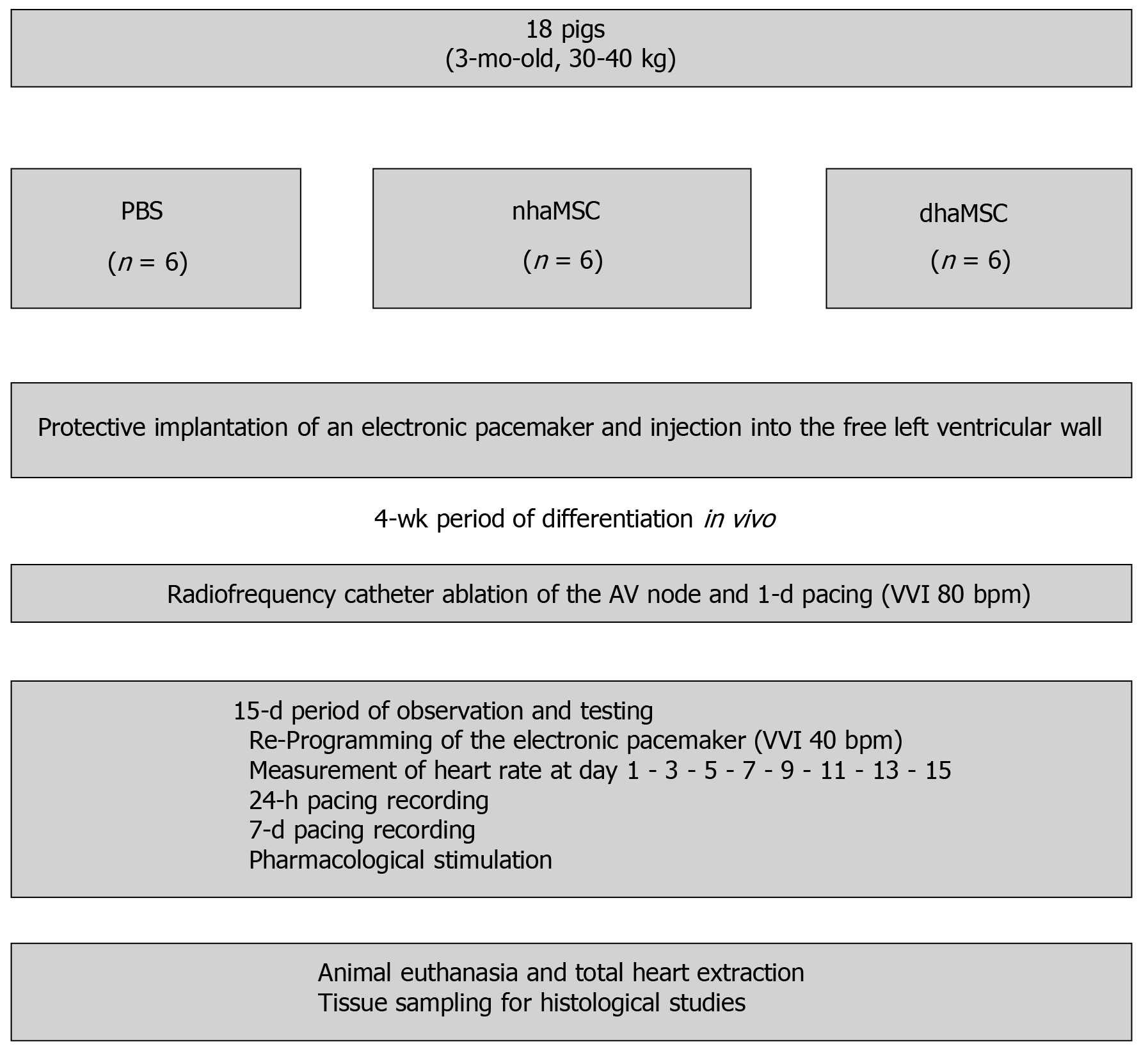
Figure 1 Schematic overview of in vivo experiments.
Eight healthy pigs (matched in sex, age and weight) were equally and randomly divided into 3 groups [phosphate-buffered saline (PBS), native human mesenchymal stem cells derived from adipose tissue, and differentiated human mesenchymal stem cells derived from adipose tissue]. Protective implantation of an electronic pacemaker was performed in each pig and cells or PBS were injected into the free left ventricular wall. After a 4-wk period, radiofrequency catheter ablation of the atrioventricular (AV) node was performed to establish third degree AV block in each animal. A 15-d period of observation and testing was followed by animal euthanasia and tissue sampling for histological studies. PBS: Phosphate-buffered saline; nhaMSC: Native human mesenchymal stem cells derived from adipose tissue; dhaMSC: Differentiated human mesenchymal stem cells derived from adipose tissue; AV: Atrioventricular.
