Published online Nov 21, 2025. doi: 10.3748/wjg.v31.i43.111876
Revised: August 7, 2025
Accepted: October 13, 2025
Published online: November 21, 2025
Processing time: 132 Days and 8.5 Hours
The unique survival environment of Helicobacter pylori (H. pylori) presents chal
To identify the optimal liquid culture medium for H. pylori using both reference and clinical strains.
Nine H. pylori strains were incubated in 10 different broth media commonly used for bacterial cultures under microaerophilic conditions. Bacterial concentrations were estimated using serial dilutions and the pour plate method. A range of H. pylori initial inoculum concentrations was tested for each strain. For growth evaluation, optical density at 600 nm was measured at 24, 48, and 72 hours. The growth trends of H. pylori were compared among strains and media.
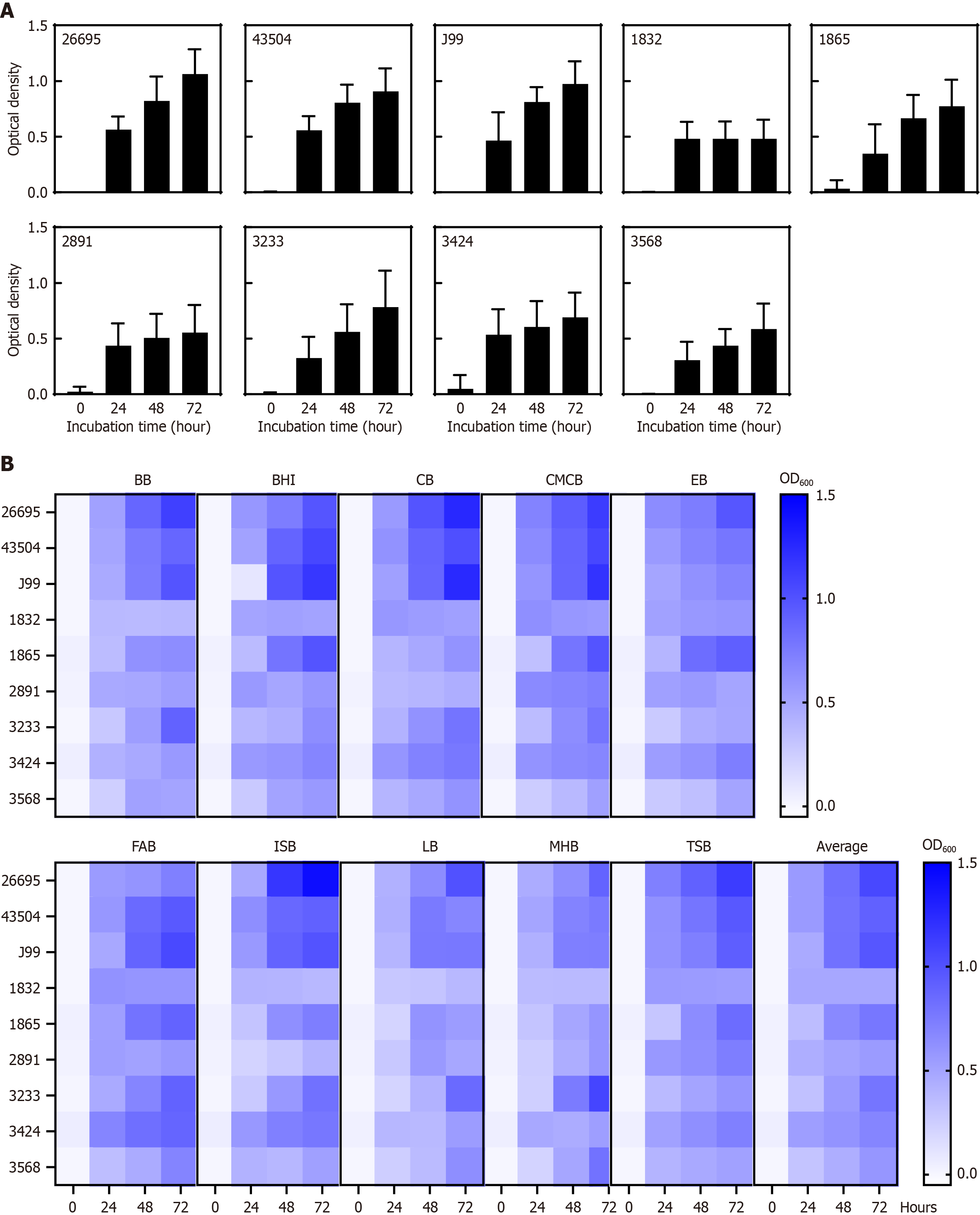
All H. pylori strains grew successfully over time, regardless of the initial inoculum concentration. The specific growth trends and the broth that yielded the highest optical density value at each time point varied with strain. The reference strains
Chopped meat carbohydrate broth was the most effective for H. pylori liquid culture, and Columbia broth and fastidious anaerobe broth also supported greater growth than Brucella broth.
