修回日期: 2025-11-07
接受日期: 2025-12-12
在线出版日期: 2025-12-28
囊性纤维化相关肝病(cystic fibrosis-related liver disease, CFLD)在我国人群中极为罕见, 临床表现常缺乏特异性. 本文报道1例以不明原因肝结节和门脉高压为主要表现, 最终通过病理活检及基因检测确诊为CFLD的中年男性患者, 以提高临床医生对该病识别与诊治的重视.
患者为中年男性, 因"不明原因肝结节及门脉高压"入院. 入院时肝功能基本正常. 影像学提示肝癌、肝硬化. 肝穿刺活检提示: 肝板排列不规整, 肝窦灶性扩张, 局灶呈结节性再生性增生样改变; 门静脉束外围薄壁小血管疝入, 直接毗邻肝细胞板. 进一步全基因组测序发现囊性纤维化跨膜传导调节因子基因杂合错义变异c.374T>C(p.Ile125Thr). 结合病理及分子学结果, 最终诊断为CFLD.
CFLD在我国临床实践中罕见, 且表现缺乏特异性, 极易被误诊. 本例提示临床医师在遇到不明原因肝结节及门脉高压时, 应关注CFLD的可能性, 并及时联合病理学与分子遗传学检测以明确诊断. 早期识别和干预对改善患者预后具有重要意义.
核心提要: 中年男性患者, 因不明原因肝结节及门脉高压入院, 肝功能基本正常.影像学表现提示肝癌或肝硬化, 经过肝穿刺、全基因组测序最终诊断为囊性纤维化相关肝病. 该病例临床表现非典型, 极易误诊. 本病例的诊治过程提示临床医师在面对不明原因肝结节和门脉高压时, 应考虑囊性纤维化相关肝病的可能性, 以提高识别和诊治水平.
引文著录: 胡克珍, 谭杰, 江晨, 毕研贞, 辛永宁. 肝结节、门脉高压为表现的不典型囊性纤维化相关肝病1例. 世界华人消化杂志 2025; 33(12): 1031-1036
Revised: November 7, 2025
Accepted: December 12, 2025
Published online: December 28, 2025
Cystic fibrosis-related liver disease (CFLD) is extremely rare in the Chinese population and often presents with nonspecific manifestations. We report a middle-aged male patient with unexplained hepatic nodules and portal hypertension, ultimately diagnosed with CFLD based on histopathology and genetic testing, to emphasize the importance of considering this entity in clinical practice.
A middle-aged man was admitted for evaluation of unexplained hepatic nodules and portal hypertension. Liver function tests were essentially normal. Liver biopsy revealed mild hepatocellular edema within lobules, focal sinusoidal dilatation with nodular regenerative hyperplasia-like changes, and no significant inflammation in portal tracts. Mild proliferation of portal vein branches, marked bile duct proliferation with collagen deposition, and smooth muscle fiber hyperplasia in vessel walls were also noted. Whole-genome sequencing identified a heterozygous missense variant in the cystic fibrosis transmembrane conductance regulator gene, c.374T>C (p.Ile125Thr). Based on pathological and molecular findings, the patient was diagnosed with CFLD.
CFLD is exceedingly rare in China and may present with atypical clinical features, often leading to misdiagnosis. This case highlights that clinicians should consider CFLD in patients with unexplained hepatic nodules and portal hypertension. Timely histopathological and genetic testing is crucial for accurate diagnosis, as early recognition may improve both patient management and outcomes.
