Published online Nov 14, 2025. doi: 10.3748/wjg.v31.i42.111708
Revised: August 6, 2025
Accepted: October 11, 2025
Published online: November 14, 2025
Processing time: 124 Days and 16.5 Hours
Yiyi Fuzi Baijiang powder (YFB), a classic Chinese medicine, significantly affects ulcerative colitis (UC). However, it remains unclear whether YFB plays a thera
To explore the mechanisms of action of YFB in treating UC.
A mouse model of UC was established by drinking 2.5% dextran sulfate sodium (DSS). Mice were treated with YFB. 16S rDNA sequencing was used to detect changes in intestinal flora and perform functional predictions. Corresponding target genes of core active ingredients in YFB and UC were obtained using mul
YFB improved DSS-UC mice by restoring body weight, reducing disease activity index, increasing water and food intake, and alleviating diarrhea and local histopathological damage. YFB enhanced beta diversity, decreased pathogenic bacteria such as Turicibacter and Clostridium_sensu_stricto_1, and increased probiotics such as unclassified_f_Lachnospiraceae and Akkermansia. However, it also reduced anaerobic probiotics such as Ruminococcus, Enterorhabdus and Bifidobacterium. Network pharmacology identified 17 pathways, with cancer and adipocytokine signaling pathways showing significant differences in predicting intestinal microbial function. Molecular docking revealed that nuclear factor kappa-B inhibitor A, RELA and NFKB1, and colchamine, morusin and orotinin had docking scores > 5.0.
YFB treats UC by reducing harmful bacteria and boosting probiotics to restore intestinal balance, while potentially influencing signaling pathways.
Core Tip: The therapeutic application of Yiyi Fuzi Baijiang powder (YFB) for ulcerative colitis (UC) extends beyond merely altering the structure and abundance of gut microbiota. Instead, it aims to restore dynamic equilibrium by comprehensively modulating the intestinal microecological balance, thereby facilitating disease recovery. Furthermore, it is hypothesized that YFB powder may exert its effects on UC through potential mechanisms involving the NFKB1, RELA, and nuclear factor kappa B inhibitor alpha regulatory pathways, as well as the cancer and adipocytokine signaling pathways.
- Citation: Zhang LK, Gu WC, Chen J. Unveiling Yiyi Fuzi Baijiang powder: Microecological and network pharmacology approach to ulcerative colitis treatment. World J Gastroenterol 2025; 31(42): 111708
- URL: https://www.wjgnet.com/1007-9327/full/v31/i42/111708.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i42.111708
Ulcerative colitis (UC) affects approximately 0.5% of the global population, with rising incidence in newly industrialized nations[1]. Patients with UC face an 18% cumulative incidence of cancer over 30 years, while the overall risk of malignant transformation ranges from 1.4% to 34%[2]. Characterized by relapsing-remitting mucosal inflammation limited to the colon, UC presents clinically with bloody diarrhea, abdominal pain, fecal urgency, and weight loss. Histopathological hallmarks include crypt architectural distortion, neutrophilic infiltration, and ulceration[3,4]. Despite substantial advances in modern medicine concerning the diagnosis and treatment of UC, challenges persist due to prolonged treatment durations, high treatment costs, and suboptimal therapeutic responses, rendering it a persistent health issue[5]. In recent years, traditional Chinese herbs have garnered increased attention for UC treatment, owing to their distinct advantages, including multi-target therapeutic efficacy and multi-channel mechanisms of action[6]. Recent studies have indicated that traditional Chinese medicine (TCM) can mitigate the risk of intestinal diseases by modulating the intestinal flora and signaling pathways, alleviating intestinal injury and inflammatory responses, and repairing the intestinal mucosal barrier[7].
Yiyi Fuzi Baijiang powder (YFB) is a traditional formulation from the Jin Gui Yao Lue (Synopsis of Prescriptions of the Golden Chamber) authored by Zhang ZJ. This formulation comprises Coicis Semen, Aconiti Lateralis Radix Praeparata, and Herba Patriniae, and is traditionally used for treatment of carbuncles. In contemporary clinical practice, YFB has been widely applied in the management of UC, demonstrating substantial therapeutic efficacy. Basic research suggests that YFB exerts its therapeutic effects through several pathways[8]. Previous research conducted by our team demonstrated that the abundance of intestinal flora in UC model mice, induced by dextran sulfate sodium (DSS), exhibited dynamic changes[9]. This suggests that an imbalance in intestinal microecology is a crucial factor in the onset and progression of UC. Building on these findings, the present study used a multi-technology integration strategy that combined network pharmacology, intestinal microecology, and molecular linkage technology. This approach aimed to systematically analyze the active components and their targets in YFB for the treatment of UC, and to investigate in depth the mechanisms by which TCM exerts its therapeutic effects. The objective was to provide new insights into the multi-component/multi-target/multi-pathway synergistic mechanisms of TCM formulations, thereby offering a significant theoretical foundation and practical guidance for the development of novel treatment strategies and drugs for UC.
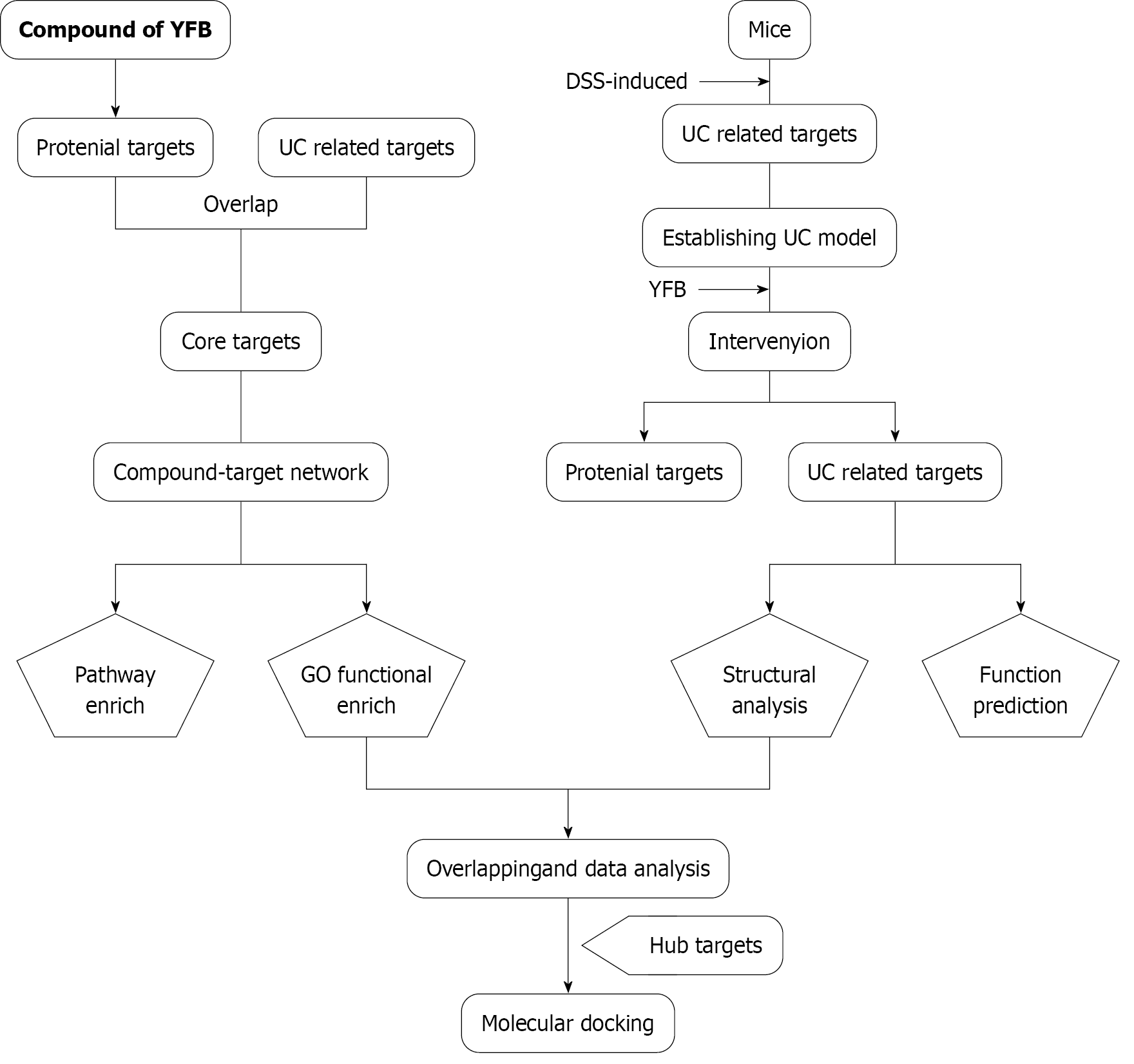
The technical strategy of this study is presented in Figure 1, and all databases, software, and tools used are listed in Supplementary Table 1.
Screening of active ingredients and target genes: The chemical constituents of YFB were collected from four databases [TCM systems pharmacology (TCMSP), TCM integrated database (TCMID), the encyclopedia of TCM (ETCM) and bioinformatics analysis tool for molecular mechanism of TCM (BATMAN-TCM)]. We obtained the potential targets of the compounds from Swiss target prediction and the UC-related targets from four databases [Common Technical Document (CTD), Online Mendelian Inheritance in Man (OMIM), Pharmacogenetics and Pharmacogenomics Knowledge Base (PharmGKB) and Therapeutic Target Database (TTD)]. The UniProt database compared the target information and gene name standardization. We selected common targets between the compounds and UC as the core targets.
Protein-protein interaction and network construction: Proteins form macromolecular complexes through interactions to perform biological functions. The core target genes of compounds and diseases were entered into STRING with a medium reliability of 0.4 to analyze the protein-protein interaction (PPI) between the target proteins. The Cytoscape software was used to visualize the networks. To determine the core targets of YFB for UC treatment, the obtained PPI network data were imported into Cytoscape 3.6.1 software to establish TCM-compound-target-disease for visualizing networks.
Enrichment analysis: We imported the screened core targets into the Database for Annotation Visualization and Integrated Discovery (DAVID) database to better understand the functions of the obtained core target genes and their roles in the signaling pathway. We used the functional annotation tool in the DAVID database to obtain results for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. The top 17 KEGG and GO results were screened into networks for further study and sorted according to the “false discovery rate” value.
Animals: C57BL/6J male mice (6 weeks old, 18-22 g) were procured from Beijing Weitong Lihua Experimental Animal Technology Co. Ltd. (SCXK 2016-0006). All animals were housed in a chamber at a temperature (22-24 °C) and humidity (50%-55%) with ample water and food. All the animal experiments were performed in strict accordance with the International Ethical Guidelines and National Institutes of Health Guide concerning the Care and Use of Laboratory Animals and with the approval of the Committee for Animal Ethics Committee of Shandong University of Traditional Chinese Medicine (No. SDUTCM20191015101).
