Published online Dec 7, 2025. doi: 10.3748/wjg.v31.i45.111643
Revised: August 22, 2025
Accepted: October 27, 2025
Published online: December 7, 2025
Processing time: 151 Days and 20.1 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) has become a major contributor to liver-related morbidity and mortality worldwide, with Egypt carrying one of the highest national burdens in the Middle East and North Africa region. This narrative review explores the epidemiological landscape, patho
Core Tip: Egypt faces one of the highest metabolic dysfunction-associated steatotic liver disease (MASLD) burdens globally, yet disease awareness and access to care remain critically limited. Recognizing MASLD as a national health priority and integrating it into non-communicable disease strategies through population-based screening, lifestyle interventions, and provider education, offers a scalable model for other high-risk, resource-constrained settings.
- Citation: Abdelhamed W, Amin M, Waked I, El-Kassas M. Metabolic dysfunction-associated steatotic liver disease in Egypt: Epidemiology, risk factors and management challenges. World J Gastroenterol 2025; 31(45): 111643
- URL: https://www.wjgnet.com/1007-9327/full/v31/i45/111643.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i45.111643
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD), encompasses a spectrum of liver conditions associated with metabolic derangements[1]. The updated nomenclature seeks to improve disease recognition and management by explicitly linking hepatic steatosis to metabolic risk factors. Globally, MASLD represents a leading cause of liver-related morbidity and mortality[2]. Approximately 5% of affected individuals progress to end-stage liver disease, positioning MASLD as a primary driver of cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation[3]. Beyond its clinical impact, MASLD poses substantial economic and health-related quality of life (HRQoL) burdens[4], with a global prevalence estimated at 38%[5]. Beyond its hepatic manifestations, MASLD is increasingly recognized for its extra-hepatic implications, particularly as a cardiovascular risk factor[6], in heart failure[7], atherosclerosis[8], cardiovascular events[9] and even Sudden cardiac death[10]. In the Middle East and North Africa (MENA) region, prevalence is even higher around 46%[11]. Egypt, in particular, bears a disproportionate burden due to high rates of obesity, type 2 diabetes mellitus (T2DM), and metabolic syndrome[2]. The disease often remains undiagnosed until late stages due to under-recognition and limited screening practices. Given Egypt’s unique combination of epidemiological, cultural, and socioeconomic factors, tailored approaches to diagnosis and mana
MASLD is projected to affect 55.4% of the global population by 2040, representing a growing public health crisis[14]. The disease’s prevalence varies widely across regions: 31.2% in North America and Australia, 28.0% in Asia Pacific, 25.1% in Western Europe, 33.1% in Southeast Asia, 29.7% in East Asia, 33.8% in South Asia, 44.4% in Latin America, and 36.5% in the MENA region[11]. The MENA region, alongside parts of Asia, is witnessing an accelerated increase in MASLD risk factors, including obesity and sedentary lifestyles[15]. According to the 2019 Global Burden of Disease (GBD) data, the global age-standardized MASLD prevalence was 15.7%, while in Arab countries it averaged 27.2%, ranging from 13.5% to 36.2%[16]. Rapid lifestyle transitions, nutritional shifts, and urbanization are key drivers of this trend[17]. Despite the high regional burden, comprehensive data on MASLD remain limited[18]. Studies evaluating prevalence of steatotic liver disease (SLD) in Egypt are scarce and included only small numbers. Between 2017 and 2020, the prevalence of obesity and MASLD rose together with about 70% of obese adolescents affected by MASLD[19]. Using liver stiffness mea
A recent analysis showed an increase in MENA MASLD prevalence from 35.4% (2008-2016) to 46.2% (2017-2020). Using GBD-2019 data, the region was estimated to have 141.5 million MASLD cases, with Egypt accounting for the highest national burden at 25.7 million cases, followed by Türkiye and Iran[5]. MASLD prevalence exceeded 40% in 10 of 21 countries, with the highest rates reported in Kuwait (45.4%), Egypt (45.0%), Qatar (44.4%), and Jordan (43.3%). This trend underscores MASLD as a major contributor to global liver disease burden[11]. Key contributors to this epidemic include coexisting metabolic conditions such as T2DM and obesity[5,21]. A recent meta-analysis indicated a 38% global prevalence between 2016 and 2019 reflecting a 50% increase compared to 1990-2006 figures[11].
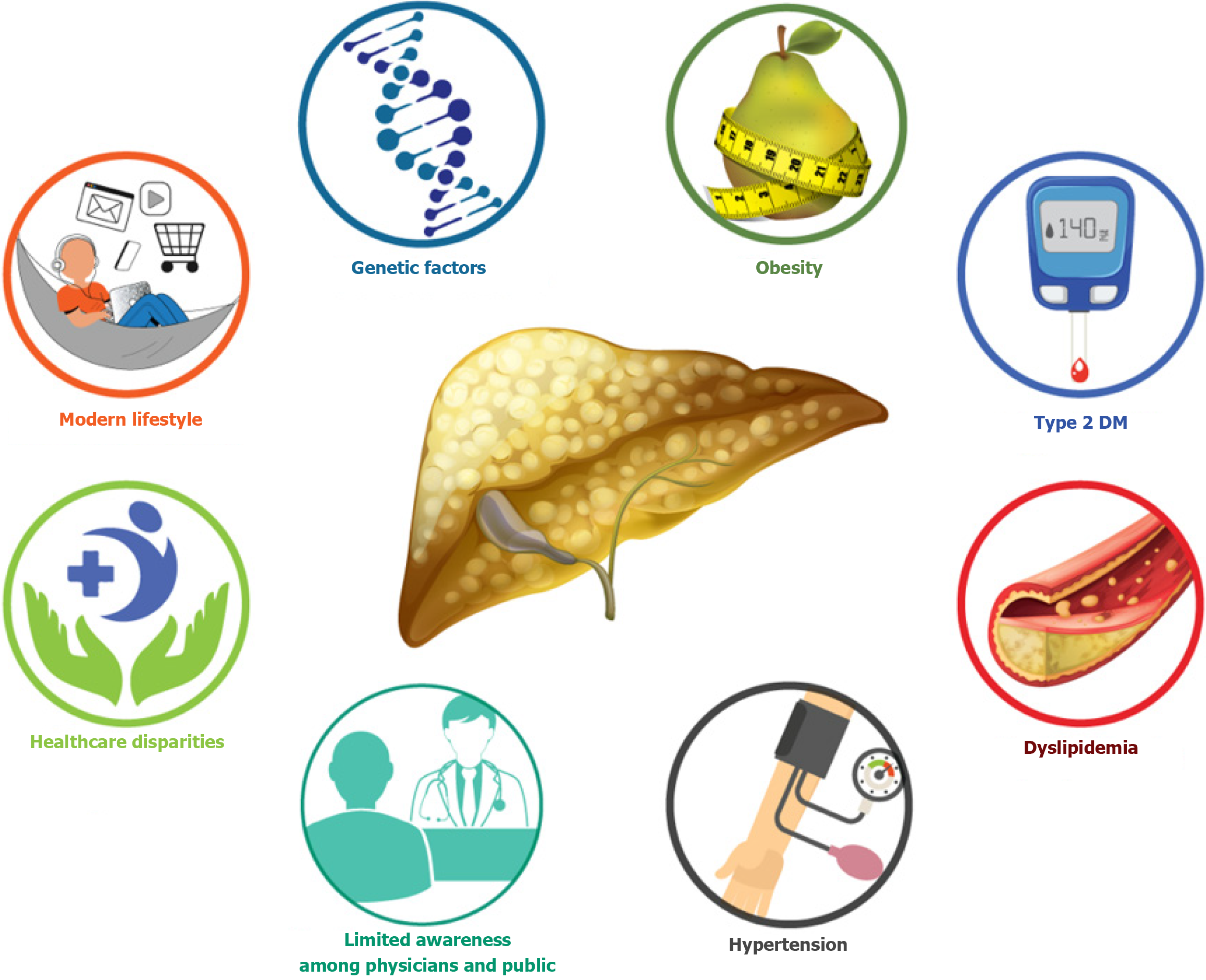
Obesity and central adiposity: MASLD is more prevalent in overweight or obese individuals, with global prevalence reaching 50% in this group and nearly 60% among individuals with T2DM[22]. Obesity is a major driver of MASLD, particularly in Egypt, which ranks fourth globally in overall obesity prevalence and first among women, excluding sparsely populated Pacific Island nations[23]. Very few studies have been published about the burden of diseases in Egypt in general, and the burden of obesity is even more complex as the impact of obesity is a result of its comorbidities[24]. In Egypt, approximately 71.2% of adult men and 79.4% of adult women are overweight, with obesity rates of 26.4% and 48.4%, respectively[20]. While obesity is a well-established MASLD risk factor, a subset of individuals with a normal body mass index (BMI) termed “lean MASLD” also develop the disease. This is particularly relevant in those with BMI < 25 kg/m2 (non-Asian) or < 23 kg/m2 (Asian)[25]. In these individuals, factors such as visceral adiposity, adipose dysfunction, sarcopenia, and early β-cell failure likely contribute to both MASLD and T2DM development[25].
