Published online Nov 21, 2025. doi: 10.3748/wjg.v31.i43.111609
Revised: August 18, 2025
Accepted: October 27, 2025
Published online: November 21, 2025
Processing time: 135 Days and 17.1 Hours
Environmental enteric dysfunction (EED) is a subclinical condition caused by fecal-oral contamination leading to enteric inflammation and dysbiosis. Bile acids serve to facilitate lipid digestion and absorption, regulate metabolic pathways associated with childhood growth and inflammation, and may be affected by EED.
To investigate bile acid metabolism in Bangladeshi children with EED and its association with growth impairment.
We conducted a cross-sectional study of 100 Bangladeshi infants (aged 6-9 months) and quantified serum and fecal bile acids using LC-MS/MS. We compared profiles to a control group of 6 American children (6-12 months) and 80 older Bangladeshi children (aged 2 years).
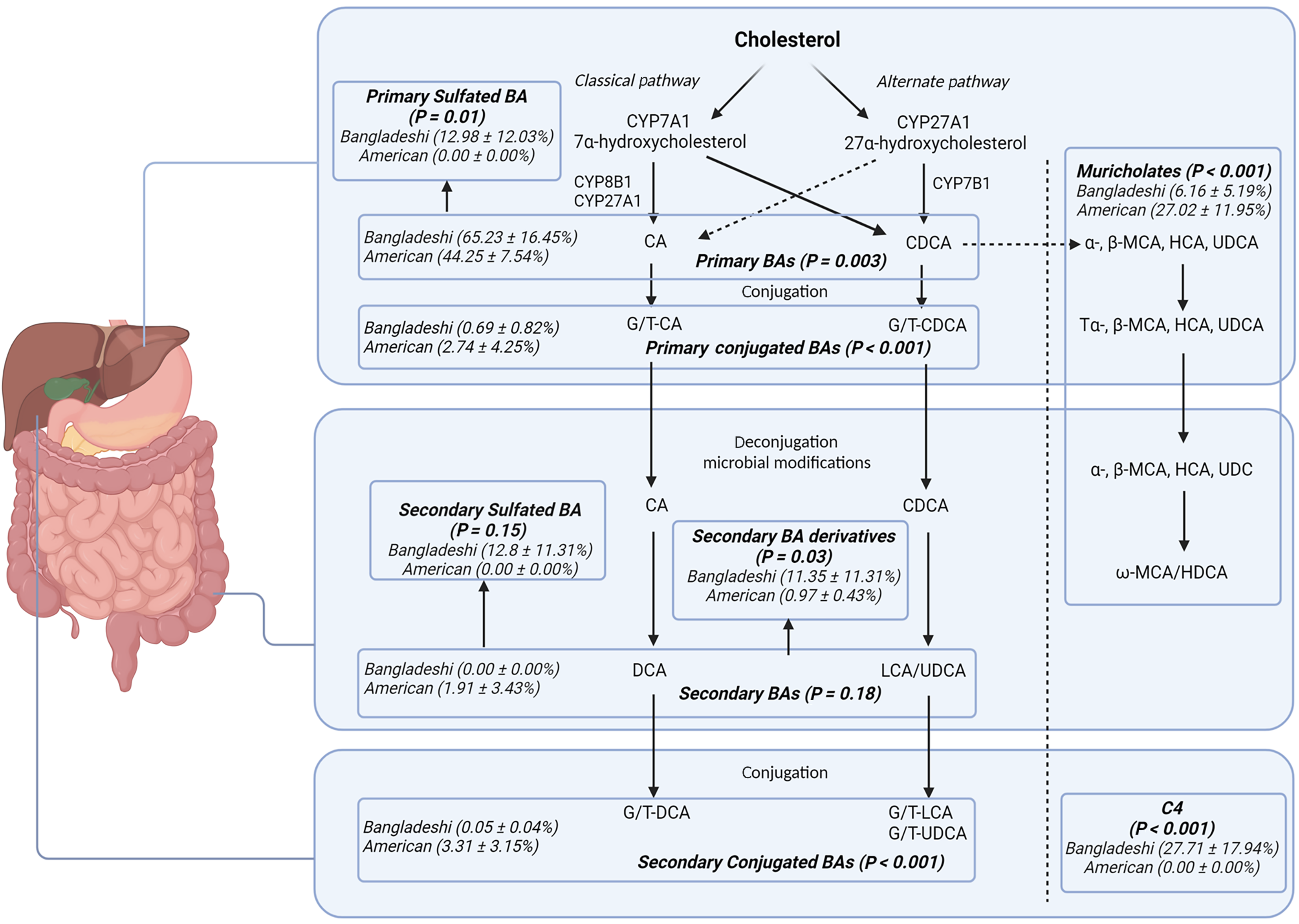
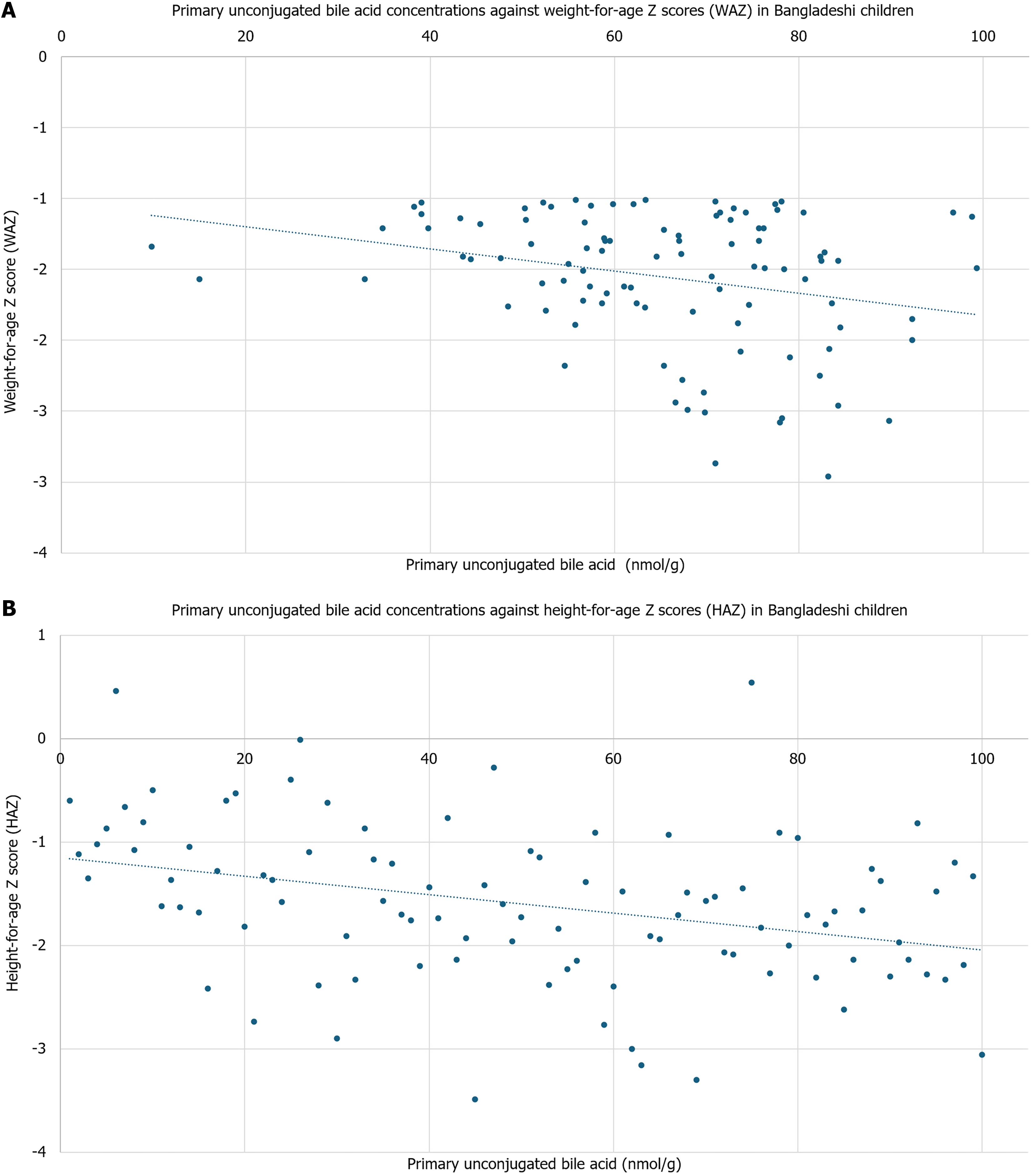
Bangladeshi infants had higher levels of plasma unconjugated primary (65.23% vs 44.25%, P = 0.003) and sulfated primary bile acids (12.98% vs < 0.001%, P = 0.01), with lower primary conjugated bile acids (0.69% vs 2.74%, P ≤ 0.001) compared to American children. Stool unconjugated primary bile acids were inversely associated with weight-for-age [regression coefficient (β) = -0.01, P = 0.01] and height-for-age Z scores (β = -0.01, P = 0.03). Con
Our data suggests an age-dependent defect in conjugation of primary bile acids in Bangladeshi children with compensatory hydrophilic shunting. Additionally, bile acid profiles are associated with intestinal overgrowth.
Core Tip: This study identifies a previously unrecognized defect in bile acid conjugation among Bangladeshi infants with environmental enteric dysfunction, linking altered bile acid metabolism to poor growth and intestinal inflammation. Elevated unconjugated primary bile acids were strongly associated with anthropometry. Further, the findings highlight a possible age-related delay in maturation of bile acid conjugation pathways in impoverished children. This study may provide the initial insights for exploring novel therapeutic targets through bile acid pathways for treating malnourished children worldwide.
- Citation: Hasan F, Hylemon PB, Haque R, Petri WA, Faruque ASG, Kirkpatrick BD, Alam M, Ferdous T, Shama T, Moreau B, Ramakrishnan G, Zhou H, Chesney A, Medrano Garcia F, Smirnova E, Prem P, Huang Y, Bojja R, Thapaliya A, Donowitz JR. Bile acid dysmetabolism in Bangladeshi infants associated with poor linear growth, enteric inflammation, and small intestine bacterial overgrowth. World J Gastroenterol 2025; 31(43): 111609
- URL: https://www.wjgnet.com/1007-9327/full/v31/i43/111609.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i43.111609
Twenty-one percent of deaths in children under the age of five can be attributed to malnutrition[1]. Pediatric malnutrition in low and middle-income countries is multifactorial with contribution from food insecurity, inadequate access to clean water, and unsanitary living conditions resulting in chronic enteric pathogen exposure. Chronic carriage of enteric pathogens leads to environmental enteric dysfunction (EED), a sub-clinical condition characterized by chronic gut inflammation, small intestinal injury, and small intestinal dysbiosis[2]. EED has been associated with deficient nutrient absorption and systemic inflammation which leads to linear growth shortfalls