Published online Dec 21, 2025. doi: 10.3748/wjg.v31.i47.113776
Revised: September 29, 2025
Accepted: October 29, 2025
Published online: December 21, 2025
Processing time: 107 Days and 21 Hours
Inflammation is closely related to survival and disease progression in patients with cancer. However, the predictive value of inflammation-based scores for survival in patients with hepatocellular carcinoma (HCC) treated with Lenvatinib has not been fully elucidated.
To compare different inflammation scores' prognostic values, and establish novel nomogram for predicting overall survival (OS) in HCC patients on Lenvatinib.
In total, 144 patients with HCC treated with Lenvatinib were enrolled in this study. The prognostic value of pre-treatment inflammation-based scores was retrospectively analyzed, including the platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, lymphocyte-to-C-reactive protein ratio, lymphocyte-to-monocyte ratio, systemic immune-inflammation index, C-reactive protein-to-albumin ratio, and prognostic nutritional index (PNI). Kaplan-Meier survival curves and time-de
All the inflammation-based scores demonstrated good discrimination in terms of OS (all P < 0.05), and the PNI emerged as an independent predictor of OS in multivariate analysis (hazard ratio = 4.097; 95% confidence interval: 1.405-11.944; P = 0.01). We selected three independent prognostic factors (macrovascular invasion, metastasis, and PNI) to generate a nomogram for OS.
The PNI is a prognostic indicator for assessing OS in patients with HCC treated with Lenvatinib and is superior to other inflammation-based scores in predicting OS.
Core Tip: This study compared the prognostic value of multiple inflammation-based scores in patients with hepatocellular carcinoma treated with Lenvatinib. The prognostic nutritional index (PNI) emerged as an independent predictor of overall survival and was superior to the other scores. A nomogram incorporating the PNI was established, facilitating personalized clinical decisions.
- Citation: Wu WJ, Wu ZY, Hu DD, Zhou ZG, Chen MS, Zhang YJ, Yang ZY, Chen JB. Comparison of the prognostic value of different inflammation-based scores in patients with hepatocellular carcinoma after Lenvatinib therapy. World J Gastroenterol 2025; 31(47): 113776
- URL: https://www.wjgnet.com/1007-9327/full/v31/i47/113776.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i47.113776
Hepatocellular carcinoma (HCC) is the most prevalent form of primary liver cancer and ranks as the third leading cause of cancer-related deaths worldwide[1]. Owing to the relatively insidious and rapid progression of the early-onset stage of HCC, most patients do not receive radical treatment at the time of diagnosis[2]. In recent years, there has been a surge in research on targeted drugs, offering a novel treatment approach for patients with HCC. Lenvatinib is an oral small molecule multireceptor tyrosine kinase inhibitor approved as first-line treatment for patients with unresectable HCC in the United States, the European Union, Japan, and China. In the REFLECT study, Lenvatinib demonstrated a higher ob
Inflammation is a hallmark of cancer, promoting the induction of angiogenesis, the activation of invasion, and me
This study aimed to directly compare the prognostic efficacy of various inflammation-based scores in patients with HCC following treatment with Lenvatinib.
This study included 144 patients diagnosed with HCC who received Lenvatinib therapy between June 2020 and September 2022 at Sun Yat-sen University Cancer Center. Patients were selected based on the following criteria: (1) Aged 18 to 75 years; (2) Diagnosed with HCC through imaging or pathology according to the American Association for the Study of Liver Diseases practice guidelines[14]; (3) Confirmed records of receiving Lenvatinib; (4) Performance status < 2; (5) No other malignant tumors; and (6) Complete medical and follow-up data. The study used retrospective anonymous clinical data obtained after each patient agreed to treatment.
Lenvatinib was administered according to the following regimen: The recommended daily dose was 8 mg once daily in patients who weighed < 60 kg and 12 mg once daily in patients who weighed ≥ 60 kg. Treatment was continued until disease progression or the occurrence of intolerable toxic effects.
Blood samples were collected 5 days before the initiation of Lenvatinib treatment. Serum biomarker levels were measured through centrifugation, and serum albumin (ALB) concentrations were determined by colorimetry using a Roche Cobas 702 automated biochemical analyzer. Neutrophil, lymphocyte, and platelet levels were assessed using a Sysmex XN-2000 automated hemocytometer. The coefficient of variation between the two laboratory tests at our center was ≤ 5%.
