Published online Jan 7, 2026. doi: 10.3748/wjg.v32.i1.112496
Revised: September 9, 2025
Accepted: November 11, 2025
Published online: January 7, 2026
Processing time: 160 Days and 15.2 Hours
The etiopathogenesis of gastrointestinal diseases is varied in nature. Various etio
Core Tip: With advancements in genetics, there are emerging trends in better understanding of diseases and diagnosis of many undiagnosed gastrointestinal disorders. This aids in the search for newer medicines, which are pivotal to the progress of precision medicine. Genetic analysis enables accurate diagnosis, risk stratification, and individualized treatment by identifying germline mutations, somatic alterations, and epigenetic changes. It also plays a crucial role in predicting treatment response and guiding targeted therapies. Gene therapy, gene editing, and clustered regularly interspaced short palindromic repeats -associated protein systems represent promising tools for managing many complex gastrointestinal disorders and also are an aid to the conventional treatment and has a very promising future.
- Citation: Kumar A, Sarangi Y, Kaw P. Gene, genetics and genetic medicines in gastroenterology: Current status and its future. World J Gastroenterol 2026; 32(1): 112496
- URL: https://www.wjgnet.com/1007-9327/full/v32/i1/112496.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i1.112496
Advances in molecular biology and genetics have revolutionized our understanding of health and disease across medical disciplines, and gastroenterology is no exception. Many gastrointestinal (GI) disorders are directly or indirectly associated with genetic influences; some are known, and many are unknown. From inherited syndromes like Lynch syndrome and familial adenomatous polyposis (FAP) to complex disorders such as inflammatory bowel disease (IBD) and colorectal cancer (CRC), genetics plays a pivotal role in the initiation, progression, and response to treatment[1-4]. Now we are in the era of precision medicine, which tailors’ disease prevention, diagnosis, and treatment to the individual characteristics of each patient. Unlike the traditional “one-size-fits-all” model, precision medicine aims to deliver the right treatment to the right person at the right time, thereby improving outcomes and minimizing unnecessary interventions[5]. The precision medicine model involves large databases of diseases of various etiological factors including genetics, multi-omics, environmental and social factors which are critically analyzed with the use of artificial intelligence (AI) and forms the basis of preventive, diagnostic and therapeutic medicine. Genetic medicine includes genetic testing, molecular diagnostics, pharmacogenomics and gene therapy, which are currently used in many diseases, including gastroenterological disorders. However, the progress also possesses several challenges, including ethical concerns, high costs, and limited equitable access for the general population. This article explores the current applications of genes, genetics, and genetic medicines in gastroenterology, and also how the future innovations are likely to shape these.
A comprehensive literature search was conducted using PubMed/MEDLINE databases using the search terms genes, genetics, precision medicine, omics, genetic testing, pharmacogenomics, targeted therapy, gene therapy, gene editing, and bullion operators like “and, or, and not”. We included only those publications relevant to disorders of gastro GI tract (GIT). Secondary sources retrieved from these publications were identified through manual searches and assessed for relevance. The results are discussed in detail.
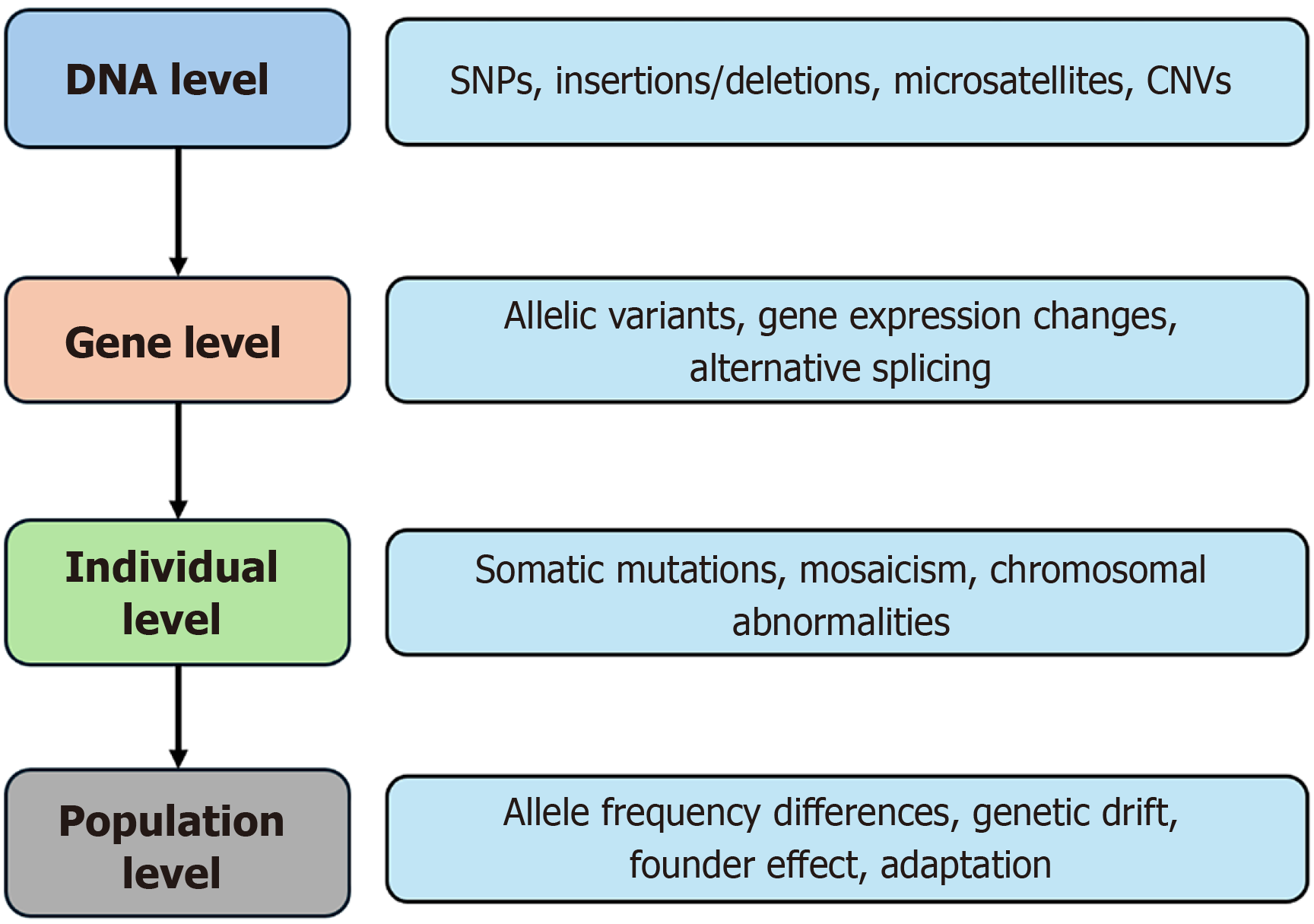
Genetic alterations in GIT disorders can be broadly classified into germline mutations, somatic mutations, and epigenetic changes. Germline mutations are inherited and present in the egg or sperm, thus passed on to offspring. Somatic mutations, on the other hand, are acquired genetic alterations that occur after conception in non-germline cells and are not inherited. These mutations commonly contribute to the pathogenesis of sporadic GI cancers and other non-hereditary GIT disorders. Epigenetic changes, including DNA methylation, histone modifications, and regulation by non-coding RNAs, alter gene expression without modifying the DNA sequence and play a significant role in both benign and malignant GIT conditions. Epigenetics refers to heritable changes in gene expression that do not involve changes to the DNA sequence itself. These modifications regulate when, where, and how much a gene is expressed, and are frequently influenced by environmental factors, aging, disease processes, or developmental stages, playing a significant role in both benign and malignant conditions of the GIT. Different types of epigenetic changes with examples are described in Table 1[6-9]. Genetic variation occurs at multiple levels. It can be observed at the DNA level (e.g., single nucleotide poly
| Mechanism | How it works | Effect on genes with example |
| DNA methylation[6] | Addition of methyl groups | MLH1 silenced: Leads to microsatellite instability increased mutation rate |
| Histone modification[7] | Acetylation, methylation, phosphorylation of histone proteins | CDH1 (E-cadherin) silenced by H3 acetylation in promoter regions of cytokine genes (e.g., TNF-α) leads to increased transcription |
| Chromatin remodeling[8] | Changing the physical structure of chromatin | Loss of ARID1A failure of chromatin remodeling improper gene silencing or activation. Progression of HCC, CRC |
| Non-coding RNAs (e.g., miRNA, lncRNA)[9] | Bind to mRNA or DNA to regulate expression | CRC (miR-21, miR-135b, lncRNA HOTAIR); gastric cancer (miR-148a, miR-21, lncRNA MALAT1, circPVT1); inflammatory bowel disease (miR-155, miR-21, lncRNA IFNG-AS1); HCC (miR-122, miR-221/222, lncRNA HULC); celiac disease (miR-449a) |
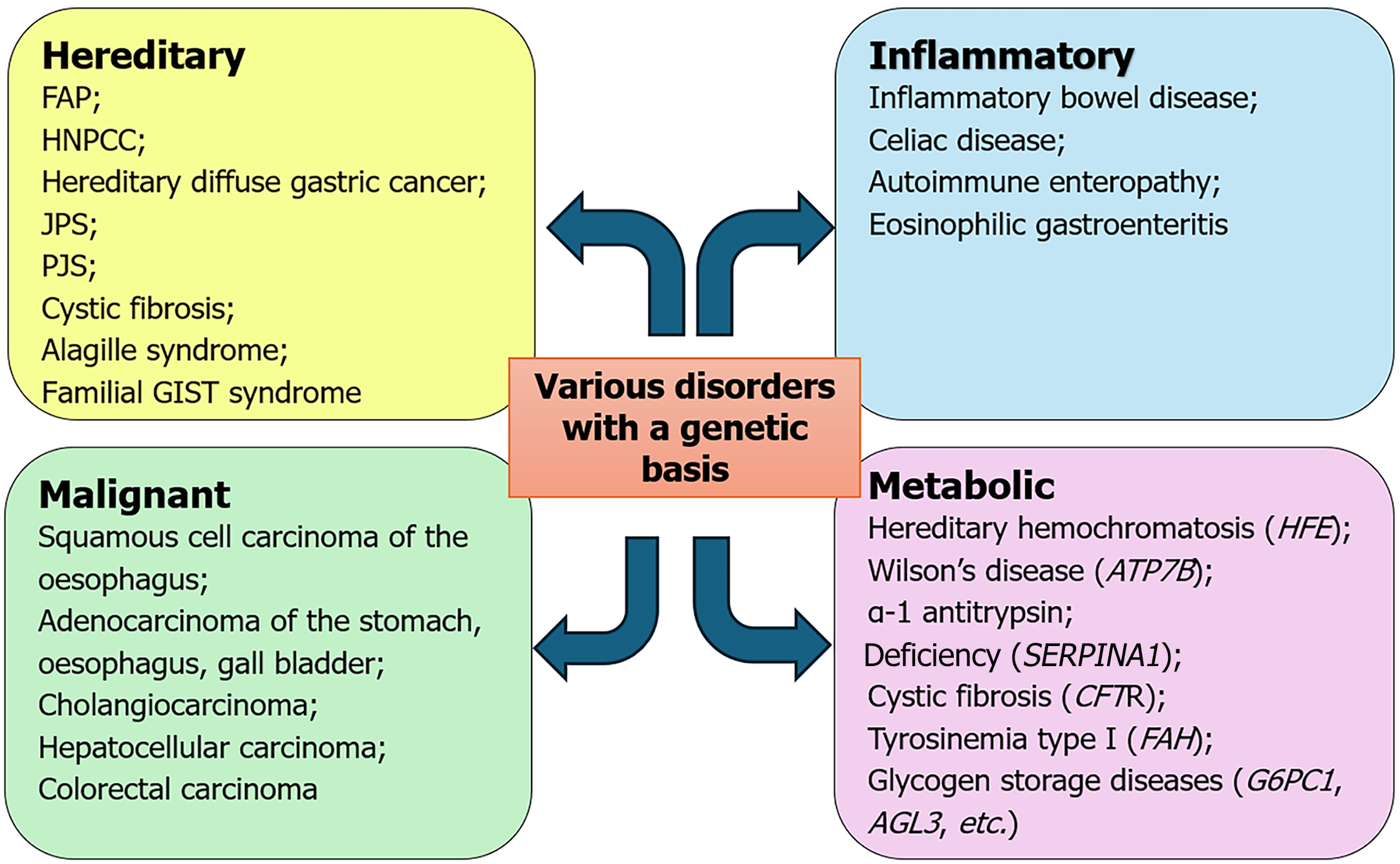
Another form of genetic variation is mosaicism, which occurs when a postzygotic genetic variant exists in only a portion of the body’s cells, meaning two or more genetically distinct cell populations within the same individual, all originating from a single zygote. Similarly, genetic disorders can be classified as monogenic, caused by mutations in a single gene, while others are polygenic, involving complex interactions among multiple genes (Table 2). Disorders of the GIT can be classified as hereditary, inflammatory, malignant and metabolic (Figure 2).
| Genetic pathway disorder | Mechanism | Examples |
| Monogenic disorders | Mutations in a single gene that often follow Mendelian inheritance patterns | Hereditary hemochromatosis (HFE gene), Wilson disease (ATP7B gene), alpha-1 antitrypsin deficiency |
| Polygenic and multifactorial disorders | Involve multiple genes and environmental interactions | Inflammatory bowel disease (over 200 loci have been identified), celiac disease (HLA-DQ2 and HLA-DQ8) |
| Cancer predisposition syndromes | Inherited mutations in tumor suppressor genes or DNA repair genes increase GI cancer risk | Lynch syndrome (HNPCC) (MLH1, MSH2), familial adenomatous polyposis (APC) gene |
| Mosaicism | Two or more genetically distinct cell populations within the same individual, derived from a single zygote | Mosaic APC gene mutations may cause attenuated forms of FAP. Very early changes in IBD |
Numerous genes implicated in these disorders are involved in key biological processes such as DNA repair, cell adhesion, maintenance of structural integrity, bile acid synthesis and transport, immune regulation, mucosal barrier function and nutrient absorption. Table 3[10-16] provides a functional classification of these genes and highlights their specific associations with various GI disorders, helping to illustrate the complexity and diversity of the genetic contributions to GIT pathology.
