Published online Dec 28, 2025. doi: 10.3748/wjg.v31.i48.114355
Revised: October 15, 2025
Accepted: November 12, 2025
Published online: December 28, 2025
Processing time: 101 Days and 15 Hours
Biologic therapies have transformed the management of inflammatory bowel disease (IBD), yet their high cost poses substantial challenges for healthcare systems. Biosimilars offer a cost-effective alternative, with extensive evidence supporting the safety and efficacy of non-medical switching for infliximab and adalimumab. However, real-world data on ustekinumab biosimilars in IBD re
To evaluate clinical efficacy, treatment persistence, biomarker activity, and ad
This was an observational study of consecutive IBD patients who underwent a biosimilar switch. Disease activity, biomarkers, drug sustainability, and adverse events were captured 8 weeks before the switch, at the time of switch (baseline), 12 weeks, and 24 weeks after the switch.
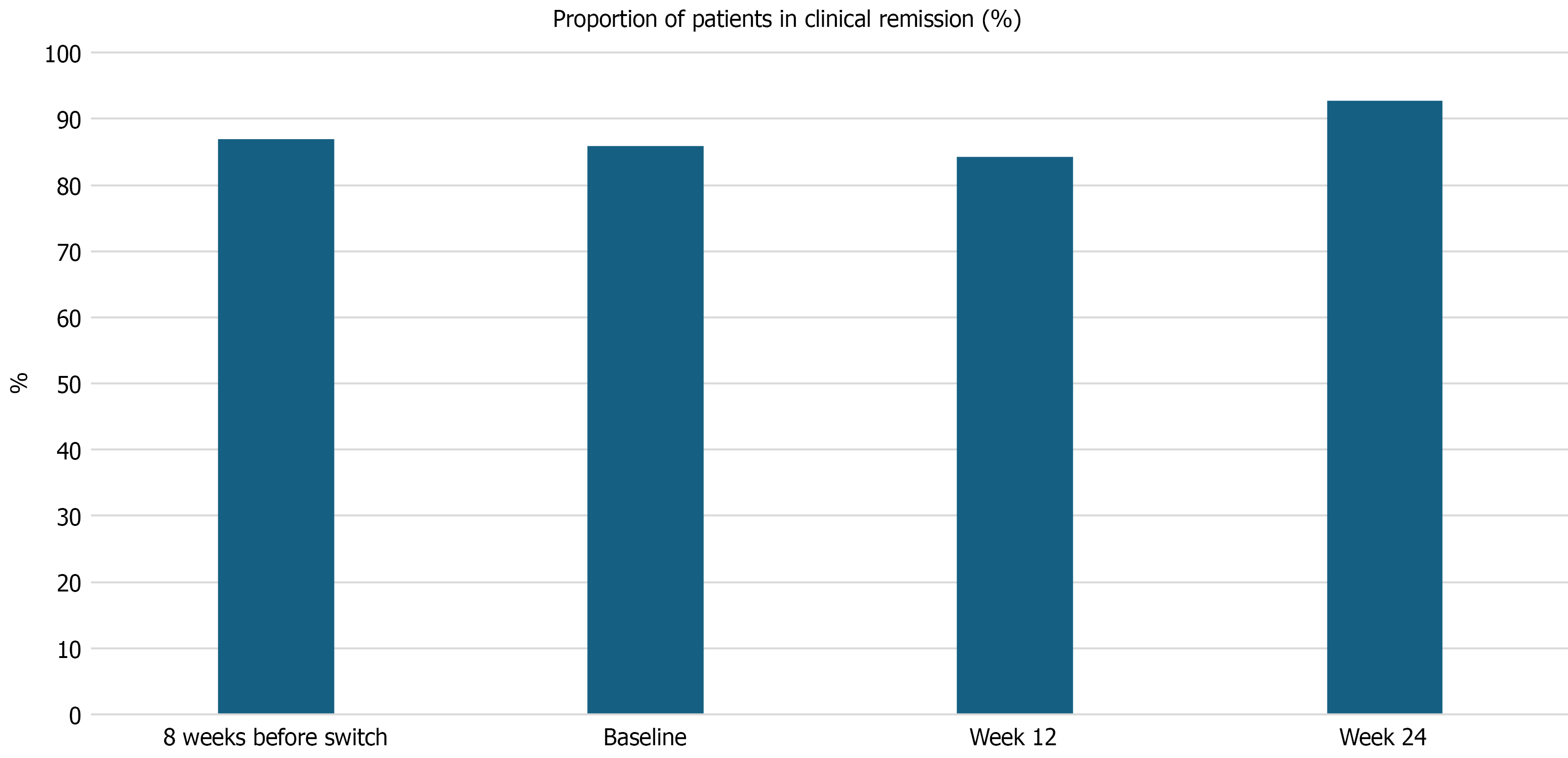
Of 81 patients were included [85.2% had Crohn’s disease, the median age at inclusion: 42 years (interquartile ranges: 29-61)]. Previous biological exposure was 82.7% and a dose optimization of the originator ustekinumab was performed in 63% before the switch. Drug sustainability at 12 weeks and 24 weeks of switch was 96.3% and 95%, regardless of disease type or phenotype. The discontinuation rate was 4.9%. There was no significant difference in the rates of clinical remission at week 8 before switch, baseline, week 12, and 24 after switch: 87%, 85.9%, 84.3%, and 92.7%, P = not statistically significant. The biomarker activity was not significantly different for C-reactive protein, hemoglobin, albumin, and fecal calprotectin (P = not statistically significant). All patients who stopped therapy after the non-medical switch needed a dose optimisation of the originator ustekinumab and had previous biological therapy prior to starting the ustekinumab originator.
