Published online Dec 7, 2025. doi: 10.3748/wjg.v31.i45.112336
Revised: October 2, 2025
Accepted: October 31, 2025
Published online: December 7, 2025
Processing time: 130 Days and 1.1 Hours
Chimeric antigen receptor (CAR)-T cell therapy has emerged as a transformative treatment option for relapsed or refractory follicular lymphoma (FL), particularly in patients in whom multiple lines of conventional therapy have failed. Among cluster of differentiation (CD) 19-targeted products, lisocabtagene maraleucel (liso-cel) offers distinct advantages owing to its defined CD4+/CD8+ composition and favorable safety profile. Compared with diffuse large B-cell lymphoma, FL patients consistently achieve higher overall response rates and exhibit lower rates of severe cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome, supporting the rationale for expanding CAR-T cell the
Core Tip: Chimeric antigen receptor (CAR)-T therapy, particularly lisocabtagene maraleucel (liso-cel), offers a promising option for patients with relapsed or refractory and relapsed follicular lymphoma (FL), demonstrating high response rates and a favorable safety profile in the TRANSCEND FL trial. Compared to other CAR-T products, liso-cel shows advantages in toxicity, logistics, and feasibility. This editorial emphasizes its relevance for gastrointestinal FL, a subgroup often underrepresented in studies, and calls for broader inclusion and real-world validation to optimize CAR-T use across FL subtypes.
- Citation: Watanabe T. Emerging role of lisocabtagene maraleucel chimeric antigen receptor-T cell in nodal and gastrointestinal follicular lymphoma. World J Gastroenterol 2025; 31(45): 112336
- URL: https://www.wjgnet.com/1007-9327/full/v31/i45/112336.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i45.112336