| Genes | Function | Associated disorders |
| Genes involved in DNA repair and genomic stability[10] | ||
| MLH1, MSH2, MSH6, PMS2, EPCAM | Mismatch repair (MMR) system | Lynch syndrome colorectal, gastric, pancreatic cancer |
| MUTYH | Base excision repair | MUTYH-associated polyposis |
| BRCA1/BRCA2, ATM, PALB2 | Double-strand break repair | Familial pancreatic and gastric cancers |
| TP53 | Tumor suppressor, DNA damage response | CRC, gastric, pancreatic, hepatocellular carcinoma |
| Genes involved in cell adhesion and structural integrity[11] | ||
| CDH1 | E-cadherin (cell-cell adhesion) | Hereditary diffuse gastric cancer |
| CTNNA1 | Catenin alpha-1 (adherens junctions) | HDGC |
| SMAD4, BMPR1A | TGF-β pathway mediators | Juvenile polyposis syndrome, pancreatic cancer |
| Genes regulating inflammation and immune response[12] | ||
| NOD2 | Innate immunity, bacterial sensing | Crohn’s disease |
| IL23R, IL10, IL12B | Cytokine signaling | IBD susceptibility |
| IRGM, ATG16 L1 | Autophagy genes | Crohn’s disease |
| HLA-DQA1/HLA-DQB1 | Antigen presentation | Celiac disease |
| TLR4, TLR9 | Pattern recognition receptors | Functional dyspepsia, IBD |
| Genes involved in bile acid transport and cholestasis[13] | ||
| ABCB11 | Bile salt export pump | PFIC2, BRIC |
| ABCC2 (MRP2) | Bile excretion | Dubin-Johnson syndrome |
| ATP8B1 | Phospholipid transporter | PFIC1 |
| TJP2 | Tight junction protein | PFIC4 |
| Genes in neuronal/gut motility and enteric nervous system[14] | ||
| RET, EDNRB, GDNF | ENS development | Hirschsprung’s disease |
| SCN5A | Sodium channel in ICCs/ENS | IBS with constipation |
| NEUROG3 | Enteroendocrine differentiation | Congenital malabsorptive diarrhoea |
| Genes affecting nutrient absorption and metabolism[15] | ||
| LCT | Lactase enzyme | Lactose intolerance |
| SAR1B | Chylomicron transport | Chylomicron retention disease |
| SLC26A3 | Cl-/HCO3- exchange | Congenital chloride diarrhea |
| SLC5A1 (SGLT1) | Glucose transport | Glucose-galactose malabsorption |
| Genes in oncogenic signaling and growth factors[15] | ||
| KRAS, NRAS | MAPK signaling | CRC, pancreatic cancer |
| BRAF | Downstream of KRAS | CRC (BRAF V600E in MSI tumors) |
| PIK3CA | PI3K/AKT pathway | CRC, gastric cancer |
| EGFR, HER2 (ERBB2) | Receptor tyrosine kinases | Gastric, colorectal cancers |
| FGFR2, IDH1/IDH2 | Growth factor pathways | Cholangiocarcinoma |
| Genes related to epigenetic and transcriptional regulation[16] | ||
| ARID1A | Chromatin remodeling | Biliary cancer, CRC, gastric |
| MLH3, MSH3 | Mismatch repair (minor MMR genes) | Polyposis syndromes |
| TET2, DNMT3A | DNA methylation regulation | CRC and inflammatory epigenetic signatures |
Advances in genomics have elucidated several critical pathways that contribute to the pathogenesis, progression, and treatment response of these disorders. Chronic inflammation in IBD can culminate in colitis-associated CRC through sequential genetic alterations involving TP53, chromosomal instability, microsatellite instability, and promoter hy
| Pathway | Key genes | Associated disorders | Mechanism/role | Ref. |
| Wnt/β-catenin | APC, CTNNB1, AXIN2 | Colorectal cancer, hepatocellular carcinoma (HCC), familial adenomatous polyposis | Controls cell proliferation and differentiation; mutation leads to uncontrolled growth | Li et al[17] |
| NF-κB | NFKB1, TNFAIP3, IKK complex | IBD (Crohn’s, UC), gastric cancer, colorectal cancer | Regulates inflammation, cell survival, immunity; chronic activation promotes inflammation and tumorigenesis | Peng et al[18] |
| TGF-β/SMAD | TGFBR2, SMAD4 | Juvenile polyposis, CRC, pancreatic cancer | Controls growth inhibition and apoptosis; mutations cause evasion of tumor suppression | Hata and Chen[19] |
| JAK/STAT | JAK2, STAT3, STAT1 | IBD, colitis-associated cancer | Regulates immune cell signaling and cytokine responses | Hu et al[20] |
| MAPK/ERK | KRAS, BRAF, EGFR | CRC, pancreatic cancer, gastric cancer | Regulates cell proliferation and survival; mutations oncogenic signaling | Guo et al[21] |
| PI3K/AKT/mTOR | PIK3CA, PTEN, AKT1, MTOR | CRC, gastric cancer, IBD | Promotes cell growth, metabolism, and angiogenesis; dysregulation contributes to tumor growth and inflammation | Glaviano et al[22] |
| Mismatch repair | MLH1, MSH2, MSH6, PMS2 | Lynch syndrome, CRC, gastric cancer | Repairs DNA replication errors; loss leads to microsatellite instability (MSI) | Li[23] |
| P53 pathway | TP53 | CRC, esophageal, gastric, HCC | Controls cell cycle arrest, apoptosis, DNA repair; mutations common in late cancer stages | Harris and Levine[24] |
| Hedgehog signaling | PTCH1, GLI1 | Gastric cancer, GI developmental disorders | Controls tissue patterning and stem cell maintenance | Briscoe and Thérond[25] |
| Notch signaling | NOTCH1, DLL1, HES1 | Colitis, CRC, esophageal cancer | Regulates differentiation, especially goblet cells; dysregulation affects intestinal homeostasis | Kopan[26] |
| Autophagy pathway | ATG16 L1, IRGM | Crohn’s disease, IBD-associated cancer | Maintains intracellular bacterial clearance and mucosal homeostasis | Yu et al[27] |
| Immune checkpoint pathway | PD-L1, CTLA4 | MSI-high CRC, gastric cancer, IBD | Immune evasion in cancer; dysregulated tolerance in autoimmune GI diseases | He and Xu[28] |
| ER stress/UPR | XBP1, IRE1, PERK | IBD, Paneth cell dysfunction, CRC | Regulates response to unfolded proteins; chronic ER stress leads to inflammation and epithelial damage | Chen et al[29] |
| IL-23/Th17 pathway | IL23R, STAT3, RORC | Crohn’s disease, UC, CRC | Inflammatory cytokine signaling driving chronic inflammation | Bunte and Beikler[30] |
| Apoptosis/FAS-FASL | FAS, BAX, CASP8 | Colitis-associated cancer, gastric cancer | Regulates programmed cell death; evasion supports tumor survival | Waring and Müllbacher[31] |
| DNA repair pathways (base/nucleotide excision) | OGG1, XPA, POLB | CRC, gastric cancer | Repair oxidative and chemical DNA damage; defects genomic instability | Kumar et al[32] |
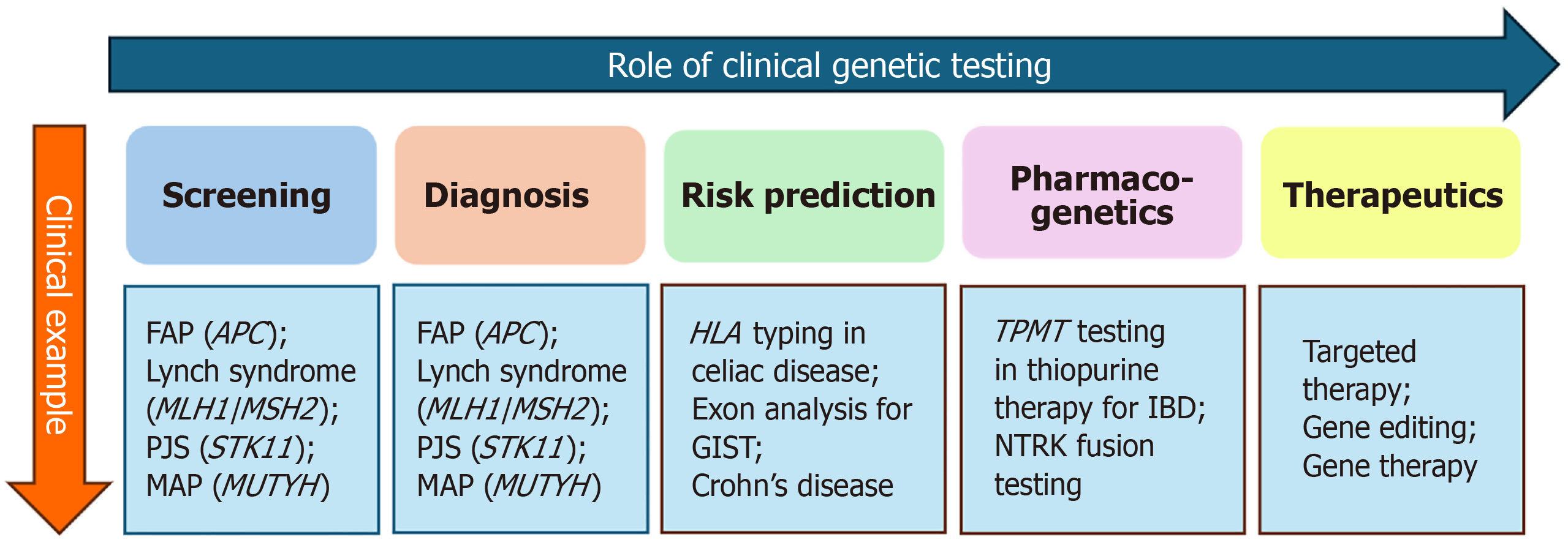
Clinical genetic testing plays a pivotal role in the screening, diagnosis, prognostication, and prediction of GI and hepatopancreatic biliary (HPB) disorders (Figure 3).
Clinical genetic testing can be classified based on the origin of the mutation as well as the clinical purpose of the test (Table 5). Based on origin, tests are categorized into germline variation testing, which detects inherited mutations present in every cell of the body, somatic variation testing, which identifies acquired mutations confined to specific tissues (commonly in tumors) and mosaicism testing, which detects mosaicism in both inherited and acquired diseases like FAP and IBD[33]. Genetic testing can be categorized by purpose into various types: Diagnosis, prognostication, prediction of treatment response, and guiding targeted therapy.
| Classification | Type | Purpose |
| Mutation origin | Germline testing | Detects inherited mutations; used for familial risk, carrier status, and predisposition |
| Somatic testing | Identifies acquired mutations in specific tissues (e.g., tumors); guides cancer therapy | |
| Mosaicism testing | Identify mosaicism in FAP, IBD | |
| Clinical purpose | Diagnostic testing | Confirms or rules out a specific genetic disorder in symptomatic individuals |
| Prognostic testing | Predicts disease course, severity, or likelihood of complications | |
| Predictive/screening | Identifies asymptomatic individuals at risk of developing a genetic disorder | |
| Carrier testing | Identifies individuals who carry one copy of a gene mutation (relevant for recessive conditions) | |
| Pharmacogenetic testing | Assesses genetic variants affecting drug metabolism and response | |
| Somatic/tumor profiling | Detects actionable mutations in cancer cells to guide targeted therapy and prognosis | |
| Newborn screening | Early identification of treatable genetic disorders in neonates |
Germline genetic testing involves the analysis of inherited DNA changes that are present in every cell of the body. These mutations are passed from parent to offspring and can predispose individuals to a range of hereditary conditions, including cancers, metabolic disorders, and autoimmune diseases, particularly those affecting the GI and HPB systems. Germline testing is typically performed using DNA extracted from saliva, blood, or buccal swabs and targets mutations that are present in all somatic and germ cells. It is especially valuable when evaluating individuals at a young age or during childhood, where early detection has significant clinical implications. Techniques such as sanger sequencing, next-generation sequencing (NGS), and multiplex gene panels are commonly used for this purpose. Germline testing plays a critical role in confirming diagnoses, guiding screening strategies, prognostication and informing cascade testing in families. It is also instrumental in identifying at-risk offspring, optimizing pharmacogenetic decisions, and supporting reproductive planning in affected families. Many genetic tests currently available for screening are summarized in Table 6.
| No. | GI disorder/syndrome | Guideline source | Genes recommended for testing | Testing criteria |
| 1 | Lynch syndrome (hereditary nonpolyposis colorectal cancer) | ACG, NCCN, ESMO | MLH1, MSH2, MSH6, PMS2, EPCAM | Personal/family history of colorectal, endometrial, or other LS-associated cancers; tumor MSI or IHC abnormality |
| 2 | Familial adenomatous polyposis (FAP) | ACG, NCCN | APC | > 100 colorectal adenomas or family history of FAP |
| 3 | Attenuated FAP | ACG | APC | Patients with 10-99 adenomas |
| 4 | MUTYH-associated polyposis | ACG | MUTYH (biallelic) | Multiple adenomas and autosomal recessive inheritance |
| 5 | Peutz-Jeghers syndrome | NCCN, ESMO | STK11 | Mucocutaneous pigmentation and hamartomatous polyps; family history |
| 6 | Juvenile polyposis syndrome | ACG, NCCN | SMAD4, BMPR1A | ≥ 5 juvenile polyps or family history |
| 7 | Cowden syndrome/PTEN hamartoma tumor syndrome | NCCN | PTEN | GI polyps with mucocutaneous lesions or macrocephaly |
| 8 | Hereditary pancreatic cancer | NCCN | BRCA1/BRCA2, PALB2, ATM, CDKN2A, STK11 | Family history of pancreatic cancer or known mutation |
| 9 | Hereditary diffuse gastric cancer | NCCN | CDH1 | Family history of diffuse gastric cancer or lobular breast cancer |
| 10 | Serrated polyposis syndrome | WHO, ACG | No known high-penetrance genes; RNF43 under investigation | Multiple serrated polyps meeting WHO criteria |
Somatic genetic testing has emerged as a cornerstone of precision medicine, particularly in oncology, by detecting non-inherited, tissue-specific genetic alterations acquired during a person’s lifetime. These somatic mutations distinct from germline mutations are most commonly found in tumors and are instrumental in guiding diagnosis, prognosis, and therapy selection. While somatic testing is predominantly used in malignant conditions, its utility is expanding into non-malignant, complex diseases. Disorders such as Celiac disease and Crohn’s disease, although primarily immune-mediated and polygenic in origin, can exhibit somatic epigenetic changes and mucosal genetic alterations that influence disease progression, therapeutic response, and relapse risk.
Genetic testing involves the analysis of DNA, or in some cases RNA transcribed from DNA, to identify variations associated with disease or an increased risk of disease. The foundation of genetic testing lies in the concept of genetic variation, which can occur at multiple levels from large chromosomal abnormalities to single-nucleotide changes.
Broadly, clinical genetic testing can be classified into two main categories: Cytogenetic testing and molecular genetic testing. Cytogenetics is a specialized branch of genetic analysis focused on examining the structure and number of chromosomes within cells to detect large-scale genetic abnormalities. Cytogenetic testing is used to identify chromosomal changes such as aneuploidy (abnormal number of chromosomes), translocations (exchange of segments between chromosomes), deletions (loss of chromosome segments), duplications (gain of additional segments), and inversions (reversal of a chromosome segment). Molecular genetic testing involves the analysis of DNA or RNA at the molecular level to identify small-scale genetic variations, such as single-nucleotide changes, insertions, deletions, or duplications that may be associated with disease. Unlike cytogenetic testing, which detects large chromosomal alterations, molecular genetic testing focuses on gene-level mutations that may not be visible under a microscope, like specific genes, exons, introns, or nucleotide sequences. In Table 7, we have described various cytogenetic and molecular genetic testing with their clinical utility. Various molecular genetic tests are sanger sequencing, NGS, etc. Sanger sequencing, also known as the chain termination method, is a technique for DNA sequencing based upon the selective incorporation of chain-terminating dideoxynucleotides by DNA polymerase during in vitro DNA replication. NGS is a high-throughput technology that enables the simultaneous analysis of multiple DNA fragments within a single sample. NGS technologies include whole-genome sequencing, targeted panel sequencing, RNA sequencing, single-cell sequencing, duplex sequencing, reduced-representation sequencing, and long-read sequencing. Various platforms are used in NGS. The most common are 454/Roche, Illumina (MiSeq/HiSeq/NextSeq), SOLiD, and Ion Torrent. Panel-based testing eliminates the time and financial strain required to sequence several individual genes until the right gene is identified. Panel-based testing is also especially helpful when any of several genes may be known to cause a specific disorder[34]. Table 7[35-55] details the various genetic testing methods.
| Test | Detects | Clinical use | Benefit | Limitation | Ref. |
| Cytogenetic testing | |||||
| Karyotyping (conventional cytogenetics) | Detects large chromosomal abnormalities: Trisomies, translocations, deletions, G-banding of metaphase chromosomes | Down syndrome, Turner syndrome | Whole-genome overview, identifies balanced/unbalanced rearrangements | Low resolution, cannot detect small deletions/duplications, requires dividing cells | Genetic Alliance[35] |
| Fluorescence in situ hybridization (FISH) | Fluorescent probes bind specific DNA sequences on chromosomes | Detects gene amplifications, deletions, rearrangements (e.g., HER2 in gastric cancer, ALK in GI stromal tumors) | Rapid, targeted, works on interphase cells | Limited to known targets, one probe/test, cannot assess whole genome | Yilmaz and Demiray[36] |
| Comparative genomic hybridization (aCGH) | DNA from patient and control hybridized to a microarray | Detects copy number variations (e.g., deletions in polyposis syndromes, microdeletion syndromes | High-resolution, genome-wide, detects sub microscopic CNV | Cannot detect balanced rearrangements (e.g., translocations), limited to CNVs only | Weiss et al[37] |
| Chromosomal microarray analysis | aCGH + SNP array | Used in syndromic GI diseases, unexplained developmental delay, congenital anomalies | Genome-wide, detects CNVs, uniparental disomy, mosaicism | Cannot detect balanced rearrangements, may report VUS | Myllykangas et al[38] |
| Spectral karyotyping | Whole chromosome painting with multicolor FISH | Identifies complex chromosomal rearrangements, often in cancers | Detects complex karyotypes, color-coded analysis | Expensive, not used for routine diagnostics, lower resolution than aCGH | Guo et al[39] |
| Molecular genetic testing | |||||
| Sanger sequencing | SNV, small insertions/deletions | Confirmatory testing (e.g., known APC, MLH1 mutations | High accuracy for point mutation or small deletion/duplication/SNV, cost effective for single genetic testing | Only identify small subset of gene or single gene, not precisely quantifiable | Herpich et al[40] |
| NGS | Panel, exome, or genome-wide variants | Multigene panels for IBD, polyposis, CRC, gastric cancer, GIST | Multiple, individually produced readings of the target area mosaism, quantitative, whole exome or genome sequencing | Limited in their ability to detect copy number variations, incidental findings need to be verified by sanger sequencing | Satam et al[41] |
| Targeted gene panels | Focused sequencing of disease-specific genes | Panel specific to GIST, IBD, hereditary colorectal cancer panel, gist panel | Accurate diagnosis focus on specific genes cost-effective and efficient: Can be customized according to disorder | Limited coverage not detect structural rearrangements or copy number variants cannot identify novel or new gene related to disease | Málaga et al[42] |
| Whole exome sequencing | All coding regions | Early-onset or monogenic IBD, congenital diarrheal disorders (e.g., DGAT1, EPCAM mutations). Hereditary pancreatitis (e.g., PRSS1, SPINK1) colorectal cancer | Cost-effective WES allows deeper sequencies WES captures approximately 85% of known disease-causing mutations | Misses non-coding variants incomplete exome coverage | Rabbani et al[43]; Uhlig et a[[44] |
| WGS | Coding and non-coding genome variant | Identification of colorectal cancer genes. Undiagnosed complex disease | Cover both coding and non-coding reason detection of structural variant both germline and somatic mutation | High cost difficult to pathogenic variant from benign variant | de Voer et al[45] |
| MLPA | Large deletions/duplications | Detects large deletions, especially EPCAM deletions causing MSH2 inactivation | Efficient CNV detection cost-effective and high throughput applicable on degraded DNA | Cannot detect point mutations or small indels limited to pre-designed probes | Kuiper et al[46]; Schouten et al[47] |
| qPCR | Copy number variations or known mutations | Rapid screening for common mutations, detects bacterial, viral, and parasitic DNA/RNA rapidly and accurately, bacterial load determination in gastro intestinal disorder | High sensitivity and specificity, rapid turnaround, quantitative | Requires prior sequence knowledge | Shah et al[48]; Bamias et al[49] |
| Array comparative genomic hybridization (aCGH) | Sub microscopic deletions/duplications, germline CNVs in genes like APC, SMAD4, and BMPR1A | Genome-wide coverage, germline CNVs in genes like APC, SMAD4 and BMPR1A | High resolution can detect CNVs as small as 50-100 kb | Inability to detect balanced chromosomal rearrangements difficulties in interpreting CNVs of uncertain significance | McKay et al[50]; Assämäki et al[51] |
| HLA typing (PCR-SSP, NGS-based) | HLA allele identification | Celiac disease, IBD pharmacogenetics IBD, primary sclerosing cholangitis drug-induced GI injury, idiosyncratic reactions to drugs causing hepatic/GI damage. Transplant compatibility | Cost-effective, simple requires minimal computational support | Limited resolution may not differentiate similar alleles. May yield ambiguous results | Megiorni and Pizzuti[52] |
| FISH | Large chromosomal rearrangements, gene fusions | In Barretts esophagus identifies chromosomal instability (e.g., 20q gain, 18q loss), and BRAF rearrangements; detection of HER2 gene amplification (ERBB2 at 17q12) predicts response to trastuzumab therapy (gastric cancer) | High specificity and sensitivity for targeted chromosomal regions | Targeted approach only. Limited genomic coverage | Brankley et al[53] |
| PCR | Specific known mutations | Quick detection (e.g., PRSS1 in hereditary pancreatitis), KRAS in CRC | High sensitivity and specificity can detect minute amounts of target DNA/RNA. Rapid turnaround time. Typically, within a few hours. Quantitative provides absolute or relative quantification | Requires prior sequence knowledge. Primers must be designed for specific known targets. Cannot differentiate live from dead organisms, detects DNA from both | Tol et al[54] |
| RNA-seq | Gene expression, fusion transcripts | Detects tumor-specific expression changes, fusion transcripts (e.g., NTRK fusions), and provides prognostic biomarkers in CRC reveals deregulated pathways (e.g., WNT, PI3K), tumor microenvironment features, and therapeutic target molecular marker of pancreatic cancer | Unbiased and comprehensive: Captures all RNA species (mRNA, lncRNA, miRNA, circular RNA), high resolution. Detects single-nucleotide changes, splicing variants, and gene fusions | Expensive and resource-intensive, requires advanced sequencing and computational infrastructure, data analysis is complex, needs bioinformatics expertise and robust pipelines | Bailey et al[55] |
Apart from its role in screening and diagnosis of various disorders, genetic testing also assists clinicians in prognostication, prediction of therapeutic response, and decision-making regarding targeted therapy. In this section, we describe the role of genetic alterations in prognostication, prediction, pharmacogenetics and targeted therapy treatment of different GI disorders.
