修回日期: 2026-01-14
接受日期: 2026-01-21
在线出版日期: 2026-01-28
胃癌(gastric cancer, GC)仍然是全球重大的健康挑战, 其中幽门螺杆菌(Helicobacter pylori, H. pylori)感染是主要的病因学因素. 本综述深入分析了幽门螺杆菌感染、TP53(p53)缺陷和CDKN2A(p16)缺失在驱动GC发生中的协同机制, 重点关注代谢重编程. H. pylori感染诱发慢性炎症和代谢改变(如瓦博格效应和谷氨酰胺成瘾), 这些改变与p53和p16缺陷相互作用, 共同营造了一个促肿瘤发生的代谢微环境. p53缺陷增强了糖酵解和谷氨酰胺代谢, 而p16缺失则通过解除细胞周期检查点促进合成代谢过程. 这些因素的整合形成了一个"致癌三角", 加速了肿瘤进展. 当前研究强调了靶向代谢的治疗策略, 包括针对p53缺陷肿瘤的谷氨酰胺酶抑制剂(如CB-839)和针对p16缺失肿瘤的CDK4/6抑制剂(如帕博西尼), 并与根除H. pylori相结合. 然而, 由于肿瘤异质性和代谢可塑性, 临床转化仍面临挑战. 未来方向包括建立前瞻性队列、开发靶向HP-p53-p16轴的联合疗法, 以及整合人工智能模型以实现个体化治疗. 本综述为理解GC发病机制提供了范式转变, 并为通过跨学科合作与创新克服治疗障碍提供了见解.
核心提要: 本文首次提出"幽门螺杆菌(Helicobacter pylori, H. pylori)感染、p53缺陷与p16缺失"构成"致癌三角", 系统阐述三者通过协同驱动代谢重编程(如Warburg效应与谷氨酰胺成瘾)促进胃癌(gastric cancer, GC)发生. 该机制整合微生物、遗传与代谢维度, 为靶向干预(如H. pylori根除联合代谢抑制剂)提供新策略, 推动GC治疗向多靶点精准模式转变.
引文著录: 戴健, 周密, 吕键宇, 夏雯慧, 李小兵. 幽门螺杆菌感染、p53缺陷与p16缺失在胃癌代谢重编程中的致癌三角: 机制与治疗策略. 世界华人消化杂志 2026; 34(1): 28-36
Revised: January 14, 2026
Accepted: January 21, 2026
Published online: January 28, 2026
Gastric cancer (GC) remains a major global health challenge, with Helicobacter pylori (H. pylori) infection serving as a primary etiological factor. This review provides an in-depth analysis of the synergistic mechanisms by which H. pylori infection, TP53 (p53) deficiency, and CDKN2A (p16) deletion drive gastric carcinogenesis, with a focus on metabolic reprogramming. H. pylori infection induces chronic inflammation and metabolic alterations (such as the Warburg effect and glutamine addiction), which interact with p53 and p16 deficiencies to collectively foster a pro-tumorigenic metabolic microenvironment. p53 deficiency enhances glycolysis and glutamine metabolism, while p16 loss promotes anabolic processes by dysregulating cell cycle checkpoints. The integration of these factors forms an "oncogenic triangle" that accelerates tumor progression. Current research highlights therapeutic strategies targeting metabolism, including glutaminase inhibitors (e.g., CB-839) for p53-deficient tumors and CDK4/6 inhibitors (e.g., palbociclib) for p16-deficient tumors, in combination with H. pylori eradication. However, clinical translation remains challenging due to tumor heterogeneity and metabolic plasticity. Future directions include establishing prospective cohorts, developing combination therapies targeting the H. pylori-p53-p16 axis, and integrating artificial intelligence models to enable personalized treatment. This review offers a paradigm shift in understanding GC pathogenesis and provides insights into overcoming therapeutic barriers through interdisciplinary collaboration and innovation.
- Citation: Dai J, Zhou M, Lv JY, Xia WH, Li XB. Oncogenic triangle of Helicobacter pylori infection, p53 deficiency, and p16 loss in gastric cancer metabolic reprogramming: Mechanisms and therapeutic strategies. Shijie Huaren Xiaohua Zazhi 2026; 34(1): 28-36
- URL: https://www.wjgnet.com/1009-3079/full/v34/i1/28.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v34.i1.28
胃癌(gastric cancer, GC)是全球公共卫生领域的重大挑战, 2022年全球新发病例约110万例, 死亡76.8万例, 居癌症相关死亡第四位[1]. 幽门螺杆菌(Helicobacter pylori, H. pylori)作为GCⅠ类致癌因子, 全球定植率超50%, 前瞻性研究证实其感染者GC发病相对危险度达5-6倍[2,3]. 但仅1%-3%感染者最终进展为GC, 提示单纯H. pylori感染不足以驱动恶性转化, 宿主遗传及表观遗传易感性(如抑癌基因失活)是关键协同因素[4,5].
在GC相关抑癌基因中, TP53(p53)与CDKN2A(p16)的失活最为突出. 多组学研究显示[3,4], 二者的突变、缺失或启动子甲基化频率沿"慢性胃炎-萎缩-肠化生-癌变"序列逐级升高. H. pylori诱导的氧化应激可选择性扩增p53功能缺陷的上皮克隆, 而p53与p16双缺失在动物及类器官模型中显著加速肿瘤形成[6]. 因此, H. pylori引发的慢性炎症与DNA损伤可视为致癌"第一击", p53/p16功能丧失则构成决定性"第二击", 共同解释了H. pylori感染相关GC的低发生率特征.
代谢重编程是GC核心生物学特征, 主要表现为有氧糖酵解(瓦博格效应)和谷氨酰胺依赖性增强. 前者可快速提供生物合成所需碳源与还原当量[7]. 后者通过补充三羧酸循环中间体、合成生物大分子及维持氧化还原稳态, 支撑肿瘤细胞快速增殖[8]. 尽管H. pylori毒力蛋白已被证实可重塑宿主能量与氨基酸代谢, 但其诱导的代谢改变与GC整体代谢重编程的因果机制仍待阐明[9].
当前研究存在显著空白: 现有综述多孤立分析H. pylori的致癌机制或p53/p16的细胞周期调控功能, 却忽视了三者在代谢重编程中的协同效应. 针对单一靶点的治疗探索较多, 但靶向"H. pylori-p53-p16三重调控"的联合策略缺乏系统性梳理. 本综述旨在: (1)解析H. pylori感染p53缺陷与p16缺失协同驱动GC代谢重编程的分子机制; 和(2)基于代谢脆弱性提出靶向该"致癌三角"的精准治疗策略, 为突破GC治疗瓶颈提供新方向.
