Published online Nov 28, 2025. doi: 10.3748/wjg.v31.i44.112187
Revised: September 10, 2025
Accepted: October 20, 2025
Published online: November 28, 2025
Processing time: 125 Days and 11.2 Hours
Amphicrine carcinoma is an extremely rare entity. In this article, we report the first known case of very-early-stage amphicrine carcinoma characterized by a mixed growth pattern with multiple pathological components.
A 59-year-old male patient underwent gastroscopy for abdominal discomfort, which revealed a 1.5 cm elevated lesion on the lower part of the anterior aspect of the gastric body close to the gastric angle. Initial biopsy indicated signet-ring cell carcinoma (SRCC) confined to the mucosa. Following endoscopic submucosal dis
Routine neuroendocrine immunohistochemical marker staining for SRCC may improve the diagnosis of amphicrine carcinoma and provide guidance for sub
Core Tip: Based on white-light endoscopy findings and biopsy pathology results, this case was initially diagnosed as pure signet-ring cell carcinoma. Subsequent narrow-band imaging magnifying endoscopy supported this diagnosis. However, post-endoscopic submucosal dissection pathology revealed a very-early-stage amphicrine carcinoma with a mixed growth pattern comprising poorly differentiated adenocarcinoma, neuroendocrine tumor, and signet-ring cell carcinoma. We subsequently re-evaluated the endoscopic manifestations of this lesion and, in conjunction with the pathological findings, proposed the endoscopic features of amphicrine carcinoma presenting with signet-ring cell morphology.
- Citation: Gao CZ, Meng Y, Du JZ, Zhu X. Very-early-stage gastric amphicrine carcinoma with mixed histology: A case report and review of literature. World J Gastroenterol 2025; 31(44): 112187
- URL: https://www.wjgnet.com/1007-9327/full/v31/i44/112187.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i44.112187
Amphicrine carcinoma refers to cells capable of both neuroendocrine and exocrine secretion. Advancements in morphological knowledge and immunohistochemical applications have led to the recognition of neoplasms with hybrid-phenotypic features in pathology. This hybrid-phenotypic nature has been ultrastructurally confirmed by the presence of both mucous and neuroendocrine granules within the cytoplasm. Amphicrine carcinoma is distinct from both adenocarcinoma and neuroendocrine neoplasms in terms of morphology, immunohistochemical features, and transcriptomic investigations[1,2]. Due to its rarity, specific incidence data for gastric amphicrine carcinoma is unavailable, with only a few cases reported - all presenting at advanced stages[3-5]. One study of pancreatic tumors reported that approximately 5.4% of neoplasms showed amphicrine differentiation[6]. Here we report the first case of very-early-stage amphicrine carcinoma, including endoscopic features. We believe that when formulating treatment plans, the occurrence of this unique neoplasm should be taken into consideration[7,8].