Preparation of YFB powder: YFB, composed of Coicis Semen (Chinese name: Yiyiren), Aconiti Lateralis Radix Praeparata (Chinese name: Fuzi), Herba Patriniae (Chinese name: Baijiangcao), was acquired from Sanjiu Medical and Pharmaceutical Co. Ltd. The details of the drugs used are listed in Table 1. Three bags of Coicis Semen, two bags of Aconiti Lateralis Radix Praeparata, and one bag of Herba Patriniae granules were dissolved in 240 mL ultrapure water and stored in a refrigerator at 4 °C.
| Medicine | Serial number | Drug specifications |
| Coicis Semen | 1908005W | 2 g (equivalent to 15 g decoction pieces) |
| Aconiti Lateralis Radix Praeparata | 1808001C | 1 g (equivalent to 3 g decoction pieces) |
| Herba Patriniae | 1904004C | 1 g (equivalent to 15 g decoction pieces) |
Experimental design: The mice were randomly divided into three groups of 10: Blank control (Con) group, model (Mod) group, and YFB group. The mice in the Con group were given normal drinking water, and the others were free to drink 2.5% DSS aqueous solution for seven consecutive days to develop UC. After the UC model was established, mice in the Con and Mod groups were administered ultrapure water, whereas mice in the YFB group were administered YFB via gastric gavage once daily.
Biological data and sample collection: All mice were weighed daily from the first to the last day of the experiment, and their weights, general conditions, and deaths were recorded daily. In addition to body weight, we recorded stool viscosity and hematochezia status on days 0, 7, 14 and 21 to calculate the disease activity index (DAI) score (Supplementary Table 2). On day 21, we collected serum, colon tissue (proximal, mid and distal), and stool samples from the mice. Proximal and distal colon tissues were fixed with 40 g/L paraformaldehyde for histological evaluation using hematoxylin-eosin (HE) staining. All other tissues were placed in a refrigerator at -80 °C, where serum and mid-colon samples were used to detect inflammatory factors by enzyme-linked immunosorbent assay, and stool samples were collected for 16S rDNA se
Histological evaluation: The proximal and distal sections of the colon were embedded in paraffin, cut into 5-μm sections, and stained with HE according to standard protocols. The sections were visualized under a microscope, photographed, and viewed at a final magnification of 200 using a Leica Application Suite/Leica DM5000B. The histopathological scores (Supplementary Table 3) were independently evaluated by two double-blind pathologists based on the colon. Discrepant results were adjudicated by a third experienced pathologist.
16S rDNA sequencing and gut microbiota analysis: DNA extraction of colon contents was performed using the E.Z.N.A.® soil DNA Kit (Omega Biotek, Norcross, GA, United States). The DNA concentration and purification levels were determined using a NanoDrop 2000 ultraviolet-visible spectrophotometer (Thermo Scientific, Wilmington, DE, United States). The V3-V4 hypervariable regions of the 16S rRNA gene were amplified with the primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) using a thermocycler polymerase chain reaction (PCR) system (GeneAmp 9700, ABI, United States). The resulting PCR products were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States), quantified using QuantiFluor™-ST (Promega, Madison, WI, United States), and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, CA, United States) according to the standard protocols of Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). Trimmomatic software was used to control the quality of the original sequencing sequence, and FLASH software was used to splice. Operational taxonomic units (OTUs) were clustered with a 97% similarity cut-off using UPARSE, and chimeric sequences were identified and removed using UCHIME. The taxonomy of each 16S rRNA gene sequence was analyzed using the Ribosomal Database Project classifier algorithm against the 16S SILVA database (Silva 138, Bremen, Germany) using a confidence threshold of 70%. KEGG functional analysis of intestinal microbes was performed using the PICRUSt2 software package.
Functional prediction of the gut microbiota and network pharmacology enrichment analysis of each obtained pathway overlapped to produce a common pathway. The abundance values of these common pathways were statistically analyzed to identify the pathways with a significant difference. The core target genes involved in the predicted common pathways and network pharmacology were searched in the Protein Data Bank database. Mol2 format files of the YFB key active ingredients were downloaded from the TCMSP platform. AutoDock Tools 1.5.6 software was used to process proteins as follows: Separating proteins, adding hydrogen, computing Gasteiger, assigning AD4 type, and setting all flexible bonds of small molecule ligands to be rotatable. The docking box was adjusted according to the original ligand coordinates to include all protein structures. Meanwhile, the receptor protein was set to rigid docking, the genetic algorithm was selected, and the maximum number was set as the medium. Docking results were obtained by running Autogrid 4 and AutoDock 4, and the binding energies were determined. A partial diagram of molecular docking was then generated using PyMol software.
Statistical analyses were performed using GraphPad Prism 9.8 software and the R statistical programming language. All experimental data are presented as mean ± SD. Intergroup comparisons between two groups were conducted using unpaired t-tests, while comparisons among three were assessed by one-way analysis of variance. P < 0.05 indicated statistical significance.
Screening of active ingredients and target genes: For the constituents of the three herbs in YFB, we collected 155 compounds in TCMSP, 169 in TCMID, 40 in ETCM, and 76 in BATMAN-TCM. We obtained 258 constituents from YFB without duplicate compounds (Table 2). Then, 258 chemical components were uploaded to Swiss target prediction to obtain the potential targets. Finally, we removed the components that did not predict the target, and the predicted value of the target was 0; 195 effective components were obtained (Supplementary Table 4), and 1052 nonrepetitive targets (Supplementary Table 5) were obtained after UniProt unification.
| Herbs | TCMSP | TCMID | ETCM | BATMAN-TCM |
| Coicis Semen | 38 | 52 | 0 | 3 |
| Aconiti Lateralis Radix Praeparata | 65 | 101 | 38 | 58 |
| Herba Patriniae | 52 | 16 | 2 | 15 |
| Union | 155 | 169 | 40 | 76 |
Potential targets of UC disease and differential gene screening: We collected potential UC-related targets in CTD, OMIM, pharmGKB and TTD and obtained 59 key genes in TTD, 182 in OMIM, 14 in pharmGKB and 34 in TTD. After UniProt unification, 258 genes or potential targets related to UC, without repetitive genes, were identified (Supplemen
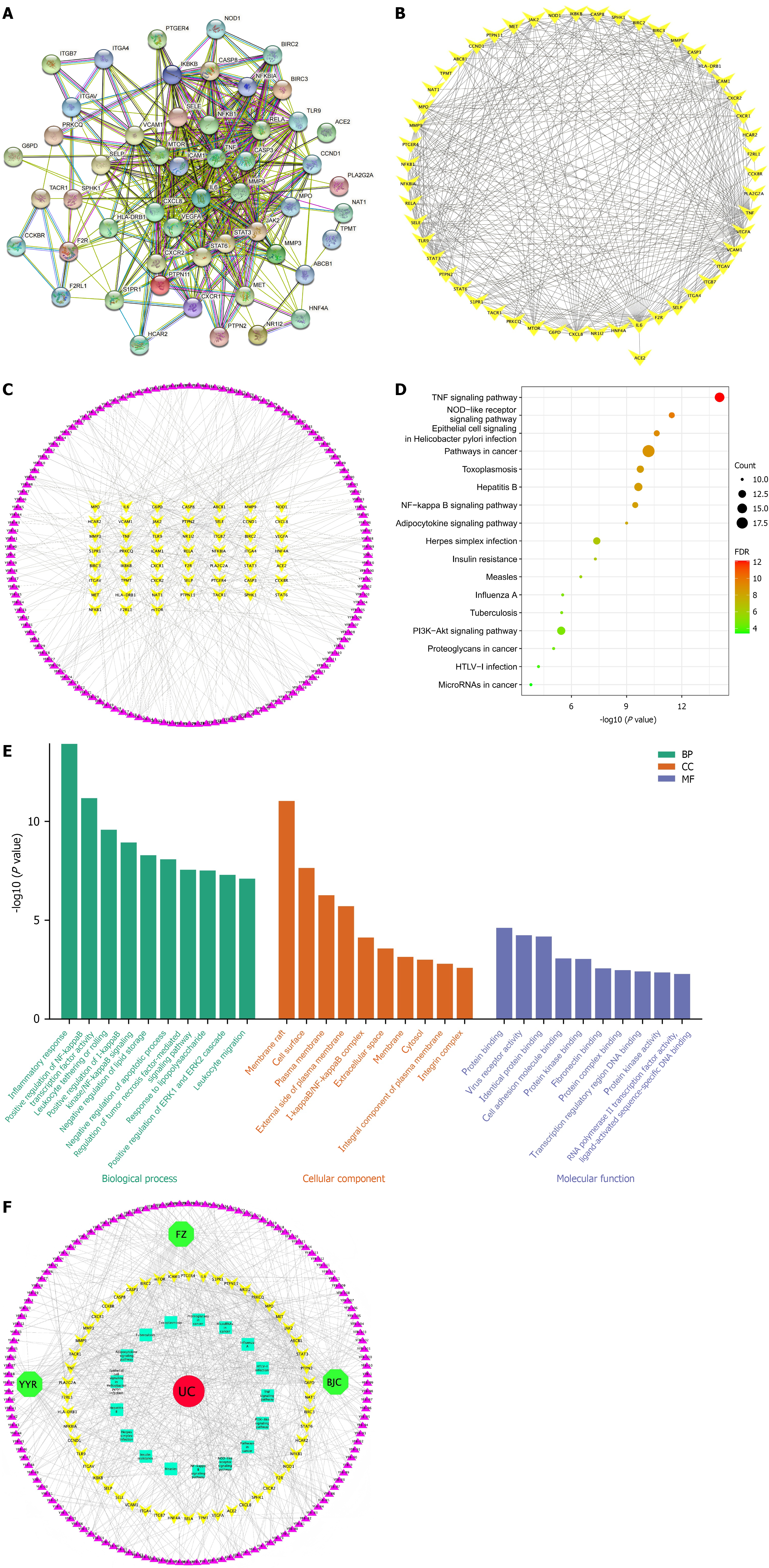
Network visualization analysis: We constructed a PPI network of 52 common genes using STRING and Cytoscape software (Figure 2A and B). The PPI network comprised 52 nodes and 396 edges. The average node degree of the network was 15.2, and the median degree of the network was 14. Interleukin (IL)-6 (degree 42), VEGFA (degree 37), tumor necrosis factor (TNF) (degree 36), CXCL8 (degree 34) and signal transducer and activator of transcription 3 (STAT3) (degree 32) were the top five with high degree and betweenness centrality in the PPI network.
