Published online Jan 14, 2026. doi: 10.3748/wjg.v32.i2.112132
Revised: September 2, 2025
Accepted: November 28, 2025
Published online: January 14, 2026
Processing time: 166 Days and 0.5 Hours
Crohn’s disease (CD) is a chronic inflammatory bowel disease with unknown etiology. Inflammatory chemical mediators synthesized from arachidonic acid, an n-6 polyunsaturated fatty acid (PUFA), have been shown to activate CD. Addi
To investigate the relationship of FADS2 polymorphisms with serum and erythro
Using previously reported findings regarding FADS2 genetic polymorphisms, the records of 52 CD patients undergoing treatment at Jikei University Kashiwa Hospital were analyzed. Mutations noted were divided into three groups; wild-type (GG), heterozygous mutants (GA), and homozygous (AA), with the activities of delta-6 and delta-5 desaturases compared using redefined d6d index (rd.d6di) and d5d index (d5di). Additionally, comparisons of serum and erythrocyte membranes for fatty acid composition, and also gene polymorphisms and CD activity index (CDAI) were performed.
The presence of the rs174538 mutation in FADS2 resulted in reduction of only rd.d6di in the erythrocyte membrane (P < 0.01). In contrast, that mutation was found to be associated with d5di induced by FADS1 in serum (P = 0.019) as well as the erythrocyte membrane (P < 0.0001), and also with reduction in the fatty acid composition of arachidonic acid in both serum (P < 0.0001) and the erythrocyte membrane (P < 0.01). Regarding disease activity, a positive correlation of CDAI score with rd.d6di in both serum (P < 0.05) and the erythrocyte membrane (P < 0.05) was found only in the rs174538 wild-type group. In contrast, there was no correction between CDAI and d5di in either serum or erythrocyte membrane samples.
The rs174538 mutation alters the fatty acid profile through strong linkage to the FADS1 gene. In wild-type indi
Core Tip: Erythrocyte membrane fatty acid composition ratios in Japanese Crohn’s disease (CD) patients are distinctive. Analysis was performed to determine effects on FADS2 genetic polymorphisms by serum and erythrocyte membrane fatty acid composition ratios, shown by delta 6 and delta 5 desaturases (D6D and D5D, respectively), and also disease activities. The FADS2 gene with the rs174538 mutation affected D6D and D5D activities, with a greater effect on D5D. However, for disease activity, wild-type rs174538 was positively correlated with D6D activity. These results indicate that confirmation of the rs174538 mutation can be used to predict disease severity in CD cases.
- Citation: Matsuzawa H, Ito Z, Uchiyama K, Motoi Y, Ohtaki Y, Iwashita Y, Suzuki S, Nakada T, Koido S, Kojima K, Murohashi K, Saruta M, Ohkusa T, Kubota T. Association of FADS2 polymorphism rs174538 with fatty acid metabolism and disease severity in Japanese patients with Crohn’s disease. World J Gastroenterol 2026; 32(2): 112132
- URL: https://www.wjgnet.com/1007-9327/full/v32/i2/112132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i2.112132
Crohn’s disease (CD) is a chronic intestinal disease with unknown cause in which inflammation is induced throughout the digestive tract, from the mouth to anus, with relapse and/or remission noted in affected individuals. The occurrence of CD is increasing not only in Asia but also Western European countries[1,2]. At the time of diagnosis, pediatric CD is often more extensive and severe than adult CD, while it has also been speculated that pediatric patients are more strongly affected by its development than adult patients[3]. Since the intestinal tract is the primary site of inflammatory bowel disease (IBD) occurrence, CD has been reported to be related to the oncostatin M receptor and autophagy associated with the intestinal barrier[4]. These events are correlated with the activation of T cells, which are immune cells, thus it is considered that immune memory influences development of IBD, though genetic factors alone cannot explain its deve