A 59-year-old male patient was admitted to our hospital because of abdominal discomfort for 1 week.
The patient’s primary symptom was dull pain in the upper abdomen.
The patient had no relevant past illness.
The patient had a 30-year drinking history, with approximately 1.5 L of beer two or three times per week.
Physical examination revealed slight tenderness in the upper abdomen without rebound tenderness. No hepatosplenomegaly, lymphadenopathy, or other abnormalities were detected.
Complete blood count, coagulation profiles, liver function tests, and renal function tests showed no abnormalities. Tumor markers, including carbohydrate antigen 19-9, carbohydrate antigen 72-4, alpha-fetoprotein, and carcinoembryonic antigen, were normal. Serum levels of gastrin-17 and pepsinogen II were normal, while pepsinogen I was elevated at 247.20 μg/L (reference range: 70-165 μg/L).
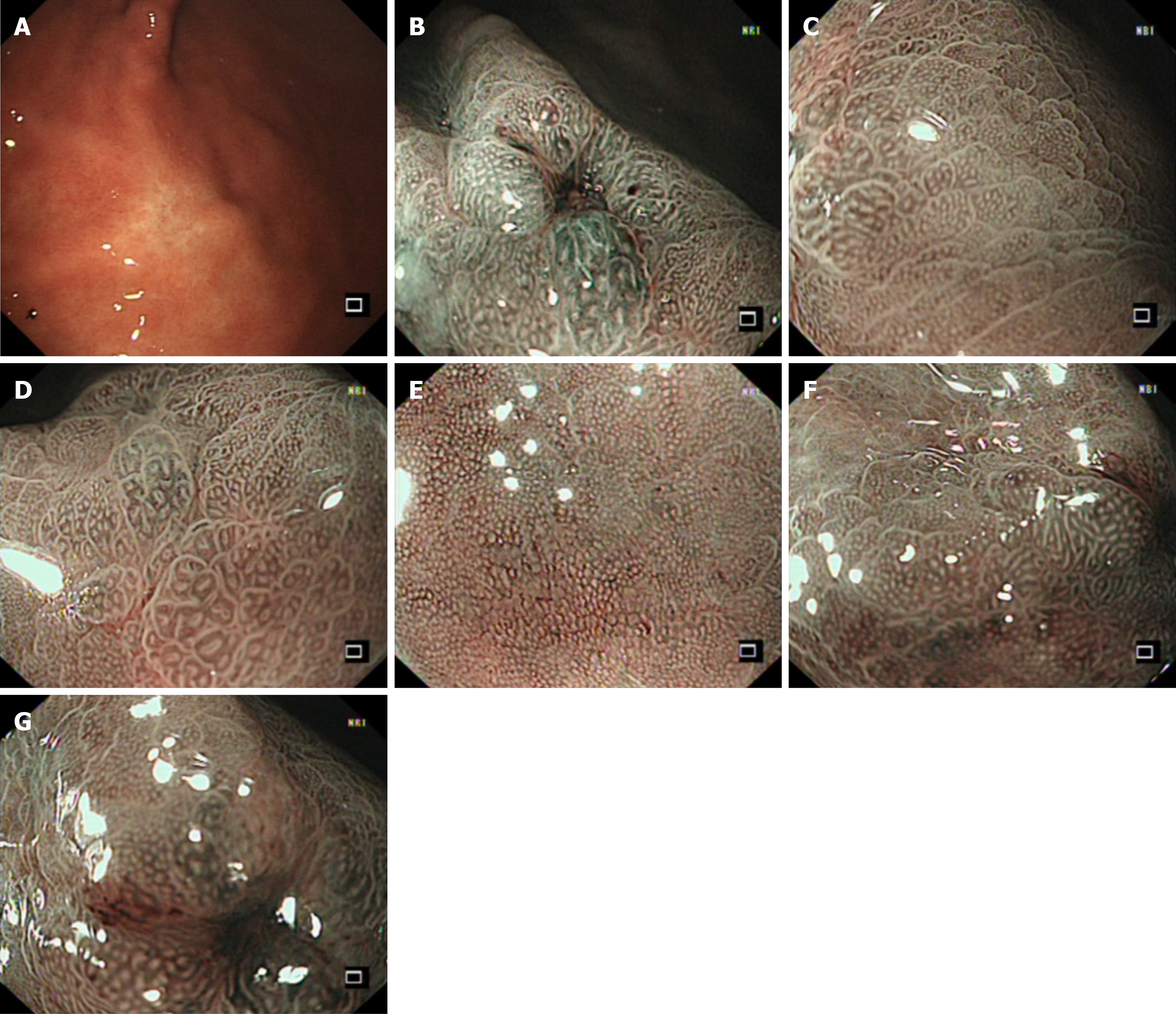
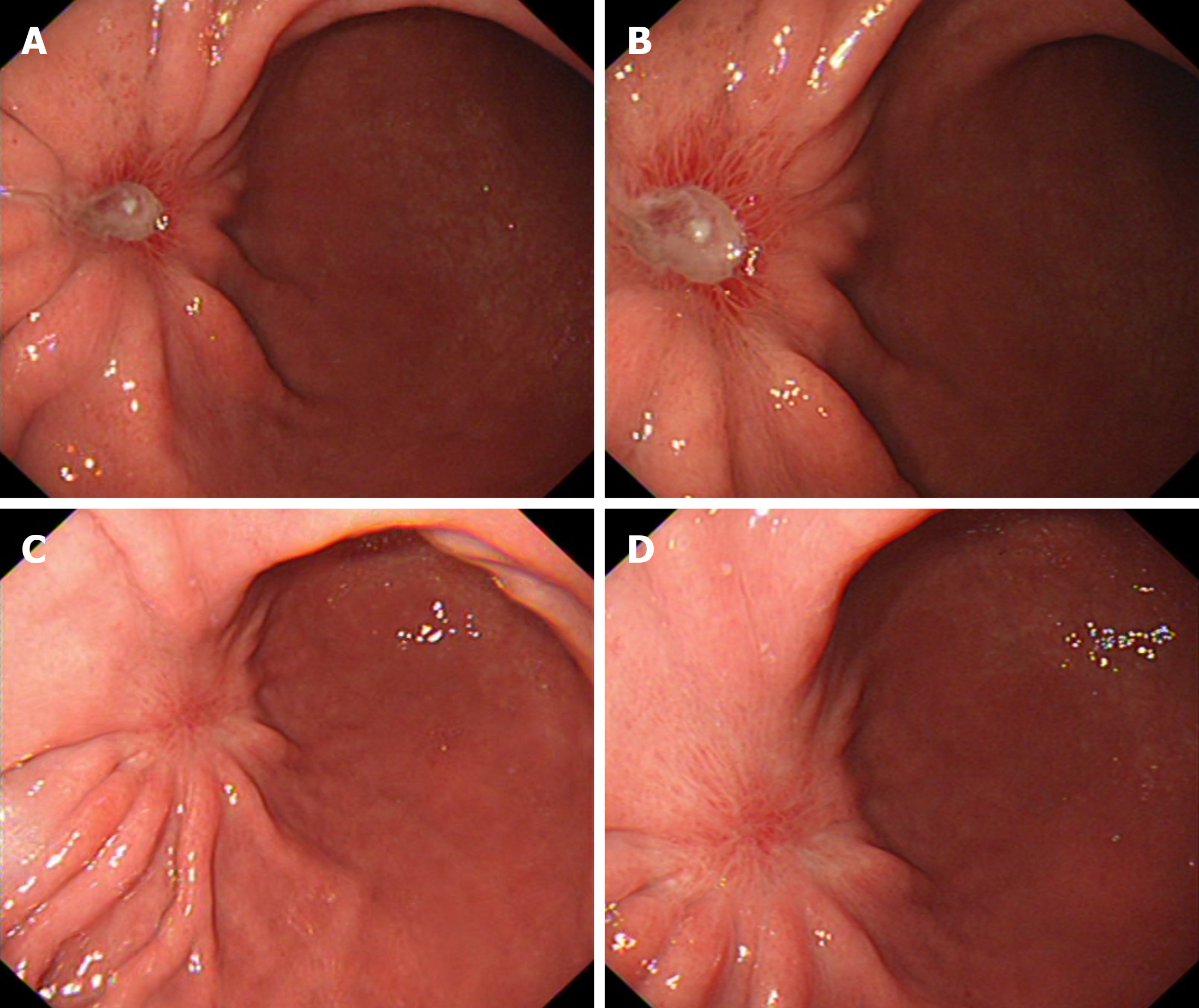
Outpatient gastroscopy revealed a flat elevated lesion (0-IIa), approximately 1.5 cm × 1.5 cm in diameter, located on the lower part of the anterior aspect of the gastric body close to the gastric angle. The lesion appeared as a submucosal tumor-like elevation with a faded surface color. The gastric folds were interrupted at the oral side of the lesion. The overlying mucosa was smooth, without evidence of ulceration (UL0) or erosion (Figure 1A). Pathology and immunohistology revealed that the biopsy specimen was cytokeratin pan (CK-P) positive, Ki-67 (approximately 1% +), focal carcinoembryonic antigen positive, S-100 negative, and periodic acid Schiff-alcian blue (AB-PAS) positive, suggesting undifferentiated carcinoma confined to the mucosa, with a possible diagnosis of signet-ring cell carcinoma (SRCC). Narrow-band imaging magnifying endoscopy revealed the fading color change, with an indistinct demarcation line and regular microvascular pattern. An ulcer-like change, likely resulting from a previous biopsy, was observed on the anterior wall side of the lesion, accompanied by distortion of adjacent glandular structures (Figure 1B-G). Enhanced abdominal and chest computed tomography (CT) scans were performed for pre-treatment staging. The scans showed no evidence of enlarged regional lymph nodes or distant metastasis. The primary gastric lesion was not clearly visible on CT images. Endoscopic submucosal dissection (ESD) was planned one week after endoscopy. Four-quadrant biopsies were taken every 1 cm from the lesion margins before the procedure. All pathology results revealed mild active inflammation.