To investigate the active components of YFB anti-UC, a compound-target network was constructed for 52 common genes and 132 related components using Cytoscape. The compound-target network was composed of 184 nodes and 395 edges (Figure 2C). The top three targets were G6PD (degree 29), PRKCQ (degree 28) and ABCB1 (degree 26). 2,7-Dideacetyl-2,7-dibenzoyl-taxayunnanine f (YFB-15), neokadsuranic acid b (YFB-98), ferulic acid (FER) (YFB-61) and karanjin (YFB-80) were the top four compounds, and their serial numbers are listed in Supplementary Table 8.
Pathway enrichment: The findings of pathway enrichment by DAVID revealed that the top 17 pathways (Figure 2D) (P < 0.05, number of common targets contained in the pathway was > 10, ranked from small to large) included pathways in cancer, TNF signaling pathway, hepatitis B, phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway, toxoplasmosis, herpes simplex infection, nucleotide-binding oligomer-zation domain-like receptor signaling pathway, epithelial cell signaling in Helicobacter pylori infection, nuclear factor kappa-B (NF-κB) signaling pathway, adipocytokine signaling pathway, insulin resistance, measles, influenza A, tuberculosis, proteoglycans in cancer, human T-lymphotropic virus-I infection, and microRNAs in cancer (Supplementary Table 9).
GO functional enrichment: GO functional enrichment analysis yielded 245 GO results (Supplementary Table 10), including 20 cell composition (CC), 193 biological processes (BP) and 32 molecular function (MF) results, accounting for 8.16%, 78.78% and 13.06%, respectively. The top-ranked GO enrichment pathways included the inflammatory response, positive regulation of NF-κB transcription factor activity, leukocyte tethering or rolling, positive regulation of inhibitor of NF-κB (IkB) kinase/NF-κB signaling, and negative regulation of lipid storage. The top 10 BP, CC and MF results in GO annotation analysis according to the P value were visualized separately using the R software package (Figure 2E).
Network construction and analysis: Cytoscape 3.8.1 software was used to draw the TCM-compound-target-pathway-disease (Figure 2F). The TCM compound-target-pathway-disease network comprised 205 nodes (three medicinal material nodes, 132 compound nodes, 52 target nodes, 17 pathway nodes, and one disease node) and 785 edges. These interactions indicated that one compound could modulate many targets and that one target could be modulated by multiple compounds simultaneously.
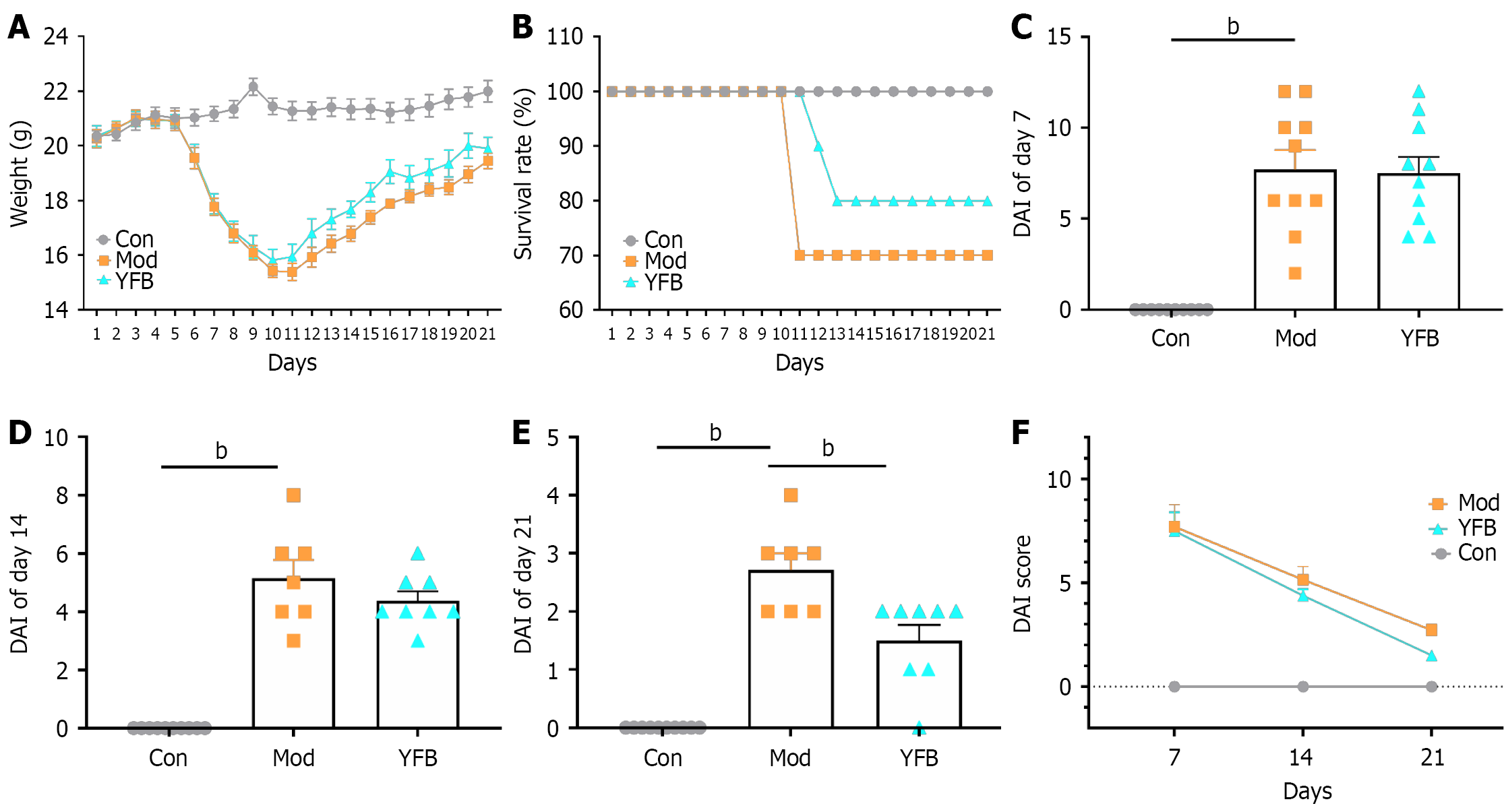
YFB powder ameliorates colitis induced by DSS: Mice in the Con group were in good condition throughout the study. However, the body weight of the mice decreased significantly after DSS i10-1duction. It tended to increase after YFB intervention (Figure 3A). Three mice in the Mod group and two in the YDB group died during treatment (Figure 3B). DSS also significantly increased DAI. In contrast, YFB improved this phenomenon on day 7 of intervention and significantly reduced DAI on day 14 (Figure 3C-F).
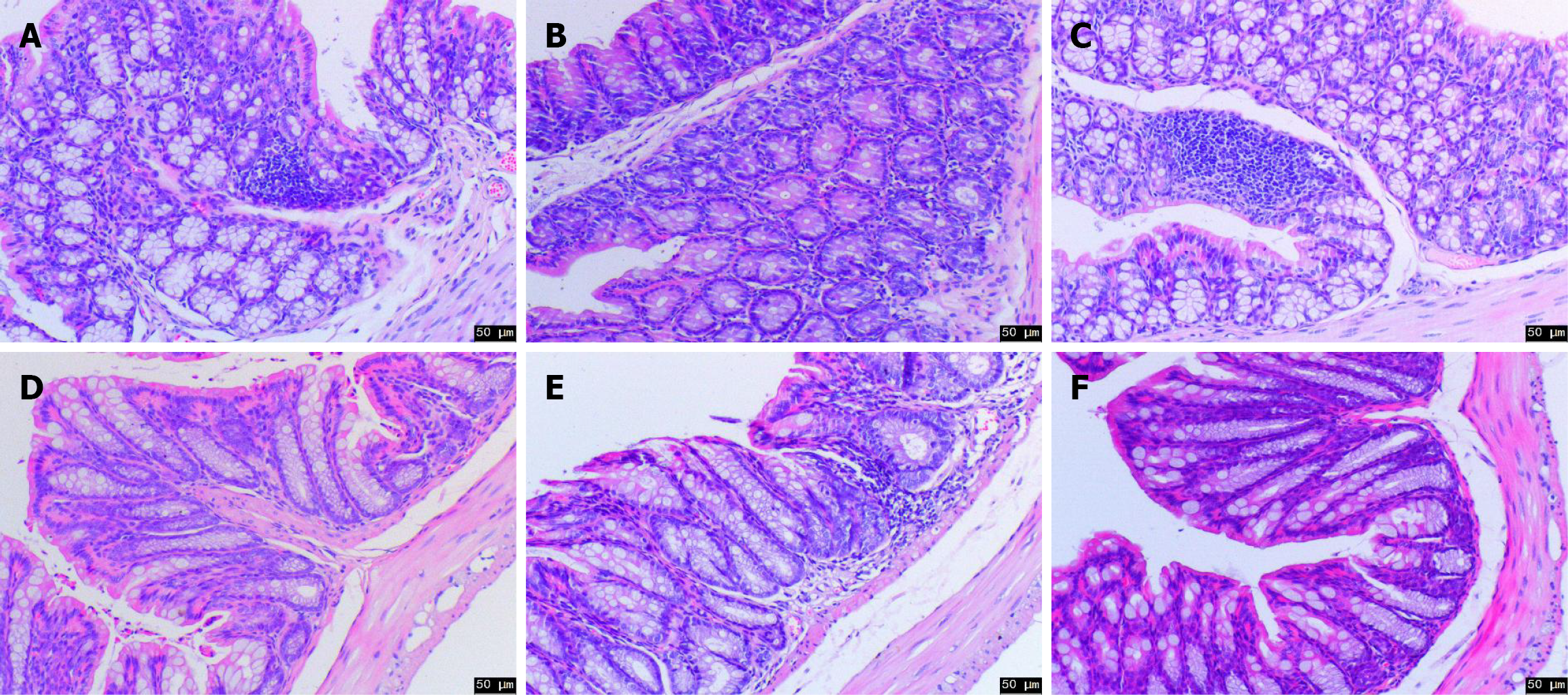
Histopathological changes: The pathological results indicated that the proximal colon of mice in the Con group was tightly arranged, the crypt structure was normal, and glandular goblet cells were tightly arranged. Conversely, the crypt structure of the Mod group was branched and distorted, and the number of goblet cells was reduced. However, the cell arrangement was normal after drug intervention, the branch of the crypt decreased, and the structure tended to be normal (Figure 4A-C). Simultaneously, the distal pathological findings revealed that the tissues of mice in the Con group were neatly structured and tightly arranged. However, the number of goblet cells in the Mod group was significantly reduced, and inflammatory infiltration was evident. After drug intervention, the epithelial cell structure gradually returned to normal, and the goblet cells were tightly arranged and tended to be normal (Figure 4D-F). These findings provide important evidence for improving the effectiveness of YFB in UC treatment.