Type 2 diabetes and insulin resistance: MASLD frequently coexists with T2DM and other cardiometabolic risk factors, reflecting their shared pathophysiological basis in insulin resistance, inflammation, and obesity[26,27]. Patients with MASLD have a 2 5-fold increased risk of developing T2DM, particularly in the presence of advanced fibrosis[28]. Conversely, individuals with T2DM and coexisting MASLD especially with fibrosis demonstrate poorer glycemic control over time, as indicated by higher glycosylated hemoglobin levels[29]. Epidemiological data show that MASLD affects up to 70% of patients with T2DM, and biopsy-based studies report prevalence rates exceeding 90%[30]. As a result, recent clinical guidelines recommend routine MASLD fibrosis screening in all T2DM patients[31,32]. It was predicted that there are 24.96 million cases of MASLD with T2D in the MENA region[5]. However, there is limited data on the extent of MASLD between diabetics in Egypt.
Dyslipidemia and hypertension: Dyslipidemia, commonly underdiagnosed in Egypt, affects up to 37% of the popu
Modern lifestyles marked by rapid eating and sedentary habits have significantly contributed to the MASLD epidemic. Several studies have shown that eating at a fast pace is associated with higher caloric intake, insulin resistance, and metabolic dysregulation[40-42]. Frequent fast eaters (≥ 2 times/week) are at 81% increased risk for MASLD, with meta-analyses confirming this association (odds ratio = 1.22; 95% confidence interval: 1.07-1.39)[43]. Ultra-processed foods (UPFs), rich in sugars, fats, and additives but low in fiber and micronutrients, also promote hepatic steatosis by enhancing de novo lipogenesis and worsening insulin resistance[44]. High UPF consumption is linked to obesity, MASLD, and related complications in both pediatric and adult populations[45,46]. The widespread adoption of sedentary lifestyles and nutrient-poor diets, combined with low physical activity levels and rising pediatric obesity, further amplifies the risk and progression of MASLD in Egypt[47]. Egypt like many developing nations is experiencing significant changes in dietary habits characterized by increased calorie-dense food consumption alongside challenges such as food prices and limited access to diverse, nutrient rich foods[48]. These shifts have profound fat consumption patterns and their health implications within the Egyptian context[49].
Ethnic variability in MASLD progression: Ethnic differences significantly affect MASLD susceptibility and outcomes. A meta-analysis of United States data revealed that Hispanic individuals had the highest MASLD prevalence (22.9%) compared to White (14.4%) and Black (13.0%) populations[50]. This disparity correlates with differences in the distribution of genetic risk alleles, including PNPLA3, TM6SF2, HSD17B13, MBOAT7, and GCKR[50,51]. A longitudinal study by Nguyen et al[52] found that while Black patients have a lower MASLD prevalence, they experience worse overall and non-liver-related mortality outcomes once diagnosed. These findings highlight the role of structural inequities, comorbid conditions, and delayed access to care[53].
Genetic predisposition in the Egyptian population: Several genetic variants are implicated in MASLD, including PNPLA3 (rs738409C>G), TM6SF2 (rs58542926C>T), HSD17B13 (rs9992651G>A), and GCKR (rs1260326T>C)[54-58]. Although the prevalence and impact of these polymorphisms in the Egyptian population remain underexplored[59], global genome-wide association studies have validated them as significant risk factors for MASLD[60]. Notably, PNPLA3 (rs738409) shows the strongest correlation with disease severity, as supported by predicted protein-protein interactions in network analyses[61].
Urban rural differences: Urbanization in Egypt has contributed significantly to the rising burden of MASLD. Rapid socioeconomic shifts, characterized by smaller household sizes, increased employment rates, and higher disposable income, have resulted in reduced time for home-cooked meals and increased reliance on processed or ready-to-eat foods[62,63]. These transitions, coupled with a sedentary lifestyle and increased consumption of calorie-dense diets, have escalated MASLD-associated metabolic risk factors[64,65]. With respect to men, women of fertile age show a lower susceptibility to develop MASLD, pointing to the relevance of the hepato-ovarian axis and of estrogen signaling in female liver physiopathology[66]. In the female liver, estrogen’s effects are mainly mediated by the estrogen receptor alpha, whose activation contributes to reducing lipid synthesis, uptake, and storage while promotes lipid catabolism and export[67]. Despite this general protection, women’s risk of developing MASLD varies throughout their reproductive lifespan[68]. Moreover, women with MASLD often exhibit different clinical and pathological features compared to men[68].
The regional burden of MASLD is modulated by disparities in food security and healthcare accessibility. In high socio-demographic index (SDI) countries, MASLD is often driven by consumption of ultra-processed, nutrient-poor diets[65]. Conversely, in low-SDI settings, underdiagnosis may explain lower reported MASLD prevalence, despite increasing exposure to risk factors[69]. Food insecurity in these populations contributes indirectly by exacerbating obesity and T2DM, both primary drivers of MASLD[70]. Furthermore, limited healthcare infrastructure and insufficient access to diagnostic tools in rural and underserved regions of Egypt may result in significant underreporting of MASLD cases[65]. Addressing these gaps through targeted health policy reforms is essential.
Low levels of awareness about MASLD among both healthcare providers and the general population remain a critical barrier. Medical education curricula often allocate minimal time to MASLD, resulting in inadequate physician preparedness[71]. Public knowledge is also limited, with surveys indicating widespread misconceptions, such as the belief that MASLD only occurs in older individuals or runs strictly in families[71-74]. A regional survey identified substantial knowledge gaps in the diagnosis and management of MASLD among healthcare professionals in Egypt, Saudi Arabia, and Türkiye[75]. In response to this global awareness deficit, a 91-country panel of over 200 multidisciplinary experts achieved consensus in 2022 on the urgent need for education, advocacy, and public engagement surrounding MASLD[18]. Figure 1 illustrates the multifactorial risk landscape contributing to MASLD in Egypt.
The pathogenesis of MASLD is underpinned by a complex interplay of genetic, metabolic, and environmental factors. At its core lies a cycle driven by insulin resistance, hyperinsulinemia, lipotoxicity, and low-grade chronic inflammation[76-78]. These mechanisms result in hepatic fat accumulation and promote cellular injury through oxidative stress and inflammatory cascades. Emerging evidence also implicates the gut-liver axis, particularly gut dysbiosis and increased intestinal permeability, in exacerbating hepatic steatosis and inflammation[79]. In individuals consuming unhealthy diets, the liver becomes overwhelmed by pro-inflammatory molecules and toxic lipid intermediates. This sets off a self-perpetuating cycle of hepatocellular injury, fibrogenesis, and metabolic dysfunction[80].
Dietary patterns and nutritional transition: Egypt faces a dual burden of malnutrition, where undernutrition (e.g., micronutrient deficiencies and stunting) coexists with high rates of obesity and overnutrition[63,81]. Over the past decades, dietary patterns have shifted markedly toward increased consumption of refined carbohydrates, sugar, and vegetable oils, largely due to subsidy-driven distortions[82]. Fructose consumption, in particular, has emerged as a key modifiable risk factor. Excessive intake of high-fructose diets has been strongly linked to obesity, insulin resistance, cardiovascular disease, MASLD, and even cancer[83,84]. These dietary imbalances have contributed to Egypt’s alarmingly high obesity rates and rising prevalence of MASLD.
Residual liver injury from chronic hepatitis C virus: Egypt’s longstanding hepatitis C virus (HCV) epidemic has left a significant imprint on the hepatic landscape. Hepatic steatosis has been reported in 30% to 70% of patients with chronic HCV, especially those infected with genotype 3[85]. Although direct-acting antivirals have successfully achieved viral eradication, the evolution of hepatic steatosis post-sustained virologic response remains contentious[86]. Notably, both pre- and post-treatment steatosis have been associated with increased risk of cirrhosis and HCC[87,88]. While some studies report resolution or reduction of steatosis following HCV clearance[89], others describe persistence or even emergence of steatosis, independent of weight changes[90]. Recent data suggest that individuals with a history of HCV infection and concurrent MASLD are at greater risk of significant liver fibrosis than those with HCV or MASLD alone. In one study, fibrosis prevalence reached 58% in the MASLD group, compared to 45% in the HCV-steatosis group and 39% in those without steatosis[91]. This co-existence likely creates a synergistic effect through mechanisms involving oxidative stress, steatosis, and hepatic metabolic dysregulation[91].
Micronutrient deficiencies (vitamin D, E, B12, folic acid): Low serum vitamin D levels have been frequently associated with increased MASLD risk[92]. Although the exact mechanisms remain under investigation, vitamin D appears to exert anti-inflammatory and anti-fibrotic effects on hepatic stellate cells, in addition to modulating insulin sensitivity and lipid metabolism[92]. Egyptian studies confirm that MASLD patients often present with deficient vitamin D levels[93]. Vitamin E, known for its antioxidant properties, has demonstrated beneficial effects in improving aminotransferase levels, hepatic steatosis, and fibrosis in MASLD[94-96]. Egyptian clinical studies corroborate these findings, showing improvement in both biochemical and histological parameters following vitamin E supplementation. Data on vitamin B12 and folate are more limited. While some studies indicate that low levels of these micronutrients may increase all-cause mortality in MASLD patients[97], interventional studies report inconsistent outcomes. For example, one Egyptian trial noted reduced fasting glucose, oxidative stress markers, and liver steatosis following vitamin B12 supplementation; however, the differences did not reach statistical significance compared to placebo[98].