Despite prior biologic exposure and frequent dose escalation, switching to ustekinumab biosimilar showed stable efficacy, unchanged biomarkers, and high treatment persistence.
Core Tip: Switching from expensive originator biologics to lower-cost biosimilars can help reduce healthcare costs, but safety and effectiveness must be proven. This is the first real-world study of ustekinumab biosimilars in inflammatory bowel disease that showed stable disease control, unchanged biomarkers, and high treatment persistence, even in patients with prior biologic use and dose intensification. Fewer than 5% discontinued therapy, and no new safety issues emerged. These findings provide reassurance that biosimilars are a safe and effective alternative, supporting their wider adoption in clinical practice while ensuring patient outcomes remain uncompromised.
- Citation: Kritzinger J, Candel I, Kotrri G, Nadeem H, Afif W, Bitton A, Wild G, Bessissow T, Lakatos PL. Clinical outcomes and drug sustainability after non-medical switch from ustekinumab originator to biosimilars in inflammatory bowel disease. World J Gastroenterol 2025; 31(48): 114355
- URL: https://www.wjgnet.com/1007-9327/full/v31/i48/114355.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i48.114355
The introduction of biologic therapies has revolutionized the management of inflammatory bowel disease (IBD), significantly improving clinical outcomes for patients with Crohn’s disease (CD) and ulcerative colitis (UC)[1,2]. Among these agents, ustekinumab - a fully human monoclonal antibody targeting the p40 subunit shared between interleukin (IL)-12 and IL-23 - has demonstrated efficacy in moderate to severe IBD and is now a cornerstone of treatment in both conditions[1,2]. However, the high cost of originator biologics has created challenges in terms of access and healthcare sustainability, particularly in publicly funded healthcare systems[3,4]. In Canada, using originator biologics for all indications was estimated to cost Canadian Dollars 7.7 billion in 2018, representing a significant financial burden on the healthcare system[5]. Furthermore, Canadians living with IBD incur substantial individual economic costs, with the combined indirect and out-of-pocket expenses estimated to be more than Canadian Dollars 2 billion in 2023[6]. As such, the high costs of biologic agents and their patent expiration date have prompted the development of highly similar versions of these drugs that are known as biosimilars.
Biosimilars are biologic agents that are highly similar to their reference product, with no clinically meaningful differences in safety, efficacy, or immunogenicity[7]. Regulatory bodies such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) allow biosimilars to be approved via an abbreviated pathway that includes extrapolation of efficacy and safety data from one indication to others held by the reference product[8,9]. In Canada, biosimilars are introduced into patient care through one of two pathways: (1) A new start (for patients requiring biologic therapy who have not previously used the originator drug); or (2) A non-medical switch (where insurers decide to no longer cover the more expensive originator drug)[10]. The expiration of patents for us
In jurisdictions such as Quebec, health policy has recently mandated non-medical switching from originator ustekinumab to biosimilars to reduce drug expenditures. Although similar transitions have been studied extensively with anti-tumor necrosis factor (TNF) biosimilars such as infliximab and adalimumab - demonstrating comparable efficacy, safety, and immunogenicity[14] - real-world data evaluating biosimilar ustekinumab use in IBD does not currently exist. Moreover, concerns persist regarding the potential for loss of response, development of anti-drug antibodies, and the nocebo effect - where patient expectations negatively influence treatment outcomes - particularly in open-label, non-medical switch scenarios[15]. To address these gaps, we conducted an observational cohort study evaluating adult patients with IBD at the tertiary IBD centre of McGill University, who underwent a mandatory non-medical switch from originator ustekinumab to a biosimilar. The primary aim was to assess the clinical efficacy, drug sustainability, loss of response, and safety of biosimilar ustekinumab in this real-world setting. These findings aim to inform clinicians, patients, and policymakers on the early experience and clinical impact of ustekinumab biosimilar adoption in routine IBD care.
The present retrospective observational study included consecutive adult IBD patients (> 18 years) treated with ustekinumab originator at the IBD Centre of the McGill University Health Centre (Montreal, Quebec, Canada), who underwent a mandatory non-medical switch between April 1, 2024, and November 6, 2024. All patients who underwent a mandatory non-medical switch to the biosimilar, based on reimbursement regulations, regardless of the actual disease activity, were included in the study. The original ustekinumab (Stellara®) was switched to ustekinumab-stba (Steqeyma, CT-P43). Dosing and intervals after the switch to the biosimilar were changed or adjusted only if this was deemed necessary due to the clinical need. Dose adjustments of the biosimilar or initiation of corticosteroid therapy were performed at the discretion of the treating physician. Clinical disease activity, laboratory parameters (including complete blood count, albumin, and inflammatory biomarkers), and adverse events (AEs) were assessed at week 8 prior to the switch, at baseline (time of switch), and at weeks 12 and 24 following the transition to the biosimilar.