Table 1 summarizes the basic statistical indicators and clinical characteristics of the patients selected in this study, including age, gender, hepatitis status, levels of alanine aminotransferase (U/L), aspartate aminotransferase (AST) (U/L), ALB (g/L), and total bilirubin (μmol/L), maximum tumor size (cm), number of tumors, presence of macrovascular invasion and lymph node metastasis, tumor-node–metastasis stage, C-reactive protein (CRP), PLR, NLR, LCR, LMR, SII, CAR, and PNI.
| Variables | n = 144 |
| Age, year | 52 (21-75) |
| Gender (male/female) | 126/18 (87.5/12.5) |
| Hepatitis (yes/no) | 128/16 (88.9/11.1) |
| ALT, U/L, (>/≤ 50) | 64/80 (44.4/55.6) |
| AST, U/L, (>/≤ 40) | 117/27 (81.3/18.7) |
| ALB, g/L, (>/≤ 35) | 125/19 (86.8/13.2) |
| TBIL, µmol/L, (>/≤ 17.1) | 62/82 (43/57) |
| Largest tumor size, cm | 11.65 (1.2-23.1) |
| Tumor number (> 1/1) | 109/35 (75.7/24.3) |
| Macrovascular invasion (yes/no) | 102/42 (70.8/29.2) |
| Lymph node metastasis (yes/no) | 39/105 (27/73) |
| Metastasis (yes/no) | 27/117 (18.7/81.3) |
| TNM stage (III-IV/II) | 125/19 (86.8/13.2) |
| CRP, mg/L (> 7.9/≤ 7.9) | 100/44 (69.4/30.6) |
| PLR (1/0) | 19/125 (13.2/86.8) |
| NLR (1/0) | 73/71 (50.7/49.3) |
| LCR (1/0) | 110/34 (76.4/23.6) |
| LMR (1/0) | 89/55 (61.8/38.2) |
| SII (1/0) | 78/66 (54.2/45.8) |
| CAR (1/0) | 101/43 (70.1/29.9) |
| PNI (1/0) | 71/73 (49.3/50.7) |
The inflammation-based scores are shown in Table 2. The radiological response was evaluated according to the Response Evaluation Criteria in Solid Tumors, v1.1, with computed tomography or magnetic resonance imaging conducted before the initiation of treatment and at 6-12 weeks after treatment or every 3 months thereafter[15].
| Variable | Score |
| C-reactive protein, mg/L | |
| ≤ 7.9 | 0 |
| > 7.9 | 1 |
| Platelet-to-lymphocyte ratio | |
| Platelet count (× 109/L): Lymphocyte count (× 109/L) ≤ 269.51 | 0 |
| Platelet count (× 109/L): Lymphocyte count (× 109/L) > 269.51 | 1 |
| Neutrophil-to-lymphocyte ratio | |
| Neutrophil count (× 109/L): Lymphocyte count (× 109/L) ≤ 3.28 | 0 |
| Neutrophil count (× 109/L): Lymphocyte count (× 109/L) > 3.28 | 1 |
| Lymphocyte-to-C-reactive protein ratio | |
| 104 × lymphocyte count (× 109/L): CRP (mg/L) > 2500 | 0 |
| 104 × lymphocyte count (× 109/L): CRP (mg/L) ≤ 2500 | 1 |
| Lymphocyte-to-monocyte ratio | |
| Lymphocyte count (× 109/L): Monocyte count (× 109/L) ≤ 2.41 | 0 |
| Lymphocyte count (× 109/L): Monocyte count (× 109/L) > 2.41 | 1 |
| Systemic immune-inflammation index | |
| Platelet count (× 109/L) × neutrophil count (× 109/L)/Lymphocyte count (× 109/L) ≤ 768.03 | 0 |
| Platelet count (× 109/L) × neutrophil count (× 109/L)/Lymphocyte count (× 109/L) > 768.03 | 1 |
| C-reactive protein-to-albumin ratio | |
| C-reactive protein (mg/L): Albumin (g/L) ≤ 0.19 | 0 |
| C-reactive protein (mg/L): Albumin (g/L) > 0.19 | 1 |
| Prognostic nutritional index | |
| Albumin (g/L) + 5 × lymphocyte count (× 109/L) > 49.1 | 0 |
| Albumin (g/L) + 5 × lymphocyte count (× 109/L) ≤ 49.1 | 1 |
Overall survival (OS) was defined as the interval from the initiation of Lenvatinib treatment to cancer-related death.
The results are presented as medians and ranges, as the data were non-normally distributed. Continuous data were compared using the Mann-Whitney U test, and categorical data were assessed using the χ2 test. Independent predictors of OS were identified by univariate and multivariate Cox regression analyses using the forward likelihood ratio method. Risk-stratified survival was represented by Kaplan-Meier curves and analyzed using the log-rank test. For single-value indicators, to avoid bias associated with the different criteria for the prognostic score cutoff values in this cohort, R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria) was used to calculate the best cutoff scores for CRP, PLR, NLR, LCR, LMR, SII, CAR, and PNI (Supplementary Figure 1). Time-dependent receiver operating characteristic (ROC) curves and area under the curve (AUC) values at 6, 12, 18, and 24 months were calculated to com
A total of 144 patients with HCC who were treated with Lenvatinib at Sun Yat-sen University Cancer Center were enrolled in this study. A total of 126 (87.5%) of these patients were male, in contrast to 18 (12.5%) who were female, and the age distribution of the cohort covered 21 to 75 years. In the same group, 128 (88.9%) patients were hepatitis B virus (HBV) carriers, and 109 (75.7%) had multiple tumors. Tumor size ranged from 1.2 cm to 23.1 cm, with a median size of 11.65 cm. A total of 102 (70.8%) patients had macrovascular invasion, and 39 (27%) had lymph node metastasis. Furthermore, 27 (18.7%) patients had metastasis. Most of these patients (n = 138) received combined treatment with Programmed Death-1 inhibitors (Supplementary Table 1). Table 1 summarizes the clinical characteristics of the patients, including the eight inflammation-based scores.