H. pylori感染, 尤其CagA阳性菌株, 通过毒力因子直接干扰宿主代谢稳态H. pylori的CagA毒力因子可激活PI3K/Akt/mTOR信号通路, 上调葡萄糖转运蛋白GLUT1表达, 增强宿主细胞葡萄糖摄取与有氧糖酵解, 为细菌定植和炎症反应提供能量支持[10,11]. 蒙古沙鼠模型证实, H. pylori感染组血清葡萄糖和糖化血红蛋白水平显著升高, 提示可能引发全身性糖代谢紊乱, 而长期感染可诱导胃黏膜上皮细胞适应性增强糖酵解通量, 为癌变奠定代谢基础[12,13].
H. pylori毒素VacA通过靶向线粒体内膜, 直接诱导线粒体膜电位崩溃, 抑制氧化磷酸化(oxidative phosphorylation, OXPHOS)并减少腺苷三磷酸(adenosine triphosphate, ATP)生成, 这一效应与VacA对ATP合成酶(ATP5A/ATP5B)的特异性抑制相关[14-16]. 同时, VacA激活线粒体分裂蛋白Drp1, 诱导线粒体碎片化, 加剧代谢功能障碍, 且在慢性感染中导致线粒体DNA含量下降及突变频率升高[16-18]. 人胃窦类器官模型显示, VacA介导的线粒体损伤主要累及黏液生成细胞, 导致黏膜屏障通透性增加, 而Aurora激酶A抑制剂可通过恢复线粒体融合与自噬功能, 部分逆转OXPHOS抑制[17].
H. pylori还通过双向调控宿主氨基酸代谢适配感染需求: CagA经PI3K/AKT通路激活mTORC1信号, 促进促炎因子(肿瘤坏死因子、白细胞介素-1β等)和抗菌肽LL37分泌, 增强炎症反应[19,20]; 而VacA通过靶向线粒体功能诱导氨基酸稳态失衡, 抑制mTORC1活性并激活自噬, 可能通过回收宿主成分支持细菌长期存活[21]. 此外, VacA导致的亮氨酸缺乏可激活GCN2通路, 提示H. pylori可能通过调控宿主氨基酸代谢获取氮源[21].
H. pylori感染通过代谢重编程与表观遗传调控的交互作用, 驱动胃黏膜细胞恶性转化. H. pylori的γ-谷氨酰转肽酶(gamma-glutamyl transferase, GGT)消耗谷氨酰胺, 降低细胞内α-酮戊二酸(alpha-ketoglutarate, α-KG)水平, 导致组蛋白H3K9me3、H3K27me3修饰增加, 进而激活PI3K/AKT通路, 促进骨髓来源间充质干细胞增殖与迁移[22]. 同时, H. pylori感染抑制S-腺苷甲硫氨酸合成, 降低DNA甲基转移酶活性, 引发全基因组甲基化异常, 包括p53等抑癌基因的沉默[2].
H. pylori感染可直接诱导p16/CDKN2A启动子区CpG岛高甲基化, 抑制其转录表达, 且该异常甲基化在癌前病变阶段即可检测, 与GC发生风险、侵袭及转移显著相关[23]. 此外, H. pylori通过调控组蛋白去甲基化酶(如KDM家族)活性, 降低H3K4me3水平, 抑制分化相关基因表达并激活促增殖通路[24]. H. pylori诱导的活性氧(reactive oxygen species, ROS)还可氧化DNA甲基化标记或直接损伤DNA, 导致基因组广泛低甲基化与局部高甲基化共存, 增加基因组不稳定性[5]. 值得注意的是, 补充α-KG可恢复组蛋白甲基化平衡, 抑制PI3K/AKT信号, 逆转间充质干细胞的恶性表型, 提示靶向代谢-表观遗传轴具有干预潜力[5,9].
p53作为关键肿瘤抑制因子, 通过多靶点、多通路精准调控细胞代谢稳态, 是维持代谢平衡的核心分子. 在糖代谢调控中, p53通过下游靶基因TIGAR(TP53诱导的糖酵解和凋亡调节因子)抑制糖酵解通量: TIGAR水解果糖-2,6-二磷酸, 降低磷酸果糖激酶-1活性, 减少糖酵解的同时, 促进葡萄糖向磷酸戊糖途径转向, 增加NADPH生成以支撑抗氧化防御和DNA修复[25,26]. 此外, TIGAR还可通过非酶功能与线粒体膜上的己糖激酶2、ATP合酶亚基相互作用, 增强己糖激酶2活性并维持线粒体膜电位, 为氧化磷酸化提供支持[27].
在线粒体稳态维持中, p53通过直接调控细胞色素C氧化酶组装蛋白SCO2的表达, 保障电子传递链复合体Ⅳ的功能. 肝癌细胞研究显示[28], p53与表观调控因子TCF19形成复合物, 动态调节SCO2转录: 短期高葡萄糖条件下激活SCO2促进氧化磷酸化, 长期高葡萄糖应激下则抑制SCO2, 导致线粒体膜电位下降. 果蝇心脏特异性SCO2同源基因敲除模型证实, 复合体Ⅳ活性降低会引发p53依赖性凋亡, 并伴随糖酵解代偿性增强[29].
p53还通过抑制谷氨酰胺代谢限制肿瘤细胞代谢可塑性. 结肠癌HCT116细胞实验表明, 低剂量紫杉醇处理可上调p53表达, 同步下调谷氨酰胺转运体SLC1A5的mRNA和蛋白水平, 减少谷氨酰胺向三羧酸循环的输入[30]. 天然化合物Lobetyolin也可通过激活p53号通路, 剂量依赖性抑制SLC1A5转录翻译, 降低细胞内谷氨酰胺、谷氨酸及α-酮戊二酸水平, 通过能量危机和氧化应激抑制肿瘤增殖, 而p53特异性抑制剂可部分逆转这一效应, 证实p53的核心调控作用[30,31].
p53功能缺陷会解除对多项代谢通路的负向调控, 显著重塑肿瘤细胞代谢表型, 为恶性增殖提供支持. 在糖代谢方面, p53失活导致TIGAR表达下调, 解除对磷酸果糖激酶-1的抑制, 糖酵解通量大幅增加, 同时丙酮酸脱氢酶活性受抑, 大部分丙酮酸经乳酸脱氢酶A转化为乳酸, 形成典型瓦博格效应[32]. 过量乳酸通过单羧酸转运体外排至肿瘤微环境, 持续酸化环境并促进基质降解、血管生成及免疫逃逸, 增强肿瘤侵袭能力[33,34].
