Published online Dec 21, 2025. doi: 10.3748/wjg.v31.i47.113381
Revised: September 27, 2025
Accepted: November 4, 2025
Published online: December 21, 2025
Processing time: 118 Days and 10.3 Hours
Despite advances in the treatment of ulcerative colitis (UC), some patients remain refractory to the currently available treatments. Dual biologic therapy (DBT) has emerged as a promising strategy for these patients.
A patient with extensive UC presented with steroid dependence and contraindications (past medical history included breast cancer and previous myocardial infarction) to treatment with tumor necrosis factor and Janus kinase inhibitors. DBT of α4β7 integrin antagonist (vedolizumab) and interleukin 23p19 inhibitor (mirikizumab) resulted in a sustained clinical and biochemical remission. No adverse events were recorded during the follow-up.
This case highlighted the challenge of managing refractory UC, especially in frail patients.
Core Tip: Dual biologic therapy (DBT) is a promising treatment option for patients with refractory ulcerative colitis (UC) who have limited therapeutic alternatives. We presented the first case of a patient with extensive UC treated with miri
- Citation: Guimarães AC, Ferreiro-Iglesias R, Calviño-Suarez C, Baston-Rey I, Barreiro-de Acosta M. Dual biologic therapy in patient with refractory ulcerative colitis and comorbidities: A case report. World J Gastroenterol 2025; 31(47): 113381
- URL: https://www.wjgnet.com/1007-9327/full/v31/i47/113381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i47.113381
Biologic therapies have revolutionized the management of inflammatory bowel diseases (IBD). Tumor necrosis factor (TNF) inhibitors are the cornerstone of biologic treatment. However, some patients remain refractory to this type of treatment[1]. Therefore, new agents targeting alternative inflammatory pathways have been explored and have led to the consideration of dual biologic therapy (DBT) for selected cases. The selective interleukin (IL)-23p19 inhibitors, risankizumab, mirikizumab and guselkumab, have shown consistent safety and efficacy in IBD[2,3]. Vedolizumab, an α4β7 integrin antagonist, also has a favorable safety profile in the treatment of IBD[4,5]. Both selective IL-23p19 inhibitors and vedolizumab may be suitable candidates for DBT in complex ulcerative colitis (UC) cases. We report herein the successful treatment of a patient with refractory UC with mirikizumab and vedolizumab.
A 52-year-old female with extensive UC presented to our clinic for a follow-up visit in March 2025 reporting clinical worsening over the prior month. She reported experiencing an increased number of bowel movements (> 15/day) that was associated with fecal urgency and cramp-like abdominal pain.
The patient had been diagnosed with UC at age 45. Initial treatment with oral/topical mesalamine and azathioprine failed, and the patient required multiple courses of corticosteroids. Remission was achieved with induction and main
Past medical history included hypertension, prior myocardial infarction treated with coronary stenting in 2017 and breast cancer diagnosed in 2022.
Fecal calprotectin: 64 µg/g; hemoglobin: 12.9 g/dL; white blood cells: 10.7 × 103/µL; platelets: 428 × 103/µL; and erythrocyte sedimentation rate: 33 mm/hour.
Treatment-refractory UC.