Follicular lymphoma (FL) is the most common subtype of indolent B-cell non-Hodgkin lymphomas in adults, characterized by slow progression but frequent relapses and eventual resistance to standard therapies[1-3]. Over the past two decades, the introduction of chemoimmunotherapy with anti-cluster of differentiation (CD) 20 monoclonal antibodies, particularly rituximab-based regimens, has significantly improved patient outcomes[4-6]. However, despite these ad
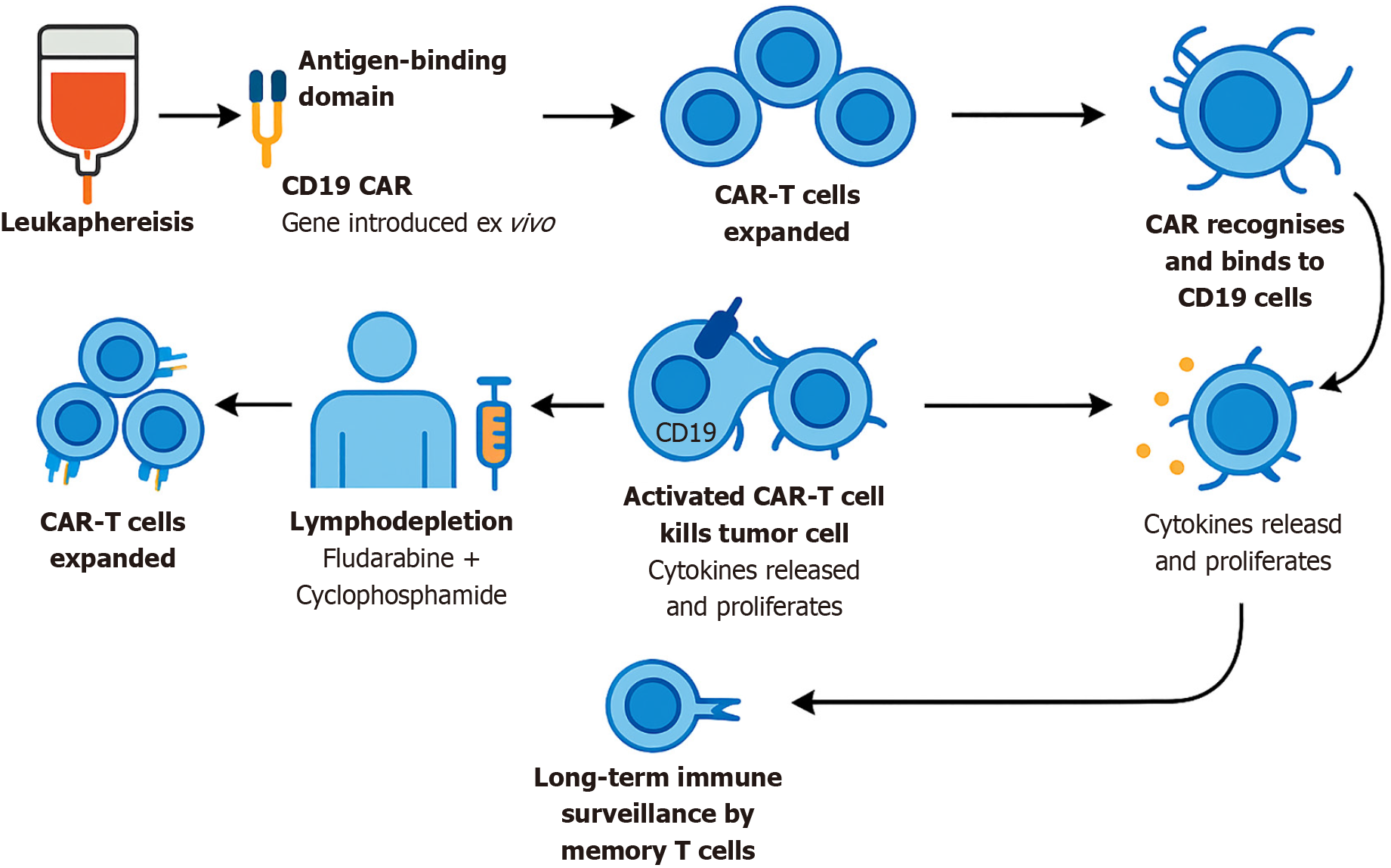
Recent years have witnessed the emergence of chimeric antigen receptor (CAR)-T cell therapy as a transformative modality for relapsed/refractory B-cell lymphomas[9-11]. Importantly, compared to diffuse large B-cell lymphoma and other B-cell malignancies, FL patients tend to achieve higher response rates and lower incidences of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) after CD19 CAR-T cell therapy (Figure 1), providing a compelling rationale for its application in this subgroup[12,13]. Among the currently available CD19 CAR-T cell products, axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel) represent distinct options, each with unique strengths and limitations[14-16].
In this editorial, we summarize the therapeutic potential of liso-cel in the treatment of nodal and gastrointestinal FL (GI-FL), compare it with other CAR-T cell products, and discuss future directions for clinical practice.
In the pivotal TRANSCEND FL (No. NCT04245839) trial, liso-cel achieved an overall response rate of 97% and a complete response rate of 94%[14]. The median age was 63 years, with the majority of patients having received ≥ 3 prior lines of therapy and nearly half presenting with high tumor burden, highlighting the refractory nature of the cohort[17]. The estimated 12-month progression-free survival and overall survival rates exceeded 85%[18,19].
One of the most critical advantages of liso-cel is its safety profile. Grade ≥ 3 CRS occurred in 0% of patients, while grade ≥ 3 ICANS occurred in only 3%[14]. A summary of adverse events, including hematologic toxicities and infections, has been incorporated into an expanded Table 1 for clarity.
