Published online Dec 7, 2025. doi: 10.3748/wjg.v31.i45.111540
Revised: September 17, 2025
Accepted: October 28, 2025
Published online: December 7, 2025
Processing time: 119 Days and 1.2 Hours
Optimal treatment strategies for small hepatocellular carcinoma (HCC) remain under investigation. Laparoscopic hepatectomy (LH) and radiofrequency ablation (RFA) are key curative modalities.
To compare the long-term survival and perioperative outcomes of LH and RFA for small HCC.
In this retrospective study, 254 patients with small HCC who were admitted to the First Affiliated Hospital of Chongqing Medical University between December 2022 and March 2025 were analyzed. Patients were divided into LH (n = 109) and RFA (n = 145) groups based on their treatment modality. Primary endpoints were 36-month overall survival (OS) and disease-free survival (DFS). Secondary end
Baseline demographic and clinical characteristics, except mean tumor size (LH: 3.05 ± 1.12 cm vs RFA: 2.48 ± 0.93 cm, P < 0.001) and platelet count (higher in LH, P = 0.008), were comparable between groups. LH was associated with longer operative time, greater blood loss, prolonged recovery, higher costs, and increased complication rates (62.39% vs 15.87% for RFA, P < 0.001). However, at 36 months, OS (85.32% vs 66.21%, P < 0.001) and DFS (64.22% vs 44.83%, P = 0.002) were significantly higher in the LH group. Multivariate Cox regression identified surgical approach (LH), lower tumor size, and lower platelet count as independent predictors for improved DFS; only LH independently improved OS (hazard ratio = 0.55; 95% confidence interval: 0.38-0.79; P < 0.001).
LH, though associated with increased perioperative morbidity, provides superior long-term survival outcomes compared with RFA in patients with small HCC. Surgical approach should be considered when selecting optimal therapy for this patient population.
Core Tip: This study demonstrates that for patients with small hepatocellular carcinoma, laparoscopic hepatectomy (LH) offers significantly better long-term survival compared to radiofrequency ablation, despite a higher risk of short-term complications. LH was associated with higher 3-year overall and disease-free survival rates. The findings suggest that LH should be the preferred curative option for suitable patients with adequate liver function, while radiofrequency ablation remains a valuable less invasive alternative for higher-risk cases.
- Citation: Lei ZL, Tan ZL, Luo YH, Yang M, Wang JL, Qin Z, Liu YY. Comparison of the efficacy of laparoscopic hepatectomy and radiofrequency ablation for small hepatocellular carcinoma: A retrospective study. World J Gastroenterol 2025; 31(45): 111540
- URL: https://www.wjgnet.com/1007-9327/full/v31/i45/111540.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i45.111540
Hepatocellular carcinoma (HCC) ranks as the sixth most common malignancy, is the third leading cause of cancer-related mortality globally, and accounts for over 800000 deaths each year[1]. Despite advances in diagnostic and therapeutic approaches, the prognosis for HCC remains poor, primarily due to late diagnosis, underlying cirrhosis, and high propensity for intrahepatic and extrahepatic recurrence[2]. Early detection programs have increased the proportion of patients diagnosed at a stage amenable to potentially curative local therapies[3]. Among these, patients presenting with small HCC, commonly defined as tumor(s) measuring < 3 cm in diameter or multiple (≤ 3) nodules, each ≤ 3 cm, re
With liver transplantation restricted by donor scarcity and eligibility constraints, two principal modalities are widely utilized for patients with small HCC and preserved liver function: Surgical resection and local ablation[5]. In particular, minimally invasive surgical techniques such as laparoscopic hepatectomy (LH) have garnered increasing favor over open resection and offers the benefits of reduced operative trauma, improved perioperative recovery, and equivalent onco
Despite the widespread use of LH and RFA, the optimal approach for the treatment of small HCC remains a subject of ongoing debate[7]. Several randomized controlled trials, retrospective cohort studies, and meta-analyses have compared the oncological outcomes and perioperative parameters of these modalities, yet the results have not been wholly consistent[8]. Many studies suggest that surgical resection may offer superior long-term oncologic control and lower local recurrence rates, particularly in patients with solitary tumors, favorable anatomy, and preserved hepatic reserve[8]. By contrast, RFA is lauded for its lower complication rates, shorter hospital stays, and comparable early survival outcomes, especially in patients with high risk for surgery, impaired liver function, or tumors in anatomically challenging locations[9]. Nevertheless, incomplete ablation and the inability of RFA to address microscopic vascular invasion or satellite nodules could result in higher recurrence rates than LH, even for small tumors[10].
Decision-making in clinical practice is further complicated by the significant heterogeneity among published studies concerning patient selection criteria, tumor morphology, procedural expertise, and perioperative care protocols[10]. Thus, a clear consensus on the relative benefits and optimal indications for LH vs RFA in small HCC is lacking, and robust, institution-specific investigations remain necessary to tailor treatment recommendations to real-world populations.
Given this context, the present retrospective cohort study was designed to compare the efficacy and safety of LH and RFA systematically in a well-characterized population of patients with small HCC. The aims of this study are to assess long-term outcomes, including disease-free survival (DFS) and overall survival (OS), as well as perioperative morbidity, hospital stay, and local recurrence. By elucidating factors that favor one modality over the other, this investigation seeks to inform individualized clinical decision-making, optimize the therapeutic algorithm for small HCC, and ultimately improve patient survival and quality of life.