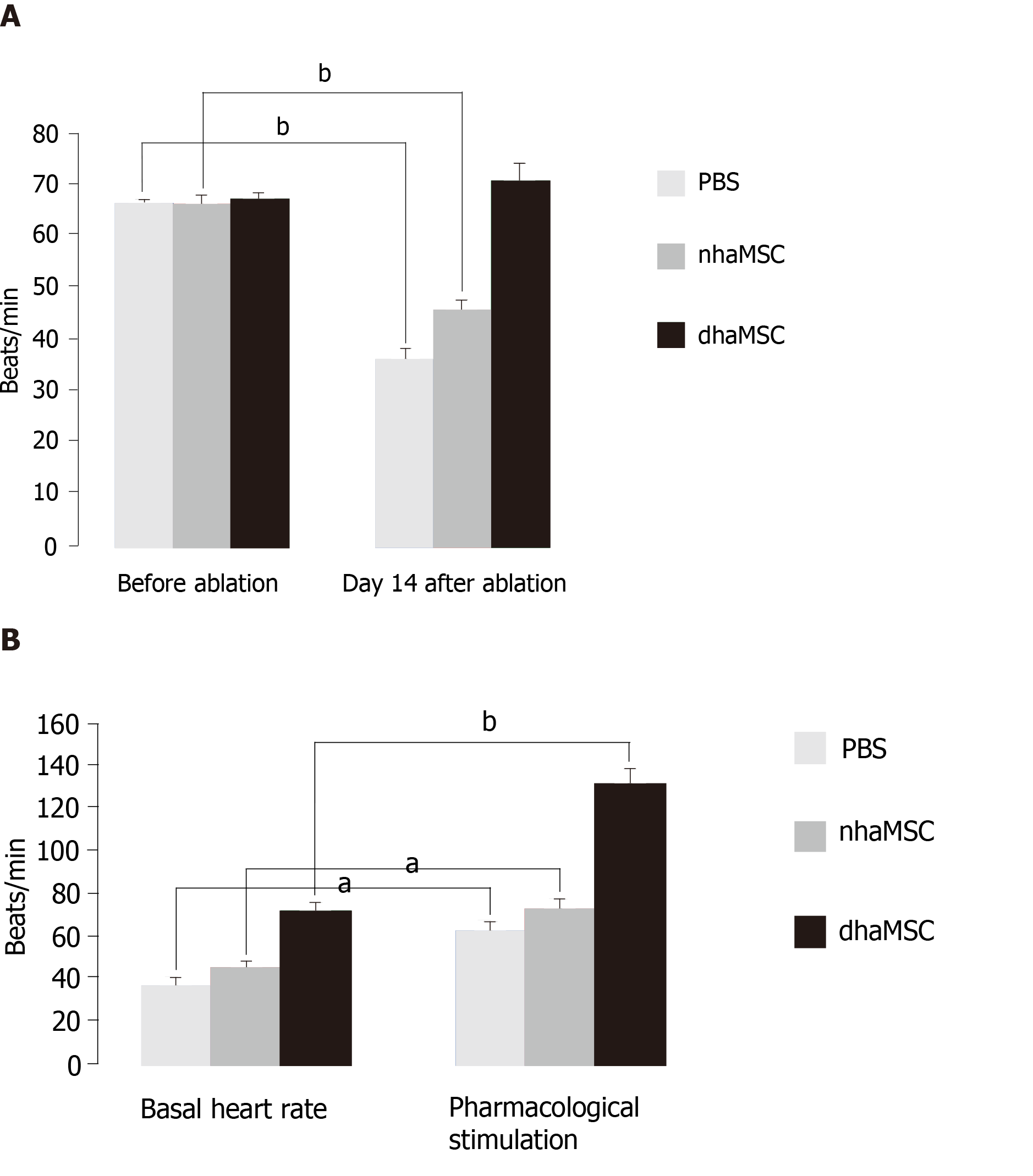
Figure 2 Heart rate assessment.
Evaluated by electrocardiogram (ECG) recordings in pigs with transplanted differentiated human mesenchymal stem cells derived from adipose tissue (green, n = 6) or transplanted native human mesenchymal stem cells derived from adipose tissue (red, n = 6) and in pigs treated with PBS (blue, n = 6). A: Intrinsic heart rate recordings before ablation and on day 14 after ablation (intervention rate of the electronic pacemaker device switched from 40 to 30 bpm during recordings); B: Basal and stimulated heart rates, recorded on day 14 after ablation. Chronotropic responsiveness was induced by simulation with epinephrine (1 mg) IV and atropine (0.5 mg) IV. aP < 0.05, bP < 0.001, showing statistical significance. PBS: Phosphate-buffered saline; nhaMSC: Native human mesenchymal stem cells derived from adipose tissue; dhaMSC: Differentiated human mesenchymal stem cells derived from adipose tissue.