- Citation: Hu KZ, Tan J, Jiang C, Bi YZ, Xin YN. Atypical cystic fibrosis-associated liver disease presenting with hepatic nodules and portal hypertension: A case report. Shijie Huaren Xiaohua Zazhi 2025; 33(12): 1031-1036
- URL: https://www.wjgnet.com/1009-3079/full/v33/i12/1031.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v33.i12.1031
囊性纤维化(cystic fibrosis, CF)是一种常染色体隐性遗传性疾病, 中国CF患者罕见, 2018年被列入中国首批罕见病目录[1]. CF由CF跨膜传导调节因子(cystic fibrosis transmembrane conductance regulator, CFTR)基因突变引起, CFTR基因变异可影响蛋白结构或功能, 从而导致多器官异常, 其中肺脏和胰腺为最常见受累器官, 肝脏受累相对少见[2]. 当CFTR蛋白功能障碍仅累及单一器官、不满足CF确诊标准时, 则称为CF相关疾病[2]. 本文报道1例以肝结节及门脉高压为主要表现的不典型CF相关肝病患者, 从CFTR相关疾病谱角度阐述其肝脏表型, 强调囊性纤维化相关肝病(cystic fibrosis-related liver disease, CFLD)的诊断依据为表型与组织学证据的综合判断, 而非经典CF的双等位基因标准, 旨在提示临床医师提高对该病变的认识, 为今后诊疗和研究提供借鉴.
患者为东亚裔中国籍男性, 50岁, 因"体检发现肝结节1 wk"入院.
患者1 wk前体检发现肝结节, 超声提示左肝低回声结节约3.9 cm × 2.2 cm, 无其他不适. 患者为求进一步诊疗, 以"肝结节"收入我科. 患者自发病以来, 神志清, 精神可, 食欲、睡眠正常, 大小便无特殊, 体重无变化.
既往有脾大、脾功能亢进病史46年, 36年前因门脉高压伴脾大、脾功能亢进及上消化道出血行脾切除术, 术后口服普萘洛尔降门脉压; 肾结石20年; 6年前行胆囊切除术. 无其他慢性病、肿瘤及传染病史, 有青霉素过敏史.
无烟酒及毒物接触史, 无相关遗传性疾病史.
体格检查显示发育正常, 未见黄疸、肝掌或蜘蛛痣, 腹部左上方可见愈合良好手术瘢痕, 无压痛及反跳痛, 肝脾未触及.
入院检查提示血常规、肾功能、电解质及凝血均正常, 肝功能轻度异常(白蛋白39.78 g/L, 谷丙转氨酶12.79 U/L, 谷草转氨酶37.46 U/L, 碱性磷酸酶124.42 U/L, γ-GT 48.62 U/L, 总胆汁酸51.72 μmol/L), 血脂中总胆固醇升高(6.30 mmol/L), D-二聚体升高(3.07 μg/mL), 促甲状腺激素轻度降低(0.3251 μIU/mL), 肝纤维化指标轻度异常[层黏蛋白75.70 ng/mL、透明质酸66.60 ng/mL(<100)、Ⅲ型胶原26.8 ng/mL(<30)、Ⅳ型胶原19.00 ng/mL(<30)], 甲胎蛋白(alpha-fetoprotein, AFP)正常. 甲肝抗体、丙肝抗体、戊肝抗体、EB-DNA、CMV-DNA均阴性. 抗核抗体+自身免疫性肝病抗体谱均阴性; 铜蓝蛋白、铁蛋白、转铁蛋白饱和度、免疫球蛋白无异常; 免疫球蛋白G4(immunoglobulin G4, IgG4)升高(2960 mg/L).