OTU sequence analysis: Illumina sequencing of the V3 and V4 regions of the 16S rRNA gene produced sequences in each OTU that were counted to obtain taxonomic information about the OTU for all fecal samples from surviving mice at different time points at a similarity level of 97%. To conduct OTU diversity analysis, all 15 microbiome samples were subsampled to a 900616 sequence and 422 average length reads. The reads included 377 OTUs belonging to 10 phyla, 13 classes, 29 orders, 46 families, 96 genera and 144 species.
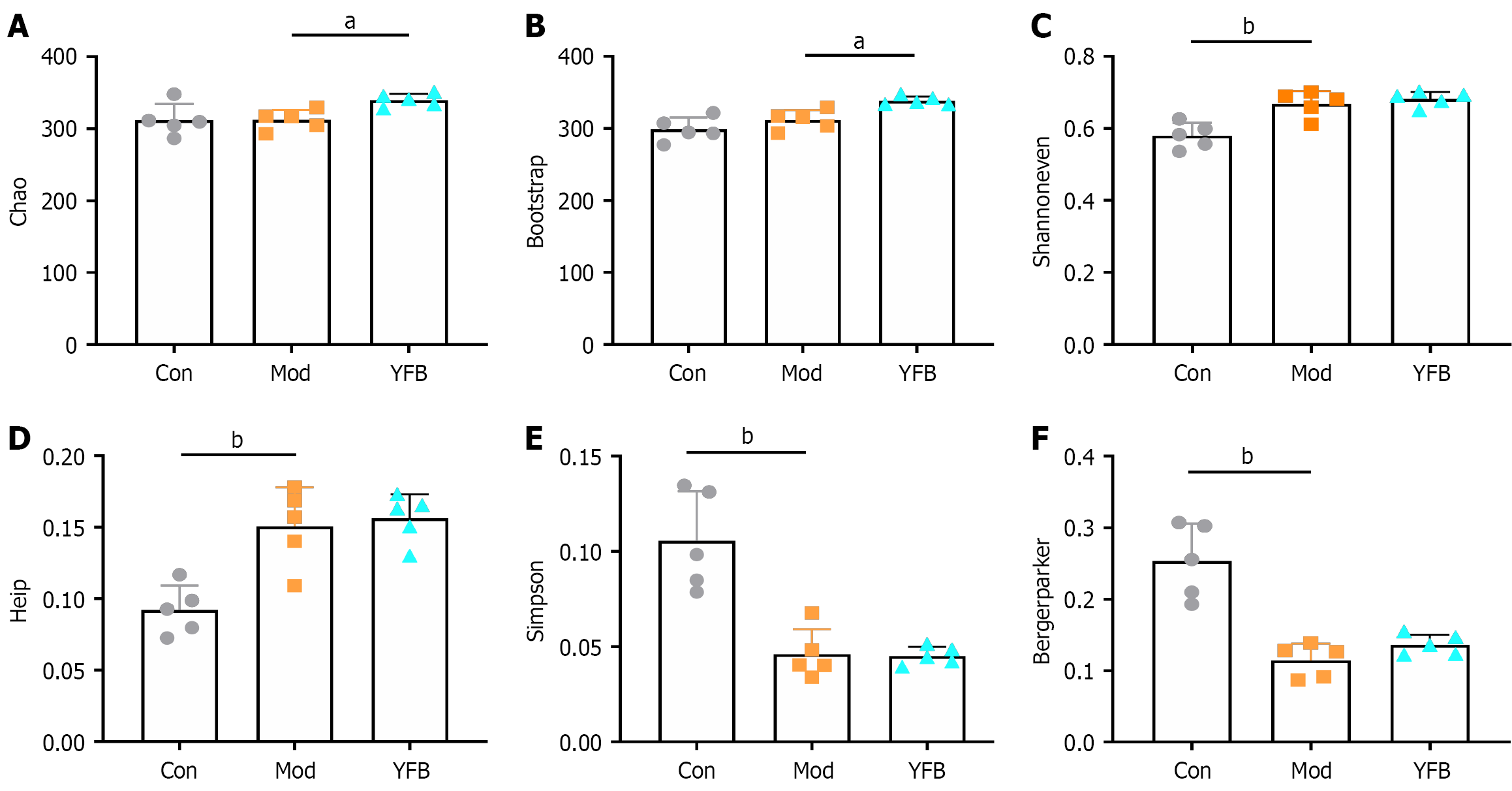
Alpha diversity analysis: Alpha diversity is primarily used in the diversity analysis of a single sample to reflect community richness, evenness, diversity and coverage of the microbiota community. In this study, the coverage index was > 0.998, indicating that > 99.8% of the OTUs were identified and analyzed, implying that the detection rate of the sample sequence was high. After DSS-induced modeling, the chao and bootstrap indices of the Mod group did not change significantly compared with those of the Con group. However, the shannoneven and heip indices were significantly increased, and the Simpson and bergerparker indices were significantly reduced. After the intervention, the chao and bootstrap indices increased significantly, but the shannoneven, heip, Simpson and bergerparker indices did not. This demonstrated that the diversity of the gut microbiota of UC mice was reduced and evenness was increased, whereas YFB increased the abundance of model mice (Figure 5).
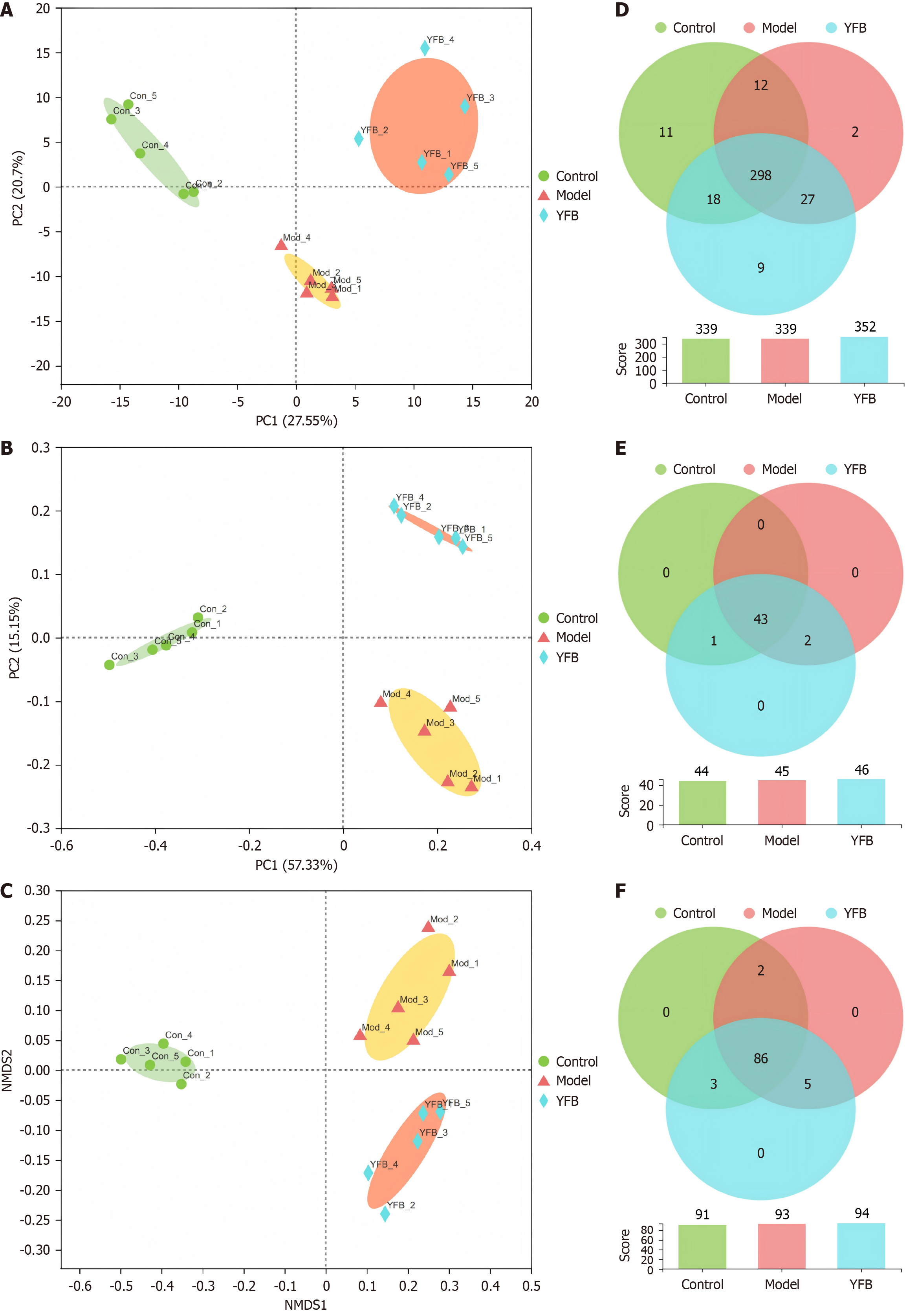
Beta diversity analysis: To investigate the influence of YFB on the overall profile of the gut microbiota in DSS-UC model mice, we performed principal component analysis (PCA), principal coordinate analysis (PCoA) and nonmetric multidimensional scale analysis (NMDS) based on the OTU results to demonstrate the degree of similarity or difference in community composition in different samples. The Con and Mod groups were separated into PCA, PCoA and NMDS (Figure 6A-C). The YFB group was distributed between the Con and Mod groups, which separated from the Mod group, indicating that beta diversity analysis was superior. Significant differences were observed in the structures of the flora groups.
Venn analysis: Venn analysis can count the number of common and unique species in multiple groups or samples. It can more intuitively illustrate the similarity and overlap of the species composition of environmental samples. At the OTU level (Figure 6D), 298 OTUs were shared by the three groups, 18 were shared by the control and YFB groups, and nine were unique to the YFB group at the family level (Figure 6E). There were 43 phyla shared by the three groups and one species shared by the Con and YFB groups (f_Aerococcaceae). At the genus level (Figure 6F), 86 were shared by the three groups, and three species were shared by the Con and YFB groups (g_UCG-009, g_Prevotellaceae_NK3B31_group, and g_Aerococcus). This demonstrated that after DSS-UC, the structure of the gut microbiota in model mice changed, and after intervention, the structure tended to recover.