In the predictive setting, genetic testing helps categorize individuals based on their inherited risk of developing diseases like cancer, IBD, and other conditions. In the prognostic field, genetic profiling especially in oncology offers information on disease severity, likely progression, and response to treatment. For example, mutations in BRCA1/BRCA2 not only indicate increased risk for breast and ovarian cancers but also influence treatment options and long-term outcomes. Similarly, tumor genomic profiling detects biomarkers linked to recurrence risk, treatment resistance, and overall survival. Table 8[56-89] describes the various genes that help in the prediction of phenotypes of disease and the prognostication of various disorders.
| Disease | Prediction and prognostication | Genes | Ref. |
| FAP | Profuse polyposis | APC codon 1250-1464, 1250-1311, 1309-1324 | Nagase et al[56]; Enomoto et al[57]; Ficari et al[58]; Walon et al[59]; Gebert et al[60] |
| Desmoid tumors | APC codon 1924, 1962, 1444-1560, 1403-1987 | Caspari et al[61] | |
| Upper gastrointestinal polyps | 1445-1578 | Davies et al[62] | |
| Gastric adenomas | 1403-1987 | Caspari et al[61] | |
| Multiple extracolonic manifestations | 3’14451995, 3’1403 | Caspari et al[61] | |
| CHRPE | 311-1444, 413-1387, 542-1309 | Caspari et al[61] | |
| Crohn’s disease | Stenotic/structuring behavior | NOD2, TLR4, IL-12B, CX3CR1, IL-10, IL-6 | Tsianos et al[63] |
| Penetrating/fistulizing behavior | NOD2, IRGM, TNF, HLADRB1, CDKAL1 | Tsianos et al[63] | |
| Inflammatory behavior | HLA | Tsianos et al[63] | |
| Granulomatous disease | TLR4/CARD15 | Tsianos et al[63] | |
| Upper gastrointestinal | NOD2, MIF | Tsianos et al[63] | |
| Ileal | IL-10, CRP, NOD2, ZNF365, STAT3 | Tsianos et al[63] | |
| Ileocolonic | ATG16 L1, TCF-4 (TCF7 L2) | Tsianos et al[63] | |
| Colonic | HLA, TLR4, TLR1, TLR2, TLR6 | ||
| Crohn’s disease activity | HSP70-2, NOD2, PAI-1, CNR1 | Tsianos et al[63] | |
| Surgery | NOD2, HLA-G | Tsianos et al[63] | |
| Dysplasia and cancer | FHIT | ||
| Extraintestinal manifestations | CARD15, FcRL3, HLADRB103 | Tsianos et al[63] | |
| Ulcerative colitis | |||
| Extensive colitis and increased colectomy risk | HLA-DRB1 alleles, CASP9 gene on 1p36, ATG16 L1 T300A | Nam et al[64] | |
| May influence severity and steroid dependence | IL23R, STAT3, HSP70-2, MDR1 | Nam et al[64] | |
| Early response to infliximab | IL23R higher gene expression IL-17A and IFN-γ | Jürgens et al[65]; Rismo et al[66] | |
| Good response to therapy | TNF ALPHA expression | Olsen et al[67] | |
| Non response to infliximab | PR3-ANCA | Yoshida et al[68] | |
| Favorable response to treatment | FCGR3A, TNFRSF1A, IL-6, and IL-1B | ||
| Failure of steroid therapy | MDR1 (ABCB1), TNFα (-308/-857 SNPs), HLA-DQA1 05/DRB1, NOD2, ATG16 L1, IL13RA2, IL6, IL11, TNFAIP6 | ||
| Unfavorable response to therapy (IBD) | TLR2 and TLR9 show a negative correlation | Sazonovs et al[69] | |
| Development of ADA against infliximab and adalimumab | HLA-DQA1 05 | Sazonovs et al[69] | |
| Development of ADA against infliximab | HLA-DRB1 | ||
| Celiac disease | |||
| Increase severity of disease | DQA1 05 and DQB1 02, homozygous for DQ2.5 haplotype, second copy of the DQB1 0201 | Murray et al[70]; Stanković et al[71] | |
| Hereditary pancreatitis | Increased risk of disease | PRSS1 pathogenic variants include p.Asn29Ile and p.Arg122His, p.Asn29Ile and p.Arg122His | Avanthi et al[72]; Whitcomb[73] |
| Increased severity and early onset of disease | SPINK1, c.101A>G p.Asn34Ser and SPINK1, c.56-37T>C | Abass et al[74] | |
| GIST | |||
| Increase severity and relapse | Exon 11, 13, 17, c-KIT mutation; SDH deficient, BRAF mutation | Zhang and Liu[75] | |
| Colorectal cancer | Increased severity and predict recurrence | P53, KRAS codon 12, loss of 18q | Andreyev et al[76]; Walther et al[77] |
| HCC | Increased severity | EZH2, STAT3, YB-1, ANLN, NLRC5 | |
| Poor prognosis | Overexpression of CDCA5 | Wang and Lai[78]; Hashemi et al[79]; Svinka et al[80]; Chao et al[81]; Jia et al[82]; Peng et al[83] | |
| Overexpression of CDCA5 | Tian et al[84] | ||
| Gall bladder cancer | Increased severity of disease | SERPINB5 (maspin) KRAS, E-cadherin/beta-catenin, PML, P53, CDKN21 loss | Kim et al[85]; Hirata et al[86]; Chang et al[87] |
| Intra hepatic cholangiocarcinoma | Increased severity and large tumor size | BRAF | Xin et al[88] |
| Pancreatic cancer | Poor prognosis | KRAS (G12D/G12V/G12R), CDKN2A (p16), SMAD4 (DPC4) | Zhou et al[89] |
Pharmacogenetics is a field that examines how genetic variations influence an individual’s response to pharmacologic agents, with the aim of optimizing drug selection and dosing for improved therapeutic efficacy and minimized adverse effects. Variants in genes involved in drug metabolism, such as DPYD, can significantly impair the breakdown of 5-fluorouracil, predisposing patients to severe toxicity, especially in CRC. Genetic alterations can also impact drug targets; for instance, KRAS mutations are well-established predictors of resistance to anti-epidermal growth factor receptor therapies (e.g., cetuximab, panitumumab) in metastatic CRC. Furthermore, polymorphisms in transporter genes like ABCB1 can affect drug absorption, distribution, and resistance. Immune-related genetic factors, such as human leukocyte antigen-B alleles, are associated with drug-induced liver injury, while HLA-DQ2/HLA-DQ8 are linked to hypersensitivity in celiac disease. Thus, pharmacogenetic testing provides valuable information for tailoring therapy and improving clinical outcomes. Genes that influence therapy to other drugs are described in Table 9[90-100]. Similarly, some genes determine the therapeutic response to targeted therapy (Table 10)[101-125].
| Disorder | Gene | Drug(s) | Clinical impact | Ref. |
| CRC, gastric, pancreatic cancers | DPYD | 5-fluorouracil, capecitabine | Deficiency life-threatening toxicity (mucositis, myelosuppression) | De Moraes et al[90]; Ruzzo et al[91] |
| CRC, pancreatic cancer | UGT1A1 | Irinotecan | UGT1A1 28/28 reduced glucuronidation increased toxicity (neutropenia, diarrhea) | Maitland et al[92] |
| IBD, autoimmune hepatitis | TPMT/NUDT15 | Azathioprine, 6-MP | TPMT or NUDT15 deficiency risk of myelosuppression | Moriyama et al[93] |
| IBD | HLA-DQA102:01, HLA-DQB102:02 | Thiopurines | Increase risk of thiopurine-induced pancreatitis | Ås et al[94] |
| IBD | HLA-DQ2 | Infliximab | Increased formation of antibody formation against infliximab | Brun et al[95] |
| GERD, H. pylori, ulcers | CYP2C19 | PPIs (omeprazole, lansoprazole) | Poor metabolizers increase drug levels; rapid metabolizers treatment failure in H. pylori | El Rouby et al[96] |
| NAFLD, metabolic syndrome | SLCO1B1 | Statins (e.g., simvastatin) | Variants statin-induced myopathy risk | SEARCH Collaborative Group[97] |
| IBD | ABCB1 | Various (e.g., corticosteroids) | Associated with glucocorticoid resistance in some patients | Li et al[98] |
| Autoimmune hepatitis, liver transplant | CYP3A5 | Tacrolimus | Expressors need higher doses; non-expressors risk overexposure | Kim et al[99] |
| IBD | G6PD deficiency | Sulfasalazine, dapsone | Increase risk of hemolysis | Dore et al[100] |
| Disorder | Gene/mutation | Role | Treatment/clinical implication | Ref. |
| Colorectal cancer | KRAS (codon 12/13) | Predicts resistance to anti-EGFR therapy | Avoid cetuximab/panitumumab in mutant cases | Zhu et al[101] |
| Colorectal cancer | NRAS mutations | Similar to KRAS | Also predicts non-response to EGFR inhibitors | Hu et al[102] |
| CRC, cholangiocarcinoma | BRAF V600E | Poor prognosis, targetable | Consider BRAF + MEK inhibitors | Rizzo et al[103] |
| Gastric, colorectal cancer | HER2 (ERBB2) amplification | Targetable mutation | Responds to trastuzumab, pertuzumab | Bang et al[104] |
| CRC, gastric, biliary | MSI-H/dMMR | Biomarker for immunotherapy. Poor response to chemotherapy in stage 2 tumor | Eligible for checkpoint inhibitors (e.g., pembrolizumab) | Le et al[105] |
| HCC | CTNNB1 (β-catenin) | Resistance to immunotherapy | Poor response to immunotherapy | Shah et al[106] |
| EZH2 | Resistance to immunotherapy | Negatively express PD-L1 | Xiao et al[107] | |
| Crohn’s disease (IBD) | SNP rs396991GG of gene FCGR3A, rs976881-AA + GA (TNFRSF1B), SNPs in loci DENND1B (rs2488397) and aryl hydrocarbon receptor (rs1077773) s1813443-CC and rs1568885-TT (CNTN5) from the immunoglobulin superfamily | Resistance to biologics | Poor response to immunotherapy | Curci et al[108]; Yoon et al[109]; Ye and McGovern[110] |
| Polymorphisms in ATG16 L1 (C11orf30; rs7927894CC, CCNY; rs12777960CC) (rs10210302) | Clinical response to adalimumab | Koder et al[111] | ||
| Crohn’s disease (IBD) | Polymorphisms in NOD2 | Loss of response to anti-TNF | Juanola et al[112] | |
| UC | Polymorphisms in IL-23R | Early response to infliximab | Jürgens et al[65]; Golan et al[113] | |
| Crohn’s disease | ATG16 L1, IRGM | Autophagy pathway genes | Predict disease course and microbiome interaction | Rioux et al[114] |
| Polymorphisms in FcγRIIIa, HLA-DRB1, HLA-DQA1 05 | Development of ADA against infliximab and adalimumab | Salvador-Martín et al[115]; Billiet et al[116] | ||
| Polymorphisms in FAS, FASL, and CASP9 (apoptotic pharmacogenetic index) | Clinical response to infliximab and adalimumab | Hlavaty et al[117] | ||
| Gene protein tyrosine phosphatase non-receptor type 2 (rs7234029AG + GG, CASP9) | Non-response to anti-TNF and ustekinumab | Hlavaty et al[117] | ||
| HCC | EZH2 | Negatively regulate PD-L1 expression. Less response to PD-L1 agonist | Meng et al[118] | |
| TOP2A, PRC1 | Resistance to chemotherapy | Meng et al[118]; Wang et al[119] | ||
| IBS | TJP1, TPH1, SERT (SLC6A4) | Serotonin signaling, barrier dysfunction | May guide use of 5-HT3 antagonists or SSRIs | Camilleri et al[120]; Kerckhoffs et al[121] |
| Hereditary pancreatitis | SPINK1, PRSS1, CTRC | Trypsin regulation defects | May influence early interventions and surveillance | Panchoo et al[122] |
| Autoimmune hepatitis | HLA-DRB103, 04 | Susceptibility and severity | May predict treatment response to steroids/immunosuppressants | |
| Gastric, pancreatic, cholangiocarcinoma | ARID1A mutations | Epigenetic dysregulation | May predict response to EZH2 inhibitors or immunotherapy | |
| Pancreatic cancer | KRAS | Anti EGFR treatment in effective | Fotopoulos et al[123] | |
| hENT1 | Good response to gemcitabine therapy | |||
| DCK | Increase active form of gemcitabine and increase survival | |||
| DPD | Low DPD level associated with increase survival | |||
| hMLLH1/2 | Pancreatic cancer with MSI associated with less response to 5-FU | |||
| TS | Lower level of TS associated with better response to capecitabine and 5-FU | |||
| WOXX | Decreased expression interferes with gemcitabine sensitivity | |||
| SMAD4 (DPC4) | Poor response to chemotherapy | |||
| GBC | ARID1A | Potential sensitivity to EZH2 inhibitors or immunotherapy | Wardell et al[124] | |
| CDKN2A loss/mutation | Resistant to chemotherapy | Nakamura et al[125] |
Targeted therapy in gastroenterology refers to the use of drugs that specifically interact with molecular or genetic abnormalities in GI and HPB disorders. These therapies target specific genetic mutations, gene fusions, or overexpressed proteins, enabling precise treatment with minimal damage to normal cells unlike conventional chemotherapy. By blocking key oncogenic pathways or modulating the immune response, targeted therapies provide a personalized approach tailored to an individual’s genetic profile. Although highly effective in selected patients, these treatments are often expensive and may not be accessible to all. Currently, most targeted therapies are used in the metastatic setting; however, emerging evidence supports their potential role in neoadjuvant and adjuvant settings. The NICHE-2 trial demonstrated the efficacy of neoadjuvant immunotherapy in locally advanced deficient mismatch repair colon cancer, with short-course nivolumab plus ipilimumab achieving a 98% pathological response rate, 68% complete pathological remission rate, and 100% 3-year disease-free survival. Similarly, the MATTERHORN trial showed improved outcomes with durvalumab plus perioperative FLOT compared to chemotherapy alone in resectable gastric and gastroesophageal junction cancer[126,127]. Together, these studies highlight the evolving role of immune checkpoint inhibitors in the neoadjuvant management of GI cancers. Table 11[128-148] details the present status of targeted therapy in disorders of GIT based on genetic testing.
| Gene/pathway | Targeted drug(s) | Clinical status and trial setting | Ref. |
| KRAS G12C | Sotorasib, adagrasib (+ cetuximab) | Colorectal cancer, FDA approved | Ros et al[128] |
| EGFR (mAB) | Cetuximab, panitumumab, necitumumab | Colorectal cancer, gastric, FDA approved | Xie et al[129] |
| EGFR TKI | Erlotinib, gefitinib, afatinib, osimertinib, amivantamab | Colorectal cancer, gastric cancer, FDA approved | Corvaja et al[130] |
| VEGF | Bevacizumab, aflibercept | Colorectal cancer, gastric cancer | Mahaki et al[131] |
| BRAF V600E | Encorafenib, dabrafenib | Colorectal cancer, gastric cancer | Elez et al[132] |
| CLDN18.2 | Zolbetuximab | Gastric/GEJ adenocarcinoma | Shitara et al[133] |
| NTRK fusion (NTRK1/NTRK2/NTRK3) | Larotrectinib, entrectinib | CRC, pancreatic, cholangiocarcinoma, gastric, others | Manea et al[134] |
| PD-1 (CD274 gene, checkpoint pathway) | Dostarlimab, camrelizumab1, nivolumab and pembrolizumab (keytruda) | Hepatocellular carcinoma, gastric and esophagogastric cancer | Abou-Alfa et al[135] |
| RET fusion | Selpercatinib, pralsetinib, avelumab | Rare GI/HPB tumors (cholangiocarcinoma, pancreatic) | Li et al[136] |
| FGFR2 fusion/rearrangement | Pemigatinib, futibatinib, infigratinib1 | Intrahepatic cholangiocarcinoma | Hyung et al[137] |
| IDH1 mutation | Ivosidenib | Cholangiocarcinoma | Carosi et al[138] |
| BRCA1/BRCA2, PALB2 (HRD pathway) | Olaparib (PARP inhibitor) | Pancreatic adenocarcinoma (germline BRCA) | Alhusaini et al[139] |
| VEGFR, FGFR, PDGFR, RAF (angiogenesis/multikinase) | Sorafenib, lenvatinib, regorafenib, cabozantinib, pazopanib | Hepatocellular carcinoma | Kim[140] |
| APC mutation/COX2 pathway | Celecoxib (COX2 inhibitor) | FAP | Steinbach et al[141] |
| NR1H4 (FXR nuclear receptor) | Obeticholic acid | Primary biliary cholangitis | Floreani et al[142] |
| AGXT mutation (glyoxylate metabolism) | Lumasiran (RNAi against glycolate oxidase) | Primary hyperoxaluria type 1 | Garrelfs et al[143] |
| SERPINA1 mutation (A1AT deficiency, liver disease) | Fazirsiran, ARO-AAT (RNAi) | Alpha-1 antitrypsin liver disease | Strnad et al[144] |
| ATP7B mutation | Chelators (penicillamine, trientine); zinc | Wilson disease | |
| Anti-TNF agents | Infliximab, adalimumab | IBD | Feng et al[145] |
| IL-12/23 pathway | Ustekinumab (anti-IL-12/23) | IBD | Feng et al[145] |
| α4β7 integrin/cell trafficking | Vedolizumab (gut-specific anti-integrin) | IBD | Feng et al[145] |
| JAK-STAT pathway | Tofacitinib (pan-JAK), upadacitinib (JAK1) | IBD | Liu et al[146] |
| PD-L1 antibody | Durvalumab (imfinzi), atezolizumab, tislelizumab | GBC, HCC | Li et al[147] |
| MET amplification/overexpression | Foretinib1, cabozantinib (multi-target TKIs), glumetinib1 | GBC, HCC, gastric, cholangiocarcinoma | Zhang et al[148] |
In this section, we describe various gene therapies, gene editing and their current status in GI disorders. We also highlight emerging treatments and discuss the limitations of gene therapy. We have also discussed various delivery systems tailored to GIT.