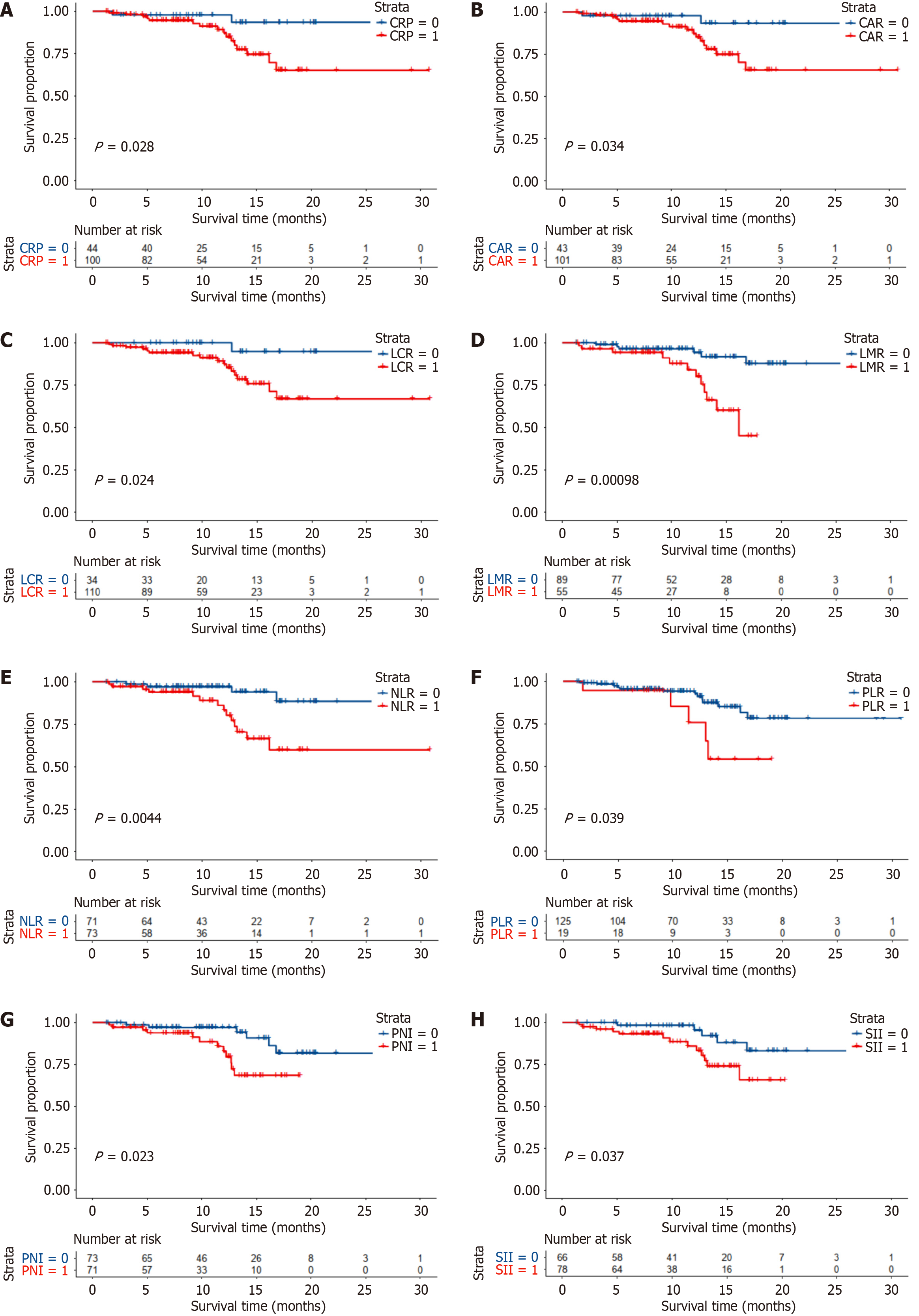
All inflammation-based scores were associated with OS in patients who received Lenvatinib (Figure 1). Low CRP, CAR, LCR, LMR, NLR, PLR, PNI, and SII scores suggested a good prognosis (all P < 0.05). In multivariate analysis, the PNI remained a significant and independent predictor of OS. The PNI was used to divide patients with HCC into two groups with different prognoses (median OS times of 12.5 months and 9.17 months, respectively).