and poor neurodevelopmental outcomes[3]. Constant exposure to fecal-oral contamination leads to enteric inflammation and subsequent dysbiosis which, in turn, leads to reduced barrier function, villous blunting, and a decreased crypt-to-villus ratio[4]. These histological changes recovered when subjects were removed from areas of poor sanitation and hygiene[4]. While intestinal biopsies are the gold standard for diagnosing EED, endoscopy is invasive and therefore various biomarkers of intestinal and systemic inflammation have been used as proxy measures of EED burden[5]. Childhood EED has been associated with growth stunting, decreased weight, and poor neurodevelopmental outcomes[4].
Among children with EED, small intestine bacterial overgrowth (SIBO) is common. SIBO was present in 11% of 18 weeks old Bangladeshi infants and the prevalence increased to 30%-40% in 1 to 2-year-olds[6]. In this population, SIBO was associated with markers of intestinal inflammation[7]. SIBO in the low-income country setting is also associated with growth stunting and language delay, independently of inflammation[6,8]. Despite the association of SIBO and poor growth being noted in several studies across several continents, the nature of this association and the pathogenesis of SIBO remains unclear. One study did, however, note that the SIBO microbiome led to decreased lipid absorption when transplanted from patients into an in vitro murine intestinal m-IcCl2 cell line[8].
EED associated gut dysbiosis has also been linked to abnormal bile acid patterns[9]. Bile acids act as detergents in the intestine to facilitate lipid digestion and absorption. They also serve as signaling molecules that regulate numerous metabolic pathways associated with childhood growth[10]. Furthermore, bile acids have been shown to play a role in host susceptibility to inflammation and serve as sensitive markers to hepatic and cholestatic function[11]. Bile acid metabolism defects can lead to fat-soluble vitamin deficiency, malnutrition, neurologic impairments, and stunted growth[12]. Lack of bile acid conjugation has even been linked to the action of the bacteria proliferating in the upper small intestine[13]. There is a paucity of literature investigating the role of bile acid metabolism in low- and middle-income country children with EED and malnutrition although preliminary evidence suggests altered bile acid metabolism in this population[9,11,14-16]. A study looking at children in rural Malawi reported that children with EED displayed altered bile acid metabolism from a young age, with a higher proportion of bile acids conjugated with taurine instead of glycine. Total serum bile acids were also approximately 12% lower in children with EED compared with children without EED, which may be reflective of impaired reuptake due to injury in the ileum[9]. The same study also inversely correlated levels of primary unconjugated bile acids with age in children with EED[9]. Another study in undernourished Pakistani children with EED found elevated levels of serum bile acids when compared to well-nourished local American children, with over 70% of the un
Based on these previous studies, we hypothesized that stunted children with EED and SIBO have altered bile acid metabolism. To test this hypothesis, we conducted a cross-sectional analysis of Bangladeshi children and compared them to American controls, investigating bile acid patterns in both the serum and stool.