Gene therapy involves modifying or manipulating gene expression to treat or prevent disease at its root cause. In the context of GIT disorders, this can include gene replacement (to restore a nonfunctional or missing gene), gene enhancement (to boost expression of beneficial genes), gene overexpression (to increase the production of protective proteins), gene function blocking (to suppress harmful gene activity), or transgenic somatic cell transplantation (inserting genetically modified cells into target tissues). The core principle of gene therapy is to deliver therapeutic genetic material directly into the body, usually in the form of DNA or RNA, to modify cellular function. This can be done by introducing DNA sequences that code for beneficial proteins or enzymes, using short hairpin RNA to silence disease-causing genes, or applying advanced tools such as clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) to precisely edit the genome at specific loci. The goal is to achieve long-lasting expression of therapeutic genes at levels sufficient to alleviate or cure disease symptoms, while simultaneously minimizing the risk of adverse effects[149]. Therapeutic gene materials for diseases can include plasmid DNA, messenger RNA (mRNA), small interfering RNA, microRNA, and short hairpin RNA. Current gene therapy strategy are of two types: Ex vivo and in vivo. Ex vivo gene therapy involves removing cells from a patient, genetically modifying them in a controlled laboratory environment, and then reintroducing the altered cells back into the body. This method is commonly applied to blood cells, stem cells, or immune cells due to their accessibility and ability to be expanded and manipulated outside the body. The typical process includes cell extraction (e.g., from bone marrow or peripheral blood), introduction of therapeutic genes using viral or non-viral vectors, expansion and rigorous testing for safety and efficacy, followed by reinfusion into the patient. One of the main advantages of ex vivo gene therapy is the high level of control it offers genetic modifications that can be precisely assessed, and risks are minimized before the cells are returned to the patient. However, it requires complex laboratory infrastructure and may not be feasible for all tissue types, especially those that are difficult to isolate or culture. In contrast, in vivo gene therapy involves the direct delivery of therapeutic genetic material into a patient’s body, targeting specific tissues or organs without the need to remove cells. This is typically achieved through viral vectors [such as adeno-associated viruses (AAV) or lentiviruses] or non-viral delivery systems like lipid nanoparticles or polymer-based carriers. The genetic payload may consist of DNA for gene replacement, RNA for gene silencing, or tools like CRISPR/Cas9 for precise genome editing.
The major advantage of the in vivo approach is that it is less invasive and technically simpler compared to ex vivo methods, making it suitable for tissues and organs that are difficult to access or manipulate outside the body. However, it offers less control over the site and extent of gene expression, and there is a higher risk of immune reactions or off-target effects. In vivo approach targets long-lived, non-dividing cells, allowing sustained gene expression as long as the introduced DNA remains stable[149]. Although various gene therapies are approved by the Food and Drug Administration for several diseases (hemgenix, zolgensma) but very few are approved and others are under trial for GIT disorders. Examples of some gene therapy for disorders of the GIT are described in Table 12[150-164].
| Therapy/product | Target | Ref. |
| Alicaforsen (antisense targeting ICAM-1) (phase III) | Pouchitis, left-sided UC | Greuter et al[150] |
| Glybera (AAV1-LPL) (withdrawn) | Lipoprotein lipase deficiency (severe pancreatitis) | Ferreira et al[151] |
| Oncolytic AAV-DC-CTL (phase 1) | Stage IV gastric cancer | Yan et al[152] |
| CRISPRedited TIL therapy (phase 1 completed) | Metastatic GI cancers (colorectal, pancreas, gallbladder, esophagus, stomach) | Lou et al[153] |
| CTX131 (allogeneic, CRISPR-engineered CD70-CAR-T) (phase 1/2 trial) | Pancreatic/oesophageal cancers | Pal et al[154] |
| CAN2409 (HSV thymidine kinase gene + pro-drug) (phase 2a) | Pancreatic cancer | Garrett Nichols et al[155] |
| Mutogene cevumeran (personalized mRNA vaccine) (phase 1b) | Pancreatic ductal adenocarcinoma | Lopez et al[156] |
| GENEGUT (preclinical settings) | Crohn’s disease | Hoffmann et al[157] |
| AAVrh.10mAnti-Eos, a serotype rh.10 AAV vector coding for an anti-Siglec-F monoclonal antibody (preclinical) | Eosinophill esophagitis | Camilleri et al[158] |
| Local delivery of an adenoviral vector expressing the HSV-tk gene (aglatimagene besadenovec, AdV-tk) followed by anti-herpetic prodrug | Pancreatic cancer | Aguilar et al[159] |
| Thymidine kinase-based gene therapy | HCC | Sangro et al[160] |
| Adenovirus-mediated double-suicide gene therapy | PDAC | Lee et al[161] |
| Oncolytic virus pelareorep (reolysin) (phase 1/2 trial) | PDAC | Noonan et al[162] |
| GVAX pancreas prime and Listeria Monocytogenes expressing mesothelin (CRS-207) boost vaccines (preclinical) | PDAC | Le et al[163] |
| TNF-erade biologic (phase 1) | Esophageal cancer | Chang et al[164] |
| GNT-0003 (phase III trial) | Crigler-Najjar syndrome | |
| Pexa-Vec (JX-594) (phase 3 trial) | HCC | |
| DTX401 (AAV8-G6Pase gene therapy) (phase 3 trial) | Glycogen storage disorder 1a | |
| DTX301 (avalotcagene ontaparvovec) (phase 3 trial) | Ornithine transcarbamylase deficiency | |
| UX701 (rivunatpagene miziparvovec) (AAV9) (phase 1/2 trial) | Wilson disease | |
| VTX-802 (preclinical study) | PFIC type 2 (BSEP) |
Gene-delivery systems currently include both viral and non-viral carriers. Viral vectors such as retroviruses, AAV, adenoviruses, and lentiviruses use their natural ability to infect cells[165,166]. Non-viral vectors include lipid-based carriers like lipid nanoparticles, polymer-based systems, naked DNA or RNA, inorganic nanoparticles, and peptide-based vectors. Lipid nanoparticles are widely used for mRNA delivery. Polymer-based vectors, such as polyethyleneimine or poly (lactic-co-glycolic acid), provide controlled release and tissue-specific delivery. Although non-viral vectors generally have lower transfection efficiency compared to viral vectors, they offer significant advantages such as low immunogenicity, ease of large-scale production, and the ability to carry larger genetic payloads, including CRISPR components[167]. Nanoparticle-mediated DNA delivery is a cost-effective alternative to AAV systems and enables gene expression without the need for knockout strategies. However, DNA delivery via lipid nanoparticles is often inefficient due to nuclear membrane barriers in resting hepatocytes[168]. In contrast, rapidly dividing enterocytes experience nuclear membrane breakdown, allowing nanoparticle-mediated gene expression in rodent models[169]. The GI tract provides unique access for gene therapy through oral, endoscopic, and rectal routes, making it an attractive target for treating GI disorders.
Hydrodynamic delivery is a non-viral gene transfer technique that uses rapid, high-volume injection to introduce nucleic acids into specific organs, most notably the liver. The method temporarily increases vascular permeability and cell membrane porosity, allowing efficient uptake of genetic material. This approach holds promise for safe, efficient, and localized gene therapy without the risks associated with viral vectors[165,166].
Gene editing refers to a set of molecular technologies that enable precise alterations in the DNA of living organisms. The most widely used tool is CRISPR-Cas9, a revolutionary system adapted from bacterial immune defense, which allows targeted cutting and modification of genes with unprecedented ease and accuracy. Recent advances include base editing and prime editing, which offer even more precise and less disruptive methods of correcting single-point mutations without causing double-strand breaks. mRNA can also express gene-editing enzymes like Cas9, which only need transient expression to modify the genome. In a recent example, base editing, achieved through modified CRISPR enzymes, has shown significant progress, knocking out PCSK9 in primate liver to upregulate LDLR and significantly decrease cholesterol levels[167]. Gene editing techniques are of two types: Ex vivo and in vivo techniques (Table 13)[168]. In the subsequent section, we describe CRISPR as a gene-editing tool and also highlight some of the newer gene-editing technologies.
| Gene editing techniques | In vivo gene editing | Ex vivo gene editing |
| Technique | CRISPR-Cas system is delivered by various vectors to disease-associated cells or organs of the body to correct the mutations or treat the cause of diseases | Targeted cells of a patient are extracted, isolated, edited, expanded, and delivered back to the same patient |
| Application | Treatment of monogenic genetic disorders | Cancer immunotherapy. Treatment of hereditary diseases. Viral infection inhibition |
CRISPR is a family of highly homologous DNA sequences found in genomes of prokaryotic organisms and archaea. Initially discovered in 1987 in Escherichia coli[150]. Later, CAS genes were found to be associated with CRISPR, shifting the focus to proteins encoded by CAS genes[169]. Cas9 protein was of particular significance in establishing the CRISPR/Cas gene editing system. This genome-editing tool is called CRISPR technology[170]. The CRISPR gene editing involves four phases: Designing the experiment, delivery of CRISPR components, induction and repair of double-strand breaks and analysis of genetic edits.
CRISPR technology has potential roles in screening, diagnosis, and treatment of various GI disorders. CRISPR/Cas9 is not only used to study known driver mutations but also to identify and functionally characterize novel driver genes. For example, Yau et al[171] performed a CRISPR/Cas9 knockout screen of 19050 human coding genes in KRAS-mutated vs wild-type HCT116 CRC cells and uncovered pathways such as NADK and fructose metabolism (KHK) as KRAS-specific vulnerabilities and potential therapeutic targets. Although most of the CRISPR-mediated gene therapy studies have been performed in organoid models, an increasing number are now being conducted directly in humans. Li et al[172] found that corrected, a mutation of the WNT pathway gene β-catenin in the human CRC cell line HCT116 with CRISPR/Cas9 resulted in increased protein phosphorylation and reduced proliferation of CRC. Zhang et al[173] found that knocking PDEF out via CRISPR/Cas9 in the AGS gastric cell line decreased proliferation, migration and invasion of the cell. Seino et al[174] found that CRISPR/Cas9-based knockdown of GATA6 resulted in WNT self-activation through upregulation of WNT7B in the previously WNT-non-producing subtype. Similar studies of CRISPR-mediated therapy are summarized in Table 14[153,175-177].
| Serial No. | Model/sample size | Disease | CRISPR target | Key findings | Ref. |
| 1 | Phase 1 trial; 12 patients with metastatic colorectal cancer | Metastatic CRC (human trial) | CISH knockout in autologous T cells | CRISPR-edited T cells were safe, feasible, and showed preliminary anti-tumor activity | Lou et al[153] |
| 2 | Phase 1 trial; 3 patients with advanced cancers (incl 1 GI malignancy) | Advanced solid tumors | Knockout of TRAC, TRBC, PD-1; insertion of NY-ESO-1 TCR | Demonstrated safety and persistence of CRISPR-edited T cells in humans; proof of feasibility | Stadtmauer et al[175] |
| 3 | Ongoing; sample size approximately 20 planned | Solid tumors (GI cancers included) | Endogenous TCR knockout + NY-ESO-1 TCR insertion | Designed to enhance adoptive T-cell therapy; early feasibility data available | Clinical trial (No. NCT03399448) |
| 4 | Human colon organoids | Colorectal cancer modeling | DNA repair genes (MLH1, MSH2, APC, TP53) | Sequential CRISPR editing in organoids recapitulated colorectal tumorigenesis | Drost et al[176] |
| 5 | Human intestinal organoids | Tumor suppressor modeling | PTEN, APC | High-efficiency CRISPR editing showed functional loss-of-gene effects; robust platform for GI cancer studies | Skoufou-Papoutsaki et al[177] |
Apart from CRISPR, several newer gene-editing tools are available, which are summarized in Table 15.
| Technique | Mechanism | GIT applications |
| CRISPR-Cas9/12/13 | DNA or RNA targeting via guide RNA and nuclease | Cancer mutations (APC, KRAS), viral hepatitis, IBD models |
| Base/prime editing | Precise base or sequence correction without DSBs | CFTR mutations, APC mutations |
| ZFNs | DNA-binding proteins fused to nucleases | HBV suppression (preclinical) |
| TALENs | TALE DNA-binding fused to nucleases | Cancer cell targeting, liver disease models |
| Epigenome editing | dCas9 fused to activators/repressors | Regulation of PD-L1, IBD immune genes |
| RNAi (siRNA, ASO) | Degrade/block specific mRNAs | Lumasiran (PH1), fazirsiran (A1AT deficiency) |
The majority of present gene therapy is via AAV, which has many limitations. Firstly, the safety of the vector. Although AAV predominantly remains episomal after entering hepatocyte nuclei, a small fraction can randomly integrate into the host genome. This integration has been linked to an increased risk of hepatocellular carcinoma (HCC) in mouse models[178,179]. Secondly, AAV can induce T-cell responses against capsid proteins, causing loss of transgene expression[180]. Third, sustained expression remains a challenge. AAV largely stays episomal and is gradually lost during cell division. This is especially problematic in the GI tract, where rapid mucosal turnover (every 2-6 days) limits gene persistence in enterocytes unless intestinal stem cells in the crypts are permanently modified[181]. Fourthly, the efficiency of delivery, as AAV delivers in a fraction of cells within an organ, and at levels often lower than the wild-type cells[182]. Regarding gene editing, safety is the prime concern, considering the possibility of off-target effects or unintended base edits, i.e., edits in the wrong place and mosaicism. Additionally, many rare genetic liver disorders are caused by hundreds of distinct mutations, making them less suitable for precise correction of a single point mutation through current gene-editing techniques[183]. Since there is a potential for misuse, genome editing should be managed through policy and regulation[184].
As we advance further into the era of precision medicine, a wide range of innovative concepts and technologies are steadily emerging. Precision medicine (also called personalized medicine) uses genomics, biomarkers, and data analytics to tailor medical care to the individual characteristics of each patient. It has 4 core components: Genomics, pharmacogenomics, biomarkers, and data analysis. Personalized to each disease, whether hereditary or somatic, precision medicine helps in incorporating genes as the basis of all disorders. It will help in screening, early diagnosis and therapeutic potential, whether target therapy or gene editing. Many newer tools have already been discussed in earlier sections; others are still in the experimental or early translational phase. These include novel approaches such as multi-omics integration (combining genomics, transcriptomics, proteomics, and metabolomics for precision medicine), spatial genomics, RNA therapies, epigenome editing, synthetic biology-based therapeutics, and next-generation RNA therapies. Advances in delivery systems, including nanoparticle-based vectors and exosome-mediated gene transfer, are also expanding therapeutic possibilities. Additionally, AI and machine learning (ML) are being increasingly applied to analyze large genomic datasets, enabling better prediction of disease risk, treatment response, and drug development. Some of these emerging concepts are summarized in Table 16[185-189].
| Area | Key advancements | Ref. |
| Multi-omics integration | Combined use of genomics, transcriptomics, proteomics, and metabolomics to understand complex GI diseases | Zhao et al[185] |
| Polygenic risk scores | Using multiple low-risk variants to predict risk of diseases like IBD, colorectal cancer | Cross et al[186] |
| Single-cell sequencing | Helps identify cell-specific pathways in diseases like IBD, gastric cancer | Misra et al[187] |
| Organoid models | Patient-derived GI organoids used for drug testing, personalized therapy, and gene editing studies | Yang et al[188] |
| Epigenomics | Studying methylation, histone modifications, especially in GI cancers (e.g., MLH1 methylation in CRC) | Struhl[189] |
| Artificial intelligence | AI-driven prediction models, imaging-genomics integration for early diagnosis and prognosis |
RNA therapies work by modulating gene expression at the RNA level either by blocking, replacing, or editing RNA messages inside cells. These therapies don’t typically alter DNA directly but instead influence how proteins are made, which can help treat diseases caused by faulty gene expression, inflammation, or toxic proteins. RNA therapies can target previously “undruggable” genes. These are highly specific and customized, with no permanent DNA change, making them safer than some gene therapies. Table 17 describes the current RNA therapies in GIT disorders[190-193].
| Type | Mechanism of action | Example use | Ref. |
| Antisense oligonucleotides | Single-stranded RNA/DNA binds mRNA blocks translation or triggers degradation (via RNase H) | Alicaforsen in IBD (targets ICAM-1 mRNA) (phase 2/3 study) | Greuter et al[150] |
| Small interfering RNA | Double-stranded RNA binds to target mRNA guides RISC complex degrades mRNA | STNM01 in Crohn’s disease (fibrosis gene CHST15) (phase 1) | Suzuki et al[190] |
| mRNA replacement therapy | Synthetic mRNA encoding a therapeutic protein is delivered translated into protein | mRNA vaccines, IL-10 mRNA for colitis. Arcturus “lunar” mRNA, IL-10 mRNA LNPs (phase 1/2 study) | Qin et a[191] |
| CRISPR-Cas9 mRNA | mRNA encodes Cas9 protein + guide RNA edits DNA directly via targeted cleavage | Casgevy (CRISPR for β-thalassemia) (FDA approved) | Parums[192] |
| RNA aptamers | Structured RNA molecules bind and inhibit specific proteins or receptors | Macugen for eye disease; potential GI targets in research (preclinical) | Nagpal et al[193] |
MRNA gene therapy is an innovative approach that involves delivering synthetic mRNA into the body to direct cells to produce specific therapeutic proteins. Unlike DNA-based therapies, mRNA functions in the cytoplasm and does not integrate into the genome, making it a safer alternative with reduced risk of permanent genetic alterations. Delivered typically via lipid nanoparticles, mRNA therapy enables transient and controlled expression of proteins, which is particularly useful for diseases requiring short-term or repeat interventions. Some examples of mRNA therapies are described in Table 18.
| Agent/platform | Target/indication (GIT) | Study type/phase |
| RNA-4157/V940 (Moderna) | Individualized neoantigen vaccine colorectal cancer | Phase 2b/3 trial undergoing |
| BioNTech iNeST/BNT-pipeline | Personalized or fixed mRNA cancer vaccines for CRC, pancreatic, HCC | Phase 1/2 trials |
| Gritstone GRANITE | Personalized neoantigen immunotherapy MSS colorectal cancer | Phase 2 trial |
| MSK/investigator-initiated mRNA vaccine | Personalized mRNA neoantigen vaccine pancreatic adenocarcinoma | Early phase trial |
| OX40 L mRNA (LNP delivery) | Immune costimulatory agonist mRNA for HCC | Preclinical |
Epigenetic alterations such as DNA methylation and histone modifications play a key role in the pathogenesis of GI cancers. These tumors often display a dual epigenetic profile: Global DNA hypomethylation alongside hypermethylation at CpG islands. Such changes are increasingly recognized for their diagnostic, prognostic, and therapeutic value. Targeting epigenetic regulators, such as histone acetyltransferase inhibitors, histone deacetylase inhibitors (HDACis), and DNA methyltransferase inhibitors (DNMTis) offers a promising therapeutic strategy. Agents like azacitidine (DNMTi), vorinostat (HDACi), hydralazine (DNMTi), and tucidinostat (HDACi) are currently in early-phase clinical trials, un
AI and ML can integrate and transform big data into clinically useful diagnostic and therapeutic tools. The term genetic AI refers to the application of AI methods to bioinformatics data, including amino acid, DNA, and RNA sequences. Several studies have demonstrated that by generating clinical, diagnostic, and therapeutic algorithms, often in the form of decision-support systems or flowcharts, genetic AI can assist clinicians in making faster and more informed decisions. AI also enables precision diagnostics by integrating genetic profiles with histopathology and imaging, thereby facilitating earlier and more accurate detection of GI disorders. In addition, AI applications aid in risk stratification, prediction of disease progression, and planning of surveillance strategies. Kang et al[195] analyzed single-nucleotide polymorphism genotype data from 337 patients with Crohn’s disease and demonstrated that a combined clinical-genetic model using ML algorithms predicted the need for early intestinal resection more accurately than clinical features alone. Garza-Hernandez et al[196], using Genome-Wide Association Studies (GWAS) datasets from the United Kingdom IBD Genetics Consortium, showed that multivariate prediction analysis can identify previously unrecognized IBD-associated loci. Schophaus et al[197], analyzing United Kingdom Biobank data, reported that ML-based analysis revealed a higher manganese intake to be associated with a lower risk of non-alcoholic fatty liver disease (NAFLD). Wang et al[198] developed a tumor-infiltrating immune cell signature score, which provided a novel prognostic tool and potential guidance for immunotherapy in esophageal squamous cell carcinoma.