| Ref. | Product | Target | Patients (n) | ORR/CR (%) | PFS/OS | Main AEs |
| Morschhauser et al[30] | Liso-cel | CD19 | 94 (FL) | 97/73 | NR/NR (30-month DOR 80%) | Neutropenia, anemia, low CRS/ICANS |
| Fowler et al[28] | Tisa-cel | CD19 | 97 (FL) | 86/69 | 12-month PFS 67%; 12-month OS 92% | Neutropenia, ICANS (grade ≥ 3: 4%) |
| Jacobson et al[14] | Axi-cel | CD19 | 146 (FL) | 94/79 | 17-month PFS 65%; 17-month OS 88% | CRS (any: 82%, ≥ 3: 7%), ICANS (≥ 3: 19%) |
| Shadman et al[21] | MB-106 | CD20 | 28 (FL) | 96/75 | NR | Low CRS; no ICANS |
Three CD19-directed CAR-T products axi-cel, tisa-cel, and liso-cel have been approved for B-cell malignancies[20]. Axi-cel demonstrated strong efficacy but at the cost of higher toxicity, with grade ≥ 3 CRS and ICANS rates up to 13% and 28%, respectively[14,21]. Tisa-cel showed favorable safety but variable efficacy and longer manufacturing times[22,23]. Liso-cel offers balanced efficacy and lower toxicity, permitting outpatient use in select centers[14,24]. These differences in efficacy, safety, and logistics underscore the importance of individualized selection among the three CAR-T products[25-27]. Furthermore, real-world data continue to validate these findings, demonstrating that liso-cel maintains comparable efficacy with reduced incidence of high-grade immune toxicities even in older or comorbid patients[28-30].
GI-FL is a relatively rare entity, usually diagnosed at early stages[31]. However, the Ann Arbor staging system often fails to capture the organ-confined and mucosal patterns typical of GI-FL[32,33]. Modified Lugano or tumor node metastasis/Paris staging systems, which account for depth of invasion and extra-nodal spread, may be more appropriate for clinical practice[34,35].
Although most GI-FL patients are managed with “watch-and-wait” or local therapy, a subset with advanced or tran
Future guidelines for GI-FL should integrate histological, anatomical, molecular, and cytogenetic perspectives through multicenter collaborations to ensure tailored treatment strategies. Efforts to reduce manufacturing time, expand out
In conclusion, liso-cel is a promising therapeutic modality for FL, with high efficacy, manageable toxicity, and potential curative benefits in advanced GI-FL. Its role should be considered alongside bispecific antibodies and targeted small molecules as part of a rapidly evolving therapeutic landscape.
The emergence of CAR-T cell therapy, particularly liso-cel, has advanced FL treatment, including select GI-FL cases[14,21,29]. Ongoing challenges include durability of response, long-term monitoring, and sequencing with other novel agents[23,25,27]. Predictive biomarkers such as baseline tumor burden, cytokine profiles, and molecular features are under investigation to guide patient selection and optimize outcomes[14,40]. Future guidelines for GI-FL should integrate histological, anatomical, molecular, and cytogenetic perspectives through multicenter collaborations to ensure tailored treatment strategies[33,35,40]. Efforts to reduce manufacturing time, expand outpatient delivery, and address cost barriers are essential for broader adoption[39,40].
Liso-cel is a promising therapeutic modality for FL, with high efficacy, manageable toxicity, and potential curative benefits in advanced GI-FL. Its role should be considered alongside bispecific antibodies and targeted small molecules as part of a rapidly evolving therapeutic landscape.
We would like to thank Dr. Watanabe T, Miss Watanabe M and Mrs. Watanabe T for their assistance in writing the manuscript and providing valuable suggestions.