We conducted a cross-sectional analysis of Bangladeshi toddlers investigating the association between SIBO, EED biomarkers, and bile acid profiles in both serum and stool. We enrolled 100 toddlers from the urban neighborhood of Mirpur in Dhaka, Bangladesh. Samples were obtained as part of a prospective efficacy study in Bangladeshi infants. All specimens were collected at baseline prior to randomization. The present work represents a secondary analysis of these existing samples from a previous study in malnourished Bangladeshi children (ClinicalTrials.gov identifier: NCT03263871). Samples were taken from the pre-intervention study visit. Enrollment was from October 2017 through July 2018. The majority of the homes in Mirpur are mud brick construction. Crowding is common with a mean of 5 people living in 1.5 rooms per dwelling. Uncovered sewers flow throughout the neighborhood with municipal water lines often running through these sewer channels. Due to the location of our study clinic, subjects tended to come from the lowest socioeconomic strata of Mirpur. Enrolled children were between age 6-9 months old with no known chronic medical problems other than mild wasting as defined by a weight-for-age Z (WAZ) score between -1 SD and -3 SD. Exclusion criteria also included milk intolerance, abnormal liver or kidney function, and exclusive breast feeding at time of enrollment.
Blood was collected by a pediatric phlebotomist in our study clinic. The stool was collected in our study clinic by field assistants. Maintaining proper cold chain, samples were transported to our laboratory within 4 hours where they were aliquoted and placed in -80 °C. One aliquot was removed for batched biomarker analysis at the completion of the study. A separate aliquot was later shipped to the United States on dry ice where samples were again stored in -80 °C until they were removed for analysis. The cold chain was monitored throughout the shipping process. We also conducted both a 2-hour dual sugar urinary lactulose-mannitol test by LC-MSMS and glucose-hydrogen breath test on subjects using methods on which we have previously published[7,17]. Pediatric urine collection bags were attached to the patients and were allowed to return to regular diet 30 minutes after LM test solution ingestion. Two mL of urine was collected at the 2-and-5-hour marks. The samples were then analyzed by the HPLC-MSMS system[17]. Glucose-hydrogen breath testing was done by QuinTron BreathTracker SC gas chromatography which collected breath via an age-appropriate anesthesia mask attached at 20-minute intervals for 3 hours. Patients fasted for 3 hours prior to and throughout testing but were allowed water. Children younger than 12 months fasted for 2 hours[7]. SIBO area under the curve (AUC) (i.e., area under the breath hydrogen curve) was calculated by summing the trapezoidal area under the glucose-hydrogen curve using methods previously published[6]. These two tests were conducted within one week of each other but not on the same day. Stool and serum were collected at the same time as glucose-hydrogen breath testing. Biomarkers were tested by commercially available ELISA and included stool regenerating family member 1 beta (Reg 1B) (TechLab, Inc. Blacksburg, VA, United States), stool myeloperoxidase (MPO) (ALPCO. Salem, NH, United States), serum C-reactive protein (CRP) (ALPCO. Salem, NH, United States), and serum soluble CD14 (sCD14) (R&D Systems, Minneapolis, MN, United States). Anthropometry was measured using calibrated infant scales and an infant measuring board by staff trained in the pro
We also obtained discarded serum samples collected and stored in EDTA from the clinical laboratories at Virginia Commonwealth University Hospital in Richmond, VA, United States. Samples were collected to be used for comparison, serving as a high-income well-nourished cohort. They were collected for clinical reasons unrelated to this study and screened prior to being discarded from the clinical lab. Samples were selected based on age with only samples from 6-12-month-old screened. Exclusion criteria included acute or chronic malnutrition, any known gastrointestinal diseases, or known metabolic disorders. Only serum was collected as paired stool samples were unavailable. Samples were collected for clinical purposes and sent within minutes of collection to the clinical laboratory where they were processed within 1 hour and stored at 4 °C for 3 days. Samples stored longer than 3 days are generally discarded. Once a sample was identified as marked for discard, having sufficient volume for bile acid testing, and from the appropriate age range, the electronic medical record was reviewed by our team (Donowitz JR) to ensure the children had no known gastrointestinal or metabolic diagnoses associated with altered bile acid metabolism.
Serum from both Bangladeshi and American children, as well as stool from Bangladeshi children underwent bile acid and 7α-hydroxy-4-cholesten-3-one (C4) profiling. C4 is a bile acid precursor, serving as an indicator of overall bile acid production. The serum samples were processed and the composition and levels of individual bile acid metabolism were measured using a Shimadzu liquid chromatography/tandem mass spectrometric 8600 system as described previously[18]. Ten isotope-labeled compounds, each sharing the same structure as the corresponding bile acid analytes, were used as internal standards. The same volume and concentration of internal standards were added to each sample for quality control (QC) and quantitative bile acid analysis. Internal standards help monitor extraction efficiency and correct for instrument variability. The assay was validated using calibration curves and QC samples prepared at various concentrations, with QC samples analyzed in every batch to ensure consistent instrument performance and accurate quantification across all runs.
We first analyzed the composition of bile acids in both the serum and stool samples from both cohorts. Individual bile acid levels were expressed as percentages of total bile acids detected, excluding C4 which was analyzed as a continuous variable. If a bile acid was undetectable, it was assigned a value of 0. If there were no detectable levels, bile acids were valued at a 0. Bile acids were grouped based on the bile acid metabolism pathway as follows: CDCA and CA were categorized as unconjugated primary bile acids, TCA, GCA, TCDCA, and GCDCA were categorized as conjugated primary bile acids, CDCA-3-S and CA-3-S were categorized as sulfated primary bile acids, UDCA, DCA, and LCA were categorized as unconjugated secondary bile acids, TUDCA, GUDCA, MDCA, GDCA, TLCA, and GLCA were categorized as conjugated secondary bile acids, 7keto-DCA, 7keto-LCA, isoDCA, isoLCA, allo-isoLCA, and 3keto-LCA were cate
As an exploratory endeavor, we then conducted a secondary analysis of data that was collected as part of two other cohorts in 2-year-old Bangladeshi children from the same neighborhood in Mirpur, Dhaka. SIBO data, collected using identical methodology to that detailed above, was also available on these samples. Eighty paired serum and stool samples collected between May 2011 and March 2016 had bile acid analysis performed by liquid chromatography-mass spectroscopy using the Biocrates AbsoluteIDQ p180 Kit and high-performance liquid chromatography column per manufacturer's protocols (Biocrates, Inc Aliso Viejo, CA, United States). Bile acids were converted into percentages of total bile acids detected. If there were no detectable levels, bile acids were valued at a 0. Then bile acids were grouped based on the bile acid metabolism pathway as follows: CDCA and CA were categorized as unconjugated primary bile acids, TCA, GCA, TCDCA, and GCDCA were categorized as conjugated primary bile acids, UDCA, DCA, and LCA were categorized as unconjugated secondary bile acids, TUDCA, GUDCA, TDCA, GDCA, TLCA, and GLCA were categorized as conjugated secondary bile acids, and TaMCA, TbMCA, aMCA, bMCA, oMCA, and HDCA were categorized as muricholates. Linear regression models were completed between these bile acid categories and SIBO AUC data. Finally, two-sample t-tests were conducted to determine if there were differences between bile acid groups of the 2-year-old cohort and the American children, as well as between the 2-year-old cohort and the original Bangladeshi cohort of 6-9-month-old children.