Univariate analyses involved prognostic factors associated with clinical characteristics, liver function, tumor burden, and the eight inflammation-based scores. All inflammation scores were significant prognostic factors for OS, in addition to liver function and tumor burden. Multivariate Cox proportional analysis showed that macrovascular invasion (P = 0.023), metastasis (P = 0.012), and PNI (P = 0.01) were significant and independent prognostic factors for OS (Table 3).
| Variables | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, year (>/≤ 50) | 0.821 | 0.325-2.075 | 0.677 | |||
| Gender (female/male) | 0.273 | 0.036-2.057 | 0.207 | |||
| Largest tumor size, cm (>/≤ 10) | 1.982 | 0.702-5.6 | 0.197 | |||
| Tumor number (> 1/1) | 8.086 | 1.073-60.944 | 0.043 | |||
| Macrovascular invasion (yes/no) | 10.399 | 1.380-78.368 | 0.023 | 10.594 | 1.394-80.525 | 0.023 |
| Lymph node metastasis (yes/no) | 1.383 | 0.490-3.908 | 0.540 | |||
| Metastasis (yes/no) | 4.393 | 1.47-13.128 | 0.008 | 4.431 | 1.395-14.073 | 0.012 |
| PT, s (>/≤ 13.5) | 1.098 | 0.251-4.799 | 0.901 | |||
| HBsAg, IU/mL (>/≤ 0.05) | 2.386 | 0.317-17.942 | 0.398 | |||
| ALB, g/L (>/≤ 35) | 1.322 | 0.382-4.569 | 0.659 | |||
| ALT, U/L (>/≤ 50) | 1.118 | 0.443-2.821 | 0.813 | |||
| APOB, g/L (>/≤ 1.10) | 0.822 | 0.308-2.196 | 0.697 | |||
| APOA1, g/L (>/≤ 1.60) | 1.166 | 0.267-5.094 | 0.838 | |||
| AST, U/L (>/≤ 40) | 27.327 | 0.145-5143.118 | 0.216 | |||
| CHO, mmol/L (>/≤ 5.69) | 1.038 | 0.402-2.683 | 0.938 | |||
| CRE, µmol/L (>/≤ 97) | 1.969 | 0.452-8.573 | 0.367 | |||
| CRP, mg/L (>/≤ 7.9) | 4.494 | 1.031-19.585 | 0.045 | |||
| GGT, U/L (>/≤ 60) | 27.116 | 0.115-6392.722 | 0.236 | |||
| TBIL, µmol/L (>/≤ 20.5) | 2.628 | 1.027-6.727 | 0.044 | |||
| MO, × 109/L (>/≤ 0.6) | 1.204 | 0.477-3.042 | 0.694 | |||
| NE, × 109/L (>/≤ 6.3) | 1.084 | 0.386-3.047 | 0.878 | |||
| WBC, × 109/L (>/≤ 9.5) | 1.067 | 0.351-3.246 | 0.909 | |||
| PLR (>/≤ 269.51) | 2.843 | 1.009-8.008 | 0.048 | |||
| NLR (>/≤ 3.28) | 3.268 | 1.161-9.195 | 0.025 | |||
| LCR (>/≤ 3185.19) | 7.315 | 0.971-55.084 | 0.053 | |||
| LMR (>/≤ 2.41) | 0.276 | 0.105-0.725 | 0.009 | |||
| SII (>/≤ 768.03) | 2.888 | 1.021-8.166 | 0.046 | |||
| CAR (>/≤ 0.19) | 2.708 | 0.782-9.374 | 0.116 | |||