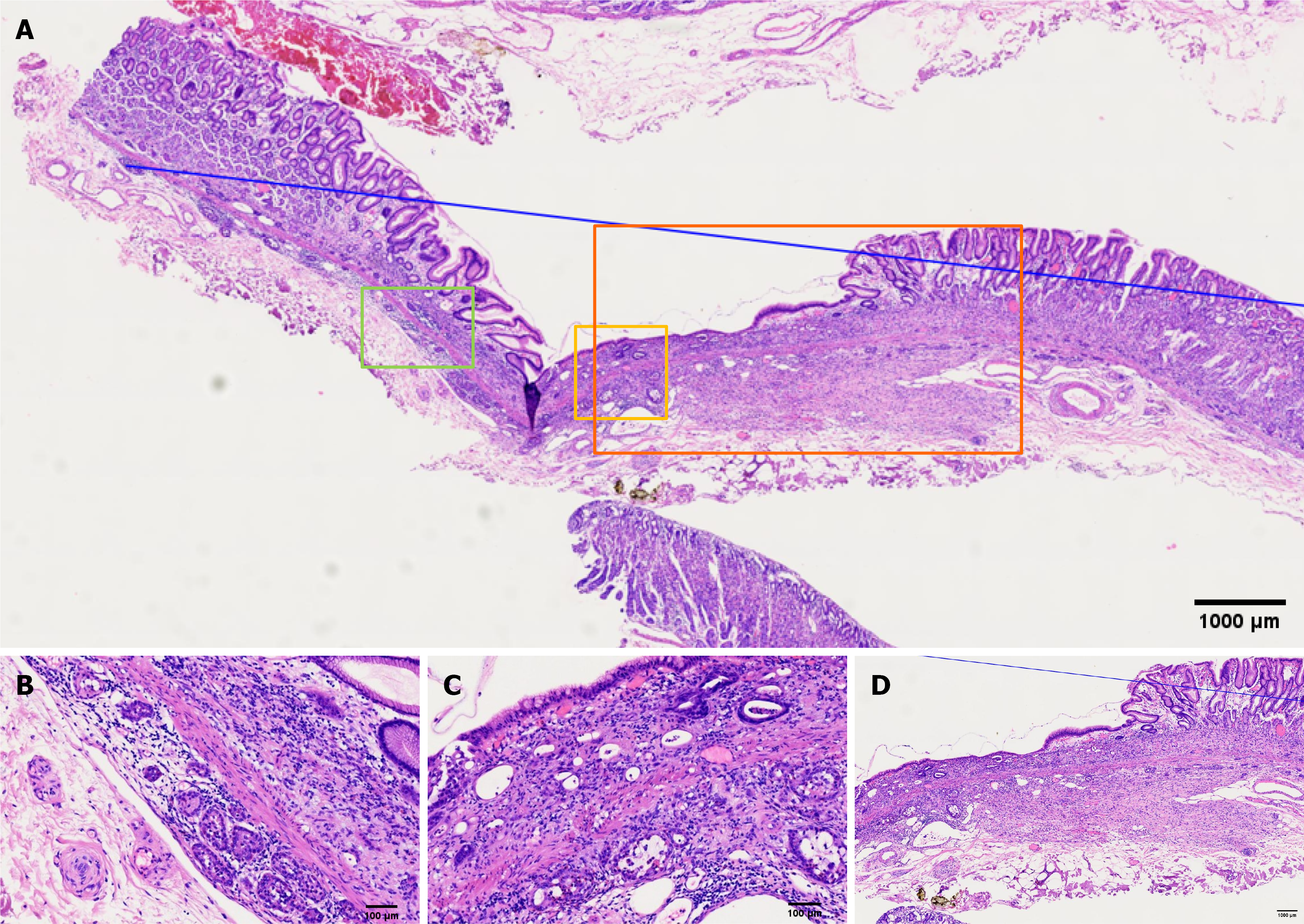
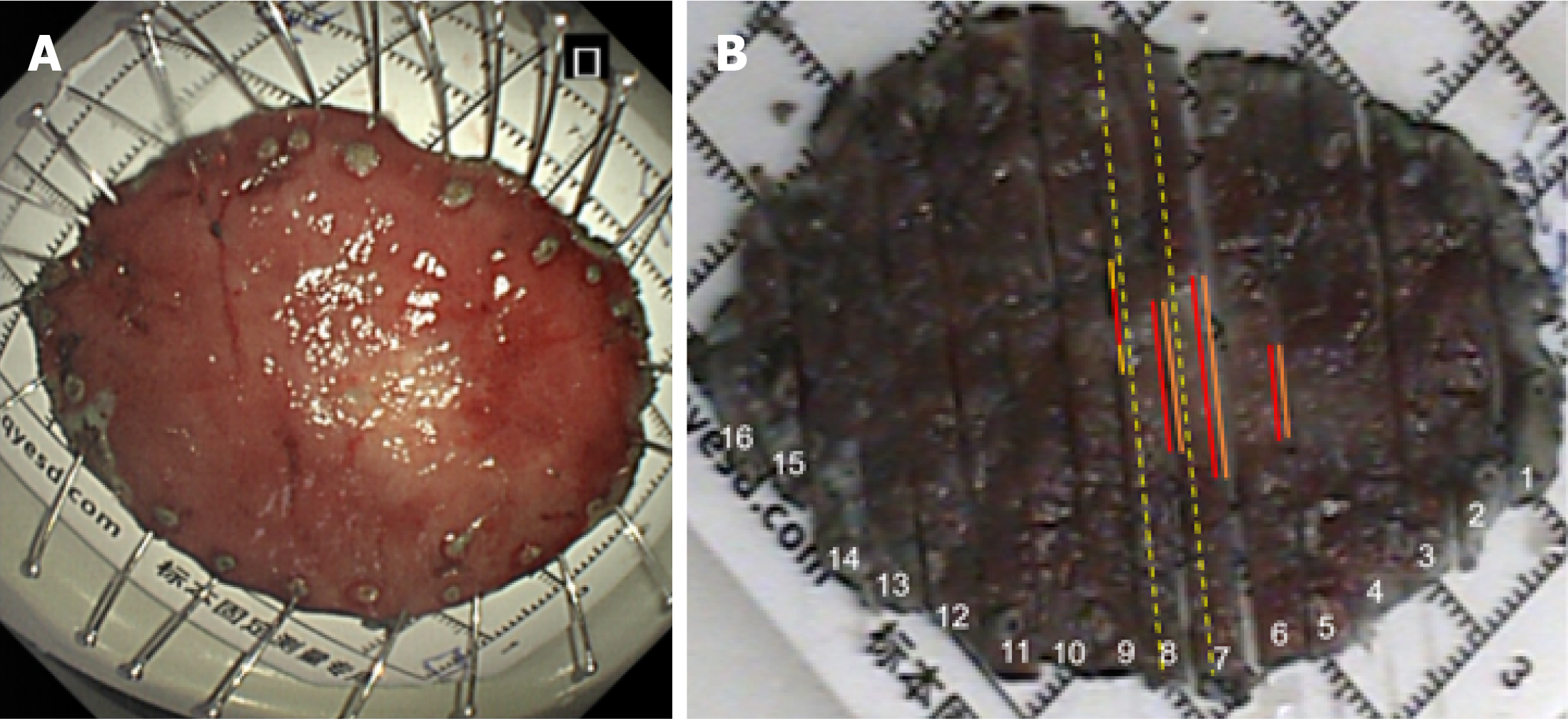
Postoperative pathological examination (Figures 2, 3 and 4) revealed a lesion measuring 1.5 cm × 1.2 cm. Strip #8 demonstrated loss of surface glandular epithelium, with mucosal and submucosal layers diffusely infiltrated by cord-like atypical cells with enlarged pleomorphic nuclei and moderate-to-abundant cytoplasm, while adjacent glands remained normal. In strips #7 and #8, tumor cells were arranged in nests and gland-like patterns, appearing as round and uniform nuclei, distributed