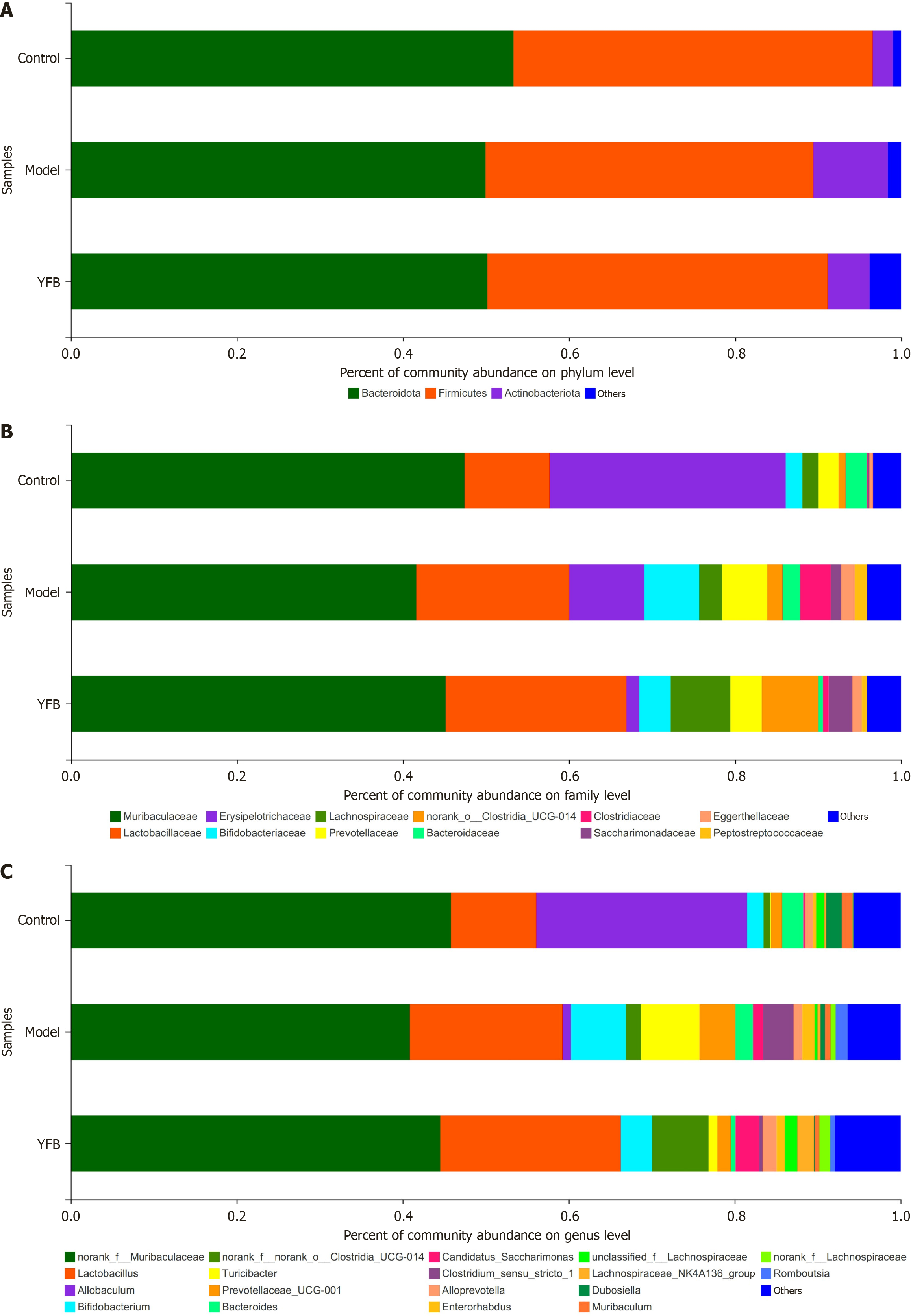
Species composition analysis: The Ribosomal Database Project classifier algorithm was used to obtain fecal samples of each group by comparing the Silva 16S rRNA database taxonomic information of the flora. At the phylum level (Figure 7A), the gut microbiota of each group of mice was primarily distributed across three phyla: Bacteroidota, Firmicutes and Actinobacteriota. Bacteroidota and Firmicutes accounted for > 80% of the mice in each group. At the family level (Figure 7B), 17 were dominant, with a relative abundance > 1%, among which the top two abundances were f_Muribaculaceae and f_Lactobacillaceae. The abundance of the dominant family in the gut microbiota of each group of mice was > 70%. Concurrently, 24 genera were dominant, with a relative abundance of > 1% at the genus level; among which, the top five abundances were g_norank_f_Muribaculaceae, g_Lactobacillus, g_Allobaculum, g_Bifidobacterium, and g_norank_f_no
| OTU ID | Control | Model | YFB |
| p_Bacteroidota | 0.5330 ± 0.0916 | 0.4995 ± 0.1713 | 0.5017 ± 0.1307 |
| f_Muribaculaceae | 0.4743 ± 0.0825 | 0.4163 ± 0.1403 | 0.4515 ± 0.1056 |
| g_norank_f_Muribaculaceae | 0.4585 ± 0.0771 | 0.4087 ± 0.1352 | 0.4452 ± 0.1033 |
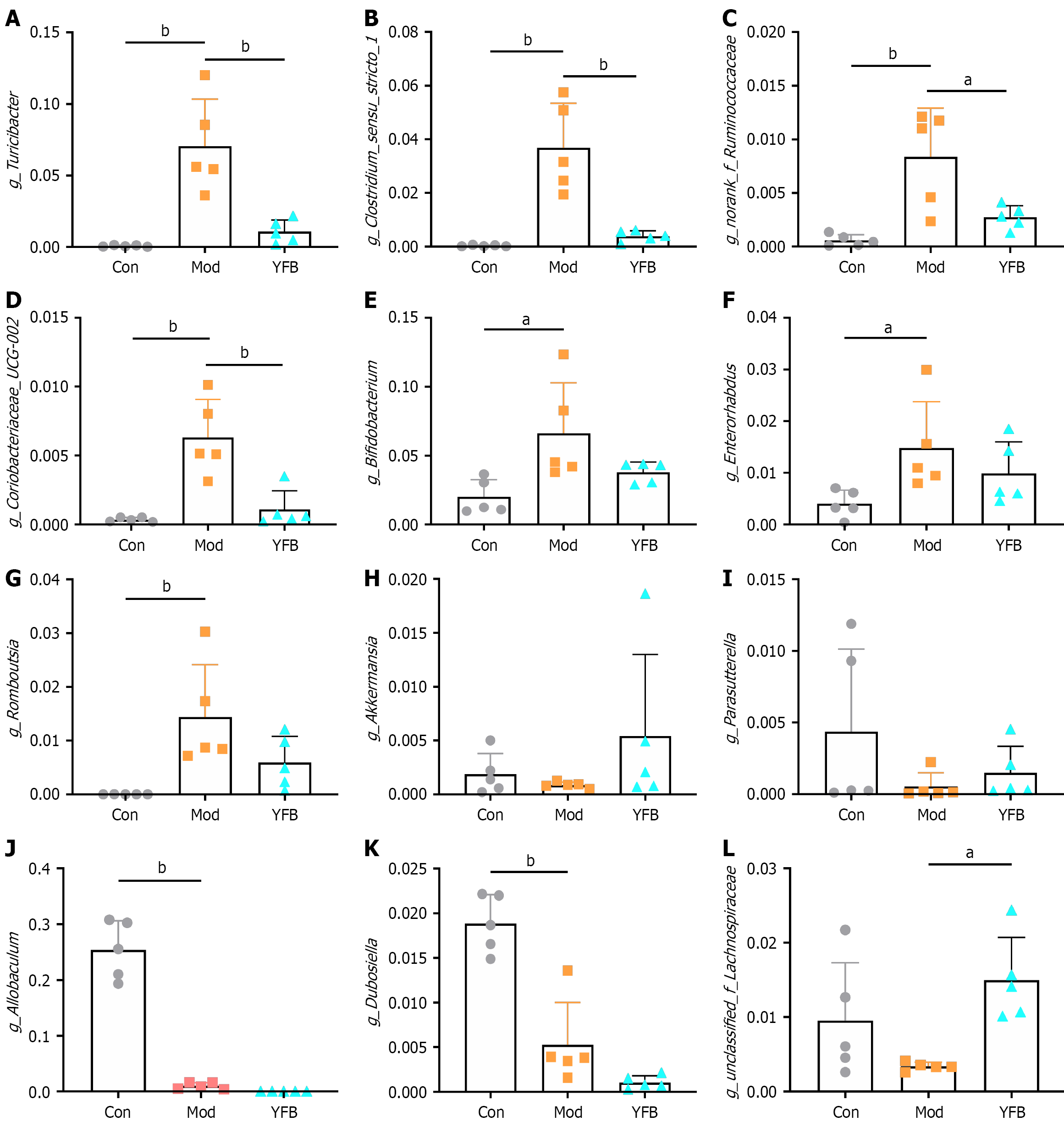
Differential strain analysis: To explore the changes in the structure of the gut microbiota of DSS-UC model mice and the impact of YFB treatment, we analyzed all samples at the genus level based on the OTU annotation information obtained by 16S rRNA sequencing. Compared with the Con group, the abundance of g_Turicibacter, g_Clostridium_sensu_stricto_1, g_norank_f_Ruminococcaceae, g_Coriobacteriaceae_UCG-002, g_Bifidobacterium, g_Enterorhabdus and g_Romboutsia in the Mod group increased significantly. After YFB intervention, g_Turicibacter, g_Clostridium_sensu_stricto_1, g_norank_f_Ruminococcaceae and g_Coriobacteriaceae_UCG-002 decreased significantly, and g_Bifidobacterium, g_Enterorhabdus and g_Romboutsia exhibited a decreasing trend, but the difference was not significant (Figure 8A-G). Compared with the Con group, the abundance of g_Akkermansia and g_Parasutterella exhibited a decreasing trend, which increased after drug intervention. However, the difference was not significant. The abundance of g_Allobaculum and g_Dubosiella decreased significantly after DSS induction, but the difference was not significant (Figure 8H-K). However, the abundance of g_unclassified_f_La
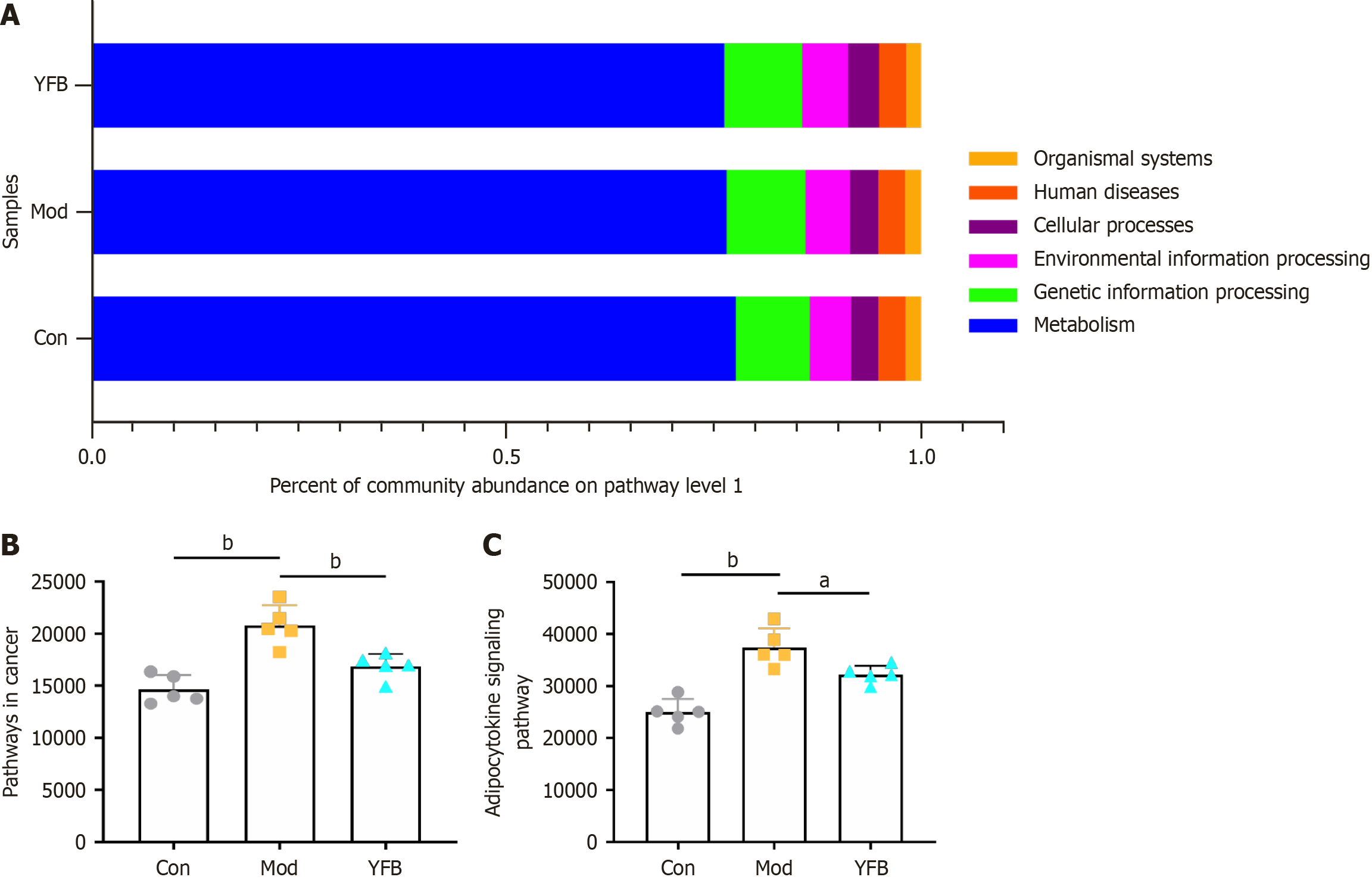
Pathways prediction results: Using the KEGG database to obtain a functional overview of the gut microbiota of different groups of mice at pathway level 1, we identified a total of six categories: Metabolism, genetic information processing, environmental information processing, cellular processes, human diseases, and organic systems (Figure 9A). At pathway level 3283 functions and their abundance in each group were obtained.