This study retrospectively analyzed the clinical data of 254 patients with small HCC who were admitted to the First Affiliated Hospital of Chongqing Medical University from December 2022 to March 2025. Patients were divided into two groups based on their treatment modality: 145 cases underwent RFA (RFA group) and 109 cases underwent LH (LH group). The protocol of this retrospective study was reviewed and approved by the Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University. Given the retrospective design of the study and the use of anonymized data, informed consent was not required.
Patients who met the following criteria were included: (1) First diagnosed with HCC and completed all treatments in our hospital; (2) Had a maximum tumor diameter of less than 3 cm and no more than three intrahepatic tumors, or a single intrahepatic lesion with a diameter of less than 5 cm; (3) Child-Pugh class A or B; (4) Barcelona Clinic Liver Cancer (BCLC) level 0 or A; (5) No intrahepatic and distant metastases; (6) No invasion of the portal vein, the hepatic vein trunk, or secondary branches; and (7) Indocyanine green retention rates of less than 30% at 15 minutes.
Patients who met the following criteria were excluded: (1) Postoperative permanent pathology results indicating benign focal nodular hyperplasia, inflammatory nodules, metastatic liver cancer, or other types of primary liver cancer; (2) Initial treatment outcomes that were suboptimal with unclear tumor boundaries, proximity to blood vessels, or poor differentiation, and subsequent receipt of additional antitumor therapies such as trans arterial chemoembolization, per
RFA group: Patients underwent comprehensive preoperative evaluation, including assessment of liver function (Child-Pugh score), cardiopulmonary fitness, and coagulation profile. Patients fasted for 8 hours prior to the procedure. Ablation was performed percutaneously under conscious sedation and local anesthesia. The patient’s position (supine, lateral decubitus) was optimized based on the tumor location under ultrasound guidance. Either Talon or Cool-tip radiofrequency needles and their associated cooling systems were used. The contrast agent for ultrasound was SonoVue. Preoperative routine ultrasound contrast was performed to confirm the size, location, blood supply, surrounding organs, and major blood vessels of the lesion. The puncture point and needle insertion path were selected, and under ultrasound guidance, the radiofrequency needle was inserted into the tumor. Depending on the size of the tumor and its surroun
LH group: Patients were administered general anesthesia. Pneumoperitoneum was established to a pressure of 12-14 mmHg via an observation port below the umbilicus, and a laparoscope was inserted. Patients were positioned in a 30° reverse Trendelenburg position. Additional 3-4 trocars and related surgical instruments were then placed in the upper abdomen. Depending on the specific situation, a hepatic pedicle occlusion band might be placed (Pringle maneuver, applied intermittently: 15-minute occlusion, 5-minute release). After marking the planned resection line, an ultrasonic scalpel was used to cut into the liver parenchyma gradually. If a regular hepatectomy was feasible, it was performed according to standard procedures. For cases where regular resection was difficult, the liver parenchyma more than 2 cm away from the tumor edge was carefully dissected. In special locations such as the caudate lobe or close to major blood vessels, where achieving a 2 cm margin was challenging, the resection margin was determined based on tumor size and location. The portal triad was then dissected and ligated using absorbable clips or Hem-o-lok clips before being divided. Bipolar coagulation or electrocautery was used to control bleeding at the resection surface. Dry gauze was used to inspect the liver resection surface carefully, and if necessary, Johnson and Johnson absorbable hemostatic fibers were applied. After confirming no significant active bleeding or bile leakage, an abdominal drain was placed, and the surgery was completed.
The main objectives of the study were to evaluate the 36-month DFS rate and OS rate. Secondary goals included assessing the total recurrence rate. DFS was calculated from the date of the procedure until the first detection of a recurrent tumor, whereas OS was measured from the date of the procedure to the time of death. Additional factors included duration of operation, volume of blood loss, necessity for blood transfusions, recovery after surgery, length of hospital stay, asso
Following any procedure, the patient’s liver function was reassessed prior to discharge. A multidetector abdominal contrast computed tomography (CT) scan was conducted four weeks post-treatment and recommended every six months for the next three years. For patients at risk of radiation damage, abdominal B-ultrasound was a suitable alternative for CT scans. Dynamic contrast magnetic resonance imaging was used when intrahepatic recurrence could not be confirmed. If symptoms suggested possible extrahepatic metastasis (such as coughing, blood-tinged sputum, or bone pain), chest CT and bone emission CT scans were conducted. During follow-up visits, routine blood tests, liver function assessments, and serum α-fetoprotein (AFP) levels were checked. Post-three-year follow-ups were scheduled every 6-12 months based on patient preference. Contact with patients was maintained through phone calls every 3-6 months to ensure they received regular medical reviews and assistance with scheduling clinic appointments. This ongoing communication helped track their liver health and manage their condition effectively. Complete surgical excision was confirmed by pathology reports showing clear resection margins. Effective RFA was indicated by the absence of peripheral enhancement in the first post-treatment contrast-enhanced CT scan. Patients diagnosed with recurrence were readmitted for subsequent treatment.
For the statistical analysis, SPSS software version 29.0 (IBM Corp., NY, United States) was used. Continuous variables were reported as mean ± SD and analyzed through independent samples t-tests. Categorical variables were expressed as n (%), and comparisons were made using χ2 tests. DFS and OS curves were generated using the Kaplan-Meier method. Univariate and multivariate Cox proportional hazards regression models were utilized to identify factors associated with DFS and OS. Variables that showed significance (P < 0.05) in univariate analysis were entered into the multivariate model to determine their independent effects on survival outcomes. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. A two-sided P value < 0.05 was considered statistically significant.
