Published online Nov 14, 2025. doi: 10.3748/wjg.v31.i42.111706
Revised: September 4, 2025
Accepted: October 13, 2025
Published online: November 14, 2025
Processing time: 128 Days and 21.6 Hours
Mast cells (MCs) under stress conditions contribute to the development of irritable bowel syndrome (IBS), yet their precise mechanisms in IBS remain un
To investigate the role of MC-derived thymosin β4 (Tβ4) in stress-induced intes
The colonic mucus Tβ4 levels in IBS patients were determined and their effects on the epithelial barrier were assessed in vitro and in vivo. Specifically, rats gene
We demonstrated that high levels of Tβ4 in IBS mucus and intestinal MCs mediate stress-associated disruptive changes to the epithelial barrier. Moreover, Tβ4 treatment of wild-type or MC-deficient Kitw-sh/w-sh mice caused a reduction in tight junction proteins and the interleukin 22 receptor A1 (IL22RA1)/Reg3γ cascade, but an increase in myosin light chain kinase. Furthermore, Tβ4-/- rats were resistant to stress, though reintroduction of Tβ4 or wt-PMCs restored stress or corticotropin-releasing hormone (CRH)-induced barrier disturbance. Consistently, Tβ4 release from MCs was dependent on the CRH receptor 1, but not degranulation. The effect of Tβ4 was accom
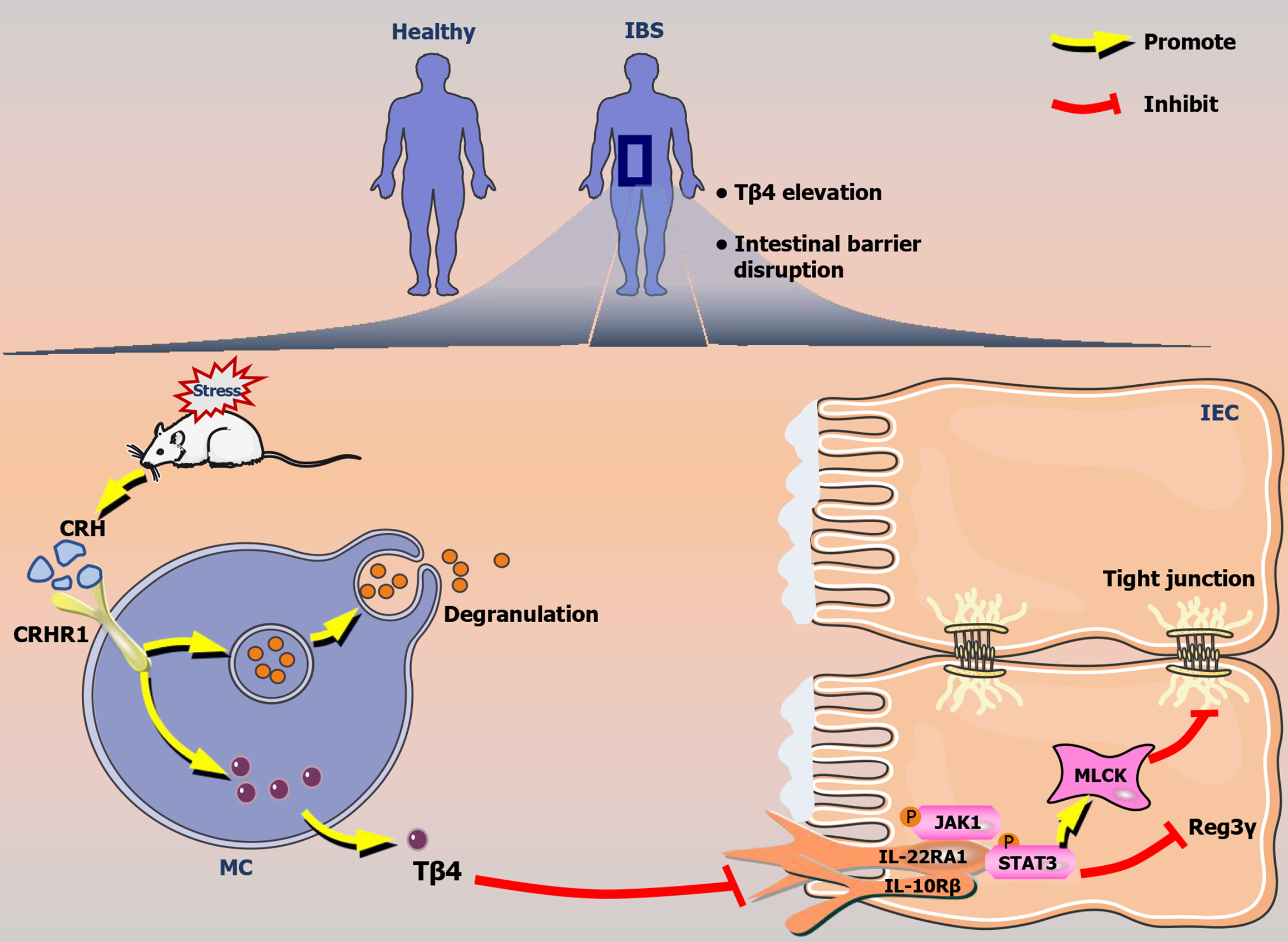
Collectively, these results suggest that Tβ4, which is abundant in IBS mucus and the secretome of MCs, plays a crucial role in the pathogenesis of IBS via IL22RA1/JAK1/STAT3 signaling, with potential implications for diagnostic and therapeutic targeting.
Core Tip: Thymosin β4 (Tβ4) is identified as a prevalent component in the intestinal mucus of irritable bowel syndrome patients that is capable of independently causing intestinal barrier disruption, even without the involvement of mast cells, when triggered by either corticotropin-releasing hormone (CRH) or stress. In addition, mast cells are the source of the Tβ4 required for intestinal barrier damage, and its release is contingent on CRH/CRH receptor 1 but not on degranulation. Mechanistically, the impact of Tβ4 on both the physical and immune barriers is mediated through the inhibition of Janus kinase 1/signal transducer and activation of transcription 3 signaling. These findings position Tβ4 as a promising diagnostic biomarker and therapeutic target for irritable bowel syndrome.
- Citation: Sun YS, Bai XQ, Sun KD, Li J, Liu L, Chen YY, Zeng ZY, Wang Q, Guo YB. Thymosin β4 released by mast cells under stress conditions impairs intestinal epithelial barrier via IL22RA1/JAK1/STAT3 signaling in irritable bowel syndrome. World J Gastroenterol 2025; 31(42): 111706
- URL: https://www.wjgnet.com/1007-9327/full/v31/i42/111706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i42.111706
The primary function of the intestinal barrier is to maintain intestinal homeostasis by providing protection against potential infections from the luminal microbiome[1]. A compromised intestinal barrier can trigger immunological responses, leading to exacerbated inflammation[2]. Furthermore, intestinal barrier dysfunction is linked to various human diseases, including systemic autoimmune and metabolic conditions such as type II diabetes, human immunodeficiency virus, and autism, as well as disorders primarily affecting the gut, including inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS)[3]. Healing of the intestinal barrier or mucosae is a typical endpoint in clinical trials and a more accurate predictor of clinical remission in IBD patients than either endoscopic or histologic remission[4,5].
The intestinal barrier is primarily composed of four sub-barriers: A physical barrier created by layers of intestinal epithelial cells (IECs), a chemical (mucus) barrier produced by the mucus released from IECs, an immune barrier consisting of immune cells within the mucosa, and a microbiome barrier made up of microorganisms[6]. A key structural component of the physical barrier is the tight junctions (TJs), which connect neighboring epithelial cells. The connections are assembled by zonula occludens (ZO), occludin, and claudins. Additionally, adhesion junctions and gap junctions bind epithelial cells together, sealing the intercellular gaps[7]. The disruption of TJs results in high permeability, driven by the activation of myosin light chain kinase (MLCK), which phosphorylates myosin light chain (MLC). Contraction of the perijunctional actin-myosin ring subsequently facilitates epithelial TJ permeability[8]. In this context, MLCK is considered a potential therapeutic target for restoring the intestinal barrier.
Interleukin 22 (IL22), an IL-10 superfamily cytokine, is produced by immune cells and plays a protective role in epithelial repair and host defense at barrier surfaces[9]. IL22 engages with a heterodimeric receptor composed of IL22 receptor A1 (IL22RA1) and IL-10 receptor 2 (IL-10R2) in IECs. This interaction orchestrates the release of mucins and antimicrobial peptides, including S100A7-9 and Reg3γ/β, through the activation of the Janus kinase 1 (JAK1)/signal transducer and activation of transcription 3 (STAT3) pathway[10]. Cell-intrinsic IL22RA1-STAT3 signaling has been showed to regulate mucosal immunity, microbiota colonization, inflammation, and tissue repair[11-13]. IL22 also en
IBS is a functional gastrointestinal disease characterized by increased intestinal permeability and mucosal infiltration of immune cells, notably mast cells (MCs)[16]. In the colonic mucosa of IBS patients, MCs are present in large numbers and exhibit activation, as evidenced by heightened degranulation[17]. This activation leads to the release of various mediators, such as tryptase, histamine, and cytokines, affecting intestinal barrier homeostasis[18]. A recognized pathway in IBS pathogenesis involves enhancement due to psychological stress of the release of peripheral corticotropin-releasing hormone (CRH), which then activates mucosal MCs, resulting in increased intestinal permeability[19]. Though MC stabilizers are thought to be promising for preventing stress-induced intestinal disorders[20], therapies targeting MC activity have only demonstrated modest benefits[21-23]. Their limited efficacy can, in part, be attributed to the inability of stabilizers to effectively block the release of active factors[23], especially those secreted independent of degranulation. Therefore, there is a pressing need for further research into the molecular mechanisms through which MCs contribute to intestinal defects.