Determination of molecular docking targets: The gut microbiota function prediction and pathways obtained by network pharmacology enrichment analysis were checked, and 42 shared pathways were identified. The abundance value of shared pathways was significant in pathways with > 10 shared targets. Compared with the Con group, pathways in cancer and adipocytokine signaling pathways in the Mod group were significantly increased, whereas they were significantly decreased after drug intervention (Figure 9B and C). Consequently, we further overlapped the common targets in the two pathways to obtain NF-κB inhibitor alpha (NFKBIA), RELA, IkB kinase subunit beta (IKBKB), mammalian target of rapamycin (mTOR), NFKB1, and STAT3 proteins, and 38 compounds were obtained from YFB. Four compounds (colchamine, FER, morusin and orotinin) had more than two common targets. AutoDock tools software was used to perform molecular docking analysis of four compounds (colchamine, FER, morusin and orotinin) in YFB with NFKBIA, RELA, IKBKB, mTOR, NFKB1 and STAT3 proteins.
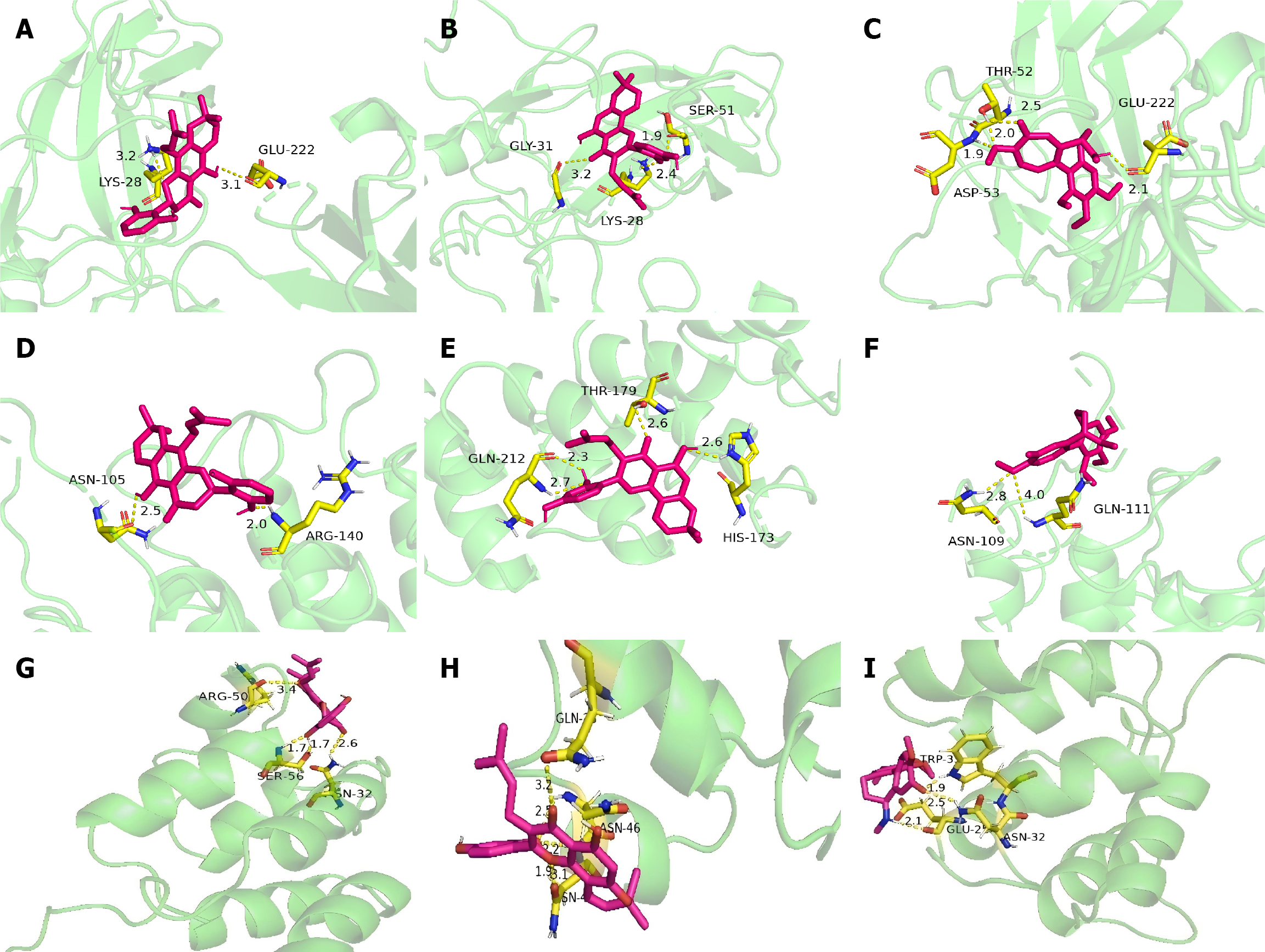
Analysis of molecular docking results: Molecular docking revealed that most of the active compounds in YFB had good binding activities with NFKBIA, RELA, IKBKB, mTOR, NFKB1 and STAT3 proteins. Table 4 presents the docking fractions of the compounds and proteins. The molecular docking of NFKB1, RELA and NFKBIA with an absolute score > 5 was colchamine, morusin and orotinin (Figure 10). Conversely, the absolute values of the molecular docking scores of other proteins and compounds were < 5, detailed information is provided in Supplementary Table 11.
| Target | Compound | Affinity (kcal/mol) |
| NFKB1 | Colchamine | -5.72 |
| FER | -4.03 | |
| Morusin | -5.56 | |
| Orotinin | -5.87 | |
| RELA | Colchamine | -6.37 |
| FER | -4.85 | |
| Morusin | -5.04 | |
| Orotinin | -5.2 | |
| IKBKB | Colchamine | -3.26 |
| FER | -2.65 | |
| Morusin | -2.48 | |
| Orotinin | -3.24 | |
| mTOR | Colchamine | -4.36 |
| FER | -4.46 | |
| Morusin | -4.08 | |
| Orotinin | -4.47 | |
| NFKBIA | Colchamine | -6.49 |
| FER | -4.32 | |
| Morusin | -6.0 | |
| Orotinin | -5.88 | |
| STAT3 | Colchamine | -3.13 |
| FER | -2.45 | |
| Morusin | -3.27 | |
| Orotinin | -3.33 |
In recent years, the gut microbiota has become a topic of interest in TCM research. Studies have demonstrated that changes in intestinal microecology are a significant contributor to the pathogenesis of UC[10,11]. Previous studies reported that the relative abundance of harmful bacteria, such as Clostridiaceae and Turicibacter in the intestines of patients with UC increased significantly, whereas the relative abundance of beneficial bacteria, including Prevotellacea, Lachno
YFB powder, derived from Zhongjing’s Jin Gui Yao Lue, has been used in the diagnosis and treatment of UC with notable therapeutic outcomes. The mechanism of action involves the upregulation of superoxide dismutase (SOD), nuclear respiratory factor 2, and heme oxygenase 1 protein expression, which collectively ameliorate oxidative stress in the intestinal tract. YFB also downregulates proinflammatory cytokines such as TNF-α, IL-1β and IL-17, while upregulating the anti-inflammatory cytokine IL-10, thereby enhancing mucosal permeability and alleviating local inflammation in the colon[15]. Research indicates that YFB alters gut microbiota composition by reducing the relative abundance of Bacteroides and Erysipelotrichaceae, while increasing Lactobacillus, Bifidobacterium, and other beneficial probiotics, thereby contributing to the treatment of intestinal adenomas and diabetes.
