修回日期: 2026-01-14
接受日期: 2026-01-26
在线出版日期: 2026-01-28
射频消融被认为是早期肝细胞癌(hepatocellular carcinoma, HCC)的根治性治疗手段之一, 但其术后复发率高于手术切除, 不完全射频消融(insufficient radiofrequency ablation, IRFA)可能是其关键因素之一. IRFA后HCC细胞表现出的增殖、侵袭、迁移能力增强, 可能导致了局部的复发及肿瘤转移. 研究表明: IRFA后出现了肿瘤微环境重塑、诱导上皮间充质转化、细胞自噬、缺氧环境形成及新生血管等病理生理改变, 这些过程与肿瘤的进展及复发关系密切. 但其促进肿瘤进展复发的具体分子机制尚不清晰, 本篇将重点关注上述问题, 对文献进行回顾, 尝试对IRFA后HCC复发、进展的研究现状进行综述.
核心提要: 不完全射频消融通过重塑肿瘤微环境(诱导髓系抑制性细胞扩增、T细胞耗竭)、激活上皮间充质转化、形成缺氧/缺氧诱导因子(hypoxia-inducible factor, HIF)信号通路及增强保护性自噬, 协同促进残留肝癌细胞增殖、侵袭与免疫逃逸. 靶向HIF、信号换能器和转录激活因子3、自噬关键节点或联合免疫检查点抑制剂, 有望逆转促复发机制.
引文著录: 付星开, 刘源. 不完全射频消融后肝细胞癌复发机制的研究进展. 世界华人消化杂志 2026; 34(1): 37-43
Revised: January 14, 2026
Accepted: January 26, 2026
Published online: January 28, 2026
Radiofrequency ablation is considered one of the curative treatments for early-stage hepatocellular carcinoma (HCC), but its postoperative recurrence rate is higher than that of surgical resection. Insufficient radiofrequency ablation (IRFA) may be a key contributing factor. Following IRFA, HCC cells exhibit enhanced proliferation, invasion, and migration capabilities, potentially leading to local recurrence and tumor metastasis. Research indicates that IRFA induces pathophysiological alterations including tumor microenvironment remodeling, epithelial-mesenchymal transition induction, cellular autophagy, hypoxic environment formation, and neovascularization. These processes are closely associated with tumor progression and recurrence. However, the specific molecular mechanisms underlying their promotion of tumor progression and recurrence remain unclear. This paper will focus on these issues by reviewing the current research state of HCC recurrence and progression after IRFA.