Thymosin β4 (Tβ4) is a peptide consisting of 43 amino acid residues that was initially identified in the thymus but is now recognized as being widely distributed across tissues[24], including the intestine and MCs[25]. In our earlier study, we investigated the effects of Tβ4 on the intestinal epithelium and demonstrated that Tβ4 impairs the mucus barrier by reducing the expression and secretion of Mucin 2 through the inhibition of autophagy in IBD mice[26]. In the current study, we further discovered that Tβ4 is released from MCs independent of degranulation via the regulation of the CRH receptor and serves as a trigger for intestinal permeability by damaging both the physical and immune barriers. Moreover, Tβ4 acts as a link between physiological stress and the development of IBS, suggesting that the CRH receptor may serve as a potential therapeutic target. Given the increased levels of Tβ4 observed in IBS patients, our results indicate that Tβ4 may also serve as a promising diagnostic marker.
This study protocol was approved by the Ethics Committee of the Third People’s Hospital of Chengdu (2024-S-212), and all patients signed written informed consent and completed IBS-symptomatic assessment. The diagnosis of IBS was based on typical clinical symptoms that fulfill the Rome IV criteria[27]. IBS patients had abdominal pain that was relieved by defecation or was associated at its onset with a change in stool frequency (either an increase or decrease) or a change in the appearance of the stool (either too loose or too hard). Patients with a family history of organic gastroenterological diseases and organic or severe psychiatric disorders that may lead to gastrointestinal symptoms were excluded. In total, 38 IBS patients and 38 healthy controls were recruited for this research. Each patient or control subject underwent colonoscopy, and Z sigmoid colon mucus was washed with N-acetylcysteine for 3 minutes to obtain mucus. Mucosal biopsies were obtained from the proximal descending colon.
Human mast HMC-1 cells and epithelial Caco2 cells were provided by the Cell Institute of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Iscove's Modified Dulbecco's Medium and RPMI-1640, respectively, which were supplemented with 10% fetal bovine serum and 1% antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin). All cells were incubated at 37 °C in a humidified atmosphere with 50 mL/L CO2.
The rates of cell mortality were determined using a cell counting kit-8 (CCK-8, Biosharp; Cat#BL1055C). Briefly, Caco2 cells at a density of 1 × 104 cells/well were treated in 96-well plates with increasing concentrations of colonic mucus (1 ng/mL, 10 ng/mL, 100 ng/mL, 1 μg/mL, 10 μg/mL) for 24 hours, followed by 10% CCK-8 reagent for 1 hour. The absorbance was detected on a Microplate Reader (PerkinElmer, Waltham, MA, United States) at 450 nm. All experiments were performed in triplicate.
Caco2 cells were harvested, and 200 μL cell suspension was seeded on the apical compartment of 12 well Transwell® Filters (1.12 cm2 area with 0.4 μm pore size, Corning; Cat#3460) at a density of 1 × 105 cells per well. In the bottom well, 1 mL RPMI-1640 medium was added. Transepithelial electrical resistance (TER) was measured with a chopstick-type probe (STX-2) connected to a Millicell ERS-2 voltmeter (Millipore, Billerica, MA, United States). Cells were incubated at room temperature for 30 minutes, and then the TER value was recorded at different time points (30, 60, 90, 120, and 150 minutes) after stabilization. Culture inserts without cells were set as a background control for all TER measurements. The TEER was calculated using the following formula: TEER = (Ω Treated - Ω background) × Area (cm2).
To assess the epithelial barrier stability, the electrical impedance signals were observed in real-time using an ACEA xCELLigence® Real-Time Cell Analyzer (RTCA) DP (Roche Diagnostics, Mannheim, Germany). Caco2 cells (5 × 103 cells/well) were seeded in “E-Plates 16”, and Tβ4 was added at different concentrations to the culture medium after the cell growth had stabilized (about 48 hours after seeding). E-Plates were locked into the RTCA DP Analyzer for a consistent track record of impedance changes at 10 kHz AC frequency, which were expressed as Cell Index (CI) values. CI is a dimensionless parameter based on the relative impedance changes referenced to the values of the cell-free electrode[28]. The peptides for CRH (Sequence: SEEPPISLDLTFHLLREVLEMARAEQLAQQAHSNRKLMEII, Modification: Ile-41 = C-terminal amide) and Tβ4 (Sequence: SDKPDMAEIE KFDKSKLKKTETQEKNPLPS KETIEQEKQAGES) were synthesized by GL Biochem (Shanghai) Ltd.
After TEER measurement, cells were washed with PBS for 30 minutes, and 100 μg/mL FITC-dextran 4000 (FD4, Sigma; Cat#60842-46-8) was added to the upper chamber for 2 hours. The fluorescence intensity of cells from the basolateral membrane was evaluated in a clear 96-well plate using a Nivo 3S plate reader (PerkinElmer, Waltham, MA, United States) with an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
After 12 hours of incubation of human mast HMC-1 cells with CRH, supernatant was collected to evaluate Tβ4 by ELISA assay (Immundiagnostik; Cat#KR9520) as described previously[29]. Additionally, clinical colon mucus was evaluated by ELISA assay to detect Tβ4. Experiments were repeated at least three times.
Fully differentiated Caco2 cell monolayers were treated with patient intestinal mucus (100 ng/mL) with or without Tβ4 neutralizing antibody. Additionally, HMC-1 cells and Tβ4 knockdown HMC-1 (HMC-1shTβ4) were pretreated with CRH and then were co-cultured with Caco2 cell monolayers. For zonulin 1 (ZO-1) and Occludin staining, the cells were fixed with 16 g/L paraformaldehyde for 20 minutes and then blocked with 50 mL/L bovine serum albumin (BSA) in PBS for 30 minutes. ZO-1 (1:1000) and Occludin (1:100) primary antibody were added overnight, followed by secondary antibody incubation. The cells were mounted with DAPI, and representative images were captured under an Olympus ScanR confocal microscope (Olympus Kogyo Corp Ltd, Tokyo, Japan).
IF staining of the 5 μm small intestine of animals was performed as described previously. After deparaffinization and rehydration, paraffin sections were boiled in EDTA antigen retrieval solution for 15 minutes. Subsequently, sections were blocked with 100 mL/L normal goat serum and stained with primary antibodies (Proteintech, ZO-1 Cat#21773-1-AP, IL22RA1 Cat#13462-1-AP, and Tβ4 Cat#19850-1-AP; Abcam, Occludin Cat#Ab216327 and MLCK Cat#Ab232949; Santa Cruz, Tryptase Cat#sc59587). Images were recorded by fluorescence microscopy (Leica DM4, Wetzlar, Germany).
As described previously[30], intestinal tissue was dewaxed with xylene and hydrated with graded ethanol (100%, 95%, 85% and 75%). Afterwards, section repair was performed using the pressure cooker antigen repair method, and the samples were blocked with 30 mL/L H2O2. The slices were incubated with primary antibody (MLCK) overnight and horseradish peroxidase-conjugated secondary antibody for 30 minutes, followed by staining with DAB and hematoxylin.
Caco2 cells were subjected to patient mucus, mucus with Tβ4-neutralizing antibody (1 µg), or Tβ4 recombinant protein (0.25, 0.5, 1, 2, 4 μmol/L) for 24 hours. The cells were harvested and lysed with RIPA buffer (Beyotime, Shanghai, China) with protease inhibitors on ice for 30 minutes. Then, the supernatants were collected by centrifugation at 4 °C and 10000 × g for 30 minutes. Equal amounts of protein were isolated by sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) followed by transfer to PVDF membranes. The membranes were incubated with primary antibodies after being blocked with 5% skimmed milk, including ZO-1 (Proteintech, Cat#21773-1-AP), Occludin (Abcam, Cat#Ab216327), IL22RA1 (Proteintech, Cat#13462-1-AP), Reg3γ (Abcam, Cat#Ab198216), β-actin (Proteintech, Cat#60008-1-Ig) and GAPDH (Proteintech, Cat#60004-1-Ig). Next, the membranes were incubated with second antibody and examined with a chemiluminescence detection kit.
The small intestines of rats were cut into pieces and prefixed with 3% glutaraldehyde. Then, the slices were fixed in 10 mL/L OsO4, dehydrated with an acetone series, infiltrated in Epox 812, and embedded in epoxy resin. The semithin sections were cut with a diamond knife and then stained with methylene blue, uranyl acetate and lead citrate. Sections were examined by Transmission Electron Microscopy (JEM-1400-FLASH, Jeol, Tokyo, Japan).
Short hairpin RNA (shRNA) lentiviruses (Tβ4 shRNA, Negative shRNA) were designed and synthesized by GenePharma (Shanghai, China). The LV3 Lentiviral vector expresses shRNA via the H1 promoter. Synthetic oligonucleotide primers were as follows: Forward, 5’-GATCCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAACTTT TTTG-3'; Reverse, 5’-AATTCAAAAAAGTTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACG-3'. The targeting sequence was 5’-GTTCTCCGAACGTGTCACGT-3’, and the specific sequence targeting Tβ4 used for shRNA interference was 5’-ATCCACTGCCTTCCAAAGAAA-3’. A nontargeting fragment (5’-TTCTCCGAACGTGTCA CGT-3’) was used as controlshRNA. The lentiviruses were transfected into HMC-1 (MOI = 30) with 2 µg/mL polybrene, which was replaced with fresh culture medium after 24 hours. Then, 72 hours later, the stably infected cells were screened using a SONY MA900 flow cytometer (SONY, San Jose, CA, United States), and the expression of Tβ4 in cells was evaluated by qPCR.