Patient demographic characteristics, detailed disease phenotype (including disease location or extent, severity at diagnosis), and treatment history (previous and concomitant medications) were collected from the electronic medical records. Disease phenotype was assessed by using the Montreal classification[16]. Harvey-Bradshaw Index (HBI) for CD and the partial Mayo (pMayo) score for UC were used to assess disease activity. Clinical assessments and biochemical test results were captured at 8 weeks before the switch, at baseline, at 12 weeks, and at 24 weeks after the switch to the biosimilar. Data on clinical flares, need for corticosteroids, hospitalization, and emergency visits were collected. In addition, dosage optimization (i.v. reinduction or change in dosing frequency) and the need to change the biological agent were assessed at week 8 before the switch, at baseline, at week 12, and at week 24 after the switch to the biosimilar. The AEs were collected from the EMRs and patients’ self-reporting. Of note, physicians were asking specific AE-related questions during every clinical visit.
The primary outcome was clinical efficacy, both clinical remission (CR) and corticosteroid-free CR in patients treated with biosimilars after the switch from the originator. The secondary outcomes included biochemical tests, drug sustainability, and AEs before the switch and during the biosimilar treatment period. CR was defined as an HBI score < 5 and/or absence of drainage from active fistula(s) in patients with CD, and as a pMayo score < 3 with no individual subscore > 1 and a rectal bleeding subscore of 0 in patients with UC. Corticosteroid-free CR was defined as CR maintained without systemic corticosteroid use for at least one week prior to the assessment. Disease flare among patients previously in remission was defined as an HBI ≥ 5 for CD or a pMayo > 3 and/or rectal bleeding subscore > 1 for UC. Patients who discontinued the biosimilar or lacked follow-up clinical data were excluded from the efficacy analyses. Biochemical disease activity was assessed using serum C-reactive protein (CRP), with a normal reference value of < 5 mg/mL, and fecal calprotectin, with a normal reference value of < 250 µg/g. Endoscopic activity, where available, was defined as a Simple Endoscopic Score for CD > 2 for CD or an endoscopic Mayo (eMayo) score > 1 for UC. Drug sustainability was defined as the median time to treatment discontinuation following the switch to the biosimilar.
Continuous variables were summarized as medians with interquartile ranges (IQRs), and comparisons were performed using the analysis of variance test with separate variance estimates, as appropriate. Categorical variables were expressed as counts and percentages, and differences between groups were assessed using the χ² test or Fisher’s exact test. Drug sustainability was analyzed using Kaplan-Meier survival curves, with comparisons evaluated by the log-rank test. Statistical analyses were conducted using SPSS software version 20.0 (Chicago, IL, United States), and a two-sided P value < 0.05 was considered statistically significant.
Ethical approval of the study was obtained from the ethical committee of the McGill University Health Centre, Montreal, Canada (No. REB 2025-11484).
Overall, 81 IBD patients (CD: 85.2%, males: 46.9%) underwent a non-medical switch to a biosimilar ustekinumab from the originator. The median age at inclusion was 42.0 years (IQR: 29.0-61.0), the median duration of IBD was 12.0 years (IQR: 8.0-27.0), with a median 58 months (IQR: 31-89) duration of ustekinumab originator treatment before switching. More than half of the CD patients had extensive disease, and almost half of the CD patients had complicated disease behavior. Extensive colitis was diagnosed in more than 80% of UC patients, while 65% had moderate to severe disease at the time of diagnosis. Half of the CD patients had previously undergone intestinal surgeries. More than 60% of patients had previous biologic exposure, and approximately 20% of patients had exposure to multiple biologics before the start of the ustekinumab originator. At the time of the switch, 63% of the ustekinumab-treated patients received an intensified dose regimen (every 4 or 6 weeks). The baseline clinical characteristics of the IBD patient cohort are shown in Table 1.
| Characteristics | IBD (n = 81) |
| CD/UC | 69/12 |
| Male gender | 38 (46.9) |
| Median age at inclusion, years, (IQR) | 42.0 (22.0-61.0) |
| Median duration of disease, years, (IQR) | 12.0 (8.0-27.0) |
| Disease phenotype of CD | |
| A1/A2/A3 | 31.9%/50.7%/17.4% |
| L1/L2/L3 | 17.4%/24.6%/58% |
| B1/B2/B3 | 53.6%/28.8%/19.7% |
| Disease phenotype of UC1 | |
| UC: E1/E2/E3 | -/16.7/83.3 |
| UC: S1/S2/S32 | 33.3/16.7/50 |
| Upper GI involvement in CD | 9 (14.0) |
| Perianal/fistulizing disease in CD | 15 (21.7) |
| Extra-intestinal manifestation3 | 22 (27.2) |
| Previous intestinal surgery in CD4 | 35 (50.7) |
| Earlier medical therapy | |
| Oral 5-ASA | 39 (48.1) |
| Corticosteroid | 72 (88.8) |
| Immunomodulators (AZA, MTX) | 34 (42)/20 (24.7) |
| Ustekinumab originator dose before switch | |
| Q8 week | 30 (37) |
| Q6 week | 2 (2.5) |
| Q4 week | 49 (60.5) |
| Median duration of originators, months (IQR) | 58 (31-89) |
| Biological exposure before ustekinumab originator | 67 (82.7) |
| Multiple biologicals | 16 (19.7) |
| Infliximab | 44 (54.3) |
| Adalimumab | 32 (39.5) |
| Vedolizumab | 7 (8.6) |
There was no significant difference in the proportion of patients who remained in CR [87%, 85.9%, 84.3%, and 92.7%, P = not statistically significant (NS)] at 8 weeks before the switch, at baseline, or at week 12, and 24 after the switch to the biosimilar (Figure 1). The rates of flares at baseline, at week 12, and 24 after the switch to the biosimilar were also not significantly different (P = NS). Similarly, the proportion of patients who needed new systemic corticosteroids for active disease or flare (P = NS) was not significantly different. One CD patient required intestinal resection due to small bowel obstruction at 6 months after the switch. The clinical efficacy before and after the switch to the biosimilar ustekinumab is shown in Table 2. There was no significant difference in median biomarker activity in IBD patients at 8 weeks before the switch, at baseline, at week 12, and 24 after the switch to the biosimilar. The biomarkers of IBD patients following the biosimilar switch are shown in Table 2.