| PNI (>/≤ 49.1) | 3.924 | 1.384-11.124 | 0.01 | 4.097 | 1.405-11.944 | 0.01 |
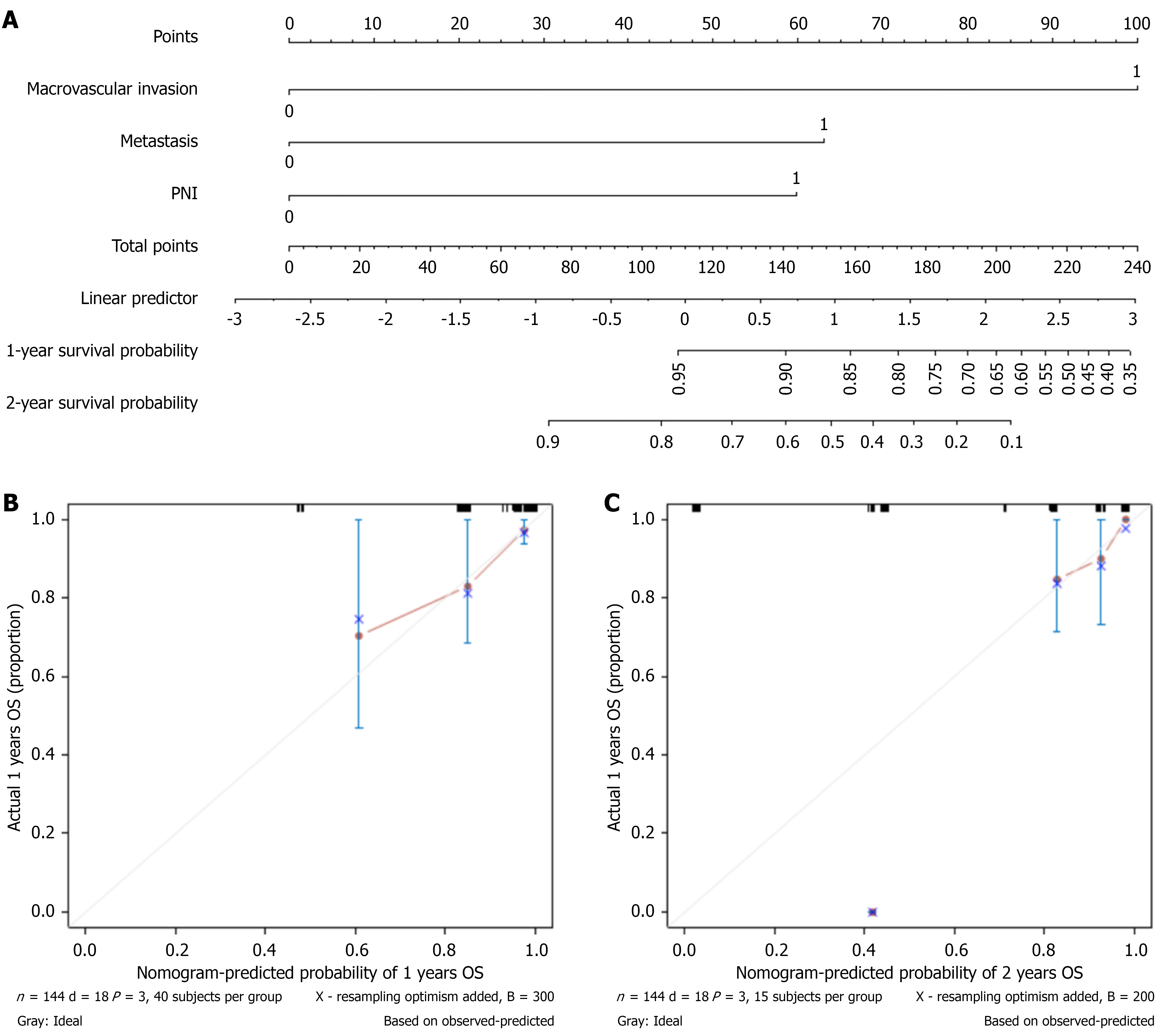
Variables derived from Cox proportional analysis were used to establish a prognostic nomogram for OS. The prognostic factors of the nomogram included three risk factors: Macrovascular invasion (yes vs no), metastasis (yes vs no), and the PNI (> 49.1 vs ≤ 49.1). Drawing a vertical line along the axis labeled “1- and 2-year OS probability” enabled us to determine the probability of the outcomes by summing the total scores of all factors and placing them on the total score scale (Figure 2A). Calibration plots showed satisfactory consistency between the nomogram-predicted OS and actual survival outcomes (Figure 2B and C). Supplementary Figure 2 illustrates the predictive performance of the nomogram when using the specific values of each variable derived from the individual study subjects. Additionally, the figure shows the scoring pathway and predicted outcomes of the subjects presented in the nomogram.
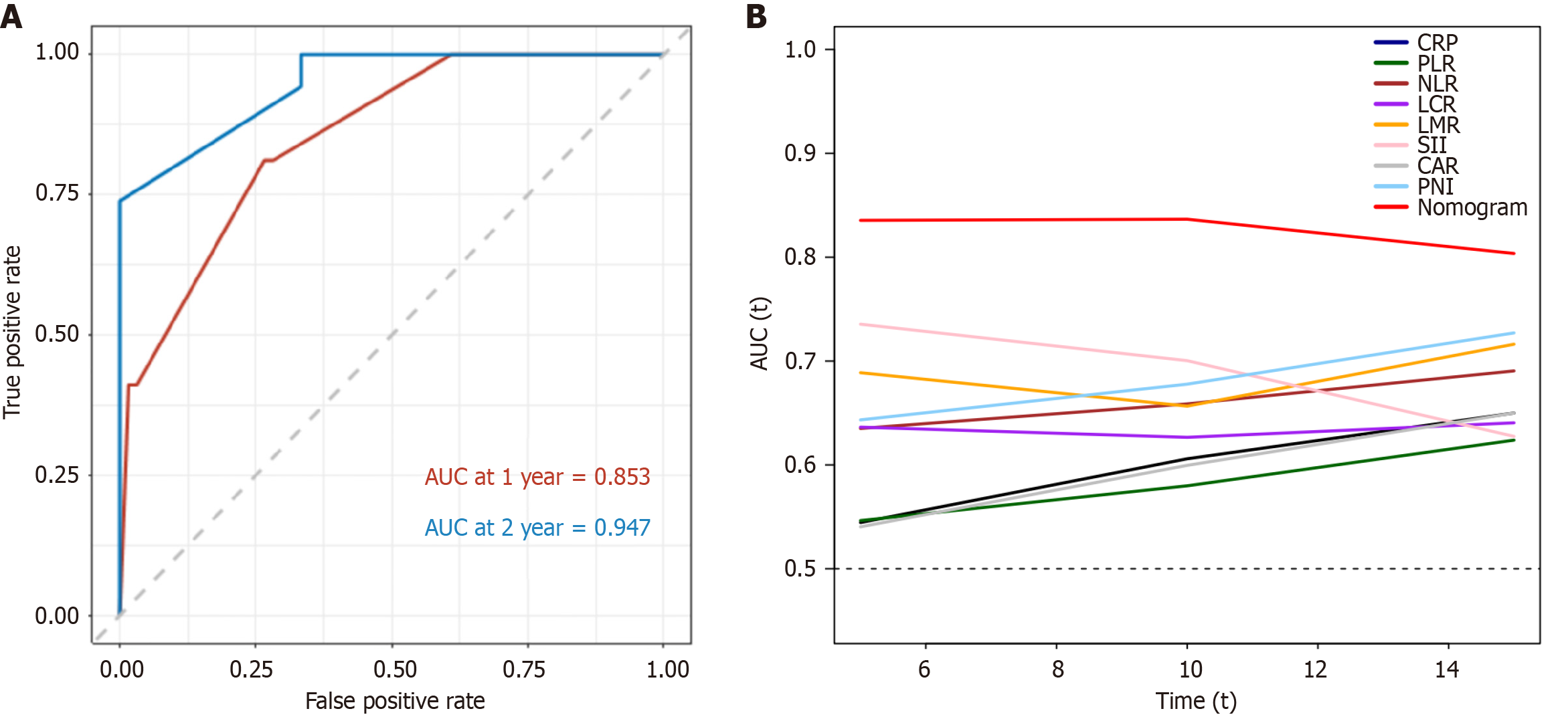
Time-dependent ROC curve analysis was performed to evaluate the predictive accuracy of the nomogram for OS. When the nomogram was used to predict 1- and 2-year survival rates, the AUC was 0.853 and 0.947, respectively (Figure 3A). Time-dependent ROC curves for 1- and 2-year OS were constructed to compare the efficacy of the nomogram and inflammation-based scores (Supplementary Figure 3), and the nomogram outperformed the other measures. The nomogram was more accurate in predicting OS based on the AUC of the time-dependent ROC curve (Figure 3B). The AUC values were calculated (Supplementary Table 2). The nomograms had consistently higher values than the other measures.
The correlations between the PNI and clinical characteristics are shown in Table 4. A higher PNI was associated with higher AST levels (P = 0.002), lower ALB levels (P < 0.001), larger tumor size (P = 0.003), an increased number of tumors (P = 0.041), and the presence of macrovascular invasion (P = 0.036).
| Variables | PNI ≤ 49.1 (n = 71) | PNI > 49.1 (n = 73) | P value |
| Age, year | 54 (29-74) | 50 (21-75) | 0.018 |
| Gender (male/female) | 64/7 (90.1/9.9) | 62/11 (84.9/15.1) | 0.345 |