Diagnosing MASLD and its progressive form, metabolic dysfunction-associated steatohepatitis (MASH), presents substantial challenges. These include low disease awareness among clinicians, limited consensus on optimal diagnostic tools, and non-availability of approved pharmacological agent like resmetirom[99-101]. Consequently, MASLD is often discovered incidentally during routine blood tests or imaging performed for unrelated reasons[102]. Traditionally described as asymptomatic, MASLD is increasingly recognized to impact patients’ HRQoL even at early stages. Some patients report subtle symptoms such as fatigue and vague abdominal discomfort, including pain localized to the right upper quadrant[103,104]. These non-specific manifestations, combined with under-recognition, contribute to delayed diagnosis and care.
Although the annual incidence of MASLD-related HCC is lower than that observed in chronic viral hepatitis (ranging from 0.7% to 2.6% in cirrhotic patients and 0.1 to 1.3 per 1000 patient-years in non-cirrhotic MASLD), the sheer size of the MASLD population suggests a rising future burden[2,105,106]. The pathogenesis of MASLD-related HCC is mult
Chronic liver disease significantly impairs HRQoL and patient-reported outcomes (PROs). A multicenter study in Saudi Arabia, Türkiye, and Egypt revealed that younger age, female sex, advanced fibrosis, comorbidities, and lack of physical activity were independently associated with worse PROs among MASLD patients[108]. MASLD patients frequently experience fatigue, emotional distress, and reduced productivity, particularly in the presence of advanced fibrosis or extrahepatic comorbidities[109]. These impairments are not always captured by traditional clinical assessments, underscoring the importance of integrating PROs into both clinical trials and real-world care settings.
While advances in non-invasive diagnostic modalities have improved the evaluation of liver fibrosis, their accessibility remains limited in many parts of Egypt. Tools such as the enhanced liver fibrosis test and FIBROSpect II go beyond routine biochemical markers and can more accurately assess fibrotic progression. However, their integration into clinical practice is constrained by cost and availability[32]. Imaging modalities including vibration-controlled transient ela
Several non-invasive scoring systems have been developed to stratify fibrosis risk in MASLD patients and reduce dependence on liver biopsy[112]. Among the most widely used are: Fibrosis-4 index: This score incorporates age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count. A value < 1.3 suggests low risk for advanced fibrosis, while a value > 2.67 is predictive of advanced disease[113,114]. However, reliance on age may result in overestimation in elderly patients due to age-related elevations[113] which led to modification of the low cutoff point in those over 65 years old to be 2 instead of 1.6. NAFLD fibrosis score (NFS): Based on age, BMI, fasting glucose, platelet count, albumin, and AST/ALT ratio, this score distinguishes between low and high-risk patients. Scores ≤ -0.640 rule out significant fibrosis, while those > -0.640 indicate higher risk[115,116]. NFS correlates with cardiovascular morbidity and mortality but performs poorly in assessing hepatic steatosis[117]. Both fibrosis-4 and NFS are endorsed by the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) as initial triage tools in primary care to identify patients who may require hepatology referral[32,118].
Ultrasound remains the frontline imaging technique for diagnosing hepatic steatosis and for HCC surveillance. In moderate to severe steatosis, ultrasound offers high sensitivity (approximately 90%) and specificity (approximately 95%), but sensitivity drops to 50%-62% in mild cases[110,119]. Diagnostic accuracy is also limited by operator dependency and reduced performance in patients with obesity or low-grade steatosis. For HCC surveillance, both AASLD and EASL recommend biannual abdominal ultrasound, with or without serum alpha-fetoprotein[120]. However, ultrasound alone may miss early-stage HCC, especially in patients with non-cirrhotic MASLD. In cases of inconclusive ultrasound, MRI or computed tomography should be considered[121]. Interestingly, recent studies suggest that patients with MASLD-related cirrhosis exhibit slower tumor growth compared to those with viral etiologies, raising concerns about appropriate surveillance intervals and diagnostic sensitivity[121].
Effective management of MASLD requires a multidisciplinary approach that encompasses prevention, early diagnosis, lifestyle modification, pharmacological treatment, comorbidity control, and public health engagement. Given Egypt’s unique socioeconomic context, tailored interventions that are locally adaptable and scalable are essential[122].
Lifestyle modification remains the foundational intervention for MASLD. The increasing prevalence of the disease is closely associated with sedentary behavior, caloric excess, and poor dietary quality[123,124]. Hypocaloric diets, which reduce overall caloric intake, have demonstrated benefit in hepatic steatosis reduction[125]. The Mediterranean diet rich in unsaturated fats, fiber, and antioxidants has shown superior outcomes in reducing hepatic fat and improving metabolic profiles[126]. Intermittent fasting, including 5:2 protocols and time-restricted eating, has also shown potential benefits for hepatic and glycemic parameters[127]. Although no single diet has been universally established as superior, all effective nutritional strategies share the aim of promoting weight loss and improving hepatic and systemic inflammation[31]. Physical activity contributes significantly to these improvements by enhancing insulin sensitivity and reducing intrahepatic lipid accumulation[128]. Nonetheless, the literature remains inconclusive on the optimal frequency, intensity, and type of exercise for MASLD, requiring personalization[129]. Although evidence suggests that a weight loss of ≥ 5% improves steatosis, ≥ 7% is needed to resolve steatohepatitis, and ≥ 10% can lead to fibrosis regression or stabilization[130], unfortunately, adherence is low; over 80% of MASLD patients fail to sustain lifestyle modifications long-term[131], highlighting the need for culturally adapted behavioral counseling and patient support programs.
While lifestyle change is essential, pharmacologic interventions are needed in patients with more advanced disease or insufficient response. Despite the failure of several drug candidates in clinical trials[132], recent progress has been made. Resmetirom, a selective thyroid hormone receptor-β agonist, was recently approved by the United States Food and Drug Administration as the first pharmacotherapy for MASH, demonstrating efficacy in reducing hepatic fat and improving fibrosis[133,134]. Some anti-diabetic medications have shown benefits in reducing liver disease progression and non-hepatic causes of morbidity and mortality, especially cardiovascular disease[135]. Pioglitazone, a peroxisome proliferator-activated receptor γ agonist, has been shown to improve steatosis, hepatocellular ballooning, and inflammation, though its effects on fibrosis are less consistent[31,136]. Its clinical use is tempered by adverse effects such as bone loss and increased fracture risk, particularly in postmenopausal women[137]. Vitamin E has also demonstrated benefit in MASLD. Meta-analyses confirm improvements in ALT, AST, steatosis, and fibrosis when administered at ≥ 600 IU/day or over ≥ 12 months[138]. In Egypt, studies have shown that vitamin E, alone or in combination with other agents, leads to favorable biochemical and histological improvements in both adult and pediatric populations[96,139].
The close relationship between MASLD and metabolic comorbidities necessitates a comprehensive treatment strategy. Statins are pivotal for managing dyslipidemia in MASLD patients and are safe even in those with elevated liver enzymes. Beyond lipid-lowering, they exhibit anti-inflammatory and anti-fibrotic properties[140-142]. Hypertension, a common comorbidity, should be treated aggressively. Blockade of the renin-angiotensin system improves adipokine profiles and inflammatory responses, potentially reducing hepatic fibrosis in MASLD[143]. In diabetic patients, glucagon-like peptide-1 receptor agonists, including liraglutide, semaglutide, and dulaglutide, are gaining prominence due to their dual benefits in glycemic control and hepatic fat reduction. These agents also provide cardiovascular protection and have been endorsed in MASLD-related guidelines for high-risk patients[144-147]. Sodium-glucose co-transporter 2 inhibitors, by improving insulin sensitivity and reducing hepatic steatosis, represent another promising option under ongoing investigation[148].