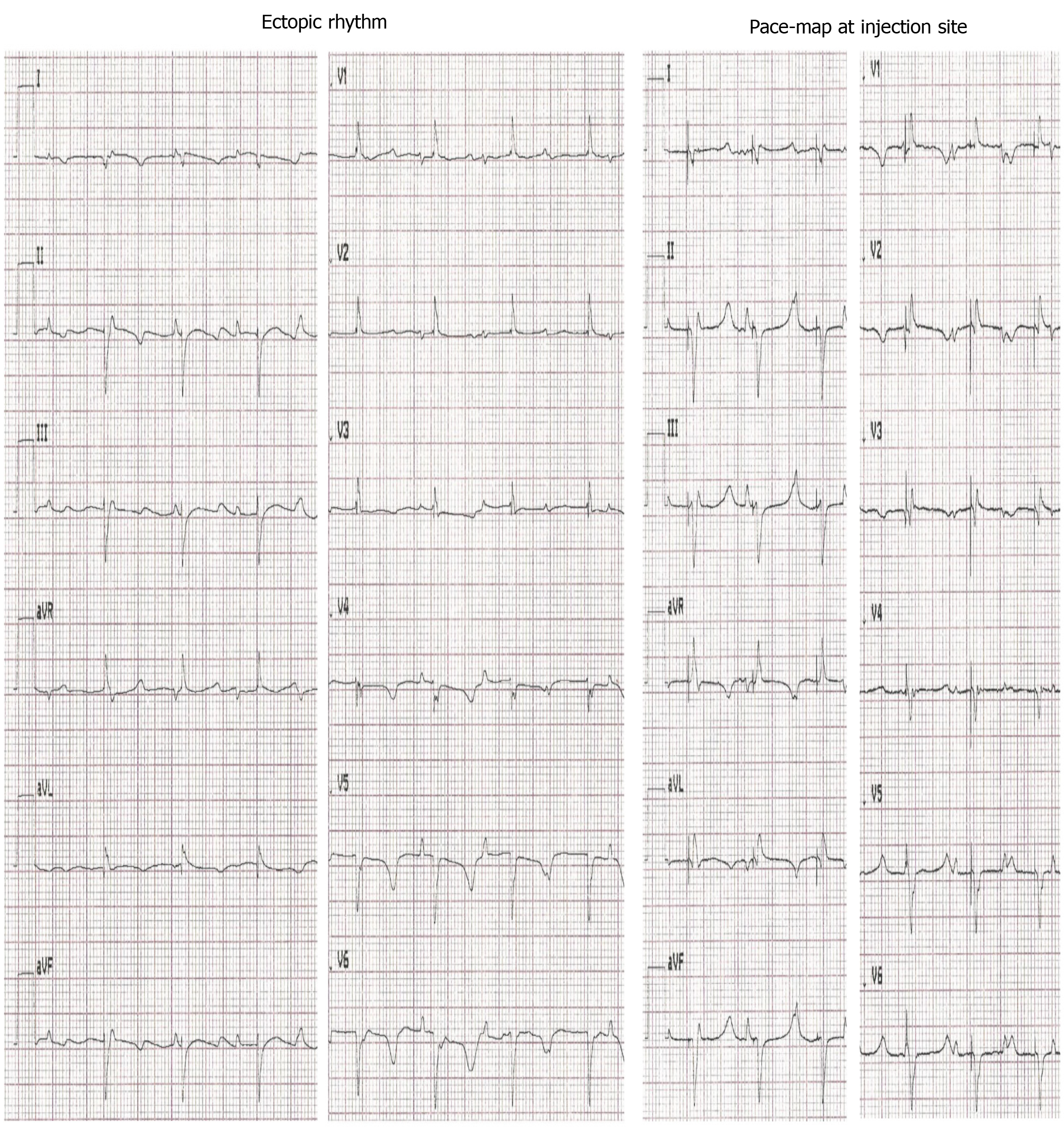
Figure 3 Electrocardiograms of escape and pace-mapped beats recorded in pigs with transplanted differentiated human mesenchymal stem cells derived from adipose tissue.
Left: 12-lead-electrocardiogram (ECG) showing escape beats (ectopic rhythm); Right: 12-lead-ECG showing pace-mapped beats (pace-map at injection site). ECGs recorded at a speed of 25 mm/s.
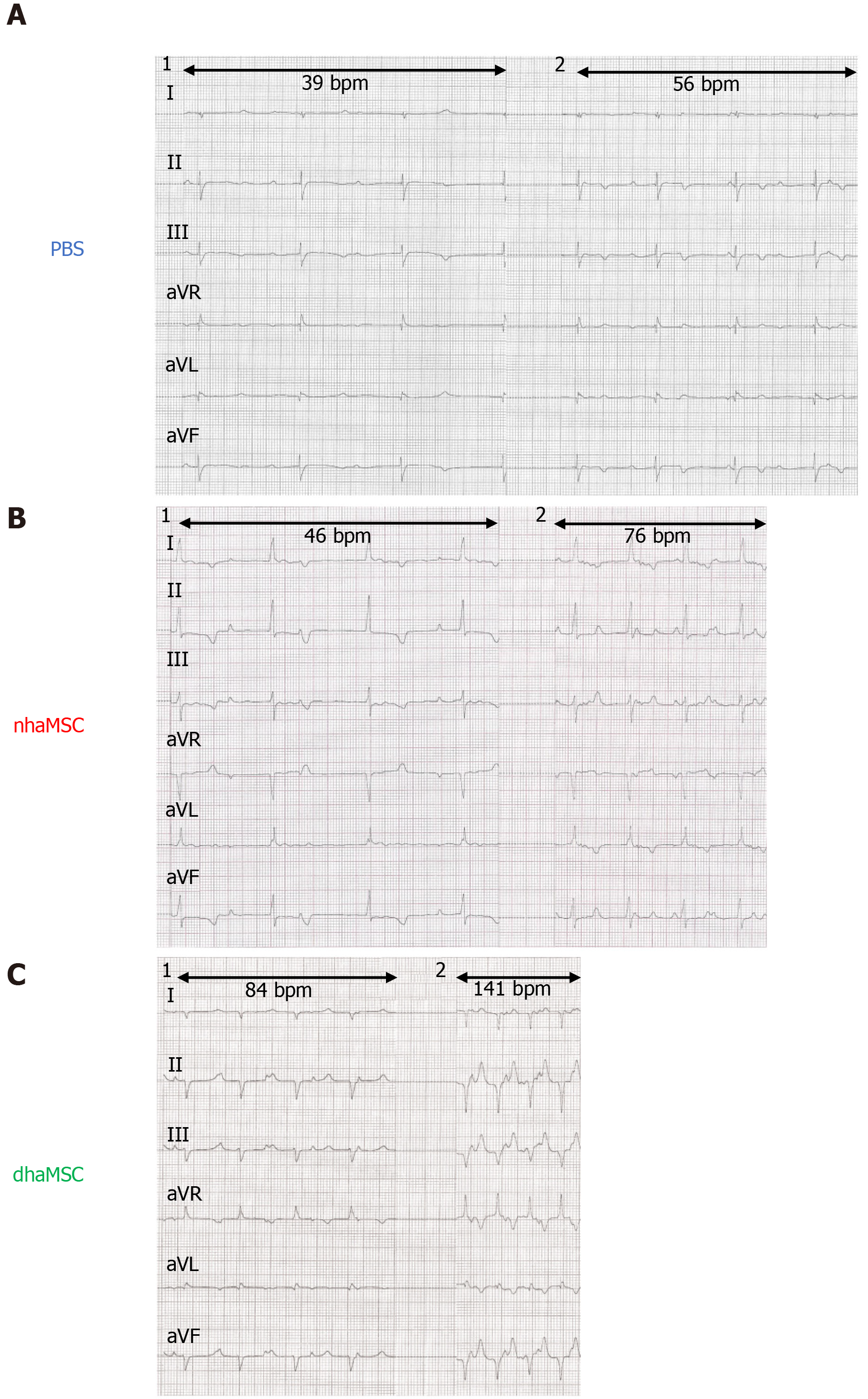
Figure 4 Electrocardiograms of ventricular escape rhythms after atrioventricular node ablation.