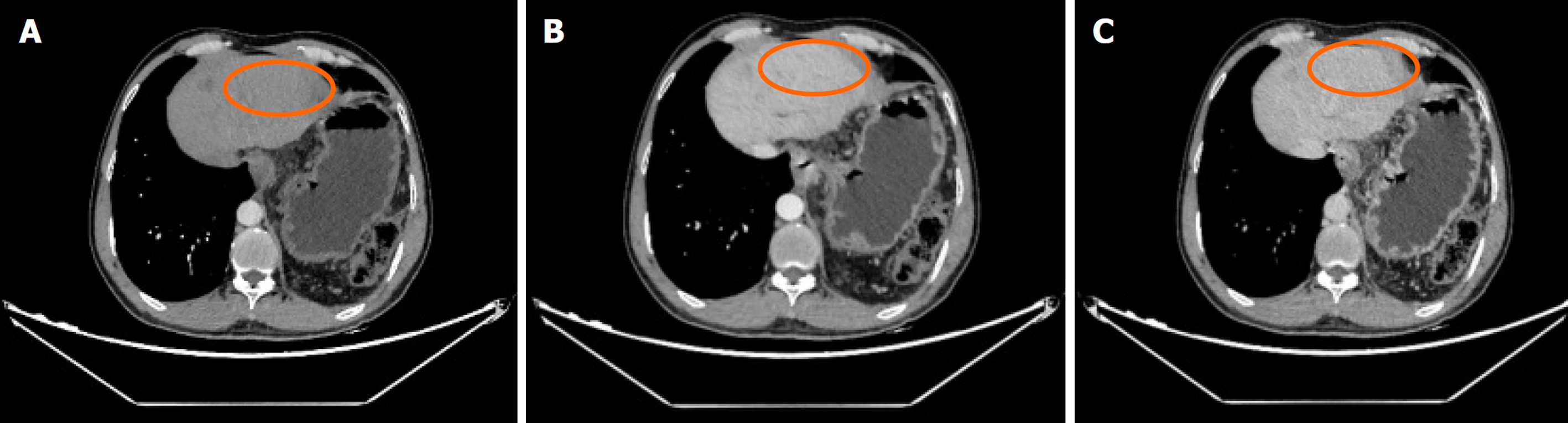
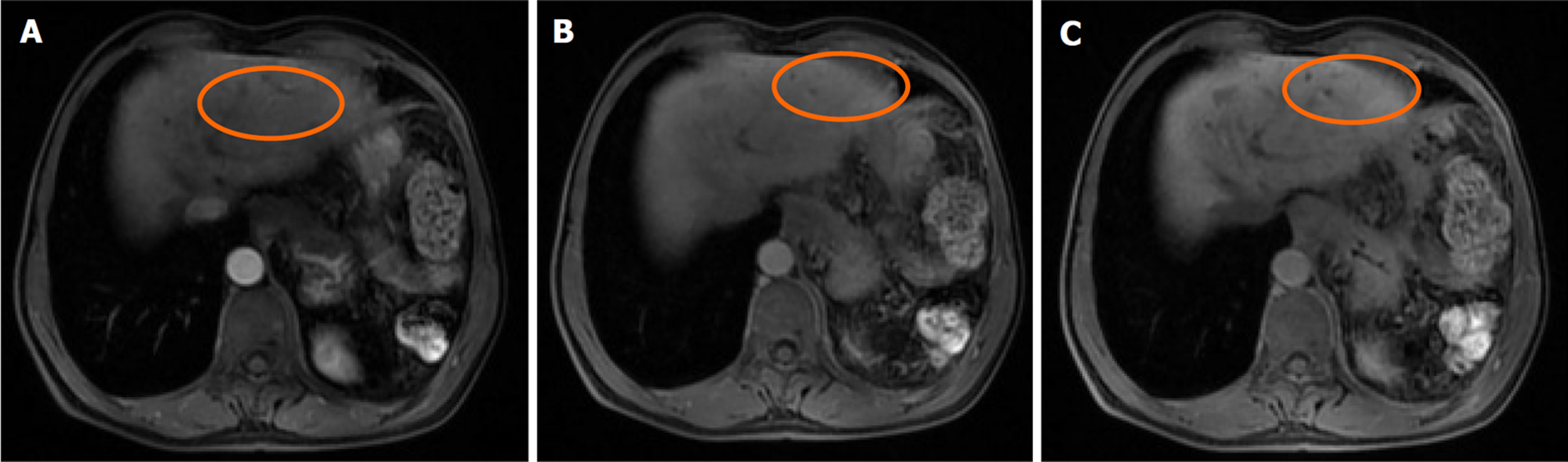
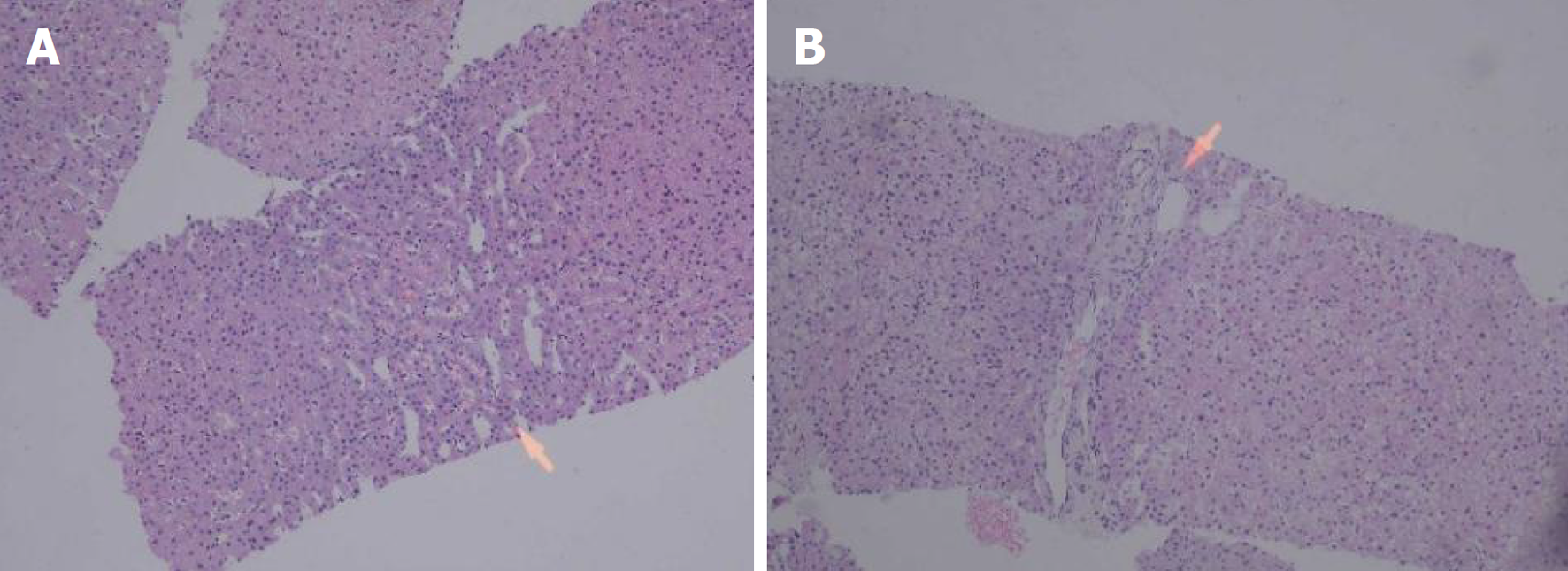
肝脏瞬时弹性测定: CAP 144 dB/m(<238), E 5.8 kPa(<7.3), 未见异常. 心脏超声: 左室假腱索, 二尖瓣+三尖瓣轻度反流. 胸部平扫计算机断层扫描(computerized tomography, CT): 双肺微小结节; 双肺条索灶; 左膈肌升高. 患者行上腹部增强CT示肝脏多发弱强化灶, 不除外肝癌(大者位于左内叶, 截面约1.3 cm×1.1 cm). 肝右叶门静脉期多发异常强化灶, 异常灌注可能. 肝硬化、门脉高压、食管胃底静脉曲张. 门静脉海绵样变. 肝多发囊肿. 胆囊术后; 脾术后. 肝内胆管扩张, 肝门区胆管壁增厚, 炎性病变?下腹部增强CT提示左肾多发结石; 左肾小囊肿; 腹膜后多发淋巴结. 盆腔增强CT提示直肠末端壁稍厚; 盆腔-直肠下段迂曲血管影(图1). 肝脏特异性对比剂增强磁共振(magnetic resonance, MR): 肝脏多发异常信号, 隔顶、左叶病变明显, 考虑肝癌可能. 肝硬化、门脉高压、食管胃底静脉曲张. 门静脉海绵样变. 肝多发囊肿. 胆囊术后、脾术后. 肝内胆管扩张, 肝门区胆管壁增厚, 炎性病变?(图2). 胃镜显示食管胃底静脉曲张(重度)及慢性非萎缩性胃窦炎, 肠镜显示直肠静脉曲张(重度)及结肠多发毛细血管扩张. 