The demographic and baseline clinical characteristics of the LH group (n = 109) and the RFA group (n = 145) are summarized in Table 1. No statistically significant differences regarding age, sex distribution, body mass index (BMI), prevalence of type II diabetes, family history of cancer, alcohol consumption, cigarette smoking, Child-Pugh classification, BCLC stage, hepatitis B surface antigen positivity, hepatitis C antibody positivity, liver cirrhosis, portal hypertension, or tumor number were observed between the LH and RFA groups (all P > 0.05). The mean age of patients was 60.62 ± 12.37 years in the LH group and 62.45 ± 11.32 years in the RFA group. The majority of patients in both groups were male (84.40% in LH and 86.21% in RFA) and had Child-Pugh class A liver function (95.41% in LH and 93.10% in RFA). The incidence of cirrhosis was comparable between groups (66.97% vs 68.97%). However, the mean tumor size was significantly larger in the LH group than in the RFA group (3.05 ± 1.12 cm vs 2.48 ± 0.93 cm, respectively; t = 4.319, P < 0.001). Other clinicopathologic features did not differ significantly between the two groups.
| Variable | LH group (n = 109) | RFA group (n = 145) | t/χ2 | P value |
| Age (years) | 60.62 ± 12.37 | 62.45 ± 11.32 | 1.229 | 0.220 |
| Male gender | 92 (84.40) | 125 (86.21) | 0.163 | 0.687 |
| BMI (kg/m2) | 23.19 ± 3.06 | 22.68 ± 3.74 | 1.189 | 0.236 |
| Type-II diabetes | 17 (15.60) | 25 (17.24) | 0.122 | 0.727 |
| Lineal descent cancer history | 27 (24.77) | 38 (26.21) | 0.067 | 0.795 |
| Alcohol use | 44 (40.37) | 56 (38.62) | 0.079 | 0.778 |
| Cigarette smoking | 43 (39.45) | 54 (37.24) | 0.129 | 0.720 |
| Child-Pugh classification ratio [A/(A + B) × 100%] | 104 (95.41) | 135 (93.10) | 0.597 | 0.440 |
| BCLC level [0/(0 + A) × 100%)] | 24 (22.02) | 36 (24.83) | 0.272 | 0.602 |
| HBsAg (+) | 89 (81.65) | 114 (78.62) | 0.356 | 0.551 |
| Hepatitis C antibody (+) | 5 (4.59) | 5 (3.45) | 0.019 | 0.892 |
| Liver cirrhosis | 73 (66.97) | 100 (68.97) | 0.114 | 0.736 |
| Portal hypertension | 5 (4.59) | 13 (8.97) | 1.812 | 0.178 |
| Tumor number | 0.422 | 0.516 | ||
| One | 101 (92.66) | 131 (90.34) | ||
| Two | 8 (7.34) | 14 (9.66) | ||
| Mean tumor size (cm) | 3.05 ± 1.12 | 2.48 ± 0.93 | 4.319 | < 0.001 |
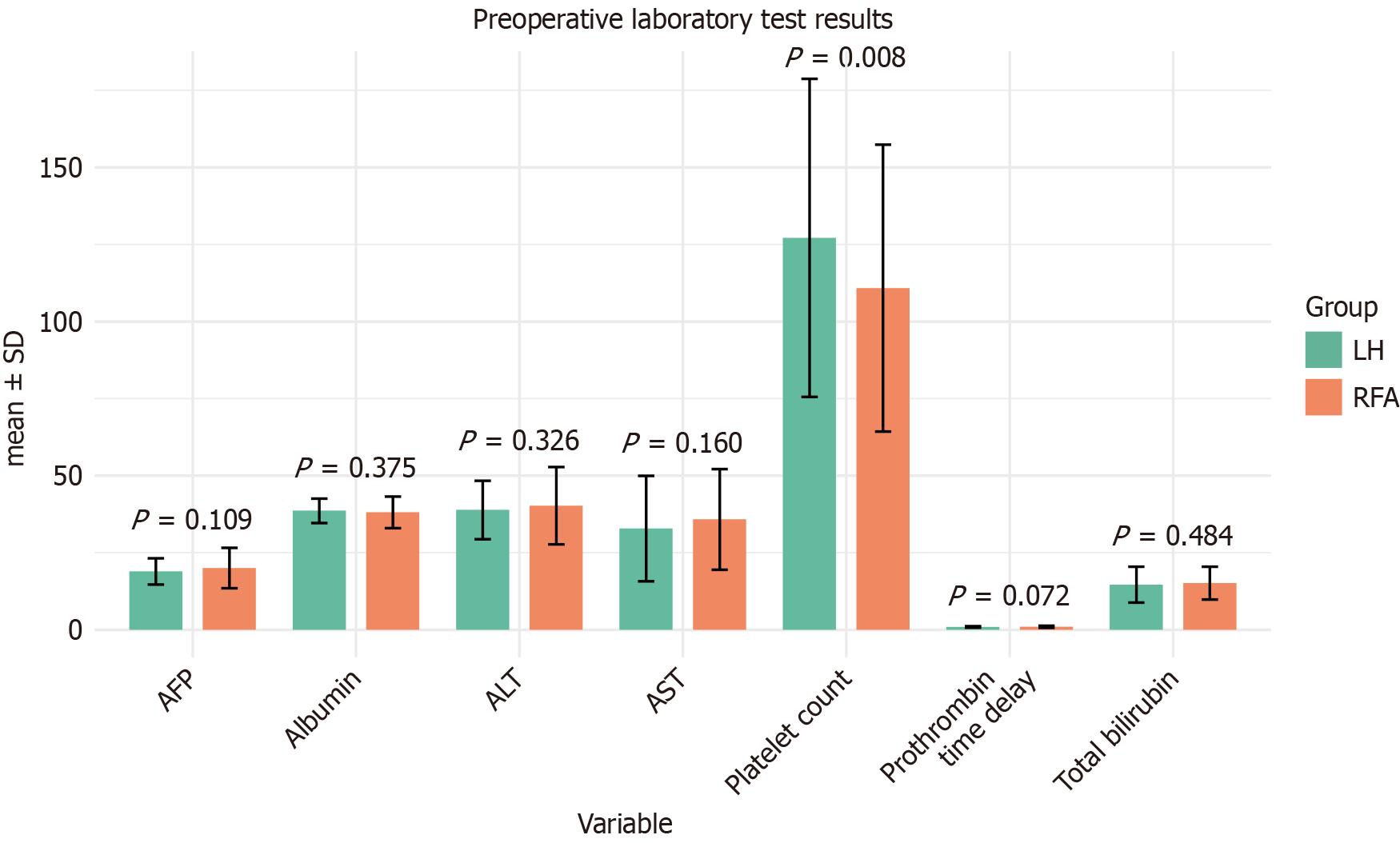