| Hepatitis (yes/no) | 58/13 (81.7/18.3) | 54/19 (74.0/26.0) | 0.265 |
| ALT, U/L | 48.1 (10-177.1) | 42.9 (0.5-320.3) | 0.248 |
| AST, U/L | 73.1 (27.1-506.7) | 69.6 (21.8-290.5) | 0.002 |
| ALB, g/L | 37.7 (28.3-46.4) | 43.9 (35.2-50.9) | < 0.001 |
| TBIL, µmol/L | 17.2 (5.3-60.9) | 15.8 (5.5-31.1) | 0.068 |
| Largest tumor size, cm | 12.4 (2.8-23.1) | 10.4 (1.2-21.5) | 0.003 |
| Tumor number (> 1/1) | 59/12 (83.1/16.9) | 50/23 (68.5/31.5) | 0.041 |
| Macrovascular invasion (yes/no) | 56/15 (78.9/21.1) | 46/27 (63.0/37.0) | 0.036 |
| Lymph node metastasis (yes/no) | 18/53 (25.4/74.6) | 21/52 (28.8/71.2) | 0.645 |
| TNM stage (III-IV/II) | 64/7 (90.1/9.9) | 61/12 (83.6/16.4) | 0.243 |
| Best tumor response | |||
| CR | 0 (0) | 0 (0) | |
| PR | 19 (26.8) | 26 (35.6) | |
| SD | 34 (47.9) | 33 (45.2) | |
| PD | 18 (25.4) | 14 (19.2) | |
| ORR | 19 (26.8) | 26 (35.6) | 0.252 |
| DCR | 53 (74.6) | 59 (80.8) | 0.373 |
Numerous studies have highlighted the associations between inflammation-based scores and cancer-specific survival. However, the inflammation-related markers that can best predict the prognosis of patients with HCC treated with Lenvatinib remain unclear. Therefore, this study comprehensively explored the correlations between inflammation-based scores and OS in patients with HCC and concluded that the PNI is superior to other inflammation-based scores in predicting OS.
HCC, which is closely related to inflammation, is a malignant tumor mainly caused by chronic HBV and/or hepatitis C virus infection. Host inflammation-related factors are important predictors of the prognosis of HCC after treatment[16]. Most cases of HCC occur because of chronic liver inflammation, fibrosis, and cirrhosis[17-21]. This study shows that the systemic inflammatory response, as indicated by the PNI, which is calculated from a combination of the serum ALB level and total peripheral lymphocyte count, is a superior tool for assessing survival in patients with HCC treated with Lenvatinib compared with other inflammation-based measures. The PNI can be measured clinically by simple, readily available, and inexpensive means to determine prognosis by dividing patients treated with Lenvatinib into different risk groups.