Core Tip: Unique survival environments for Helicobacter pylori (H. pylori) present challenges for establishing appropriate in vitro culture conditions. This study aimed to identify the optimal liquid culture medium for H. pylori using both reference and clinical strains. The choice of liquid medium significantly influenced H. pylori growth. Chopped meat carbohydrate broth was found to be the most effective medium for H. pylori liquid culture. Columbia broth and fastidious anaerobe broth supported greater growth than Brucella broth.
- Citation: Kim SM, Rahaman MI, Bang CS, Lee S, Kim HW, Kim SW, Ahn JY, Jung HY, Kim YH, Gong EJ. Optimal liquid culture media for Helicobacter pylori: Strain-specific growth in different broth formulations. World J Gastroenterol 2025; 31(43): 111876
- URL: https://www.wjgnet.com/1007-9327/full/v31/i43/111876.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i43.111876
Helicobacter pylori (H. pylori) is a Gram-negative bacterium that primarily colonizes the human stomach[1]. H. pylori infection is well known to be associated with various gastrointestinal diseases, including chronic gastritis, peptic ulcer disease, gastric mucosa-associated lymphoid tissue lymphoma, and non-cardia adenocarcinoma[2]. To investigate the pathogenesis of H. pylori, a fundamental understanding of its characteristics is necessary. Currently, bacterial culture is the most specific method for diag
Although solid culture media are sufficient for the identification and antimicrobial susceptibility testing of H. pylori, biochemical analyses cannot be readily performed on colonies grown on solid agar[4]. In contrast, liquid culture yields larger quantities of bacteria and allows for quantitative analysis of bacterial growth, which requires la
Although methods for primary isolation and subculture of H. pylori using solid media are well established, achieving reliable growth in liquid media remains a challenge[9,10]. Several nutrient-rich broths, such as Brucella broth (BB), brain heart infusion (BHI) broth, and Mueller-Hinton broth (MHB), have been used for H. pylori culture[6,8-10]. However, the selection of a broth is usually made empirically based on previous protocols rather than on comparative data identifying the optimal liquid medium for H. pylori. Several previous studies have focused on the effects of specific supplements or microaerophilic conditions using reference strains, but few have comprehensively examined clinical isolates[9,11]. Moreover, comparative studies that systematically evaluate the growth-supporting capacity across multiple broth types are lacking, leaving a knowledge gap in media selection for H. pylori. Selecting an optimal medium for H. pylori culture may play a critical role in improving the accuracy of diagnosis, treatment, prevention, and related research outcomes. Additionally, determining the bacterial inoculum concentration and incubation duration at which H. pylori thrives is crucial for optimizing the experimental conditions. Therefore, this study aimed to identify the optimal liquid culture medium for H. pylori growth using a diverse set of strains, including both reference strains and clinical isolates.
Nine H. pylori strains were used to establish culture conditions, including three reference strains (H. pylori 26695, ATCC 43504, and J99) and six clinical strains (1832, 1865, 2891, 3233, 3424, and 3568). The clinical strains were isolated from gastric mucosal biopsy specimens obtained from Korean patients with chronic atrophic gastritis. Each biopsy specimen was inoculated onto Brucella agar supplemented with 7% defibrinated sheep blood (MB-M1005-P50, Kisanbio) and antibiotics (vancomycin 10 μg/mL, trimethoprim 5 μg/mL, polymyxin B 2.5 IU/mL, and amphotericin B 5 μg/mL). Plates were incubated at 37 °C under a microaerophilic atmosphere (5% O2, 10% CO2, and 85% N2) for 5-7 days. Suspected H. pylori colonies were identified based on their typical morphology, Gram-negative staining, and positive urease, catalase, and oxidase tests. The confirmed H. pylori isolates were subcultured and stored at -80 °C in tryptic soy broth (TSB) containing 15% glycerol.