The preoperative laboratory test results of patients who underwent LH and those treated with RFA were compared in detail (Figure 1). The tests included alanine aminotransferase, aspartate aminotransferase (AST), AFP, albumin levels, platelet count, prothrombin time delay, and total bilirubin. Error bars represent SD, and P values indicate statistical significance. No significant differences were observed for alanine aminotransferase, AST, total bilirubin, albumin, prothrombin time delay, or AFP levels between the two groups (all P > 0.05). However, the LH group exhibited a significantly higher platelet count than the RFA group (P = 0.008). These findings suggest that baseline liver function and tumor markers, except platelet counts, were comparable between the two cohorts.
The intraoperative and postoperative outcomes of the LH and RFA groups were analyzed (Table 2). Compared with the RFA group, the LH group had significantly longer operating time (120.52 ± 33.19 minutes vs 43.68 ± 8.48 minutes), greater intraoperative blood loss (240.05 ± 95.19 mL vs 14.95 ± 4.67 mL), and higher need for blood transfusion (23.85% vs 3.45%), and all P < 0.001. Postoperative recovery was also less favorable in the LH group, with significantly higher pain scores on postoperative day 1 (3.14 ± 0.93 vs 0.95 ± 0.32), delayed ambulation (2.32 ± 0.94 days vs 0.76 ± 0.27 days), and postponed first oral intake (2.42 ± 0.60 days vs 1.12 ± 0.14 days), and all P < 0.001. Additionally, the LH group exhibited higher AST levels on postoperative day 2 (155.03 ± 57.40 IU/L vs 78.35 ± 37.74 IU/L, P < 0.001), longer postoperative hospital stays (10.42 ± 4.51 days vs 6.30 ± 2.90 days, P < 0.001), and higher hospitalization costs (30563.72 ± 3447.61 Chinese Yuan vs 22872.80 ± 2059.51 Chinese Yuan, P < 0.001). All differences between the two groups were statistically significant.
| Variable | LH group (n = 109) | RFA group (n = 145) | t/χ2 | P value |
| Operating time (minutes) | 120.52 ± 33.19 | 43.68 ± 8.48 | 23.598 | < 0.001 |
| Blood loss (mL) | 240.05 ± 95.19 | 14.95 ± 4.67 | 24.665 | < 0.001 |
| Pain score on postoperative day 1 (points) | 3.14 ± 0.93 | 0.95 ± 0.32 | 23.372 | < 0.001 |
| Time to ambulation after surgery (days) | 2.32 ± 0.94 | 0.76 ± 0.27 | 16.808 | < 0.001 |
| Time to first oral intake after surgery (days) | 2.42 ± 0.60 | 1.12 ± 0.14 | 22.103 | < 0.001 |
| AST on postoperative day 2 (IU/L) | 155.03 ± 57.40 | 78.35 ± 37.74 | 12.117 | < 0.001 |
| Need for blood transfusion | 26 (23.85) | 5 (3.45) | 24.179 | < 0.001 |
| Hospital stay (days) | 10.42 ± 4.51 | 6.30 ± 2.90 | 8.327 | < 0.001 |
| Hospital costs (Chinese Yuan) | 30563.72 ± 3447.61 | 22872.80 ± 2059.51 | 20.681 | < 0.001 |
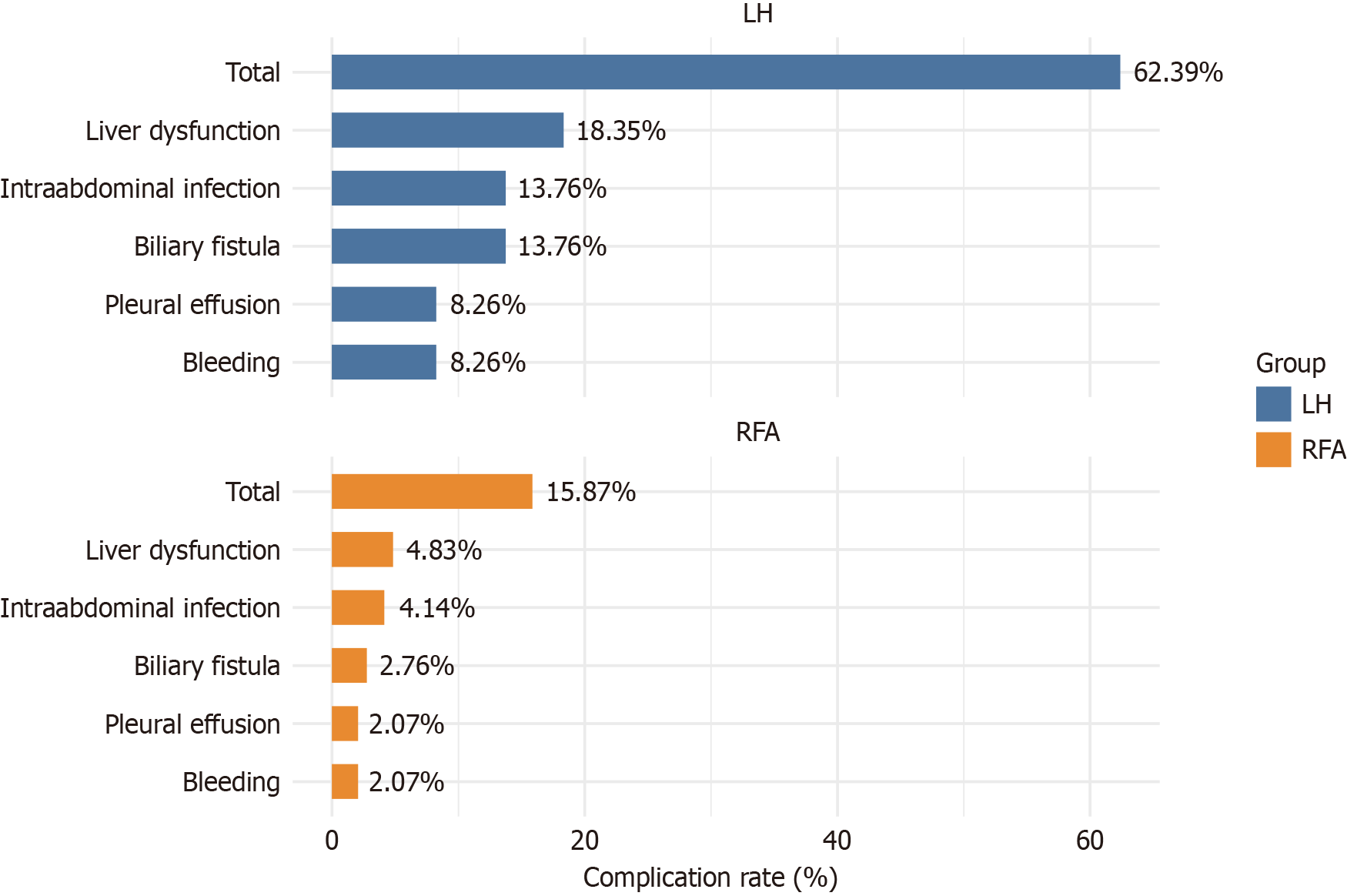