| 8 weeks before the switch | At switch (baseline) | Week 12 after the switch | Week 24 after the switch | P value | |
| Clinical activity | |||||
| Clinical remission | 87 | 85.9 | 84.3 | 92.7 | NS1 |
| IBD flares | NA | 7.4 | 2.7 | 3.8 | NS1 |
| Need for systemic corticosteroid | 3.7 | 3.1 | 1.7 | 3.8 | NS1 |
| Ustekinumab dose modification | NA | 2.5 | 1.4 | 0 | NS1 |
| Need for intestinal surgery | 0 | 0 | 0 | 1.7 | NS1 |
| Biomarker activity | |||||
| Median CRP levels, mg/mL (IQR) | 2.8 (1.1-4.5) | 2.5 (1.1-4.7) | 2.0 (1.0-4.2) | 2.7 (0.7-7.8) | NS1 |
| Median FCAL levels mcg/g (IQR) | 97.0 (46.0-353.0) | 100.0 (74.0-342.3) | 103.0 (71.5-246.0) | 128.6 (58.6-262.5) | NS1 |
| Median hemoglobin level mg/mL (IQR) | 135.0 (125.0-145.0) | 138.0 (130.0-151.0) | 139.0 (127.0-152.0) | 136.0 (129.0-149.0) | NS1 |
| Median albumin level mg/mL (IQR) | 42.0 (38.0-45.0) | 41.0 (39.0 -45.0) | 41.0 (39.0-43.0) | 42.0 (38.0-46.0) | NS1 |
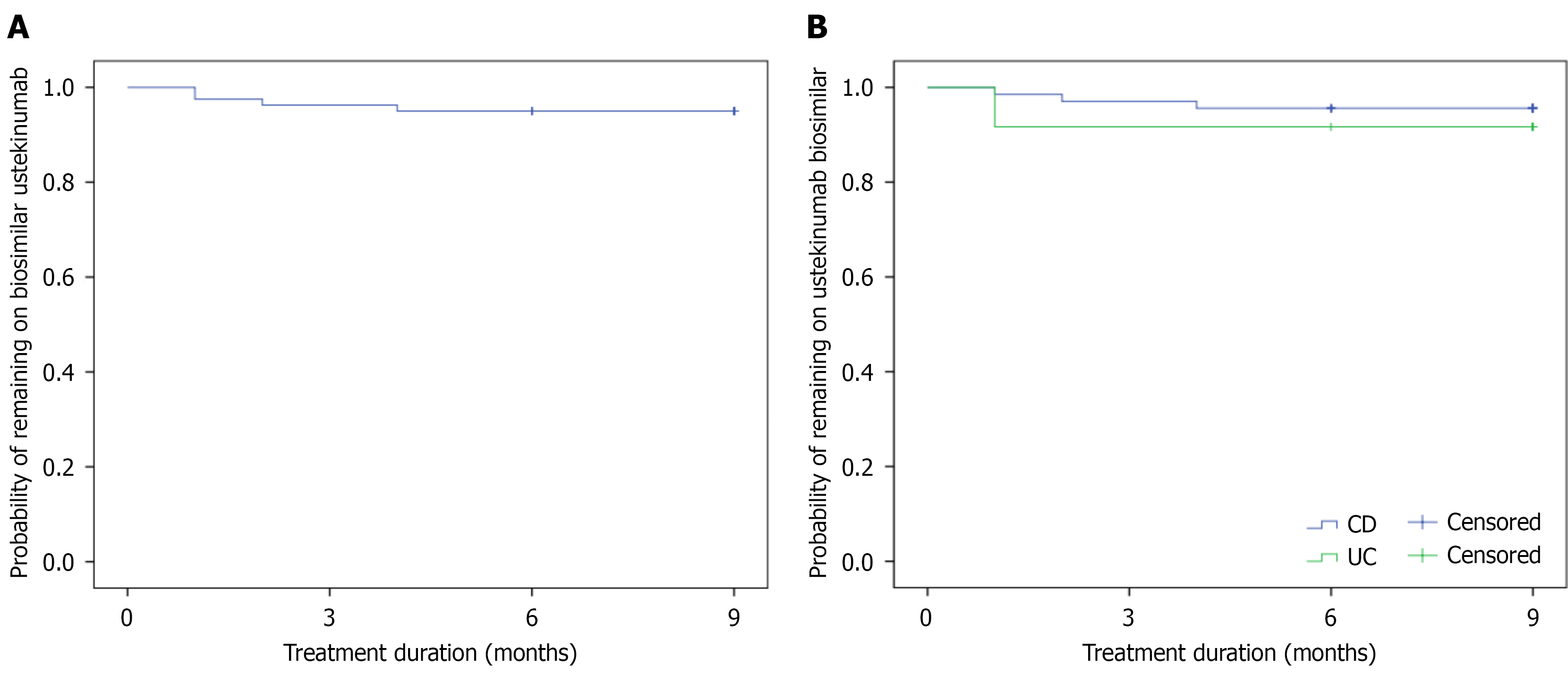
The probability of remaining on the biosimilar treatment after 12 and 24 weeks after the switch to the biosimilar ustekinumab was 96.3% (95% confidence interval: 94.2%-98.4%) and 95% (95% confidence interval: 92.6%-97.4%) (Figure 2). There was no significant difference in the probability of drug sustainability between the CD and UC patients in Kaplan-Meier analysis (Log-rank = 0.554). We assessed possible risk factors for stopping biosimilar ustekinumab therapy. There was no association with disease type, gender, age groups, location, behavior, perianal fistula in CD, extent of UC, or being in CR before or at the time of the switch in univariate analysis. There was a trend for association with needing dose optimisation before the switch and the need for previous biological therapy. Ustekinumab originator was dose optimised in all patients who stopped after the switch [probability of stopping: 8% (4/50) vs 0% (0/30), P = 0.11], and all patients had a previous biological therapy before the originator ustekinumab therapy [6.1% (4/66) vs 0% (0/14), P = 0.3].