谷氨酰胺代谢层面, p53缺陷通过miR145-5p/c-Myc通路解除对谷氨酰胺酶1的抑制, 显著上调其表达, 加速谷氨酰胺分解为谷氨酸[35]. 谷氨酸经转氨酶转化为α-酮戊二酸, 回补三羧酸循环中间体, 为核苷酸、脂质等生物大分子合成提供原料, 满足肿瘤细胞快速增殖需求[36]. 此外, p53缺陷还会抑制SLC7A11(胱氨酸/谷氨酸逆向转运体亚基)表达, 削弱谷胱甘肽合成能力, 使细胞对铁死亡的敏感性显著增加[37].
p53缺陷还会导致细胞抗氧化防御系统崩溃. 在葡萄糖剥夺等代谢应激下, p53缺陷细胞无法通过上调谷氨酰胺酶2和谷氨酸脱氢酶有效利用谷氨酰胺代谢生成NADPH[38]. 尽管戊糖磷酸途径活性会暂时增强以维持NADPH供应, 但长期葡萄糖剥夺最终会耗尽NADPH储备, 丧失抵抗氧化应激的关键还原力[38]. 同时, p53缺陷解除对NADPH氧化酶的负调控, 导致超氧阴离子持续爆发, 放大氧化损伤, 这也使得p53缺陷细胞对三氧化二砷等氧化应激诱导剂及放疗高度敏感[38,39].
p16(CDKN2A)作为细胞周期关键调控因子, 其缺失通过多重信号网络参与代谢重编程, 是连接细胞周期与代谢稳态的核心分子. p16通过调控RB-E2F通路发挥代谢调控作用, 其缺失或功能失活会解除RB1对E2F1的抑制, 导致通路异常激活, 这一机制在胃肠道间质瘤中已得到证实-p16低表达与E2F1、CDK4高表达显著相关, 直接驱动细胞周期进展并增强肿瘤侵袭性[40,41].
p16对AMPK/mTORC1信号平衡的调控同样关键: p16缺失导致CDK4/6持续激活, 抑制AMPK通路并促进mTORC1介导的合成代谢[42]. 在衰老细胞中, 这种持续的mTORC1激活(通过磷酸化p70S6K和S6蛋白)会驱动核糖体生物合成与细胞肥大, 促进不可逆衰老表型[43]. 单细胞及空间转录组分析进一步揭示, p16高表达区域常伴随Wnt信号激活和上皮-间质转化特征, 形成代谢重编程与恶性表型的协同效应[40].
p16缺失通过代谢-细胞周期耦合及微环境重塑, 形成特征性代谢表型. 在代谢-周期协同方面, p16缺失导致CDK4/6持续活化, 解除G1/S期阻滞, 同时通过MTOR和MEK/ERK信号维持高代谢活性. 这一改变在胰腺导管腺癌中尤为显著: p16缺失与致癌性Kras突变协同上调NOX4表达, 通过氧化NADH生成NAD+维持糖酵解通量(GAPDH活性), 增强葡萄糖摄取和乳酸生成, 为S期核苷酸合成提供核糖-5-磷酸前体[44]. 在胃肠道间质瘤中, p16缺失通过RB-E2F1通路激活DNA复制相关基因(如核苷酸合成酶), 其高表达与肿瘤高增殖指数及不良预后直接相关[41].
在肿瘤微环境代谢互作方面, p16低表达肿瘤会招募大量SPP1+巨噬细胞, 这些巨噬细胞通过MMP7介导的基质重塑, 创造有利于肿瘤生长的代谢微环境[40]. 同时, 基于Treg细胞功能对脂肪酸氧化的依赖性, 以及p16对AMPK信号的调控作用, 推测p16缺失可能通过AMPK-FAO轴增强Treg细胞免疫抑制功能, 参与肿瘤免疫逃逸, 这种代谢-免疫交互作用, 为理解p16缺失肿瘤的进展机制提供了新视角[45,46].
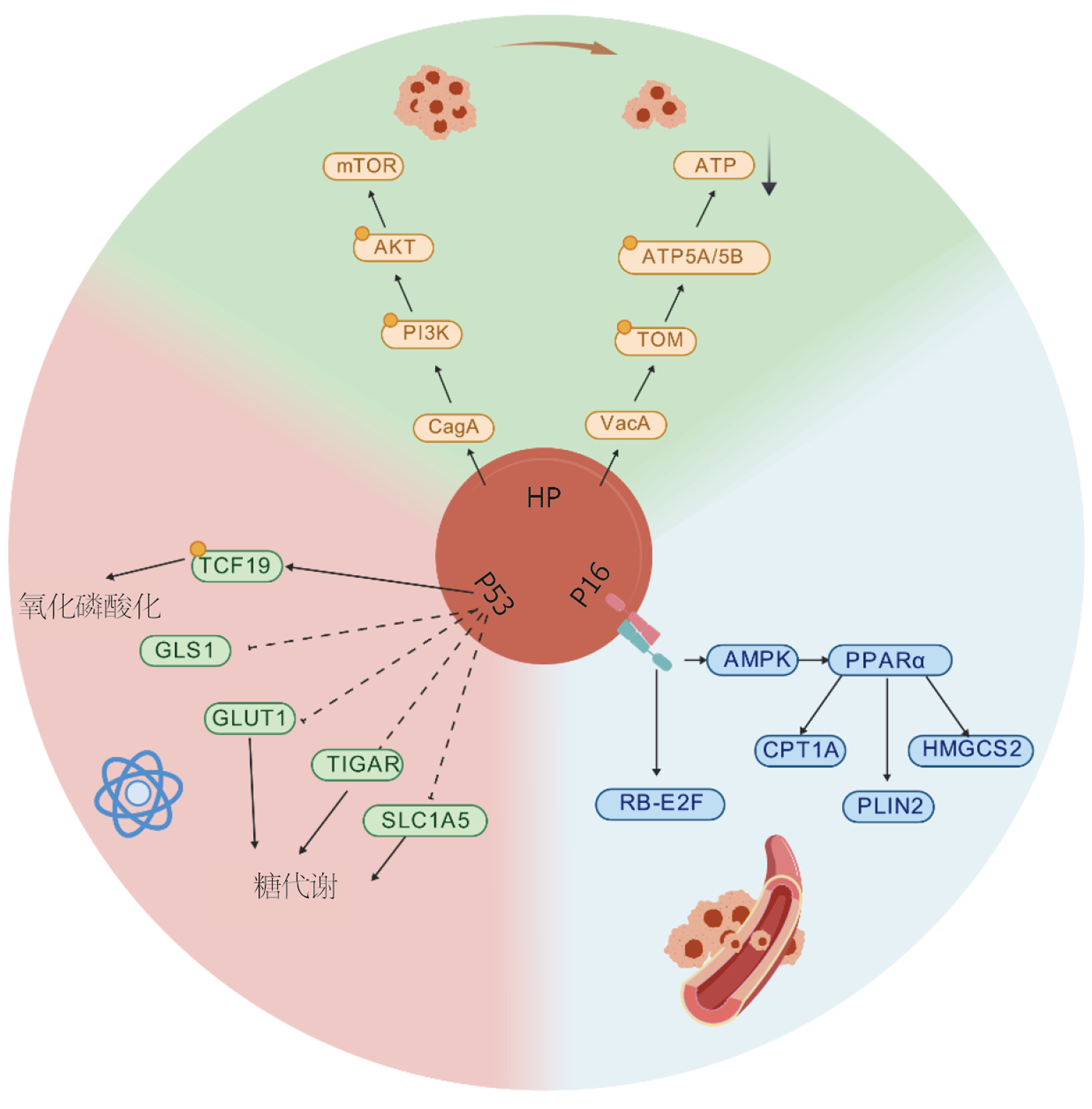
图1中核心展示了H. pylori、p53和p16在GC代谢重编程中的核心作用. H. pylori通过其毒力因子(如CagA和VacA)直接干扰宿主代谢, 激活PI3K/AKT/mTOR信号通路, 并促进糖酵解和谷氨酰胺代谢. p53缺陷增强糖酵解(通过GLUT1和TIGAR)和谷氨酰胺代谢(通过GLS1), 而p16缺失则通过解除CDK4/6抑制, 激活AMPK/PPARα信号通路, 促进脂肪酸代谢和细胞周期进程(通过RB-E2F通路). 这些相互作用形成了一个"致癌三角", 通过代谢重编程(如糖酵解、谷氨酰胺代谢和脂肪酸代谢)以及调控OXPHOS, 促进GC细胞的增殖和侵袭. 此外, H. pylori感染和p53/p16缺陷还通过表观遗传机制(如DNA甲基化)进一步破坏代谢稳态, 驱动GC的发展[10,14,45-47].