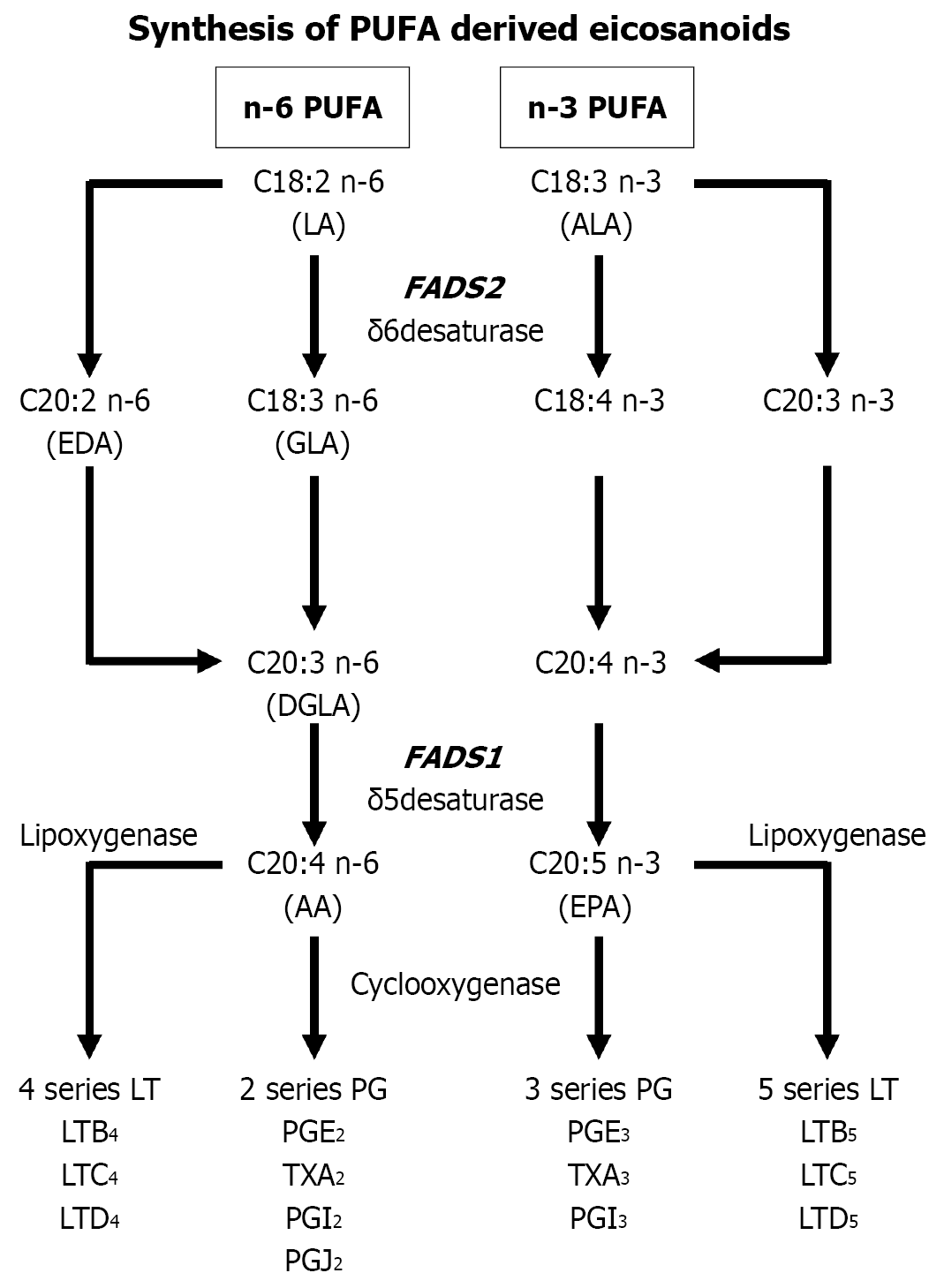
Arachidonic acid (AA) is supplied by animal fats and oils, while it is produced endogenously from linoleic acid (LA), which is found primarily in large amounts in various vegetable oils. LA is metabolized to AA by delta 6 and delta 5 desaturases (D6D and D5D, respectively) via γ-linolenic acid (GLA) and dihomo-GLA (DGLA). Each of these fatty acids (FAs) are considered to be essential n-6 polyunsaturated FAs (PUFAs). AA produces 2-series prostaglandins (PGI2 and PGE2, respectively) and thromboxane (TXA2) by cyclooxygenase (COX), as well as 4-series leukotrienes (LTB4 and LTC4, respectively) by 5-lipoxygenase (LOX) inflammatory mediators.
Additionally, n-3 PUFAs, also essential FAs, include α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). In the pathway from ALA to EPA (D6D, D5D, COX, LOX, etc.), metabolic enzymes are involved in formation of EPA-derived eicosanoids (3-series PG, TX, 5-series LT), indicating that n-6 and n-3 PUFAs are in a competitive antagonistic relationship based on metabolism. As a result, n-3 PUFAs inhibit LA metabolism and the AA cascade, causing inhibition of synthesis of AA-derived inflammatory mediators, such as prostaglandin E2 (PGE2) and thromboxane A2 (TXA2), which exert anti-inflammatory effects (Figure 1). Several studies have suggested that a high dietary ratio of n-6/n-3 PUFAs may be associated with increased risk of cardiovascular and inflammatory diseases[5,6]. Conversely, increased intake of n-3 PUFAs has been reported to prevent IBD relapse[7,8]. Furthermore, recent studies have shown that in children who are CYP4F3 and FADS2 gene variant carriers, higher dietary ratios of n-6/n-3 PUFAs render them more susceptible to CD[9].
We previously analyzed erythrocyte membrane FA composition in adult CD patients and calculated the ratio of (DGLA + AA)/LA as the d6d index (d6di) using the weight percentages of LA, DGLA, and AA, and reported findings showing their characteristic FA composition[10]. In addition, in another study we analyzed the FADS2 gene, which encodes a FA-metabolizing enzyme, in patients with CD[11]. However, it remains unclear whether the characteristic FA composition in CD patients is genetic or related to disease activity. For the present study, the patient database accessed for our previous genetic analysis to examine the association between FA composition and FA metabolic enzymes was analyzed to determine FA metabolism FADS2 genetic polymorphisms in CD patients. To exclude the influence of D5D activity due to the FADS1 gene, d6di was redefined for the present study as only weight percentages of LA and GLA, while the ratio of GLA/LA was calculated as redefined d6d index (rd.d6di). Additionally, the association of FADS2 genetic polymorphisms with disease activity in CD patients was examined.
All of the present subjects were recruited from CD patients being treated at the Jikei University Kashiwa Hospital, with diagnosis based on CD criteria established for Japanese patients[12]. Patients under 18 years of age or with evidence of a severe medical comorbidity were excluded. To reduce the effects of geographical conditions, genetic background, and diet, all recruited participants were Japanese and consumed a diet typical for Japan. Information regarding clinical features, including age, sex, body mass index, disease duration, location of disease, medication, and Crohn’s disease activity index (CDAI), was obtained from medical records. A total of 52 CD patients were enrolled, with details presented in Table 1.