- Citation: Fu XK, Liu Y. Advances in understanding of mechanism of recurrence of hepatocellular carcinoma after insufficient radiofrequency ablation. Shijie Huaren Xiaohua Zazhi 2026; 34(1): 37-43
- URL: https://www.wjgnet.com/1009-3079/full/v34/i1/37.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v34.i1.37
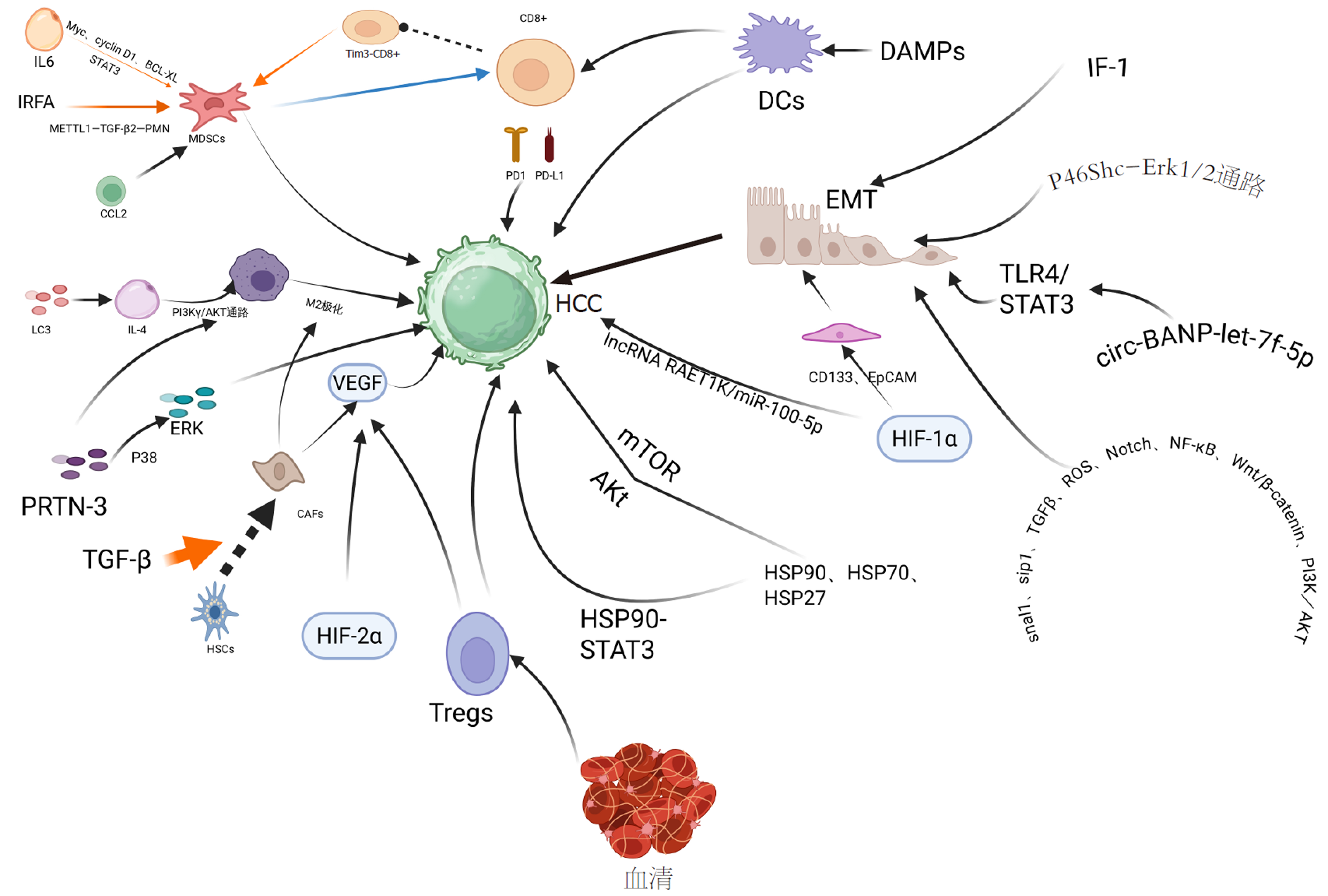
肝细胞癌(hepatocellular carcinoma, HCC)是我国第4位常见恶性肿瘤及第5位肿瘤致死病因[1], 严重威胁我国人民的生命安全健康. 近年来, 肿瘤热消融治疗作为一种局部治疗手段, 在HCC的治疗中发挥着重要作用, 被认为是早期HCC的根治性治疗手段之一. 热消融治疗是指在医学影像技术引导下, 引入消融针或消融天线穿刺到肿瘤内, 通过局部温度升高, 直接杀死肿瘤组织的治疗方法. 目前热消融的主要技术手段有射频消融(radiofrequency ablation, RFA)、微波消融(microwave ablation, MWA)等, 其中RFA通过离子摩擦加热目标组织, 使局部温度升高超过60 ℃, 然后引起组织细胞凝固性坏死. 局部消融治疗比肝切除术具有更安全、并发症少、恢复时间短等优势; RFA联合其他治疗, 能获得更好的疗效[2,3]. 然而, 研究发现[4]RFA后HCC的复发和转移率均高于手术切除, 这可能与热消融后局部仍有残存的肿瘤组织相关. 尽管MWA在理论上能降低因热沉降效应导致的不完全消融风险, 但在处理邻近大血管的肿瘤或形态不规则的病灶时, 同样面临消融不彻底的可能. RFA后肿瘤消融区域在组织学上可分为三个区域: 中心区域: 组织发生凝固性坏死; 亚致死温度移行区域: 组织呈亚致死性应激状态; 肿瘤周围温度区域: 组织未受累. 在亚致死性区, 肿瘤细胞遭受可逆损伤并最终存活, 导致肿瘤在应激状态下加速进展. 该"移行区"的概念在不同热消融技术中普遍存在, 是理解不完全消融后肿瘤复发机制的关键. 在临床中, 为保证消融手术安全, 可能会导致局部肿瘤消融不完全, 使残余HCC受到亚致死性温度刺激, 出现可逆性损伤并存活, 称之为不完全射频消融(insufficient radiofrequency ablation, IRFA). IRFA在肿瘤的快速进展中起着重要作用[5]. IRFA可导致肿瘤微环境(tumor microenvironment, TME)改变、上皮间充质转化(epithelial-mesenchtmal transition, EMT)、缺氧、细胞自噬、热休克蛋白(heat shock protein, HSP)表达变化. 这些改变共同导致残留肿瘤细胞侵袭迁移性增强、加速细胞增殖. 但其促进肿瘤进展复发的具体分子机制尚未完全明确, 本文将重点探讨IRFA诱导的肿瘤促进机制, 尝试阐述IRFA后HCC复发的机制.
TME由细胞和非细胞成分组成. 除了肿瘤细胞外, HCC微环境的细胞成分还包括免疫细胞[如肿瘤浸润淋巴细胞、髓系抑制性细胞(myeloid-derived suppressor cells, MDSCs)、肝星状细胞(hepatic stellate cells, HSCs)和内皮细胞]. 非细胞成分包括细胞外基质(extracellular matrix, ECM)、细胞因子、血管和淋巴网络. 它们相互作用, 形成动态TME, 通过各种机制调节肿瘤存活、传播和进展, 影响肿瘤的结果.