H. pylori感染与p53缺陷通过代谢通路交叉、氧化应激叠加及表观遗传调控, 形成强效协同致癌效应. 在代谢重编程层面, H. pylori通过VacA毒素靶向线粒体, 抑制氧化磷酸化并迫使细胞依赖糖酵解供能[14]; 而p53缺陷进一步解除对糖酵解关键酶(如PFK-1)的抑制, 同时增强谷氨酰胺代谢, 形成"糖酵解-谷氨酰胺成瘾"正反馈环路, 固化肿瘤代谢表型[10,25]. H. pylori的CagA毒力因子还可激活PI3K/Akt/mTOR通路, 协同上调GLUT1、SLC1A5等转运蛋白, 放大代谢重编程效应[30]. 值得注意的是, H. pylori的CagA蛋白可与凋亡刺激蛋白ASPP2结合, 阻碍其与p53的相互作用, 加剧p53降解并抑制凋亡应答, 这一过程与p16启动子甲基化的诱导密切相关[48].
在基因组稳定性方面, 二者的协同作用尤为显著: H. pylori通过VacA、GGT等毒力因子诱导线粒体功能障碍, 引发ROS爆发; p53缺陷则通过下调TIGAR和GLS2表达, 减少NADPH生成并削弱谷胱甘肽依赖性抗氧化防御, 同时解除对NADPH氧化酶的负调控, 进一步放大ROS累积[9,39,49]. H. pylori感染还可通过激活AKT信号磷酸化MDM2, 加速p53泛素化降解, 而p14ARF对p53的保护作用会被H. pylori的CagA蛋白显著削弱, 形成"H. pylori→p14ARF失活→p53降解→ROS清除障碍"的级联反应[48]. 持续的氧化应激通过氧化DNA碱基、诱导双链断裂直接损伤基因组, 临床前模型证实, H. pylori感染合并p53缺陷的胃黏膜中双链断裂标志物γH2AX水平显著升高[2,50].
H. pylori感染还通过表观遗传机制加剧p53功能丧失: H. pylori感染导致USF1蛋白核质转运异常, 使其无法结合p53并阻断HDM2介导的泛素化降解, 最终引发p53蛋白降解[2]. TCGA数据分析显示, H. pylori阳性GC患者中USF1与TP53低表达高度相关, 且与不良预后相关, 形成"代谢异常-表观遗传紊乱"恶性循环.
H. pylori感染与p16缺失通过表观遗传修饰与细胞周期调控的交互, 推动GC进展. H. pylori引发的慢性炎症可诱导p16/CDKN2A启动子区CpG岛高甲基化, 沉默其转录表达, 该异常甲基化在癌前病变阶段即可检测, 且与GC发生风险、局部侵袭及淋巴结转移显著相关[51,52]. 临床样本研究证实[53], H. pylori阳性的慢性萎缩性胃炎、肠化生及异型增生组织中, p16阳性表达率显著低于H. pylori阴性组织, 而Bcl-2、COX-2表达显著升高, 提示H. pylori感染通过调控p16及相关通路启动早期癌变. Meta分析证实, p16甲基化在低分化GC和EBV阳性GC中更为常见, 提示不同致癌因素可能协同作用于p16的表观遗传沉默[52].
p16缺失通过RB-E2F-DREAM复合体形成正反馈环路: p16缺失解除对CDK4/6的抑制, 促进RB蛋白磷酸化并释放E2F转录因子, 激活BRCA1、RAD51等DNA修复基因, 修复mTORC1活性增强引发的转录相关DNA损伤, 同时支持增殖所需的代谢适应性[54]. DREAM复合体还可下调组蛋白H1表达并促进异染色质形成, 进一步强化p16表观遗传沉默, 加剧细胞周期失调[55]. H. pylori感染可通过激活NF-κB通路上调COX-2表达, 进一步强化p16缺失介导的合成代谢激活, 形成"H. pylori→NF-κB/COX-2→p16甲基化→RB-E2激活"的协同调控[53]. 此外, H. pylori感染诱导的ROS可通过氧化修饰DNA甲基化标记, 加剧p16启动子高甲基化, 而p53缺陷会进一步增强这一效应, 因p53可通过调控DNMTs活性间接抑制p16甲基化[48].
p53缺陷与p16缺失在GC发展中呈现明确的时空互补性, 共同驱动代谢重编程的动态演变. 肿瘤发生早期, p53功能丧失主导代谢适应启动: 其引发的瓦博格效应和谷氨酰胺成瘾, 为细胞增殖提供快速能量与碳源基础[32,33]; 同时, p53缺失的胃干细胞在致癌物暴露下耐受DNA损伤, 获得选择性生长优势, 促进不典型增生形成[35,36,56]. 体外实验证实, H. pylori感染可同时上调GES-1细胞中p53与p16的表达, 且二者表达水平与细胞迁移、侵袭能力呈正相关, 提示H. pylori感染早期即启动p53-p16通路的协同激活[3].
随着肿瘤进展, p16缺失的作用逐渐凸显: p16低表达导致CDK4/6持续激活, 解除G1/S期阻滞, 同时激活NOX4-NAD+通路, 维持糖酵解通量以保障S期核苷酸合成所需的核糖-5-磷酸前, E2F1转录因子还可直接驱动胸苷激酶、核糖核苷酸还原酶等基因表达, 满足DNA复制的高需求[41,44]. 动物实验证实, p53与p16双缺陷小鼠的肿瘤发生率显著高于单一缺陷小鼠, 且更易发展为侵袭性血管肉瘤, H. pylori感染可进一步缩短肿瘤潜伏期[57]. 临床数据显示, p16纯合性缺失在肠型进展期GC中更为频繁(15.6% vs 弥漫型3.6%), 且与肿瘤高增殖指数相关, 体现其在晚期合成代谢中的关键作用[58].