The collection and analysis of Bangladeshi samples was approved by the Ethics and Research Review Committees at the ICDDR,B and the Institutional Review Board at the University of Virginia. Reliance agreements between the University of Virginia and the ICDDR,B with Virginia Commonwealth University were also established for this study. Informed consent for participation was received by both parents of enrolled children. For the American control group, the study was approved by the Institutional Review Board at Virginia Commonwealth University. A waiver of consent was obtained to use discarded samples.
The cohort of 100 6-9-month-old Bangladeshi consisted of 51 females and 49 males. The average age was 229 days (range: 185-265 days). The average (and SD) of WHZ was -0.75 SD (± 0.69 SD), HAZ was -1.60 SD (± 0.76 SD), WAZ was -1.55 SD (± 0.47 SD), and BAZ was -0.83 SD (± 0.67 SD) (Table 1).
| Characteristics | Value | |
| Female, n | 51 | 51% |
| Age (days) | 229 | 21 |
| Weight-for-height Z-score | -0.75 | 0.69 |
| Height-for-age Z-score | -1.60 | 0.76 |
| Weight-for-age Z-score | -1.55 | 0.47 |
| BMI-for-age Z-score | -0.83 | 0.67 |
In the serum samples, the Bangladeshi children averaged 216540 nmol/L unconjugated primary bile acids, 1537 nmol/L conjugated primary bile acids, 23185 nmol/L primary sulfated bile acids, 4413 nmol/L secondary bile acids, 93 nmol/L secondary conjugated bile acids, 774 nmol/L secondary sulfated bile acids, 23998 nmol/L secondary bile acid derivatives, and 14033 nmol/L muricholates. They had a total average bile acid serum sample concentration of 11956 nmol/L. In comparison, the American group has an average serum distribution of 1097 nmol/L unconjugated primary bile acids, 74 nmol/L conjugated primary bile acids, 0 primary sulfated bile acids, 0 secondary bile acids, 57 nmol/L secondary conjugated bile acids, 0 secondary sulfated bile acids, 20 nmol/L secondary bile acid derivatives, and 695 nmol/L muricholates. They had a total average bile acid serum sample concentration of 2570 nmol/L. For analysis purposes between the two cohorts, the percentage distribution of each category was calculated and then compared. C4 Levels were also obtained in both groups, with Bangladeshi children having an average of 28 nmol/L and American children of 0 nmol/L (Supplementary Table 1).
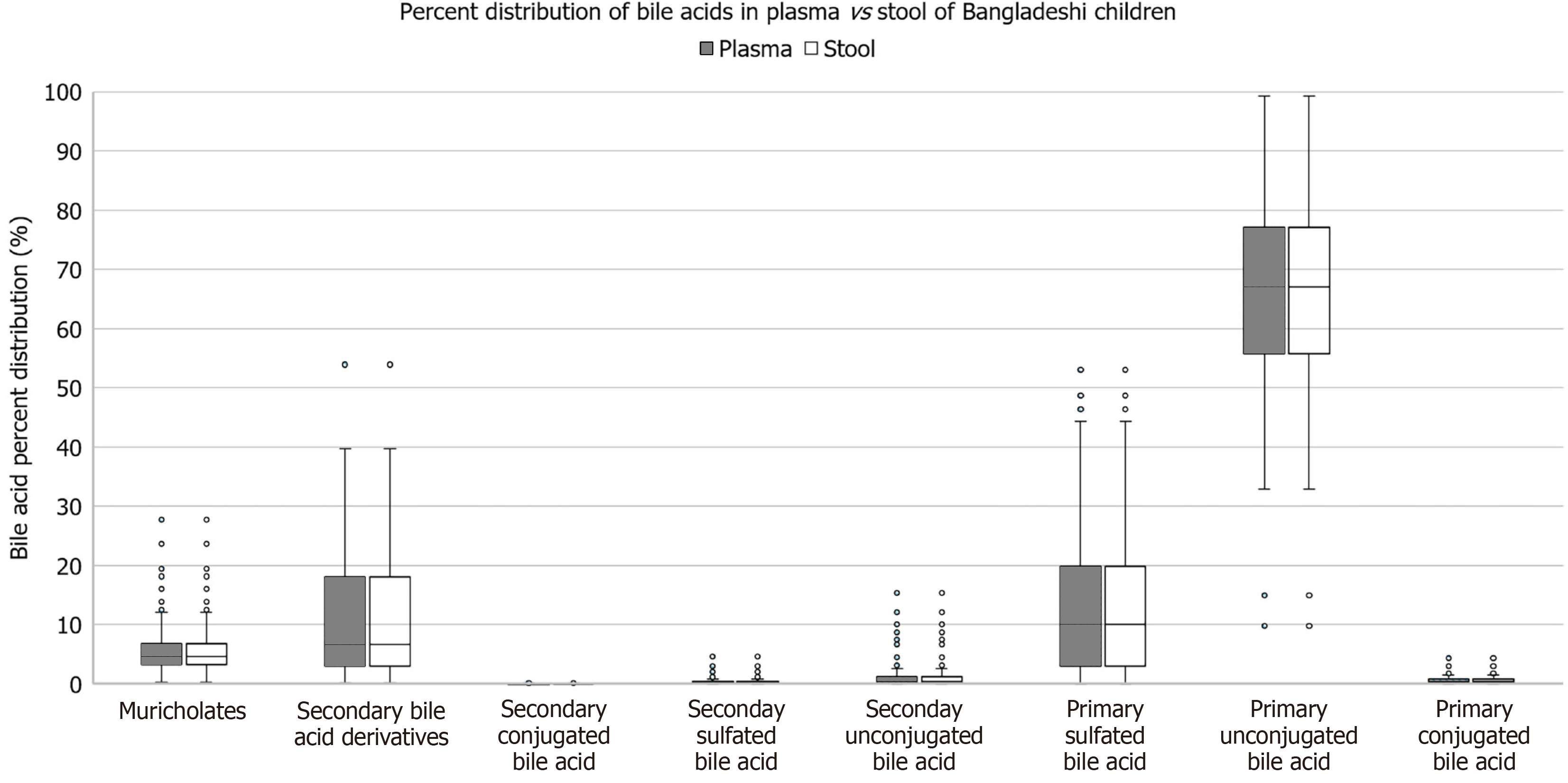
As compared to American children, Bangladeshi children’s serum had higher levels of primary unconjugated bile acids (65.23% vs 44.25%, P = 0.003), primary sulfated bile acids (12.98% vs < 0.001%, P = 0.01), and secondary bile acids derivatives (11.35% vs 0.97%, P = 0.03). Bangladeshi children had lower percentages of muricholates (6.16% vs 27.02%, P ≤ 0.001), primary conjugated bile acids (0.69% vs 2.74%, P ≤ 0.001), and secondary conjugated bile acids (0.05% vs 3.31%, P ≤ 0.001) compared to American children. There was no significant difference in percentage of unconjugated secondary bile acids, percentage of secondary sulfated bile acids, or total bile acids between the two groups (Figure 1). There was no significant difference between percentage of muricholates, unconjugated primary bile acids, conjugated primary bile acids, sulfated primary bile acids, unconjugated secondary bile acids, conjugated secondary bile acids, sulfated secondary bile acids, or secondary bile acid metabolite levels in the stool and serum of the 6-9-month-old Bangladeshi children (Figure 2).