患者AFP正常, 影像学呈非典型多灶表现且合并显著门脉高压. 经多学科团队诊疗讨论, 为排除肝细胞癌并厘清基础肝病实体[门窦血管病变(porto-sinusoidal vascular disorder, PSVD)/结节性再生性增生(nodular regenerative hyperplasia, NRH)], 决定行影像引导下肝穿刺活检. 病理示肝板排列不规整, 肝窦灶性扩张, 局灶呈NRH样改变; 静脉束外围薄壁小血管疝入, 直接毗邻肝细胞板(图3). 免疫组化示CD10、CD34、CK19、CK8/18阳性; Masson染色显示汇管区及大血管周围胶原纤维增生, 弹力染色示血管壁弹力纤维增生, 网状纤维染色示局灶肝窦周网状纤维增生. 全基因组测序发现CFTR基因杂合错义变异c.374T>C(p.Ile125Thr).
CFLD, PSVD(导致非肝硬化性门脉高压); 肝多发结节, NRH; 食管胃底静脉曲张; 直肠静脉曲张; 肝内胆管扩张; 脾切除术后; 胆囊切除术后; 肾结石; 肺结节; 慢性胃炎; 上消化道出血个人史.
给予患者普萘洛尔降低门脉压力治疗; 向患者做好宣教: 避免便秘、剧烈咳嗽及呕吐以避免加重门脉压力, 谨慎使用非甾体抗炎药减少出血风险, 避免大量饮酒和过度运动. 患者在接受保守治疗后出院.