值得注意的是, p53缺陷可通过Ets1蛋白稳定化上调p16表达, 形成代偿机制, 而H. pylori感染可通过抑制p14ARF活性阻断这一补偿, 导致p53与p16同时失活[48,59]. 二者的协同作用贯穿GC全程, 形成"代谢适应-增殖加速"正反馈环路: p53缺陷主导的早期代谢重编程为恶性转化奠定基础, p16缺失驱动的核苷酸合成增强则支持晚期肿瘤快速增殖与侵袭, 为针对不同疾病阶段的联合治疗提供了理论依据[41,44].
GC治疗正逐步迈向基于代谢特征和分子分型的精准医疗, 核心思路是靶向"H. pylori-p53-p16"致癌三角介导的代谢脆弱性, 结合多模态评估与联合干预, 提升治疗有效性.
联合检测H. pylori感染状态、p53突变和p16甲基化状态可有效预测肿瘤代谢亚型, 为个体化治疗提供依据. 研究表明[60,61], CagA阳性H. pylori菌株通过激活NF-κB和HIF-1α通路促进糖酵解并增加ROS水平, 而p53突变导致TIGAR下调和GLS上调, 分别增强糖酵解和谷氨酰胺分解. p16甲基化则通过CDK4/6-RB-mTOR信号轴加剧代谢失衡[62]. 临床检测可采用¹³C-尿素呼气试验(H. pylori)、免疫组化/基因测序(p53)和甲基化特异性PCR(p16), 结合18F-FDG PET和18F-Gln PET显像区分代谢表型[6,63]. 这种多模态评估方法有望实现更精准的疗效预测.
靶向代谢通路的合成致死策略展现出良好的临床应用前景. 对于H. pylori感染合并p53缺陷的患者, 谷氨酰胺酶抑制剂CB-839可有效抑制肿瘤生长[64]. 其作用机制基于p53缺陷导致的谷氨酰胺依赖性增强: p53野生型细胞可通过p21抑制GLS1维持代谢平衡, 而p53缺陷细胞则因GLS1上调形成合成致死窗口[65]. 对于p16缺失患者, CDK4/6抑制剂Palbociclib通过阻断RB-E2F1通路抑制糖酵解和核苷酸合成[66]. 值得注意的是, GC细胞可能通过代谢适应产生耐药性, 如激活AMPK信号或增强自噬[67]. 因此, 联合用药策略值得探索, 如Palbociclib与mTOR抑制剂联用可克服耐药[62].
三者协同效应的时空动态调控机制尚未完全阐明: 在GC不同发展阶段(早期癌变、局部侵袭、远处转移), H. pylori感染、p53/p16缺陷对代谢重编程的调控权重存在显著差异, 其动态交互模式仍需系统解析[9]. 代谢产物与信号通路的反馈调控网络也有待深入挖掘, 例如乳酸是否通过稳定HIF-1α间接抑制p53活性, α-酮戊二酸如何通过调控组蛋白及DNA甲基化影响p16表达, 这些关键科学问题尚未形成明确结论. 现有实验模型的局限性同样突出, 多数研究聚焦单一因素干预[40,73], 缺乏同时整合H. pylori感染与p53/p16基因编辑的类器官或动物模型, 难以真实模拟人体内"感染-基因-代谢"的复杂交互作用.
临床转化相关技术仍存在明显短板: 人胃类器官培养体系难以长期维持H. pylori感染状态, 体外培养条件下细菌毒力因子(如CagA、VacA)的表达水平与临床分离株存在差异, 导致实验结果的临床关联性不足[60]. 代谢成像技术的空间分辨率有限, 无法在单细胞水平精准捕捉H. pylori感染引发的局部代谢异质性, 难以实现代谢表型的精准量化[74]. 此外, 临床样本的多组学数据(基因组、代谢组、表观组)整合分析方法尚不成熟, 限制了从海量数据中筛选关键生物标志物及治疗靶点的效率[6], 亟需开发标准化的数据分析流程[6,9,73].
未来研究应重点关注以下方向: 建立更贴近临床的前瞻性队列, 系统分析H. pylori感染患者的p53/p16突变谱与代谢特征关联[9]; 开发靶向"致癌三角"的联合治疗策略, 如H. pylori根除联合代谢调节剂(谷氨酰胺酶抑制剂)和表观遗传药物(去甲基化剂)[63]; 探索基于人工智能的疗效预测模型, 整合代谢影像(如18F-FDG/18F-Gln PET)、基因检测和病理特征优化治疗分层[9]. 这些突破将推动GC治疗从单一靶向向多维度精准干预的转变.
GC的发生发展是H. pylori感染、p53/p16缺陷与代谢重编程协同作用的结果, 三者通过代谢交互形成"致癌三角", 为GC发病机制提供了新范式. H. pylori作为始动因素, 不仅直接诱导慢性炎症与代谢紊乱(瓦博格效应、谷氨酰胺成瘾), 还通过表观遗传修饰和蛋白调控加剧p53/p16功能丧失. p53缺陷主导早期代谢适应, 增强糖酵解与谷氨酰胺代谢; p16缺失则强化合成代谢, 支撑肿瘤晚期增殖与侵袭, 二者时空互补推动癌前病变向侵袭性癌演进.
基于代谢脆弱性的精准治疗策略已展现应用前景, 包括针对p53缺陷的谷氨酰胺酶抑制剂 CB-839、靶向p16缺失的CDK4/6抑制剂帕博西尼, 联合H. pylori根除治疗构成多靶点干预核心. 但肿瘤异质性与代谢可塑性导致的耐药问题, 仍是临床转化的主要瓶颈.