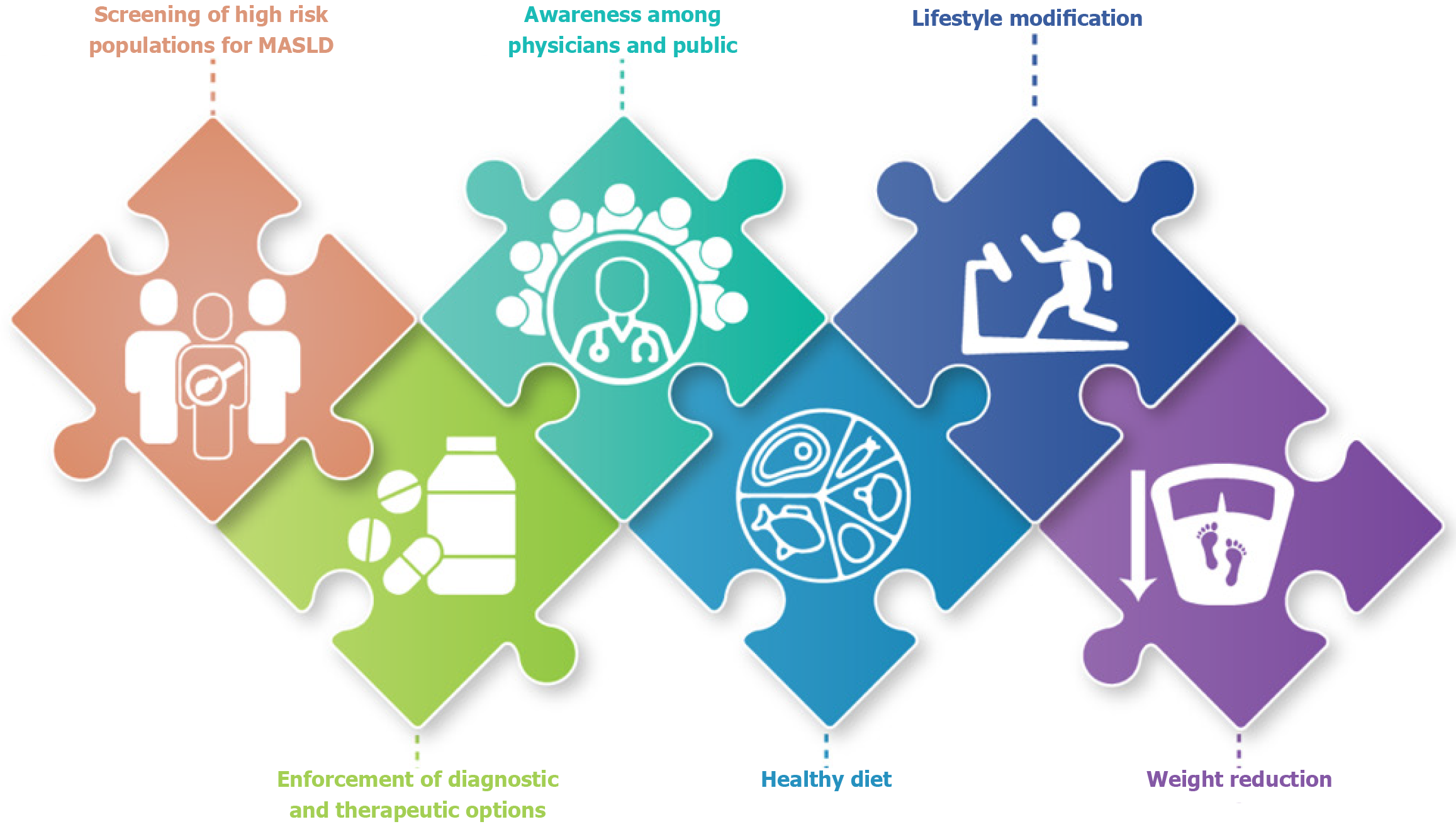
Bariatric surgery is a highly effective intervention for obese patients with advanced MASLD or MASH. It leads to substantial and sustained weight loss, reduces hepatic steatosis, improves insulin resistance, and has been associated with histological improvements. Studies report that 80% of patients experience resolution of MASH within one-year post-surgery, and 30%-40% show regression in liver fibrosis[149,150]. Sustained weight loss of up to 30% can be achieved through surgical intervention, far exceeding outcomes of lifestyle therapy alone[151]. Despite these benefits, long-term data on the safety and efficacy of different bariatric procedures in MASLD are limited, particularly in patients with advanced fibrosis or cirrhosis. Therefore, surgery should be considered following a thorough benefit-risk evaluation by a multidisciplinary team, ideally involving hepatologists, bariatric surgeons, and endocrinologists[32]. Figure 2 shows suggested public health framework for MASLD prevention in Egypt.
A recent global survey highlighted that the stigma associated with MASLD is largely rooted in its link to obesity, with 38% of healthcare providers perceiving the disease as stigmatizing compared to only 8% of patients[152]. The perception of MASLD-related stigma varies across patient groups, medical specialties, and geographic regions, further complicating its recognition and management[152]. Another factor contributing to limited awareness was the absence of approved pharmacological therapies for MASLD, which diminishes both urgency and attention to the disease[153]. Compounding these issues is a persistent knowledge gap in the identification, diagnosis, and management of MASLD, as observed in several high-burden countries including Egypt, Saudi Arabia, and Türkiye[75]. In Egypt, these challenges are exacerbated by the lack of a national screening program and the absence of locally adapted clinical guidelines. Diagnostic and therapeutic options remain limited, particularly outside urban tertiary centers, resulting in delayed diagnosis and fragmented care. In addition, lack of clinical trials assessing the current situation in Egypt is still a barrier for well-defined data analysis and outcomes.
Future directions should prioritize the integration of MASLD into Egypt’s national non-communicable disease agenda. A comprehensive national strategy is urgently needed one that incorporates population-level screening for high-risk groups, standardized non-invasive diagnostic pathways, and evidence-based, locally adapted clinical guidelines. Efforts must also be directed toward strengthening provider education, expanding public health campaigns to reduce stigma and promote lifestyle change, and investing in accessible diagnostic and therapeutic infrastructure beyond tertiary care settings.
Research priorities should include longitudinal cohort studies to better characterize MASLD progression in the Egyptian population, genome-wide studies to elucidate ethnic and genetic determinants, and implementation science to evaluate the effectiveness of policy interventions. Egypt’s successful model of HCV elimination demonstrates that with political will, multisectoral collaboration, and a clear roadmap, scalable and impactful liver health strategies are achie
MASLD represents an urgent yet underrecognized public health challenge in Egypt. With one of the highest national burdens in the MENA region, Egypt faces a convergence of risk factors including obesity, diabetes, sedentary lifestyles, and nutritional transitions that have amplified the prevalence and clinical impact of MASLD. The legacy of chronic HCV, widespread micronutrient deficiencies, and socioeconomic disparities further complicate the pathophysiological landscape. Despite increasing global attention, awareness of MASLD among both healthcare providers and the public remains limited, and diagnostic and treatment strategies are often fragmented, inaccessible, or inconsistently applied.
| 1. | Diaz Torres JV, Villanueva Guerrero VR, Vargas Gómez JP, Pacheco Miranda FJ, Orozco Álvarez LR, León Insignares JD, Mares M, Rodríguez Ortiz HM, Mendoza-Torres E. Metabolic Dysfunction Associated to Steatotic Liver Disease: A Review. Metab Syndr Relat Disord. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Lin Z, Zhang R, Ren S, He T, Mi H, Jiang W, Su C. Global burden of metabolic dysfunction-associated steatotic liver disease from 1990 to 2021 and the prediction for the next 10 years. Prev Med Rep. 2025;59:103248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Stepanova M, Al Shabeeb R, Eberly KE, Shah D, Nguyen V, Ong J, Henry L, Alqahtani SA. The changing epidemiology of adult liver transplantation in the United States in 2013-2022: The dominance of metabolic dysfunction-associated steatotic liver disease and alcohol-associated liver disease. Hepatol Commun. 2024;8:e0352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 80] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 4. | Stepanova M, Henry L, Younossi ZM. Economic Burden and Patient-Reported Outcomes of Nonalcoholic Fatty Liver Disease. Clin Liver Dis. 2023;27:483-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Golabi P, Paik J, Owrangi S, Yilmaz Y, El-Kassas M, Alswat K, Alqahtani SA. Prevalence of metabolic dysfunction-associated steatotic liver disease in the Middle East and North Africa. Liver Int. 2024;44:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 6. | Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. BMJ. 2014;349:g4596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Mantovani A, Petracca G, Csermely A, Beatrice G, Bonapace S, Rossi A, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut. 2022;gutjnl-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 8. | Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, O'Donnell CJ, Speliotes EK. Hepatic steatosis and cardiovascular disease outcomes: An analysis of the Framingham Heart Study. J Hepatol. 2015;63:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, Khan SS, Coresh J, Baker-Smith CM, Carnethon MR, Després JP, Ho JE, Joseph JJ, Kernan WN, Khera A, Kosiborod MN, Lekavich CL, Lewis EF, Lo KB, Ozkan B, Palaniappan LP, Patel SS, Pencina MJ, Powell-Wiley TM, Sperling LS, Virani SS, Wright JT, Rajgopal Singh R, Elkind MSV, Rangaswami J; American Heart Association. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement From the American Heart Association. Circulation. 2023;148:1636-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 639] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 10. | Vo J, Truyen TTTT, Uy-Evanado A, Sargsyan A, Chugh H, Young C, Hurst S, Miyake CY, Reinier K, Chugh SS. Sudden cardiac death associated with fatty liver disease. Int J Cardiol Heart Vasc. 2025;56:101602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 2006] [Article Influence: 668.7] [Reference Citation Analysis (3)] |
| 12. | Bassyouni M, Mysara M, Wohlers I, Busch H, Saber-Ayad M, El-Hadidi M. A comprehensive analysis of genetic risk for metabolic syndrome in the Egyptian population via allele frequency investigation and Missense3D predictions. Sci Rep. 2023;13:20517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Attia A, Labib NA, Abdelzaher NEE, Musa S, Atef M. Lifestyle determinants as predictor of severity of metabolic associated fatty liver disease (MAFLD). Egypt Liver J. 2023;13:47. [DOI] [Full Text] |
| 14. | Le MH, Yeo YH, Zou B, Barnet S, Henry L, Cheung R, Nguyen MH. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin Mol Hepatol. 2022;28:841-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 218] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 15. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. 2024;403:1027-1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 934] [Cited by in RCA: 1125] [Article Influence: 562.5] [Reference Citation Analysis (0)] |
| 16. | Yang E, Chen F, Yang Y, Zhang Y, Lin H, Zhang Y, Chu M. Global trends in depressive disorder prevalence and DALYs among young populations: a comprehensive analysis from 1990 to 2021. BMC Psychiatry. 2024;24:943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 17. | Sarkoohi Z, Bastan MM, Khajuei Gharaei MA, Iranmanesh M, Adinepour A, Khajezade R, Bahri F, Akhlaghi F, Kadkhodamanesh A, Pourghadamyari H, Sharifi H, Eslami O, Nejadghaderi SA. Epidemiological trends and burden of metabolic dysfunction-associated steatotic liver disease in the Middle East and North Africa region: a 32-year analysis of health impact. J Health Popul Nutr. 2025;44:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, Cortez-Pinto H, Crespo J, Cusi K, Dirac MA, Francque S, George J, Hagström H, Huang TT, Ismail MH, Kautz A, Sarin SK, Loomba R, Miller V, Newsome PN, Ninburg M, Ocama P, Ratziu V, Rinella M, Romero D, Romero-Gómez M, Schattenberg JM, Tsochatzis EA, Valenti L, Wong VW, Yilmaz Y, Younossi ZM, Zelber-Sagi S; NAFLD Consensus Consortium. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19:60-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 510] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 19. | Sun M, Sun H. Recent prevalence and trends of obesity and metabolic dysfunction-associated steatotic liver disease (MASLD) among US adolescents: 1999 to 2020. Pediatr Obes. 2025;20:e70003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 20. | Tomah S, Hamdy O, Abuelmagd MM, Hassan AH, Alkhouri N, Al-Badri MR, Gardner H, Eldib AH, Eid EA. Prevalence of and risk factors for non-alcoholic fatty liver disease (NAFLD) and fibrosis among young adults in Egypt. BMJ Open Gastroenterol. 2021;8:e000780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Nowak K, Paluch M, Cudzik M, Syska K, Gawlikowska W, Janczura J. From steatosis to cirrhosis: the role of obesity in the progression of liver disease. J Diabetes Metab Disord. 2025;24:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 5842] [Article Influence: 1460.5] [Reference Citation Analysis (37)] |