A: Phosphate-buffered saline group; B: Native human mesenchymal stem cells derived from adipose tissue group; C: Differentiated human mesenchymal stem cells derived from adipose tissue group. 1: Baseline ventricular escape rhythms. 2: Stimulated ventricular escape rhythm by epinephrine (1 mg) IV and atropine (0.5 mg) IV. Electrocardiograms recorded at a speed of 25 mm/s. PBS: Phosphate-buffered saline; nhaMSC: Native human mesenchymal stem cells derived from adipose tissue; dhaMSC: Differentiated human mesenchymal stem cells derived from adipose tissue.
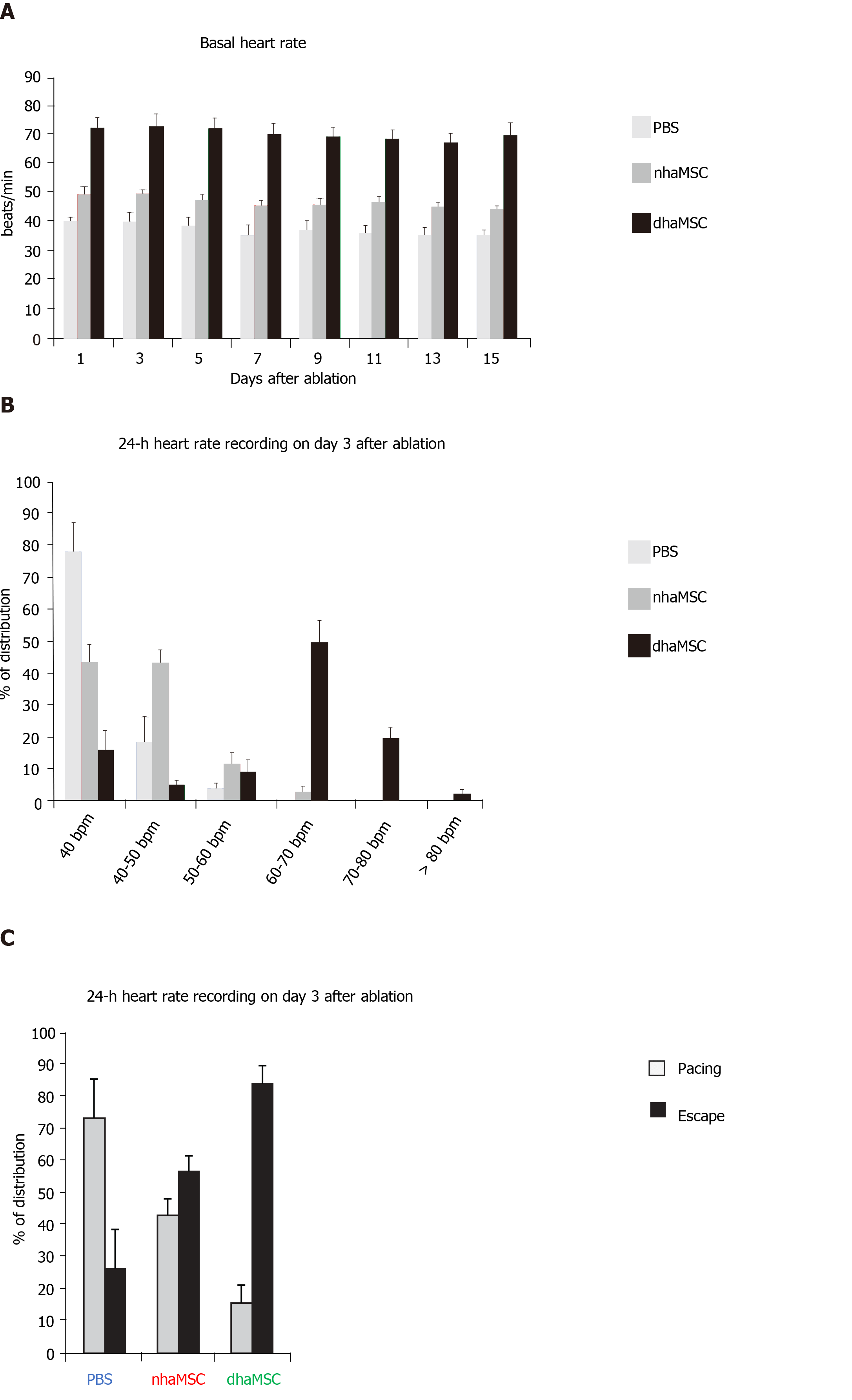
Figure 5 Paced and spontaneous ventricular rhythm recordings after atrioventricular node ablation.