未来需通过跨学科合作, 构建整合H. pylori感染与p53/p16基因编辑的临床前模型, 优化代谢影像指导的联合疗法临床试验设计, 并整合多组学与人工智能技术开发个体化方案. 对"H. pylori-p53-p16"致癌三角的深入解析, 不仅为GC研究提供新视角, 也为其他感染相关癌症的联合治疗提供重要参考.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [PubMed] [DOI] |
| 2. | Costa L, Corre S, Michel V, Le Luel K, Fernandes J, Ziveri J, Jouvion G, Danckaert A, Mouchet N, Da Silva Barreira D, Torres J, Camorlinga M, D'Elios MM, Fiette L, De Reuse H, Galibert MD, Touati E. USF1 defect drives p53 degradation during Helicobacter pylori infection and accelerates gastric carcinogenesis. Gut. 2020;69:1582-1591. [PubMed] [DOI] |
| 3. | Wang J, Yao Y, Zhang Q, Li S, Tang L. Inflammatory responses induced by Helicobacter pylori on the carcinogenesis of gastric epithelial GES1 cells. Int J Oncol. 2019;54:2200-2210. [PubMed] [DOI] |
| 4. | Salvatori S, Marafini I, Laudisi F, Monteleone G, Stolfi C. Helicobacter pylori and Gastric Cancer: Pathogenetic Mechanisms. Int J Mol Sci. 2023;24:2895. [PubMed] [DOI] |
| 5. | Deng M, Zhang L, Zheng W, Chen J, Du N, Li M, Chen W, Huang Y, Zeng N, Song Y, Chen Y. Helicobacter pylori-induced NAT10 stabilizes MDM2 mRNA via RNA acetylation to facilitate gastric cancer progression. J Exp Clin Cancer Res. 2023;42:9. [PubMed] [DOI] |
| 6. | Duan Y, Xu Y, Dou Y, Xu D. Helicobacter pylori and gastric cancer: mechanisms and new perspectives. J Hematol Oncol. 2025;18:10. [PubMed] [DOI] |
| 7. | Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211-218. [PubMed] [DOI] |
| 8. | Fang L, Huang H, Lv J, Chen Z, Lu C, Jiang T, Xu P, Li Y, Wang S, Li B, Li Z, Wang W, Xu Z. m5C-methylated lncRNA NR_033928 promotes gastric cancer proliferation by stabilizing GLS mRNA to promote glutamine metabolism reprogramming. Cell Death Dis. 2023;14:520. [PubMed] [DOI] |
| 9. | Jiang X, Wang W, Wang Z, Wang Z, Shi H, Meng L, Pang S, Fan M, Lin R. Gamma-glutamyl transferase secreted by Helicobacter pylori promotes the development of gastric cancer by affecting the energy metabolism and histone methylation status of gastric epithelial cells. Cell Commun Signal. 2024;22:402. [PubMed] [DOI] |
| 10. | Liu Y, Zhang Z, Wang J, Chen C, Tang X, Zhu J, Liu J. Metabolic reprogramming results in abnormal glycolysis in gastric cancer: a review. Onco Targets Ther. 2019;12:1195-1204. [PubMed] [DOI] |
| 11. | Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53:667-682. [PubMed] [DOI] |
| 12. | Yang Z, Li W, He C, Xie C, Zhu Y, Lu NH. Potential effect of chronic Helicobacter pylori infection on glucose metabolism of Mongolian gerbils. World J Gastroenterol. 2015;21:12593-12604. [PubMed] [DOI] |
| 13. | Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196-202. [PubMed] [DOI] |
| 14. | Domańska G, Motz C, Meinecke M, Harsman A, Papatheodorou P, Reljic B, Dian-Lothrop EA, Galmiche A, Kepp O, Becker L, Günnewig K, Wagner R, Rassow J. Helicobacter pylori VacA toxin/subunit p34: targeting of an anion channel to the inner mitochondrial membrane. PLoS Pathog. 2010;6:e1000878. [PubMed] [DOI] |
| 15. | Galmiche A, Rassow J, Doye A, Cagnol S, Chambard JC, Contamin S, de Thillot V, Just I, Ricci V, Solcia E, Van Obberghen E, Boquet P. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361-6370. [PubMed] |
| 16. | Jain P, Luo ZQ, Blanke SR. Helicobacter pylori vacuolating cytotoxin A (VacA) engages the mitochondrial fission machinery to induce host cell death. Proc Natl Acad Sci U S A. 2011;108:16032-16037. [PubMed] [DOI] |
| 17. | Son YS, Kwon YH, Lee MS, Kwon O, Jeong YJ, Mun SJ, Jeon S, Park JH, Han MH, Bae JS, Hur K, Jang AR, Park JH, Cho HS, Jung CR, Ryu CM, Son MJ, Park DS, Son MY. Helicobacter pylori VacA-induced mitochondrial damage in the gastric pit cells of the antrum and therapeutic rescue. Biomaterials. 2025;314:122842. [PubMed] [DOI] |
| 18. | Chatre L, Fernandes J, Michel V, Fiette L, Avé P, Arena G, Jain U, Haas R, Wang TC, Ricchetti M, Touati E. Helicobacter pylori targets mitochondrial import and components of mitochondrial DNA replication machinery through an alternative VacA-dependent and a VacA-independent mechanisms. Sci Rep. 2017;7:15901. [PubMed] [DOI] |
| 19. | Feng GJ, Chen Y, Li K. Helicobacter pylori promote inflammation and host defense through the cagA-dependent activation of mTORC1. J Cell Physiol. 2020;235:10094-10108. [PubMed] [DOI] |
| 20. | Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015;25:545-555. [PubMed] [DOI] |
| 21. | Kim IJ, Lee J, Oh SJ, Yoon MS, Jang SS, Holland RL, Reno ML, Hamad MN, Maeda T, Chung HJ, Chen J, Blanke SR. Helicobacter pylori Infection Modulates Host Cell Metabolism through VacA-Dependent Inhibition of mTORC1. Cell Host Microbe. 2018;23:583-593.e8. [PubMed] [DOI] |
| 22. | Wang Z, Wang W, Shi H, Meng L, Jiang X, Pang S, Fan M, Lin R. Gamma-glutamyltransferase of Helicobacter pylori alters the proliferation, migration, and pluripotency of mesenchymal stem cells by affecting metabolism and methylation status. J Microbiol. 2022;60:627-639. [PubMed] [DOI] |
| 23. | Dong CX, Deng DJ, Pan KF, Zhang L, Zhang Y, Zhou J, You WC. Promoter methylation of p16 associated with Helicobacter pylori infection in precancerous gastric lesions: a population-based study. Int J Cancer. 2009;124:434-439. [PubMed] [DOI] |
| 24. | Shin CM, Kim N, Jung Y, Park JH, Kang GH, Kim JS, Jung HC, Song IS. Role of Helicobacter pylori infection in aberrant DNA methylation along multistep gastric carcinogenesis. Cancer Sci. 2010;101:1337-1346. [PubMed] [DOI] |
| 25. | Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107-120. [PubMed] |
| 26. | Li M, Sun M, Cao L, Gu JH, Ge J, Chen J, Han R, Qin YY, Zhou ZP, Ding Y, Qin ZH. A TIGAR-regulated metabolic pathway is critical for protection of brain ischemia. J Neurosci. 2014;34:7458-7471. [PubMed] [DOI] |