The bar chart illustrates the incidence rates of postoperative complications following LH and RFA (Figure 2). The overall complication rate was significantly higher in the LH group (62.39%) than in the RFA group (15.87%, χ2 = 58.579, P < 0.001). Specific complications such as biliary fistula (13.76% vs 2.76%, P < 0.001), pleural effusion and postoperative bleeding (both at 8.26% vs 2.07%, P = 0.021), intra-abdominal infection (13.76% vs 4.14%, P = 0.006), and liver dysfunction (18.35% vs 4.83%, P < 0.001) were all significantly more common in the LH group. These data highlight the increased risk profile associated with the surgical approach vs the minimally invasive technique.
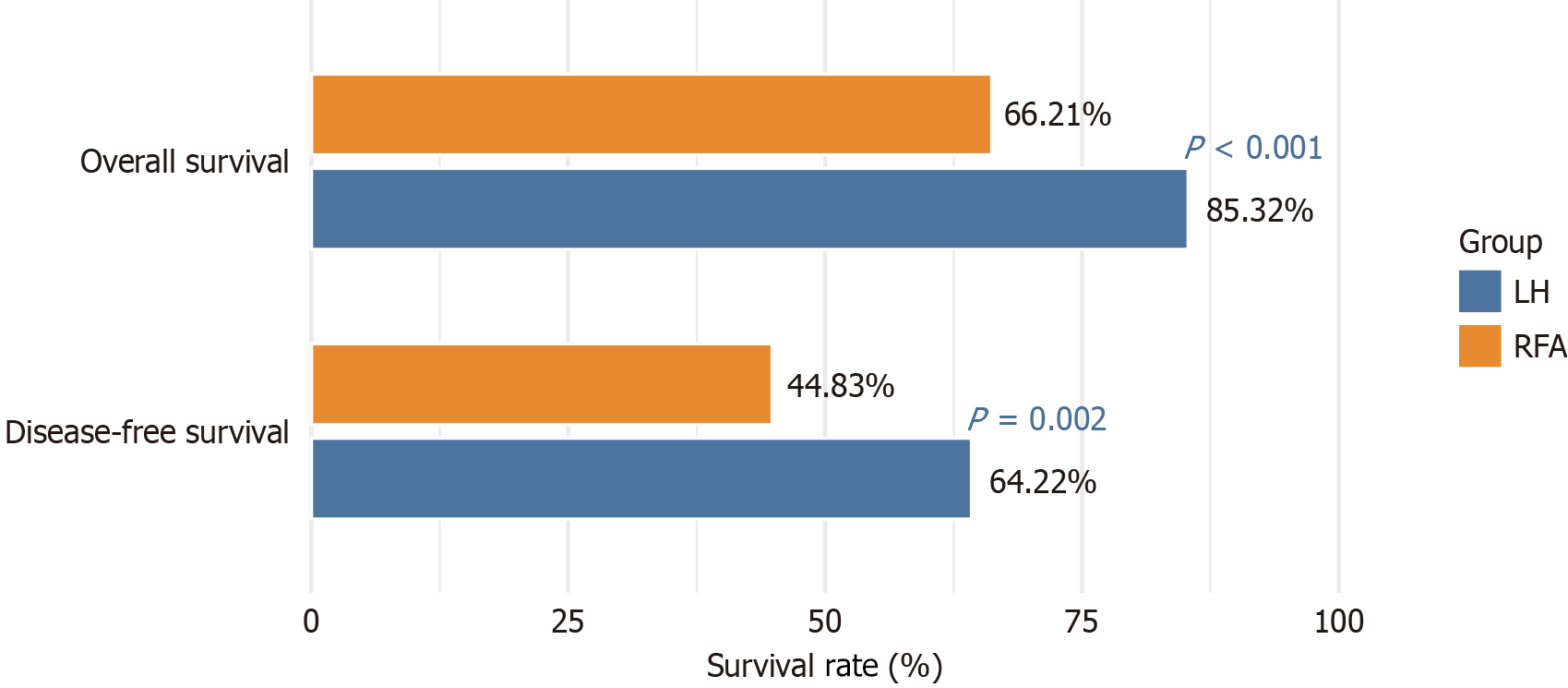
The survival outcomes for LH and RFA treatments for small HCC were compared (Table 2 and Figure 3). The OS and DFS rates at 36 months revealed that the LH group demonstrated significantly better OS (85.32% vs 66.21%, χ2 = 11.939, P < 0.001) and DFS (64.22% vs 44.83%, χ2 = 9.398, P = 0.002) than the RFA group. These findings indicate superior long-term survival outcomes for patients undergoing LH compared with RFA.
Cox regression analysis identified mean tumor size, platelet count, and surgical approach as significant factors in
| Variable | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Mean tumor size (≥ 2.84 vs < 2.84) | 0.500 (0.310-0.810) | 0.005 | 0.600 (0.370-0.970) | 0.037 |
| Platelet count (≤ 125.42 vs > 125.42) | 0.667 (0.470-0.947) | 0.022 | 0.700 (0.490-0.990) | 0.045 |
| Surgical approach (LH vs RFA) | 0.550 (0.380-0.790) | 0.001 | 0.500 (0.340-0.730) | < 0.001 |
In univariate Cox regression analysis, mean tumor size (≥ 2.84 cm vs < 2.84 cm: HR = 0.55; 95%CI: 0.35-0.86; P = 0.008), platelet count (≤ 125.42 vs > 125.42 × 109/L: HR = 0.70; 95%CI: 0.50-0.98; P = 0.038), and surgical approach (LH vs RFA: HR = 0.60; 95%CI: 0.42-0.86; P = 0.004) were significant factors influencing OS (Table 4). Multivariate Cox regression analysis demonstrated that the surgical approach was independently associated with OS, and LH conferred a signi
| Variable | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Mean tumor size (≥ 2.84 vs < 2.84) | 0.550 (0.350-0.860) | 0.008 | 0.650 (0.400-1.060) | 0.085 |
| Platelet count (≤ 125.42 vs > 125.42) | 0.700 (0.500-0.980) | 0.038 | 0.750 (0.520-1.080) | 0.120 |
| Surgical approach (LH vs RFA) | 0.600 (0.420-0.860) | 0.004 | 0.550 (0.380-0.790) | < 0.001 |
The present retrospective study provides further insight into the comparative efficacy and safety of LH and RFA for small HCC. Rather than simply summarizing the observed outcomes, this work discusses the mechanisms, clinical rationale, and broader oncological context that likely underlie these findings. One of the most striking observations in the field, consistently substantiated by the current study and mounting external evidence, is the superior long-term DFS and OS associated with LH compared with RFA for patients with small HCC[11]. This effect is most plausibly rooted in fun