截至2025-08-01未见病情进展. 由于自确诊至今约1年, 患者尚未按期门诊复诊, 我们已再次电话提醒其尽快复诊, 并给出分层随访方案: 建议患者每1-2年胃镜评估食管/胃底静脉曲张进展; 每6 mo行腹部超声+AFP检查, 如出现可疑肝结节, 行肝特异性对比剂磁共振或增强CT进一步评估进行肿瘤监测; 每12 mo复查腹部影像(含门静脉多普勒)与脾径, 必要时补充弹性成像评估门脉系统.
仅发现单等位基因CFTR变异并不能满足CF的分子学标准, 亚洲人群中已报道在仅检出单等位CFTR变异的情况下, 结合表型与功能学证据仍可确立CF/CFTR相关疾病的病例-例如中国青少年携带c.106G>A(p.Asp36Asn)的杂合变异而被诊断为CF的个案[3]. 我们的病例不排除存在深度内含子/调控区/拷贝数或复合等位等隐匿变异; 结合组织学与临床表型, 我们将本例定位为CFTR相关肝病. 患者血清IgG4升高, 但肝穿刺病理未见IgG4相关疾病的特征性表现, 如车轮样纤维化、闭塞性静脉炎或显著浆细胞团簇[4]. 影像学未发现IgG4相关硬化性胆管炎特有的多段胆管狭窄和环形/偏心性强化表现, 且胰腺、泌腺等器官无受累征象[5]. 因此综合临床、影像和病理证据, 可以排除IgG4相关疾病, 而考虑到门静脉束外围薄壁小血管疝入, 直接毗邻肝细胞板, 这更符合PSVD. CT/MR显示脾静脉、门静脉主干及肠系膜上静脉腔畅通、对比剂充盈均匀, 未见截断征或充盈缺损, 排除了脾静脉栓塞或门静脉血栓等肝前型病因; 肝静脉及下腔静脉口径正常、无狭窄或血栓, 肝实质无肝后性回流受阻灌注模式, 排除了布加综合征等肝后型病因[6].