A total of 10 different commercially available broths with varying nutritional compositions were evaluated: BB, BHI, Columbia broth (CB), chopped meat carbohydrate broth (CMCB), Eugon broth (EB), fastidious anaerobe broth (FAB), Iso-Sensitest broth (ISB), Luria Bertani broth (LB, low salt), MHB, and TSB. The detailed composition of each broth is presented in Supplementary Table 1. Each broth was prepared by dissolving the specified amount of powder in 1 L of distilled water and autoclaving at 121 °C for 15 minutes (e.g., 28.1 g BB; 37.0 g BHI; 47.5 g CMCB; 35.0 g CB; 30.4 g EB; 33.7 g FAB; 23.4 g ISB; 20.0 g LB; 21.0 g MHB; 30.0 g TSB; all products from Kisanbio, Korea). After sterilization, fetal bovine serum (FBS; Gibco, United States) was heat-inactivated at 56 °C for 30 minutes and added to the broth to achieve a final concentration of 10%.
The bacterial inoculum for liquid culture was prepared by harvesting 3-day-old cultures on agar plates. Briefly, H. pylori was inoculated onto blood agar supplemented with 5% defibrinated sheep blood and incubated at 37 °C in a 10% CO2 for recovery. After achieving sufficient bacterial growth, the bacterial colonies were harvested by scraping the bacterial growth with a sterile swab and transferred into BB with 10% FBS. The media was incubated in upright 25T flasks, shaking at 100 revolutions per minute (rpm), within a shaking incubator (SI-30, U1TECH, Korea) set at 10% CO2, 37 °C, and a humidified environment. The microaerophilic conditions used in this study were selected based on previously validated protocols for the optimal H. pylori growth[10].
Mueller-Hinton agar (MB-M1033, Kisanbio) plates (90 mm dishes) were used to measure bacterial colony-forming units (CFUs). The cultures were serially diluted 10-fold, and 100 μL of each dilution was spread on the agar surface using the spread plating method. To ensure distinct colony formation, an agar overlay technique was used after seeding. Briefly, cooled Mueller-Hinton agar at 50 °C was poured onto the inoculated plates and immediately tilted back and forth to form a thin layer. After the overlay solidified, the plates were inverted and incubated at 37 °C under 10% CO2 for 48 hours in a humidified atmosphere. Following colony formation, the number of colonies per 100 μL was counted, and CFU/mL was calculated based on the dilution. Throughout the CFU counting process, the remaining bacterial suspensions were stored at 4 °C.
Serial dilutions of each H. pylori strain were prepared at final concentrations ranging from 1 × 103 CFU/mL to 5 × 106 CFU/mL. Aliquots of 200 μL from each dilution were inoculated into wells of a 96-well plate (91096, SPL, Korea) containing the various broth media. All nine H. pylori strains (26695, ATCC 43504, J99, 1832, 1865, 2891, 3233, 3424, and 3568) were tested in all 10 broths (BB, BHI, CB, CMCB, EB, FAB, ISB, LB, MHB, and TSB) with FBS and without additional supplements or antibiotics. The plates were incubated at 37 °C in a humidified 10% CO2 atmosphere with shaking at 100 rpm. The optical density at a wavelength of 600 nm in a 1 cm light path (OD600) was measured at 0, 24, 48, and 72 hours using a microplate spectrophotometer (Epoch, BioTek, Unite States)[12]. Each experiment was performed in triplicate, and the data are presented as mean ± SD.
To confirm the identity of the cultured H. pylori, two strains were selected at random time points during cultivation for additional testing. Streptococcus pneumoniae (S. pneumoniae), which was negative for catalase, oxidase, and urease tests and glmM gene, was used as a negative control. Each selected H. pylori strain and S. pneumoniae were cultured in BB and CMCB, harvested, and centrifuged at 3000 rpm for 3 minutes to remove the broth. The resulting pellet was resuspended in distilled water and used for biochemical testing and polymerase chain reaction (PCR) (Supplementary Figure 1).
The catalase test involved diluting 35% hydrogen peroxide (H2O2) solution (Sam-Hyun Pharm, Korea) to a final concentration of 3%[13]. Subsequently, 1 mL of the prepared H2O2 was mixed with the bacterial sample on a microscope slide to observe the bubble formation. The oxidase test utilized oxidase test disc (70439-50DISKS-F, Millipore, United States); 20 μL of the prepared sample were applied to a disc and the color change was observed for 10 minutes at room temperature[13]. The urease test employed a commercial kit (ASAN Helicobacter Test, Asan Pharm, Korea); 20 µL of the prepared sample was injected into the kit’s urea-containing gel, which was then incubated at room temperature for 10 minutes and observed for a color change from yellow to red[13,14].