Differentiated monolayers of Caco2 cells (5 × 105 cells/well) were co-cultured with MCs that had been pretreated with CRH (1 μmol/L) or JAK1/STAT3A inhibitor (2.5 μmol/L, SHR0302; Selleck; Cat#E0324). HMC-1 and HMC-1shTβ4 cells were adaptively cultured in RPMI-1640 medium for one week, and the cells were then seeded onto a 6-well plate at a density of 5 × 105 cells/well for 12 hours. After intervention, MCs were resuspended in fresh culture medium and added to Transwell® Filters (24 mm diameter with 0.4 μm pore size, Corning, Cat#3450) for co-culture with epithelial cells.
Total RNA was extracted from the small intestines of rats (or cells) according to the instructions of the RNAsimple Total RNA Kit (TIANGEN, Beijing, China). One microgram of RNA (measured using a NanoDrop spectrophotometer; Thermo Fisher Scientific, Waltham, MA, United States) was reversed transcribed with HiScript® Q RT SuperMix, and qPCR (Vazyme; Cat#R223-01) was performed using ChamQ Universal SYBR qPCR Master Mix (Vazyme; Cat#Q711-02). The primers are as follows: IL22RA1 (rat): 5’-ATGACCTGTTCTACCGCTTAAA-3’ (forward), 5’-GCCAAGGAACTCATACT CTCTC-3’ (reverse); Reg3γ (rat): 5’-CCCAGGTTCACAAGACAGACA-3’ (forward), 5’-AATAGGAGCCATAGGCACGC-3’ (reverse); GAPDH (rat): 5’-TGATGGGTGTGAACCACGAG-3’ (forward), 5’-AGTGATGGCATGGACTGTGG-3’ (re
β-Hexosaminidase is generally used as a marker of MC degranulation[18]. We performed the β-hexosaminidase release assay essentially as described previously[31] to measure MC degradation. In brief, HMC-1 cells were incubated with Tyrode’s solution at a density of 1 × 105 cells/well in 24-well plates for 0.5 hours. Subsequently, the supernatant was replaced with Tyrode’s solution containing C48/80, CRH or DSCG for 10 minutes. The supernatants were incubated with 5 mmol/L 4-nitrophenyl N-acetyl-β-D-glucosamine (Sigma; Cat#N9376) dissolved with 0.05 M citric acid/sodium citrate buffer (pH 4.5) at 37 °C for 1.5 hours, and then the reaction was terminated with stop buffer (0.1 M Na2CO3/NaHCO3, pH 10.7). The absorbance was measured at 405 nm with a Microplate Reader (PerkinElmer, Waltham, MA, United States).
Rats were obtained from Chengdu Dossy Experimental Animals Co., LTD. (Chengdu, Sichuan). MC-deficient mutants (KitW-sh/W-sh mice) were obtained from Cyagen Biosciences (Guangzhou, China). Wistar male rats (180-220 g) were used for water avoidance stress (WAS)[31], and C57BL/6 male mice (18-20 g) were used for Tβ4 injection experiments. Experimental animals were maintained on a 12-hour light/12-hour dark cycle, with free access to pure water and standard chow under room temperature (22-24 °C). The animals were maintained in accordance with internationally accepted principles for laboratory animal use. All experiments were approved by the Ethics Committee of Southwest Jiaotong University Approval (Agreement No. SWJTU-2013-026).
Tmsb4x knockout rats (Tβ4-/-) were generated using the CRISPR/Cas9 approach. Briefly, two sgRNAs were designed using a CRISPR design tool (http://crispr.mit.edu) to target the region upstream or downstream of exon 2-3. The resulting mutants then were screened for on-target activity using a Universal CRISPR Activity Assay (UCATM, Biocytogen Inc., Beijing). The T7 promoter sequence was added to the Cas9 or sgRNA template by in vitro PCR amplification. Cas9 mRNA and sgRNAs were co-injected into the cytoplasm of one-cell stage fertilized SD eggs. The injected zygotes were transferred into oviducts of SD pesudopregnant females to generate F0 rats. The F0 rats with the expected genotype, confirmed by tail genomic DNA PCR and sequencing, were mated with SD rats to establish germline-transmitted F1 heterozygous rats, which were genotyped by tail genomic PCR and DNA sequencing. Because female animals' hormone levels fluctuate more in response to stress than males, only male mice were used in the animal IBS model. Before conducting experiments, each animal was assigned at random by body weight.
Adult rats were exposed to WAS as described previously[31]. Briefly, fourteen male Wistar rats were randomly divided into two groups (n = 7 per group), including negative control and WAS groups. The WAS group was placed on a platform surrounded by water for 1 hour/day (9:00-10:00 AM) for 10 consecutive days. Subsequently, additional stress at random was applied to the rats, including ultrasonic interference for 1 hour, water deprivation for 24 hours, simulation of abdominal injection, tilting the cage for 12 hours, clamping the tail for 2 minutes, and ice for 5 minutes. The WAS device was composed of a bucket (65 cm × 47 cm × 41 cm) and a drying platform at the bottom with a diameter of 9 cm and a height of 12 cm. The bucket was filled with water to a level 1 cm below the platform. Rats in the control group were placed on the platform for 1 hour in the absence of water.
EUB338 probe labeled with CY3 was prepared using the EUB338-I Fluorescence in situ hybridization (FISH) Probe Kit from the Focobio Corporation (Cat#FB-0010B). The FISH assay was performed according to the manufacturer’s in
Colonic perfusion was performed as described previously[31]. Briefly, adult male SD rats (200-250 g, n = 6 per group) were anesthetized with phenobarbital after 24 hours of starvation. The mucus (10 µg) of healthy individual (NC mucus) and IBS (IBS mucus) were transplanted overnight into the colons of rats 3 cm from the anus. Intestinal tissues were collected before sacrificing rats for subsequent experiments.
Peritoneum-derived MC (PMC) were isolated from SD rats according to previous methods[32-35]. In brief, adult male SD rats (200-250 g) were sacrificed, and 40 mL sterile PBS was injected intraperitoneally. After slowly shaking the abdomen for 90 seconds, the PBS containing peritoneal cells was collected into sterile Eppendorf tubes. The cells were centrifuged at 200 × g at room temperature for 5 minutes, and then the supernatant was removed, leaving 5 mL of liquid in the bottom of the tube. To produce a density gradient solution, 5 mL of 40% BSA solution was added to the tube, which was carefully layered with 5 mL of the 30% BSA solution. The BSA phase was removed after centrifugation at 200 × g for 5 minutes at room temperature, leaving 2 mL. Finally, the cells were washed twice with PBS and then cultured in RPMI-1640 medium containing 4 mmol/L glutamine, 20 ng/mL stem cell factor, 200 U/mL penicillin and 200 µg/mL st
Caco2 cells were treated with Tβ4 (400 nmol/L) for 24 hours. The cells (5 × 105) were dissolved in Trizol reagent to obtain total RNA. Subsequently, the RNA Nano 6000 Assay Kit was used to estimate the RNA integrity on a Bioanalyzer 2100 system (Agilent Technologies, CA, United States). After cluster generation, the library preparations were sequenced on an Illumina Novaseq platform to generate 150 bp paired-end reads at the Novogene Bioinformatics Institute (Novogene Biotech Co., Ltd., Beijing, China).
All data were analyzed by one-way ANOVA using GraphPad Prism version 8.0.1 (GraphPad Software, Inc., La Jolla, CA, United States). P < 0.05 was considered statistically significant. All experiments were independently performed in triplicate.