A complex interplay between human gut microbiome, brain and gut is known to play a significant role in the pa
Several polygenic risk scoring (PRS) systems have been developed for CRC screening[203,204]. These scores can help identify individuals who may benefit from a colonoscopy at a younger age. Beyond screening, PRS can also be applied to stratify disease severity in CRC. Similarly, PRS has been shown to predict disease severity in chronic liver disease[205]. In IBD, Gettler et al[206] designed a PRS using genomic data from 32595 individuals, demonstrating its potential clinical utility. De Vincentis et al[207] demonstrated that incorporating PRS provides prognostic insights beyond established clinical and biochemical parameters in NAFLD. Similarly, Thomas et al[208] reported that combining PRS with hepatic fat content enhances the prediction of HCC risk. PRS quantifies an individual’s genetic predisposition to various diseases by analyzing multiple genetic variants, which in turn helps in risk assessment and genetic counselling[209]. There are various limitation of PRS. Firstly, most PRS are based on European ancestry GWAS datasets and accuracy drops significantly in non-European populations due to allele frequency differences and linkage disequilibrium patterns[210]. Secondly, PRS requires large sample sizes and complex computational methods to achieve reliable prediction. Thirdly, it does not account for environmental and lifestyle factors, limiting real-world applicability. Fourthly, its predictive accuracy often falls short of clinical utility. Transferability across ancestries is inconsistent, influenced by trait heritability and the diversity of training data, with admixed populations posing additional challenges. Moreover, the lack of standardization hampers implementation. Finally, PRS is dynamic and its stability is affected as new genetic discoveries continuously reshape risk estimates[211].
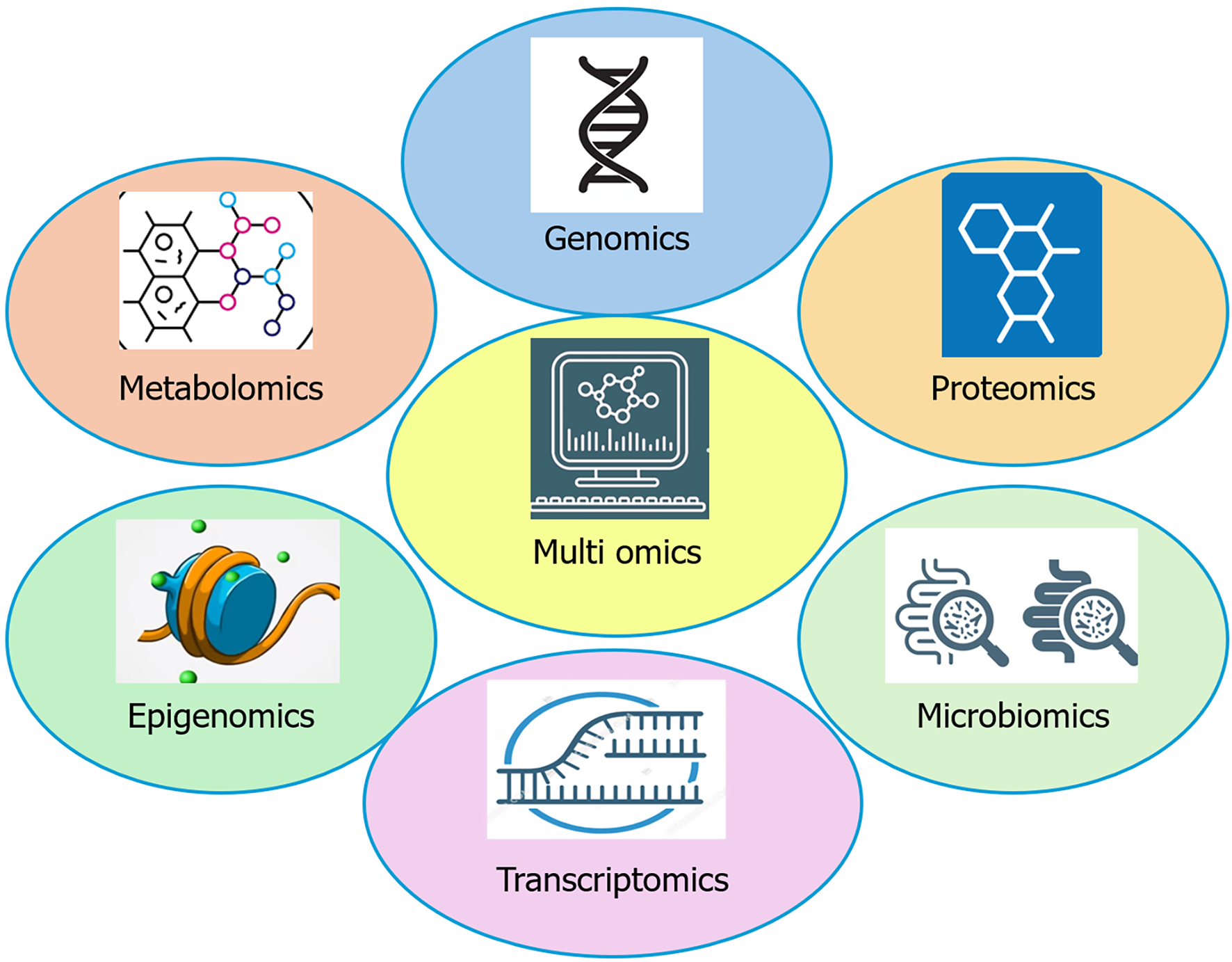
Multi-omics techniques refer to the integrated analysis of multiple layers of biological data such as genomics, transcriptomics, proteomics, metabolomics, epigenomics, and microbiomics to gain a comprehensive understanding of disease mechanisms (Figure 4)[212]. In GIT diseases, such as CRC, IBD, IBS, and celiac disease, multi-omics helps identify disease-associated genetic variants, altered gene expression patterns, epigenetic modifications, and dysregulated metabolic pathways. For instance, integrating genomic and transcriptomic data can reveal mutations (like in KRAS or APC) and corresponding changes in gene expression that drive colorectal carcinogenesis[213]. Multi-omics database is a structured repository that integrates multiple layers of biological information collected from patients, healthy controls, or model systems. Some of the databases are IBD multi-omics database (IBD), the Cancer Genome Atlas (CRC, gastric cancer, liver cancer), clinical proteomic tumor analysis consortium (CRC proteogenomics), international cancer genome consortium (liver/HCC) and Human Microbiome Project (HMP) 2/gutMGene/GMrepo (microbiome-host integration). These databases provide valuable insights into how different components of multi-omics contribute to understanding disease mechanisms and guiding management. The HMP2 multi-omics study is a landmark effort that offered a comprehensive view of how the gut microbiome and its interactions with the host are altered in IBDs[214].
The emerging role of genetic medicine; poses ethical issues and several other issues of concern. Various issues and challenges are discussed below.
The liver acts as a major sink for systemically delivered AAV vectors, making hepatotoxicity the most frequent adverse event after intravenous administration. Recent reports of severe, immune-mediated liver injury, including fatalities at high doses, underscore vector-related immunotoxicity as a critical barrier to liver-directed gene therapies in GIT disorders[215].
Since the technology is new and unpredictable, these errors are bound to occur and carry fatal consequences if germline mutations occur, affecting the individual and future generations. Off-target mutation i.e., insertion of mutagenic gene. These off-target integrations can occur due to inaccurate gene delivery by the viral vector systems. Refinement of the vector systems may reduce this error[216,217]. Genetic mosaicism i.e., different cells carry genetic information as a result of genetic mutations during the early development of an organism. This occurs when the vector can persist and transcribe, making it possible to further introduce the Cas protein into parts of already engineered cells and potentially initiate another cleavage, leading to mosaicism. This may impair embryo maturation[218].
All humans have the right to informed consent and privacy. Although it is feasible in the case of somatic gene therapy to have regulated informed consent. However, germline gene therapy raises complex regulatory challenges, particularly around who holds the authority to make informed decisions on behalf of future generations. Whether the consent of a future generation is required and, if so, who should express consent because embryos cannot consent to germline in
Gene enhancement, i.e., manipulating genes to improve the characteristics of an individual according to the interests of the person, remains a legitimate concern surrounding the application of gene therapy. Examples include the injection of recombinant human growth hormone, human recombinant erythropoietin. However, if the injection to children of normal height in an attempt to make them taller may create ethical issues[221]. The distinction between therapy and enhancement is often context-dependent and must be clearly defined.
Disparities exist in access to clinical genetic services and in the efficacy of those services. For instance, African-American women have poor access to BRCA1 genetic testing than white women. As well, they are likely to receive ambiguous genetic test results after exome sequencing, or be told that they have variants of unknown significance[222]. Although the global market for genetic testing is expanding rapidly, translating this progress into routine clinical practice remains limited and uneven across regions. Accessibility continues to be a significant barrier many healthcare systems, particularly in low- and middle-income countries, lack the infrastructure and trained personnel to offer advanced genetic services. Even in developed regions, disparities in availability between urban and rural healthcare centers persist.
For those who do gain access, affordability presents another major hurdle. Advanced genetic tests, multi-omics profiling, and personalized gene therapies are often prohibitively expensive, placing them out of reach for a large portion of the population and potentially widening health inequities.
Genomic sequencing frequently uncovers actionable secondary findings (e.g., BRCA, Lynch genes) that have implications beyond the original indication. One should follow the guidelines from professional bodies to what to do with secondary findings.
High-profile events (Jesse Gelsinger’s death, the He Jiankui’s embryo edits) and recent AAV toxicities have driven stricter oversight and international governance recommendations[223]. For GIT applications where the liver is a common target, and tumor/germline testing often uncovers actionable hereditary findings, researchers must prioritize rigorous preclinical safety, robust consent and counselling, inclusive trial design, privacy protections, and long-term registries to ethically translate genomic advances.
With the development era of precision medicine, the genes and genetic medicine is going to take center stage in the management of many GI (GIT) disorders. The integration of genetic diagnostic testing, multi-omics approaches, targeted therapy, gene therapy, and gene editing has already transformed the landscape of gastroenterology. These advances enable a deeper understanding of disease mechanisms at the molecular level, allowing for more accurate diagnosis, individualized risk prediction, and tailored therapeutic strategies. In this evolving paradigm, it is imperative for gastroenterologists to keep pace with developments in genetic and genomic medicine, ensuring they remain at the forefront of patient care. Embracing precision medicine not only enhances clinical outcomes but also aligns with the future of personalized, predictive, and preventive gastroenterology.
| 1. | Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1920] [Cited by in RCA: 1874] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 2. | Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1636] [Cited by in RCA: 1565] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 3. | Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2346] [Cited by in RCA: 2104] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 4. | Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5632] [Cited by in RCA: 5235] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 5. | Naithani N, Sinha S, Misra P, Vasudevan B, Sahu R. Precision medicine: Concept and tools. Med J Armed Forces India. 2021;77:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Jasmine F, Haq Z, Kamal M, Raza M, da Silva G, Gorospe K, Paul R, Strzempek P, Ahsan H, Kibriya MG. Interaction between Microsatellite Instability (MSI) and Tumor DNA Methylation in the Pathogenesis of Colorectal Carcinoma. Cancers (Basel). 2021;13:4956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Chen HM, Fang JY. Epigenetic Biomarkers for the Early Detection of Gastrointestinal Cancer. Gastrointest Tumors. 2014;1:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Angelico G, Attanasio G, Colarossi L, Colarossi C, Montalbano M, Aiello E, Di Vendra F, Mare M, Orsi N, Memeo L. ARID1A Mutations in Gastric Cancer: A Review with Focus on Clinicopathological Features, Molecular Background and Diagnostic Interpretation. Cancers (Basel). 2024;16:2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 9. | Sahu A, Singhal U, Chinnaiyan AM. Long noncoding RNAs in cancer: from function to translation. Trends Cancer. 2015;1:93-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 10. | Chae YK, Anker JF, Carneiro BA, Chandra S, Kaplan J, Kalyan A, Santa-Maria CA, Platanias LC, Giles FJ. Genomic landscape of DNA repair genes in cancer. Oncotarget. 2016;7:23312-23321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Petruzzelli L, Takami M, Humes HD. Structure and function of cell adhesion molecules. Am J Med. 1999;106:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Saklatvala J, Dean J, Clark A. Control of the expression of inflammatory response genes. Biochem Soc Symp. 2003;95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Henkel AS. Genetic Disorders of Bile Acid Transport. Clin Liver Dis (Hoboken). 2021;18:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Roy-Carson S, Natukunda K, Chou HC, Pal N, Farris C, Schneider SQ, Kuhlman JA. Defining the transcriptomic landscape of the developing enteric nervous system and its cellular environment. BMC Genomics. 2017;18:290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Charcosset M, Sassolas A, Peretti N, Roy CC, Deslandres C, Sinnett D, Levy E, Lachaux A. Anderson or chylomicron retention disease: molecular impact of five mutations in the SAR1B gene on the structure and the functionality of Sar1b protein. Mol Genet Metab. 2008;93:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Tripathi SK, Lahesmaa R. Transcriptional and epigenetic regulation of T-helper lineage specification. Immunol Rev. 2014;261:62-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Li N, Liu J, Li B, Bai L, Feng Y, Liu J, Zhang L, Yang H, Liu Y. Personalized Flow Division Method Based on the Left-Right Coronary Cross-Sectional Area. J Mech Med Biol. 2022;22:2250008. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Peng C, Ouyang Y, Lu N, Li N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front Immunol. 2020;11:1387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 19. | Hata A, Chen YG. TGF-β Signaling from Receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8:a022061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 708] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 20. | Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 1695] [Article Influence: 339.0] [Reference Citation Analysis (0)] |
| 21. | Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997-2007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 857] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 22. | Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, Kulke MH, Baird RD, Prabhu JS, Carbone D, Pecoraro C, Teh DBL, Sethi G, Cavalieri V, Lin KH, Javidi-Sharifi NR, Toska E, Davids MS, Brown JR, Diana P, Stebbing J, Fruman DA, Kumar AP. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 1264] [Article Influence: 421.3] [Reference Citation Analysis (0)] |
| 23. | Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 1026] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 24. | Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1399] [Cited by in RCA: 1436] [Article Influence: 68.4] [Reference Citation Analysis (1)] |
| 25. | Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1167] [Cited by in RCA: 1408] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 26. | Kopan R. Notch signaling. Cold Spring Harb Perspect Biol. 2012;4:a011213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 27. | Yu L, Chen Y, Tooze SA. Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 2018;14:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 1142] [Article Influence: 126.9] [Reference Citation Analysis (0)] |
| 28. | He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 967] [Article Influence: 161.2] [Reference Citation Analysis (0)] |
| 29. | Chen X, Shi C, He M, Xiong S, Xia X. Endoplasmic reticulum stress: molecular mechanism and therapeutic targets. Signal Transduct Target Ther. 2023;8:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 590] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 30. | Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci. 2019;20:3394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 386] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 31. | Waring P, Müllbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 240] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Kumar N, Moreno NC, Feltes BC, Menck CF, Houten BV. Cooperation and interplay between base and nucleotide excision repair pathways: From DNA lesions to proteins. Genet Mol Biol. 2020;43:e20190104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Jansen AML, Goel A. Mosaicism in Patients With Colorectal Cancer or Polyposis Syndromes: A Systematic Review. Clin Gastroenterol Hepatol. 2020;18:1949-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Pereira F, Teixeira MR, Dinis Ribeiro M, Brandão C. Multi-Gene Panel Testing in Gastroenterology: Are We Ready for the Results? GE Port J Gastroenterol. 2021;28:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Understanding Genetics: A New York, Mid-Atlantic Guide for Patients and Health Professionals. Washington (DC): Genetic Alliance, 2009 . [PubMed] |