| 23. | Esmat G, Zaid H, Hassany M, Abdel-Razek W, El-Serafy M, El Akel W, Salah A, Kamal E, Elshishiney G, Ammar I, Kabil K, Abdallah M, Saeed R, Saad T, Omar Y, Dabbous H, El-Sayed MH, El Shazly Y, Doss W, Waked I. Obesity prevalence in adults and patients with hepatitis C: results from screening a population of 50 million in Egypt. Egypt Liver J. 2024;14:22. [DOI] [Full Text] |
| 24. | Abolfotouh MA, Soliman LA, Mansour E, Farghaly M, El-Dawaiaty AA. Central obesity among adults in Egypt: prevalence and associated morbidity. East Mediterr Health J. 2008;14:57-68. [PubMed] |
| 25. | Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, Yang H, Liu C, Kam LY, Tan XXE, Chien N, Trinh S, Henry L, Stave CD, Hosaka T, Cheung RC, Nguyen MH. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 635] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 26. | Lazarus JV, Newsome PN, Francque SM, Kanwal F, Terrault NA, Rinella ME. Reply: A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2024;79:E93-E94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 140] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 27. | Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10:284-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 395] [Article Influence: 98.8] [Reference Citation Analysis (1)] |
| 28. | Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 350] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 29. | Mantovani A, Taverna A, Cappelli D, Beatrice G, Csermely A, Sani E, Byrne CD, Targher G. Long-Term Adverse Effect of Liver Stiffness on Glycaemic Control in Type 2 Diabetic Patients with Nonalcoholic Fatty Liver Disease: A Pilot Study. Int J Mol Sci. 2022;23:12481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (1)] |
| 30. | Ajmera V, Cepin S, Tesfai K, Hofflich H, Cadman K, Lopez S, Madamba E, Bettencourt R, Richards L, Behling C, Sirlin CB, Loomba R. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol. 2023;78:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 210] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 31. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 680] [Article Influence: 170.0] [Reference Citation Analysis (1)] |
| 32. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1465] [Cited by in RCA: 1585] [Article Influence: 528.3] [Reference Citation Analysis (1)] |
| 33. | Taha HSED, Badran HM, Kandil H, Farag N, Oraby A, El Sharkawy M, Shokry K, Fawzy F, Mahrous H, Bahgat J, Samy M, Shaker MM. Egyptian practical guidance in lipid management 2020. Egypt Heart J. 2021;73:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, Kassir R, Singhal R, Mahawar K, Ramnarain D. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 566] [Article Influence: 141.5] [Reference Citation Analysis (1)] |
| 35. | Yuan M, He J, Hu X, Yao L, Chen P, Wang Z, Liu P, Xiong Z, Jiang Y, Li L. Hypertension and NAFLD risk: Insights from the NHANES 2017-2018 and Mendelian randomization analyses. Chin Med J (Engl). 2024;137:457-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 36. | Lorbeer R, Bayerl C, Auweter S, Rospleszcz S, Lieb W, Meisinger C, Heier M, Peters A, Bamberg F, Hetterich H. Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. J Hypertens. 2017;35:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Long MT, Zhang X, Xu H, Liu CT, Corey KE, Chung RT, Loomba R, Benjamin EJ. Hepatic Fibrosis Associates With Multiple Cardiometabolic Disease Risk Factors: The Framingham Heart Study. Hepatology. 2021;73:548-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 38. | Nabi O, Lacombe K, Boursier J, Mathurin P, Zins M, Serfaty L. Prevalence and Risk Factors of Nonalcoholic Fatty Liver Disease and Advanced Fibrosis in General Population: the French Nationwide NASH-CO Study. Gastroenterology. 2020;159:791-793.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, Asch SM, El-Serag HB. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:808-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 40. | Barrea L, Vetrani C, Verde L, Napolitano B, Savastano S, Colao A, Muscogiuri G. "Forever young at the table": metabolic effects of eating speed in obesity. J Transl Med. 2021;19:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Kolay E, Bykowska-Derda A, Abdulsamad S, Kaluzna M, Samarzewska K, Ruchala M, Czlapka-Matyasik M. Self-Reported Eating Speed Is Associated with Indicators of Obesity in Adults: A Systematic Review and Meta-Analysis. Healthcare (Basel). 2021;9:1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Saito Y, Kajiyama S, Nitta A, Miyawaki T, Matsumoto S, Ozasa N, Kajiyama S, Hashimoto Y, Fukui M, Imai S. Eating Fast Has a Significant Impact on Glycemic Excursion in Healthy Women: Randomized Controlled Cross-Over Trial. Nutrients. 2020;12:2767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Zhang M, Sun X, Zhu X, Zheng L, Bi Y, Li Q, Sun L, Di F, Xu Y, Zhu D, Gao Y, Bao Y, Wang Y, He L, Fan C, Gao X, Gao J, Xia M, Bian H. Association between fast eating speed and metabolic dysfunction-associated steatotic liver disease: a multicenter cross-sectional study and meta-analysis. Nutr Diabetes. 2024;14:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Monda A, de Stefano MI, Villano I, Allocca S, Casillo M, Messina A, Monda V, Moscatelli F, Dipace A, Limone P, Di Maio G, La Marra M, Di Padova M, Chieffi S, Messina G, Monda M, Polito R. Ultra-Processed Food Intake and Increased Risk of Obesity: A Narrative Review. Foods. 2024;13:2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 45. | Calcaterra V, Cena H, Rossi V, Santero S, Bianchi A, Zuccotti G. Ultra-Processed Food, Reward System and Childhood Obesity. Children (Basel). 2023;10:804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 46. | Henney AE, Gillespie CS, Alam U, Hydes TJ, Cuthbertson DJ. Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis. Nutrients. 2023;15:2266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 47. | Mambrini SP, Grillo A, Colosimo S, Zarpellon F, Pozzi G, Furlan D, Amodeo G, Bertoli S. Diet and physical exercise as key players to tackle MASLD through improvement of insulin resistance and metabolic flexibility. Front Nutr. 2024;11:1426551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 48. | The double burden of malnutrition. Case studies from six developing countries. FAO Food Nutr Pap. 2006;84:1-334. [PubMed] |
| 49. | Ibrahim MM, Rizk H, Appel LJ, el Aroussy W, Helmy S, Sharaf Y, Ashour Z, Kandil H, Roccella E, Whelton PK. Hypertension prevalence, awareness, treatment, and control in Egypt. Results from the Egyptian National Hypertension Project (NHP). NHP Investigative Team. Hypertension. 1995;26:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Kubiliun MJ, Cohen JC, Hobbs HH, Kozlitina J. Contribution of a genetic risk score to ethnic differences in fatty liver disease. Liver Int. 2022;42:2227-2236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Vilar-Gomez E, Pirola CJ, Sookoian S, Wilson LA, Liang T, Chalasani N. PNPLA3 rs738409 and risk of fibrosis in NAFLD: Exploring mediation pathways through intermediate histological features. Hepatology. 2022;76:1482-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Nguyen VH, Le I, Ha A, Le RH, Rouillard NA, Fong A, Gudapati S, Park JE, Maeda M, Barnett S, Cheung R, Nguyen MH. Differences in liver and mortality outcomes of non-alcoholic fatty liver disease by race and ethnicity: A longitudinal real-world study. Clin Mol Hepatol. 2023;29:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential Clinical Characteristics and Mortality Outcomes in Persons With NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19:2172-2181.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 54. | Unalp-Arida A, Ruhl CE. Patatin-Like Phospholipase Domain-Containing Protein 3 I148M and Liver Fat and Fibrosis Scores Predict Liver Disease Mortality in the U.S. Population. Hepatology. 2020;71:820-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 55. | Anstee QM, Darlay R, Cockell S, Meroni M, Govaere O, Tiniakos D, Burt AD, Bedossa P, Palmer J, Liu YL, Aithal GP, Allison M, Yki-Järvinen H, Vacca M, Dufour JF, Invernizzi P, Prati D, Ekstedt M, Kechagias S, Francque S, Petta S, Bugianesi E, Clement K, Ratziu V, Schattenberg JM, Valenti L, Day CP, Cordell HJ, Daly AK; EPoS Consortium Investigators. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort(☆). J Hepatol. 