Differences in the biology and microenvironment of HCC further magnify the inherent limitations of RFA[16]. HCC is well known for its high propensity for microvascular invasion and the development of satellite nodules adjacent to the main lesion[16]. LH can remove these foci by virtue of resecting a margin of nontumorous tissue alongside the index tumor; RFA is fundamentally incapable of targeting disease beyond the ablation zone, particularly if microscopic extensions breach the periphery of the intended treatment field[16]. Thus, while ablation may be sufficient for small, well-demarcated tumors that lack microinvasion, the inability to address multifocal or infiltrative disease may lead to earlier recurrence in a non-negligible proportion of patients[17].
Factors such as tumor size further tip the balance in favor of surgery because the efficacy of RFA diminishes substantially as the lesion approaches a diameter of 3 cm or greater. Larger tumors pose a higher risk of incomplete ablation due to thermal gradients and variability in tissue conduction properties in addition to possible microvascular invasion that extends beyond imaging resolution. By contrast, LH, when feasible, is not constrained in margin width by heat sink ef
Platelet count, an independent prognostic factor emerging from this and other studies, may be seen as a proxy for tumor biology and underlying hepatic substrate[18,19]. Elevated platelet counts, particularly in the context of preserved liver function, have been associated with an increased risk of early HCC recurrence, plausibly due to their role in fa
Addressing the higher perioperative complication rates observed with LH compared with RFA is also important[25]. This disparity is fundamentally attributed to the invasiveness of surgery, which, despite advances in laparoscopic te
Synthesizing these mechanistic considerations, the current study’s findings reinforce the growing consensus in the literature that LH should be considered the first-line curative modality for small HCC in patients with adequate hepatic reserve. RFA, while offering clear benefits of minimal invasiveness and rapid convalescence, may optimally serve as an alternative for those with significant comorbidities, high surgical risk, or poor liver function. The interplay between tumor size, biology, and patient background is paramount in individualized treatment selection.