Inflammation is closely related to the occurrence and development of tumors. Various cells in the tumor immune microenvironment play a major role in the processes of inflammation, tumor progression, and metastasis[22]. In the inflammatory process associated with cancers, continuous angiogenesis is considered to be one of the key mechanisms of tumorigenesis and progression[4]. The vascular endothelial growth factor (VEGF) receptor pathway is one of the most important regulatory pathways of tumor angiogenesis, in which VEGF-A plays a more important role. VEGF can also directly participate in the immune escape mechanism of tumors, inhibit the extravasation of immune cells into tumor tissue, and reduce the presentation of tumor antigens by inhibiting the maturation of dendritic cells. Lenvatinib inhibits tumor angiogenesis and regulates the tumor immune microenvironment by comprehensively blocking the VEGF signaling pathway, resulting in the inhibition or death of tumor cells[23]. Moreover, Lenvatinib plays an immunomodulatory role by inhibiting the fibroblast growth factor/fibroblast growth factor receptor pathway, thereby reducing tumor Programmed Death-Ligand 1 expression and inhibiting regulatory T cell differentiation[24]. We established a connection between Lenvatinib treatment and inflammation-based scores and then explored their interrelationship.
Numerous studies have demonstrated the efficacy of the PNI as a predictor for various digestive system tumors, including HCC[25], although the specific mechanism has not been fully elucidated. The PNI was originally proposed to assess perioperative immune-nutrition status and surgical risk in patients undergoing gastrointestinal surgery. However, with the increase in studies, the PNI has gradually been used to evaluate the immune nutrition of patients with different types of cancer. Liver cancer are closely related to nutritional status and potential cirrhosis.
Malnutrition and cirrhosis may compromise the anti-tumor and anti-metastatic responses of the body. A low PNI indicates relatively poor nutritional status and lymphopenia. Many studies have demonstrated the close association between PNI and the occurrence and progression of various malignant tumors[26-28]. Lymphopenia indicates a weakened immune response in the body[29]. In contrast, serum ALB levels reflect liver function, which greatly affects the survival and prognosis of patients with HCC undergoing similar treatments. Serum ALB is an indicator of nutritional status. Low level of serum ALB indicates malnutrition, which compromises the cellular and humoral immune response, phagocytosis, and other host defense mechanisms among patients with cancers[30].
Several limitations exist in this study. Firstly, as a retrospective study based on a cohort from a single-center in China, its findings cannot be extrapolated to other regions or countries. Although many cases of liver cancer in China are associated with HBV infection, the predominant causes in the United States, Japan, and other countries include hepatitis C virus infection, excessive alcohol consumption, and an imbalanced diet. Therefore, the findings of this study need to be validated in other patients with diverse disease backgrounds. Second, we enrolled patients who received combination therapy in addition to Lenvatinib during treatment, which inevitably introduced bias. Finally, the potential regulatory mechanisms of serum ALB and peripheral lymphocyte count in the context of Lenvatinib therapy remain incompletely understood and require further research efforts.
Our study demonstrates that the PNI is an independent prognostic indicator for patients with HCC treated with Lenvatinib and performs better compared to other inflammation-based scores. It is an easy-to-use risk stratification tool that allows physicians to make more case-specific decisions about Lenvatinib use in patients with HCC.