The biosimilar ustekinumab was discontinued in 4.9% of the patients. In total, 4 patients discontinued the biosimilar after the switch, 2 had a loss of treatment response, 1 had a flare, and 1 had an adverse event - new extra-intestinal manifestation (uveitis). Three patients were started on rizankizumab, and one patient with severe UC was started on Upadacitinib. Any AE was observed in 6 patients (7.4%) during the biosimilar treatment period. There was no signal for new AEs. The patient with uveitis switched therapies due to the development of a new extra-intestinal manifestation.
This is the first real-world observational study reporting the outcomes after a mandatory non-medical switch from originator ustekinumab to a biosimilar in patients with IBD. Despite a high rate of prior biological exposure, we observed preserved clinical efficacy, high drug sustainability, and no new safety signals over 24 weeks of follow-up, with more than 95% of patients remaining on the biosimilar therapy. Biosimilars have become integral to the management of IBD, offering treatments with comparable efficacy, safety, and immunogenicity to their originator biologics while significantly reducing healthcare costs. Since the approval of infliximab biosimilars in 2013, extensive clinical trials and real-world data have demonstrated sustained remission, high treatment persistence, and no new safety concerns, including in switch and multiple-switch settings[17]. Adalimumab biosimilars have similarly shown robust effectiveness and safety in both naïve patients and those undergoing non-medical switching. More recently, ustekinumab biosimilars have entered the therapeutic landscape, with approvals in IBD based on extrapolated evidence from other immune-mediated diseases[18]. Although long-term IBD-specific data are still emerging, early adoption aligns with the strong precedent set by anti-TNF biosimilars. Collectively, biosimilars have improved access to advanced therapies, enabled cost savings, and fostered confidence among patients, clinicians, and policymakers, making them a cornerstone of sustainable gastroenterology care.
In the present real-world observational study, we found that a mandatory non-medical switch from originator ustekinumab to a biosimilar in patients with IBD was associated with preserved clinical efficacy, high treatment persistence, and no new safety signals over 24 weeks of follow-up. Despite a high rate of prior biologic exposure and frequent dose intensification with the originator, more than 95% of patients remained on biosimilar therapy, with discontinuations occurring in fewer than 5%. Rates of CR and biomarker activity remained stable across all time points, supporting the therapeutic equivalence of biosimilar ustekinumab in this context. These findings are consistent with prior reports of non-medical switches in IBD with anti-TNF biosimilars, which have demonstrated comparable outcomes in terms of efficacy, immunogenicity, and retention[14,17,18]. Importantly, our study provides the first real-world evidence for ustekinumab biosimilars in IBD, suggesting that these agents can be safely adopted into routine care without compromising patient outcomes, while potentially alleviating financial pressures on healthcare systems.
Comparable evidence from adalimumab biosimilar switch studies further supports our observations. Large multicenter cohorts, including the Hungarian prospective study of 276 IBD patients, demonstrated no significant differences in remission rates, CRP, or drug survival after mandatory non-medical switches from originator to biosimilar adalimumab, with persistence rates exceeding 85% at 40 weeks[17]. Similarly, the TABLET registry in Italy reported maintenance of remission in 89% of patients switched to Stanford-Binet Fifth Edition, with no emergence of antidrug antibodies, while the nationwide Italian multicenter study of 533 patients confirmed remission was maintained in over 80% of switchers, regardless of which biosimilar was used[19,20]. Additional real-world data from the SPOSAB study in Sicily and the Edinburgh IBD unit corroborated high drug persistence and safety after originator-to-Adalimumab Biosimilar (ABP 501) or Stanford-Binet Fifth Edition switches (90% persistence at 48 weeks, and 70% persistence at one year, respectively)[21,22]. Among patients within the SPOSAB study, the group who switched from the originator to ABP showed higher treatment persistence than those naïve to adalimumab, an expected finding in patients who were responders to the originator drug. Most recently, Canadian and Eastern European cohorts similarly found long-term treatment persistence above 85% and no new safety signals after mandatory adalimumab switches[17,18,23]. Taken together, these studies demonstrate a consistent pattern across anti-TNF and anti-IL-12/23 pathways: Non-medical biosimilar switching is safe, preserves clinical efficacy, and sustains drug retention in IBD, reinforcing the generalizability of our findings with ustekinumab.