IRFA诱导的免疫抑制微环境是复发的重要驱动力, 其特点是抑制性免疫细胞增多和效应性免疫细胞功能受损. (1)MDSCs扩增: MDSCs是一类具有显著的抑制免疫应答能力的异质性骨髓来源细胞. 研究发现通过增加肿瘤浸润的MDSCs和减少T细胞介导的抗肿瘤免疫反应可加速残余肿瘤的进展[6]. IRFA后MDSCs增加, 通过激活METTL1-TGF-β2-PMN-MDSC轴、减少CD8+T细胞来抑制抗肿瘤免疫反应, 促进肿瘤生长及转移增强[7]. 此外白介素-6(interleukin-6, IL-6)通过信号换能器和转录激活因子3(signal transducer and activator of transcription 3, STAT3)促进MDSCs的增殖, 并通过诱导Myc、cyclin D1和B细胞淋巴瘤XL的表达促进MDSCs的存活, 诱导TME中MDSCs的增殖[8,9], 抑制免疫应答, 促进肿瘤存活. Shi等[10]发现IRFA可诱导CCL2分泌, 招募单核细胞和MDSCs, 抑制免疫应答细胞功能[11]; (2)研究表明, 消融治疗后复发的HCC患者周围血、瘤周组织及肿瘤组织中Tim-3-CD8+T 细胞的比例增加[12]. Tim-3在复发患者肿瘤组织中的表达显著高于未复发组, 且与早期复发(<1年)显著相关(P<0.01). Tim-3是介导免疫抑制微环境的形成和参与肿瘤免疫逃逸的关键免疫检查点分子, 其通过激活核因子-κB(nuclear factor kappaB, NF-κB)通路诱导IL-6的分泌, 从而促进肿瘤进展[13,14]. 使用抗Tim-3抗体可有效逆转CD8+T细胞的消耗, 同时诱导IRFA后适应性程序性死亡受体-1(programmed death 1, PD-1)上调, 联合阻断Tim-3和PD-1显示出明确的协同抗肿瘤疗效[15]; (3)肿瘤相关巨噬细胞M2极化: Liu等[16]指出热处理后巨噬细胞通过LC3蛋白相关吞噬, 激活PI3Kγ/AKT通路, 增强了IL-4介导的巨噬细胞M2编程, 抑制T细胞增殖, 形成免疫抑制微环境; (4)其他机制: IRFA后Sumo2表达增加激活SUMO化(small ubiquitin -like-modifier小泛素样修饰蛋白), 阻碍了IFN-1信号传导进而阻碍了树突状细胞及细胞毒性T淋巴细胞介导的抗肿瘤免疫, 临床分析同样证实Sumo2在IRFA后复发患者的肿瘤组织中表达上调, 与CD8+T细胞浸润负相关(r = -0.68, P<0.05)[17]. IRFA激活caspase-3/GSDME, 导致细胞程序性死亡-细胞焦亡, 并增加耗竭性T细胞(CD3+PD-1+/CTLA-4+)导致PD-L1抑制剂耐药[18]. 增强自噬诱导的免疫原性细胞死亡(immunogenic cell death, ICD)可促进损伤相关分子模式(damage-associated molecular pattern, DAMPs)释放, 激活树突状细胞成熟, 效应CD8+T细胞浸润及细胞因子分泌, 启动特异性免疫, 结合抗PD-L1治疗可有效抑制残留原发灶及转移灶[19].
HSCs在TGF-β作用下转化为癌症相关成纤维细胞, 招募M2巨噬细胞并分泌血管内皮生长因子(vascular endithelial growth factor, VEGF), 增强免疫抑制[20,21]. 另一方面, 肿瘤内皮细胞是TME中促进肿瘤进展和转移的关键部分, 参与血管生成并为癌细胞播散提供途径. IRFA后肿瘤内皮细胞的血管生成能力增强. 肿瘤内皮细胞中ICAM-1的表达升高[22], 激活血小板, 增加内皮通透性, 这与IRFA后HCC细胞的生长和转移有关. 此外, IRFA导致残留肿瘤中ATP酶抑制因子(ATPase inhibitory factor 1, IF1)、CD31和N-钙粘蛋白的表达增加[23]. 抑制IF1可减少血管生成, 增加IF1的表达诱导的血管生成.
RFA在破坏肿瘤细胞的同时释放肿瘤碎片, 理论上刺激机体的抗肿瘤免疫反应[24]. 然而其所诱导的免疫原性通常较弱, 无法产生持续的抗肿瘤免疫反应, 无法有效抑制残余肿瘤的复发[25]. TILs在IRFA后与血管生成相关因子及ECM产生"延迟效应"进而未能立即增加并促进肿瘤细胞扩散[26]. ECM重塑在肿瘤侵袭和转移中发挥着至关重要的作用. IRFA后, Zhang等[27]观察到在消融周围区内Ⅰ型胶原沉积增加, 诱导残留HCC细胞中细胞外调解蛋白激酶(extracellular protein kinase, ERK)磷酸化的激活. 其磷酸化可促进残留HCC增殖. Shi等[26]的研究证实IRFA诱导的HCC微环境中存在差异表达基因(differentially expressed gene, DEG)及差异表达蛋白质(differentially expressed protein, DEP). DEP-DEG主要参与免疫炎症反应、癌症进展和代谢过程. 蛋白酶-3被确定为IRFA之后持续上调的DEP之一. 通过多种致癌因子以及PI3K/AKT和P38/ERK信号通路促进肿瘤生长. RFA后血清成分具有双重作用: 一方面其可增强单核细胞抗原呈递能力, 另一方面其抑制CD8+T细胞功能, 降低CD161/CD5表达, 并促进Tregs/B细胞分泌VEGF来促进免疫抑制微环境[28].
EMT是HCC转移的核心机制, 与胚胎发育、器官发生、损伤修复、恶性肿瘤的迁移和侵袭密切相关[29]. 肿瘤上皮细胞在受到一定刺激后发生表型转化, 转变为具有迁移能力的间充质细胞.
EMT的激活: 热处理后的肿瘤细胞中IL-6分泌增加, 通过Jak2和STAT3通路激活诱发EMT[30]. IF-1则在HCC细胞中通过上调N-cadherin、snail和Vimentin的表达, 下调E-cadherin[31]表达促进EMT. Ikemoto等[32]和Yoshida等[33]的实验证明: 亚致死热处理, 使HCC细胞更易发生EMT, 其转化为具有更高的增殖潜力的祖细胞样状态, 这一过程由p46Shc-Erk1/2通路驱动的, 加速HCC的生长和扩散. p46-Shc表达及其磷酸化水平可能是HCC恶性转化、肿瘤侵袭和转移的一重要预测指标, 并且其下游效应物Erk1/2是EMT样变化的关键, 将其作为治疗靶点, 可能会为防止IRFA后复发提供新的方向. Li等[34]指出circ-BANP会上调let-7f-5p, 过度激活TLR4/STAT3信号通路促进RFA不充分后残留的HCC细胞的增殖、迁移和EMT形成. EMT赋予残留HCC细胞更强的迁移、侵袭、增殖能力和干性特征, 使其具有强大的转移潜力和对化疗的耐药性, 是临床控制IRFA后复发的重大挑战.