A: Assessment of intrinsic heart rate every second day (intervention rate of electronic pacemaker devices was switched from 40 to 30 bpm); B: 24-h heart rate recordings stratified by heart rate (intervention rate of electronic pacemaker devices was left at 40 bpm); C: 24-h heart rate recordings stratified by percentage of paced and ventricular escape rhythms (intervention rate of electronic pacemaker devices was left at 40 bpm). PBS: Phosphate-buffered saline; nhaMSC: Native human mesenchymal stem cells derived from adipose tissue; dhaMSC: Differentiated human mesenchymal stem cells derived from adipose tissue.
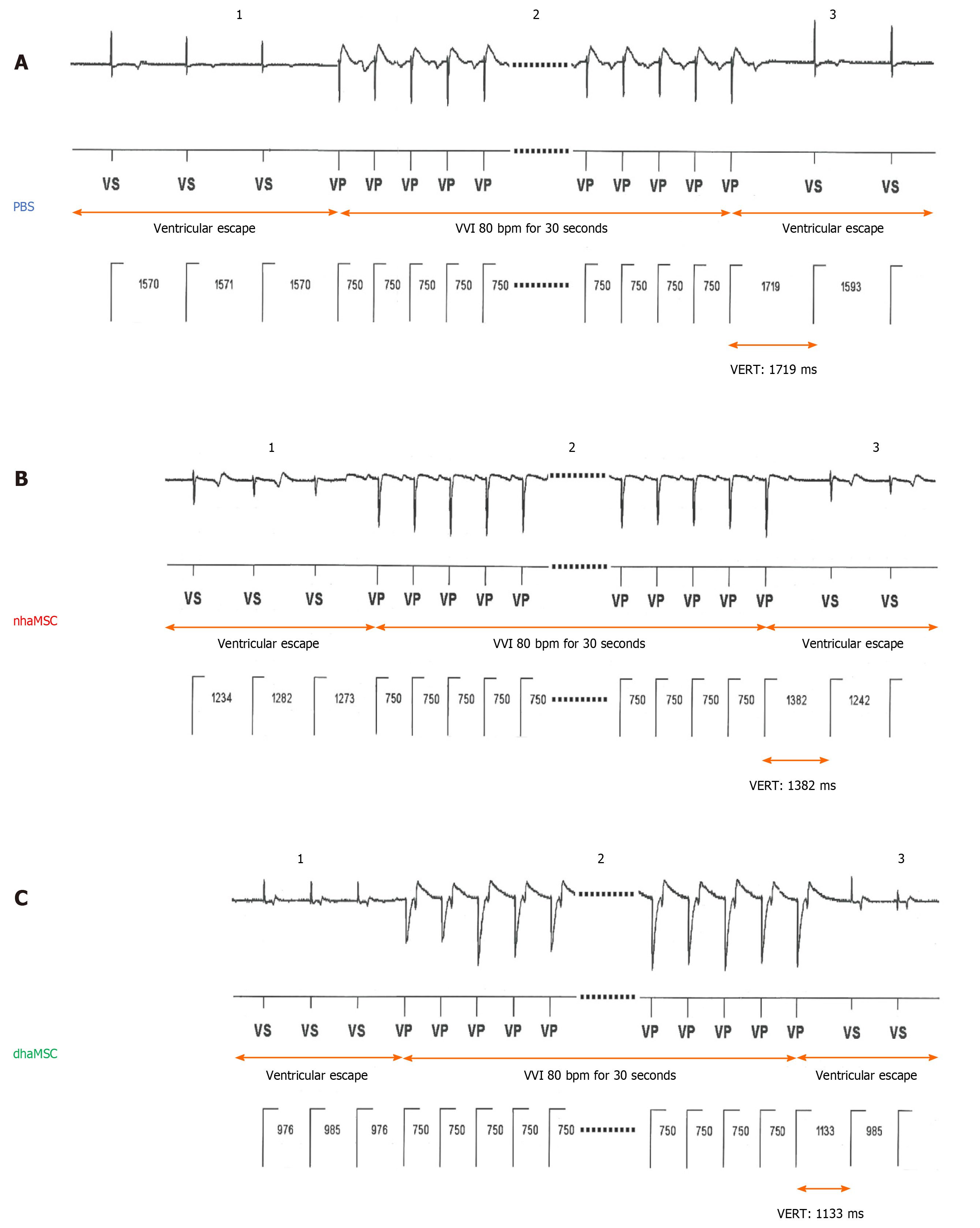
Figure 6 Ventricular escape rhythm recovery time after atrioventricular node ablation.