We thank Dr. Teng Long for critical evaluation of the manuscript and work.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12654] [Article Influence: 6327.0] [Reference Citation Analysis (6)] |
| 2. | Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, Schwartz M, Han G, Izzo F, Chen M, Blanc JF, Johnson P, Kudo M, Roberts LR, Sherman M. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 338] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 3. | Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: A Review in Hepatocellular Carcinoma. Drugs. 2019;79:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 4. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48749] [Article Influence: 3249.9] [Reference Citation Analysis (12)] |
| 5. | Akkız H, Şimşek H, Balcı D, Ülger Y, Onan E, Akçaer N, Delik A. Inflammation and cancer: molecular mechanisms and clinical consequences. Front Oncol. 2025;15:1564572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 6. | Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 948] [Article Influence: 86.2] [Reference Citation Analysis (1)] |
| 7. | Zhao Z, Xu H, Ma B, Dong C. Prognostic value of platelet to lymphocyte ratio (PLR) in breast cancer patients receiving neoadjuvant therapy: a systematic review and meta-analysis. Front Immunol. 2025;16:1658571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Ciobotariu I, Ortega-Berbel LA, Calderón-Mollinedo A, Aguerralde-Martin M, Requena C, Moro R, Manrique-Silva E, Traves V, Nagore E. Neutrophil-to-Lymphocyte Ratio Associated With Worse Melanoma Specific-Survival and Distant Metastasis Free Survival in Localized (Stage I and II) Cutaneous Melanoma: An Observational Retrospective Study on 988 Patients. Int J Dermatol. 2025;64:1855-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Altaf A, Baldo A, Khalil M, Rashid Z, Akabane M, Zindani S, Sarfraz A, Ruzzenente A, Aldrighetti L, Bauer TW, Marques HP, Martel G, Popescu I, Weiss MJ, Kitago M, Poultsides G, Maithel SK, Lam V, Hugh T, Gleisner A, Shen F, Cauchy F, Koerkamp BG, Endo I, Pawlik TM. Lymphocyte-C-Reactive Protein Ratio: Impact on Prognosis of Patients Following Resection of Primary Liver Cancer. World J Surg. 2025;49:2195-2206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Özkan O, Peker P, Geçgel A, Göker E. Prognostic Value of Preoperative Lymphocyte-to-Monocyte Ratio in Patients with Recurrent Colorectal Cancer. Medicina (Kaunas). 2025;61:707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Huang Z, Wang J, Zhou B, Zhang X, Liao R, Xu J, Guo P, Liu Z, You N, Tao R, Hu Q. Application of systemic immune-inflammation index in survival prediction after radical resection of gallbladder cancer: A multicenter retrospective clinical study. Eur J Surg Oncol. 2025;51:110267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Zheng T, Zheng Y, Zhou Z, Ye R, Jia H, Chen W, Zheng M, Chen Y. C-reactive protein-to-albumin ratio predicts outcome of neoadjuvant chemotherapy for colorectal liver metastases: A multicenter prediction model. Surgery. 2025;182:109334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | Dudzic W, Kimilu N, Kapała A, Świerblewski M, Połom K, Kobiela J, Girnyi S, Folwarski M. Preoperative prognostic nutritional index and albumin levels are associated with cancer staging in patients undergoing gastrectomy. Surgery. 2025;187:109659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1315] [Article Influence: 131.5] [Reference Citation Analysis (3)] |
| 15. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 494] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 16. | Chen L, Zhang Q, Chang W, Du Y, Zhang H, Cao G. Viral and host inflammation-related factors that can predict the prognosis of hepatocellular carcinoma. Eur J Cancer. 2012;48:1977-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Chamseddine S, Mohamed YI, Lee SS, Yao JC, Hu ZI, Tran Cao HS, Xiao L, Sun R, Morris JS, Hatia RI, Hassan M, Duda DG, Diab M, Mohamed A, Nassar A, Datar S, Amin HM, Kaseb AO. Clinical and Prognostic Biomarker Value of Blood-Circulating Inflammatory Cytokines in Hepatocellular Carcinoma. Oncology. 2023;101:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Keenan BP, Fong L, Kelley RK. Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer. 2019;7:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 19. | Lawal G, Xiao Y, Rahnemai-Azar AA, Tsilimigras DI, Kuang M, Bakopoulos A, Pawlik TM. The Immunology of Hepatocellular Carcinoma. Vaccines (Basel). 2021;9:1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Seyhan D, Allaire M, Fu Y, Conti F, Wang XW, Gao B, Lafdil F. Immune microenvironment in hepatocellular carcinoma: from pathogenesis to immunotherapy. Cell Mol Immunol. 2025;22:1132-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 21. | Farbod Y, Kankouni H, Moini M, Fung S. Hepatitis B-Induced Hepatocellular Carcinoma: Understanding Viral Carcinogenesis and Disease Management. J Clin Med. 2025;14:2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2340] [Reference Citation Analysis (0)] |
| 23. | Kudo M. Lenvatinib May Drastically Change the Treatment Landscape of Hepatocellular Carcinoma. Liver Cancer. 2018;7:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, Lin J, Zhang J, Zhu W, Jia H, Qin L, Lu L, Chen J. Lenvatinib Targets FGF Receptor 4 to Enhance Antitumor Immune Response of Anti-Programmed Cell Death-1 in HCC. Hepatology. 2021;74:2544-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 25. | Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 26. | Hong S, Zhou T, Fang W, Xue C, Hu Z, Qin T, Tang Y, Chen Y, Ma Y, Yang Y, Hou X, Huang Y, Zhao H, Zhao Y, Zhang L. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36:3389-3397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Jiang N, Deng JY, Liu Y, Ke B, Liu HG, Liang H. The role of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers. 2014;19:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 29. | Luo Y, Xie Y, Zhang W, Lin Q, Tang G, Wu S, Huang M, Yin B, Huang J, Wei W, Yu J, Hou H, Mao L, Liu W, Wang F, Sun Z. Combination of lymphocyte number and function in evaluating host immunity. Aging (Albany NY). 2019;11:12685-12707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Nakanishi Y, Masuda T, Yamaguchi K, Sakamoto S, Horimasu Y, Mimae T, Nakashima T, Miyamoto S, Tsutani Y, Iwamoto H, Fujitaka K, Miyata Y, Hamada H, Okada M, Hattori N. Albumin-globulin ratio is a predictive biomarker of antitumor effect of anti-PD-1 antibody in patients with non-small cell lung cancer. Int J Clin Oncol. 2020;25:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/