The results indicated that YFB significantly restored body weight, reduced DAI, increased the amount of water and diet, relieved diarrhea and blood in stools, and improved the overall state of DSS-UC model mice. YFB powder significantly reduced edema and congestion. Yiyiren extract effectively inhibits histamine release and IL-31 production, suppresses mast cell activity, and reduces nitric oxide, highlighting its anti-inflammatory properties. It also modulates gut microbiota, reduces IL-6, increases IL-10, and aids in repairing mucosal damage, alleviating UC symptoms[16,17]. Processed Fuzi is one of the most commonly used drugs for the treatment of UC, which functions by regulating the immune system, inhibiting cell apoptosis, and resisting vascular endothelial damage[18-21]. Baijiangcao has a broad-spectrum antibacterial effect that inhibits the effects of typhoid bacilli, Escherichia coli and Staphylococcus aureus. Its ethanolic extract and volatile oils have clear sedative effects. Recent studies have demonstrated that total saponins can enhance SOD expression in local diseased tissues in patients with UC, reduce myeloperoxidase (MPO) activity, scavenge oxygen free radicals, reduce IL-1β and TNF-α levels, and alleviate inflammatory infiltration to treat UC[22,23]. In recent years, research on gut microbiota has emerged as a focal point in understanding the pathogenesis and treatment of UC[24,25]. The maintenance of a dynamic equilibrium within the intestinal microecology is crucial for sustaining human health, and its disruption can precipitate the onset and progression of various disease[26,27]. Lactic acid, produced by Turicibacter, plays a crucial role in modulating the inflammatory response in inflamed tissues, as evidenced by its positive correlation with proinflammatory cytokines, including IL-1β, IL-6 and TNF-α[28]. YFB may attenuate local inflammation in the colon of DSS-UC model mice and safeguard the intestinal mucosa by reducing the relative abundance of Turicibacter and its lactic acid production. Clostridium sensu stricto 1 is commonly linked to heightened intestinal inflammation and a notable decrease in oxygen levels in mouse models induced with DSS[29]. Contrary to expectations, our observations revealed a significant increase in the relative abundance of Clostridium sensu stricto 1 following DSS induction. As an anaerobic pathogen, Clostridium sensu stricto 1 thrives under these conditions; the DSS intervention exacerbates intestinal inflammation, leading to reduced intestinal oxygen content and consequently promoting the proliferation of anaerobic bacteria. Unclassified_f_Lachnospiraceae, a prevalent probiotic bacterium, is known for its production of short-chain fatty acids (SCFAs)[30]. SCFAs are metabolites derived from intestinal microorganisms, with acetic, propionic and butyric acids being the primary constituents[31]. Butyric acid serves as the principal energy source for colonic epithelial cells and plays a crucial role in preventing the release of inflammatory mediators. It maintains the integrity of the intestinal epithelial barrier by facilitating the absorption of various electrolytes and promoting the production of antimicrobial peptides[32]. The findings suggest that YFB can effectively ameliorate UC by counteracting the DSS-induced reduction in the abundance of unclassified_f_Lachnospiraceae, enhancing SCFA levels in the intestine, promoting epithelial barrier integrity, inhibiting inflammatory factor release, and modulating the local inflammatory response. Akkermansia, a gram-negative bacterium, is a probiotic that protects the integrity of intestinal epithelial cells and regulates T regulatory cells and nonclassical Toll-like receptors by inducing FOXP3[33]. YFB can reduce the symptoms of UC by increasing its relative abundance to exert anti-inflammatory effects. In summary, YFB powder may be used to improve UC by reducing the relative abundance of harmful bacteria Turicibacter and Clostridium_sensu_stricto_1 and increasing the relative abundance of probiotics unclassified_f_Lachnospiraceae and Akkermansia. Bifidobacterium, a common probiotic, is commonly used for the microbiota treatment of UC. It inhibits the reproduction of spoilage and pathogenic bacteria, improves the permeability of the intestinal mucosa, enhances the defense ability of the intestine, and induces an immunoregulatory effect[34]. Similarly, studies have demonstrated a positive correlation between the relative abundance of the intestinal bacteria Ruminococcus and Enterorhabdus and tryptophan content in humans[35,36]. Under the control of intestinal microorganisms, tryptophan is metabolized and decomposed into indole and indole acid derivatives, which further activate the intestinal immune system, protect the intestinal epithelial barrier, and exert an anti-inflammatory effect, thereby promoting the recovery of UC[37].
However, it is worth noting that the relative abundance of the probiotics Ruminococcus, Enterorhabdus and Bifidobacterium increased in DSS-UC model mice and significantly decreased after intervention with YFB powder in this study, which is different from the findings of previous studies[38,39]. In-depth analysis revealed that the probiotics Rumi
We explored the possible mechanism through combined gut microbiota function prediction and network pharmacology. Through network pharmacology research, we analyzed 52 core targets obtained by overlapping the targets predicted by YFB powder with those associated with UC. Date demonstrate that they contained core targets ≥ 10 and predicted 17 associated pathways. Studies have demonstrated that UC can be treated by NF-κB regulation and PI3K/Akt signaling pathway, which is consistent with the conclusion of existing studies[42-44]. Critically, the constructed TCM-compound-target-disease interaction network, consisting of 205 nodes (132 compounds, 52 targets and 17 pathways) and 785 edges, elucidated the distinctive polypharmacological properties of YFB. Firstly, the high network connectivity indicated the synergistic modulation of UC-related targets by multiple compounds, exemplified by the association of colchamine with 29 targets. Secondly, pathway convergence, as demonstrated through KEGG enrichment analysis, revealed that diverse compounds co-regulated core pathways associated with UC, such as the NF-κB signaling pathway, which was simultaneously targeted by morusin, orotinin and colchamine. This systems-level analysis substantiates multi-component, multi-target and multi-pathway mechanism of YFB; a characteristic feature of traditional herbal formulations used for complex diseases like UC.
By overlapping the prediction of gut microbiota function with network pharmacology function during data analysis, we identified that cancer and adipocytokine signaling pathways exhibited significant differences in predicting gut microbiota function. This phenomenon can be mechanistically elucidated through two well-established facts: (1) YFB has demonstrated efficacy against various cancers by targeting the same biological pathways[45]; and (2) UC is a well-documented precursor to colorectal cancer (CRC), with chronic inflammation driving genomic instability and carcinogenesis via sustained activation of pathways such as NF-κB, TNF and STAT3[46,47]. Our identified core targets are shared regulators of both UC inflammation and CRC progression, explaining the enrichment of cancer-related pathways. The enrichment of cancer pathways should not be misinterpreted as off-target effects. Instead, it underscores the potential of YFB to break the UC-CRC continuum by modulating shared nodes like NF-κB, which is a key advantage for chronic UC management. The common targets of the two pathways underwent molecular docking, revealing that the docking scores for NFKBIA, RELA, NFKB1, as well as the compounds colchamine, morusin and orotinin, exceeded 5.0. These findings suggest that YFB powder has therapeutic potential for UC through these specific targets. The hyperactivation of the NF-κB signaling pathway serves as the central driving force behind the inflammatory cascade in UC. Within this pathway, RELA functions as the principal transcriptional activation subunit. Upon phosphorylation, RELA translocates to the nucleus in the intestinal mucosa of UC patients, where it directly upregulates the expression of proinflammatory cytokines such as TNF-α, IL-1β and IL-6. This upregulation facilitates the recruitment of neutrophils, leading to their infiltration and subsequent compromise of the intestinal barrier integrity. Concurrently, NFKB1 interacts with RELA to form heterodimers, which synergistically amplify the inflammatory response. The deficiency of p50/p50 anti-inflammatory homodimers exacerbates immune imbalance, fostering the progression from chronic inflammation to CRC. Additionally, NFKBIA, a critical negative regulator, becomes ineffective in UC due to IkB kinase-mediated phosphory
This study had several limitations. The effective components of TCM were obtained by using public databases, and high performance liquid chromatography was not used to clarify the components. While database screening efficiently prioritizes mechanistically relevant targets, future studies will integrate high performance liquid chromatography-mass spectrometry to quantify specific compounds in YFB extracts, experimentally elucidate its molecular mechanisms against UC through cellular and animal models, and correlate compound levels with in vivo efficacy.
The mechanism of YFB powder to treat UC based on intestinal microecology is not simply to reduce the relative abundance of harmful bacteria Turicibacter and Clostridium_sensu_stricto_1, and increasing the abundance of probiotics unclassified_f_Lachnospiraceae and Akkermansia, but to achieve dynamic balance through the overall adjustment of intestinal microecological balance to disease recovery. We predicted that another potential mechanism of YFB powder in treating UC might involve the NFKB1, RELA and NFKBIA regulatory pathways in cancer and adipocytokine signaling pathways.
The authors would like to sincerely thank Shandong University of Traditional Chinese Medicine for their participation.