| 1. | The world health organization classification of malignant lymphomas in japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int. 2000;50:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 345] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Ramsower CA, Wright G, Li H, Cerhan JR, Maurer MJ, Mwangi R, Rosenthal AC, Novak AJ, Link BK, Witzig TE, Habermann TM, Kridel R, LeBlanc ML, Shadman M, Smith SM, Friedberg JW, Scott DW, Steidl C, Staudt LM, Rimsza LM. Development and Validation of a Gene Expression Signature to Predict Early Events in Patients with Follicular Lymphoma. Blood Adv. 2025;bloodadvances.2025016827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, Tomotaka S, Morton LM, Weisenburger DD, Matsuo K. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 4. | Yamamoto S, Nakase H, Yamashita K, Matsuura M, Takada M, Kawanami C, Chiba T. Gastrointestinal follicular lymphoma: review of the literature. J Gastroenterol. 2010;45:370-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Watanabe T, Suda T, Hirono H, Hasegawa K, Soga K, Shibasaki K, Umezu H. Successful treatment of mucosa-associated lymphoid tissue lymphoma in a patient with gastric and rectal lesions with metachronous and ectopic development. Rare Tumors. 2011;3:e24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Watanabe T, Homma N, Ogata N, Saito H, Kanefuji T, Hasegawa K, Soga K, Shibasaki K, Endo T, Ajioka Y. Complete response in a patient with colonic mantle cell lymphoma with multiple lymphomatous polyposis treated with combination chemotherapy using anti-CD20 antibody and cladribine. Gut. 2007;56:449-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Watanabe T. Recent advances in treatment of nodal and gastrointestinal follicular lymphoma. World J Gastroenterol. 2023;29:3574-3594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (11)] |
| 8. | Watanabe T. Recent advances in treatment of follicular lymphoma: efficacy of PI3Kα/δ inhibitor (TQ-B3525). Signal Transduct Target Ther. 2024;9:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 9. | Morschhauser F, Flinn IW, Advani R, Sehn LH, Diefenbach C, Kolibaba K, Press OW, Salles G, Tilly H, Chen AI, Assouline S, Cheson BD, Dreyling M, Hagenbeek A, Zinzani PL, Jones S, Cheng J, Lu D, Penuel E, Hirata J, Wenger M, Chu YW, Sharman J. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol. 2019;6:e254-e265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 10. | Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, Assouline S, Kim TM, Kim WS, Ozcan M, Hirata J, Penuel E, Paulson JN, Cheng J, Ku G, Matasar MJ. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2020;38:155-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 601] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 11. | Terui Y, Rai S, Izutsu K, Yamaguchi M, Takizawa J, Kuroda J, Ishikawa T, Kato K, Suehiro Y, Fukuhara N, Ohmine K, Goto H, Yamamoto K, Kanemura N, Ueda Y, Ishizawa K, Kumagai K, Kawasaki A, Saito T, Hashizume M, Shibayama H. A phase 2 study of polatuzumab vedotin + bendamustine + rituximab in relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci. 2021;112:2845-2854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Tsukamoto T, Tokuda Y, Nakano M, Tashiro K, Kuroda J. Expression of activated B-cell gene signature is predictive of the outcome of follicular lymphoma. Blood Adv. 2022;6:1932-1936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Fujii M, Takata K, Chuang SS, Miyata-Takata T, Ando M, Sato Y, Yoshino T. A20 (TNFAIP3) Alterations in Primary Intestinal Diffuse Large B-cell Lymphoma. Acta Med Okayama. 2018;72:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, Munshi PN, Casulo C, Maloney DG, de Vos S, Reshef R, Leslie LA, Yakoub-Agha I, Oluwole OO, Fung HCH, Rosenblatt J, Rossi JM, Goyal L, Plaks V, Yang Y, Vezan R, Avanzi MP, Neelapu SS. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23: 91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 466] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 15. | Xu W, Xue L, Sun Y, Henry A, Battle JM, Micault M, Morris SW. Bcl10 is an essential regulator for A20 gene expression. J Physiol Biochem. 