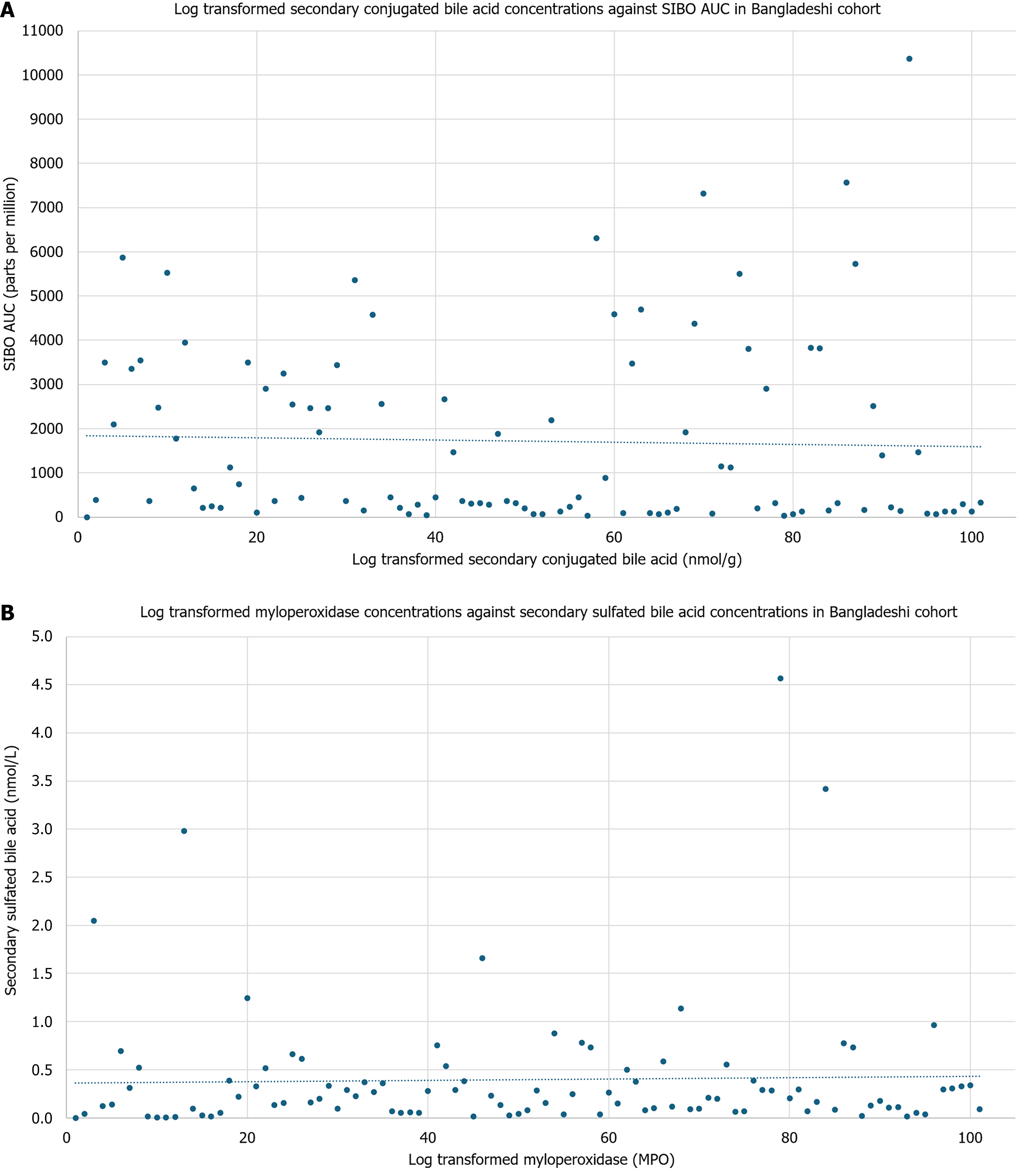
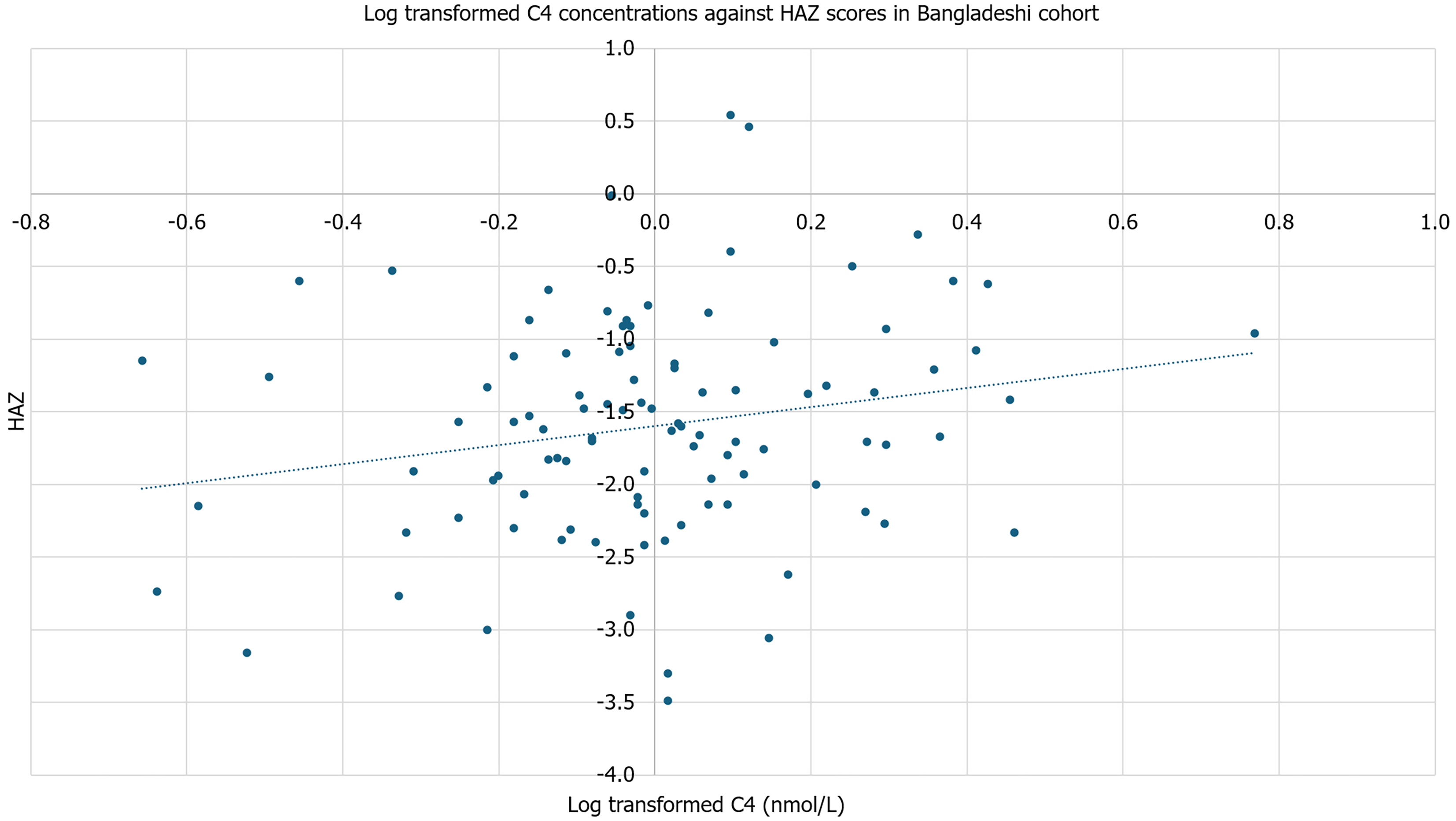
In the stool of Bangladeshi children, conjugated secondary bile acids had an inverse relationship with SIBO AUC [regression coefficient (β) = -1096.68, P = 0.05], while MPO had an inverse association with sulfated secondary bile acids (β = -0.40, P = 0.04; Figure 3). No other groups of bile acids were significantly associated with inflammatory biomarkers. Unconjugated primary bile acids were associated with weight-for-age (β = -0.27, P = 0.01) and HAZ (β = -0.01, P = 0.03; Figure 4). There was no significant association against weight-for-height or body mass index (BMI)-for-age. Further, C4 concentrations were associated with HAZ (β = 0.65, P = 0.04; Figure 5). There was no significant association with C4 with weight-for-age, weight-for-height, or BMI-for-age. No other bile acids were significantly associated with weight-for-age, height-for age, weight-for-height, or BMI-for-age.