| 27. | Geng J, Wei M, Yuan X, Liu Z, Wang X, Zhang D, Luo L, Wu J, Guo W, Qin ZH. TIGAR regulates mitochondrial functions through SIRT1-PGC1α pathway and translocation of TIGAR into mitochondria in skeletal muscle. FASEB J. 2019;33:6082-6098. [PubMed] [DOI] |
| 28. | Mondal P, Gadad SS, Adhikari S, Ramos EI, Sen S, Prasad P, Das C. TCF19 and p53 regulate transcription of TIGAR and SCO2 in HCC for mitochondrial energy metabolism and stress adaptation. FASEB J. 2021;35:e21814. [PubMed] [DOI] |
| 29. | Martínez-Morentin L, Martínez L, Piloto S, Yang H, Schon EA, Garesse R, Bodmer R, Ocorr K, Cervera M, Arredondo JJ. Cardiac deficiency of single cytochrome oxidase assembly factor scox induces p53-dependent apoptosis in a Drosophila cardiomyopathy model. Hum Mol Genet. 2015;24:3608-3622. [PubMed] [DOI] |
| 30. | Lv C, Qu H, Zhu W, Xu K, Xu A, Jia B, Qing Y, Li H, Wei HJ, Zhao HY. Low-Dose Paclitaxel Inhibits Tumor Cell Growth by Regulating Glutaminolysis in Colorectal Carcinoma Cells. Front Pharmacol. 2017;8:244. [PubMed] [DOI] |
| 31. | He W, Tao W, Zhang F, Jie Q, He Y, Zhu W, Tan J, Shen W, Li L, Yang Y, Cheng H, Sun D. Lobetyolin induces apoptosis of colon cancer cells by inhibiting glutamine metabolism. J Cell Mol Med. 2020;24:3359-3369. [PubMed] [DOI] |
| 32. | Johnson RF, Perkins ND. Nuclear factor-κB, p53, and mitochondria: regulation of cellular metabolism and the Warburg effect. Trends Biochem Sci. 2012;37:317-324. [PubMed] [DOI] |
| 33. | Liu Y, Gu W. The complexity of p53-mediated metabolic regulation in tumor suppression. Semin Cancer Biol. 2022;85:4-32. [PubMed] [DOI] |
| 34. | Liao M, Yao D, Wu L, Luo C, Wang Z, Zhang J, Liu B. Targeting the Warburg effect: A revisited perspective from molecular mechanisms to traditional and innovative therapeutic strategies in cancer. Acta Pharm Sin B. 2024;14:953-1008. [PubMed] [DOI] |
| 35. | Smith B, Schafer XL, Ambeskovic A, Spencer CM, Land H, Munger J. Addiction to Coupling of the Warburg Effect with Glutamine Catabolism in Cancer Cells. Cell Rep. 2016;17:821-836. [PubMed] [DOI] |
| 36. | Ye C, Liu S, Li N, Zuo S, Niu Y, Lin Q, Liang H, Luo X, Fu X. Mandarin Fish (Siniperca chuatsi) p53 Regulates Glutaminolysis Induced by Virus via the p53/miR145-5p/c-Myc Pathway in Chinese Perch Brain Cells. Microbiol Spectr. 2022;10:e0272721. [PubMed] [DOI] |
| 37. | Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369-379. [PubMed] [DOI] |
| 38. | Chang HW, Lee M, Lee YS, Kim SH, Lee JC, Park JJ, Nam HY, Kim MR, Han MW, Kim SW, Kim SY. p53-dependent glutamine usage determines susceptibility to oxidative stress in radioresistant head and neck cancer cells. Cell Signal. 2021;77:109820. [PubMed] [DOI] |
| 39. | Kim SJ, Wong PKY. p53 as a retrovirus-induced oxidative stress modulator. J Gen Virol. 2015;96:144-149. [PubMed] [DOI] |
| 40. | Cheng X, Yang F, Li Y, Cao Y, Zhang M, Ji J, Bai Y, Li Q, Yu Q, Gao D. The crosstalk role of CDKN2A between tumor progression and cuproptosis resistance in colorectal cancer. Aging (Albany NY). 2024;16:10512-10538. [PubMed] [DOI] |
| 41. | Haller F, Gunawan B, von Heydebreck A, Schwager S, Schulten HJ, Wolf-Salgó J, Langer C, Ramadori G, Sültmann H, Füzesi L. Prognostic role of E2F1 and members of the CDKN2A network in gastrointestinal stromal tumors. Clin Cancer Res. 2005;11:6589-6597. [PubMed] |
| 42. | Martell E, Kuzmychova H, Senthil H, Kaul E, Chokshi CR, Venugopal C, Anderson CM, Singh SK, Sharif T. Compensatory cross-talk between autophagy and glycolysis regulates senescence and stemness in heterogeneous glioblastoma tumor subpopulations. Acta Neuropathol Commun. 2023;11:110. [PubMed] [DOI] |
| 43. | Leontieva OV, Blagosklonny MV. CDK4/6-inhibiting drug substitutes for p21 and p16 in senescence: duration of cell cycle arrest and MTOR activity determine geroconversion. Cell Cycle. 2013;12:3063-3069. [PubMed] [DOI] |
| 44. | Ju HQ, Ying H, Tian T, Ling J, Fu J, Lu Y, Wu M, Yang L, Achreja A, Chen G, Zhuang Z, Wang H, Nagrath D, Yao J, Hung MC, DePinho RA, Huang P, Xu RH, Chiao PJ. Mutant Kras- and p16-regulated NOX4 activation overcomes metabolic checkpoints in development of pancreatic ductal adenocarcinoma. Nat Commun. 2017;8:14437. [PubMed] [DOI] |
| 45. | Deleye Y, Cotte AK, Hannou SA, Hennuyer N, Bernard L, Derudas B, Caron S, Legry V, Vallez E, Dorchies E, Martin N, Lancel S, Annicotte JS, Bantubungi K, Pourtier A, Raverdy V, Pattou F, Lefebvre P, Abbadie C, Staels B, Haas JT, Paumelle R. CDKN2A/p16INK4a suppresses hepatic fatty acid oxidation through the AMPKα2-SIRT1-PPARα signaling pathway. J Biol Chem. 2020;295:17310-17322. [PubMed] [DOI] |
| 46. | Xu L, Huang Q, Wang H, Hao Y, Bai Q, Hu J, Li Y, Wang P, Chen X, He R, Li B, Yang X, Zhao T, Zhang Y, Wang Y, Ou J, Liang H, Wu Y, Zhou X, Ye L. The Kinase mTORC1 Promotes the Generation and Suppressive Function of Follicular Regulatory T Cells. Immunity. 2017;47:538-551.e5. [PubMed] [DOI] |
| 47. | Minami JK, Morrow D, Bayley NA, Fernandez EG, Salinas JJ, Tse C, Zhu H, Su B, Plawat R, Jones A, Sammarco A, Liau LM, Graeber TG, Williams KJ, Cloughesy TF, Dixon SJ, Bensinger SJ, Nathanson DA. CDKN2A deletion remodels lipid metabolism to prime glioblastoma for ferroptosis. Cancer Cell. 2023;41:1048-1060.e9. [PubMed] [DOI] |
| 48. | Li N, Xie C, Lu NH. p53, a potential predictor of Helicobacter pylori infection-associated gastric carcinogenesis? Oncotarget. 2016;7:66276-66286. [PubMed] [DOI] |
| 49. | Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y, Sugano S, Sato E, Nagao T, Yokote K, Tatsuno I, Prives C. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl Acad Sci U S A. 2010;107:7461-7466. [PubMed] [DOI] |