CF是CFTR基因突变导致的常染色体隐性遗传病, 由美国学者Dorothy Andersen在1938年首次发现[7]. 其临床表现复杂多样, 可累及呼吸、消化、内分泌及生殖系统. 目前文献中报道的CF病例以儿童呼吸系统症状为主, 而肝脏受累在成人中更易被忽视. 在肝胆系统, CFTR异常使胆管上皮离子/水通量及胆汁成分失衡, 诱发胆管微结构损伤与轻度胆汁淤积; 其后胆管周围微环境(成纤维化信号上调、胆管反应)被激活, 形成胆管周围胶原沉积与结缔组织重塑. 上述改变在汇管区附近对门静脉终末小分支形成外压性受压与不规则狭窄, 即使未出现肝硬化结节, 也足以造成肝内窦前性阻力升高, 呈PSVD/NRH样改变并出现门脉高压体征[8]. 2025年Hepatology综述指出, CF患者中PSVD是非肝硬化性门脉高压的主要驱动因素, PSVD患者往往肝脏硬度正常而脾脏硬度增高, 临床上呈现脾肿大、食管胃底静脉曲张等表现[9]. 本例患者的临床及病理特点符合该机制.
本例患者的影像学表现提示肝癌或肝硬化, 但肝穿刺病理未见假小叶, 而显示NRH样改变, 提示CFLD合并PSVD. NRH表现为直径≤3 mm的再生结节, 纤维化轻微或缺如, 常由门静脉-动脉血流失衡所致[10], 是PSVD的典型组织学特征[11]. 与肝硬化不同, PSVD患者早期肝功能常保持正常, 诊断高度依赖于肝活检[12]. 近年来, 影像学弹性成像被提出用于鉴别诊断, 如肝脏硬度测值(liver stiffness measurement, LSM)<10 kPa可排除肝硬化并提示PSVD, 特异性可达97%[13]. 本例患者LSM为5.8 kPa, 与病理学结果一致. 因此, 对于出现脾大或食管胃底静脉曲张的中年CF患者, 应考虑PSVD, 而不仅仅局限于典型肝硬化.
中国及东亚人群CFTR突变谱与白种人不同. 系统综述表明, c.1766+5G>T和c.2909G>A等突变在中国患者中较常见, 而F508del在中国罕见[14,15]. 2025年回顾研究分析了15例中国患儿, 发现共27种CFTR变异, 其中c.374T>C变异出现2例, 提示其在中国人群中仍属罕见, 且超过半数突变的致病性仍不明确[16]. 提示了c.374T>C变异与CF的关联性, 但仍需更多功能学与家系学证据; 临床上应降低漏诊阈值, 在不明原因门脉高压与NRH样改变中合并考虑CFTR相关肝病. 基因型与临床表型间可能存在差异, 已有研究表明, 相同CFTR基因型患者间肝脏损害程度差异显著, 这可能与环境因素及修饰基因相关[17,18]. 其中Bartlett等[19]、Boëlle等[20]、Stonebraker等[21]团队先后证明了SEPRINA1(编码α-1抗胰蛋白酶蛋白)是影响CFLD患者肝病严重程度的重要修饰因子. 该发现提示, 对于非典型CF病例, 基因学检测必须结合临床与病理学综合分析, 方能实现精准诊断.
治疗方面, 目前尚无特异性药物疗法可显著改善预后. 熊去氧胆酸对CF相关肝胆疾病疗效有限, 无症状者一般无需干预[2]. Baveno共识建议, 非选择性β受体阻滞剂用于门静脉高压并发症的一级预防, 内窥镜静脉曲张结扎术或经颈静脉肝内门体分流术用于二级预防或难治性并发症[22]. CFTR调节剂虽可改善肺部疾病, 但对CFLD疗效尚未明确, 部分患者甚至存在肝毒性风险[23,24]. 因此, 定期监测(如AFP、影像学评估及胃镜检查)对CFLD患者更为关键.