缺氧环境是IRFA后HCC进展的另一重要因素. 缺氧是实体瘤的一大特征, 缺氧在某些情况下对肿瘤增殖有负面影响, 但其主要是使肿瘤细胞适应缺氧和营养缺乏, 从而通过激活自噬, 抑制细胞凋亡来诱导癌细胞存活. 缺氧通过氧化还原效应和缺氧诱导因子(hypoxia-inducible factor, HIF)的稳定作用影响细胞生长的诸多方面. HIF由α和β亚基组成. HIF通过促进血管生成、促进糖酵解, 免疫逃避等方式影响IRFA后HCC进展[35].
大多数实体瘤都有一定程度的缺氧. 增强渗透滞留效应(enhanced permeation retention, EPR)是指纳米材料更易渗透进入肿瘤并长期滞留的现象. 缺氧促进血管生成, 广泛的血管生成提高了ERP, 促进砷负载的ZIF-8纳米颗粒在残余肿瘤中的富集, 可显著抑制残留肿瘤生长、转移及EMT[36]. 在缺氧条件下肿瘤细胞可通过snal1、sip1、TGFβ、ROS、Notch、NF-κB、Wnt/β-catenin、PI3K/AKT等途径的活化来诱导EMT, 上皮细胞转变为可移动的基质细胞, 获得迁移到远处部位的能力[31]. 有研究发现[37]参与ECM沉积和重塑的多种酶受缺氧因子调节, 通过HIF-1α依赖性机制, 可下调细胞中金属蛋白酶2组织抑制剂的表达. 另外, HIF-2α通过增加残留组织血管生成, 促进缺血或缺氧条件下肿瘤细胞存活和肿瘤复发, 这是通过HIF-2α/VEGFA/EphA2途径表达上调调控[38]. 在缺氧环境下, HIF-1α通过HIF-1α/lncRNA RAET1K/miR-100-5p轴的调节诱导HCC细胞糖酵解进而影响肿瘤进展[39].
缺氧使HIF-1α诱导CD133及EpCAM上调导致肿瘤干细胞扩增, 其通过EMT增强肿瘤向远程转移的能力[29]. HIF-1、HIF-2、HIF-3之间是否存在一些相互调控作用及其具体机制?如果存在相互调控作用, 靶向HIF-1α治疗及HIF-1α评估预后的同时, 是否需评估HIF-2和HIF-3的影响?这些问题有待更多深入研究阐明.
自噬是一种高度保守的细胞内自我保护过程, 对于受损细胞器和细胞质物质的降解至关重要. 在肿瘤中, 自噬扮演着"双刃剑"的角色. HSP是一类在应激条件下(如高热)被强烈诱导表达的分子伴侣蛋白.
研究表明[40,41], IRFA后亚致死热应激主要诱导残留HCC细胞发生低水平自噬激活. 这种保护性自噬有利于肿瘤细胞在应激下存活, 清除受损细胞器, 维持能量供应. 更重要的是, 低水平自噬限制了细胞的免疫原性, 并有助于形成免疫抑制微环境: 支持调节性T细胞存活和促进肿瘤相关巨噬细胞向M2型极化[33,42]. 这导致肿瘤对免疫治疗产生抵抗. 研究还发现[43], 亚致死热处理后的肿瘤细胞对自噬的依赖性可能更高. 与保护性自噬相反, 高水平或持续性自噬可导致肿瘤细胞死亡. 特别重要的是, 某些强烈的自噬诱导可触发ICD. ICD过程中, 死亡细胞释放DAMPs, 如ATP、HMGB1、钙网蛋白等. DAMPs可有效激活树突状细胞, 促进其成熟和抗原呈递, 进而激活效应CD8+T细胞浸润和细胞因子分泌, 启动特异性抗肿瘤免疫应答[44,45]. 增强IRFA诱导的自噬至ICD水平, 并结合抗PD-L1治疗, 被证明能有效抑制残留原发灶及转移灶[19,41]. IRFA后, 细胞内AMP与ATP比值升高, 激活AMP依赖的蛋白激酶(AMP-activated protein kinase, AMPK)通路[42]. AMPK磷酸化可抑制下游哺乳动物雷帕霉素靶蛋白信号, 从而诱导细胞自噬.
IRFA的高温强烈诱导多种HSPs(如HSP27, HSP70, HSP90)表达[33,46]. 临床研究显示[47], HSP70和HSP90在IRFA后复发患者的肿瘤组织中表达显著升高, 且高表达与术后早期复发风险增加相关(P<0.05). HSPs作为分子伴侣, 协助蛋白质正确折叠, 保护细胞免受热损伤. 应激诱导磷酸化蛋白1调节HSP70和HSP90的功能, 促进其复合物组装. 研究发现热处理可导致STAT3表达增加. STAT3能与HSP90形成复合物, 通过稳定其客户蛋白(包括多种促生存和促癌蛋白), 促进残留肿瘤细胞的存活[34,48]. 应用HSP90抑制剂(如XL888, 17-AAG)可破坏这些复合物, 促进肿瘤细胞凋亡[48]. 少数临床个案报道提示, HSP90抑制剂联合RFA可延缓复发进程, 值得进一步临床验证. HSP表达与自噬活性存在关联. Chen等[47]指出HSP90、HSP70和HSP27表达上调常伴随Akt和mTOR磷酸化增强, 导致自噬减少和细胞凋亡增加. 这种关联提示联合靶向HSP和自噬可能是有效策略. Chen等[49]发现, 联合应用HSP90抑制剂17-AAG与自噬抑制剂3-甲基腺嘌呤(3-MA), 比单药治疗能更显著地促进IRFA后残留HCC细胞的凋亡. IRFA也被发现通过调节CD133(一种肿瘤干细胞标志物)影响自噬水平(如抑制LC3B-Ⅱ表达, 诱导p62表达), 形成一个促进HCC细胞快速生长和侵袭的反馈回路[50,51].