| Variable | Value |
| Age, years, median (min-max) | 33.4 ± 13.2 (18-73) |
| Female | 15 (28.8) |
| BMI, median (min-max) | 21.0 (14.9-31.6) |
| Disease location | |
| Ileum-colon type | 36 (69.2) |
| Ileum type | 9 (17.3) |
| Colon type | 7 (13.5) |
| Disease duration, year | |
| < 1 | 13 (25.0) |
| 1-3 | 11 (21.2) |
| > 3 | 28 (53.8) |
| Medications, n | |
| TNF-α inhibitor | 30 (IFX: 17, ADA: 13) |
| Azathioprine | 17 |
| Aminosalicylate | 51 |
| Corticosteroids | 1 |
| CDAI, median ± SD | 114.9 ± 90.6 |
| Surgery | 15 (28.8) |
| Endoscopic activity | |
| Remission | 10 (19.2) |
| Mild | 13 (25.0) |
| Moderate | 18 (34.6) |
| Severe | 3 (5.8) |
| ND | 8 (15.4) |
All subjects provided written informed consent, and the study was approved by the Clinical Research Ethics Committee of the Jikei University School of Medicine and the Jikei University Kashiwa Hospital (No. 26-363-7869), as well as the Clinical Research Ethics Committee of Niigata University of Pharmacy and Applied Life Sciences (No. H27-005). This study was conducted in compliance with the Declaration of Helsinki.
After fasting, venous blood samples were collected from all subjects for FA analysis. Venous blood samples taken from the peripheral vein were centrifuged at 3000 rpm for 10 minutes at 4 °C and stored at -10 °C until analysis. Plasma and erythrocyte membranes were analyzed using a previously reported method[10]. The weight percent of each FA was calculated (Table 2). Total saturated FA (SFA), monounsaturated FA (MUFA), n-6 and n-3 polyunsaturated FA (PUFA n-6 and PUFA n-3, respectively), and trans FA levels were calculated. In our previous study of erythrocyte membrane FA composition in adult CD patients, the d6d index (d6di) was calculated as the ratio of (DGLA + AA)/LA, based on the weight percentages of LA, DGLA, and AA[10]. For the present study, to exclude the influence of D5D activity due to the FADS1 gene, d6di was redefined as only weight percentages of LA and GLA, while the ratio of GLA/LA was calculated as redefined d6d index (rd.d6di), and D5D activity was calculated using the ratio of DGLA to AA (AA/DGLA) and referred to as d5d index (d5di). Results of our analysis of FADS2 genetic polymorphisms encoding FA metabolizing enzymes in CD patients noted in that previous study were used to examine the relationships of rd.d6di and d5di with various FAs, disease activity, and FADS2 genetic polymorphisms.
| Plasma | median ± SD | RBC | median ± SD |
| SFA (%) | SFA (%) | ||
| C16:0 PA | 24.4 ± 2.05 | C16:0 PA | 23.1 ± 1.94 |
| C18:0 SA | 6.97 ± 0.83 | C18:0 SA | 17.3 ± 0.86 |
| C20:0 AdA | 0.27 ± 0.05 | C20:0 AdA | 0.42 ± 0.05 |
| C24:0 LgA | 0.52 ± 0.14 | C24:0 LgA | 5.89 ± 0.53 |
| n-9 (%) | n-9 (%) | ||
| C16:1 PtA | 2.82 ± 1.61 | C16:1 PtA | 0.32 ± 0.17 |
| C18:1 OA | 23.3 ± 4.05 | C18:1 OA | 12.4 ± 1.07 |
| C20:1 EA | 0.15 ± 0.05 | C20:1 EA | 0.21 ± 0.04 |
| C22:1 EcA | 0.02 ± 0.03 | C22:1 EcA | 0.09 ± 0.02 |
| C24:1 NA | 1.33 ± 0.40 | C24:1 NA | 6.25 ± 0.57 |
| n-3 (%) | n-3 (%) | ||
| C18:3 ALA | 0.79 ± 0.82 | C18:3 ALA | 0.09 ± 0.06 |
| C20:5 EPA | 1.71 ± 1.69 | C20:5 EPA | 1.08 ± 0.97 |
| C22:5 DPA | 0.60 ± 0.29 | C22:5 DPA | 1.82 ± 0.56 |
| C22:6 DHA | 3.74 ± 1.53 | C22:6 DHA | 6.31 ± 2.02 |
| n-6 (%) | n-6 (%) | ||
| C18:2 LA | 22.9 ± 6.81 | C18:2 LA | 6.86 ± 1.54 |
| C20:2 EDA | 0.20 ± 0.04 | C20:2 EDA | 0.22 ± 0.04 |
| C18:3 GLA | 0.46 ± 0.43 | C18:3 GLA | 0.55 ± 0.03 |
| C20:3 DGLA | 1.33 ± 0.43 | C20:3 DGLA | 1.39 ± 0.29 |
| C20:4 AA | 6.54 ± 1.75 | C20:4 AA | 12.0 ± 1.67 |
| C22:4 DTA | 0.21 ± 0.09 | C22:4 DTA | 2.05 ± 0.61 |
| d5di: AA/DGLA | 5.26 ± 1.78 | d5di: AA/DGLA | 8.95 ± 1.88 |
| rd.d6di: GLA/LA | 0.03 ± 0.05 | rd.d6di: GLA/LA | 0.01 ± 0.01 |
Statistical analysis was performed using Microsoft Excel statistical tools, and included Student’s t-test and Welch’s t-test, analysis of variance and Tukey’s test, and Pearson’s correlation coefficient. All reported P values are two-sided, with values < 0.05 considered significant. All data are expressed as mean ± SD.
This study included 52 CD patients, 37 males and 15 females, with an average age of 33.4 ± 13.2 years. The average duration of illness from the time of CD diagnosis was 92.4 ± 194.0 months. CD type was small/Large intestinal type in 36 (70%), small intestinal type in 9 (17%), and large intestinal type in 7 (13%). The average CDAI value was 114.9 ± 90.6, with the majority of patients in remission and their disease activity mild. Other clinical features presented in Table 1.