In our multivariable model with HAZ as outcome, the model only retained unconjugated primary bile acids (β = -0.01, P = 0.05), age (β = -0.01, P = 0.08), female (β = 0.28, P = 0.05), and Reg 1B (β = -0.00, P = 0.02) as significant predictors. In our multivariable model with WAZ as outcome, the model only retained unconjugated primary bile acids (β = -0.01, P = 0.01) and CRP (β = -0.00, P = 0.01) as significant predictors.
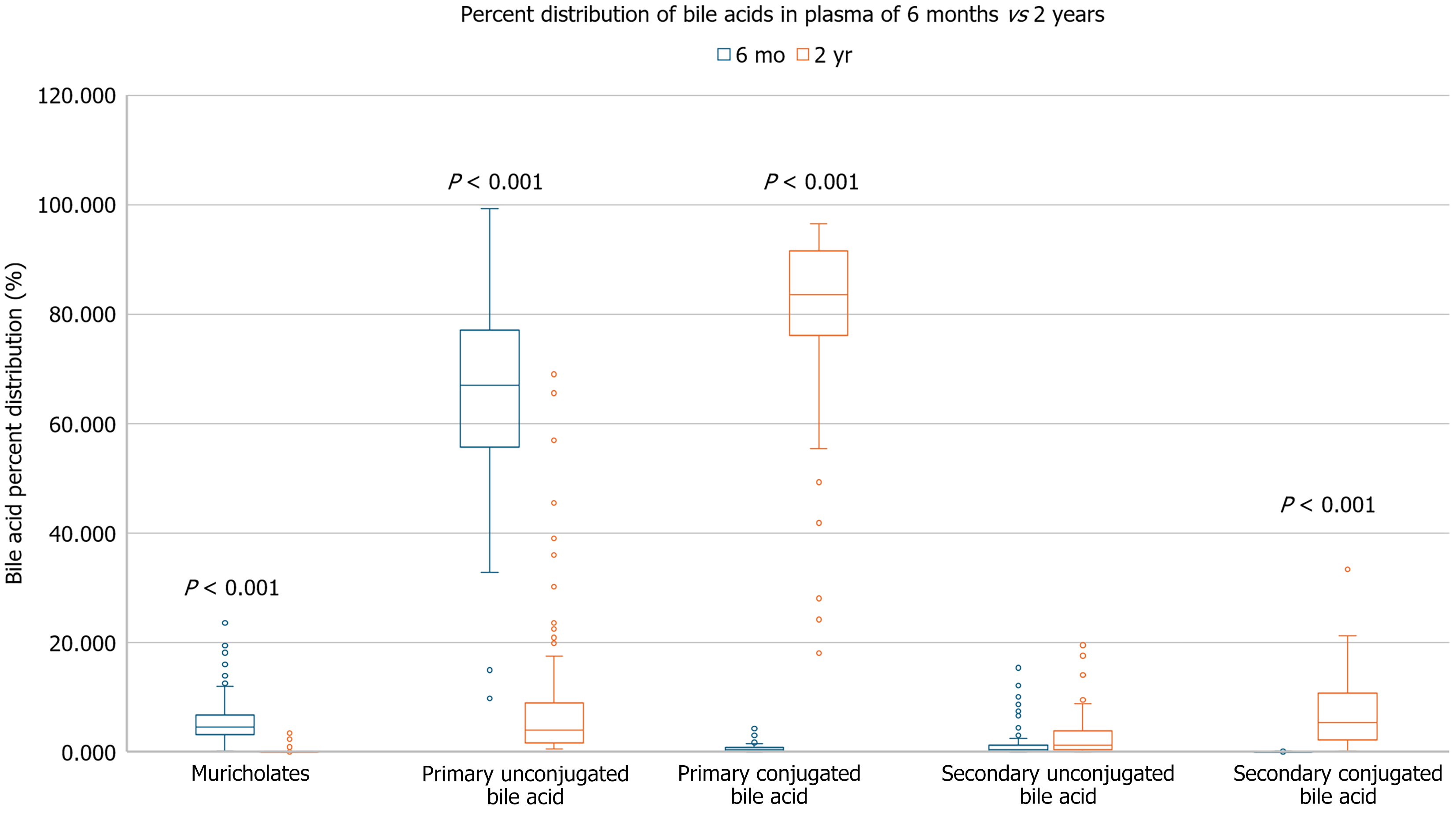
The plasma samples of the 6-9-month-old Bangladeshi children were compared to the plasma samples of 80 2-year-old Bangladeshi children. As compared to the 2-year-old children, the 6-9-month-old Bangladeshi children’s serum had higher levels of unconjugated primary bile acids (65.23% vs 9.20%, P ≤ 0.001) and muricholates (6.16% vs 0.26%, P ≤ 0.001). They had lower percentages of primary conjugated bile acids (0.69% vs 80.38%, P ≤ 0.001), and conjugated secondary bile acids (0.05% vs 7.24%, P ≤ 0.001) compared to the 2-year-old children. There was no significant difference in percentage of unconjugated secondary bile acids between the two groups (Figure 6). There were no significant associations between the older children’s bile acid group percentages and SIBO AUC.
Finally, when compared to the American children, the 2-year-old Bangladeshi children had higher levels of unconjugated secondary bile acids (2.93% vs < 0.001%, P ≤ 0.001) but lower levels of muricholates (0.26% vs 27.02%, P = 0.02). There was no significant difference in percentage of unconjugated primary bile acids, conjugated primary bile acids, or conjugated secondary bile acids between the two groups.