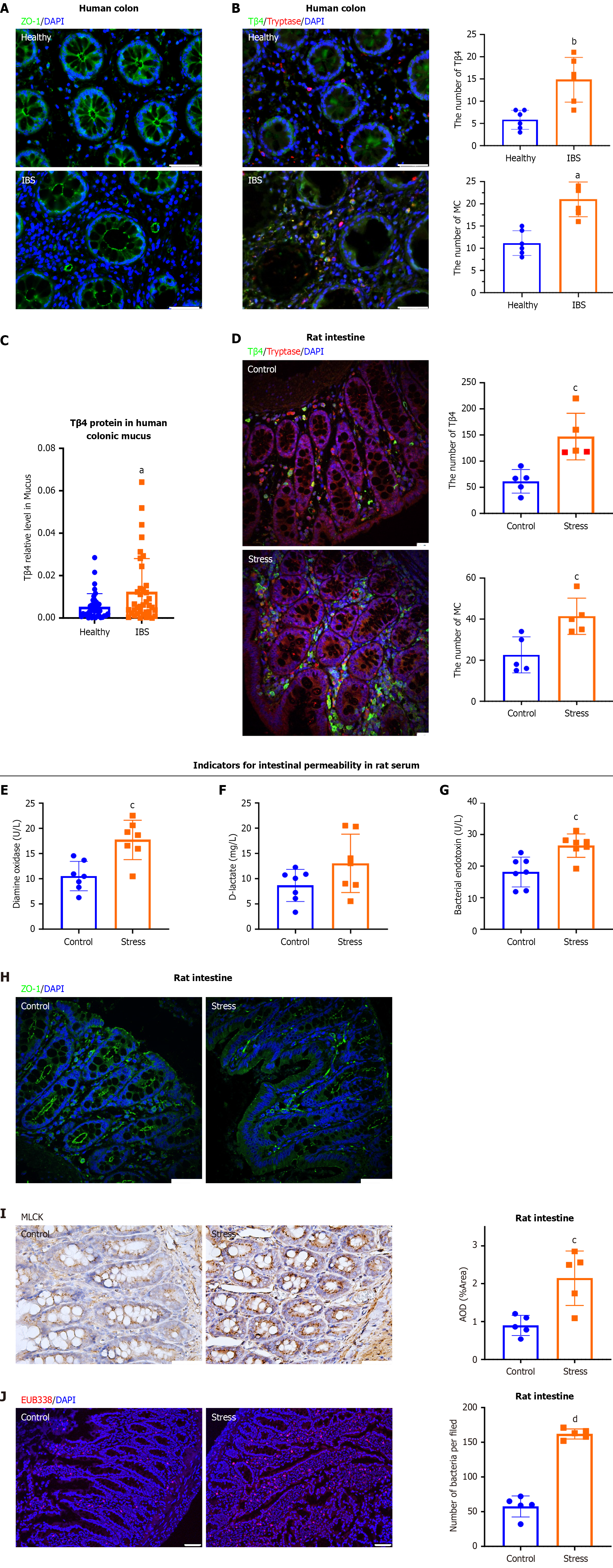
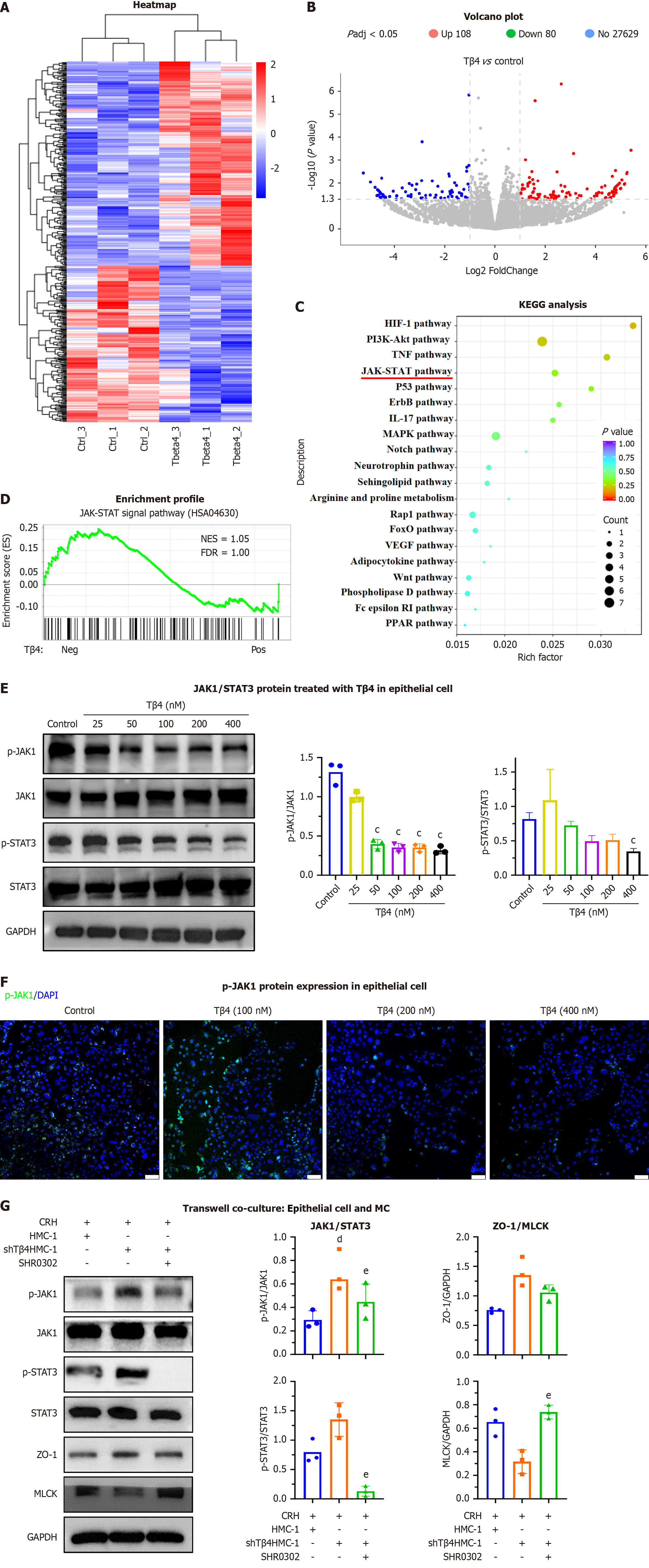
To elucidate the molecular mechanisms regulating TJs in IBS patients and the potential role of MCs, we performed immunostaining of the colonic mucosa. The results demonstrated that basic physical barrier TJs, identified by ZO-1, were significantly compromised in IBS patients in comparison to healthy individuals (Figure 1A). Additionally, mucosal MCs, marked with tryptase, showed elevated expression of Tβ4 in IBC patients relative to healthy subjects (Figure 1B). Notably, the patients (n = 38) also exhibited a significant increase in Tβ4 Levels within the colonic mucus (Figure 1C).
As previously reported, IBS can be mirrored in animal models under psychological stress caused by a combination of WAS and random stimulation[31]. The WAS rats exhibited typical symptoms of IBS, such as visceral pain, which was assessed through behavioral observations, and increased CRH in colonial mucosa[37]. After ten days of psychological stress, the amount of MCs and Tβ4 secretion was obviously enhanced in the colons of rats (Figure 1D). To evaluate the effects of stress induction on intestinal barrier integrity, we measured the serum levels of diamine oxidase, D-lactate and bacterial endotoxin[38]. ELISA results demonstrated a dramatic upregulation of each of these proteins in the stress group, thus verifying that stress induction increased the intestinal permeability (Figure 1E-G). Consistent with the results from IBS patients, the TJs were disrupted with a loss of ZO-1 in the colonic mucosa of stressed rats (Figure 1H). Furthermore, immunohistochemistry revealed a significant increase in MLCK, which promotes the opening of TJs by phosphorylating MLC[39], in colon mucosa of stressed rats compared with control rats (Figure 1I). This was accompanied by a marked increase in bacterial infiltration, as determined by FISH (Figure 1J), thus verifying the stress damage to the intestinal barrier.
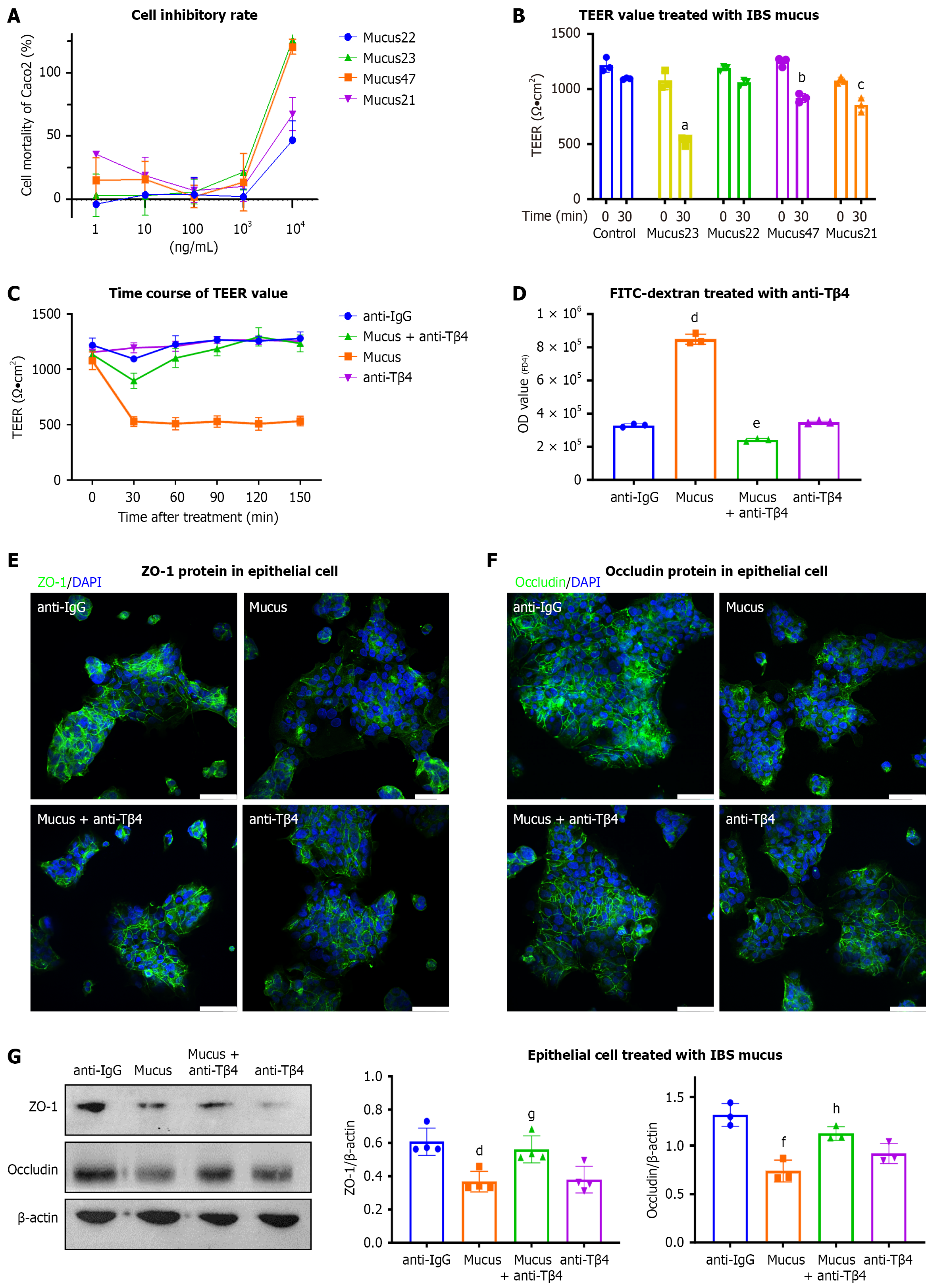
To investigate the role of Tβ4 in the mucus of IBS patients, we conducted in vitro tests on IEC cell monolayers. Initially, we tested a range of concentrations of mucus from four randomly selected cases, which indicated that 1 μg/mL of mucus had a minimal effect on the mortality of epithelial Caco2 cells (Figure 2A). Next, we used TEER and fluorescein iso