Additionally, PCR was performed after bacterial DNA extraction to target the glmM gene. The forward primer 5′-AAGCTTTTAGGGGTGTTAGGGGTTT-3′ and reverse primer 5′-AAGCTTACTTTCTAACACTAACGC-3′ were used to amplify a 294 bp fragment of glmM[15]. PCR was carried out on a thermal cycler (SimpliAmp, Thermo Fisher Scientific, United States) with the following conditions: Initial denaturation at 95 °C for 2 minutes; 32 cycles of denaturation at 95 °C for 20 seconds, annealing at 58 °C for 40 seconds, polymerization at 72 °C for 1 minute; and a terminal polymerization at 72 °C for 5 minutes. PCR products were subjected to electrophoresis (Mupid-exU, Takara, Japan) at 100 V for 25 minutes and visualized using an iBright imaging system (Thermo Fisher Scientific).
Growth data are presented as mean ± SD. An unpaired t-test was used to compare the mean OD values between groups. A P value of < 0.05 was considered statistically significant. To assess the effects of broth type and incubation time on H. pylori growth, two-way analysis of variance (ANOVA) was conducted, treating broth type as the inter-group factor and time as the intra-group factor. Interactions between these two variables were also evaluated. The percentage contributions to the total variation were also reported to indicate the relative influence of each factor. Where appropriate, post-hoc analyses were not performed because of the large sample size (n = 243 per group), and the focus was placed on the main and interaction effects. Graphs were generated using GraphPad Prism (version 10).
Figure 1 shows the optical density-based growth profiles of the nine H. pylori strains cultured in 10 different broths. Supplementary Tables 2 and 3 summarize the average OD600 of each strain across all media at 24, 48, and 72 hours. The three reference strains (26695, 43504, and J99) consistently exhibited more robust growth than the clinical strains in all media tested. Notably, the two clinical strains demonstrated distinct media preferences: Strain 1865 thrived in BHI, CMCB, EB, FAB, and TSB, whereas strain 3233 showed favorable growth in BB, CB, CMCB, FAB, ISB, LB, and MHB. Strain 2891 grew well in most broths except CB, ISB, and MHB during the first 24 hours, with particularly good growth in LB for up to 48 hours, after which its growth decelerated. This pattern was also observed in strain 3424.
Regarding the inoculum concentration, some strains (1865, 2831, 3233, and 3424) achieved higher OD values when the initial inoculum concentration was higher, whereas others (26695, 43504, J99, 1832, and 3568) grew to similar densities regardless of the initial inoculum (Supplementary Figure 2). Although some strains (1865, 3233, and 3568) showed minimal growth at 24 hours, all strains exhibited substantial growth by 72 hours even at the lowest inoculum concentration of 1000 CFU/mL.