The extensive real-world experience with infliximab biosimilars provides additional context for our findings. The pivotal randomized NOR-SWITCH trial and its extensions demonstrated that switching from originator infliximab to subcutaneous infliximab (CT-P13) was not associated with loss of efficacy, increased immunogenicity, or new safety concerns across immune-mediated diseases, including IBD[24]. Subsequent evidence from a randomised, multicentre, double-blind, phase 3 non-inferiority study confirmed non-inferiority of subcutaneous infliximab (CT-P13) to originator infliximab in biologic-naïve CD patients, including switch subgroups at week 30, with similar CD activity index responses, biomarker trajectories, and endoscopic outcomes[25]. Observational cohorts, such as the 12-month Dutch prospective study of 133 IBD patients, similarly reported stable infliximab trough levels, disease activity, and CRP after switching, with discontinuations largely driven by subjective worsening rather than objective loss of response[26]. Longer-term studies, including the Erlangen SB2 switch cohort (144 patients, up to 80 weeks) and the Phoenix study in Japan (117 patients, up to 5 years), confirmed high persistence rates above 75%-80% and no unexpected safety events[27,28]. Furthermore, multiple sequential switches across infliximab biosimilars have been shown to maintain remission and drug survival[29]. Collectively, this body of evidence parallels our results with ustekinumab, demonstrating that non-medical switching across biologic classes is safe, effective, and sustainable in IBD care.
An important consideration in the interpretation of biosimilar switch studies is the potential for a nocebo effect, where negative expectations contribute to perceived loss of efficacy or intolerance. In a recent prospective Canadian cohort, nocebo effects were reported in approximately 13% of patients following a mandatory non-medical switch, often mani
Our study has limitations that warrant consideration. First, as an observational analysis, missing data points for clinical scores and biomarkers reduced the completeness of follow-up assessments and may have introduced bias (e.g., see availability of biochemical data). As a conservative approach, we considered patients as not being in remission if data were not available or treatment was stopped. As for biochemical data, many patients were receiving ustekinumab for a long period before the switch, and they were in clinical/biochemical remission; thus, patients may have missed their biochemical analysis voluntarily since they were in continued remission. Although overall persistence was high, discontinuations may sometimes be attributed to subjective symptoms without objective evidence of disease activity, reflecting the challenge of distinguishing true loss of response from nocebo-related effects. In addition, the cohort included patients undergoing a mandatory non-medical switch, some of whom did not have fully stable disease at baseline. As a result, outcomes may have been confounded by attributing subsequent disease activity to the biosimilar rather than the natural disease course. This should be considered when generalizing our results among IBD patient populations who do not need to undergo a mandatory non-medical switch, but switch to the biosimilar voluntarily. Finally, the relatively short follow-up period (3 months before and 6 months after the switch) limits conclusions about long-term efficacy, immunogenicity, and safety. Of note, there is no consistent data available on the utility of drug monitoring in patients with Ustekinumab.
Despite these limitations, our study provides important strengths. It represents the first real-world data examining the clinical efficacy, safety, and drug sustainability of ustekinumab biosimilars in IBD, addressing a critical evidence gap. The inclusion of a large, unselected cohort undergoing a mandatory switch reflects routine clinical practice and increases generalizability. Rigorous monitoring of clinical outcomes, biomarkers, and persistence allowed for a comprehensive assessment of biosimilar performance. Importantly, most patients had prior biologic exposure and dose intensification with the originator, underscoring the validity of the findings in a challenging, treatment-experienced population. High persistence and remission rates, combined with the absence of new safety signals, reinforce the therapeutic equivalence of biosimilar ustekinumab and support its integration into routine IBD care.
Our study suggests switching from originator ustekinumab to a biosimilar in patients with IBD was associated with preserved clinical efficacy, high treatment persistence, and no new safety concerns. These findings are consistent with the broader biosimilar experience in IBD and provide the first evidence to support ustekinumab biosimilars in routine clinical practice. While limitations include missing data and the inclusion of patients not always in stable remission at the time of switch, the overall outcomes highlight the therapeutic equivalence of biosimilar ustekinumab. As biosimilar use continues to expand within the Canadian healthcare system, these results provide reassurance for patients, clinicians, and policymakers that non-medical switching can be implemented safely and effectively, with the potential to reduce costs while maintaining high standards of care.