in linear or micronodular patterns along both sides of the muscularis mucosa, reaching to the lateral margin. In strip #8, some atypical cells with signet-ring cell morphology were arranged in nested clusters within the mucosa and submucosa, alongside goblet cell-like cells forming tubular structures. In strips #5 to #7, abundant signet-ring-like atypical cells were observed, with mucin-rich cytoplasm pushing nuclei to the periphery.
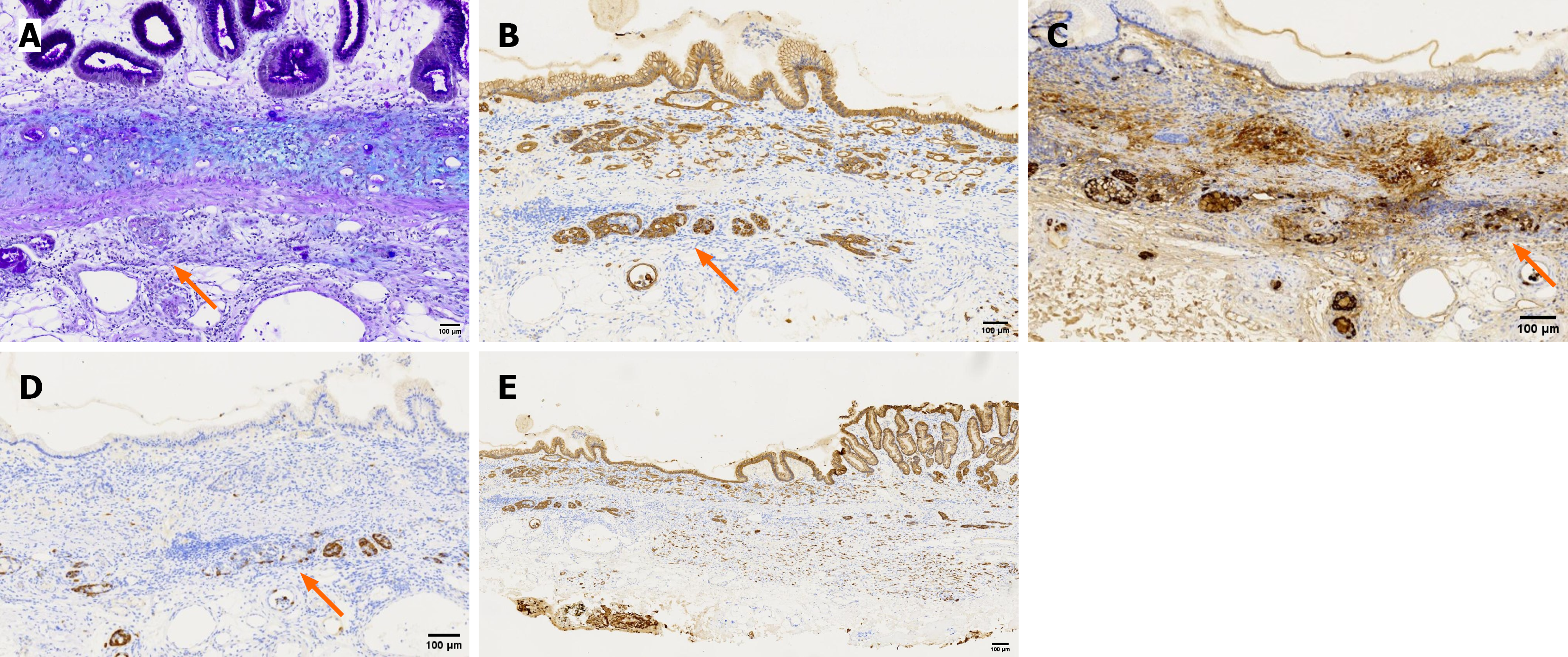
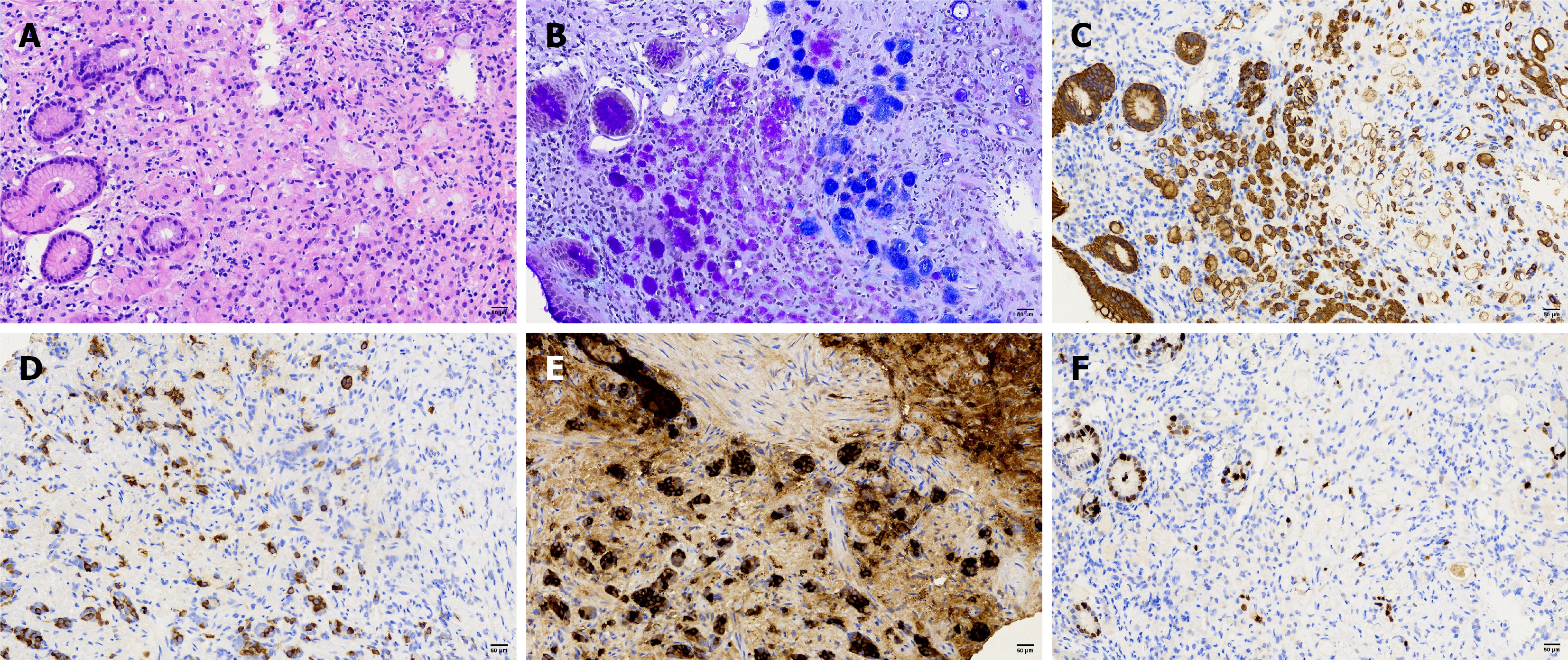
Immunohistochemistry results: Poorly differentiated carcinoma: P53 (20% +), Ki-67 (10% +), synaptophysin (Syn) (-), mucin 5AC (partial +), vascular endothelial markers CD34 (+) and lymphatic endothelial marker D2-40 (+), and no tumor emboli were found.
Invasion depth: 600 μm into the submucosa, and no carcinoma was found at the vertical margin (300 μm clear).
Neuroendocrine tumor: P53 (20% +), Ki-67 (≤ 2% +), Syn (+), mucin 5AC (-), CD34 (+) and D2-40 (+), and no tumor emboli were found.
Signet-ring cell nested clusters: AB-PAS (+), CK-P (+), Syn (+), partially expressing chromogranin A (CgA) (+).