| 36. | Yilmaz O, Demiray E. Clinical role and importance of fluorescence in situ hybridization method in diagnosis of H pylori infection and determination of clarithromycin resistance in H pylori eradication therapy. World J Gastroenterol. 2007;13:671-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Weiss MM, Hermsen MA, Meijer GA, van Grieken NC, Baak JP, Kuipers EJ, van Diest PJ. Comparative genomic hybridisation. Mol Pathol. 1999;52:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Myllykangas S, Junnila S, Kokkola A, Autio R, Scheinin I, Kiviluoto T, Karjalainen-Lindsberg ML, Hollmén J, Knuutila S, Puolakkainen P, Monni O. Integrated gene copy number and expression microarray analysis of gastric cancer highlights potential target genes. Int J Cancer. 2008;123:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Guo B, Han X, Wu Z, Da W, Zhu H. Spectral karyotyping: an unique technique for the detection of complex genomic rearrangements in leukemia. Transl Pediatr. 2014;3:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 40. | Herpich CM, Gomes CAFP, Dibai-Filho AV, Politti F, Souza CDS, Biasotto-Gonzalez DA. Correlation Between Severity of Temporomandibular Disorder, Pain Intensity, and Pressure Pain Threshold. J Manipulative Physiol Ther. 2018;41:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Satam H, Joshi K, Mangrolia U, Waghoo S, Zaidi G, Rawool S, Thakare RP, Banday S, Mishra AK, Das G, Malonia SK. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology (Basel). 2023;12:997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 511] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 42. | Málaga DR, Brusius-Facchin AC, Siebert M, Pasqualim G, Saraiva-Pereira ML, Souza CFM, Schwartz IVD, Matte U, Giugliani R. Sensitivity, advantages, limitations, and clinical utility of targeted next-generation sequencing panels for the diagnosis of selected lysosomal storage disorders. Genet Mol Biol. 2019;42:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Rabbani B, Tekin M, Mahdieh N. The promise of whole-exome sequencing in medical genetics. J Hum Genet. 2014;59:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 44. | Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, Ouahed J, Wilson DC, Travis SP, Turner D, Klein C, Snapper SB, Muise AM; COLORS in IBD Study Group and NEOPICS. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990-1007.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 501] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 45. | de Voer RM, Hahn MM, Weren RD, Mensenkamp AR, Gilissen C, van Zelst-Stams WA, Spruijt L, Kets CM, Zhang J, Venselaar H, Vreede L, Schubert N, Tychon M, Derks R, Schackert HK, Geurts van Kessel A, Hoogerbrugge N, Ligtenberg MJ, Kuiper RP. Identification of Novel Candidate Genes for Early-Onset Colorectal Cancer Susceptibility. PLoS Genet. 2016;12:e1005880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Kuiper RP, Vissers LE, Venkatachalam R, Bodmer D, Hoenselaar E, Goossens M, Haufe A, Kamping E, Niessen RC, Hogervorst FB, Gille JJ, Redeker B, Tops CM, van Gijn ME, van den Ouweland AM, Rahner N, Steinke V, Kahl P, Holinski-Feder E, Morak M, Kloor M, Stemmler S, Betz B, Hutter P, Bunyan DJ, Syngal S, Culver JO, Graham T, Chan TL, Nagtegaal ID, van Krieken JH, Schackert HK, Hoogerbrugge N, van Kessel AG, Ligtenberg MJ. Recurrence and variability of germline EPCAM deletions in Lynch syndrome. Hum Mutat. 2011;32:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 47. | Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1811] [Cited by in RCA: 1870] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 48. | Shah A, Talley NJ, Koloski N, Macdonald GA, Kendall BJ, Shanahan ER, Walker MM, Keely S, Jones MP, Morrison M, Holtmann GJ. Duodenal bacterial load as determined by quantitative polymerase chain reaction in asymptomatic controls, functional gastrointestinal disorders and inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Bamias G, Goukos D, Laoudi E, Balla IG, Siakavellas SI, Daikos GL, Ladas SD. Comparative study of candidate housekeeping genes for quantification of target gene messenger RNA expression by real-time PCR in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2840-2847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | McKay SC, Unger K, Pericleous S, Stamp G, Thomas G, Hutchins RR, Spalding DR. Array comparative genomic hybridization identifies novel potential therapeutic targets in cholangiocarcinoma. HPB (Oxford). 2011;13:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Assämäki R, Sarlomo-Rikala M, Lopez-Guerrero JA, Lasota J, Andersson LC, Llombart-Bosch A, Miettinen M, Knuutila S. Array comparative genomic hybridization analysis of chromosomal imbalances and their target genes in gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2007;46:564-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Megiorni F, Pizzuti A. HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: practical implications of the HLA molecular typing. J Biomed Sci. 2012;19:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 53. | Brankley SM, Halling KC, Jenkins SM, Timmer MR, Iyer PG, Smyrk TC, Barr Fritcher EG, Voss JS, Kipp BR, Campion MB, Lutzke LS, Minot DM, Wang KK. Fluorescence in situ hybridization identifies high risk Barrett's patients likely to develop esophageal adenocarcinoma. Dis Esophagus. 2016;29:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Tol J, Dijkstra JR, Vink-Börger ME, Nagtegaal ID, Punt CJ, Van Krieken JH, Ligtenberg MJ. High sensitivity of both sequencing and real-time PCR analysis of KRAS mutations in colorectal cancer tissue. J Cell Mol Med. 2010;14:2122-2131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM; Australian Pancreatic Cancer Genome Initiative, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2713] [Article Influence: 271.3] [Reference Citation Analysis (7)] |
| 56. | Nagase H, Miyoshi Y, Horii A, Aoki T, Ogawa M, Utsunomiya J, Baba S, Sasazuki T, Nakamura Y. Correlation between the location of germ-line mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res. 1992;52:4055-4057. [PubMed] |
| 57. | Enomoto M, Konishi M, Iwama T, Utsunomiya J, Sugihara KI, Miyaki M. The relationship between frequencies of extracolonic manifestations and the position of APC germline mutation in patients with familial adenomatous polyposis. Jpn J Clin Oncol. 2000;30:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Ficari F, Cama A, Valanzano R, Curia MC, Palmirotta R, Aceto G, Esposito DL, Crognale S, Lombardi A, Messerini L, Mariani-Costantini R, Tonelli F, Battista P. APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis. Br J Cancer. 2000;82:348-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Walon C, Kartheuser A, Michils G, Smaers M, Lannoy N, Ngounou P, Mertens G, Verellen-Dumoulin C. Novel germline mutations in the APC gene and their phenotypic spectrum in familial adenomatous polyposis kindreds. Hum Genet. 1997;100:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Gebert JF, Dupon C, Kadmon M, Hahn M, Herfarth C, von Knebel Doeberitz M, Schackert HK. Combined molecular and clinical approaches for the identification of families with familial adenomatous polyposis coli. Ann Surg. 1999;229:350-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Caspari R, Olschwang S, Friedl W, Mandl M, Boisson C, Böker T, Augustin A, Kadmon M, Möslein G, Thomas G. Familial adenomatous polyposis: desmoid tumours and lack of ophthalmic lesions (CHRPE) associated with APC mutations beyond codon 1444. Hum Mol Genet. 1995;4:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 228] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 62. | Davies DR, Armstrong JG, Thakker N, Horner K, Guy SP, Clancy T, Sloan P, Blair V, Dodd C, Warnes TW. Severe Gardner syndrome in families with mutations restricted to a specific region of the APC gene. Am J Hum Genet. 1995;57:1151-1158. [PubMed] |
| 63. | Tsianos EV, Katsanos KH, Tsianos VE. Role of genetics in the diagnosis and prognosis of Crohn's disease. World J Gastroenterol. 2012;18:105-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 64. | Nam SY, Kim N, Kim JS, Lim SH, Jung HC, Song IS. Heat shock protein gene 70-2 polymorphism is differentially associated with the clinical phenotypes of ulcerative colitis and Crohn's disease. J Gastroenterol Hepatol. 2007;22:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Jürgens M, Laubender RP, Hartl F, Weidinger M, Seiderer J, Wagner J, Wetzke M, Beigel F, Pfennig S, Stallhofer J, Schnitzler F, Tillack C, Lohse P, Göke B, Glas J, Ochsenkühn T, Brand S. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am J Gastroenterol. 2010;105:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 66. | Rismo R, Olsen T, Cui G, Christiansen I, Florholmen J, Goll R. Mucosal cytokine gene expression profiles as biomarkers of response to infliximab in ulcerative colitis. Scand J Gastroenterol. 2012;47:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Olsen T, Goll R, Cui G, Christiansen I, Florholmen J. TNF-alpha gene expression in colorectal mucosa as a predictor of remission after induction therapy with infliximab in ulcerative colitis. Cytokine. 2009;46:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Yoshida A, Matsuoka K, Ueno F, Morizane T, Endo Y, Hibi T. Serum PR3-ANCA Is a Predictor of Primary Nonresponse to Anti-TNF-α Agents in Patients with Ulcerative Colitis. Inflamm Intest Dis. 2021;6:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 69. | Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, Bewshea CM, Chanchlani N, Walker GJ, Perry MH, McDonald TJ, Lees CW, Cummings JRF, Parkes M, Mansfield JC, Irving PM, Barrett JC, McGovern D, Goodhand JR, Anderson CA, Ahmad T; PANTS Consortium. HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn's Disease. Gastroenterology. 2020;158:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 70. | Murray JA, Moore SB, Van Dyke CT, Lahr BD, Dierkhising RA, Zinsmeister AR, Melton LJ 3rd, Kroning CM, El-Yousseff M, Czaja AJ. HLA DQ gene dosage and risk and severity of celiac disease. Clin Gastroenterol Hepatol. 2007;5:1406-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Stanković B, Radlović N, Leković Z, Ristić D, Radlović V, Nikčević G, Kotur N, Vučićević K, Kostić T, Pavlović S, Zukic B. HLA genotyping in pediatric celiac disease patients. Bosn J Basic Med Sci. 2014;14:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Avanthi SU, Ravi Kanth VV, Agarwal J, Lakhtakia S, Gangineni K, Rao GV, Reddy DN, Talukdar R. Association of claudin2 and PRSS1-PRSS2 polymorphisms with idiopathic recurrent acute and chronic pancreatitis: A case-control study from India. J Gastroenterol Hepatol. 2015;30:1796-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Whitcomb DC. PRSS1-Related Hereditary Pancreatitis. Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. [PubMed] |
| 74. | Abass MK, Al Shamsi A, Jan I, Masalawala MSY, Deeb A. Combined SPINK1 mutations induce early-onset severe chronic pancreatitis in a child with severe obesity. Endocrinol Diabetes Metab Case Rep. 2022;2022:22-0273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 75. | Zhang H, Liu Q. Prognostic Indicators for Gastrointestinal Stromal Tumors: A Review. Transl Oncol. 2020;13:100812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 76. | Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter "RASCAL" study. J Natl Cancer Inst. 1998;90:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 549] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 77. | Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009;9:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 518] [Article Influence: 30.5] [Reference Citation Analysis (4)] |
| 78. | Wang X, Lai Y. When SLC46A2 delivers cargo into keratinocytes. Immunity. 2023;56:897-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Hashemi M, Sabouni E, Rahmanian P, Entezari M, Mojtabavi M, Raei B, Zandieh MA, Behroozaghdam M, Mirzaei S, Hushmandi K, Nabavi N, Salimimoghadam S, Ren J, Rashidi M, Raesi R, Taheriazam A, Alexiou A, Papadakis M, Tan SC. Deciphering STAT3 signaling potential in hepatocellular carcinoma: tumorigenesis, treatment resistance, and pharmacological significance. Cell Mol Biol Lett. 2023;28:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 80. | Svinka J, Mikulits W, Eferl R. STAT3 in hepatocellular carcinoma: new perspectives. Hepat Oncol. 2014;1:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Chao HM, Huang HX, Chang PH, Tseng KC, Miyajima A, Chern E. Y-box binding protein-1 promotes hepatocellular carcinoma-initiating cell progression and tumorigenesis via Wnt/β-catenin pathway. Oncotarget. 2017;8:2604-2616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 82. | Jia H, Gao Z, Yu F, Guo H, Li B. Actin-binding protein anillin promotes the progression of hepatocellular carcinoma in vitro and in mice. Exp Ther Med. 2021;21:454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 83. | Peng YY, He YH, Chen C, Xu T, Li L, Ni MM, Meng XM, Huang C, Li J. NLRC5 regulates cell proliferation, migration and invasion in hepatocellular carcinoma by targeting the Wnt/β-catenin signaling pathway. Cancer Lett. 2016;376:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 84. | Tian Y, Wu J, Chagas C, Du Y, Lyu H, He Y, Qi S, Peng Y, Hu J. CDCA5 overexpression is an Indicator of poor prognosis in patients with hepatocellular carcinoma (HCC). BMC Cancer. 2018;18:1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 85. | Kim JH, Kim HN, Lee KT, Lee JK, Choi SH, Paik SW, Rhee JC, Lowe AW. Gene expression profiles in gallbladder cancer: the close genetic similarity seen for early and advanced gallbladder cancers may explain the poor prognosis. Tumour Biol. 2008;29:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Hirata K, Ajiki T, Okazaki T, Horiuchi H, Fujita T, Kuroda Y. Frequent occurrence of abnormal E-cadherin/beta-catenin protein expression in advanced gallbladder cancers and its association with decreased apoptosis. Oncology. 2006;71:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Chang HJ, Yoo BC, Kim SW, Lee BL, Kim WH. Significance of PML and p53 protein as molecular prognostic markers of gallbladder carcinomas. Pathol Oncol Res. 2007;13:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Xin HY, Sun RQ, Zou JX, Wang PC, Wang JY, Ye YH, Liu KX, Hu ZQ, Zhou ZJ, Fan J, Zhou J, Zhou SL. Association of BRAF Variants With Disease Characteristics, Prognosis, and Targeted Therapy Response in Intrahepatic Cholangiocarcinoma. JAMA Netw Open. 2023;6:e231476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 89. | Zhou J, Hui X, Mao Y, Fan L. Identification of novel genes associated with a poor prognosis in pancreatic ductal adenocarcinoma via a bioinformatics analysis. Biosci Rep. 2019;39:BSR20190625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 90. | de Moraes FCA, de Almeida Barbosa AB, Sano VKT, Kelly FA, Burbano RMR. Pharmacogenetics of DPYD and treatment-related mortality on fluoropyrimidine chemotherapy for cancer patients: a meta-analysis and trial sequential analysis. BMC Cancer. 2024;24:1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 91. | Ruzzo A, Graziano F, Galli F, Galli F, Rulli E, Lonardi S, Ronzoni M, Massidda B, Zagonel V, Pella N, Mucciarini C, Labianca R, Ionta MT, Bagaloni I, Veltri E, Sozzi P, Barni S, Ricci V, Foltran L, Nicolini M, Biondi E, Bramati A, Turci D, Lazzarelli S, Verusio C, Bergamo F, Sobrero A, Frontini L, Menghi M, Magnani M. Dihydropyrimidine dehydrogenase pharmacogenetics for predicting fluoropyrimidine-related toxicity in the randomised, phase III adjuvant TOSCA trial in high-risk colon cancer patients. Br J Cancer. 2017;117:1269-1277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 92. | Maitland ML, Vasisht K, Ratain MJ. TPMT, UGT1A1 and DPYD: genotyping to ensure safer cancer therapy? Trends Pharmacol Sci. 2006;27:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, Lin TN, Hoshitsuki K, Nersting J, Kihira K, Hofmann U, Komada Y, Kato M, McCorkle R, Li L, Koh K, Najera CR, Kham SK, Isobe T, Chen Z, Chiew EK, Bhojwani D, Jeffries C, Lu Y, Schwab M, Inaba H, Pui CH, Relling MV, Manabe A, Hori H, Schmiegelow K, Yeoh AE, Evans WE, Yang JJ. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 379] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 94. | Ås J, Bertulyte I, Eriksson N, Magnusson PKE, Wadelius M, Hallberg P. HLA variants associated with azathioprine-induced pancreatitis in patients with Crohn's disease. Clin Transl Sci. 2022;15:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Brun MK, Bjørlykke KH, Viken MK, Stenvik GE, Klaasen RA, Gehin JE, Warren DJ, Sexton J, Sandanger Ø, Kvien TK, Mørk C, Haavardsholm EA, Jahnsen J, Goll GL, Lie BA, Bolstad N, Jørgensen KK, Syversen SW. HLA-DQ2 is associated with anti-drug antibody formation to infliximab in patients with immune-mediated inflammatory diseases. J Intern Med. 2023;293:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 96. | El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol. 2018;14:447-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 97. | SEARCH Collaborative Group; Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1693] [Cited by in RCA: 1462] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 98. | Li Z, Zhao D, Wang B. ABCB1 gene polymorphisms and glucocorticoid-induced avascular necrosis of the femoral head susceptibility: a meta-analysis. Med Sci Monit. 2014;20:2811-2816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Kim JM, Ryu JH, Lee KW, Hong SK, Yang K, Choi GS, Kim YA, Lee JY, Yi NJ, Kwon CHD, Chu CW, Suh KS, Joh JW. Effect of CYP3A5 on the Once-Daily Tacrolimus Conversion in Stable Liver Transplant Patients. J Clin Med. 2020;9:2897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Dore MP, Tomassini G, Rocchi C, Bulajic M, Carta M, Errigo A, Dimaggio A, Padedda F, Pes GM. Risk of Hemolytic Anemia in IBD Patients with Glucose-6-Phosphate Dehydrogenase Deficiency Treated with Mesalamine: Results of a Retrospective-Prospective and Ex Vivo Study. J Clin Med. 2023;12:4797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 101. | Zhu G, Pei L, Xia H, Tang Q, Bi F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer. 2021;20:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 318] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 102. | Hu Y, Tao SY, Deng JM, Hou ZK, Liang JQ, Huang QG, Li LH, Li HB, Chen YM, Yi H, Chen XL, Liu H. Prognostic Value of NRAS Gene for Survival of Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Asian Pac J Cancer Prev. 2018;19:3001-3008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Rizzo A, Federico AD, Ricci AD, Frega G, Palloni A, Pagani R, Tavolari S, Marco MD, Brandi G. Targeting BRAF-Mutant Biliary Tract Cancer: Recent Advances and Future Challenges. Cancer Control. 2020;27:1073274820983013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5528] [Article Influence: 345.5] [Reference Citation Analysis (3)] |
| 105. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 5175] [Article Influence: 575.0] [Reference Citation Analysis (0)] |
| 106. | Shah PM, Peersen A, Jatoi A, Mcwilliams RR, Jin Z, Torbenson MS, Halfdanarson TR, Babiker HM, Conboy C, Kankeu Fonkoua LA, Washburn L, Roberts LR, Gores GJ, Bekaii-Saab TS, Borad MJ, Ou F, Tran NH. Prognostication of ß-catenin (CTNNB1) in patients with HCC treated with first line immunotherapy. J Clin Oncol. 2024;42:e15146. [DOI] [Full Text] |