A: Phosphate-buffered saline (PBS) group; B: Native human mesenchymal stem cells derived from adipose tissue (nhaMSC) group; C: Differentiated human mesenchymal stem cells derived from adipose tissue (dhaMSC) group. 1: Ventricular escape rhythm before overstimulation by electronic pacemaker. 2: Overstimulation with VVI 80 bpm for 30 s by electronic pacemaker. 3: Ventricular escape rhythm after overstimulation by electronic pacemaker. Depicted ventricular escape rhythm recovery time values are exemplary for one pig of each group (dhaMSC, nhaMSC or PBS), average values of n = 6 pigs are included in the text. Electrocardiograms recorded at a speed of 25 mm/s. PBS: Phosphate-buffered saline; nhaMSC: Native human mesenchymal stem cells derived from adipose tissue; dhaMSC: Differentiated human mesenchymal stem cells derived from adipose tissue; VERT: Ventricular escape rhythm recovery time.
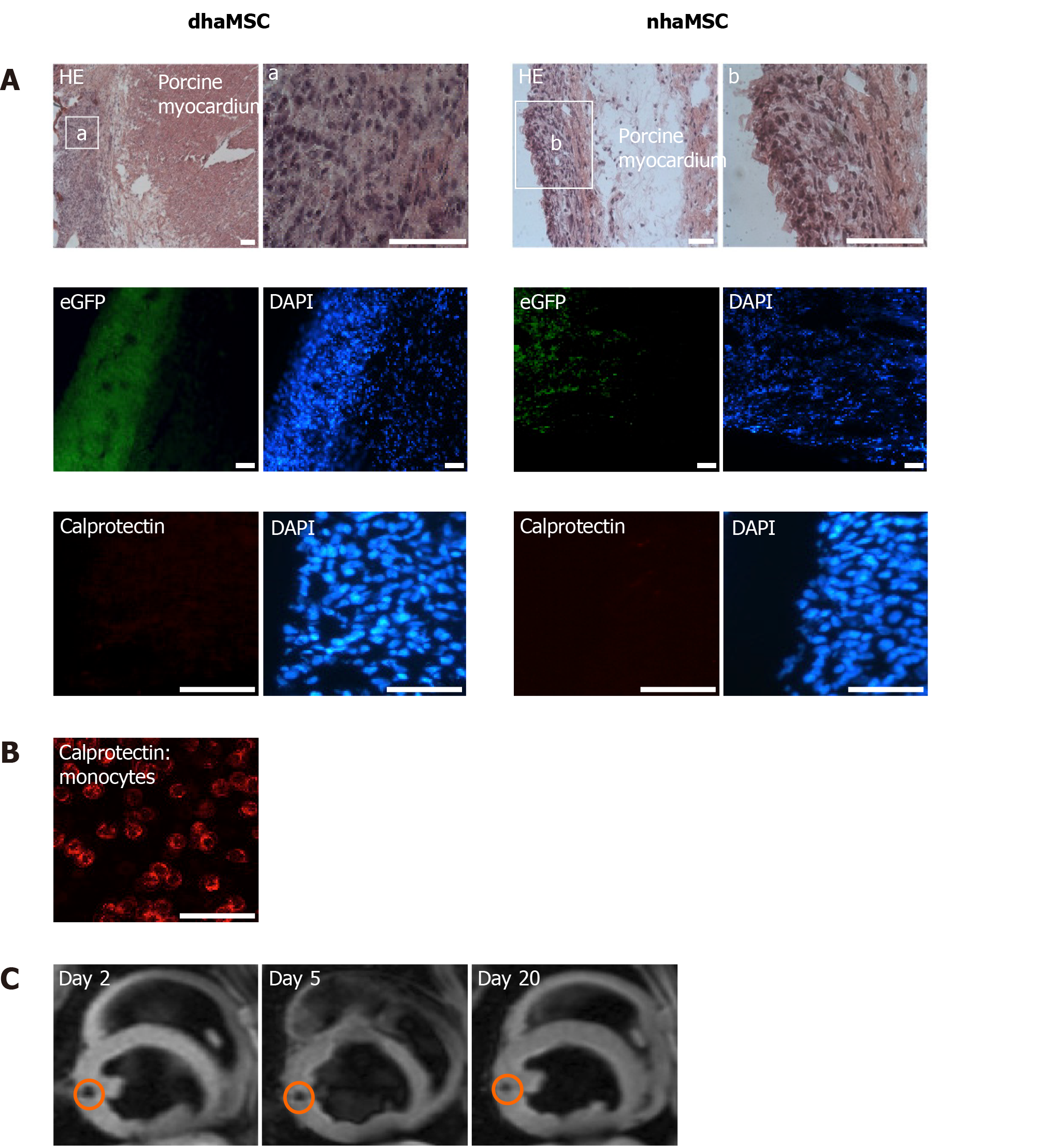
Figure 7 Localization and immunogenicity of xenotransplanted differentiated human mesenchymal stem cells derived from adipose tissue and native human mesenchymal stem cells derived from adipose tissue in the left ventricle of porcine myocardium.