Signet-ring cells with focal areas of diffuse infiltration: AB-PAS (+), CK-P (+), Syn (+), CgA (+).
Gastric adenocarcinoma (poorly differentiated adenocarcinoma and signet-ring cells); well-differentiated neuroendocrine tumor (G1 grade); amphicrine carcinoma. Reconstructed pathology (Figure 5) indicated that strips #5 to #7 demonstrated diffusely infiltrating SRCC, with focal areas exhibiting an endocrine-exocrine co-expression feature, specifically amphicrine carcinoma. Poorly differentiated adenocarcinoma infiltrating into the submucosa was found in site #8, possibly explaining the submucosal tumor-like appearance. Amphicrine carcinoma was present at both oral and anal sides of poorly differentiated adenocarcinoma of approximately 2 mm. Neuroendocrine tumor cells distributed linearly along the muscularis mucosae were observed in pathological tissue strips #7 and #8, reaching the lateral margin.
Following ESD, additional surgical procedures were required. However, the patient declined further surgical intervention based on personal preference after informed consent. To date, the patient has not received any additional treatment. Given the non-curative nature of the ESD and the patient’s decision to decline surgery, a strict surveillance protocol was recommended. This strategy included intensive endoscopic monitoring of the post-ESD scar with biopsies every 3-6 months for the first two years, along with regular contrast-enhanced CT of the chest and abdomen to screen for any locoregional recurrence or distant metastasis.
Post-ESD follow-up endoscopic examinations at 2 months and 6 months showed healing with S1-stage ulcer scarring (Figure 6). Also, the biopsy specimen revealed chronic inflammation only.
The coexistence of exocrine and endocrine secretory products within single cells was first reported by Feyrter[9] in 1938. Based on the 2022 World Health Organization Classification of Neuroendocrine Tumors[10], the diagnostic criteria for amphicrine carcinoma are: Neuroendocrine markers (Syn, CgA, and insulinoma associated protein 1) and non-neuroendocrine markers positive in the same epithelial neoplastic cells. The terminology for tumors exhibiting this dual secretion phenomenon remains controversial. Nevertheless, tumors sharing this characteristic include goblet cell adenocarcinoma[11-15]. According to the 2019 World Health Organization Classification of Tumors of the Digestive System[16], only appendiceal-origin tumors with dual secretion are termed goblet cell adenocarcinoma. Amphicrine carcinoma is of monoclonal origin, and evidence suggests its molecular profile is more aligned with adenocarcinoma than with neuroendocrine neoplasms; this is supported by pan-cancer transcriptome analyses, which show a closer genetic link to adenocarcinoma[17]. Hanamatsu et al[18] reported that amphicrine carcinoma cells immunohistochemically expressed CD44v9 - a functional cancer stem cell marker. This result supports the hypothesis that amphicrine carcinoma originates from multipotent stem cells. Signet-ring cells intrinsically exhibit a high positivity rate for neuroendocrine markers. Bartley et al[19] demonstrated that 40% of signet-ring cells showed positive expression of neuroendocrine markers. Fujiyoshi and Eimoto[20] found that 37.3% of signet-ring cells showed diffuse or focal positivity for CgA staining, and in 6% of cases, > 50% tumor cell positivity. Huang et al[17] demonstrated that four of 10 patients with amphicrine carcinoma exhibited coexisting components of adenocarcinoma or neuroendocrine carcinoma, indicating that mixed growth patterns are not uncommon in these tumors. Special characteristics from recently reported cases of gastric amphicrine carcinoma are shown in Table 1[3,17,18,21,22].
| Ref. | Age/sex | Tumor location | Size/stage | Histological components & growth pattern | IHC findings | Treatment | Outcome/follow-up |
| Current case | 59/male | Gastric body | 1.5 cm/T1b (SM) | Poorly differentiated adenocarcinoma, NET, SRCC | Syn (+), CgA (+) | ESD only | Alive, no recurrence at 6 months |
| Huang et al[17], 2019 | 61-69/male (10 cases) | Stomach (8), intestine (2) | 2-5.5 cm/ | Tubular, fusion, or single-file growth of goblet/SRCC-like cells. Mixed with adenoma or NEC in 4 cases | Syn (+), CgA (+) | Surgery +/- chemo/radio | 3/10 DOD. High-grade worse prognosis |
| Hanamatsu et al[18], 2019 | 60/female | Gastric corpus | 5.0 cm/ | Poorly cohesive carcinoma with SRCC | Syn (+), CgA (+), CD56 (+) | Total gastrectomy | Not specified |
| Qian and Feng[21], 2022 | 69/male | Gastric body/antrum | 8.5 cm/ | Tubular, solid nests, cribriform, single-file patterns | Syn (+), CgA (-) | Surgery + chemotherapy (adenocarcinoma regimen) | Alive, no recurrence at 6 months |
| Sciarra et al[22], 2023 | 63/male | Gastric cardia | 1.2 cm/T1b (SM) | Nests, trabeculae, acinar structures. Pancreatic acinar differentiation | Syn (+), CgA (+), trypsin (+), BCL10 (+) | Wedge resection | Alive, no recurrence at 18 months |
| Ebedes et al[3], 2024 | 55/female | Gastric body (lesser curvature) | 2.6 cm/T2 (MP) | Nested and glandular patterns with intracytoplasmic mucin | Syn (+), CgA (+) | Total gastrectomy | Not specified |
Amphicrine carcinoma is a rare entity with no specific clinical manifestations, and is definitively diagnosed only through pathology. In our case, the patient underwent gastroscopy due to dull pain in the upper abdomen. Based on the white-light endoscopic findings and application of the four-angle theory of gastric cancer, the lesion was primarily diagnosed as pure SRCC confined within the mucosal layer[23]. Since this was a very-early-stage lesion, narrow-band imaging magnifying endoscopy revealed no irregular microvascular or destruction of surface glandular structures of undifferentiated carcinoma. According to the Guidelines for Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Early Gastric Cancer (second edition)[24], undifferentiated-type adenocarcinoma without ulceration (UL0), confinement of the lesion to the mucosa, and diameter ≤ 2 cm constitutes an absolute indication for ESD. In addition, the latest international guidelines, including those from the European Society of Gastrointestinal Endoscopy and the Japanese Gastric Cancer Association, also provide comprehensive recommendations for endoscopic treatment of early gastric cancer, further supporting the clinical rationale for ESD in this case[25,26]. The patient underwent ESD. The lesion was classified as eCura C-2 based on the eCura system[27-29]. Zhang et al[30] demonstrated a 15% risk of lymph node metastasis for lesions measuring 1-2 cm, ulcer-negative, with submucosal invasion in undifferentiated carcinoma.