2020;73:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 56. | Motomura T, Amirneni S, Diaz-Aragon R, Faccioli LAP, Malizio MR, Coard MC, Kocas-Kilicarslan ZN, Frau C, Haep N, Ostrowska A, Florentino RM, Soto-Gutierrez A. Is HSD17B13 Genetic Variant a Protector for Liver Dysfunction? Future Perspective as a Potential Therapeutic Target. J Pers Med. 2021;11:619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Mu T, Peng L, Xie X, He H, Shao Q, Wang X, Zhang Y. Single Nucleotide Polymorphism of Genes Associated with Metabolic Fatty Liver Disease. J Oncol. 2022;2022:9282557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Li J, Zhao Y, Zhang H, Hua W, Jiao W, Du X, Rui J, Li S, Teng H, Shi B, Yang X, Zhu L. Contribution of Rs780094 and Rs1260326 Polymorphisms in GCKR Gene to Non-alcoholic Fatty Liver Disease: A Meta-Analysis Involving 26,552 Participants. Endocr Metab Immune Disord Drug Targets. 2021;21:1696-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Riazi K, Swain MG, Congly SE, Kaplan GG, Shaheen AA. Race and Ethnicity in Non-Alcoholic Fatty Liver Disease (NAFLD): A Narrative Review. Nutrients. 2022;14:4556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 60. | Fairfield CJ, Drake TM, Pius R, Bretherick AD, Campbell A, Clark DW, Fallowfield JA, Hayward C, Henderson NC, Joshi PK, Mills NL, Porteous DJ, Ramachandran P, Semple RK, Shaw CA, Sudlow CLM, Timmers PRHJ, Wilson JF, Wigmore SJ, Harrison EM, Spiliopoulou A. Genome-Wide Association Study of NAFLD Using Electronic Health Records. Hepatol Commun. 2022;6:297-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 61. | Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT, Pyysalo S, Bork P, Jensen LJ, von Mering C. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51:D638-D646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1815] [Cited by in RCA: 5199] [Article Influence: 1733.0] [Reference Citation Analysis (0)] |
| 62. | Colozza D, Wang YC, Avendano M. Does urbanisation lead to unhealthy diets? Longitudinal evidence from Indonesia. Health Place. 2023;83:103091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 63. | Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2020;395:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 867] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 64. | Kamani L, Rahat A, Yilmaz Y. Addressing the looming epidemic of metabolic dysfunction-associated steatotic liver disease in Pakistan: A call for action. Hepatol Forum. 2024;5:1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 65. | Younossi ZM, Zelber-Sagi S, Kuglemas C, Lazarus JV, Paik A, de Avila L, Gerber L, Paik JM. Association of food insecurity with MASLD prevalence and liver-related mortality. J Hepatol. 2025;82:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 66. | Della Torre S. Beyond the X Factor: Relevance of Sex Hormones in NAFLD Pathophysiology. Cells. 2021;10:2502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 67. | Meda C, Barone M, Mitro N, Lolli F, Pedretti S, Caruso D, Maggi A, Della Torre S. Hepatic ERα accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol Metab. 2020;32:97-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 68. | Cherubini A, Della Torre S, Pelusi S, Valenti L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol Med. 2024;30:1126-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 69. | Golovaty I, Tien PC, Price JC, Sheira L, Seligman H, Weiser SD. Food Insecurity May Be an Independent Risk Factor Associated with Nonalcoholic Fatty Liver Disease among Low-Income Adults in the United States. J Nutr. 2020;150:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 70. | Paik A, Henry L, de Avila L, AlQahtani S, Nader F, Paik JM, Younossi ZM. Food Swamps and Food Deserts Impact on Metabolic Dysfunction-Associated Steatotic Liver Disease Mortality in US Counties. Clin Gastroenterol Hepatol. 2025;23:997-1007.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Patel-Sanchez N, Discepolo V, Asfour N, Azzam RK. Preparedness of Residents to Manage Pediatric Nonalcoholic Fatty Liver Disease: A National Survey. JPGN Rep. 2022;3:e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 72. | Lee JH, Jung JH, Park H, Oh JH, Ahn SB, Yoon EL, Jun DW. A survey on the awareness, current management, and barriers for non-alcoholic fatty liver disease among the general Korean population. Sci Rep. 2023;13:15205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Tincopa MA, Wong J, Fetters M, Lok AS. Patient disease knowledge, attitudes and behaviours related to non-alcoholic fatty liver disease: a qualitative study. BMJ Open Gastroenterol. 2021;8:e000634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 74. | Hegazy MA, Elshafei A, Salem MR, Ashoush O, Abdelghani A. Non-alcoholic fatty liver disease related knowledge among a sample of Egyptians: an exploratory cross-sectional study. Front Public Health. 2023;11:1290842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Alqahtani SA, Yilmaz Y, El-Kassas M, Alswat K, Mawardi M, Sanai FM, Abaakhail F, Alghamdi S, Al-Hamoudi WK, Nader F, Stepanova M, Younossi ZM; Global NASH Council. Knowledge about metabolic dysfunction-associated steatotic liver disease among the medical professionals from countries in the MENA region. Ann Hepatol. 2025;30:101569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Bansal SK, Bansal MB. Pathogenesis of MASLD and MASH - role of insulin resistance and lipotoxicity. Aliment Pharmacol Ther. 2024;59 Suppl 1:S10-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 77. | Abdelhameed F, Kite C, Lagojda L, Dallaway A, Chatha KK, Chaggar SS, Dalamaga M, Kassi E, Kyrou I, Randeva HS. Non-invasive Scores and Serum Biomarkers for Fatty Liver in the Era of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD): A Comprehensive Review From NAFLD to MAFLD and MASLD. Curr Obes Rep. 2024;13:510-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 95] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 78. | Ramírez-Mejía MM, Jiménez-Gutiérrez C, Eslam M, George J, Méndez-Sánchez N. Breaking new ground: MASLD vs. MAFLD-which holds the key for risk stratification? Hepatol Int. 2024;18:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 79. | Vallianou NG, Kounatidis D, Psallida S, Vythoulkas-Biotis N, Adamou A, Zachariadou T, Kargioti S, Karampela I, Dalamaga M. NAFLD/MASLD and the Gut-Liver Axis: From Pathogenesis to Treatment Options. Metabolites. 2024;14:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 80. | Abdelhameed F, Mustafa A, Kite C, Lagojda L, Dallaway A, Than NN, Kassi E, Kyrou I, Randeva HS. Gut Microbiota and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Emerging Pathogenic Mechanisms and Therapeutic Implications. Livers. 2025;5:11. [DOI] [Full Text] |
| 81. | Al-Jawaldeh A, Taktouk M, Nasreddine L. Food Consumption Patterns and Nutrient Intakes of Children and Adolescents in the Eastern Mediterranean Region: A Call for Policy Action. Nutrients. 2020;12:3345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 82. | Aboulghate M, Elaghoury A, Elebrashy I, Elkafrawy N, Elshishiney G, Abul-Magd E, Bassiouny E, Toaima D, Elezbawy B, Fasseeh A, Abaza S, Vokó Z. The Burden of Obesity in Egypt. Front Public Health. 2021;9:718978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 83. | Elseweidy MM, Elesawy AE, Sobh MS, Elnagar GM. Ellagic acid ameliorates high fructose-induced hyperuricemia and non-alcoholic fatty liver in Wistar rats: Focusing on the role of C1q/tumor necrosis factor-related protein-3 and ATP citrate lyase. Life Sci. 2022;305:120751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 84. | Yu S, Li C, Ji G, Zhang L. The Contribution of Dietary Fructose to Non-alcoholic Fatty Liver Disease. Front Pharmacol. 2021;12:783393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 85. | Siphepho PY, Liu YT, Shabangu CS, Huang JF, Huang CF, Yeh ML, Yu ML, Wang SC. The Impact of Steatosis on Chronic Hepatitis C Progression and Response to Antiviral Treatments. Biomedicines. 2021;9:1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Cespiati A, Coelho Rodrigues I, Santos I, Policarpo S, Carvalhana S, Fracanzani AL, Cortez-Pinto H. Effect of HCV eradication by DAAs on liver steatosis, carotid atherosclerosis, and associated metabolic comorbidities: A systematic review. Liver Int. 2024;44:1075-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 87. | Lee MH, Chen YT, Huang YH, Lu SN, Yang TH, Huang JF, Yin SC, Yeh ML, Huang CF, Dai CY, Chuang WL, Yu ML, Yang HI, Chen HY, Chen CJ. Chronic Viral Hepatitis B and C Outweigh MASLD in the Associated Risk of Cirrhosis and HCC. Clin Gastroenterol Hepatol. 2024;22:1275-1285.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 88. | Sano T, Amano K, Ide T, Isoda H, Honma Y, Morita Y, Yano Y, Nakamura H, Itano S, Miyajima I, Shirachi M, Kuwahara R, Ohno M, Kawaguchi T, Tsutsumi T, Nakano D, Arinaga-Hino T, Kawaguchi M, Eguchi Y, Torimura T, Takahashi H, Harada M, Kawaguchi T; SAKS Study Group. Metabolic management after sustained virologic response in elderly patients with hepatitis C virus: A multicenter study. Hepatol Res. 2024;54:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 89. | Tokuchi Y, Suda G, Kawagishi N, Ohara M, Kohya R, Sasaki T, Yoda T, Maehara O, Ohnishi S, Kubo A, Yoshida S, Fu Q, Yang Z, Hosoda S, Kitagataya T, Suzuki K, Nakai M, Sho T, Natsuizaka M, Ogawa K, Sakamoto N. Hepatitis C virus eradication by direct-acting antivirals causes a simultaneous increase in the prevalence of fatty liver and hyper low-density lipoprotein cholesterolemia without an increase in body weight. Hepatol Res. 2023;53:595-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 90. | Cespiati A, Petta S, Lombardi R, Di Marco V, Calvaruso V, Bertelli C, Pisano G, Fatta E, Sigon G, Iuculano F, Crapanzano L, Gibilaro G, Francione P, Craxì A, Fargion S, Fracanzani AL. Metabolic comorbidities and male sex influence steatosis in chronic hepatitis C after viral eradication by direct-acting antiviral therapy (DAAs): Evaluation by the controlled attenuation parameter (CAP). Dig Liver Dis. 2021;53:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | Elgretli W, Shengir M, Sasson S, Ramanakumar AV, Cinque F, Ballestreros LER, Deschenes M, Wong P, Chen T, Kronfli N, Saeed S, Keeshan A, Tandon S, Cooper C, Sebastiani G. Association of MASLD Phenotypes With Liver Fibrosis in Hepatitis C: The Role of Cardiometabolic Risk Factors. J Viral Hepat. 