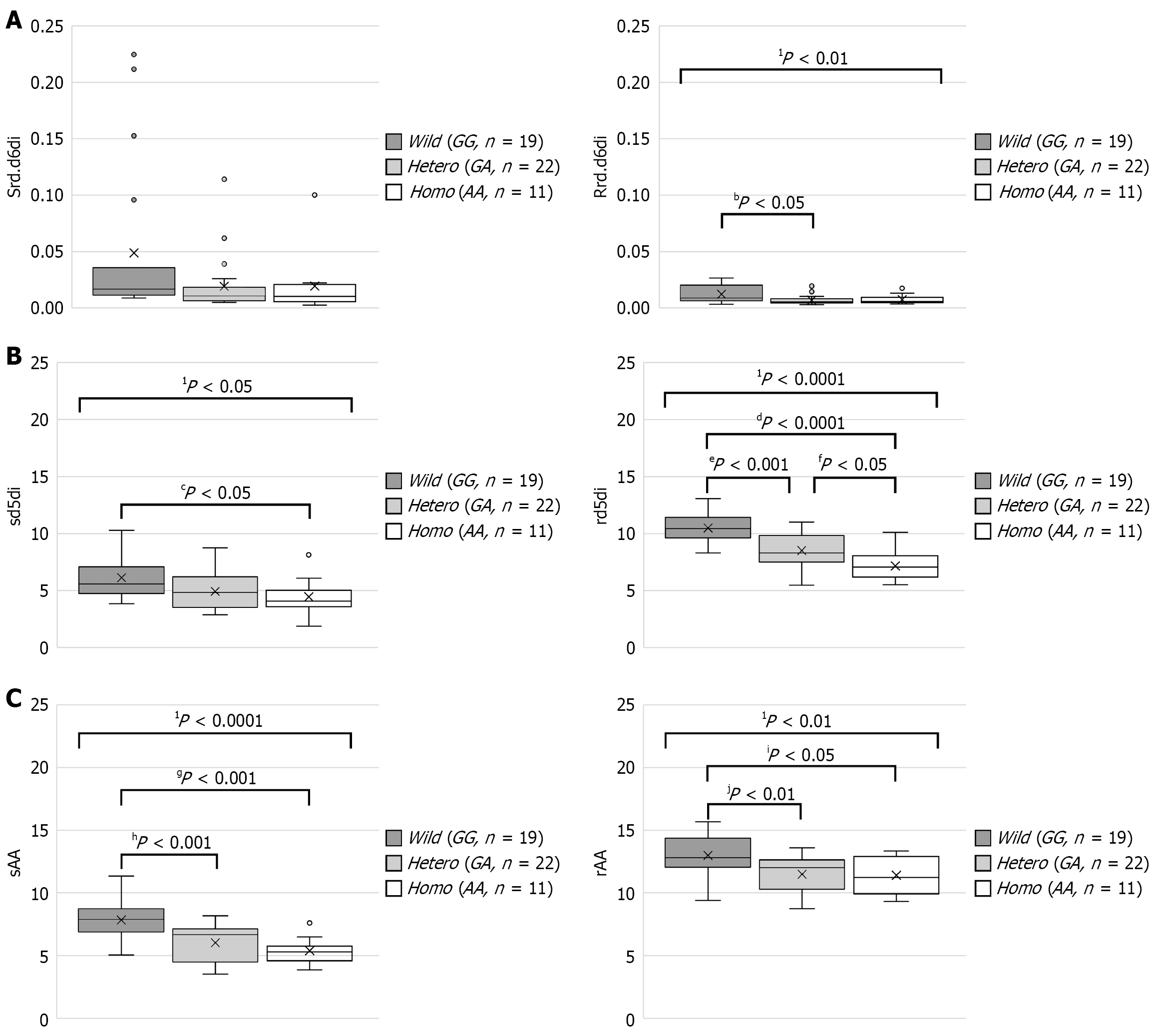
The serum and erythrocyte membrane FA composition for each of the CD patients is shown in Table 2. We previously reported that 19 FADS2 mutations were found in in CD patients[11]. Multiple regression analysis showed an association of the rs174538 mutation with rd.d6di value. To evaluate the relationship between that mutation and FA composition in the present CD patients, three groups, wild-type (GG), heterozygous (GA), and homozygous (AA), were compared. A rs174538 mutation in FADS2 reduced rd.d6di in only the erythrocyte membrane (Wild-type 0.012 ± 0.008, heterozygous 0.007 ± 0.004, homozygous 0.007 ± 0.004; P < 0.01; Figure 2A). On the other hand, d5di was significantly different regarding the rs174538 mutation in serum and erythrocyte membrane samples (serum: Wild-type 6.13 ± 1.75, heterozygous 4.92 ± 1.64, homozygous 4.45 ± 1.63, P = 0.019; erythrocyte membrane: Wild-type 10.49 ± 1.31, heterozygous 8.52 ± 1.49, homozygous 7.16 ± 1.35, P < 0.0001; Figure 2B). In both serum and erythrocyte membrane samples, the presence of rs174538 mutant alleles was associated with significantly decreased percentage weights of AA (serum: Wild-type 7.83 ± 1.56, heterozygous 6.01 ± 1.54, homozygous 4.45 ± 5.38, P < 0.0001; erythrocyte membrane: Wild-type 12.99 ± 1.62, heterozygous 11.49 ± 1.46, homozygous 11.40 ± 1.49, P < 0.01), as well as increased percentage weights of eicosadienoic acid (EDA) (serum: Wild-type 0.18 ± 0.03, heterozygous 0.19 ± 0.03, homozygous 0.23 ± 0.05, P < 0.05; erythrocyte membrane: Wild-type 0.21 ± 0.03, heterozygous 0.21 ± 0.03, homozygous 0.25 ± 0.04, P < 0.01; Figure 2C).