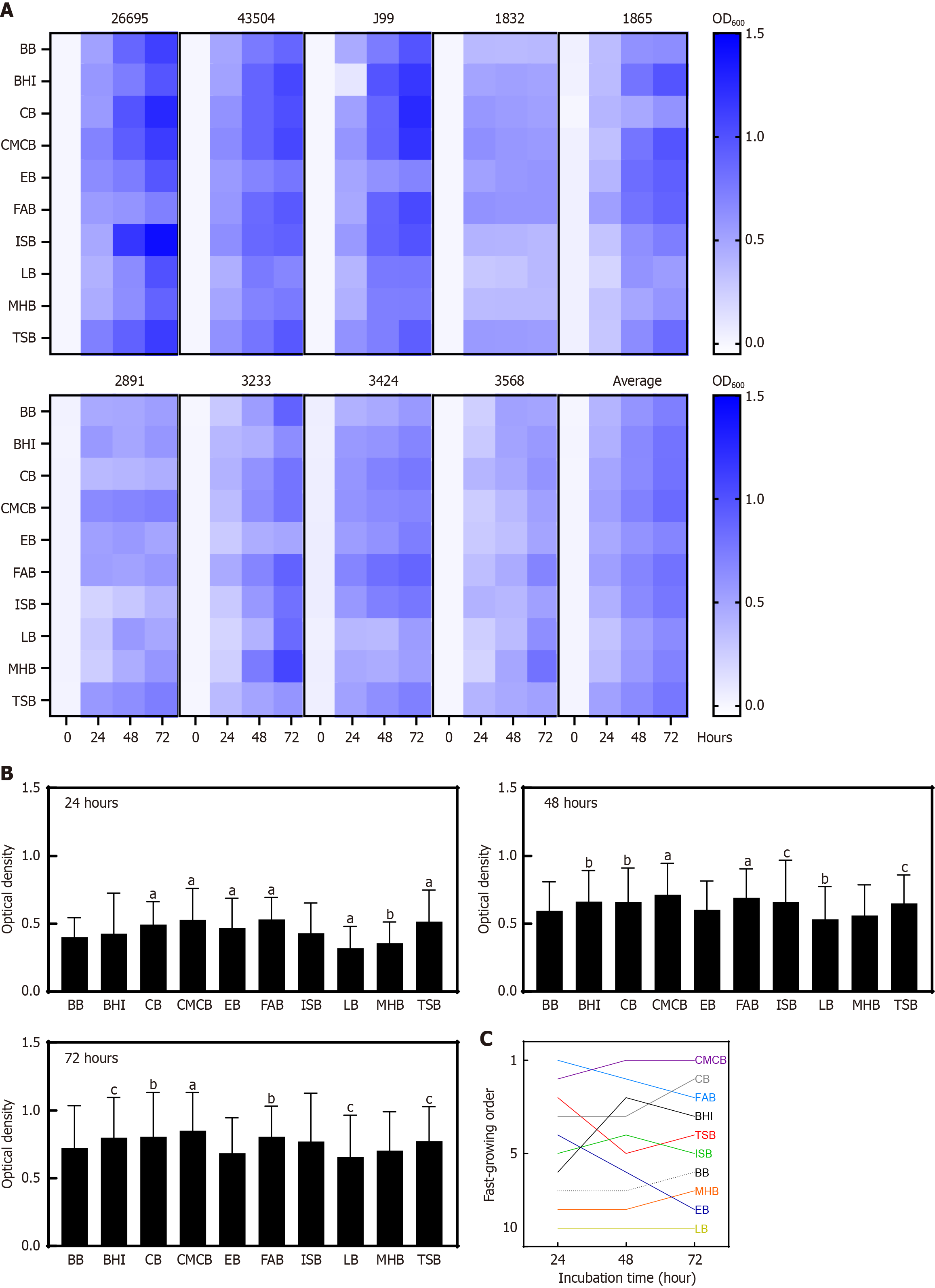
To identify the optimal broth for H. pylori culture, the growth trends of each H. pylori strain across different media were analyzed (Figure 2). Even the same strain showed different growth patterns depending on broth type and incubation duration. Notably, H. pylori 26695 showed good growth in TSB and CMCB at 24 hours, whereas it showed higher growth in ISB and CB at 48 hours. Strain 43504 grew well in the BHI medium at all time points, whereas strain J99 exhibited substantial growth in the BHI medium after 48 hours. The clinical strain 3568 displayed a complex pattern, with different broths being the most effective at different time points during its growth.
Figure 2B and Supplementary Table 4 show the average OD values of all strains for each broth at 24, 48, and 72 hours. Figure 2C shows the ranking of the broths based on their overall performance at each time point. Across the 72-hour incubation, CMCB, CB, and FAB supported significantly greater H. pylori growth than BB (Supplementary Table 5). To further assess the effects of broth type and time on H. pylori growth, two-way ANOVA was performed (Supplementary Table 6). The analysis revealed significant effects of both incubation time (P < 0.0001) and broth (P < 0.0001), as well as a significant interaction between the two factors (P < 0.0001), indicating that the growth response over time differed across media.
In this study, we evaluated the growth of H. pylori in 10 different broths. We found that all the tested strains, including the reference and clinical strains, could be cultured in liquid media under microaerophilic conditions, and their growth profiles were highly medium- and strain-dependent. These findings address the long-standing gap in H. pylori culture methodology: Although H. pylori have been cultured on various solid and liquid media since its discovery, no single liquid medium has emerged as a standard for optimal growth.
H. pylori is well known to be a fastidious bacterium that requires rich nutrients and a microaerophilic environment for growth, making its isolation and culture challenging in routine laboratories[1,10,16]. Several solid and liquid media have been evaluated for H. pylori culture, but those studies were limited to a few reference strains and a narrow range of media[6,17-21]. Consequently, the choice of liquid culture medium in practice remains largely empirical. By including a broad panel of media and both reference and clinical strains, our study provides comparative data that clearly indicate that media composition significantly influences H. pylori growth in liquid cultures.