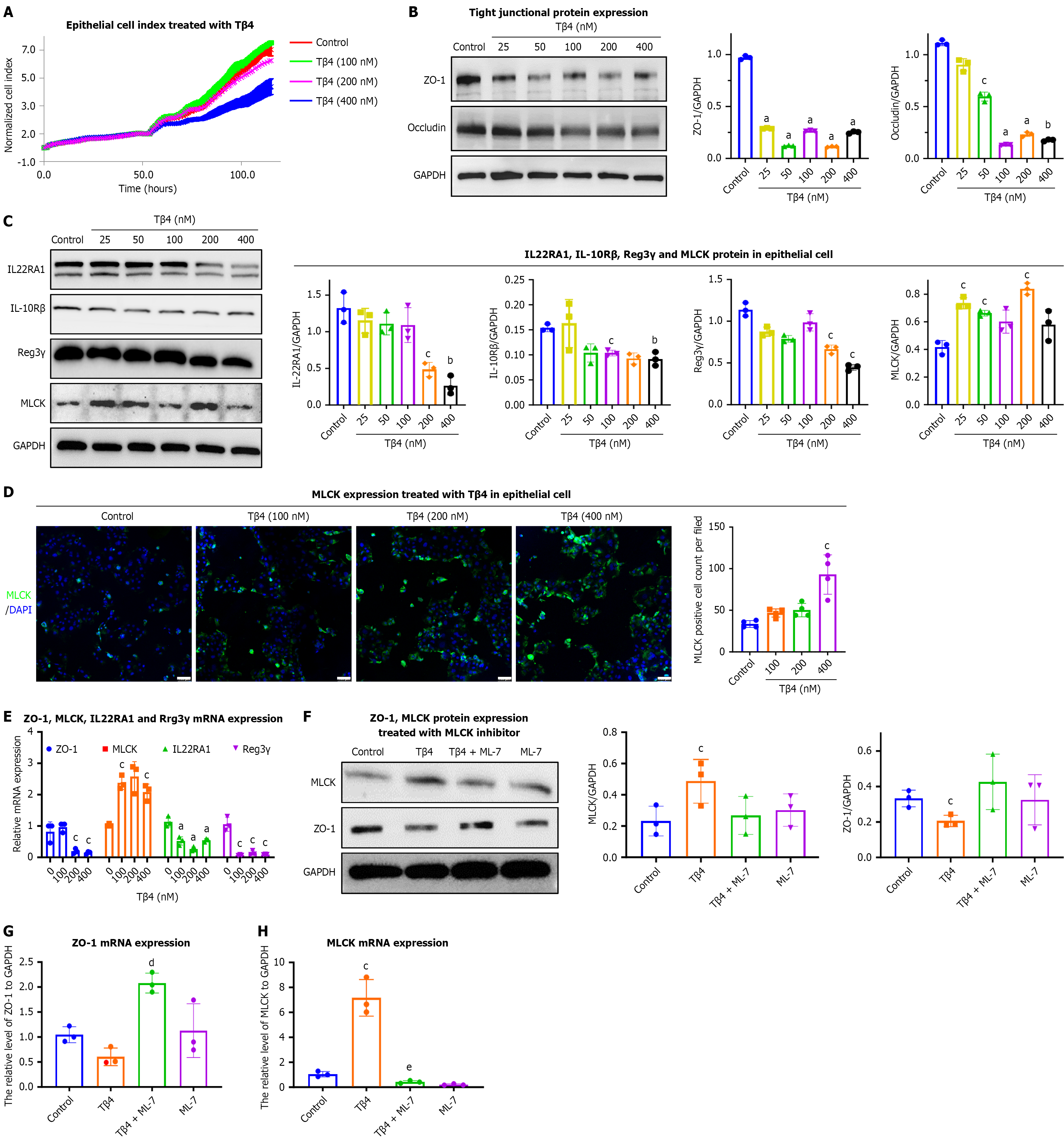
To determine the direct effects of Tβ4 on epithelial barrier function, we applied synthetic Tβ4 to Caco2 cell monolayers. Changes in permeability were assessed using impedance-based analysis with the xCELLigence® system. The results show a concentration-dependent decline in the normalized CI due to Tβ4 treatment (Figure 3A). Consequently, Tβ4 signi
Beyond their role in maintaining the physical barrier, epithelial cells also contribute to the immune barrier through the production of immune factors and effectors. IL22, which binds to IL22R (comprising IL22RA1 and IL-10Rβ) on IECs, prompts the production of antimicrobial peptides (e.g., Reg3γ) and mucin, aiding in the segregation of microorganisms from the intestinal mucosa[40,41]. Our results demonstrated that Tβ4 upregulates MLCK expression while decreasing the protein and mRNA levels of IL22RA1 and Reg3γ in Caco2 cells (Figure 3C-E). This suggests a complex effect of Tβ4 on the integrity and functionality of epithelial barriers. Notably, pre-treatment with ML-7, an MLCK inhibitor, alleviated the decrease in ZO-1 Levels induced by Tβ4 (Figure 3F-H).
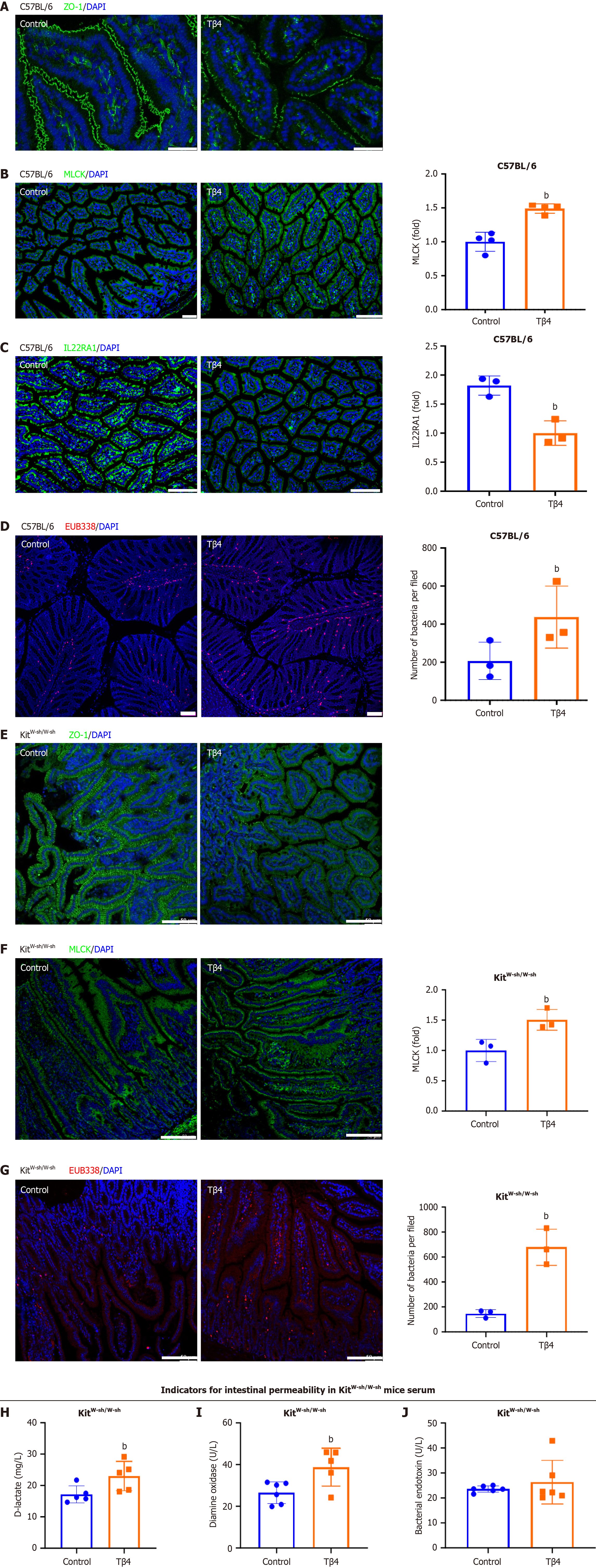
To further support these findings, we performed in vivo experiments. C57BL/6 mice treated with Tβ4 via intraperitoneal injection exhibited increased intestinal permeability, evidenced by disrupted TJ integrity (Figure 4A) and enhanced MLCK activation (Figure 4B). Furthermore, the levels of IL22RA1 in the intestine were reduced after Tβ4 administration (Figure 4C). As a result, a more significant bacterial presence was detected beneath the mucosa in Tβ4-treated mice compared to the controls (Figure 4D), suggesting that Tβ4 promotes the development of dysbiosis within the compro
Given the established role of Tβ4 in activating MCs[42,43], which are key players in disrupting the intestinal barrier, we further investigated whether the barrier dysfunction induced by Tβ4 was MC-dependent. To this end, we treated MC-deficient mice (KitW-sh/W-sh) with Tβ4. The results demonstrated that Tβ4 also induced intestinal permeability and dysbiosis in KitW-sh/W-sh mice (Figure 4E-J), as evidenced by disrupted TJ, activated-MLCK, and enhanced EUB338. These results suggest that the impact of Tβ4 on the intestinal barrier occurs independently of MC-mediated mechanisms.