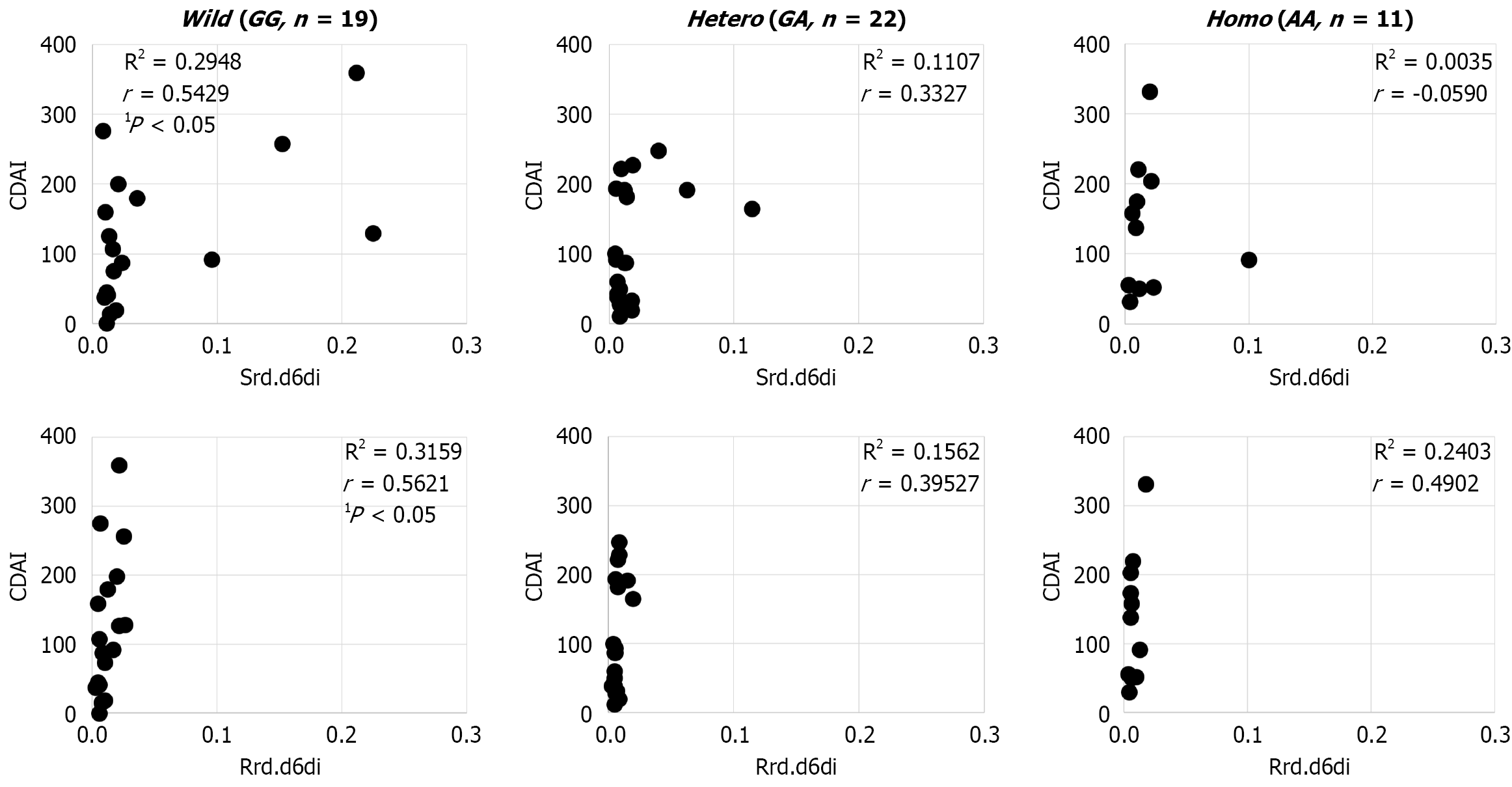
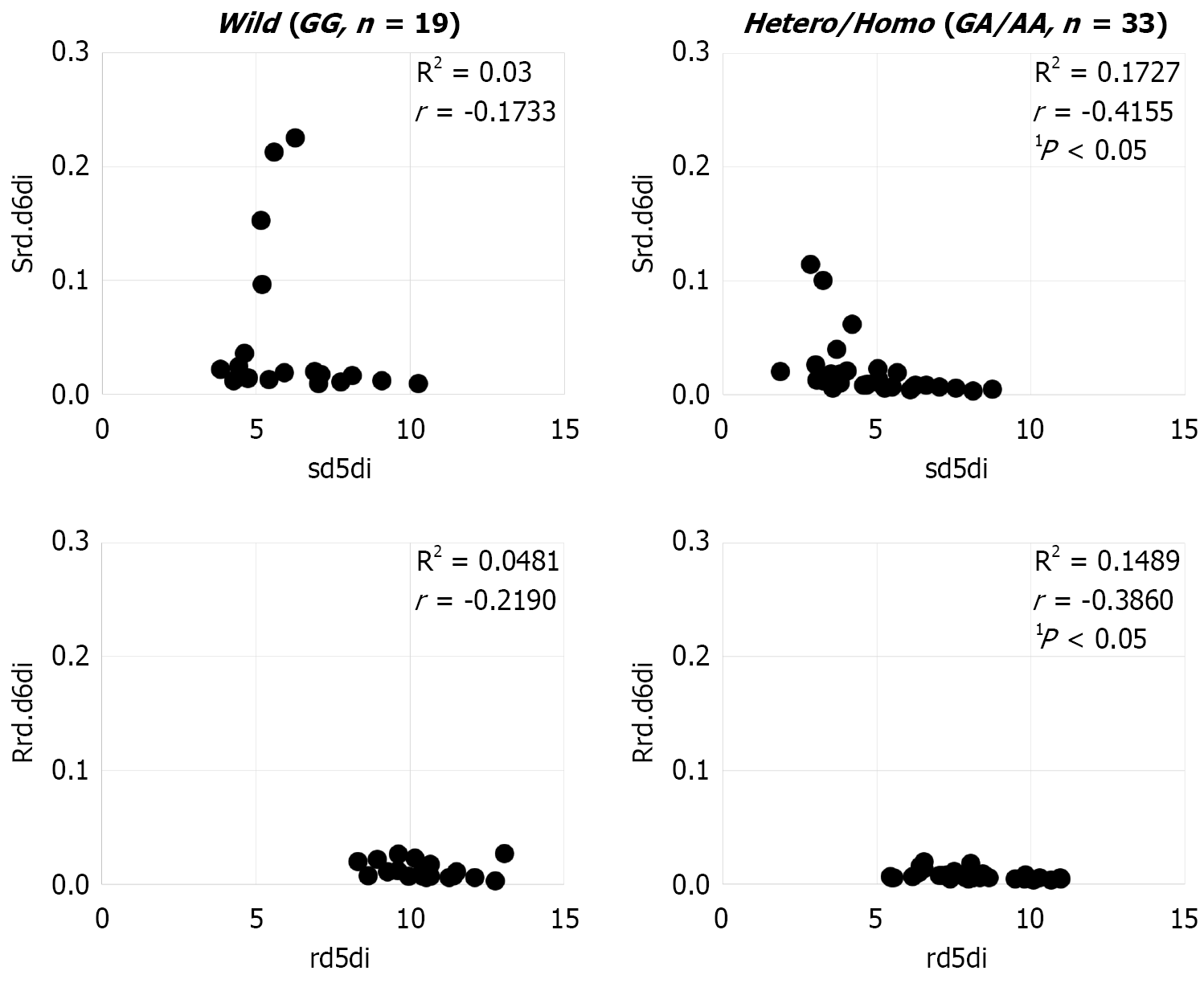
As for disease activity, there were no significant differences noted for Hb, leukocytes, C-reactive protein (CRP), equivalent series resistance (ESR), or CDAI due to the rs174538 mutation (Table 3). In the wild-type cases alone, CDAI was found to be positively correlated with rd.d6di in both serum and erythrocyte membrane samples (Figure 3), while there was no correlation between CDAI and d5di noted in either.
| rs174538 in FADS2 gene | Wild-type (GG) | With mutant allele (GA or AA) | P value | Mean difference (95%CI) |
| Hemoglobin, g/dL | 13.1 | 13.4 | 0.535 | -1.3002 to 0.6833 |
| Leukocytes, count/μL | 5547 | 6203 | 0.206 | -1684.2 to 372.83 |
| CRP, g/dL | 0.51 | 0.84 | 0.231 | -0.8884 to 0.2202 |
| ESR | 20.1 | 22.1 | 0.707 | -12.693 to 8.669 |
| CDAI | 115.2 | 114.6 | 0.983 | -52.381 to 53.511 |
In our previous study, erythrocyte membranes in patients with CD were found to have a low LA composition and high AA content[10]. Therefore, we speculated that the FADS2 gene involved in FA metabolism is related to CD development, though there were no findings indicating involvement of that gene in the pathogenesis of CD. Therefore, the relationship of the FADS2 gene with FA composition in serum and erythrocyte membranes sampled from CD patients was examined. Multiple regression analysis implicated a relationship of rs174538 within the FADS2 gene with rd.d6di. The rs174538 mutation caused a decrease in rd.d6di only in erythrocyte membrane samples. On the other hand, the mutant allele reduced AA constituent FAs in both serum and erythrocyte membranes. Furthermore, d5di induced by the FADS1 gene was also decreased in both serum and erythrocyte membrane samples due to the mutant allele. Thus, the rs174538 mutation was found to have a greater effect on d5di than rd.d6di.