Our study demonstrates that dysfunction in bile acid metabolism is associated with stunting and wasting in Bangladeshi children with EED. Overall, when compared to age-matched American children, the Bangladeshi children showed increased levels of unconjugated primary bile acids and sulfated primary bile acids, suggesting arrested metabolism. This dysregulation likely stems from defective hepatic conjugation pathways, as evidenced by significantly reduced levels of conjugated primary bile acids and diminished downstream bile acids. Under physiological conditions, primary bile acids synthesized in the liver are rapidly conjugated, primarily with glycine or taurine, by bile acid-CoA: Amino acid N-acyltransferase (BAAT). Conjugated bile acids, which are more hydrophilic, play a crucial role in nutrient absorption. Conjugation with glycine or taurine enhances solubility and absorption of hydrophobic nutrients such as long chain fatty acids, cholesterol and fat-soluble vitamins by forming mixed micelles in the small intestine[21]. In contrast, hydrophobic bile acids have potent inflammatory properties that can damage the liver, intestine, and other tissues, whereas hy
Younger Bangladeshi children demonstrated a conjugation defect while older children did not, as shown by the elevated levels of primary and secondary conjugated bile acids in the 2-year-olds. This suggests that impoverished Bangladeshi children may have a delay in maturation of their conjugation pathways rather than an intrinsic deficit as seen in patients with BAAT and bile acid-CoA ligase (SLC27A5) gene defects[12]. This finding corroborates a previous study of Malawian children that inversely correlated levels of CA and CDCA with age in children with EED, specifically r = -0.45 for CA (P = 0.02) and r = -0.39 for CDCA (P = 0.05)[9]. These negative correlations also suggested that as the children aged, their serum levels of CA and CDCA decreased, a correlation with the delay we observed in the Bang
Muricholic acids are more common in mice but found only in small amounts in humans. While it is normal to have higher levels of muricholates during infancy, it will usually drop to undetectable levels by adulthood[28]. Our findings showed a higher percentage of muricholates in the older Bangladeshi children compared to the younger infants. Notably, the American cohort had a higher percentage of muricholates compared to the Bangladeshi infants, despite being an older cohort on average. However, this finding may be skewed given the overall significant drop in percentage of downstream bile acids due to the sequestration of primary bile acids in these children. Further research is needed to clarify this finding.
Younger Bangladeshi children demonstrated very little difference in the bile acid profile patterns between their stool and serum. In normal physiology children reabsorb their bile acids in their ileum for transport back to the liver via portal blood circulation to initiate negative feedback on subsequent bile acid synthesis, and therefore we expected a difference in bile acid levels between the stool and serum samples[29]. Specifically, we would have expected to see a significantly decreased level of bile acids in the stool in comparison, which we did not. However, it is possible that the intestinal barrier breakdown associated with EED is sufficient to equilibrate the serum and stool bile acid levels.
Currently, there is no known treatment for EED. Our data suggests correction of the early life conjugation defect could present a therapeutic opportunity. A study targeting defective bile acid amidation due to a deficiency in BAAT showed improvement in fat-soluble vitamin absorption after treatment with GCA[12]. Patients in the study demonstrated a lack of conjugated primary bile acids at baseline, similar to the children in our study. Oral GCA (15 mg/kg) was well tolerated and effectively incorporated into bile, with over 60% of biliary bile acids becoming conjugated and most of those as GCA. Treatment improved absorption of fat-soluble vitamins (D2, E) and promoted growth in prepubertal children with prior delays, demonstrating that GCA supplementation can restore key bile acid functions and support nutritional recovery in amidation defects[12]. While this study enrolled only children with genetically confirmed BAAT deficiency, GCA supplementation may hold promise beyond strictly defined genetic cases. Similarly, another review highlighted the multifaceted therapeutic potential of bile acid supplementation for treating components of metabolic syndrome[30]. Animal models demonstrated that combining probiotics with bile acids produced stronger symptom alleviation than either intervention alone. This likely stems from modulation of the gut microbiota, which in turn influences bile acid signaling pathways, specifically FXR and TGR5[30]. Overall, these works highlight the importance and foundational groundwork needed for future research into bile acid supplementation that may be of benefit for impoverished children.
The 100 6-9-month-old Bangladeshi children also demonstrated an inverse relationship with increased levels of secondary conjugated bile acids correlating with lower levels of SIBO AUC. Small intestinal bacterial overgrowth is already independently linked to growth stunting[6] but this study explores the potential role of SIBO on bile acid me
Our study had several notable strengths. First, the study conducted rigorous field collection with concomitant stool and serum samples. Second, we utilized an expanded bile acid profile to understand the accessory pathways being utilized in Bangladeshi children in the absence of effective primary bile acid conjugation. The inclusion of a wide range of bile acids in the analysis, including sulfated forms and muricholates, provides a more detailed understanding of bile acid metabolism and their implications. Finally, we sampled American children of similar age as a high-income country control.
This study also had several notable limitations that should be considered when interpreting its findings. First, as a cross-sectional study, it lacks longitudinal data on the enrolled children. Although we included a separate cohort of older children from the same impoverished neighborhood, these participants were not the same individuals as those in the younger cohort. This discrepancy limits our ability to assess individual-level influences or developmental trajectories in bile acid metabolism over time. Further, the original study did not exclude enrollment based on factors that could have influenced bile acid metabolism. Another key limitation is the relatively small sample size of the American cohort. While the comparison provides valuable insights, the limited size and diversity of the American group may not fully capture the heterogeneity present in a broader population. Also, as a cross-sectional study we are not able to infer causation but only detect association. Finally, results may not be representative of larger populations and diverse settings as the main participants were among rural Bangladeshi children. This highlights a broader gap in the literature, as few studies have attempted to characterize normal variations in bile acid metabolism across genetically and socioeconomically diverse pediatric populations at different developmental stages.
This study identified a correlation between conjugation defects and growth deficits in young Bangladeshi children. Further research is needed to better understand the impact of bile acid metabolic dysfunction in impoverished children from low-income countries and to explore potential avenues for novel interventions aimed at mitigating growth impairments.