| 1. | González-Lama Y, Ricart E, Cábez A, Fortes P, Gómez S, Casellas F. Medical consultation in ulcerative colitis: Key elements for improvement. World J Gastroenterol. 2023;29:917-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2130] [Article Influence: 85.2] [Reference Citation Analysis (2)] |
| 3. | Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, Bernstein CN, Danese S, Peyrin-Biroulet L, Hibi T. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 1158] [Article Influence: 193.0] [Reference Citation Analysis (0)] |
| 4. | Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 1325] [Article Influence: 165.6] [Reference Citation Analysis (1)] |
| 5. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2719] [Article Influence: 302.1] [Reference Citation Analysis (2)] |
| 6. | Xu H, Zhu J, Lin X, Chen C, Tao J. A Comprehensive Review of Traditional Chinese Medicine in the Management of Ulcerative Colitis. Am J Chin Med. 2025;53:435-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Zhu M, Dai Y, Gao L, Cheng L. Research Progress in Ulcerative Colitis: The Role of Traditional Chinese Medicine on Gut Microbiota and Signaling Pathways. Am J Chin Med. 2024;52:2277-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Liu M, Wang Z, Liu X, Xiao H, Liu Y, Wang J, Chen C, Wang X, Liu W, Xiang Z, Yue D. Therapeutic effect of Yiyi Fuzi Baijiang formula on TNBS-induced ulcerative colitis via metabolism and Th17/Treg cell balance. J Ethnopharmacol. 2023;309:116301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 9. | Gu W, Zhang L, Han T, Huang H, Chen J. Dynamic Changes in Gut Microbiome of Ulcerative Colitis: Initial Study from Animal Model. J Inflamm Res. 2022;15:2631-2647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Galipeau HJ, Caminero A, Turpin W, Bermudez-Brito M, Santiago A, Libertucci J, Constante M, Raygoza Garay JA, Rueda G, Armstrong S, Clarizio A, Smith MI, Surette MG, Bercik P; CCC Genetics, Environmental, Microbial Project Research Consortium, Croitoru K, Verdu EF. Novel Fecal Biomarkers That Precede Clinical Diagnosis of Ulcerative Colitis. Gastroenterology. 2021;160:1532-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 11. | Chen K, Wang H, Yang Y, Tang C, Sun X, Zhou J, Liu S, Li Q, Zhao L, Gao Z. Common mechanisms of Gut microbe-based strategies for the treatment of intestine-related diseases: based on multi-target interactions with the intestinal barrier. Cell Commun Signal. 2025;23:288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 12. | Schierová D, Březina J, Mrázek J, Fliegerová KO, Kvasnová S, Bajer L, Drastich P. Gut Microbiome Changes in Patients with Active Left-Sided Ulcerative Colitis after Fecal Microbiome Transplantation and Topical 5-aminosalicylic Acid Therapy. Cells. 2020;9:2283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Yao S, Zhao Z, Wang W, Liu X. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. J Immunol Res. 2021;2021:8030297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 14. | Pang B, Jin H, Liao N, Li J, Jiang C, Shao D, Shi J. Lactobacillus rhamnosus from human breast milk ameliorates ulcerative colitis in mice via gut microbiota modulation. Food Funct. 2021;12:5171-5186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Chen L, Zhang C, Cao J, Bei G, Wang X, Miao Z. Yiyi Fuzi Baijiang Decoction Alleviates Ulcerative Colitis Partly by Regulating TLR4-Mediated PI3K/Akt and NF-κB Pathways. Evid Based Complement Alternat Med. 2022;2022:8780514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Lee ES, Kim YI, Lee JH, Kim JH, Kim YG, Han KS, Yoon YH, Cho BO, Cho JS. Anti-Pruritic and Immunomodulatory Effects of Coix [Coix lacryma-jobi L. var. ma-yuen (Rom. Caill.) Stapf.] Sprouts Extract. Int J Mol Sci. 2024;25:11828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Zhang YX, Wan H, Shan GY, Cheng JY, Liu YY, Shi WN, Li HJ. Pharmacological role of Herba Patriniae and Coix seed in colorectal cancer. World J Gastrointest Oncol. 2025;17:99673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 18. | Huang C, Dong J, Jin X, Ma H, Zhang D, Wang F, Cheng L, Feng Y, Xiong X, Jiang J, Hu L, Lei M, Wu B, Zhang G. Intestinal anti-inflammatory effects of fuzi-ganjiang herb pair against DSS-induced ulcerative colitis in mice. J Ethnopharmacol. 2020;261:112951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Li Y, Tian Y, Zhu L, Lin H, Zhao X, Liu C, Lv Y, Wang Z, Zuo Z, Wang J, Wang Z. Fuzi Lizhong Pill inhibited inflammatory response and promoted colon mucosal healing in dextran sulfate sodium-induced ulcerative colitis mice by down-regulating PI3K/AKT/NF-κB signaling pathway. J Ethnopharmacol. 2025;343:119483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Huang Y, Lin X, Wu Q, Wu X, Yang S, Dong Y, Fu C, Lin W, Zhang Z. Integrated serum pharmacochemistry and network pharmacology to reveal the kernel material basis and underlying mechanisms of the fuzi-lizhong pill for ulcerative colitis. J Tradit Complement Med. 2025;15:307-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Zhou YL, Wu J, Wang HL, Feng WW, Peng F, Zhang RQ, Yan HL, Liu J, Tan YZ, Peng C. Fuzi lizhong pills alter microbial community compositions and metabolite profiles in ulcerative colitis rat with spleen-kidney yang deficiency syndrome. J Ethnopharmacol. 2024;335:118645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Sun C, Jiang Y, Wang X. Fuzi-Baijiangcao Herb Pair Alleviates DSS-induced Ulcerative Colitis in Mice via Inhibiting the p38 MAPK/NF-κB/HIF-1α Signaling Pathway. Comb Chem High Throughput Screen. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Li J, Shang L, Zhou F, Wang S, Liu N, Zhou M, Lin Q, Zhang M, Cai Y, Chen G, Yang S. Herba Patriniae and its component Isovitexin show anti-colorectal cancer effects by inducing apoptosis and cell-cycle arrest via p53 activation. Biomed Pharmacother. 2023;168:115690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 24. | Scaldaferri F, D'Onofrio AM, Chiera E, Gomez-Nguyen A, Ferrajoli GF, Di Vincenzo F, Petito V, Laterza L, Pugliese D, Napolitano D, Schiavoni E, Spagnolo G, Ferrarese D, Putignani L, Lopetuso LR, Cammarota G, Cominelli F, Gasbarrini A, Sani G, Camardese G. Impact of Psychopathology and Gut Microbiota on Disease Progression in Ulcerative Colitis: A Five-Year Follow-Up Study. Microorganisms. 2025;13:1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Geese T, Bang C, Franke A, Lieb W, Dempfle A. [1]The human gut microbiota in IBD, characterizing hubs, the core microbiota and terminal nodes: a network-based approach. BMC Microbiol. 2025;25:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Lee JWJ, Plichta D, Hogstrom L, Borren NZ, Lau H, Gregory SM, Tan W, Khalili H, Clish C, Vlamakis H, Xavier RJ, Ananthakrishnan AN. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe. 2021;29:1294-1304.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 27. | Bruellman R, Llorente C. A Perspective Of Intestinal Immune-Microbiome Interactions In Alcohol-Associated Liver Disease. Int J Biol Sci. 2021;17:307-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Zhao P, Cai Z, He P, Wang J, He H, Zhu Z, Guo X, Ma K, Peng K, Zhao J. Buqi-Huoxue-Tongnao decoction drives gut microbiota-derived indole lactic acid to attenuate ischemic stroke via the gut-brain axis. Chin Med. 2024;19:126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 29. | Chen Y, Li M, Deng L, Zhu Y, Mu Y, Wang C, Xia L, Wang R, Zhou M. Orally administered Chrysophyta polysaccharide ameliorates DSS-induced colitis via intestinal barrier improvement, oxidative stress regulation, NF-κB pathway inhibition, and gut microbiota modulation. Int J Biol Macromol. 2025;315:144500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Zhou Y, Wang M, Wang Z, Qiu J, Wang Y, Li J, Dong F, Huang X, Zhao J, Xu T. Polysaccharides from hawthorn fruit alleviate high-fat diet-induced NAFLD in mice by improving gut microbiota dysbiosis and hepatic metabolic disorder. Phytomedicine. 2025;139:156458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 31. | Ye X, Wu K, Xu L, Cen Y, Ni J, Chen J, Zheng W, Liu W. Methanol extract of Inonotus obliquus improves type 2 diabetes mellitus through modifying intestinal flora. Front Endocrinol (Lausanne). 2022;13:1103972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Xu HM, Huang HL, Xu J, He J, Zhao C, Peng Y, Zhao HL, Huang WQ, Cao CY, Zhou YJ, Zhou YL, Nie YQ. Cross-Talk Between Butyric Acid and Gut Microbiota in Ulcerative Colitis Following Fecal Microbiota Transplantation. Front Microbiol. 2021;12:658292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Yan Y, Li K, Jiang J, Jiang L, Ma X, Ai F, Qiu S, Si W. Perinatal tissue-derived exosomes ameliorate colitis in mice by regulating the Foxp3 + Treg cells and gut microbiota. Stem Cell Res Ther. 2023;14:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 34. | Kaźmierczak-Siedlecka K, Roviello G, Catalano M, Polom K. Gut Microbiota Modulation in the Context of Immune-Related Aspects of Lactobacillus spp. and Bifidobacterium spp. in Gastrointestinal Cancers. Nutrients. 2021;13:2674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Zakerska-Banaszak O, Ladziak K, Kruszka D, Maciejewski K, Wolko L, Krela-Kazmierczak I, Zawada A, Vibeke Vestergaard M, Dobrowolska A, Skrzypczak-Zielinska M. New potential biomarkers of ulcerative colitis and disease course - integrated metagenomic and metabolomic analysis among Polish patients. J Gastroenterol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Li J, Zang C, Li P, Sheng D, Xiao Z, Xiao B, Xia J, Zhou L. Investigating the role of gut microbiota in hemorrhagic stroke: Evidence from causal analysis. J Stroke Cerebrovasc Dis. 2025;34:108131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Chen LM, Bao CH, Wu Y, Liang SH, Wang D, Wu LY, Huang Y, Liu HR, Wu HG. Tryptophan-kynurenine metabolism: a link between the gut and brain for depression in inflammatory bowel disease. J Neuroinflammation. 2021;18:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 38. | Cui L, Guan X, Ding W, Luo Y, Wang W, Bu W, Song J, Tan X, Sun E, Ning Q, Liu G, Jia X, Feng L. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int J Biol Macromol. 2021;166:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 332] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 39. | Chen P, Xu H, Tang H, Zhao F, Yang C, Kwok LY, Cong C, Wu Y, Zhang W, Zhou X, Zhang H. Modulation of gut mucosal microbiota as a mechanism of probiotics-based adjunctive therapy for ulcerative colitis. Microb Biotechnol. 2020;13:2032-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Shimizu Y, Isoda K, Taira Y, Taira I, Kondoh M, Ishida I. Anti-tumor effect of a recombinant Bifidobacterium strain secreting a claudin-targeting molecule in a mouse breast cancer model. Eur J Pharmacol. 2020;887:173596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Badri M, Nilson B, Ragnarsson S, Senneby E, Rasmussen M. Clinical and microbiological features of bacteraemia with Gram-positive anaerobic cocci: a population-based retrospective study. Clin Microbiol Infect. 2019;25:760.e1-760.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Ali FEM, Ibrahim IM, Ghogar OM, Abd-Alhameed EK, Althagafy HS, Hassanein EHM. Therapeutic interventions target the NLRP3 inflammasome in ulcerative colitis: Comprehensive study. World J Gastroenterol. 2023;29:1026-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 43. | Abou Zaid ES, Mansour SZ, El-Sonbaty SM, Moawed FS, Kandil EI, Haroun RA. Boswellic acid coated zinc nanoparticles attenuate NF-κB-mediated inflammation in DSS-induced ulcerative colitis in rats. Int J Immunopathol Pharmacol. 2023;37:3946320221150720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 44. | Xiong Y, Huang F, Li X, Chen Z, Feng D, Jiang H, Chen W, Zhang X. CCL21/CCR7 interaction promotes cellular migration and invasion via modulation of the MEK/ERK1/2 signaling pathway and correlates with lymphatic metastatic spread and poor prognosis in urinary bladder cancer. Int J Oncol. 2017;51:75-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Zhang Y, Zhang L, Chai N, Wan Z, Sui H. Inspired by an ancient Chinese Medicine prescription: the modern significance and potential of Yiyi Fuzi Baijiang San in treating diseases. Front Pharmacol. 2024;15:1465387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Pan Z, Lin H, Fu Y, Zeng F, Gu F, Niu G, Fang J, Gu B. Identification of gene signatures associated with ulcerative colitis and the association with immune infiltrates in colon cancer. Front Immunol. 2023;14:1086898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 47. | Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014;20:16389-16397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/