Multiple factors such as the choice of basal broth, presence of supplements, and bacterial strains affect growth of H. pylori in vitro[11,22-24]. To facilitate the establishment of a reliable liquid culture method for this organism, we sought to identify an optimal broth that would support robust growth of H. pylori. In this study, we compared the performance of 10 different broths and found that CB, CMCB, and FAB were the most supportive of H. pylori growth, whereas BB, LB, and MHB were the least effective. These findings are in line with those of a previous report that a particular broth formulation was the best basal broth medium for H. pylori, whereas BB was the poorest[25].
Among the media tested, CMCB supported the highest overall H. pylori growth, making it the most effective medium. This medium, originally formulated for cultivating anaerobes, is rich in proteinaceous substrates and contains complex nutrients that are likely to benefit H. pylori. The superior performance of CMCB is a novel finding as this medium is not commonly used in H. pylori research, suggesting that certain components of CMCB more effectively fulfill nutritional and microaerophilic growth requirements of H. pylori. In contrast, LB, a simple nutrient broth for enteric bacteria, was distinctly inferior for H. pylori. This result is consistent with the known fastidious requirements of H. pylori; a rich medium with multiple growth factors is necessary for robust proliferation[26]. Nutrient-rich media such as BB or agar supplemented with blood or serum have traditionally been used for H. pylori cultures[18,25]. Our results confirmed that without enrichment, H. pylori struggled to multiply efficiently.
Interestingly, other media also showed favorable performance in H. pylori liquid culture. Both CB and FAB supported better H. pylori growth than commonly used BB (Figure 2B and Supplementary Table 4), indicating that these for
In addition to the culture medium, we observed substantial differences in the growth responses of the strains. All nine H. pylori strains achieved measurable growth in every tested broth after 72 hours; however, the growth yields and kinetics varied widely. Notably, the reference strains grew robustly in nearly all media, whereas the clinical strains showed specific media preferences. This strain-specific variability is consistent with reports that clinical H. pylori strains can exhibit diverse growth characteristics, with some strains growing rapidly and others being more fastidious[24,28]. It has been suggested that clinical isolates are generally more fastidious than laboratory-adapted or culture-collected strains[28]. Our findings reinforce that H. pylori is not monolithic in its growth requirements; individual strains may have distinct metabolic needs or tolerances, reflecting the high genetic diversity of this species and its adaptation to different host environments. It is likely that the laboratory-adapted reference strains, after repeated in vitro passages, may have evolved to grow more efficiently under standard conditions, whereas the newly isolated strains retain a wider range of nutritional dependencies. From a practical endpoint, these strain differences underscore the value of testing more than one medium when culturing new H. pylori isolates, because a medium that is suboptimal for one strain may be effective for another.
Our findings also highlight the effects of inoculum size on H. pylori growth. Certain strains (1865, 3233, and 3424) showed markedly better growth when the initial inoculum concentration was high, whereas other strains, including the reference strains, reached similar final densities regardless of the initial inoculum. This suggests that some H. pylori strains may benefit from a threshold population density to establish robust growth, possibly because of collective metabolic byproducts or protection from oxidative stress at higher cell densities. Consistent with our observations, a previous study found that H. pylori growth kinetics in liquid media are significantly influenced by inoculum size[9]. A larger inoculum may maintain microaerobic conditions better and produce sufficient urease activity to buffer the medium, thereby facilitating exponential growth. It is possible that the strains that did not show an inoculum-dependent effect possessed more efficient stress responses or lower nutrient thresholds, allowing even small populations to initiate growth. Importantly, all the strains in our study grew within 72 hours, even from the lowest inoculum, which is encouraging for culture reliability.
This study had some limitations. First, we quantified growth only by OD measurement at 24-hour interval and did not directly determine viable bacterial counts. Although OD is a convenient proxy for biomass, it does not distinguish between viable spiral bacteria and non-viable coccoid forms. H. pylori is known to transform into a non-culturable coccoid form after 2-3 days of incubation, a process that occurs faster and is more prominent in supplement-free media[20,23]. Therefore, our OD-based growth assessment may have overestimated the actual live bacterial population, especially at 72 hours. A quantitative approach at each time point in future studies would strengthen our conclusions. Second, we did not capture the initial lag phase of growth because the first OD measurement was at 24 hours, at which time the bacteria had already entered exponential growth. Although we did not specifically measure the lag phase in this study, our design focused on comparing the relative growth performance across different broth media at fixed time intervals, which is a commonly accepted approach in similar studies[11,23]. Under these conditions, the lag duration was not critical to achieving the study's aim of identifying media that supported the robust overall growth of H. pylori. However, we acknowledge that analyzing lag phase dynamics can be essential in other experimental contexts such as assessing bacterial adaptation under stress, comparing strain-specific growth kinetics, and evaluating metabolic activity during early culture[29]. Future studies aimed at investigating such parameters should include earlier and more frequent time-point measurements to capture the lag phase in detail.