尽管MWA可利用高频电磁波使组织内极性分子(主要是水分子)发生高速旋转摩擦而生热, 其加热效率高、受热沉降效应影响小, 理论上能产生更大、更均匀的消融区. 但不完全微波消融也可引发类似的TME改变、EMT、缺氧、细胞自噬及HSP表达变化. 例如, 有研究表明[52,53]IMWA后免疫细胞减少(CD4+T、CD8+T、自然杀伤细胞、巨噬细胞等), MDSCs增加. IMWA后复发患者外周血中MDSCs比例显著升高, 且与Tim-3+T细胞比例正相关. 此外IMWA同样可通过激活TGF-β通路, 激活残留肿瘤组织中的细胞增殖潜能、肿瘤生长、EMT以及浸润的Treg细胞增加[54]. IMWA后, HIF-1α同样被稳定并激活, 通过HIF-1α/lncRNA RAET1K/miR-100-5p轴等途径诱导糖酵解重编程和肿瘤干细胞扩增[29,40]. MWA更快速的升温可能导致更急剧的缺氧反应, 进而更强烈地诱导肿瘤干细胞, 但这仍需更多直接比较研究证实. 当消融不完全时, IRFA和IMWA最终都可能导向免疫抑制微环境的建立、EMT的诱导. 因此, 未来研究在深入探索IRFA特异性机制的同时, 应加强对不同消融技术间横向比较的研究.
RFA作为HCC的重要治疗手段, 其术后肿瘤复发问题不容忽视. 尽管RFA能有效局部灭活肿瘤, 但不IRFA导致的亚致死热应激可重塑TME、诱导EMT、形成缺氧微环境、调控自噬平衡和HSP表达, 最终赋予残留HCC细胞更强的增殖、侵袭、迁移和免疫逃逸能力, 显著增加局部复发和远处转移风险. 本文系统综述了IRFA后促进HCC复发的主要机制, 包括免疫抑制性TME形成(MDSCs, 肿瘤相关巨噬细胞, T细胞耗竭)、EMT转化、缺氧/HIF信号激活及肿瘤干细胞扩增、以及自噬/HSPs的双重角色及其相互作用. IRFA后触发的各种促瘤机制并非孤立存在, 而是构成一个复杂且相互关联的网络, 共同驱动HCC的复发与转移(图1). 缺氧是此网络中的核心调控因素. HIF-1α不仅能直接诱导EMT和肿瘤干细胞特性[31,42], 还能转录上调自噬关键基因如BNIP3和Beclin-1, 从而在缺氧环境下激活保护性自噬, 促进肿瘤细胞存活[42,55]. 值得注意的是, 这种保护性自噬会抑制ICD的发生, 减少DAMPs的释放, 进而削弱抗肿瘤免疫应答, 有助于免疫抑制微环境的形成[19,42]. 此外, 缺氧和自噬共同调节免疫细胞功能. 例如, HIF-1α可促进肿瘤相关巨噬细胞向M2型极化, 而M2型肿瘤相关巨噬细胞又可分泌TGF-β等因子进一步促进EMT和血管生成, 形成正反馈循环[8,16]. 另一方面, EMT过程与获得干细胞特性密切相关, 而肿瘤干细胞通常处于高度自噬状态以维持其干性, 并对化疗耐药[29,50]. 深入理解这些复杂且相互关联的机制网络, 对于优化RFA治疗策略、降低复发率至关重要. 通过本篇综述应指出在未来的研究中应聚焦于: (1)机制探索: 进一步阐明IRFA特异性触发的关键分子开关及信号通路: 不同HIF亚型间的互作、特定免疫检查点分子的时序性表达、自噬与免疫微环境/EMT/缺氧的精确串扰机制, 寻找核心调控节点; (2)RFA技术优化: 改进RFA设备和技术以扩大消融范围, 精确调控消融温度, 减少亚致死性区域, 减少IRFA发生; (3)联合治疗方案的优化: ①靶向分子通路: 靶向交叉通路中的关键节点(如HIF-1α、STAT3或特定自噬阶段)可能比针对单一通路产生更有效的抗肿瘤效果. 开发靶向IRFA后关键促瘤通路(如HIF、STAT3、特定HSPs、EMT相关因子)的小分子抑制剂或单克隆抗体. ②免疫治疗增效: 探索RFA联合免疫检查点抑制剂(抗PD-1/PD-L1, 抗Tim-3, 抗CTLA-4)、免疫激动剂(如TLR激动剂)、过继性细胞治疗(如CAR-T, TILs)的策略, 逆转IRFA诱导的免疫抑制. 利用增强自噬诱导ICD的策略联合免疫治疗具有广阔前景[19,41]. ③调节自噬: 深入研究IRFA后自噬的持续时间及影响水平, 开发能精准将保护性自噬转换为细胞毒性自噬或ICD的药物(如自噬增效剂或保护性自噬抑制剂)及其信号传导通路的更多靶点. ④新型药物的研发: 开发清除或抑制IRFA后激活的肿瘤干细胞药物. 新型纳米药物与递送系统: 利用IRFA后增强的EPR效应和改变的TME特性(如缺氧、特定酶表达), 实现药物在残留肿瘤部位的高效靶向富集和控释, 提高疗效并降低全身毒性[36]. 鉴于不完全消融后促复发机制的高度重叠(IRFA、IMWA), 许多联合治疗策略(如免疫检查点抑制剂、自噬调节、靶向HIF通路等)可能对不同技术所致的残留肿瘤均具有普适性. 然而, 仍需验证同一策略在应对IRFA与IMWA残留肿瘤时的疗效是否存在细微差异, 以实现真正的精准联合治疗.