| 50. | Murata-Kamiya N, Hatakeyama M. Helicobacter pylori-induced DNA double-stranded break in the development of gastric cancer. Cancer Sci. 2022;113:1909-1918. [PubMed] [DOI] |
| 51. | Maeda M, Moro H, Ushijima T. Mechanisms for the induction of gastric cancer by Helicobacter pylori infection: aberrant DNA methylation pathway. Gastric Cancer. 2017;20:8-15. [PubMed] [DOI] |
| 52. | Spagnol LW, Polettini J, Silveira DA, Wegner GRM, Paiva DFF. P16 gene promoter methylation is associated with oncogenesis and progression of gastric carcinomas: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2022;180:103843. [PubMed] [DOI] |
| 53. | Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions:H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848-854. [PubMed] |
| 54. | Daigh LH, Saha D, Rosenthal DL, Ferrick KR, Meyer T. Uncoupling of mTORC1 from E2F activity maintains DNA damage and senescence. Nat Commun. 2024;15:9181. [PubMed] [DOI] |
| 55. | Kandhaya-Pillai R, Miro-Mur F, Alijotas-Reig J, Tchkonia T, Schwartz S, Kirkland JL, Oshima J. Key elements of cellular senescence involve transcriptional repression of mitotic and DNA repair genes through the p53-p16/RB-E2F-DREAM complex. Aging (Albany NY). 2023;15:4012-4034. [PubMed] [DOI] |
| 56. | Sethi NS, Kikuchi O, Duronio GN, Stachler MD, McFarland JM, Ferrer-Luna R, Zhang Y, Bao C, Bronson R, Patil D, Sanchez-Vega F, Liu JB, Sicinska E, Lazaro JB, Ligon KL, Beroukhim R, Bass AJ. Early TP53 alterations engage environmental exposures to promote gastric premalignancy in an integrative mouse model. Nat Genet. 2020;52:219-230. [PubMed] [DOI] |
| 57. | Sharpless NE, Alson S, Chan S, Silver DP, Castrillon DH, DePinho RA. p16(INK4a) and p53 deficiency cooperate in tumorigenesis. Cancer Res. 2002;62:2761-2765. [PubMed] |
| 58. | Wu MS, Shun CT, Sheu JC, Wang HP, Wang JT, Lee WJ, Chen CJ, Wang TH, Lin JT. Overexpression of mutant p53 and c-erbB-2 proteins and mutations of the p15 and p16 genes in human gastric carcinoma: with respect to histological subtypes and stages. J Gastroenterol Hepatol. 1998;13:305-310. [PubMed] |
| 59. | Leong WF, Chau JF, Li B. p53 Deficiency leads to compensatory up-regulation of p16INK4a. Mol Cancer Res. 2009;7:354-360. [PubMed] [DOI] |
| 60. | Seidlitz T, Merker SR, Rothe A, Zakrzewski F, von Neubeck C, Grützmann K, Sommer U, Schweitzer C, Schölch S, Uhlemann H, Gaebler AM, Werner K, Krause M, Baretton GB, Welsch T, Koo BK, Aust DE, Klink B, Weitz J, Stange DE. Human gastric cancer modelling using organoids. Gut. 2019;68:207-217. [PubMed] [DOI] |
| 61. | Huang SC, Chang IY, Chen TC, Lin HC, Tsai CY, Hsu JT, Yeh CN, Chang SC, Yeh TS. Redefining aberrant P53 expression of gastric cancer and its distinct clinical significance among molecular-histologic subtypes. Asian J Surg. 2024;47:4699-4705. [PubMed] [DOI] |
| 62. | Kumari S, Kumar P, Kumar M, Singh S, Narayan G. Expression of p27 and p16 and their clinical significance in gastric cancer. Clin Transl Oncol. 2021;23:856-865. [PubMed] [DOI] |
| 63. | Sharafutdinov I, Tegtmeyer N, Linz B, Rohde M, Vieth M, Tay AC, Lamichhane B, Tuan VP, Fauzia KA, Sticht H, Yamaoka Y, Marshall BJ, Backert S. A single-nucleotide polymorphism in Helicobacter pylori promotes gastric cancer development. Cell Host Microbe. 2023;31:1345-1358.e6. [PubMed] [DOI] |
| 64. | Bi H, Shang J, Zou X, Xu J, Han Y. Palbociclib induces cell senescence and apoptosis of gastric cancer cells by inhibiting the Notch pathway. Oncol Lett. 2021;22:603. [PubMed] [DOI] |
| 65. | Scott GK, Yau C, Becker BC, Khateeb S, Mahoney S, Jensen MB, Hann B, Cowen BJ, Pegan SD, Benz CC. Targeting Mitochondrial Proline Dehydrogenase with a Suicide Inhibitor to Exploit Synthetic Lethal Interactions with p53 Upregulation and Glutaminase Inhibition. Mol Cancer Ther. 2019;18:1374-1385. [PubMed] [DOI] |
| 66. | Li P, Zhang X, Gu L, Zhou J, Deng D. P16 methylation increases the sensitivity of cancer cells to the CDK4/6 inhibitor palbociclib. PLoS One. 2019;14:e0223084. [PubMed] [DOI] |
| 67. | Hamada S, Matsumoto R, Tanaka Y, Taguchi K, Yamamoto M, Masamune A. Nrf2 Activation Sensitizes K-Ras Mutant Pancreatic Cancer Cells to Glutaminase Inhibition. Int J Mol Sci. 2021;22:1870. [PubMed] [DOI] |
| 68. | Liu J, Xie YS, Wang FL, Zhang LJ, Zhang Y, Luo HS. Cytotoxicity of 5-Aza-2'-deoxycytidine against gastric cancer involves DNA damage in an ATM-P53 dependent signaling pathway and demethylation of P16(INK4A). Biomed Pharmacother. 2013;67:78-87. [PubMed] [DOI] |
| 69. | Kim N. Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism. J Gastroenterol Hepatol. 2019;34:1287-1295. [PubMed] [DOI] |
| 70. | de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, Neves PHM, de Melo FF. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25:5578-5589. [PubMed] [DOI] |
| 71. | Toh JWT, Wilson RB. Pathways of Gastric Carcinogenesis, Helicobacter pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals. Int J Mol Sci. 2020;21:6451. [PubMed] [DOI] |
| 72. | Chen L, Xu W, Lee A, He J, Huang B, Zheng W, Su T, Lai S, Long Y, Chu H, Chen Y, Wang L, Wang K, Si J, Chen S. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine. 2018;35:87-96. [PubMed] [DOI] |
| 73. | Yu YY, Zhu YJ, Xiao ZZ, Chen YD, Chang XS, Liu YH, Tang Q, Zhang HB. The pivotal application of patient-derived organoid biobanks for personalized treatment of gastrointestinal cancers. Biomark Res. 2022;10:73. [PubMed] [DOI] |
学科分类: 胃肠病学和肝病学
手稿来源地: 浙江省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B
C级 (良好): C
D级 (一般): D
E级 (差): 0
科学编辑: 刘继红 制作编辑:张砚梁