2013;69:821-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Zhang J, Dominguez-Sola D, Hussein S, Lee JE, Holmes AB, Bansal M, Vlasevska S, Mo T, Tang H, Basso K, Ge K, Dalla-Favera R, Pasqualucci L. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med. 2015;21:1190-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 371] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 17. | Knutson SK, Kawano S, Minoshima Y, Warholic NM, Huang KC, Xiao Y, Kadowaki T, Uesugi M, Kuznetsov G, Kumar N, Wigle TJ, Klaus CR, Allain CJ, Raimondi A, Waters NJ, Smith JJ, Porter-Scott M, Chesworth R, Moyer MP, Copeland RA, Richon VM, Uenaka T, Pollock RM, Kuntz KW, Yokoi A, Keilhack H. Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Ther. 2014;13:842-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 447] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 18. | Izutsu K, Ando K, Nishikori M, Shibayama H, Teshima T, Kuroda J, Kato K, Imaizumi Y, Nosaka K, Sakai R, Hojo S, Nakanishi T, Rai S. Phase II study of tazemetostat for relapsed or refractory B-cell non-Hodgkin lymphoma with EZH2 mutation in Japan. Cancer Sci. 2021;112:3627-3635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Morschhauser F, Tilly H, Chaidos A, McKay P, Phillips T, Assouline S, Batlevi CL, Campbell P, Ribrag V, Damaj GL, Dickinson M, Jurczak W, Kazmierczak M, Opat S, Radford J, Schmitt A, Yang J, Whalen J, Agarwal S, Adib D, Salles G. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 2020;21:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 442] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 20. | Kelly MJ, Alberghina F, McCabe P, Goldberg CJ, Fogarty EE, Dowling FE, O'Toole P, Noël J, Kiely PJ, Moore DP, Kennedy JF. Functional Outcomes of Congenital Scoliosis at a Mean 35-Year Follow-up Post In Situ Fusion. Revisiting Patients From the 2002 Goldberg et al Study. J Pediatr Orthop. 2024;44:e381-e388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Shadman M, Caimi PF, O’Brien SM, Reagan PM, Dezube B, Navaratnarajah P, Gaur T, Petrossian S, Germani A, Till BG, Abramson JS. Efficacy and Safety of a Third Generation CD20 CAR-T (MB-106) for Treatment of Relapsed/Refractory Indolent B-Cell Non-Hodgkin Lymphoma: Phase-1 Results from a Multicenter Trial. Blood. 2023;142 Suppl 1:2102. [DOI] [Full Text] |
| 22. | Jain MD, Smith M, Shah NN. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood. 2023;141:2430-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 23. | Pinnix CC, Gunther JR, Dabaja BS, Strati P, Fang P, Hawkins MC, Adkins S, Westin J, Ahmed S, Fayad L, Lee HJ, Nair R, Steiner RE, Iyer SP, Rodriguez MA, Wang M, Flowers C, Neelapu SS, Nastoupil LJ. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4:2871-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 24. | Torrente MC, Tamblay N, Herrada J, Maass JC. Prevalence and incidence of hearing loss in school-aged children in Santiago, Chile. Acta Otolaryngol. 2025;1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Zou Y, Xie X. Effect of ac4C acetyltransferase NAT10 on tumor progression via ATF4/ASNS mediated asparagine biosynthesis in osteosarcoma. J Clin Oncol. 2024;42:234-234. [DOI] [Full Text] |
| 26. | Tang J, Li W, Zhou Q, Fang Z, Lin Y, Xu S, Feng B, Zhuo Y, Jiang X, Zhao H, Wu D, Trabalza-Marinucci M, Che L. Effect of heating, microbial fermentation, and enzymatic hydrolysis of soybean meal on growth performance, nutrient digestibility, and intestinal microbiota of weaned piglets. J Anim Sci. 2023;101:skad384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Cavo M, Tacchetti P, Zamagni E. Front-line treatment of multiple myeloma. Hemasphere. 2019;3:127-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, Ghosh M, Popplewell L, Chavez JC, Bachy E, Kato K, Harigae H, Kersten MJ, Andreadis C, Riedell PA, Ho PJ, Pérez-Simón JA, Chen AI, Nastoupil LJ, von Tresckow B, Ferreri AJM, Teshima T, Patten PEM, McGuirk JP, Petzer AL, Offner F, Viardot A, Zinzani PL, Malladi R, Zia A, Awasthi R, Masood A, Anak O, Schuster SJ, Thieblemont C. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 377] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 29. | Piepiora P. Personality profile of individual sports champions. Brain Behav. 