| 1. | Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, Warusavitarne J, Agrawal M, Allocca M, Atreya R, Battat R, Bettenworth D, Bislenghi G, Brown SR, Burisch J, Casanova MJ, Czuber-Dochan W, de Groof J, El-Hussuna A, Ellul P, Fidalgo C, Fiorino G, Gisbert JP, Sabino JG, Hanzel J, Holubar S, Iacucci M, Iqbal N, Kapizioni C, Karmiris K, Kobayashi T, Kotze PG, Luglio G, Maaser C, Moran G, Noor N, Papamichael K, Peros G, Reenaers C, Sica G, Sigall-Boneh R, Vavricka SR, Yanai H, Myrelid P, Adamina M, Raine T. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2024;18:1531-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 193] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 2. | Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, Bachmann O, Bettenworth D, Chaparro M, Czuber-Dochan W, Eder P, Ellul P, Fidalgo C, Fiorino G, Gionchetti P, Gisbert JP, Gordon H, Hedin C, Holubar S, Iacucci M, Karmiris K, Katsanos K, Kopylov U, Lakatos PL, Lytras T, Lyutakov I, Noor N, Pellino G, Piovani D, Savarino E, Selvaggi F, Verstockt B, Spinelli A, Panis Y, Doherty G. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohns Colitis. 2022;16:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 677] [Article Influence: 169.3] [Reference Citation Analysis (1)] |
| 3. | Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, Boonen A; Working Group ‘Equity in access to treatment of rheumatoid arthritis in Europe’. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73:198-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 4. | van der Valk ME, Mangen MJ, Severs M, van der Have M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, van der Woude CJ, Romberg-Camps MJ, Clemens CH, Jansen JM, van de Meeberg PC, Mahmmod N, van der Meulen-de Jong AE, Ponsioen CY, Bolwerk C, Vermeijden JR, Siersema PD, Leenders M, Oldenburg B; COIN study group and the Dutch Initiative on Crohn and Colitis. Evolution of Costs of Inflammatory Bowel Disease over Two Years of Follow-Up. PLoS One. 2016;11:e0142481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Government of Canada. Canada, Patented Medicine Prices Review Board, Annual Report 2020. Available from: https://www.canada.ca/en/patented-medicine-prices-review/services/annual-reports/annual-report-2020.html. |
| 6. | Kuenzig ME, Im JHB, Coward S, Windsor JW, Kaplan GG, Murthy SK, Benchimol EI, Bernstein CN, Bitton A, Jones JL, Lee K, Peña-Sánchez JN, Rohatinsky N, Ghandeharian S, Jones May T, Tabatabavakili S, Jogendran R, Weinstein J, Khan R, Hazan E, Browne M, Davis T, Goddard Q, Gorospe J, Latos K, Mason K, Kerr J, Balche N, Sklar A, Targownik LE. The 2023 Impact of Inflammatory Bowel Disease in Canada: Indirect (Individual and Societal) and Direct Out-of-Pocket Costs. J Can Assoc Gastroenterol. 2023;6:S16-S22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 7. | World Health Organization. Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs). [cited 3 August 2025]. Available from: https://cdn.prod.website-files.com/666d0695ca3ba7fa496a5068/66bb07c7504d59e3f6c6df97_who_guideline_01.pdf. |
| 8. | European Medicines Agency. Biosimilar medicines: marketing authorization. 2016. [cited 3 August 2025]. Available from: https://www.ema.europa.eu/en/human-regulatory-overview/marketing-authorisation/biosimilar-medicines-marketing-authorisation. |
| 9. | United States Food and Drug Administration. Biosimilar Product Information, 2022. [cited 3 August 2025]. Available from: https://www.fda.gov/drugs/biosimilars/review-and-approval#approval%20process. |
| 10. | Crohn’s and Colitis Canada. Biotherapies. [cited 3 August 2025]. Available from: https://crohnsandcolitis.ca/About-Crohn-s-Colitis/IBD-Journey/Treatment-and-Medications/Biotherapies?gad_source=1&gclid=CjwKCAiA9bq6BhAKEiwAH6bqoNaI5IfaRIyZt2fLFO6xH6OA6xBqIX0x4HcMbJVXf13AEVU9uceBoCBgQQAvD_BwE#medications-available-in-canada. |
| 11. | Australian Government, Department of Health and Aged Care. Australian Public Assessment Report for WEZLANA [cited 3 August 2025]. Available from: https://www.tga.gov.au/sites/default/files/2024-06/auspar-wezlana-240618.pdf. |
| 12. | Feldman SR, Narbutt J, Girolomoni G, Brzezicki J, Reznichenko N, Zegadło-Mylik MA, Pulka G, Dmowska-Stecewicz M, Kłujszo E, Rekalov D, Rajzer L, Lee J, Lee M, Rho YH. A randomized, double-blind, phase III study assessing clinical similarity of SB17 (proposed ustekinumab biosimilar) to reference ustekinumab in subjects with moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2024;91:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Blair HA. AVT04: An Ustekinumab Biosimilar. Clin Drug Investig. 2024;44:549-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Hoang TT, Reid J, Galorport C, Bressler B, Leung Y, Rosenfeld G. Outcomes of a mandatory non-medical switch of infliximab to a biosimilar for inflammatory bowel disease in British Columbia, Canada. J Can Assoc Gastroenterol. 2024;7:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Wetwittayakhlang P, Karkout K, Wongcha-Um A, Tselekouni P, Al-Jabri R, Afif W, Wild G, Bitton A, Bessissow T, Lakatos PL. Clinical efficacy and nocebo effect following non-medical biosimilar switch in patients with inflammatory bowel disease: A prospective observational study. Dig Liver Dis. 2024;56:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV Jr, Peña AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 2431] [Article Influence: 202.6] [Reference Citation Analysis (1)] |