A: Localization of enhanced green fluorescent protein-transfected differentiated human mesenchymal stem cells derived from adipose tissue (dhaMSC) and native human mesenchymal stem cells derived from adipose tissue (nhaMSC) within porcine myocardium. dhaMSC and nhaMSC are negative for calprotectin, a marker of immune reaction; B: Control test with monocytes, which are positive for calprotectin. Scale bars: 50 nm; C: Magnetic resonance imaging of superparamagnetic iron oxides-labeled dhaMSC (orange circle) transplanted into porcine myocardium, performed on days 2, 5 and 20 after cell injection. dhaMSC: Differentiated human mesenchymal stem cells derived from adipose tissue; nhaMSC: Native human mesenchymal stem cells derived from adipose tissue; eGFP: Enhanced green fluorescent protein; DAPI: 4’,6-diamidino-2-phenylindole.
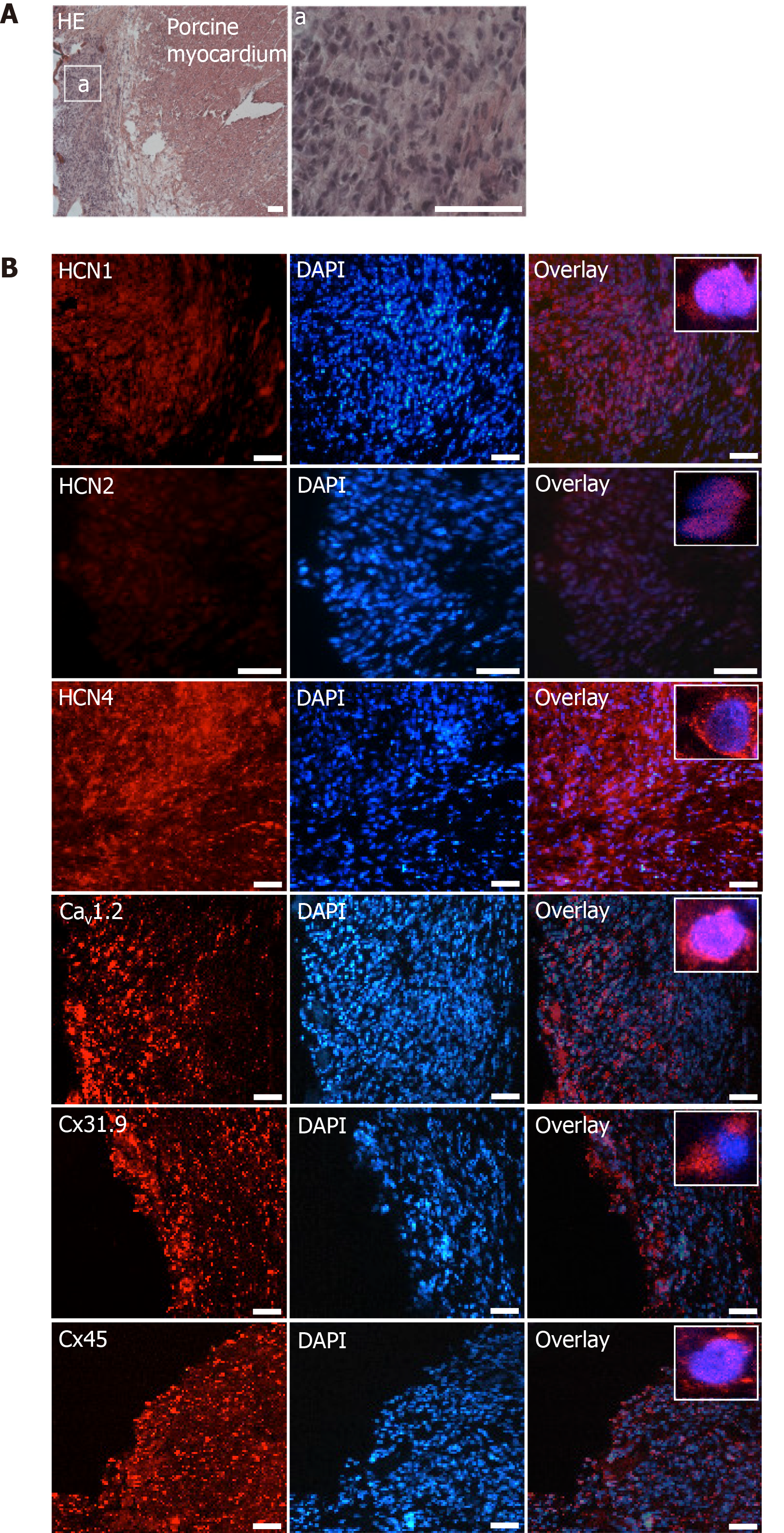
Figure 8 Microscopic analysis of transplanted differentiated human mesenchymal stem cells derived from adipose tissue within porcine myocardium.