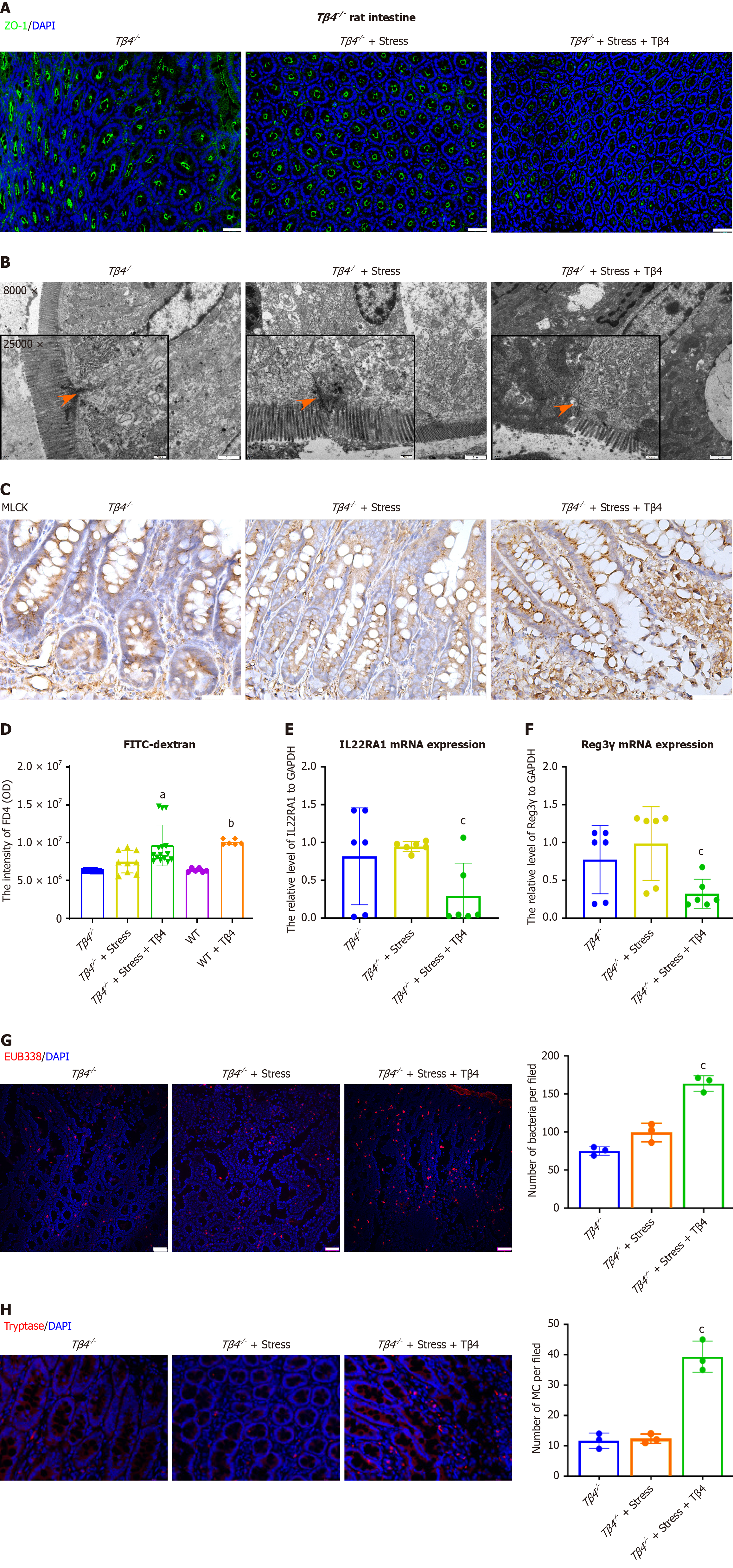
Next, to evaluate the role of Tβ4 in the onset of intestinal permeability, we subjected Tβ4 knock-out rats to IBS stress-induction. Immunofluorescence (IF) and qPCR assays confirmed the lack of Tβ4 expression in Tβ4-/- rats (Supplementary Figure 2). When subjected to psychological stress, the integrity of the intestinal barrier in the Tβ4-/- rats showed no significant deterioration, with an intact TJ lining at the interepithelial cell interface (Figure 5A and B), as well as normal MLCK expression levels (Figure 5C). However, reintroduction of Tβ4 to the Tβ4-/- rats reinstated the impairment of the intestinal barrier (Figure 5A-C, third column). Accordingly, Tβ4-deficient rats responded to stress induction only when Tβ4 was administered in the trans-epithelial permeability assay (FD4) (Figure 5D) and in assessments of IL22RA1 (Figure 5E) and Reg3γ (Figure 5F) expression reduction. Furthermore, the submucosal bacterial infiltration levels (Figure 5G) and the number of MCs (Figure 5H) in stress-induced Tβ4-/- rats were similar to those in Tβ4-/- rats without stress induction, whereas the addition of Tβ4 restored the stress-induced increases in both infiltration and MCs. These results verify the role of Tβ4 in compromising intestinal and immune barrier integrity and suggest that Tβ4 represents a potential target for interventions aimed at protecting or restoring the integrity of the intestinal barrier.
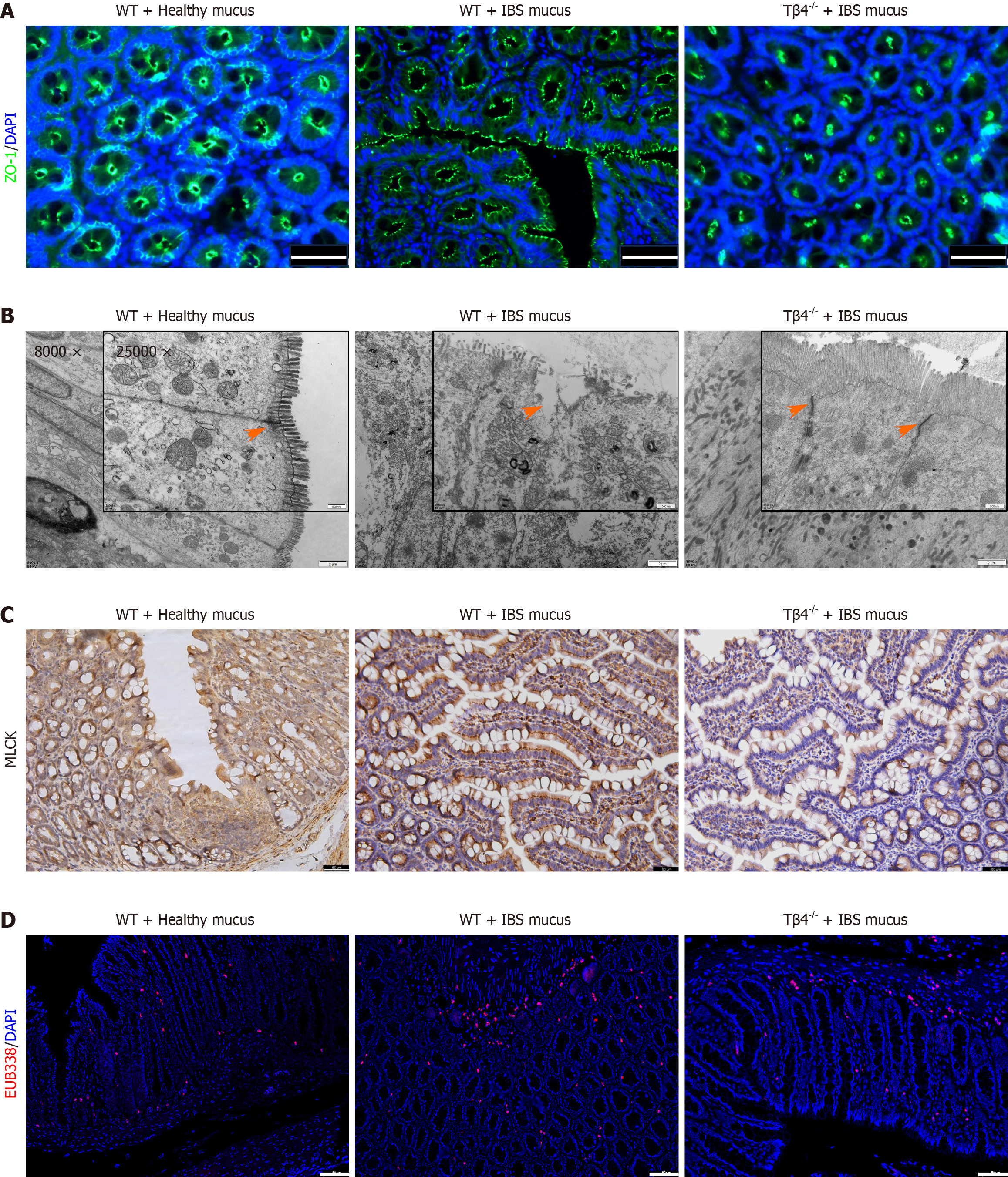
To further explore the in vivo effects of IBS patient mucus on epithelial barrier damage, we transplanted patient mucus into the colons of wild-type and Tβ4-/- rats via enemas. Detrimental effects of IBS patient mucus on the epithelial intercellular TJs were observed in wild-type rats, but not in Tβ4 knockout (Tβ4-/-) rats (Figure 6A-C) or in rats that were exposed to mucus from healthy individuals (Supplementary Figure 3). Consequently, the bacterial infiltration level in Tβ4-/- rats treated with IBS patient mucus was low (Figure 6D). These findings suggest that the absence of Tβ4 sufficiently counteracts the barrier damage induced by IBS patient mucus.