| 17. | Balogh F, Angyal D, Varga A, Gonczi L, Lontai L, Ilias A, Lakatos PL. Efficacy and safety of biosimilars in gastroenterology: a focus on inflammatory bowel disease management. Expert Opin Biol Ther. 2025;25:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Liu Chen Kiow J, Hoang T, Bedi HK, Majdzadeh Ardekani Z, Rosenfeld D, Reise-Filteau M, Bressler B, Leung Y, Rosenfeld G. Real-world experience and long-term outcomes of a mandatory non-medical switch of adalimumab originator to biosimilars in inflammatory bowel disease. World J Gastroenterol. 2024;30:4904-4913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Tapete G, Bertani L, Pieraccini A, Lynch EN, Giannotta M, Morganti R, Biviano I, Naldini S, Mumolo MG, De Nigris F, Calella F, Bagnoli S, Minciotti M, Maltinti S, Rentini S, Ceccarelli L, Lionetti P, Milla M, Costa F. Effectiveness and Safety of Nonmedical Switch From Adalimumab Originator to SB5 Biosimilar in Patients With Inflammatory Bowel Diseases: Twelve-Month Follow-Up From the TABLET Registry. Inflamm Bowel Dis. 2022;28:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Tursi A, Mocci G, Allegretta L, Aragona G, Bianco MA, Colucci R, Cuomo A, Della Valle N, Ferronato A, Forti G, Gaiani F, Giorgetti G, Graziani MG, Lofano K, Lorenzetti R, Larussa T, Penna A, Pica R, Pranzo G, Rodino' S, Scarcelli A, Zampaletta C, Bassotti G, Cazzato AI, Chiri S, Clemente V, Cocco A, De' Angelis G, Donnarumma L, Faggiani R, Graziosi C, Le Grazie M, Luzza F, Meucci C, Monterubbianesi R, Pagnini C, Perazzo P, Picchio M, Sacco R, Sebkova L, Serio M, Napolitano D, Pugliese D, Scaldaferri F, Schiavoni E, Turchini L, Armuzzi A, Elisei W, Maconi G, Papa A. Comparison of Performances of Adalimumab Biosimilars SB5, ABP501, GP2017, and MSB11022 in Treating Patients with Inflammatory Bowel Diseases: A Real-Life, Multicenter, Observational Study. Inflamm Bowel Dis. 2023;29:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Macaluso FS, Cappello M, Busacca A, Fries W, Viola A, Costantino G, Magnano A, Vinci E, Ferracane C, Privitera AC, Piccillo G, Belluardo N, Giangreco E, Romano C, Citrano M, Graziano F, Garufi S, Bertolami C, Ventimiglia M, Scrivo B, Teresi G, Renna S, Rizzuto G, Casà A, Orlando A; Sicilian Network for Inflammatory Bowel Disease (SN-IBD). SPOSAB ABP 501: A Sicilian Prospective Observational Study of Patients with Inflammatory Bowel Disease Treated with Adalimumab Biosimilar ABP 501. J Gastroenterol Hepatol. 2021;36:3041-3049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Derikx LAAP, Dolby HW, Plevris N, Lucaciu L, Rees CS, Lyons M, Siakavellas SI, Constantine-Cooke N, Jenkinson P, Su S, O'Hare C, Kirckpatrick L, Merchant LM, Noble C, Arnott ID, Jones GR, Lees CW. Effectiveness and Safety of Adalimumab Biosimilar SB5 in Inflammatory Bowel Disease: Outcomes in Originator to SB5 Switch, Double Biosimilar Switch and Bio-Naïve SB5 Observational Cohorts. J Crohns Colitis. 2021;15:2011-2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Spataru T, Popescu R, State M, Pahomeanu M, Mateescu B, Negreanu L. The Efficacy, Safety, and Persistence of Therapy after Non-Medical Switching from an Originator Adalimumab in Inflammatory Bowel Disease: Real-Life Experience from Two Tertiary Centres. Pharmaceuticals (Basel). 2024;17:1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Lichtenstein GR, Soonasra A, Latymer M, Singh S, Feagan BG. Systematic review: effectiveness and safety of switching between originator infliximab and biosimilar infliximab in patients with inflammatory bowel disease. Expert Opin Biol Ther. 2024;24:691-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Ye BD, Pesegova M, Alexeeva O, Osipenko M, Lahat A, Dorofeyev A, Fishman S, Levchenko O, Cheon JH, Scribano ML, Mateescu RB, Lee KM, Eun CS, Lee SJ, Lee SY, Kim H, Schreiber S, Fowler H, Cheung R, Kim YH. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn's disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019;393:1699-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 26. | Schmitz EMH, Boekema PJ, Straathof JWA, van Renswouw DC, Brunsveld L, Scharnhorst V, van de Poll MEC, Broeren MAC, Derijks LJJ. Switching from infliximab innovator to biosimilar in patients with inflammatory bowel disease: a 12-month multicentre observational prospective cohort study. Aliment Pharmacol Ther. 2018;47:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Fischer S, Cohnen S, Klenske E, Schmitt H, Vitali F, Hirschmann S, Ramming A, Zundler S, Rath T, Krebs S, Dörje F, Uter W, Nagore D, Meyer S, Neurath MF, Atreya R. Long-term effectiveness, safety and immunogenicity of the biosimilar SB2 in inflammatory bowel disease patients after switching from originator infliximab. Therap Adv Gastroenterol. 2021;14:1756284820982802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Kazama T, Ando K, Ueno N, Fujiya M, Ito T, Maemoto A, Ishigami K, Nojima M, Nakase H. Long-term effectiveness and safety of infliximab-biosimilar: A multicenter Phoenix retrospective cohort study. PLoS One. 2023;18:e0288393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Hanzel J, Jansen JM, Ter Steege RWF, Gecse KB, D'Haens GR. Multiple Switches From the Originator Infliximab to Biosimilars Is Effective and Safe in Inflammatory Bowel Disease: A Prospective Multicenter Cohort Study. Inflamm Bowel Dis. 2022;28:495-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/