| 1. | 邢 家利, 金 保, 桑 新亭, 毛 一雷, 杜 顺达. 原发性肝癌的综合治疗研究进展. 肿瘤综合治疗电子杂志. 2023;9:1-7. [DOI] |
| 3. | Jang SY, Park SY, Kweon YO, Lee YR, Ryeom HK, Cha JG, Kim S, Lee WK, Jo AJ, Tak WY. Temporal trends and long-term outcomes of radiofrequency ablation for hepatocellular carcinoma within the Milan criteria. Sci Rep. 2024;14:19815. [PubMed] [DOI] |
| 4. | Jiang C, Feng Q, Zhang Z, Qiang Z, Du A, Xu L, Li J. Radiofrequency ablation versus laparoscopic hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. World J Surg Oncol. 2024;22:188. [PubMed] [DOI] |
| 5. | Guo Y, Ren Y, Dong X, Kan X, Zheng C. An Overview of Hepatocellular Carcinoma After Insufficient Radiofrequency Ablation. J Hepatocell Carcinoma. 2022;9:343-355. [PubMed] [DOI] |
| 6. | Wu H, Li SS, Zhou M, Jiang AN, He Y, Wang S, Yang W, Liu H. Palliative Radiofrequency Ablation Accelerates the Residual Tumor Progression Through Increasing Tumor-Infiltrating MDSCs and Reducing T-Cell-Mediated Anti-Tumor Immune Responses in Animal Model. Front Oncol. 2020;10:1308. [PubMed] [DOI] |
| 7. | Zeng X, Liao G, Li S, Liu H, Zhao X, Li S, Lei K, Zhu S, Chen Z, Zhao Y, Ren X, Su T, Cheng AS, Peng S, Lin S, Wang J, Chen S, Kuang M. Eliminating METTL1-mediated accumulation of PMN-MDSCs prevents hepatocellular carcinoma recurrence after radiofrequency ablation. Hepatology. 2023;77:1122-1138. [PubMed] [DOI] |
| 8. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [PubMed] [DOI] |
| 9. | Tang Y, Shu Z, Zhu M, Li S, Ling Y, Fu Y, Hu Z, Wang J, Yang Z, Liao J, Xu L, Yu M, Peng Z. Size-Tunable Nanoregulator-Based Radiofrequency Ablation Suppresses MDSCs and Their Compensatory Immune Evasion in Hepatocellular Carcinoma. Adv Healthc Mater. 2023;12:e2302013. [PubMed] [DOI] |
| 10. | Shi L, Wang J, Ding N, Zhang Y, Zhu Y, Dong S, Wang X, Peng C, Zhou C, Zhou L, Li X, Shi H, Wu W, Long X, Wu C, Liao W. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat Commun. 2019;10:5421. [PubMed] [DOI] |
| 11. | Sun T, Sun B, Cao Y, Liu J, Chen J, Liang B, Zheng C, Kan X. Synergistic effect of OK-432 in combination with an anti-PD-1 antibody for residual tumors after radiofrequency ablation of hepatocellular carcinoma. Biomed Pharmacother. 2023;166:115351. [PubMed] [DOI] |
| 12. | Ghelfi J, Macek Jilkova Z, Sengel C, Brusset B, Teyssier Y, Costentin C, Mercey-Ressejac M, Dumolard L, Manceau M, Mathieu E, Bricault I, Decaens T. PD1 and TIM3 Expression is Associated with Very Early Hepatocellular Carcinoma Recurrence After Percutaneous Thermal Ablation. J Hepatocell Carcinoma. 2024;11:39-50. [PubMed] [DOI] |
| 13. | Zhang H, Song Y, Yang H, Liu Z, Gao L, Liang X, Ma C. Tumor cell-intrinsic Tim-3 promotes liver cancer via NF-κB/IL-6/STAT3 axis. Oncogene. 2018;37:2456-2468. [PubMed] [DOI] |
| 14. | Guo Q, Shen S, Guan G, Zhu C, Zou C, Cao J, Cheng W, Xu X, Yu J, Lin Z, Wang G, Chen L, Cheng P, Wu A. Cancer cell intrinsic TIM-3 induces glioblastoma progression. iScience. 2022;25:105329. [PubMed] [DOI] |
| 15. | Wu N, Pei X, Cai W, Ye X, Lu W. Blocking Tim-3 enhances CD8(+) T cell activity to inhibit hepatocellular carcinoma recurrence post-radiofrequency ablation. Int J Hyperthermia. 2025;42:2516502. [PubMed] [DOI] |
| 16. | Liu X, Zhang W, Xu Y, Xu X, Jiang Q, Ruan J, Wu Y, Zhou Y, Saw PE, Luo B. Targeting PI3Kγ/AKT Pathway Remodels LC3-Associated Phagocytosis Induced Immunosuppression After Radiofrequency Ablation. Adv Sci (Weinh). 2022;9:e2102182. [PubMed] [DOI] |
| 17. | Liu J, Li X, Chen J, Guo J, Guo H, Zhang X, Fan J, Zhang K, Mao J, Zhou B. Targeting SUMOylation with an injectable nanocomposite hydrogel to optimize radiofrequency ablation therapy for hepatocellular carcinoma. J Nanobiotechnology. 2024;22:338. [PubMed] [DOI] |
| 18. | Liang X, Liu Q, Zhu S, Li Z, Chen H, Su Z. GSDME has prognostic and immunotherapeutic significance in residual hepatocellular carcinoma after insufficient radiofrequency ablation. Transl Oncol. 2024;39:101796. [PubMed] [DOI] |
| 19. | Dumolard L, Ghelfi J, Roth G, Decaens T, Macek Jilkova Z. Percutaneous Ablation-Induced Immunomodulation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21:4398. [PubMed] [DOI] |