Additionally, we used a single reference strain inoculum to normalize the growth curves across media. While this approach enables internal comparisons, the absolute OD values may not directly correspond to the same CFU/mL in different broths owing to differences in medium composition and optical properties. To overcome this limitation, a direct quantification method such as a time-kill assay is recommended. However, time-kill assays are highly labor-intensive and, thus, not practical for large-scale screening of multiple media and supplement combinations, as performed in the present study. Future studies incorporating CFU counts for each broth condition would allow for a more accurate assessment of the absolute growth-supporting capacity of each medium. Moreover, our study was conducted in a controlled laboratory setting using fresh subcultures of H. pylori, which limits the generalizability of our findings. These results may not be fully extrapolated to the primary isolation or subculture of clinical strains, where factors such as transport time, sample contamination, and antibiotic carry-over can affect culture success[30]. Nonetheless, the trends observed have provided a baseline for the media formulations that are most supportive of H. pylori and could inform clinical culture protocols. Finally, although PCR targeting the glmM gene successfully confirmed strain identity in our study, we did not perform a sequence variability analysis across the tested strains. However, glmM is highly conserved and is widely used as a molecular target for H. pylori detection[31], and successful amplification was observed in randomly selected strains tested in this study.
Despite these limitations, our study has implications for H. pylori culture methodology in both research and clinical contexts. Liquid culture of H. pylori offers the advantage of yielding a large amount of biomass for downstream applications, such as high-throughput studies. Our findings suggest that optimizing liquid culture conditions could facilitate the broader use of H. pylori cultures. For example, a more efficient broth could improve the success rate of isolating H. pylori from biopsy samples by providing a more supportive environment for the few bacteria present in clinical specimens[32]. Although this study did not address primary isolation, it reinforces the principle that richer media can enhance H. pylori growth. In a research setting, switching to a medium, such as CMCB or CB, may shorten the time required to expand cultures or achieve detectable growth. Moreover, the ability of certain media to support high population densities may be useful for studying H. pylori pathophysiology.
In summary, this study demonstrates that the choice of liquid culture medium has a significant impact on the growth of H. pylori and that no single broth is optimal for all strains. Although we do not necessarily recommend adopting CB, CMCB, or FAB for routine liquid cultures of H. pylori, these media may be particularly useful when large quantities of H. pylori are needed. Our findings provide practical guidance for researchers cultivating H. pylori, suggesting that using enriched media can improve yields and that media should be selected or even customized with specific strain variability. Ultimately, optimizing culture conditions and potentially incorporating tailored supplements or defined components will enhance our ability to grow H. pylori in vitro for both research and clinical applications.
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3311] [Article Influence: 78.8] [Reference Citation Analysis (2)] |
| 2. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1934] [Article Influence: 80.6] [Reference Citation Analysis (3)] |
| 3. | Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 565] [Article Influence: 188.3] [Reference Citation Analysis (1)] |
| 4. | Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18:613-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 5. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;gutjnl-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 842] [Article Influence: 210.5] [Reference Citation Analysis (0)] |
| 6. | Bhadange Y, Sharma S, Das S, Sahu SK. Role of liquid culture media in the laboratory diagnosis of microbial keratitis. Am J Ophthalmol. 2013;156:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Mirzaee H, Ariens E, Blaskovich MAT, Clark RJ, Schenk PM. Biostimulation of Bacteria in Liquid Culture for Identification of New Antimicrobial Compounds. Pharmaceuticals (Basel). 2021;14:1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Murray EM, Allen CF, Handy TE, Huffine CA, Craig WR, Seaton SC, Wolfe AL. Development of a Robust and Quantitative High-Throughput Screening Method for Antibiotic Production in Bacterial Libraries. ACS Omega. 2019;4:15414-15420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Xia HX, English L, Keane CT, O'Morain CA. Enhanced cultivation of Helicobacter pylori in liquid media. J Clin Pathol. 1993;46:750-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Shahamat M, Mai UE, Paszko-Kolva C, Yamamoto H, Colwell RR. Evaluation of liquid media for growth of Helicobacter pylori. J Clin Microbiol. 