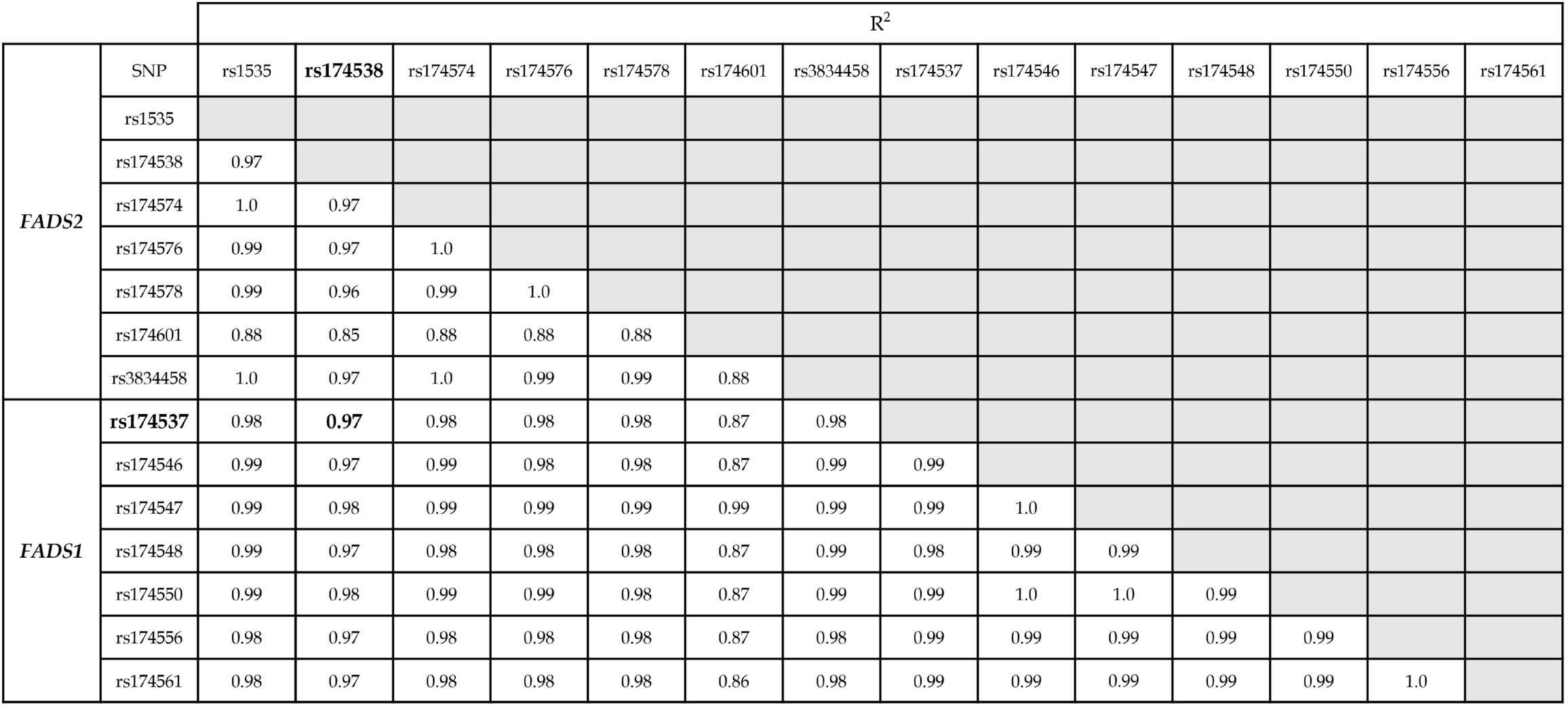
An examination of the Genotype-Tissue Expression Portal (https://www.gtexportal.org/) showed that the presence of the rs174538 mutation in whole blood results in increased activity of the FADS2 gene. Therefore, we expected that rd.d6di would increase with the presence of that mutation. However, the opposite was found, as rd.d6di was shown to be decreased in association with that mutation, indicating its greater effect on the FADS1 gene. In addition to rs174538, other mutations involved in FADS that are high in LA and low in AA in the composition of FA have been reported, including rs1535, rs174574, rs174537, and rs174546[13-17]. The Japanese Multi Omics Reference Panel (jMorp) shows rs174538 to be linked (R2 ≥ 0.85) with the examined genes (Figure 4). Among the reported genes, the present study focused on the rs174537 polymorphism related to the FADS1 gene. Previous studies have noted that rs174537 mutations lowered AA in adipose tissue, serum, and erythrocyte membranes[13-15]. The variation co-occurrence of rs174538 and rs174537 in the Japanese population noted in jMorp is R2 = 0.97, indicating that these are linked.
To confirm linkage occurrence, serum and erythrocyte membranes were examined for an association between rd.d6di and d5di with and without the rs174538 mutation. A negative correlation was observed only in regard to the mutant allele (serum: R = -0.416, P < 0.05; erythrocyte membrane: R = -0.386, P < 0.05), indicating that rs174538 and rs174537 are linked (Figure 5). Thus, the FA composition of serum and erythrocyte membranes is altered by the rs174538 mutation, and affected more by FADS1 than FADS2 due to the linked mutations.
An examination of the relationship between rs174538 and disease activity showed no significant difference related to the mutation, while a positive correlation between rd.d6di and CDAI was found only in the wild-type cases. Other than CDAI, there was no correlation of rd.d6di with CRP, Hb, ESR, or leukocytes noted. Interestingly, we expected that d5di would be shown to be involved in CD disease activity, because it decreases AA in serum and erythrocyte membranes, however, no findings indicating such involvement were noted. CD is exacerbated by inflammatory cytokines and leukotrienes synthesized from AA. Most of the CD patients enrolled in the present study were undergoing 5-ASA treatment to suppress inflammation, which may have influenced the findings regarding disease activity[18].
Factors that influence FA composition include both dietary intake and metabolic pathways. Although FAs noted in serum reflect the influence of diet consumption within the few days immediately preceding the test, those in erythrocyte membranes reflect the habitual diet of the individual over a longer period of time. Regardless, anemia produces a rapid metabolic turnover of erythrocytes, which may affect the FA fraction of erythrocyte membranes. The mean CDAI score of the CD patients in this study was 114.9 ± 90.6, and the majority of those were in remission or had mild disease. There were no cases of anemia due to active bleeding associated with worsening of CD and the effect of the disease on FA metabolism was minimal, suggesting that the evaluation was highly accurate. Taken together, the present findings indicate that the rs174538 mutation is useful to predict the severity of disease activity in CD patients.
EDA for the mutant allele was different in both serum (P < 0.05) and erythrocyte membrane (P < 0.01) samples. Since the activity of rd.d6di decreases in association with the rs174538 mutation, an increase in EDA was expected. However, there was no correlation of EDA with rd.d6d observed, indicating that EDA is metabolized to DGLA by delta 8 desaturase in addition to D6D. Furthermore, EDA was not found to contribute to disease activity in CD cases[19,20].