2025;32:e70004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 92. | Aggeletopoulou I, Tsounis EP, Triantos C. Vitamin D and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Novel Mechanistic Insights. Int J Mol Sci. 2024;25:4901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 93. | Gad AI, Elmedames MR, Abdelhai AR, Marei AM. The association between vitamin D status and non-alcoholic fatty liver disease in adults: a hospital-based study. Egypt Liver J. 2020;10:25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Chee NM, Sinnanaidu RP, Chan WK. Vitamin E improves serum markers and histology in adults with metabolic dysfunction-associated steatotic liver disease: Systematic review and meta-analysis. J Gastroenterol Hepatol. 2024;39:2545-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 95. | Vadarlis A, Antza C, Bakaloudi DR, Doundoulakis I, Kalopitas G, Samara M, Dardavessis T, Maris T, Chourdakis M. Systematic review with meta-analysis: The effect of vitamin E supplementation in adult patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2021;36:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 96. | Abdel-Maboud M, Menshawy A, Menshawy E, Emara A, Alshandidy M, Eid M. The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Therap Adv Gastroenterol. 2020;13:1756284820974917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 97. | Zhu J, Liao X, Du L, Lv P, Deng J. Associations of serum folate and vitamin B(12) levels with all-cause mortality among patients with metabolic dysfunction associated steatotic liver disease: a prospective cohort study. Front Endocrinol (Lausanne). 2024;15:1426103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 98. | Talari HR, Molaqanbari MR, Mokfi M, Taghizadeh M, Bahmani F, Tabatabaei SMH, Sharifi N. The effects of vitamin B12 supplementation on metabolic profile of patients with non-alcoholic fatty liver disease: a randomized controlled trial. Sci Rep. 2022;12:14047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 99. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1298] [Article Influence: 259.6] [Reference Citation Analysis (1)] |
| 100. | Younossi ZM, Wong VW, Anstee QM, Romero-Gomez M, Trauner MH, Harrison SA, Lawitz EJ, Okanoue T, Camargo M, Kersey K, Myers RP, Goodman Z, Stepanova M. Fatigue and Pruritus in Patients with Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: The Impact on Patient-Reported Outcomes. Hepatol Commun. 2020;4:1637-1650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 101. | Campbell P, Symonds A, Barritt AS 4th. Therapy for Nonalcoholic Fatty Liver Disease: Current Options and Future Directions. Clin Ther. 2021;43:500-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 102. | Nagra N, Penna R, La Selva D, Coy D, Siddique A, Burman B. Tagging incidental finding of fatty liver on ultrasound: A novel intervention to improve early detection of liver fibrosis. J Clin Transl Res. 2021;7:641-647. [PubMed] |
| 103. | Yamamura S, Nakano D, Hashida R, Tsutsumi T, Kawaguchi T, Okada M, Isoda H, Takahashi H, Matsuse H, Eguchi Y, Sumida Y, Nakajima A, Gerber L, Younossi ZM, Torimura T. Patient-reported outcomes in patients with non-alcoholic fatty liver disease: A narrative review of Chronic Liver Disease Questionnaire-non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2021;36:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 104. | Doward LC, Balp MM, Twiss J, Slota C, Cryer D, Brass CA, Anstee QM, Sanyal AJ. Development of a Patient-Reported Outcome Measure for Non-Alcoholic Steatohepatitis (NASH-CHECK): Results of a Qualitative Study. Patient. 2021;14:533-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 105. | Ong J, Alswat K, Hamid S, El-Kassas M. Nonalcoholic Fatty Liver Disease in Asia, Africa, and Middle East Region. Clin Liver Dis. 2023;27:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 106. | Benhammou JN, Lin J, Aby ES, Markovic D, Raman SS, Lu DS, Tong MJ. Nonalcoholic fatty liver disease-related hepatocellular carcinoma growth rates and their clinical outcomes. Hepatoma Res. 2021;7:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Vitale A, Svegliati-Baroni G, Ortolani A, Cucco M, Dalla Riva GV, Giannini EG, Piscaglia F, Rapaccini G, Di Marco M, Caturelli E, Zoli M, Sacco R, Cabibbo G, Marra F, Mega A, Morisco F, Gasbarrini A, Foschi FG, Missale G, Masotto A, Nardone G, Raimondo G, Azzaroli F, Vidili G, Oliveri F, Pelizzaro F, Ramirez Morales R, Cillo U, Trevisani F, Miele L, Marchesini G, Farinati F; Italian Liver Cancer (ITA. LI.CA) group. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002-2033: the ITA.LI.CA database. Gut. 2023;72:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 126] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 108. | Alqahtani SA, Yilmaz Y, El-Kassas M, Alswat K, Sanai F, AlZahrani M, Abaalkhail F, AlShaikh M, Al-Hamoudi WK, Nader F, Stepanova M, Younossi ZM; Global NASH Council. Clinical and patient-reported outcomes in patients with chronic hepatitis B and C and non-alcoholic fatty liver disease from real-world practices in Saudi Arabia, Turkey and Egypt. J Viral Hepat. 2024;31:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 109. | Younossi ZM, Yilmaz Y, Yu ML, Wai-Sun Wong V, Fernandez MC, Isakov VA, Duseja AK, Mendez-Sanchez N, Eguchi Y, Bugianesi E, Burra P, George J, Fan JG, Papatheodoridis GV, Chan WK, Alswat K, Saeed HS, Singal AK, Romero-Gomez M, Gordon SC, Roberts SK, El Kassas M, Kugelmas M, Ong JP, Alqahtani S, Ziayee M, Lam B, Younossi I, Racila A, Henry L, Stepanova M; Global NASH Council. Clinical and Patient-Reported Outcomes From Patients With Nonalcoholic Fatty Liver Disease Across the World: Data From the Global Non-Alcoholic Steatohepatitis (NASH)/ Non-Alcoholic Fatty Liver Disease (NAFLD) Registry. Clin Gastroenterol Hepatol. 2022;20:2296-2306.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 110. | Jang W, Song JS. Non-Invasive Imaging Methods to Evaluate Non-Alcoholic Fatty Liver Disease with Fat Quantification: A Review. Diagnostics (Basel). 2023;13:1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 111. | Tun KM, Noureddin N, Noureddin M. Noninvasive tests in the evaluation of nonalcoholic fatty liver disease: A review. Clin Liver Dis (Hoboken). 2023;22:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 112. | Cataldo I, Sarcognato S, Sacchi D, Cacciatore M, Baciorri F, Mangia A, Cazzagon N, Guido M. Pathology of non-alcoholic fatty liver disease. Pathologica. 2021;113:194-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 113. | Huang C, Seah JJ, Tan CK, Kam JW, Tan J, Teo EK, Kwek A, Wong YJ, Tan M, Ang TL, Kumar R. Modified AST to platelet ratio index improves APRI and better predicts advanced fibrosis and liver cirrhosis in patients with non-alcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. 2021;45:101528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 114. | Aydın MM, Akçalı KC. Liver fibrosis. Turk J Gastroenterol. 2018;29:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 115. | Cheng CH, Chu CY, Chen HL, Lin IT, Wu CH, Lee YK, Hu PJ, Bair MJ. Subgroup analysis of the predictive ability of aspartate aminotransferase to platelet ratio index (APRI) and fibrosis-4 (FIB-4) for assessing hepatic fibrosis among patients with chronic hepatitis C. J Microbiol Immunol Infect. 2020;53:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 116. | Lee YS, Yoo YJ, Jung YK, Kim JH, Seo YS, Yim HJ, Kim IH, Lee SY, Kim BH, Kim JW, Lee CH, Yeon JE, Kwon SY, Um SH, Byun KS. Multiparametric MR Is a Valuable Modality for Evaluating Disease Severity of Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2020;11:e00157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Lee CO, Li HL, Tsoi MF, Cheung CL, Cheung BMY. Association between the liver fat score (LFS) and cardiovascular diseases in the national health and nutrition examination survey 1999-2016. Ann Med. 2021;53:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 967] [Article Influence: 483.5] [Reference Citation Analysis (1)] |