A: HE staining of cryosections showing the engraftment of injected differentiated human mesenchymal stem cells derived from adipose tissue (dhaMSC) into porcine myocardium. Overview and dhaMSC area (a); B left: Immunohistochemistry of HCN1, HCN2, HCN4, Cav1.2, Cx31.9 and Cx45. B middle: Nuclei counterstained with 4′,6-diamidino-2-phenylindole (DAPI). B: Right, overlay of immunohistochemistry and DAPI counterstain. Scale bars: 50 nm. DAPI: 4’,6-diamidino-2-phenylindole.
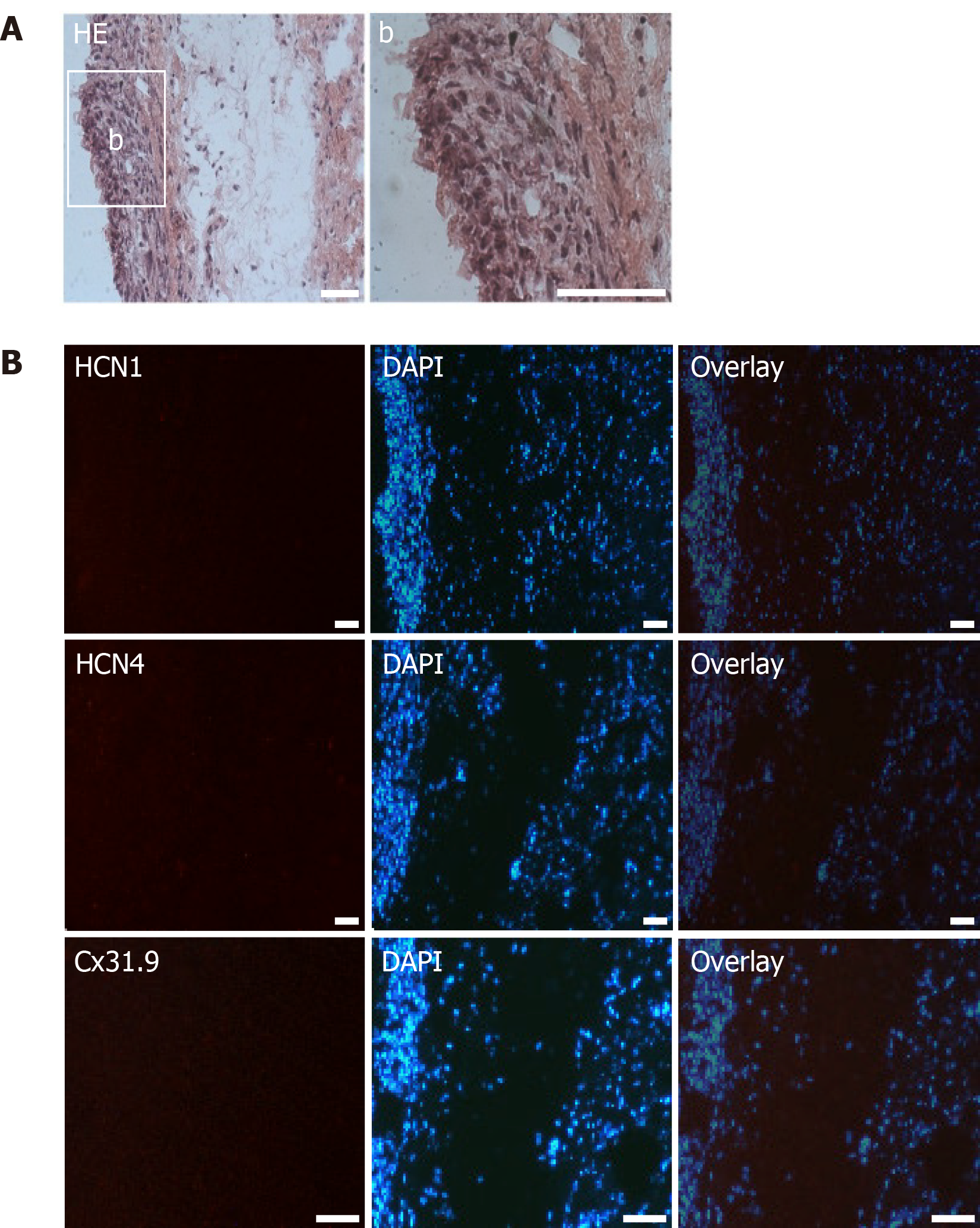
Figure 9 Microscopic analysis of transplanted native human mesenchymal stem cells derived from adipose tissue within porcine myocardium.
A: HE staining of cryosections showing the engraftment of injected native human mesenchymal stem cells derived from adipose tissue (nhaMSC) into porcine myocardium. Overview and nhaMSC area (a); B left: Immunohistochemistry of HCN1, HCN4 and Cx31.9. B middle: Nuclei counterstained with 4′,6-diamidino-2-phenylindole (DAPI). B: right, overlay of immunohistochemistry and DAPI counterstain. Scale bars: 50 nm. DAPI: 4’,6-diamidino-2-phenylindole.
- Citation: Darche FF, Rivinius R, Rahm AK, Köllensperger E, Leimer U, Germann G, Reiss M, Koenen M, Katus HA, Thomas D, Schweizer PA. In vivo cardiac pacemaker function of differentiated human mesenchymal stem cells from adipose tissue transplanted into porcine hearts. World J Stem Cells 2020; 12(10): 1133-1151
- URL: https://www.wjgnet.com/1948-0210/full/v12/i10/1133.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i10.1133