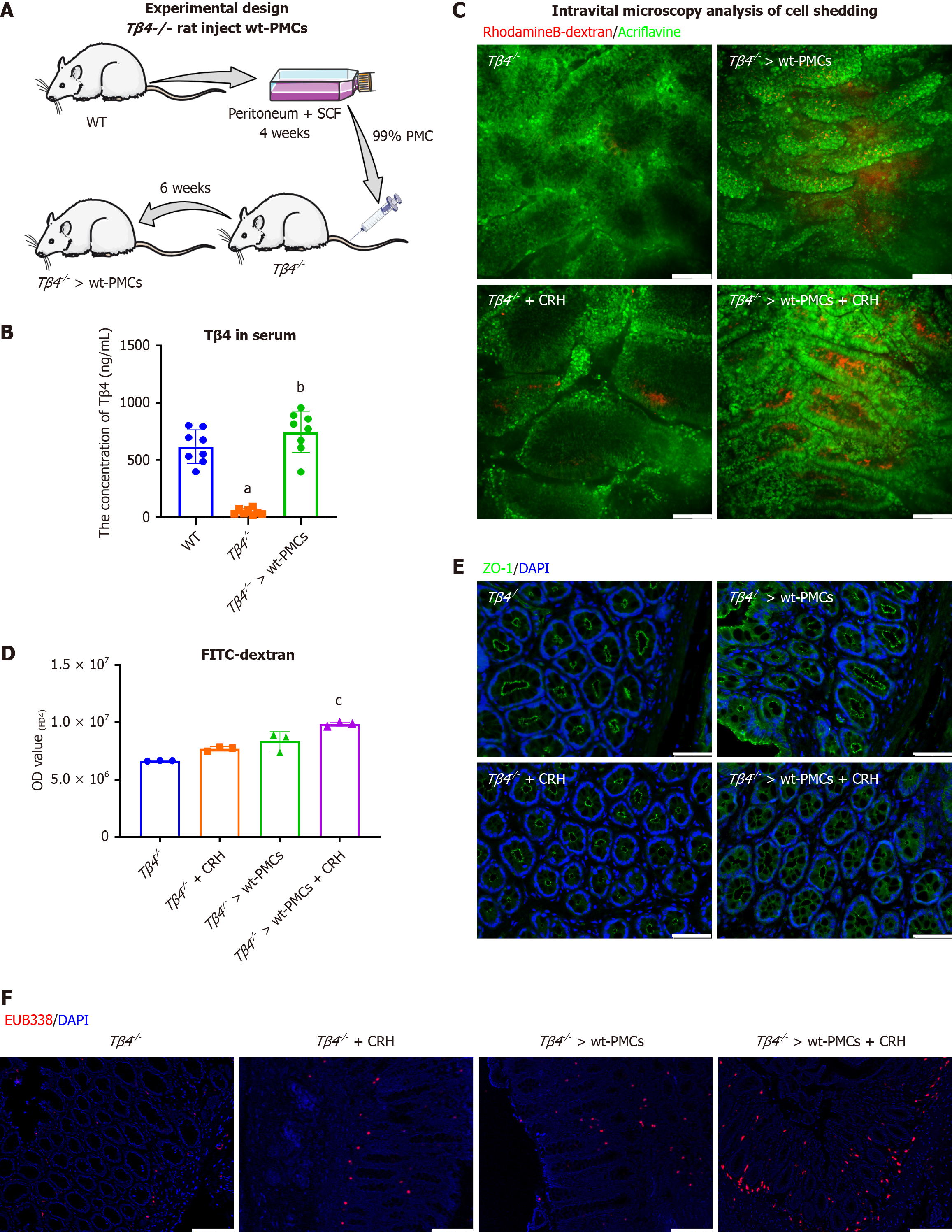
Given the high Tβ4 expression in the mucosal MCs of IBS patients (Figure 1B), we hypothesized that MCs could be a significant source of Tβ4. To explore this possibility, we introduced PMCs from wild-type rats into Tβ4 knockout (Tβ4-/-) rats (Figure 7A). Approximately 99% of the extracted PMCs were viable prior to transplantation, as determined by safranin O staining (Supplementary Figure 4A). Reconstitution with PMCs derived from WT rat (“Tβ4-/- > wt-PMCs”) significantly reinstated the serum and intestinal Tβ4 to normal levels (Figure 7B and Supplementary Figure 4B). These findings suggest that Tβ4 from MCs contributes to the levels of Tβ4 found in circulation and, to some extent, within intestinal tissue environments.
Next, we evaluated whether the reconstituted rats exhibit similar responses to stimulation with CRH, which is known to increase intestinal permeability in an MC-dependent manner[44]. Intravital microscopy demonstrated that the colons of CRH-treated rats had increased cell shedding, indicative of epithelial leakage, and that the level of shedding was even greater for “Tβ4-/- > wt-PMCs” rats (Figure 7C). Further evidence of increased barrier permeability in “Tβ4-/- > wt-PMCs” rats was demonstrated by increased serum FD4 (Figure 7D), disruption of TJ (Figure 7E) and significant infiltration of submucosal bacteria (Figure 7F). Overall, these results suggest that the Tβ4 released by activated MC is a key factor to mediate intestinal barrier impairment.
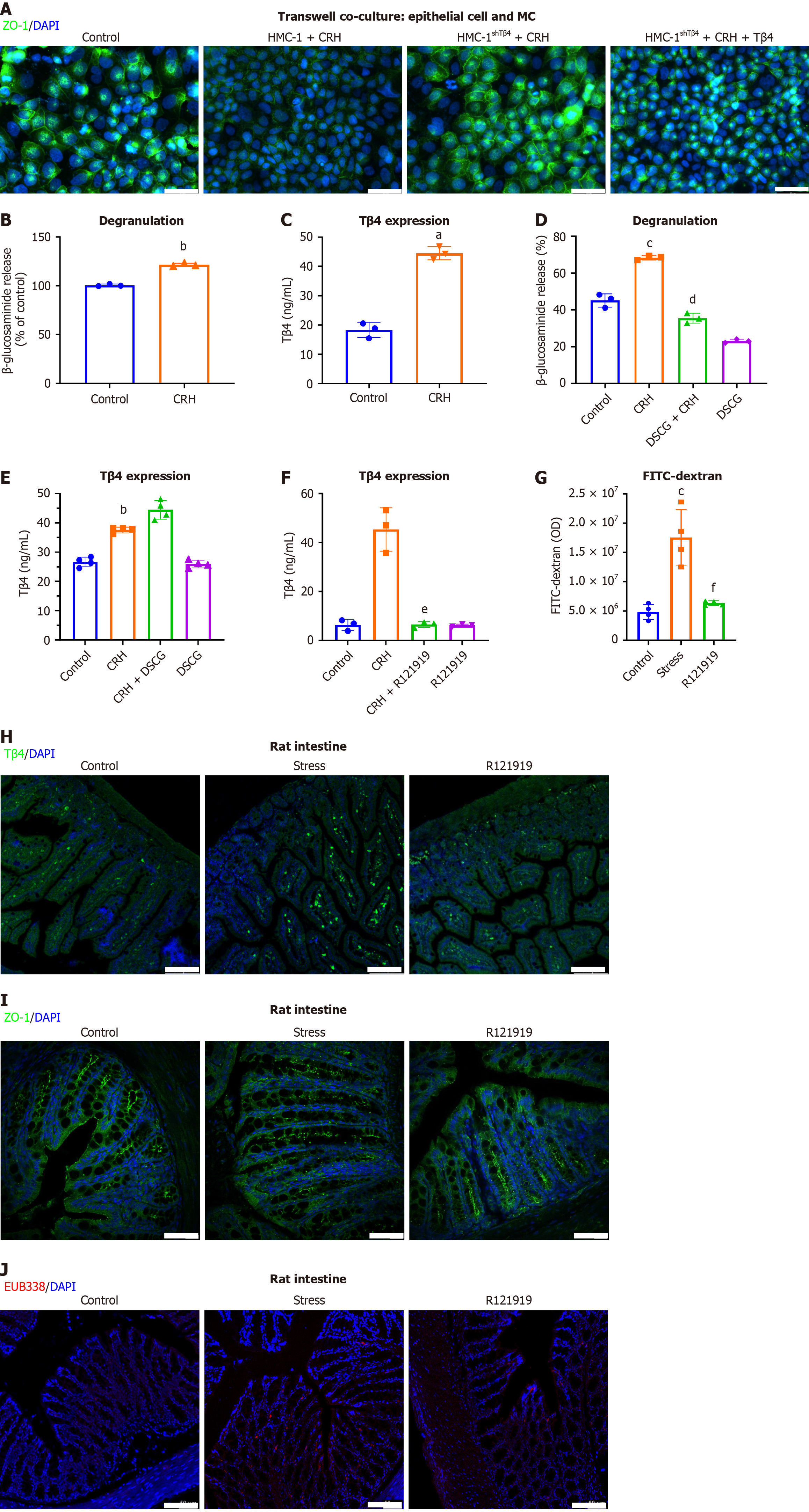
To pinpoint the specific role of Tβ4 within the MC secretome in compromising the epithelial barrier, we co-cultured Caco2 cells with CRH and either wild-type or Tβ4-knockdown HMC-1 cells (HMC-1shTβ4). Our results revealed that CRH treatment of wild-type HMC-1 cells, but not HMC-1shTβ4 cells, led to the disruption of TJs (Figure 8A), further confirming that MC-derived Tβ4 is integral in causing damage to the intestinal barrier. To determine whether Tβ4 is released from MCs via the secretory pathway, we analyzed the secretion of Tβ4 in MCs after activation with CRH. Our findings showed that CRH triggered MC degranulation, as evidenced by the release of β-hexosaminidase (Figure 8B), and also significantly increased the levels of Tβ4 release (Figure 8C). Surprisingly, however, the MC stabilizer DSCG, which is a specific degranulation inhibitor (Figure 8D), did not inhibit Tβ4 secretion (Figure 8E). This suggests that the release of Tβ4 occurs independently of degranulation.
Nevertheless, Tβ4 release was inhibited by R121919, a CRH receptor 1 (CRHR1) antagonist, pointing to CRH signaling as a specific mechanism for Tβ4 release (Figure 8F). Given the prior application of CRHR1 antagonist in mitigating the hallmark IBS symptom of visceral hypersensitivity[45], we explored its therapeutic efficacy in the stress-induced IBS model. Intraperitoneal administration of R121919 over three days blocked FD4 infiltration into peripheral blood (Figure 8G). Moreover, R121919 treatment promoted a decrease in Tβ4 Levels within the intestinal mucosa (Figure 8H) and the normalization of TJs (Figure 8I). Additionally, R121919 blocked stress-induced submucosal bacterial infiltration (Figure 8J). These findings suggest that targeting the CRH/CRHR1 signaling pathway reduces the release of Tβ4 in MCs and offers protective benefits in the stress-induced IBS model.