Given that the endoscopic features of very-early-stage amphicrine carcinoma have not been previously reported, a detailed analysis of the lesion’s appearance in the context of its underlying pathological components was warranted. Pathological findings revealed that the cellular morphology of the amphicrine carcinoma mainly presents as signet-ring cells, with molecular pathological characteristics approximating those of adenocarcinoma. Therefore, the endoscopic presentation of this lesion more closely resembled that of undifferentiated carcinoma. The lesion also contained components of SRCC, and poorly differentiated carcinoma that infiltrated both mucosal and submucosal layers, with epithelial erosion-like changes. Neuroendocrine tumor components were linearly distributed along both sides of the muscularis mucosae. Both poorly differentiated adenocarcinoma and neuroendocrine tumor components lacked characteristic endoscopic features in this lesion. Therefore, we reasonably conclude that amphicrine carcinoma with signet-ring cell morphology demonstrates endoscopic findings identical to those of pure SRCC, manifesting as mucosal faded color change.
This study has some limitations. This is the first report detailing the endoscopic features of the very-early-stage gastric amphicrine carcinoma in a single case. Therefore, these findings may not be generalizable to all such cases. Further accumulation and analysis of more cases are essential to validate our observations and establish a more definitive endoscopic profile for this rare entity.
Gastric amphicrine carcinoma is a rare entity with distinct biological and histological features[31]. This study analyzed its potential endoscopic manifestations and summarized both the endoscopic appearance and the mixed growth patterns of amphicrine carcinoma. Routine neuroendocrine immunohistochemical marker staining for signet-ring cells may improve the diagnosis of amphicrine carcinoma and provide guidance for treatment.
| 1. | Bellur S, Van der Kwast T, Mete O. Evolving concepts in prostatic neuroendocrine manifestations: from focal divergent differentiation to amphicrine carcinoma. Hum Pathol. 2019;85:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Chen H, Chen J, Ge R. Gastrointestinal Amphicrine Carcinoma: A Clinicopathologic Study of Five Patients. Int J Surg Pathol. 2025;33:1713-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Ebedes DM, Ganam S, Sujka JA, DuCoin CG. Double Digest: A Rare Case Report of Amphicrine Gastric Carcinoma Co-occurring With Papillary Thyroid Carcinoma. Cureus. 2024;16:e59205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Reis-Filho JS, Schmitt FC. Amphicrine gastric carcinoma. Arch Pathol Lab Med. 2001;125:1513-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Boşoteanu M, Boşoteanu C, Deacu M, Aşchie M. Morphological and immunohistochemical characteristics of a gastric amphicrine tumor: differential diagnosis considerations. Rom J Morphol Embryol. 2011;52:485-488. [PubMed] |
| 6. | Gonzalez de la Vega CF, Huether S, Lee W. 1597 Pancreatic Amphicrine Carcinoma: Underrecognized Entity Bridging Neuroendocrine Neoplasm and Acinar Cell Carcinoma with Potential Clinical Significance. Lab Investig. 2025;105:103840. [DOI] [Full Text] |
| 7. | Sabella G, Centonze G, Lagano V, Scardino A, Belli F, Garzone G, Pardo C, Galbiati D, Pusceddu S, Mangogna A, Angerilli V, Fassan M, Vingiani A, Agnelli L, Pruneri G, Gobba S, Uccella S, La Rosa S, Sessa F, Capella C, Milione M, Milione M. Unveiling the Prognostic Role of Synaptophysin in Conventional Colorectal Carcinomas. Neuroendocrinology. 2025;115:632-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Tamura N, Honma Y, Sekine S, Tsukamoto S, Hirano H, Okita N, Shoji H, Iwasa S, Takashima A, Kato K, Boku N. Case report: potential treatment of metastatic amphicrine carcinoma of the rectum with FOLFOXIRI chemotherapy. Oxf Med Case Reports. 2020;2020:omaa097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Feyrter F. Über diffuse endokrine epitheliale organe. Zbl Inn Med. 1938;. |
| 10. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 623] [Article Influence: 155.8] [Reference Citation Analysis (2)] |
| 11. | Bell PD, Pai RK. Goblet cell adenocarcinoma of the appendix: an update and practical approach to diagnosis and grading. Hum Pathol. 2023;132:183-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Salesse J, Chicaud M, Braham H, Taconet S. [Appendiceal goblet cell adenocarcinoma: Has the controversy come to an end?]. Ann Pathol. 2025;45:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Toshima T, Inada R, Sakamoto S, Takeda E, Yoshioka T, Kumon K, Mimura N, Takata N, Tabuchi M, Oishi K, Sato T, Sui K, Okabayashi T, Ozaki K, Nakamura T, Shibuya Y, Matsumoto M, Iwata J. Goblet cell carcinoid of the appendix: Six case reports. World J Clin Cases. 2024;12:5217-5224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Shiota T, Murata K, Kishimoto M, Yao T, Noura S, Morita S, Akiyoshi T, Okamura S, Imasato M, Furuhata T, Suto T, Takemasa I, Shingai T, Ueda M, Mizuno H, Hisamatsu Y, Takeda T, Fujii M, Kagawa Y, Sugihara K; Study Group of Appendiceal Neoplasms from the Japan Society of Colorectal Cancer Research Group. Clinicopathological features of appendiceal goblet cell adenocarcinoma in Japan: a multicenter retrospective study. Surg Today. 2023;53:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Yozu M, Johncilla ME, Srivastava A, Ryan DP, Cusack JC, Doyle L, Setia N, Yang M, Lauwers GY, Odze RD, Misdraji J. Histologic and Outcome Study Supports Reclassifying Appendiceal Goblet Cell Carcinoids as Goblet Cell Adenocarcinomas, and Grading and Staging Similarly to Colonic Adenocarcinomas. Am J Surg Pathol. 2018;42:898-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2759] [Article Influence: 459.8] [Reference Citation Analysis (3)] |