| 107. | Xiao G, Jin LL, Liu CQ, Wang YC, Meng YM, Zhou ZG, Chen J, Yu XJ, Zhang YJ, Xu J, Zheng L. EZH2 negatively regulates PD-L1 expression in hepatocellular carcinoma. J Immunother Cancer. 2019;7:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 108. | Curci D, Lucafò M, Cifù A, Fabris M, Bramuzzo M, Martelossi S, Franca R, Decorti G, Stocco G. Pharmacogenetic variants of infliximab response in young patients with inflammatory bowel disease. Clin Transl Sci. 2021;14:2184-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 109. | Yoon SM, Haritunians T, Chhina S, Liu Z, Yang S, Landers C, Li D, Ye BD, Shih D, Vasiliauskas EA, Ippoliti A, Rabizadeh S, Targan SR, Melmed GY, McGovern DPB. Colonic Phenotypes Are Associated with Poorer Response to Anti-TNF Therapies in Patients with IBD. Inflamm Bowel Dis. 2017;23:1382-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Ye BD, McGovern DP. Genetic variation in IBD: progress, clues to pathogenesis and possible clinical utility. Expert Rev Clin Immunol. 2016;12:1091-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 111. | Koder S, Repnik K, Ferkolj I, Pernat C, Skok P, Weersma RK, Potočnik U. Genetic polymorphism in ATG16L1 gene influences the response to adalimumab in Crohn's disease patients. Pharmacogenomics. 2015;16:191-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 112. | Juanola O, Moratalla A, Gutiérrez A, Sempere L, Zapater P, Giménez P, Almenta I, Peiró G, González-Navajas JM, Such JF, Francés R. Anti-TNF-alpha loss of response is associated with a decreased percentage of FoxP3+ T cells and a variant NOD2 genotype in patients with Crohn's disease. J Gastroenterol. 2015;50:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1765] [Article Influence: 252.1] [Reference Citation Analysis (0)] |
| 114. | Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, Shugart YY, Griffiths AM, Targan SR, Ippoliti AF, Bernard EJ, Mei L, Nicolae DL, Regueiro M, Schumm LP, Steinhart AH, Rotter JI, Duerr RH, Cho JH, Daly MJ, Brant SR. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1435] [Cited by in RCA: 1401] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 115. | Salvador-Martín S, López-Cauce B, Nuñez O, Laserna-Mendieta EJ, García MI, Lobato E, Abarca-Zabalía J, Sanjurjo-Saez M, Lucendo AJ, Marín-Jiménez I, Menchén LA, López-Fernández LA. Genetic predictors of long-term response and trough levels of infliximab in crohn's disease. Pharmacol Res. 2019;149:104478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Billiet T, Vande Casteele N, Van Stappen T, Princen F, Singh S, Gils A, Ferrante M, Van Assche G, Cleynen I, Vermeire S. Immunogenicity to infliximab is associated with HLA-DRB1. Gut. 2015;64:1344-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 117. | Hlavaty T, Ferrante M, Henckaerts L, Pierik M, Rutgeerts P, Vermeire S. Predictive model for the outcome of infliximab therapy in Crohn's disease based on apoptotic pharmacogenetic index and clinical predictors. Inflamm Bowel Dis. 2007;13:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Meng J, Wei Y, Deng Q, Li L, Li X. Study on the expression of TOP2A in hepatocellular carcinoma and its relationship with patient prognosis. Cancer Cell Int. 2022;22:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 119. | Wang Y, Shi F, Xing GH, Xie P, Zhao N, Yin YF, Sun SY, He J, Wang Y, Xuan SY. Protein Regulator of Cytokinesis PRC1 Confers Chemoresistance and Predicts an Unfavorable Postoperative Survival of Hepatocellular Carcinoma Patients. J Cancer. 2017;8:801-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 120. | Camilleri M, Carlson P, Valentin N, Acosta A, O'Neill J, Eckert D, Dyer R, Na J, Klee EW, Murray JA. Pilot study of small bowel mucosal gene expression in patients with irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol. 2016;311:G365-G376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 121. | Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1053-G1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 122. | Panchoo AV, VanNess GH, Rivera-Rivera E, Laborda TJ. Hereditary pancreatitis: An updated review in pediatrics. World J Clin Pediatr. 2022;11:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 123. | Fotopoulos G, Syrigos K, Saif MW. Genetic factors affecting patient responses to pancreatic cancer treatment. Ann Gastroenterol. 2016;29:466-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 124. | Wardell CP, Fujita M, Yamada T, Simbolo M, Fassan M, Karlic R, Polak P, Kim J, Hatanaka Y, Maejima K, Lawlor RT, Nakanishi Y, Mitsuhashi T, Fujimoto A, Furuta M, Ruzzenente A, Conci S, Oosawa A, Sasaki-Oku A, Nakano K, Tanaka H, Yamamoto Y, Michiaki K, Kawakami Y, Aikata H, Ueno M, Hayami S, Gotoh K, Ariizumi SI, Yamamoto M, Yamaue H, Chayama K, Miyano S, Getz G, Scarpa A, Hirano S, Nakamura T, Nakagawa H. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol. 2018;68:959-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (1)] |
| 125. | Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, Hiraoka N, Ojima H, Shimada K, Okusaka T, Kosuge T, Miyagawa S, Shibata T. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 1003] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 126. | Chalabi M, Verschoor YL, Tan PB, Balduzzi S, Van Lent AU, Grootscholten C, Dokter S, Büller NV, Grotenhuis BA, Kuhlmann K, Burger JW, Huibregtse IL, Aukema TS, Hendriks ER, Oosterling SJ, Snaebjornsson P, Voest EE, Wessels LF, Beets-Tan RG, Van Leerdam ME, Schumacher TN, van den Berg JG, Beets GL, Haanen JB. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair-Deficient Colon Cancer. N Engl J Med. 2024;390:1949-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 222] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 127. | Janjigian YY, Al-Batran SE, Wainberg ZA, Muro K, Molena D, Van Cutsem E, Hyung WJ, Wyrwicz L, Oh DY, Omori T, Moehler M, Garrido M, Oliveira SCS, Liberman M, Oliden VC, Smyth EC, Stein A, Bilici M, Alvarenga ML, Kozlov V, Rivera F, Kawazoe A, Serrano O, Heilbron E, Negro A, Kurland JF, Tabernero J; MATTERHORN Investigators. Perioperative Durvalumab in Gastric and Gastroesophageal Junction Cancer. N Engl J Med. 2025;393:217-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 101] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 128. | Ros J, Vaghi C, Baraibar I, Saoudi González N, Rodríguez-Castells M, García A, Alcaraz A, Salva F, Tabernero J, Elez E. Targeting KRAS G12C Mutation in Colorectal Cancer, A Review: New Arrows in the Quiver. Int J Mol Sci. 2024;25:3304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 129. | Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 905] [Cited by in RCA: 1144] [Article Influence: 190.7] [Reference Citation Analysis (0)] |
| 130. | Corvaja C, Passaro A, Attili I, Aliaga PT, Spitaleri G, Signore ED, de Marinis F. Advancements in fourth-generation EGFR TKIs in EGFR-mutant NSCLC: Bridging biological insights and therapeutic development. Cancer Treat Rev. 2024;130:102824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 131. | Mahaki H, Nobari S, Tanzadehpanah H, Babaeizad A, Kazemzadeh G, Mehrabzadeh M, Valipour A, Yazdinezhad N, Manoochehri H, Yang P, Sheykhhasan M. Targeting VEGF signaling for tumor microenvironment remodeling and metastasis inhibition: Therapeutic strategies and insights. Biomed Pharmacother. 2025;186:118023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 132. | Elez E, Yoshino T, Shen L, Lonardi S, Van Cutsem E, Eng C, Kim TW, Wasan HS, Desai J, Ciardiello F, Yaeger R, Maughan TS, Morris VK, Wu C, Usari T, Laliberte R, Dychter SS, Zhang X, Tabernero J, Kopetz S; BREAKWATER Trial Investigators. Encorafenib, Cetuximab, and mFOLFOX6 in BRAF-Mutated Colorectal Cancer. N Engl J Med. 2025;392:2425-2437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 133. | Shitara K, Shah MA, Lordick F, Van Cutsem E, Ilson DH, Klempner SJ, Kang YK, Lonardi S, Hung YP, Yamaguchi K, Enzinger P, Nakajima T, Matsangou M, Cao Y, Li R, Moran D, Pophale R, Oh M, Ranganath R, Ajani JA, Xu RH. Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma. N Engl J Med. 2024;391:1159-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 134. | Manea CA, Badiu DC, Ploscaru IC, Zgura A, Bacinschi X, Smarandache CG, Serban D, Popescu CG, Grigorean VT, Botnarciuc V. A review of NTRK fusions in cancer. Ann Med Surg (Lond). 2022;79:103893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 135. | Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De Toni EN, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam VC, Mody K, Thungappa SC, Ostapenko Y, Yau T, Azevedo S, Varela M, Cheng AL, Qin S, Galle PR, Ali S, Marcovitz M, Makowsky M, He P, Kurland JF, Negro A, Sangro B. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1:EVIDoa2100070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 859] [Article Influence: 214.8] [Reference Citation Analysis (0)] |
| 136. | Li AY, McCusker MG, Russo A, Scilla KA, Gittens A, Arensmeyer K, Mehra R, Adamo V, Rolfo C. RET fusions in solid tumors. Cancer Treat Rev. 2019;81:101911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 137. | Hyung S, Han B, Jung J, Kim ST, Hong JY, Park SH, Zang DY, Park JO, Park YS, Kim KM, Kang WK, Lee J. Incidence of FGFR2 Amplification and FGFR2 Fusion in Patients with Metastatic Cancer Using Clinical Sequencing. J Oncol. 2022;2022:9714570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Carosi F, Broseghini E, Fabbri L, Corradi G, Gili R, Forte V, Roncarati R, Filippini DM, Ferracin M. Targeting Isocitrate Dehydrogenase (IDH) in Solid Tumors: Current Evidence and Future Perspectives. Cancers (Basel). 2024;16:2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 139. | Alhusaini A, Cannon A, Maher SG, Reynolds JV, Lynam-Lennon N. Therapeutic Potential of PARP Inhibitors in the Treatment of Gastrointestinal Cancers. Biomedicines. 2021;9:1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 140. | Kim BK. The Position of Multikinase Inhibitors in the Era of Immune-Checkpoint Inhibitors for Hepatocellular Carcinoma. Gut Liver. 2024;18:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 141. | Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B, Godio L, Patterson S, Rodriguez-Bigas MA, Jester SL, King KL, Schumacher M, Abbruzzese J, DuBois RN, Hittelman WN, Zimmerman S, Sherman JW, Kelloff G. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1815] [Cited by in RCA: 1726] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 142. | Floreani A, Gabbia D, De Martin S. Obeticholic Acid for Primary Biliary Cholangitis. Biomedicines. 2022;10:2464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 143. | Garrelfs SF, Frishberg Y, Hulton SA, Koren MJ, O'Riordan WD, Cochat P, Deschênes G, Shasha-Lavsky H, Saland JM, Van't Hoff WG, Fuster DG, Magen D, Moochhala SH, Schalk G, Simkova E, Groothoff JW, Sas DJ, Meliambro KA, Lu J, Sweetser MT, Garg PP, Vaishnaw AK, Gansner JM, McGregor TL, Lieske JC; ILLUMINATE-A Collaborators. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N Engl J Med. 2021;384:1216-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 380] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 144. | Strnad P, Mandorfer M, Choudhury G, Griffiths W, Trautwein C, Loomba R, Schluep T, Chang T, Yi M, Given BD, Hamilton JC, San Martin J, Teckman JH. Fazirsiran for Liver Disease Associated with Alpha(1)-Antitrypsin Deficiency. N Engl J Med. 2022;387:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 145. | Feng Z, Kang G, Wang J, Gao X, Wang X, Ye Y, Liu L, Zhao J, Liu X, Huang H, Cao X. Breaking through the therapeutic ceiling of inflammatory bowel disease: Dual-targeted therapies. Biomed Pharmacother. 2023;158:114174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 146. | Liu E, Aslam N, Nigam G, Limdi JK. Tofacitinib and newer JAK inhibitors in inflammatory bowel disease-where we are and where we are going. Drugs Context. 2022;11:2021-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 147. | Li Q, Han J, Yang Y, Chen Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front Immunol. 2022;13:1070961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 148. | Zhang J, Guo L, Liu X, Li W, Ying J. MET overexpression, gene amplification and relevant clinicopathological features in gastric adenocarcinoma. Oncotarget. 2017;8:10264-10273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 149. | High KA, Roncarolo MG. Gene Therapy. N Engl J Med. 2019;381:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 389] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 150. | Greuter T, Biedermann L, Rogler G, Sauter B, Seibold F. Alicaforsen, an antisense inhibitor of ICAM-1, as treatment for chronic refractory pouchitis after proctocolectomy: A case series. United European Gastroenterol J. 2016;4:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 151. | Ferreira V, Petry H, Salmon F. Immune Responses to AAV-Vectors, the Glybera Example from Bench to Bedside. Front Immunol. 2014;5:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 152. | Yan Y, Li S, Jia T, Du X, Xu Y, Zhao Y, Li L, Liang K, Liang W, Sun H, Li R. Combined therapy with CTL cells and oncolytic adenovirus expressing IL-15-induced enhanced antitumor activity. Tumour Biol. 2015;36:4535-4543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 153. | Lou E, Choudhry MS, Starr TK, Folsom TD, Bell J, Rathmann B, DeFeo AP, Kim J, Slipek N, Jin Z, Sumstad D, Klebanoff CA, Ladner K, Sarkari A, McIvor RS, Murray TA, Miller JS, Rao M, Jensen E, Ankeny J, Khalifa MA, Chauhan A, Spilseth B, Dixit A, Provenzano PP, Pan W, Weber D, Byrne-Steele M, Henley T, McKenna DH, Johnson MJ, Webber BR, Moriarity BS. Targeting the intracellular immune checkpoint CISH with CRISPR-Cas9-edited T cells in patients with metastatic colorectal cancer: a first-in-human, single-centre, phase 1 trial. Lancet Oncol. 2025;26:559-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 154. | Pal SK, Reimers MA, Garmezy B, Xu W, Hoimes CJ, Weinstein M, Cullingford EL, Ma A, Williams LM, Dar H, Keegan A, Srour SA. A phase 1/2, open-label, multicenter, dose escalation and cohort expansion study of the safety and efficacy of anti-CD70 allogeneic CRISPR-Cas9–engineered T cells (CTX131) in adult patients with relapsed or refractory solid tumors. J Clin Oncol. 2024;42:TPS2676. [DOI] [Full Text] |
| 155. | Garrett Nichols W, Bloomston M, Huitzil D, Rosas-Camargo V, Webber E, Ramirez-Marquez M, Murillo-Cordova A, Swan R, Dwyer J, Barone F, Tak PP. 653 Neoadjuvant CAN-2409+Prodrug plus chemoradiation for borderline resectable or locally advanced non-metastatic pancreatic adenocarcinoma (PDAC). J Immunother Cancer. 2023;11. [DOI] [Full Text] |
| 156. | Lopez J, Powles T, Braiteh F, Siu LL, LoRusso P, Friedman CF, Balmanoukian AS, Gordon M, Yachnin J, Rottey S, Karydis I, Fisher GA, Schmidt M, Schuler M, Sullivan RJ, Burris HA, Galvao V, Henick BS, Dirix L, Jaeger D, Ott PA, Wong KM, Jerusalem G, Schiza A, Fong L, Steeghs N, Leidner RS, Rittmeyer A, Laurie SA, Gort E, Aljumaily R, Melero I, Sabado RL, Rhee I, Mancuso MR, Muller L, Fine GD, Yadav M, Kim L, Leveque VJP, Robert A, Darwish M, Qi T, Zhu J, Zhang J, Twomey P, Rao GK, Low DW, Petry C, Lo AA, Schartner JM, Delamarre L, Mellman I, Löwer M, Müller F, Derhovanessian E, Cortini A, Manning L, Maurus D, Brachtendorf S, Lörks V, Omokoko T, Godehardt E, Becker D, Hawner C, Wallrapp C, Albrecht C, Kröner C, Tadmor AD, Diekmann J, Vormehr M, Jork A, Paruzynski A, Lang M, Blake J, Hennig O, Kuhn AN, Sahin U, Türeci Ö, Camidge DR. Autogene cevumeran with or without atezolizumab in advanced solid tumors: a phase 1 trial. Nat Med. 2025;31:152-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 49] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 157. | Hoffmann SV, O'Shea JP, Galvin P, Jannin V, Griffin BT. State-of-the-art and future perspectives in ingestible remotely controlled smart capsules for drug delivery: A GENEGUT review. Eur J Pharm Sci. 2024;203:106911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 158. | Camilleri AE, Nag S, Russo AR, Stiles KM, Crystal RG, Pagovich OE. Gene therapy for a murine model of eosinophilic esophagitis. Allergy. 2021;76:2740-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 159. | Aguilar LK, Shirley LA, Chung VM, Marsh CL, Walker J, Coyle W, Marx H, Bekaii-Saab T, Lesinski GB, Swanson B, Sanchez D, Manzanera AG, Aguilar-Cordova E, Bloomston M. Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma. Cancer Immunol Immunother. 2015;64:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 160. | Sangro B, Mazzolini G, Ruiz M, Ruiz J, Quiroga J, Herrero I, Qian C, Benito A, Larrache J, Olagüe C, Boan J, Peñuelas I, Sádaba B, Prieto J. A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010;17:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 161. | Lee JC, Shin DW, Park H, Kim J, Youn Y, Kim JH, Kim J, Hwang JH. Tolerability and safety of EUS-injected adenovirus-mediated double-suicide gene therapy with chemotherapy in locally advanced pancreatic cancer: a phase 1 trial. Gastrointest Endosc. 2020;92:1044-1052.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 162. | Noonan AM, Farren MR, Geyer SM, Huang Y, Tahiri S, Ahn D, Mikhail S, Ciombor KK, Pant S, Aparo S, Sexton J, Marshall JL, Mace TA, Wu CS, El-Rayes B, Timmers CD, Zwiebel J, Lesinski GB, Villalona-Calero MA, Bekaii-Saab TS. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol Ther. 2016;24:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 163. | Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T Jr, Brockstedt DG, Jaffee EM. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 490] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 164. | Chang KJ, Reid T, Senzer N, Swisher S, Pinto H, Hanna N, Chak A, Soetikno R. Phase I evaluation of TNFerade biologic plus chemoradiotherapy before esophagectomy for locally advanced resectable esophageal cancer. Gastrointest Endosc. 2012;75:1139-46.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 165. | Shahriar SMS, An JM, Hasan MN, Surwase SS, Kim YC, Lee DY, Cho S, Lee YK. Plasmid DNA Nanoparticles for Nonviral Oral Gene Therapy. Nano Lett. 2021;21:4666-4675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (13)] |