| 20. | Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27-42. [PubMed] [DOI] |
| 21. | Affo S, Filliol A, Gores GJ, Schwabe RF. Fibroblasts in liver cancer: functions and therapeutic translation. Lancet Gastroenterol Hepatol. 2023;8:748-759. [PubMed] [DOI] |
| 22. | Kong J, Yao C, Dong S, Wu S, Xu Y, Li K, Ji L, Shen Q, Zhang Q, Zhan R, Cui H, Zhou C, Niu H, Li G, Sun W, Zheng L. ICAM-1 Activates Platelets and Promotes Endothelial Permeability through VE-Cadherin after Insufficient Radiofrequency Ablation. Adv Sci (Weinh). 2021;8:2002228. [PubMed] [DOI] |
| 23. | Kong J, Yao C, Ding X, Dong S, Wu S, Sun W, Zheng L. ATPase Inhibitory Factor 1 Promotes Hepatocellular Carcinoma Progression After Insufficient Radiofrequency Ablation, and Attenuates Cell Sensitivity to Sorafenib Therapy. Front Oncol. 2020;10:1080. [PubMed] [DOI] |
| 24. | Yin L, Li XY, Zhu LL, Chen GL, Xiang Z, Wang QQ, Bi JW, Wang Q. Clinical application status and prospect of the combined anti-tumor strategy of ablation and immunotherapy. Front Immunol. 2022;13:965120. [PubMed] [DOI] |
| 25. | Tian Z, Hong B, Chen J, Tang Z. Combination of Radiofrequency Ablation With Resiquimod to Treat Hepatocellular Carcinoma Via Inflammation of Tumor Immune Microenvironment and Suppression of Angiogenesis. Front Oncol. 2022;12:891724. [PubMed] [DOI] |
| 26. | Shi ZR, Duan YX, Cui F, Wu ZJ, Li MP, Song PP, Peng QL, Ye WT, Yin KL, Kang MQ, Yu YX, Yang J, Tang W, Liao R. Integrated proteogenomic characterization reveals an imbalanced hepatocellular carcinoma microenvironment after incomplete radiofrequency ablation. J Exp Clin Cancer Res. 2023;42:133. [PubMed] [DOI] |
| 27. | Zhang R, Ma M, Lin XH, Liu HH, Chen J, Chen J, Gao DM, Cui JF, Ren ZG, Chen RX. Extracellular matrix collagen I promotes the tumor progression of residual hepatocellular carcinoma after heat treatment. BMC Cancer. 2018;18:901. [PubMed] [DOI] |
| 28. | Zhao Y, Yang T, Ouyang Y, Rao W, Liu K, Zheng J, Lv F, Shi Y, Wang F, Liu D, Qiao L, Xia Z, Zhang Y, Chen D, Wang W. Radiofrequency ablation plays double role in immunosuppression and activation of PBMCs in recurrent hepatocellular carcinoma. Front Immunol. 2024;15:1339213. [PubMed] [DOI] |
| 29. | Niu Q, Ye S, Zhao L, Qian Y, Liu F. The role of liver cancer stem cells in hepatocellular carcinoma metastasis. Cancer Biol Ther. 2024;25:2321768. [PubMed] [DOI] |
| 30. | Zhou T, Liu B, Wang Y, Wang W, Chang H, Li D, Li Y, Song Z. Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition mediated by interleukin-6/signal transducer and activator of transcription 3/Snail pathway in the H22 cells. J Cancer Res Ther. 2020;16:1112-1118. [PubMed] [DOI] |
| 31. | Khalaf K, Hana D, Chou JT, Singh C, Mackiewicz A, Kaczmarek M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front Immunol. 2021;12:656364. [PubMed] [DOI] |
| 32. | Ikemoto T, Shimada M, Yamada S. Pathophysiology of recurrent hepatocellular carcinoma after radiofrequency ablation. Hepatol Res. 2017;47:23-30. [PubMed] [DOI] |
| 33. | Yoshida S, Kornek M, Ikenaga N, Schmelzle M, Masuzaki R, Csizmadia E, Wu Y, Robson SC, Schuppan D. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013;58:1667-1680. [PubMed] [DOI] |
| 34. | Li G, Kong J, Dong S, Niu H, Wu S, Sun W. Circular BANP knockdown inhibits the malignant progression of residual hepatocellular carcinoma after insufficient radiofrequency ablation. Chin Med J (Engl). 2022;135:1578-1587. [PubMed] [DOI] |
| 35. | Méndez-Blanco C, Fernández-Palanca P, Fondevila F, González-Gallego J, Mauriz JL. Prognostic and clinicopathological significance of hypoxia-inducible factors 1α and 2α in hepatocellular carcinoma: a systematic review with meta-analysis. Ther Adv Med Oncol. 2021;13:1758835920987071. [PubMed] [DOI] |
| 36. | Chen X, Huang Y, Chen H, Chen Z, Chen J, Wang H, Li D, Su Z. Correction to: Augmented EPR effect post IRFA to enhance the therapeutic efficacy of arsenic loaded ZIF-8 nanoparticles on residual HCC progression. J Nanobiotechnology. 2022;20:175. [PubMed] [DOI] |