1991;29:2835-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Kitsos CM, Stadtländer CT. Helicobacter pylori in liquid culture: evaluation of growth rates and ultrastructure. Curr Microbiol. 1998;37:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Fujita Y, Yamaguchi K, Kamegaya T, Sato H, Semura K, Mutoh K, Kashimoto T, Ohori H, Mukai T. A novel mechanism of autolysis in Helicobacter pylori: possible involvement of peptidergic substances. Helicobacter. 2005;10:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Mujtaba A, Ibrahim MS, Parveen S, Sarwar N, Alsagaby SA, Raza MA, Abdelgawad MA, Ghoneim MM, El-Ghorab AH, Selim S, Al Abdulmonem W, Hussain M, Fenta Yehuala T. Comparative Analysis of Diagnostic Techniques for Helicobacter pylori Infection: Insights for Effective Therapy. J Cell Mol Med. 2025;29:e70487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Lee H, Hwang HS, Chung JW, Kim KA, Kim ST. Development of a New Liquid Type Rapid Urease Test Kit (Helicotest(®)): Comparison with Other Commercial Kits. Korean J Gastroenterol. 2023;81:209-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Bickley J, Owen RJ, Fraser AG, Pounder RE. Evaluation of the polymerase chain reaction for detecting the urease C gene of Helicobacter pylori in gastric biopsy samples and dental plaque. J Med Microbiol. 1993;39:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Goodwin CS, Armstrong JA, Marshall BJ. Campylobacter pyloridis, gastritis, and peptic ulceration. J Clin Pathol. 1986;39:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 418] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Morgan DR, Freedman R, Depew CE, Kraft WG. Growth of Campylobacter pylori in liquid media. J Clin Microbiol. 1987;25:2123-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Buck GE, Smith JS. Medium supplementation for growth of Campylobacter pyloridis. J Clin Microbiol. 1987;25:597-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Westblom TU, Gudipatí S, Midkiff BR. Enhanced growth of Helicobacter pylori using a liquid medium supplemented with human serum. Eur J Clin Microbiol Infect Dis. 1995;14:155-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Andersen AP, Elliott DA, Lawson M, Barland P, Hatcher VB, Puszkin EG. Growth and morphological transformations of Helicobacter pylori in broth media. J Clin Microbiol. 1997;35:2918-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Lee JH, Park J, Park MR, Na YH, Cho S. A Comparative Study of Helicobacter pylori Growth on Different Agar-based Media. Korean J Helicobacter Up Gastrointest Res. 2017;17:208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Vega AE, Cortiñas TI, Mattana CM, Silva HJ, Puig De Centorbi O. Growth of Helicobacter pylori in medium supplemented with cyanobacterial extract. J Clin Microbiol. 2003;41:5384-5388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Douraghi M, Kashani SS, Zeraati H, Esmaili M, Oghalaie A, Mohammadi M. Comparative evaluation of three supplements for Helicobacter pylori growth in liquid culture. Curr Microbiol. 2010;60:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Jiang X, Doyle MP. Growth supplements for Helicobacter pylori. J Clin Microbiol. 2000;38:1984-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Walsh EJ, Moran AP. Influence of medium composition on the growth and antigen expression of Helicobacter pylori. J Appl Microbiol. 1997;83:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Cover TL. Perspectives on methodology for in vitro culture of Helicobacter pylori. Methods Mol Biol. 2012;921:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Albertson N, Wenngren I, Sjöström JE. Growth and survival of Helicobacter pylori in defined medium and susceptibility to Brij 78. J Clin Microbiol. 1998;36:1232-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Hazell SL, Markesich DC, Evans DJ, Evans DG, Graham DY. Influence of media supplements on growth and survival of Campylobacter pylori. Eur J Clin Microbiol Infect Dis. 1989;8:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Bertrand RL. Lag Phase Is a Dynamic, Organized, Adaptive, and Evolvable Period That Prepares Bacteria for Cell Division. J Bacteriol. 2019;201:e00697-e00618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Gong EJ, Ahn JY, Jung DK, Lee SM, Pih GY, Kim GH, Na HK, Lee JH, Jung HY, Kim JM. Isolation of Helicobacter pylori using leftover tissue in the rapid urease test kit. Helicobacter. 2020;25:e12733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Shahamat M, Alavi M, Watts JE, Gonzalez JM, Sowers KR, Maeder DW, Robb FT. Development of two PCR-based techniques for detecting helical and coccoid forms of Helicobacter pylori. J Clin Microbiol. 2004;42:3613-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Sainsus N, Cattori V, Lepadatu C, Hofmann-Lehmann R. Liquid culture medium for the rapid cultivation of Helicobacter pylori from biopsy specimens. Eur J Clin Microbiol Infect Dis. 2008;27:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/