| 166. | Herweijer H, Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene Ther. 2007;14:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 167. | Preta G. Development of New Genome Editing Tools for the Treatment of Hyperlipidemia. Cells. 2023;12:2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 168. | Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J. Don't edit the human germ line. Nature. 2015;519:410-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 169. | Haft DH, Selengut J, Mongodin EF, Nelson KE. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol. 2005;1:e60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 711] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 170. | Boti MA, Athanasopoulou K, Adamopoulos PG, Sideris DC, Scorilas A. Recent Advances in Genome-Engineering Strategies. Genes (Basel). 2023;14:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 171. | Yau EH, Kummetha IR, Lichinchi G, Tang R, Zhang Y, Rana TM. Genome-Wide CRISPR Screen for Essential Cell Growth Mediators in Mutant KRAS Colorectal Cancers. Cancer Res. 2017;77:6330-6339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 172. | Li Y, Li X, Qu J, Luo D, Hu Z. Cas9 Mediated Correction of β-catenin Mutation and Restoring the Expression of Protein Phosphorylation in Colon Cancer HCT-116 Cells Decrease Cell Proliferation in vitro and Hamper Tumor Growth in Mice in vivo. Onco Targets Ther. 2020;13:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 173. | Zhang YQ, Pei JH, Shi SS, Guo XS, Cui GY, Li YF, Zhang HP, Hu WQ. CRISPR/Cas9-mediated knockout of the PDEF gene inhibits migration and invasion of human gastric cancer AGS cells. Biomed Pharmacother. 2019;111:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 174. | Seino T, Kawasaki S, Shimokawa M, Tamagawa H, Toshimitsu K, Fujii M, Ohta Y, Matano M, Nanki K, Kawasaki K, Takahashi S, Sugimoto S, Iwasaki E, Takagi J, Itoi T, Kitago M, Kitagawa Y, Kanai T, Sato T. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell. 2018;22:454-467.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 485] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 175. | Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, Mangan PA, Kulikovskaya I, Gupta M, Chen F, Tian L, Gonzalez VE, Xu J, Jung IY, Melenhorst JJ, Plesa G, Shea J, Matlawski T, Cervini A, Gaymon AL, Desjardins S, Lamontagne A, Salas-Mckee J, Fesnak A, Siegel DL, Levine BL, Jadlowsky JK, Young RM, Chew A, Hwang WT, Hexner EO, Carreno BM, Nobles CL, Bushman FD, Parker KR, Qi Y, Satpathy AT, Chang HY, Zhao Y, Lacey SF, June CH. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367:eaba7365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1058] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 176. | Drost J, van Boxtel R, Blokzijl F, Mizutani T, Sasaki N, Sasselli V, de Ligt J, Behjati S, Grolleman JE, van Wezel T, Nik-Zainal S, Kuiper RP, Cuppen E, Clevers H. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science. 2017;358:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 344] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 177. | Skoufou-Papoutsaki N, Adler S, D'Santos P, Mannion L, Mehmed S, Kemp R, Smith A, Perrone F, Nayak K, Russell A, Zilbauer M, Winton DJ. Efficient genetic editing of human intestinal organoids using ribonucleoprotein-based CRISPR. Dis Model Mech. 2023;16:dmm050279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 178. | Sabatino DE, Bushman FD, Chandler RJ, Crystal RG, Davidson BL, Dolmetsch R, Eggan KC, Gao G, Gil-Farina I, Kay MA, McCarty DM, Montini E, Ndu A, Yuan J; American Society of Gene and Cell Therapy (ASGCT) Working Group on AAV Integration. Evaluating the state of the science for adeno-associated virus integration: An integrated perspective. Mol Ther. 2022;30:2646-2663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 179. | Ferla R, Alliegro M, Dell'Anno M, Nusco E, Cullen JM, Smith SN, Wolfsberg TG, O'Donnell P, Wang P, Nguyen AD, Chandler RJ, Chen Z, Burgess SM, Vite CH, Haskins ME, Venditti CP, Auricchio A. Low incidence of hepatocellular carcinoma in mice and cats treated with systemic adeno-associated viral vectors. Mol Ther Methods Clin Dev. 2021;20:247-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 180. | Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 699] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 181. | Kruse RL, Huang Y, Kumbhari V. How to Embrace Gene Therapy in Gastroenterology. Gastroenterology. 2022;162:1019-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 182. | Yao S, Rong W, Yuan Y. Optimization of adeno-associated virus (AAV) gene delivery into human bone marrow stem cells (hBMSCs). Stem Cell Investig. 2023;10:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 183. | Chehelgerdi M, Chehelgerdi M, Khorramian-Ghahfarokhi M, Shafieizadeh M, Mahmoudi E, Eskandari F, Rashidi M, Arshi A, Mokhtari-Farsani A. Comprehensive review of CRISPR-based gene editing: mechanisms, challenges, and applications in cancer therapy. Mol Cancer. 2024;23:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 125] [Article Influence: 62.5] [Reference Citation Analysis (1)] |
| 184. | Hampton T. Ethical and Societal Questions Loom Large as Gene Editing Moves Closer to the Clinic. JAMA. 2016;315:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 185. | Zhao H, Zhang Z, Liu H, Ma M, Sun P, Zhao Y, Liu X. Multi-omics perspective: mechanisms of gastrointestinal injury repair. Burns Trauma. 2025;13:tkae057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 186. | Cross B, Turner R, Pirmohamed M. Polygenic risk scores: An overview from bench to bedside for personalised medicine. Front Genet. 2022;13:1000667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 187. | Misra P, Jadhav AR, Bapat SA. Single-cell sequencing: A cutting edge tool in molecular medical research. Med J Armed Forces India. 2022;78:S7-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 188. | Yang S, Hu H, Kung H, Zou R, Dai Y, Hu Y, Wang T, Lv T, Yu J, Li F. Organoids: The current status and biomedical applications. MedComm (2020). 2023;4:e274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 141] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 189. | Struhl K. The distinction between epigenetics and epigenomics. Trends Genet. 2024;40:995-997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 190. | Suzuki K, Yokoyama J, Kawauchi Y, Honda Y, Sato H, Aoyagi Y, Terai S, Okazaki K, Suzuki Y, Sameshima Y, Fukushima T, Sugahara K, Atreya R, Neurath MF, Watanabe K, Yoneyama H, Asakura H. Phase 1 Clinical Study of siRNA Targeting Carbohydrate Sulphotransferase 15 in Crohn's Disease Patients with Active Mucosal Lesions. J Crohns Colitis. 2017;11:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 191. | Qin S, Tang X, Chen Y, Chen K, Fan N, Xiao W, Zheng Q, Li G, Teng Y, Wu M, Song X. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct Target Ther. 2022;7:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 470] [Article Influence: 117.5] [Reference Citation Analysis (6)] |
| 192. | Parums DV. Editorial: First Regulatory Approvals for CRISPR-Cas9 Therapeutic Gene Editing for Sickle Cell Disease and Transfusion-Dependent β-Thalassemia. Med Sci Monit. 2024;30:e944204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 193. | Nagpal M, Nagpal K, Nagpal PN. A comparative debate on the various anti-vascular endothelial growth factor drugs: pegaptanib sodium (Macugen), ranibizumab (Lucentis) and bevacizumab (Avastin). Indian J Ophthalmol. 2007;55:437-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 194. | Wang Y, Liu H, Zhang M, Xu J, Zheng L, Liu P, Chen J, Liu H, Chen C. Epigenetic reprogramming in gastrointestinal cancer: biology and translational perspectives. MedComm (2020). 2024;5:e670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 195. | Kang EA, Jang J, Choi CH, Kang SB, Bang KB, Kim TO, Seo GS, Cha JM, Chun J, Jung Y, Kim HG, Im JP, Kim S, Ahn KS, Lee CK, Kim HJ, Kim MS, Park DI. Development of a Clinical and Genetic Prediction Model for Early Intestinal Resection in Patients with Crohn's Disease: Results from the IMPACT Study. J Clin Med. 2021;10:633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 196. | Garza-Hernandez D, Estrada K, Trevino V. Multivariate genome-wide association study models to improve prediction of Crohn's disease risk and identification of potential novel variants. Comput Biol Med. 2022;145:105398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 197. | Schophaus S, Creasy KT, Koop PH, Clusmann J, Jaeger J, Punnuru V, Koch A, Trautwein C, Loomba R, Luedde T, Schneider KM, Schneider CV. Machine learning uncovers manganese as a key nutrient associated with reduced risk of steatotic liver disease. Liver Int. 2024;44:2807-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 198. | Wang H, Ma S, Yang Z, Niu R, Zhu H, Li S, Gao S, Li Z, Tian Y. Revolutionizing ESCC prognosis: the efficiency of tumor-infiltrating immune cells (TIIC) signature score. Discov Oncol. 2025;16:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 199. | DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. 2016;22:1137-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 744] [Article Influence: 74.4] [Reference Citation Analysis (23)] |
| 200. | Cahana I, Iraqi FA. Impact of host genetics on gut microbiome: Take-home lessons from human and mouse studies. Animal Model Exp Med. 2020;3:229-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 201. | Jiang L, Fan JG. Gut microbiota in gastrointestinal diseases: Insights and therapeutic strategies. World J Gastroenterol. 2024;30:4329-4332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 202. | Tabrett A, Horton MW. The influence of host genetics on the microbiome. F1000Res. 2020;9:F1000 Faculty Rev-F1000 Faculty R84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 203. | Mallabar-Rimmer B, Merriel SWD, Webster AP, Jackson L, Wood AR, Barclay M, Tyrrell J, Ruth KS, Thirlwell C, Oram R, Weedon MN, Bailey SER, Green HD. Colorectal cancer risk stratification using a polygenic risk score in symptomatic primary care patients-a UK Biobank retrospective cohort study. Eur J Hum Genet. 2024;32:1456-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 204. | Huyghe JR, Harrison TA, Bien SA, Hampel H, Figueiredo JC, Schmit SL, Conti DV, Chen S, Qu C, Lin Y, Barfield R, Baron JA, Cross AJ, Diergaarde B, Duggan D, Harlid S, Imaz L, Kang HM, Levine DM, Perduca V, Perez-Cornago A, Sakoda LC, Schumacher FR, Slattery ML, Toland AE, van Duijnhoven FJB, Van Guelpen B, Agudo A, Albanes D, Alonso MH, Anderson K, Arnau-Collell C, Arndt V, Banbury BL, Bassik MC, Berndt SI, Bézieau S, Bishop DT, Boehm J, Boeing H, Boutron-Ruault MC, Brenner H, Brezina S, Buch S, Buchanan DD, Burnett-Hartman A, Caan BJ, Campbell PT, Carr PR, Castells A, Castellví-Bel S, Chan AT, Chang-Claude J, Chanock SJ, Curtis KR, de la Chapelle A, Easton DF, English DR, Feskens EJM, Gala M, Gallinger SJ, Gauderman WJ, Giles GG, Goodman PJ, Grady WM, Grove JS, Gsur A, Gunter MJ, Haile RW, Hampe J, Hoffmeister M, Hopper JL, Hsu WL, Huang WY, Hudson TJ, Jenab M, Jenkins MA, Joshi AD, Keku TO, Kooperberg C, Kühn T, Küry S, Le Marchand L, Lejbkowicz F, Li CI, Li L, Lieb W, Lindblom A, Lindor NM, Männistö S, Markowitz SD, Milne RL, Moreno L, Murphy N, Nassir R, Offit K, Ogino S, Panico S, Parfrey PS, Pearlman R, Pharoah PDP, Phipps AI, Platz EA, Potter JD, Prentice RL, Qi L, Raskin L, Rennert G, Rennert HS, Riboli E, Schafmayer C, Schoen RE, Seminara D, Song M, Su YR, Tangen CM, Thibodeau SN, Thomas DC, Trichopoulou A, Ulrich CM, Visvanathan K, Vodicka P, Vodickova L, Vymetalkova V, Weigl K, Weinstein SJ, White E, Wolk A, Woods MO, Wu AH, Abecasis GR, Nickerson DA, Scacheri PC, Kundaje A, Casey G, Gruber SB, Hsu L, Moreno V, Hayes RB, Newcomb PA, Peters U. Genetic architectures of proximal and distal colorectal cancer are partly distinct. Gut. 2021;70:1325-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 205. | De Vincentis A, Tavaglione F, Jamialahmadi O, Picardi A, Antonelli Incalzi R, Valenti L, Romeo S, Vespasiani-Gentilucci U. A Polygenic Risk Score to Refine Risk Stratification and Prediction for Severe Liver Disease by Clinical Fibrosis Scores. Clin Gastroenterol Hepatol. 2022;20:658-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 206. | Gettler K, Levantovsky R, Moscati A, Giri M, Wu Y, Hsu NY, Chuang LS, Sazonovs A, Venkateswaran S, Korie U, Chasteau C; UK IBD Genetics Consortium, National Institute of Diabetes, Digestive and Kidney Diseases Inflammatory Bowel Disease Genetics Consortium, Duerr RH, Silverberg MS, Snapper SB, Daly MJ, McGovern DP, Brant SR, Rioux JD, Kugathasan S, Anderson CA, Itan Y, Cho JH. Common and Rare Variant Prediction and Penetrance of IBD in a Large, Multi-ethnic, Health System-based Biobank Cohort. Gastroenterology. 2021;160:1546-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 207. | De Vincentis A, D'Amato D, Cristoferi L, Gerussi A, Malinverno F, Lleo A, Colapietro F, Marra F, Galli A, Fiorini C, Coco B, Brunetto M, Niro GA, Cotugno R, Saitta C, Cozzolongo R, Losito F, Giannini EG, Labanca S, Marzioni M, Marconi G, Morgando A, Pellicano R, Vanni E, Cazzagon N, Floreani A, Chessa L, Morelli O, Muratori L, Pellicelli A, Pompili M, Ponziani F, Tortora A, Rosina F, Russello M, Cannavò M, Simone L, Storato S, Viganò M, Abenavoli L, D'Antò M, De Gasperi E, Distefano M, Scifo G, Zolfino T, Calvaruso V, Cuccorese G, Palitti VP, Sacco R, Bertino G, Frazzetto E, Alvaro D, Mulinacci G, Palermo A, Scaravaglio M, Terracciani F, Galati G, Ronca V, Zuin M, Claar E, Izzi A, Picardi A, Invernizzi P, Vespasiani-Gentilucci U, Carbone M; on behalf of the Club Epatologi Ospedalieri (CLEO)/Associazione Italiana Gastroenterologi ed Endoscopisti Digestivi Ospedalieri (AIGO) PBC Study Group and, the Italian PBC Registry. Predictors of serious adverse events and non-response in cirrhotic patients with primary biliary cholangitis treated with obeticholic acid. Liver Int. 2022;42:2453-2465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 208. | Thomas CE, Diergaarde B, Kuipers AL, Adibi JJ, Luu HN, Chang X, Dorajoo R, Heng CK, Khor CC, Wang R, Jin A, Koh WP, Yuan JM. NAFLD polygenic risk score and risk of hepatocellular carcinoma in an East Asian population. Hepatol Commun. 2022;6:2310-2321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 209. | Slunecka JL, van der Zee MD, Beck JJ, Johnson BN, Finnicum CT, Pool R, Hottenga JJ, de Geus EJC, Ehli EA. Implementation and implications for polygenic risk scores in healthcare. Hum Genomics. 2021;15:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 210. | Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1905] [Article Influence: 272.1] [Reference Citation Analysis (0)] |
| 211. | Jayasinghe D, Eshetie S, Beckmann K, Benyamin B, Lee SH. Advancements and limitations in polygenic risk score methods for genomic prediction: a scoping review. Hum Genet. 2024;143:1401-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 212. | Chen C, Wang J, Pan D, Wang X, Xu Y, Yan J, Wang L, Yang X, Yang M, Liu GP. Applications of multi-omics analysis in human diseases. MedComm (2020). 2023;4:e315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 232] [Reference Citation Analysis (0)] |
| 213. | Fatfat Z, Hussein M, Fatfat M, Gali-Muhtasib H. Omics technologies as powerful approaches to unravel colorectal cancer complexity and improve its management. Mol Cells. 2025;48:100200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (8)] |
| 214. | Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, Casero D, Courtney H, Gonzalez A, Graeber TG, Hall AB, Lake K, Landers CJ, Mallick H, Plichta DR, Prasad M, Rahnavard G, Sauk J, Shungin D, Vázquez-Baeza Y, White RA 3rd; IBDMDB Investigators, Braun J, Denson LA, Jansson JK, Knight R, Kugathasan S, McGovern DPB, Petrosino JF, Stappenbeck TS, Winter HS, Clish CB, Franzosa EA, Vlamakis H, Xavier RJ, Huttenhower C. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2111] [Reference Citation Analysis (0)] |
| 215. | Duan D. Lethal immunotoxicity in high-dose systemic AAV therapy. Mol Ther. 2023;31:3123-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 216. | Chandler RJ, LaFave MC, Varshney GK, Trivedi NS, Carrillo-Carrasco N, Senac JS, Wu W, Hoffmann V, Elkahloun AG, Burgess SM, Venditti CP. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest. 2015;125:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 325] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 217. | Atkins A, Chung CH, Allen AG, Dampier W, Gurrola TE, Sariyer IK, Nonnemacher MR, Wigdahl B. Off-Target Analysis in Gene Editing and Applications for Clinical Translation of CRISPR/Cas9 in HIV-1 Therapy. Front Genome Ed. 2021;3:673022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 218. | Hennig SL, Owen JR, Lin JC, Young AE, Ross PJ, Van Eenennaam AL, Murray JD. Evaluation of mutation rates, mosaicism and off target mutations when injecting Cas9 mRNA or protein for genome editing of bovine embryos. Sci Rep. 2020;10:22309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 219. | Niemiec E, Howard HC. Ethical issues related to research on genome editing in human embryos. Comput Struct Biotechnol J. 2020;18:887-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 220. | Shinwari ZK, Tanveer F, Khalil AT. Ethical Issues Regarding CRISPR Mediated Genome Editing. Curr Issues Mol Biol. 2018;26:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 221. | Graber E, Reiter EO, Rogol AD. Human Growth and Growth Hormone: From Antiquity to the Recominant Age to the Future. Front Endocrinol (Lausanne). 2021;12:709936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 222. | Ansah EO. Ethical Challenges and Controversies in the Practice and Advancement of Gene Therapy. Adv Cell Gene Ther. 2022;2022:1-5. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/