本病例缺乏早年病史和影像资料, 无法追溯门脉高压的起始病因, 本次结合PSVD组织学、更偏向肝内窦前性阻力机制, 但不据此对既往进行追溯性定论; 汗氯测试和鼻腔电位差检查未进行, 限制了CFTR功能的评估; 未行IgG4免疫组化计数; 未同期完成PIVKAⅡ与超声造影检查. 尽管如此, 结合肝活检明确的门窦前病变和临床表现, 仍支持CFLD合并PSVD的诊断. 本例提示, 成人患者的CFLD在亚洲人群中可能被低估或误诊, 应提高警惕, 并结合肝活检及非侵入性指标进行早期识别.
本例通过全基因组测序明确了CFTR杂合突变, 为成人非典型CFLD的诊断提供依据. 提示在中年患者出现多发肝结节、NRH样改变及门静脉高压时, 应高度怀疑CFLD, 避免误诊为肝癌或肝硬化. 基因学检测结合影像学及病理学是实现精准识别的关键. 本病例拓展了成人CFLD的临床谱系, 为临床诊治及鉴别提供重要参考.
| 1. | 郭 小贝, 田 欣伦. 囊性纤维化. 中国第一批罕见病目录释义. 北京: 人民卫生出版社 2018; 315-317. |
| 2. | 囊性纤维化诊断与治疗中国专家共识编写组; 中国罕见病联盟呼吸病学分会; 中国支气管扩张症临床诊治与研究联盟. 囊性纤维化诊断与治疗中国专家共识(2023版). 中华结核和呼吸杂志. 2023;46:352-372. [DOI] |
| 3. | Kan YT, Wu WF, Yang YF. [A case of cystic fibrosis with cirrhosis as the main manifestation]. Zhonghua Gan Zang Bing Za Zhi. 2023;31:1087-1089. [PubMed] [DOI] |
| 4. | Chen LYC, Mattman A, Seidman MA, Carruthers MN. IgG4-related disease: what a hematologist needs to know. Haematologica. 2019;104:444-455. [PubMed] [DOI] |
| 5. | Kamisawa T, Nakazawa T, Tazuma S, Zen Y, Tanaka A, Ohara H, Muraki T, Inui K, Inoue D, Nishino T, Naitoh I, Itoi T, Notohara K, Kanno A, Kubota K, Hirano K, Isayama H, Shimizu K, Tsuyuguchi T, Shimosegawa T, Kawa S, Chiba T, Okazaki K, Takikawa H, Kimura W, Unno M, Yoshida M. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2019;26:9-42. [PubMed] [DOI] |
| 6. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179-202. [PubMed] [DOI] |
| 7. | Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475-482. [PubMed] [DOI] |
| 8. | Colombo C, Lanfranchi C, Tosetti G, Corti F, Primignani M. Management of liver disease and portal hypertension in cystic fibrosis: a review. Expert Rev Respir Med. 2024;18:269-281. [PubMed] [DOI] |
| 9. | Colecchia L, Ravaioli F, Marasco G, Dajti E, Colecchia A. New insights on portal hypertension's screening in people with cystic fibrosis. Hepatobiliary Surg Nutr. 2024;13:894-897. [PubMed] [DOI] |
| 10. | Kleiner DE. Noncirrhotic portal hypertension: Pathology and nomenclature. Clin Liver Dis (Hoboken). 2015;5:123-126. [PubMed] [DOI] |
| 11. | Verheij J, Schouten JN, Komuta M, Nevens F, Hansen BE, Janssen HL, Roskams T. Histological features in western patients with idiopathic non-cirrhotic portal hypertension. Histopathology. 2013;62:1083-1091. [PubMed] [DOI] |
| 12. | Mironova M, Gopalakrishna H, Koh C, Kleiner DE, Heller T. Portal sinusoidal vascular diseases: Assessment and therapy. Hepatology. 2025. [PubMed] [DOI] |
| 13. | Dajti E, Ravaioli F, Paiola G, Volpi S, Colecchia L, Ferrarese A, Alemanni LV, Cusumano C, Di Biase AR, Marasco G, Vestito A, Festi D, Rautou PE, Cipolli M, Colecchia A. The non-invasive evaluation of liver involvement in patients with cystic fibrosis: A prospective study. Liver Int. 2023;43:2492-2502. [PubMed] [DOI] |