Despite the strengths of our study, several limitations should be acknowledged: As a retrospective study, potential for selection bias and confounding factors that were not controlled for is inherent. Future prospective randomized trials would provide more robust evidence. The decision to undergo either LH or RFA was made based on clinical judgment and patient-specific factors, which could introduce bias. Although the sample size was substantial, it may still be insufficient to detect subtle differences in outcomes between the two modalities, especially for subgroups with specific tumor characteristics or comorbidities. LH and RFA techniques have evolved over time, and variations in operator experience and institutional protocols could influence outcomes. This study primarily focused on DFS and OS but did not comprehensively evaluate quality of life, which is an important aspect of treatment decision-making.
Ongoing advances in ablative technologies (such as microwave ablation) and surgical techniques (including robotic and anatomic resections) may further refine the risk-benefit ratio of each approach. Improving preprocedural risk stratification by integrating radiomic, hematological, and molecular markers will likely play an increasing role in guiding personalized therapy. Moreover, recurrence after either treatment underscores the need for vigilant post-treatment surveillance and potentially, adjuvant or salvage strategies that address the residual risk of intrahepatic or extrahepatic spread.
The latent mechanisms driving superior long-term outcomes for LH relative to RFA in small HCC fundamentally derive from the greater completeness of tumor and peritumoral tissue removal, along with the technical and oncological limitations of localized thermal ablation. Individualization of management, balancing oncological efficacy, procedural risk, and patient-specific factors, remains the cornerstone of optimal care in this challenging, evolving field.
| 1. | Seki T, Tsukagoshi M, Harimoto N, Araki K, Watanabe A, Ishii N, Hagiwara K, Hoshino K, Muranushi R, Kakizaki S, Ogawa Y, Handa H, Shirabe K. Laparoscopic hepatectomy for hepatocellular carcinoma in a patient with congenital factor V deficiency: a case report. Surg Case Rep. 2022;8:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Shen ZF, Liang X. Current status of radical laparoscopy for treating hepatocellular carcinoma with portal hypertension. World J Clin Cases. 2021;9:2419-2432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Wang J, Luo H, Yi L, Yang P, Zeng X. Downstaging and laparoscopic hepatectomy plus intraoperative radiofrequency ablation for the treatment of initially unresectable multifocal hepatocellular carcinomas. Front Surg. 2023;10:1340657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Xu H, Zhou L, Jin Q. The effects of ultrasound-guided radiofrequency ablation and laparoscopic hepatectomy in the treatment of small hepatocellular carcinoma: a retrospective analysis. Transl Cancer Res. 2021;10:4794-4801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Cheng KC, Ho KM. Pure laparoscopic liver resection versus percutaneous radiofrequency ablation for small hepatocellular carcinoma: a propensity score and multivariate analysis. Transl Cancer Res. 2022;11:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Wang Z, Ouyang J, Jia B, Zhou Y, Yang Y, Li X, Li Q, Zhou J. Laparoscopic liver resection versus radiofrequency ablation for caudate lobe solitary hepatocellular carcinoma: A propensity score matching study. Cancer Med. 2024;13:e7068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Xu L, Lin Z, Chen D, Huang Z, Huang X, Che X. Laparoscopic liver resection versus radiofrequency ablation for hepatocellular carcinoma within Milan criteria: a meta-analysis and systematic review. Front Oncol. 2024;14:1442499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Jung RH, Kim HW, Yoon SY. Video-assisted transthoracic liver resection in patients with marginal liver function: a retrospective cohort study. Korean J Clin Oncol. 2021;17:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Chen W, Lin X, Wu Z, Pan W, Ke Q, Chen Y. Laparoscopic liver resection is superior to radiofrequency ablation for small hepatocellular carcinoma: a systematic review and meta-analysis of propensity score-matched studies. Hepatol Int. 2024;18:998-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Ma Z, Lin X, Zhang J, Song X, Yan M, Guo L, Xue J, Lu C, Shi J, Cheng S, Guo W. Repeat laparoscopic hepatectomy versus radiofrequency ablation for recurrent hepatocellular carcinoma: A multicenter, propensity score matching analysis. Biosci Trends. 2025;18:563-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Xiong D, Li J, Yuan S. Is laparoscopic hepatectomy superior to radiofrequency ablation in treating small hepatocellular carcinoma? Hepatol Int. 2024;18:1815-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Jiang C, Feng Q, Zhang Z, Qiang Z, Du A, Xu L, Li J. Radiofrequency ablation versus laparoscopic hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. World J Surg Oncol. 2024;22:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Shaaban Abdelgalil M, Amer BE, Yasen N, El-Samahy M, Awad AK, Elfakharany B, Saeed O, Abd-ElGawad M. Efficacy and safety of laparoscopic liver resection versus radiofrequency ablation in patients with early and small hepatocellular carcinoma: an updated meta-analysis and meta-regression of observational studies. World J Surg Oncol. 