Authors would like to acknowledge the children and families of Mirpur Dhaka without whom this research would not have been possible. ICDDR,B acknowledges its core donors, the Government of Bangladesh, and the Government of Canada for providing unrestricted support and commitment to ICDDR,B’s research effort.
| 1. | Korpe PS, Petri WA Jr. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 2. | Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut--a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 429] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 3. | Bartelt LA, Bolick DT, Guerrant RL. Disentangling Microbial Mediators of Malnutrition: Modeling Environmental Enteric Dysfunction. Cell Mol Gastroenterol Hepatol. 2019;7:692-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Tickell KD, Atlas HE, Walson JL. Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies. BMC Med. 2019;17:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Jimenez L, Duggan CP. Biomarkers of Environmental Enteric Dysfunction: The Good, the Bad, and the Ugly. J Pediatr Gastroenterol Nutr. 2017;65:4-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Donowitz JR, Pu Z, Lin Y, Alam M, Ferdous T, Shama T, Taniuchi M, Islam MO, Kabir M, Nayak U, Faruque ASG, Haque R, Ma JZ, Petri WA Jr. Small Intestine Bacterial Overgrowth in Bangladeshi Infants Is Associated With Growth Stunting in a Longitudinal Cohort. Am J Gastroenterol. 2022;117:167-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Donowitz JR, Haque R, Kirkpatrick BD, Alam M, Lu M, Kabir M, Kakon SH, Islam BZ, Afreen S, Musa A, Khan SS, Colgate ER, Carmolli MP, Ma JZ, Petri WA Jr. Small Intestine Bacterial Overgrowth and Environmental Enteropathy in Bangladeshi Children. mBio. 2016;7:e02102-e02115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Vonaesch P, Araújo JR, Gody JC, Mbecko JR, Sanke H, Andrianonimiadana L, Naharimanananirina T, Ningatoloum SN, Vondo SS, Gondje PB, Rodriguez-Pozo A, Rakotondrainipiana M, Kandou KJE, Nestoret A, Kapel N, Djorie SG, Finlay BB, Wegener Parfrey L, Collard JM, Randremanana RV, Sansonetti PJ; Afribiota Investigators. Stunted children display ectopic small intestinal colonization by oral bacteria, which cause lipid malabsorption in experimental models. Proc Natl Acad Sci U S A. 2022;119:e2209589119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 9. | Semba RD, Gonzalez-Freire M, Moaddel R, Trehan I, Maleta KM, Khadeer M, Ordiz MI, Ferrucci L, Manary MJ. Environmental Enteric Dysfunction Is Associated With Altered Bile Acid Metabolism. J Pediatr Gastroenterol Nutr. 2017;64:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Moreau GB, Ramakrishnan G, Cook HL, Fox TE, Nayak U, Ma JZ, Colgate ER, Kirkpatrick BD, Haque R, Petri WA Jr. Childhood growth and neurocognition are associated with distinct sets of metabolites. EBioMedicine. 2019;44:597-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Zhao X, Setchell KDR, Huang R, Mallawaarachchi I, Ehsan L, Dobrzykowski Iii E, Zhao J, Syed S, Ma JZ, Iqbal NT, Iqbal J, Sadiq K, Ahmed S, Haberman Y, Denson LA, Ali SA, Moore SR. Bile Acid Profiling Reveals Distinct Signatures in Undernourished Children with Environmental Enteric Dysfunction. J Nutr. 2021;151:3689-3700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Heubi JE, Setchell KD, Jha P, Buckley D, Zhang W, Rosenthal P, Potter C, Horslen S, Suskind D. Treatment of bile acid amidation defects with glycocholic acid. Hepatology. 2015;61:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Rosenberg IH, Hardison WG, Bull DM. Abnormal bile-salt patterns and intestinal bacterial overgrowth associated with malabsorption. N Engl J Med. 1967;276:1391-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 41] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Mehta HC, Saini AS, Singh H, Dhatt PS. Biochemical aspects of malabsorption in marasmus. Br J Nutr. 1984;51:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Schneider RE, Viteri FE. Luminal events of lipid absorption in protein-calorie malnourished children; relationship with nutritional recovery and diarrhea. I. Capacity of the duodenal content to achieve micellar solubilization of lipids. Am J Clin Nutr. 1974;27:777-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Zhang L, Voskuijl W, Mouzaki M, Groen AK, Alexander J, Bourdon C, Wang A, Versloot CJ, Di Giovanni V, Wanders RJ, Bandsma R. Impaired Bile Acid Homeostasis in Children with Severe Acute Malnutrition. PLoS One. 2016;11:e0155143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Musa MA, Kabir M, Hossain MI, Ahmed E, Siddique A, Rashid H, Mahfuz M, Mondal D, Ahmed T, Petri WA, Haque R. Measurement of intestinal permeability using lactulose and mannitol with conventional five hours and shortened two hours urine collection by two different methods: HPAE-PAD and LC-MSMS. PLoS One. 2019;14:e0220397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Tai YL, Zhao D, Zhang Y, Yan J, Kakiyama G, Wang X, Gurley EC, Liu J, Liu J, Liu J, Lai G, Hylemon PB, Pandak WM, Chen W, Zhou H. Berberine Prevents Disease Progression of Nonalcoholic Steatohepatitis through Modulating Multiple Pathways. Cells. 2021;10:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Fleishman JS, Kumar S. Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2024;9:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 221] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 20. | Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1268] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 21. | Marschall HU, Beuers U. When bile acids don't get amidated. Gastroenterology. 2013;144:870-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 1050] [Article Influence: 80.8] [Reference Citation Analysis (1)] |
| 23. | Lin S, Wang S, Wang P, Tang C, Wang Z, Chen L, Luo G, Chen H, Liu Y, Feng B, Wu D, Burrin DG, Fang Z. Bile acids and their receptors in regulation of gut health and diseases. Prog Lipid Res. 2023;89:101210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 76] [Reference Citation Analysis (0)] |
| 24. | Bhatt AP, Arnold JW, Awoniyi M, Sun S, Santiago VF, Quintela PH, Walsh K, Ngobeni R, Hansen B, Gulati A, Carroll IM, Azcarate-Peril MA, Fodor AA, Swann J, Bartelt LA. Giardia Antagonizes Beneficial Functions of Indigenous and Therapeutic Intestinal Bacteria during Malnutrition. bioRxiv. 2024;2024.01.22.575921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann Hepatol. 2017;16:S21-S26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 215] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 26. | Chibuye M, Mende DR, Spijker R, Simuyandi M, Luchen CC, Bosomprah S, Chilengi R, Schultsz C, Harris VC. Systematic review of associations between gut microbiome composition and stunting in under-five children. NPJ Biofilms Microbiomes. 2024;10:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 27. | Out C, Patankar JV, Doktorova M, Boesjes M, Bos T, de Boer S, Havinga R, Wolters H, Boverhof R, van Dijk TH, Smoczek A, Bleich A, Sachdev V, Kratky D, Kuipers F, Verkade HJ, Groen AK. Gut microbiota inhibit Asbt-dependent intestinal bile acid reabsorption via Gata4. J Hepatol. 2015;63:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. 2021;9:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 514] [Article Influence: 102.8] [Reference Citation Analysis (1)] |
| 29. | Setchell KD, Heubi JE, Shah S, Lavine JE, Suskind D, Al-Edreesi M, Potter C, Russell DW, O'Connell NC, Wolfe B, Jha P, Zhang W, Bove KE, Knisely AS, Hofmann AF, Rosenthal P, Bull LN. Genetic defects in bile acid conjugation cause fat-soluble vitamin deficiency. Gastroenterology. 2013;144:945-955.e6; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Ðanić M, Stanimirov B, Pavlović N, Goločorbin-Kon S, Al-Salami H, Stankov K, Mikov M. Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome. Front Pharmacol. 2018;9:1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 31. | Bala L, Ghoshal UC, Ghoshal U, Tripathi P, Misra A, Gowda GA, Khetrapal CL. Malabsorption syndrome with and without small intestinal bacterial overgrowth: a study on upper-gut aspirate using 1H NMR spectroscopy. Magn Reson Med. 2006;56:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/