| 14. | Ni Q, Chen X, Zhang P, Yang L, Lu Y, Xiao F, Wu B, Wang H, Zhou W, Dong X. Systematic estimation of cystic fibrosis prevalence in Chinese and genetic spectrum comparison to Caucasians. Orphanet J Rare Dis. 2022;17:129. [PubMed] [DOI] |
| 15. | Chen Q, Shen Y, Zheng J. A review of cystic fibrosis: Basic and clinical aspects. Animal Model Exp Med. 2021;4:220-232. [PubMed] [DOI] |
| 16. | Guo X, Liu K, Liu Y, Situ Y, Tian X, Xu KF, Zhang X. Clinical and genetic characteristics of cystic fibrosis in CHINESE patients: a systemic review of reported cases. Orphanet J Rare Dis. 2018;13:224. [PubMed] [DOI] |
| 17. | Csanády L, Vergani P, Gadsby DC. Structure, gating, and regulation of the CFTR anion channel. Physiol Rev. 2019;99:707-738. [PubMed] [DOI] |
| 18. | Butnariu LI, Țarcă E, Cojocaru E, Rusu C, Moisă ȘM, Leon Constantin MM, Gorduza EV, Trandafir LM. Genetic Modifying Factors of Cystic Fibrosis Phenotype: A Challenge for Modern Medicine. J Clin Med. 2021;10:5821. [PubMed] [DOI] |
| 19. | Bartlett JR, Friedman KJ, Ling SC, Pace RG, Bell SC, Bourke B, Castaldo G, Castellani C, Cipolli M, Colombo C, Colombo JL, Debray D, Fernandez A, Lacaille F, Macek M, Rowland M, Salvatore F, Taylor CJ, Wainwright C, Wilschanski M, Zemková D, Hannah WB, Phillips MJ, Corey M, Zielenski J, Dorfman R, Wang Y, Zou F, Silverman LM, Drumm ML, Wright FA, Lange EM, Durie PR, Knowles MR; Gene Modifier Study Group. Genetic modifiers of liver disease in cystic fibrosis. JAMA. 2009;302:1076-1083. [PubMed] [DOI] |
| 20. | Boëlle PY, Debray D, Guillot L, Corvol H; French CF Modifier Gene Study Investigators. SERPINA1 Z allele is associated with cystic fibrosis liver disease. Genet Med. 2019;21:2151-2155. [PubMed] [DOI] |
| 21. | Stonebraker JR, Pace RG, Gallins PJ, Dang H, Aksit MA, Faino AV, Gordon WW, MacParland S, Bamshad MJ, Gibson RL, Cutting GR, Durie PR, Wright FA, Zhou YH, Blackman SM, O'Neal WK, Ling SC, Knowles MR. Genetic variation in severe cystic fibrosis liver disease is associated with novel mechanisms for disease pathogenesis. Hepatology. 2024;80:1012-1025. [PubMed] [DOI] |
| 22. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [PubMed] [DOI] |
| 23. | Quon BS, Rowe SM. New and emerging targeted therapies for cystic fibrosis. BMJ. 2016;352:i859. [PubMed] [DOI] |
| 24. | Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar JL; VX16-445-001 Study Group. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N Engl J Med. 2018;379:1612-1620. [PubMed] [DOI] |
学科分类: 胃肠病学和肝病学
手稿来源地: 山东省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): 0
C级 (良好): C, C
D级 (一般): D
E级 (差): 0
科学编辑: 刘继红 制作编辑:张砚梁