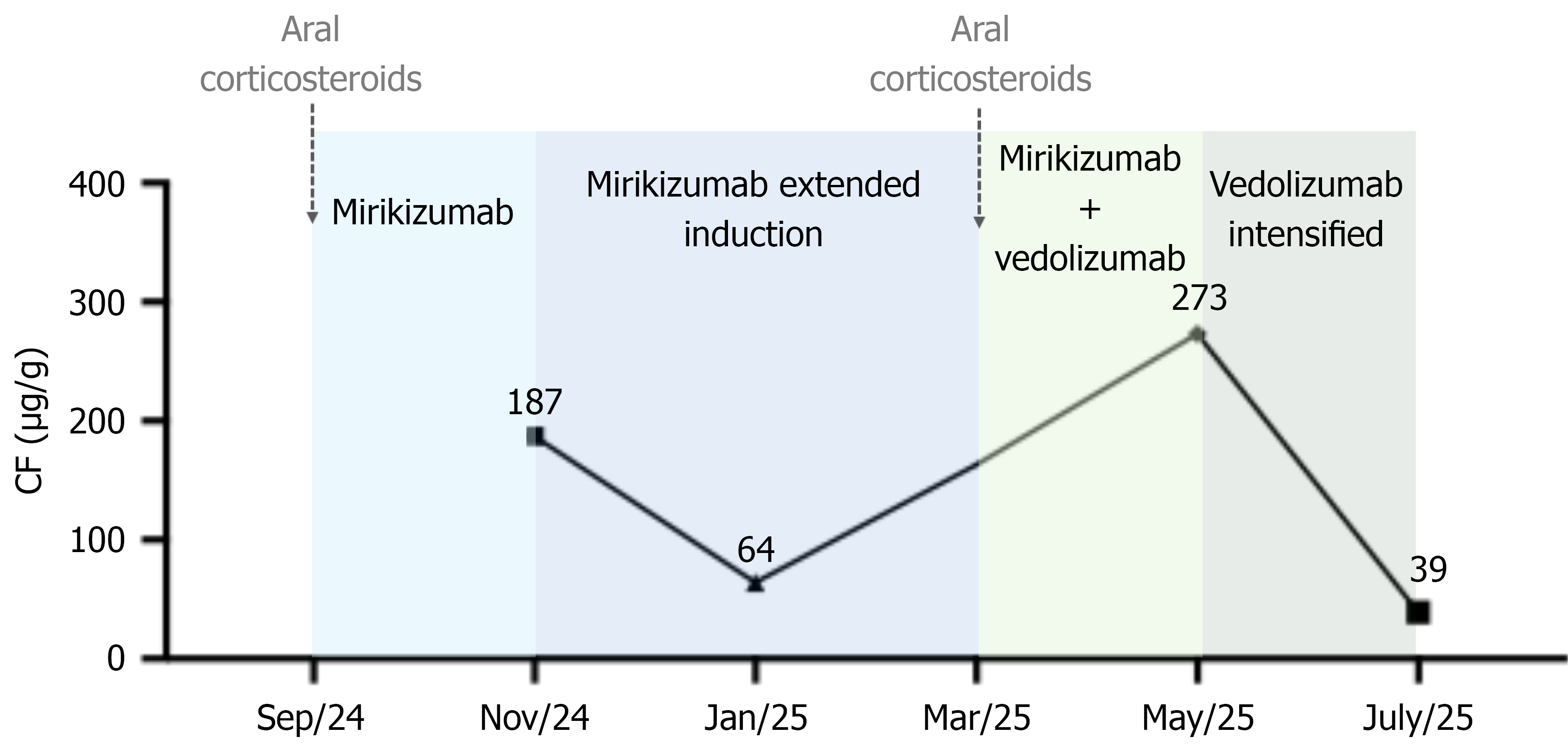
Combination therapy of mirikizumab and vedolizumab was initiated in addition to a new course of corticosteroids. Two months later, although the patient showed clinical improvement, fecal calprotectin level increased again (273 µg/g). Symptom exacerbation occurred prior to the scheduled 8-week dose. Therefore, vedolizumab administration was optimized to every 4 weeks.
Thus far, the patient has received DBT for 6 months, consisting of vedolizumab (300 mg intravenously every 4 weeks) and mirikizumab (200 mg subcutaneously every 4 weeks), administered every 2 weeks in alternating fashion. Therapeutic modifications since the initiation of mirikizumab are summarized in Figure 1. At the most recent follow-up visit, she was in a steroid-free clinical and biochemical remission with fecal calprotectin level normalization (39 µg/g). She reported no adverse events.