2021;11:e02145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Morschhauser F, Dahiya S, Palomba ML, Martin Garcia-Sancho A, Reguera Ortega JL, Kuruvilla J, Jäger U, Cartron G, Izutsu K, Dreyling M, Kahl B, Ghesquieres H, Ardeshna K, Goto H, Barbui AM, Abramson JS, Borchmann P, Fleury I, Mielke S, Skarbnik A, de Vos S, Kamdar M, Karmali R, Viardot A, Farazi T, Fasan O, Lymp J, Vedal M, Nishii R, Avilion A, Papuga J, Kumar J, Nastoupil LJ. Lisocabtagene maraleucel in follicular lymphoma: the phase 2 TRANSCEND FL study. Nat Med. 2024;30:2199-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 31. | Chiariello GA, Bruno P, Pavone N, Calabrese M, D'Avino S, Ferraro F, Nesta M, Farina P, Cammertoni F, Pasquini A, Montone RA, Montini L, Massetti M. Bleeding Complications in Patients With Perioperative COVID-19 Infection Undergoing Cardiac Surgery: A Single-Center Matched Case-Control Study. J Cardiothorac Vasc Anesth. 2022;36:1919-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Ptak B, Knosala E, Wąsik G, Białynicki-Birula R, Baran W, Batycka-Baran A. Linear IgA bullous dermatosis with Koebner phenomenon in a liver transplant patient. Postepy Dermatol Alergol. 2022;39:629-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, Beauvais D, Roulin L, Gros FX, Rubio MT, Bories P, Bay JO, Llorente CC, Choquet S, Casasnovas RO, Mohty M, Guidez S, Joris M, Loschi M, Carras S, Abraham J, Chauchet A, Drieu La Rochelle L, Deau-Fischer B, Hermine O, Gastinne T, Tudesq JJ, Gat E, Broussais F, Thieblemont C, Houot R, Morschhauser F. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28:2145-2154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 282] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 34. | Daniel Y, Mohamed I, Wheeler AP. Triad of Terror: Rapidly Progressive Austrian Syndrome in a 62-Year-Old Female. R I Med J (2013). 2024;107:7-9. [PubMed] |
| 35. | Lee SY, Lin YC, Chen CP, Cheng SH, Chang SY, Ku SY, Cheng CY. Assessment of risk factors for virological nonsuppression following switch to dolutegravir and lamivudine, or bictegravir, emtricitabine, and tenofovir alafenamide fumarate in a real-world cohort of treatment-experienced adults living with HIV. PLoS One. 2024;19:e0314003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Goff JL, Wang Y, Boyanov MI, Yu Q, Kemner KM, Fein JB, Yee N. Tellurite Adsorption onto Bacterial Surfaces. Environ Sci Technol. 2021;55:10378-10386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Paganoni S, Macklin EA, Hendrix S, Berry JD, Elliott MA, Maiser S, Karam C, Caress JB, Owegi MA, Quick A, Wymer J, Goutman SA, Heitzman D, Heiman-Patterson T, Jackson CE, Quinn C, Rothstein JD, Kasarskis EJ, Katz J, Jenkins L, Ladha S, Miller TM, Scelsa SN, Vu TH, Fournier CN, Glass JD, Johnson KM, Swenson A, Goyal NA, Pattee GL, Andres PL, Babu S, Chase M, Dagostino D, Dickson SP, Ellison N, Hall M, Hendrix K, Kittle G, McGovern M, Ostrow J, Pothier L, Randall R, Shefner JM, Sherman AV, Tustison E, Vigneswaran P, Walker J, Yu H, Chan J, Wittes J, Cohen J, Klee J, Leslie K, Tanzi RE, Gilbert W, Yeramian PD, Schoenfeld D, Cudkowicz ME. Trial of Sodium Phenylbutyrate-Taurursodiol for Amyotrophic Lateral Sclerosis. N Engl J Med. 2020;383:919-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 382] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 38. | Samra R, Hankivsky O. Adopting an intersectionality framework to address power and equity in medicine. Lancet. 2021;397:857-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 39. | Smith J, Guapo F, Strasser L, Millán-Martín S, Milian SG, Snyder RO, Bones J. Development of a Rapid Adeno-Associated Virus (AAV) Identity Testing Platform through Comprehensive Intact Mass Analysis of Full-Length AAV Capsid Proteins. J Proteome Res. 2023;23:161-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 40. | Nikolaou PE, Lambrinidis G, Georgiou M, Karagiannis D, Efentakis P, Bessis-Lazarou P, Founta K, Kampoukos S, Konstantin V, Palmeira CM, Davidson SM, Lougiakis N, Marakos P, Pouli N, Mikros E, Andreadou I. Hydrolytic Activity of Mitochondrial F(1)F(O)-ATP Synthase as a Target for Myocardial Ischemia-Reperfusion Injury: Discovery and In Vitro and In Vivo Evaluation of Novel Inhibitors. J Med Chem. 2023;66:15115-15140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/