| 119. | Petzold G. Role of Ultrasound Methods for the Assessment of NAFLD. J Clin Med. 2022;11:4581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 120. | Natarajan Y, Kramer JR, Yu X, Li L, Thrift AP, El-Serag HB, Kanwal F. Risk of Cirrhosis and Hepatocellular Cancer in Patients With NAFLD and Normal Liver Enzymes. Hepatology. 2020;72:1242-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 121. | Tayob N, Corley DA, Christie I, Almers L, Rahal AK, Richardson P, White DL, Davila J, Kanwal F, El-Serag HB. Validation of the Updated Hepatocellular Carcinoma Early Detection Screening Algorithm in a Community-Based Cohort of Patients With Cirrhosis of Multiple Etiologies. Clin Gastroenterol Hepatol. 2021;19:1443-1450.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 122. | Das S, Agarwal K, Kapoor N, Lakhani OJ, Das Gupta A. Emerging concepts in the diagnosis and management of metabolically associated steatotic liver disease. Curr Opin Endocrinol Diabetes Obes. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 123. | Stefano JT, Duarte SMB, Ribeiro Leite Altikes RG, Oliveira CP. Non-pharmacological management options for MAFLD: a practical guide. Ther Adv Endocrinol Metab. 2023;14:20420188231160394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 124. | Pathak MP, Pathak K, Saikia R, Gogoi U, Patowary P, Chattopadhyay P, Das A. Therapeutic potential of bioactive phytoconstituents found in fruits in the treatment of non-alcoholic fatty liver disease: A comprehensive review. Heliyon. 2023;9:e15347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 125. | Guveli H, Ozlu T, Ersoy Tasar B, Batuhan Kenger E, Kaya E. Sustainability of diet-based moderate calorie restriction among obese patients with metabolic-associated fatty liver disease. Hepatol Forum. 2021;2:97-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 126. | Xiao Y, Zhang X, Yi D, Qiu F, Wu L, Tang Y, Wang N. Mediterranean diet affects the metabolic outcome of metabolic dysfunction-associated fatty liver disease. Front Nutr. 2023;10:1225946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 127. | Wang YY, Tian F, Qian XL, Ying HM, Zhou ZF. Effect of 5:2 intermittent fasting diet versus daily calorie restriction eating on metabolic-associated fatty liver disease-a randomized controlled trial. Front Nutr. 2024;11:1439473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 128. | von Loeffelholz C, Roth J, Coldewey SM, Birkenfeld AL. The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease. Biomedicines. 2021;9:1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 129. | Słomko J, Zalewska M, Niemiro W, Kujawski S, Słupski M, Januszko-Giergielewicz B, Zawadka-Kunikowska M, Newton J, Hodges L, Kubica J, Zalewski P. Evidence-Based Aerobic Exercise Training in Metabolic-Associated Fatty Liver Disease: Systematic Review with Meta-Analysis. J Clin Med. 2021;10:1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 130. | Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2021;160:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (2)] |
| 131. | Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, Jang BK, Kim SG, Ahn SB, Kim H, Jun DW, Choi JI, Song DS, Kim W, Jeong SW, Kim MY, Koh H, Jeong S, Lee JW, Cho YK; Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2021;27:363-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 132. | Zhu S, Wu Z, Wang W, Wei L, Zhou H. A revisit of drugs and potential therapeutic targets against non-alcoholic fatty liver disease: learning from clinical trials. J Endocrinol Invest. 2024;47:761-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Petta S, Targher G, Romeo S, Pajvani UB, Zheng MH, Aghemo A, Valenti LVC. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024;44:1526-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 134. | Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW, Rinella ME, Anstee QM, Abdelmalek MF, Younossi Z, Baum SJ, Francque S, Charlton MR, Newsome PN, Lanthier N, Schiefke I, Mangia A, Pericàs JM, Patil R, Sanyal AJ, Noureddin M, Bansal MB, Alkhouri N, Castera L, Rudraraju M, Ratziu V; MAESTRO-NASH Investigators. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med. 2024;390:497-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1118] [Article Influence: 559.0] [Reference Citation Analysis (0)] |
| 135. | Ivancovsky Wajcman D, Byrne CJ, Dillon JF, Brennan PN, Villota-Rivas M, Younossi ZM, Allen AM, Crespo J, Gerber LH, Lazarus JV. A narrative review of lifestyle management guidelines for metabolic dysfunction-associated steatotic liver disease. Hepatology. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 136. | Genua I, Cusi K. Pharmacological Approaches to Nonalcoholic Fatty Liver Disease: Current and Future Therapies. Diabetes Spectr. 2024;37:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 137. | Nesti L, Tricò D, Mengozzi A, Natali A. Rethinking pioglitazone as a cardioprotective agent: a new perspective on an overlooked drug. Cardiovasc Diabetol. 2021;20:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 138. | Wang MY, Prabahar K, Găman MA, Zhang JL. Vitamin E supplementation in the treatment on nonalcoholic fatty liver disease (NAFLD): Evidence from an umbrella review of meta-analysis on randomized controlled trials. J Dig Dis. 2023;24:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 139. | Fouda A, Abdelaziz AE, Hussien M, Ali AA, Abdelkawy KS, Elbarbry F. A randomized controlled trial comparing the effects of Vitamin E, Ursodeoxycholic acid and Pentoxifylline on Egyptian non-alcoholic steatohepatitis patients. Eur Rev Med Pharmacol Sci. 2021;25:7449-7459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 140. | Chen JYS, Chua D, Lim CO, Ho WX, Tan NS. Lessons on Drug Development: A Literature Review of Challenges Faced in Nonalcoholic Fatty Liver Disease (NAFLD) Clinical Trials. Int J Mol Sci. 2022;24:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 141. | Lampsas S, Xenou M, Oikonomou E, Pantelidis P, Lysandrou A, Sarantos S, Goliopoulou A, Kalogeras K, Tsigkou V, Kalpis A, Paschou SA, Theofilis P, Vavuranakis M, Tousoulis D, Siasos G. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules. 2023;28:969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 142. | Perdomo C, D'Ingianna P, Escalada J, Petta S, Romero Gómez M, Ampuero J. Nonalcoholic fatty liver disease and the risk of metabolic comorbidities: how to manage in clinical practice. Pol Arch Intern Med. 2020;130:975-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 143. | Prentza V, Pavlidis G, Ikonomidis I, Pililis S, Lampsas S, Kountouri A, Pliouta L, Korakas E, Thymis J, Palaiodimou L, Tsegka A, Markakis K, Halvatsiotis P, Tsivgoulis G, Lambadiari V. Antidiabetic Treatment and Prevention of Ischemic Stroke: A Systematic Review. J Clin Med. 2024;13:5786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 144. | Roumane A, Mcilroy GD, Sommer N, Han W, Heisler LK, Rochford JJ. GLP-1 receptor agonist improves metabolic disease in a pre-clinical model of lipodystrophy. Front Endocrinol (Lausanne). 2024;15:1379228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 145. | Boutari C, DeMarsilis A, Mantzoros CS. Obesity and diabetes. Diabetes Res Clin Pract. 2023;202:110773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 146. | Brouwers B, Rao G, Tang Y, Rodríguez Á, Glass LC, Hartman ML. Incretin-based investigational therapies for the treatment of MASLD/MASH. Diabetes Res Clin Pract. 2024;211:111675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 147. | Abushamat LA, Shah PA, Eckel RH, Harrison SA, Barb D. The Emerging Role of Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Metabolic Dysfunction-Associated Steatohepatitis. Clin Gastroenterol Hepatol. 2024;22:1565-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 148. | Mo M, Huang Z, Liang Y, Liao Y, Xia N. The safety and efficacy evaluation of sodium-glucose co-transporter 2 inhibitors for patients with non-alcoholic fatty liver disease: An updated meta-analysis. Dig Liver Dis. 2022;54:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 149. | Young EN, Dogan M, Watkins C, Bajwa A, Eason JD, Kuscu C, Kuscu C. A Review of Defatting Strategies for Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2022;23:11805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 150. | Mellemkjær A, Kjær MB, Haldrup D, Grønbæk H, Thomsen KL. Management of cardiovascular risk in patients with metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med. 2024;122:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 151. | Jeeyavudeen MS, Khan SKA, Fouda S, Pappachan JM. Management of metabolic-associated fatty liver disease: The diabetology perspective. World J Gastroenterol. 2023;29:126-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 152. | Younossi ZM, Alqahtani SA, Alswat K, Yilmaz Y, Keklikkiran C, Funuyet-Salas J, Romero-Gómez M, Fan JG, Zheng MH, El-Kassas M, Castera L, Liu CJ, Wai-Sun Wong V, Zelber-Sagi S, Allen AM, Lam B, Treeprasertsuk S, Hameed S, Takahashi H, Kawaguchi T, Schattenberg JM, Duseja A, Newsome PN, Francque S, Spearman CW, Castellanos Fernández MI, Burra P, Roberts SK, Chan WK, Arrese M, Silva M, Rinella M, Singal AK, Gordon S, Fuchs M, Alkhouri N, Cusi K, Loomba R, Ranagan J, Eskridge W, Kautz A, Ong JP, Kugelmas M, Eguchi Y, Diago M, Yu ML, Gerber L, Fornaresio L, Nader F, Henry L, Racila A, Golabi P, Stepanova M, Carrieri P, Lazarus JV; Global NASH Council. Global survey of stigma among physicians and patients with nonalcoholic fatty liver disease. J Hepatol. 2024;80:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 153. | Zhang C, Yang M. Current Options and Future Directions for NAFLD and NASH Treatment. Int J Mol Sci. 2021;22:7571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/