| 37. | Takemoto R, Kamiya T, Atobe T, Hara H, Adachi T. Regulation of lysyl oxidase expression in THP-1 cell-derived M2-like macrophages. J Cell Biochem. 2021;122:777-786. [PubMed] [DOI] |
| 38. | Wu L, Zhou J, Zhou W, Huang XF, Chen Q, Wang W, Zhai L, Li S, Tang Z. Sorafenib blocks the activation of the HIF-2α/VEGFA/EphA2 pathway, and inhibits the rapid growth of residual liver cancer following high-intensity focused ultrasound therapy in vivo. Pathol Res Pract. 2021;220:153270. [PubMed] [DOI] |
| 39. | Zhou Y, Huang Y, Hu K, Zhang Z, Yang J, Wang Z. HIF1A activates the transcription of lncRNA RAET1K to modulate hypoxia-induced glycolysis in hepatocellular carcinoma cells via miR-100-5p. Cell Death Dis. 2020;11:176. [PubMed] [DOI] |
| 40. | Xu M, Hu Y, Ding W, Li F, Lin J, Wu M, Wu J, Wen LP, Qiu B, Wei PF, Li P. Rationally designed rapamycin-encapsulated ZIF-8 nanosystem for overcoming chemotherapy resistance. Biomaterials. 2020;258:120308. [PubMed] [DOI] |
| 41. | Zhang S, Huang Y, Pi S, Chen H, Ye F, Wu C, Li L, Ye Q, Lin Y, Su Z. Autophagy-amplifying nanoparticles evoke immunogenic cell death combined with anti-PD-1/PD-L1 for residual tumors immunotherapy after RFA. J Nanobiotechnology. 2023;21:360. [PubMed] [DOI] |
| 42. | Jin Z, Sun X, Wang Y, Zhou C, Yang H, Zhou S. Regulation of autophagy fires up the cold tumor microenvironment to improve cancer immunotherapy. Front Immunol. 2022;13:1018903. [PubMed] [DOI] |
| 43. | Li X, Wang ZG, Zhu H, Wen HP, Ning D, Liu HY, Pang DW, Liu SL. Inducing Autophagy and Blocking Autophagic Flux via a Virus-Mimicking Nanodrug for Cancer Therapy. Nano Lett. 2022;22:9163-9173. [PubMed] [DOI] |
| 44. | Zhu S, Wang Y, Tang J, Cao M. Radiotherapy induced immunogenic cell death by remodeling tumor immune microenvironment. Front Immunol. 2022;13:1074477. [PubMed] [DOI] |
| 45. | Catanzaro E, Feron O, Skirtach AG, Krysko DV. Immunogenic Cell Death and Role of Nanomaterials Serving as Therapeutic Vaccine for Personalized Cancer Immunotherapy. Front Immunol. 2022;13:925290. [PubMed] [DOI] |
| 46. | Wu S, Zhao Y, Wang D, Chen Z. Mode of Action of Heat Shock Protein (HSP) Inhibitors against Viruses through Host HSP and Virus Interactions. Genes (Basel). 2023;14:792. [PubMed] [DOI] |
| 47. | Chen F, Bao H, Xie H, Tian G, Jiang T. Heat shock protein expression and autophagy after incomplete thermal ablation and their correlation. Int J Hyperthermia. 2019;36:95-103. [PubMed] [DOI] |
| 48. | Sun C, Bai M, Ke W, Wang X, Zhao X, Lu Z. The HSP90 inhibitor, XL888, enhanced cell apoptosis via downregulating STAT3 after insufficient radiofrequency ablation in hepatocellular carcinoma. Life Sci. 2021;282:119762. [PubMed] [DOI] |
| 49. | Chen F, Xie H, Bao H, Violetta L, Zheng S. Combination of HSP90 and autophagy inhibitors promotes hepatocellular carcinoma apoptosis following incomplete thermal ablation. Mol Med Rep. 2020;22:337-343. [PubMed] [DOI] |
| 50. | Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:12. [PubMed] [DOI] |
| 51. | Lin F, Zhu YT, Qin ZH. Biomarkers of Autophagy. Adv Exp Med Biol. 2021;1208:265-287. [PubMed] [DOI] |
| 52. | Huang T, Cao H, Dai S, Zhu Y, Liu H, Zhu S, Lu Z, Liu C, Lv C, Li Z, Song J, Zhuo H. Gr-1 blockade remodels the immunosuppressive microenvironment induced by incomplete microwave ablation of hepatocellular carcinoma. Cancer Cell Int. 2024;24:395. [PubMed] [DOI] |
| 53. | Liu QQ, Li HZ, Li SX, Bao Y, Wang TC, Hu C, Xiao YD. CD36-mediated accumulation of MDSCs exerts abscopal immunosuppressive responses in hepatocellular carcinoma after insufficient microwave ablation. Biochim Biophys Acta Mol Basis Dis. 2024;1870:167493. [PubMed] [DOI] |
| 54. | Ju S, Duan X, Wang Y, Zhang M, Bai Y, He X, Wang C, Liu J, Yao W, Zhou C, Xiong B, Zheng C. Blocking TGFβR synergistically enhances anti-tumor effects of anti-PD-1 antibody in a mouse model of incomplete thermal ablation. Int Immunopharmacol. 2024;138:112585. [PubMed] [DOI] |
| 55. | Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560-575. [PubMed] [DOI] |
学科分类: 胃肠病学和肝病学
手稿来源地: 四川省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): 0
C级 (良好): C, C, C
D级 (一般): 0
E级 (差): 0
科学编辑: 刘继红 制作编辑:张砚梁