The present study has some limitations, including being conducted at a single institution and the low number of patients with CD analyzed. Nevertheless, few studies have reported simultaneous evaluations of FA and FA metabolism genes in erythrocyte membranes and serum obtained from CD patients. Despite the limited number of enrolled patients, the novelty of the present findings is the characteristics of genes related to FA metabolism, which were shown by analyzing background factors in CD patients treated at a Japanese institution.
The rs174538 mutation has effects on FA composition ratio in serum and red blood cell membranes, though the genetic effect of linkage is greater for d5di induced by FADS1 as compared to rd.d6di induced by FADS2. Even in wild-type group, CD disease activity was found to have a correlation with rd.d6di, suggesting that it may be useful as a predictor of disease activity as well as treatment response.
The authors would like to express our deepest appreciation to all of the CD patients and healthy controls who parti
| 1. | Shamoon M, Martin NM, O'Brien CL. Recent advances in gut Microbiota mediated therapeutic targets in inflammatory bowel diseases: Emerging modalities for future pharmacological implications. Pharmacol Res. 2019;148:104344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2254] [Article Influence: 132.6] [Reference Citation Analysis (10)] |
| 3. | Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J, Buderus S, Martín-de-Carpi J, De Ridder L, Fagerberg UL, Hugot JP, Kierkus J, Kolacek S, Koletzko S, Lionetti P, Miele E, Navas López VM, Paerregaard A, Russell RK, Serban DE, Shaoul R, Van Rheenen P, Veereman G, Weiss B, Wilson D, Dignass A, Eliakim A, Winter H, Turner D; European Crohn's and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8:1179-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 871] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 4. | Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018;64:20-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (1)] |
| 5. | Martinelli N, Girelli D, Malerba G, Guarini P, Illig T, Trabetti E, Sandri M, Friso S, Pizzolo F, Schaeffer L, Heinrich J, Pignatti PF, Corrocher R, Olivieri O. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am J Clin Nutr. 2008;88:941-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Kwak JH, Paik JK, Kim OY, Jang Y, Lee SH, Ordovas JM, Lee JH. FADS gene polymorphisms in Koreans: association with ω6 polyunsaturated fatty acids in serum phospholipids, lipid peroxides, and coronary artery disease. Atherosclerosis. 2011;214:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Uchiyama K, Nakamura M, Odahara S, Koido S, Katahira K, Shiraishi H, Ohkusa T, Fujise K, Tajiri H. N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1696-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Scaioli E, Sartini A, Bellanova M, Campieri M, Festi D, Bazzoli F, Belluzzi A. Eicosapentaenoic Acid Reduces Fecal Levels of Calprotectin and Prevents Relapse in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2018;16:1268-1275.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Merino DM, Johnston H, Clarke S, Roke K, Nielsen D, Badawi A, El-Sohemy A, Ma DW, Mutch DM. Polymorphisms in FADS1 and FADS2 alter desaturase activity in young Caucasian and Asian adults. Mol Genet Metab. 2011;103:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Ito Z, Uchiyama K, Odahara S, Takami S, Saito K, Kobayashi H, Koido S, Kubota T, Ohkusa T, Saruta M. Fatty Acids as Useful Serological Markers for Crohn's Disease. Dig Dis. 2018;36:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Motoi Y, Ito Z, Suzuki S, Takami S, Matsuo K, Sato M, Ota Y, Tsuruta M, Kojima M, Noguchi M, Uchiyama K, Kubota T. FADS2 and ELOVL6 mutation frequencies in Japanese Crohn's disease patients. Drug Discov Ther. 2019;13:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Ueno F, Matsui T, Matsumoto T, Matsuoka K, Watanabe M, Hibi T; Guidelines Project Group of the Research Group of Intractable Inflammatory Bowel Disease subsidized by the Ministry of Health, Labour and Welfare of Japan and the Guidelines Committee of the Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for Crohn's disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013;48:31-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Klingel SL, Valsesia A, Astrup A, Kunesova M, Saris WHM, Langin D, Viguerie N, Mutch DM. FADS1 genotype is distinguished by human subcutaneous adipose tissue fatty acids, but not inflammatory gene expression. Int J Obes (Lond). 2019;43:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Al-Hilal M, Alsaleh A, Maniou Z, Lewis FJ, Hall WL, Sanders TA, O'Dell SD; MARINA study team. Genetic variation at the FADS1-FADS2 gene locus influences delta-5 desaturase activity and LC-PUFA proportions after fish oil supplement. J Lipid Res. 2013;54:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D, Tsai MY, Ferrucci L. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009;5:e1000338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Schuchardt JP, Köbe T, Witte V, Willers J, Gingrich A, Tesky V, Pantel J, Rujescu D, Illig T, Flöel A, Hahn A. Genetic Variants of the FADS Gene Cluster Are Associated with Erythrocyte Membrane LC PUFA Levels in Patients with Mild Cognitive Impairment. J Nutr Health Aging. 2016;20:611-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Rabehl M, Wei Z, Leineweber CG, Enssle J, Rothe M, Jung A, Schmöcker C, Elbelt U, Weylandt KH, Pietzner A. Effect of FADS1 SNPs rs174546, rs174547 and rs174550 on blood fatty acid profiles and plasma free oxylipins. Front Nutr. 2024;11:1356986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Wiese DM, Horst SN, Brown CT, Allaman MM, Hodges ME, Slaughter JC, Druce JP, Beaulieu DB, Schwartz DA, Wilson KT, Coburn LA. Serum Fatty Acids Are Correlated with Inflammatory Cytokines in Ulcerative Colitis. PLoS One. 2016;11:e0156387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Lee JM, Lee H, Kang S, Park WJ. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients. 2016;8:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 20. | Schaeffer L, Gohlke H, Müller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006;15:1745-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 415] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/