Combination therapy for the treatment of IBD was first investigated in 2010 in the Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease. This landmark trial demonstrated that patients with moderate Crohn’s disease treated with a combination of infliximab and azathioprine were more likely to achieve corticosteroid-free remission compared with those receiving azathioprine alone[6]. In 2014 the UC-SUCCESS study included patients with moderate-to-severe UC and demonstrated that combination therapy with infliximab plus azathioprine was more effective in achieving corticosteroid-free remission compared with either agent alone[7]. Recently, DBT has been proposed as a therapeutic strategy for two clinical scenarios of IBD: (1) Patients with well-controlled luminal disease but persistent, uncontrolled extraintestinal manifestations; and (2) Patients with refractory, poorly controlled intestinal disease despite standard biologic monotherapy[5]. However, there is no consensus regarding the optimal patient selection criteria, biologic combinations, or long-term safety of DBT. Most evidence[1,5] has been limited to case reports and small case series, primarily focusing on TNF and Janus kinase inhibitor combinations, which are limited due to safety concerns[8-11]. This case highlights the successful use of DBT with mirikizumab and vedolizumab in a patient with refractory UC and multiple therapeutic limitations. In this case, the switch from ustekinumab to mirikizumab was prompted by an inadequate clinical response despite dose optimization. Mirikizumab, a selective anti-IL-23p19 antibody, was considered a rational alternative due to its distinct mechanism of action compared with ustekinumab (anti-IL-12/23p40), allowing for more specific targeting of the IL-23 pathway, which has been associated with sustained efficacy in IBD[2,3]. Vedolizumab was further incorporated into DBT in view of the patient’s previous partial response and its well-established safety profile. The strengths of this report lie in the rationale for drug selection, the achievement of clinical and biochemical remission after failure of several advanced therapies and the favorable safety profile observed. Importantly, the patient tolerated the regimen well, and no adverse events were reported during 6 months of therapy, adding further reassurance regarding safety.
We demonstrated the feasibility, potential effectiveness, and apparent safety of DBT involving IL-23p19 inhibitors and vedolizumab. Our report adds to the growing evidence of alternative therapeutic combinations that may benefit patients with difficult-to-treat IBD. However, prospective studies with large sample sizes are needed to define the optimal patient selection, timing, and long-term safety.
| 1. | Yang E, Panaccione N, Whitmire N, Dulai PS, Vande Casteele N, Singh S, Boland BS, Collins A, Sandborn WJ, Panaccione R, Battat R. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn's disease. Aliment Pharmacol Ther. 2020;51:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (2)] |
| 2. | Bourgonje AR, Ungaro RC, Mehandru S, Colombel JF. Targeting the Interleukin 23 Pathway in Inflammatory Bowel Disease. Gastroenterology. 2025;168:29-52.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 43] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 3. | D'Haens G, Dubinsky M, Kobayashi T, Irving PM, Howaldt S, Pokrotnieks J, Krueger K, Laskowski J, Li X, Lissoos T, Milata J, Morris N, Arora V, Milch C, Sandborn W, Sands BE; LUCENT Study Group. Mirikizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2023;388:2444-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 220] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 4. | Takatsu N, Hisabe T, Higashi D, Ueki T, Matsui T. Vedolizumab in the Treatment of Ulcerative Colitis: An Evidence-Based Review of Safety, Efficacy, and Place of Therapy. Core Evid. 2020;15:7-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | McCormack MD, Wahedna NA, Aldulaimi D, Hawker P. Emerging role of dual biologic therapy for the treatment of inflammatory bowel disease. World J Clin Cases. 2023;11:2621-2630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (2)] |
| 6. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2452] [Article Influence: 153.3] [Reference Citation Analysis (1)] |
| 7. | Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, van Hoogstraten HJ, Chen AC, Zheng H, Danese S, Rutgeerts P. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392-400.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 716] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 8. | Ahmed W, Galati J, Kumar A, Christos PJ, Longman R, Lukin DJ, Scherl E, Battat R. Dual Biologic or Small Molecule Therapy for Treatment of Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:e361-e379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (1)] |
| 9. | Elmoursi A, Barrett TA, Perry C. Double Biologic Therapy for Refractory Stricturing Crohn's Disease: A Successful Case of Deep Remission with Ustekinumab and Vedolizumab. Inflamm Bowel Dis. 2020;26:e62-e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Eronen H, Kolehmainen S, Koffert J, Koskinen I, Oksanen P, Jussila A, Huhtala H, Sipponen T, Ilus T. Combining biological therapies in patients with inflammatory bowel disease: a Finnish multi-centre study. Scand J Gastroenterol. 2022;57:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Meng MJ, Le PH, Kuo CJ, Lai MW, Chiu CT. P1118 Advanced Combination Therapy with Biologics and Upadacitinib in Refractory Inflammatory Bowel Disease: A Retrospective Study from Taiwan. J Crohns Colitis. 2025;19:i2052-i2053. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/