| 17. | Huang D, Ren F, Ni S, Tan C, Weng W, Zhang M, Xu M, Wang L, Xu Q, Sheng W. Amphicrine carcinoma of the stomach and intestine: a clinicopathologic and pan-cancer transcriptome analysis of a distinct entity. Cancer Cell Int. 2019;19:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Hanamatsu Y, Saigo C, Asano N, Kito Y, Nakada K, Takeda Y, Takeuchi T. A Case of Gastric Amphicrine Signet-Ring Cell Carcinoma. Clin Pathol. 2019;12:2632010X19880535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Bartley AN, Rashid A, Fournier KF, Abraham SC. Neuroendocrine and mucinous differentiation in signet ring cell carcinoma of the stomach: evidence for a common cell of origin in composite tumors. Hum Pathol. 2011;42:1420-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Fujiyoshi Y, Eimoto T. Chromogranin A expression correlates with tumour cell type and prognosis in signet ring cell carcinoma of the stomach. Histopathology. 2008;52:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Qian SM, Feng MB. [Amphicrine carcinoma of the stomach: report of a case]. Zhonghua Bing Li Xue Za Zhi. 2022;51:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Sciarra A, Uccella S, Hiroz P, Fournier I, Soubeyran V, Finzi G, La Rosa S. Gastric Amphicrine Carcinoma Showing Neuroendocrine and Pancreatic Acinar Cell Differentiation. Lesson from a Challenging Case Opening New Perspectives in the Diagnostic Work-Up of Gastric Neuroendocrine Neoplasms. Endocr Pathol. 2023;34:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Phalanusitthepha C, Grimes KL, Ikeda H, Sato H, Sato C, Hokierti C, Inoue H. Endoscopic features of early-stage signet-ring-cell carcinoma of the stomach. World J Gastrointest Endosc. 2015;7:741-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 363] [Article Influence: 72.6] [Reference Citation Analysis (1)] |
| 25. | Dinis-Ribeiro M, Libânio D, Uchima H, Spaander MCW, Bornschein J, Matysiak-Budnik T, Tziatzios G, Santos-Antunes J, Areia M, Chapelle N, Esposito G, Fernandez-Esparrach G, Kunovsky L, Garrido M, Tacheci I, Link A, Marcos P, Marcos-Pinto R, Moreira L, Pereira AC, Pimentel-Nunes P, Romanczyk M, Fontes F, Hassan C, Bisschops R, Feakins R, Schulz C, Triantafyllou K, Carneiro F, Kuipers EJ. Management of epithelial precancerous conditions and early neoplasia of the stomach (MAPS III): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG) and European Society of Pathology (ESP) Guideline update 2025. Endoscopy. 2025;57:504-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 55] [Article Influence: 55.0] [Reference Citation Analysis (2)] |
| 26. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 842] [Article Influence: 280.7] [Reference Citation Analysis (2)] |
| 27. | Mao S, Li W, Pan Y, Wu H, Xiang Y, Liu M, Zhao T, Tao H, Wang L, Xu G. Long-term outcomes of additional surgery vs. observation after noncurative endoscopic submucosal dissection for early gastric cancer and application value of the eCura scoring system: a propensity score-matched study. J Gastrointest Surg. 2025;29:102030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Yabuuchi Y, Masui Y, Kumagai K, Iwagami H, Murai K, Setoyama T, Tochio T, Utsumi T, Yoshikawa T, Araki O, Murakami S, Kitami M, Matsuura K, Kanda N, Hishitani E, Tanaka J, Marui S, Ikuta K, Yoshida H, Nishikawa Y, Nakanishi Y, Seno H; KONOE Project. External validation of the eCura system and comparison with the W-eCura score for predicting lymph node metastasis after non-curative endoscopic submucosal dissection for early gastric cancer: a multicenter retrospective cohort study. J Gastroenterol. 2025;60:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Yang HJ, Lee H, Kim TJ, Jung DH, Choi KD, Ahn JY, Lee WS, Jeon SW, Kim JH, Kim GH, Park JM, Kim SG, Shin WG, Kim YI, Choi IJ. A Modified eCura System to Stratify the Risk of Lymph Node Metastasis in Undifferentiated-Type Early Gastric Cancer After Endoscopic Resection. J Gastric Cancer. 2024;24:172-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Zhang P, Xu T, Feng H, Zhu Z, Wang J, Wang Y. Risk of lymph node metastasis and feasibility of endoscopic submucosal dissection in undifferentiated-type early gastric cancer. BMC Gastroenterol. 2023;23:175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Sun L, Wang C, Zhang J, Shao B, Zhao S, Guo Y, Li X, Sun Y. Genetic alterations in gastric amphicrine carcinomas and comparison with gastric mixed neuroendocrine-non-neuroendocrine neoplasms. Mod Pathol. 2022;35:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/