2024;22:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Ko SE, Lee MW, Ahn S, Rhim H, Kang TW, Song KD, Kim JM, Choi GS, Cha DI, Min JH, Sinn DH, Choi MS, Lim HK. Laparoscopic Hepatic Resection Versus Laparoscopic Radiofrequency Ablation for Subcapsular Hepatocellular Carcinomas Smaller Than 3 cm: Analysis of Treatment Outcomes Using Propensity Score Matching. Korean J Radiol. 2022;23:615-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 15. | Ogiso S, Seo S, Eso Y, Yoh T, Kawai T, Okumura S, Ishii T, Fukumitsu K, Taura K, Seno H, Uemoto S. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small hepatocellular carcinoma. HPB (Oxford). 2021;23:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Chen YC, Soong RS, Chiang PH, Chai SW, Chien CY. Reappraisal of safety and oncological outcomes of laparoscopic repeat hepatectomy in patients with recurrent hepatocellular carcinoma: it is feasible for the pioneer surgical team. BMC Surg. 2024;24:373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Conticchio M, Delvecchio A, Ratti F, Gelli M, Anelli FM, Laurent A, Vitali GC, Magistri P, Assirati G, Felli E, Wakabayashi T, Pessaux P, Piardi T, Di Benedetto F, de'Angelis N, Javier Briceno DF, Rampoldi AG, Adam R, Cherqui D, Aldrighetti L, Memeo R. Laparoscopic surgery versus radiofrequency ablation for the treatment of single hepatocellular carcinoma ≤3 cm in the elderly: a propensity score matching analysis. HPB (Oxford). 2022;24:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Liu F, Tan L, Luo L, Pan JJ. Comparison of laparoscopic hepatectomy and percutaneous radiofrequency ablation for the treatment of small hepatocellular carcinoma: a meta-analysis. BMC Surg. 2024;24:83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Kim S, Yoon CJ, Cho JY, Han HS, Yoon YS, Lee HW, Lee JS, Kim M, Lee B, Ahn S. Comparative long-term outcomes of laparoscopic hepatectomy and radiofrequency ablation for hepatocellular carcinoma located in the anterolateral segments of the liver. J Hepatobiliary Pancreat Sci. 2022;29:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Liu J, Zhao J, Gu HAO, Zhu Z. Repeat hepatic resection VS radiofrequency ablation for the treatment of recurrent hepatocellular carcinoma: an updated meta-analysis. Minim Invasive Ther Allied Technol. 2022;31:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Delvecchio A, Conticchio M, Casella A, Ratti F, Gelli M, Anelli FM, Laurent A, Vitali GC, Magistri P, Felli E, Wakabayashi T, Pessaux P, Piardi T, Di Benedetto F, de'Angelis N, Briceño-Delgado J, Rampoldi A, Adam R, Cherqui D, Aldrighetti L, Memeo R. Open, laparoscopic liver resection and percutaneous thermal ablation in elderly patients with hepatocellular carcinoma: outcomes and therapeutic strategy. Surg Endosc. 2024;38:6700-6710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Lee B, Han HS, Yoon YS, Cho JY, Lee HW, Lee JH, Park Y, Kang M, Kim J. Treatment strategies for solitary hepatocellular carcinoma: comparative outcomes of radiofrequency ablation vs. laparoscopic liver resection based on tumor location. Surg Endosc. 2025;39:2175-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Sakai H, Goto Y, Fukutomi S, Akashi M, Sato T, Nomura Y, Arai S, Kanno H, Hashimoto K, Akiba J, Hisaka T, Akagi Y, Okuda K. [Laparoscopic Extirpation of Peritoneal Dissemination of Hepatocellular Carcinoma Using ICG Imaging]. Gan To Kagaku Ryoho. 2021;48:1697-1699. [PubMed] |
| 24. | Park Y, Cho JY, Han HS, Yoon YS, Lee HW, Lee B, Kang M, Kim J. Comparison of Open versus Laparoscopic Approaches in Salvage Hepatectomy for Recurrent Hepatocellular Carcinoma after Radiofrequency Ablation. Medicina (Kaunas). 2023;59:1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Park Y, Han HS, Lim SY, Joo H, Kim J, Kang M, Lee B, Lee HW, Yoon YS, Cho JY. Evolution of Liver Resection for Hepatocellular Carcinoma: Change Point Analysis of Textbook Outcome over Twenty Years. Medicina (Kaunas). 2024;61:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Santambrogio R, Barabino M, D'Alessandro V, Iacob G, Opocher E, Gemma M, Zappa MA. Micronvasive behaviour of single small hepatocellular carcinoma: which treatment? Updates Surg. 2021;73:1359-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Wang X, Chai X, Tang R, Xu Y, Chen Q. Comparison of laparoscopic hepatectomy and radiofrequency ablation for small hepatocellular carcinoma patients: a SEER population-based propensity score matching study. Updates Surg. 2024;76:2755-2766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Wang Z, Liu M, Zhang DZ, Wu SS, Hong ZX, He GB, Yang H, Xiang BD, Li X, Jiang TA, Li K, Tang Z, Huang F, Lu M, Chen JA, Lin YC, Lu X, Wu YQ, Zhang XW, Zhang YF, Cheng C, Ye HL, Wang LT, Zhong HG, Zhong JH, Wang L, Chen M, Liang FF, Chen Y, Xu YS, Yu XL, Cheng ZG, Liu FY, Han ZY, Tang WZ, Yu J, Liang P. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3-5-cm HCC. Hepatology. 2022;76:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 29. | Zhu F, Chang Q, Duan S, Leng W. Efficacy and safety of radiofrequency ablation versus laparoscopic hepatectomy for small hepatocellular carcinoma: A protocol for a randomized controlled trial. Medicine (Baltimore). 2021;100:e23678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/