To identify the molecular mechanism by which Tβ4 damages the epithelial barrier, we analyzed the effects of Tβ4 on the transcriptome of Caco2 cells by RNA sequencing. A total of 243 genes were up-regulated and 186 were down-regulated after Tβ4 treatment (Figure 9A and B). KEGG analysis demonstrated enrichment in many biologically important signaling pathways, including the HIF-1, PI3K/Akt, TNF and JAK/STAT signaling pathways (Figure 9C). Furthermore, the negative effect of Tβ4 on the JAK/STAT pathway was verified by gene set enrichment analysis (GSEA, Figure 9D), western blotting (Figure 9E) and IF assay (Figure 9E and F). Co-culture of CRH-activated HMC-1shTβ4, as compared to wild-type HMC-1 cells, promoted the phosphorylation of JAK1/STAT3 and the expression of ZO-1 in Caco2 cells, while reducing the expression of MLCK, ZO-1, and these effects were reversed by JAK1/STAT3 inhibitor (SHR0302) (Figure 9G). These results indicate that Tβ4 affects MLCK/ZO-1 by inhibiting the JAK1/STAT3 signaling pathway.
A meta-system analysis of 27 studies on IBS has revealed that intestinal barrier dysfunction is prevalent in a significant majority of adults and all pediatric cases, displaying associations with symptoms like abdominal pain, bowel disturbances, overall symptom severity, depression, anxiety, and a decrease in the quality of life[46]. These observations indicate that barrier dysfunction constitutes a critical component in the development and advancement of IBS. Moreover, the secretome derived from the intestinal mucosa of IBS patients can induce barrier disruption in vivo[47]. In this study, we verified that colonic mucus from IBS patients, but not from healthy controls, has the capacity to disrupt the intestinal barrier and increase permeability in normal rats in vivo and in epithelial cells in vitro, and we further demonstrated that Tβ4 is a key agent in this process.
Our results suggest that Tβ4 is significantly more abundant in IBS mucus than in healthy control mucus. Moreover, the intestinal barrier was compromised in healthy rats in vivo when exposed to IBS mucus. However, when the rats were pretreated with a Tβ4-neutralizing antibody, the detrimental effects of the mucus were blocked. Additionally, treatment of IECs with synthetic Tβ4 resulted in disruption of the epithelial barrier. Likewise, intraperitoneal injection of Tβ4 heightened intestinal permeability, facilitating significant bacterial infiltration into the submucosa, and this occurred independent of MC involvement. Importantly, the effects of Tβ4 were found to be mediated by an increase in MLCK, as MLCK blockade with the antagonist ML7 reversed these effects. These results suggest that Tβ4 targets the core of the TJ regulation cascade.
Notably, our results suggest that Tβ4 in IBS mucus is derived from intestinal MCs, which are known to be critical in the development of IBS. We identified Tβ4-positive MCs from intestinal mucosa of IBS patients and the stressed rat model. Furthermore, the MCs secreted abundant Tβ4 when stimulated by CRH, a key factor in the pathogenesis of stress-induced IBS[44,48]. Importantly, this process occurred independently of MC stabilizers, indicating that Tβ4 secretion does not necessitate degranulation and that Tβ4’s barrier-disrupting effects are MC-independent. Further exploration revealed that blockade of the CRH receptor CRHR1 effectively prevented Tβ4 secretion. The use of the CRHR1 antagonist R121919 protected the intestinal barrier in our stressed rat model, highlighting a critical role for Tβ4 within the stress-CRH-MC axis in IBS pathogenesis. This is corroborated by a recent study demonstrating that psychological stress exacerbates intestinal inflammation and barrier dysfunction via the CRH-MC axis, though these effects were suppressed by a CRHR1 antagonist[49]. Although the therapeutic potential of CRHR1 antagonist for IBS has been noted for about two decades[50], their clinical translation faces several hurdles. Human trials have demonstrated promising effects, including reduced abdominal pain and suppressed colonic motility in IBS patients[51,52], but no CRHR1 antagonist is currently has been approved. Key challenges include suboptimal physicochemical properties and pharmacokinetics (e.g., a short half-life) limiting sustained target engagement[53], alongside the need for a clearer understanding of CRHR1 targets. Encouragingly, clinical data from studies in other conditions, such as depression, suggest that R121919 at therapeutic doses has an acceptable safety profile without endocrine-related harmful endocrine effects at therapeutic doses[54]. Therefore, our data provide new perspectives for advancing this therapeutic approach in IBS.
To further investigate the role of Tβ4 in stress-induced IBS, we generated Tβ4 knockout rats. Tβ4 knockout effectively obviated the onset of intestinal permeability induced by IBS mucus, stress, or CRH. Additionally, reintroducing Tβ4 in these rats restored the susceptibility of the intestinal barrier to stress-induced damage. We also reconstituted Tβ4 knockout rats with wild-type MCs, which suggested that MC-derived Tβ4 is sufficient to mediate stress-induced IBS development.
At the epithelial surface, IL-22 safeguards the intestinal barrier by binding to IL22RA1/IL-10RB on IECs[55]. Previous studies using intestinal injury models have highlighted the importance of antimicrobial peptides in regulating mucosal immunity via the JAK/STAT signaling cascade[56]. As a homeostatic cytokine, IL22 promotes the protection and regeneration of barrier organs such as the gastrointestinal tract. It activates the JAK/STAT pathway through a hetero
In summary, Tβ4, a key component of IBS mucus, effectively triggers intestinal barrier disruption, whether induced by CRH or stress (Figure 10). MCs are the primary source of Tβ4, and its release is contingent on CRHR1 but not on degranulation. The effects of Tβ4 are dependent on the downregulation of IL22RA1/JAK1/STAT3 signaling. These findings offer fresh perspectives on the advancement of therapies targeting MCs for IBS, highlighting Tβ4 production and its potential as a diagnostic marker.
| 1. | Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21:739-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 632] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 2. | Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 680] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 3. | Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 1324] [Article Influence: 165.5] [Reference Citation Analysis (1)] |
| 4. | Rath T, Atreya R, Bodenschatz J, Uter W, Geppert CE, Vitali F, Fischer S, Waldner MJ, Colombel JF, Hartmann A, Neurath MF. Intestinal Barrier Healing Is Superior to Endoscopic and Histologic Remission for Predicting Major Adverse Outcomes in Inflammatory Bowel Disease: The Prospective ERIca Trial. Gastroenterology. 2023;164:241-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 5. | Neurath MF, Vieth M. Different levels of healing in inflammatory bowel diseases: mucosal, histological, transmural, barrier and complete healing. Gut. 2023;72:2164-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 86] [Reference Citation Analysis (0)] |
| 6. | Di Tommaso N, Gasbarrini A, Ponziani FR. Intestinal Barrier in Human Health and Disease. Int J Environ Res Public Health. 2021;18:12836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (1)] |
| 7. | Yeste J, Illa X, Alvarez M, Villa R. Engineering and monitoring cellular barrier models. J Biol Eng. 2018;12:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Groulx S, Limburg H, Doull M, Klarenbach S, Singh H, Wilson BJ, Thombs B; pour le Groupe d’étude canadien sur les soins de santé préventifs. Ligne directrice sur le dépistage de l’adénocarcinome œsophagien chez les patients atteints de reflux gastro-œsophagien chronique. CMAJ. 2020;192:E1597-E1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Gao B, Xiang X. Interleukin-22 from bench to bedside: a promising drug for epithelial repair. Cell Mol Immunol. 2019;16:666-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Dong Y, Hu C, Huang C, Gao J, Niu W, Wang D, Wang Y, Niu C. Interleukin-22 Plays a Protective Role by Regulating the JAK2-STAT3 Pathway to Improve Inflammation, Oxidative Stress, and Neuronal Apoptosis following Cerebral Ischemia-Reperfusion Injury. Mediators Inflamm. 2021;2021:6621296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Gaudino SJ, Beaupre M, Lin X, Joshi P, Rathi S, McLaughlin PA, Kempen C, Mehta N, Eskiocak O, Yueh B, Blumberg RS, van der Velden AWM, Shroyer KR, Bialkowska AB, Beyaz S, Kumar P. IL-22 receptor signaling in Paneth cells is critical for their maturation, microbiota colonization, Th17-related immune responses, and anti-Salmonella immunity. Mucosal Immunol. 2021;14:389-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Pham TA, Clare S, Goulding D, Arasteh JM, Stares MD, Browne HP, Keane JA, Page AJ, Kumasaka N, Kane L, Mottram L, Harcourt K, Hale C, Arends MJ, Gaffney DJ; Sanger Mouse Genetics Project, Dougan G, Lawley TD. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe. 2014;16:504-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Kim DK, Jo A, Lim HS, Kim JY, Eun KM, Oh J, Kim JK, Cho SH, Kim DW. Enhanced Type 2 Immune Reactions by Increased IL-22/IL-22Ra1 Signaling in Chronic Rhinosinusitis With Nasal Polyps. Allergy Asthma Immunol Res. 2020;12:980-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Liu S, Wang Y, Hu H, Li L, Wu Y, Cao D, Cai Y, Zhang J, Zhang X. Interleukin22 regulates the homeostasis of the intestinal epithelium during inflammation. Int J Mol Med. 2019;43:1657-1668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Villablanca EJ, Selin K, Hedin CRH. Mechanisms of mucosal healing: treating inflammatory bowel disease without immunosuppression? Nat Rev Gastroenterol Hepatol. 2022;19:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 16. | Cheng L, Luo QQ, Chen SL. The role of intestinal mast cell infiltration in irritable bowel syndrome. J Dig Dis. 2021;22:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | D'Costa S, Ayyadurai S, Gibson AJ, Mackey E, Rajput M, Sommerville LJ, Wilson N, Li Y, Kubat E, Kumar A, Subramanian H, Bhargava A, Moeser AJ. Mast cell corticotropin-releasing factor subtype 2 suppresses mast cell degranulation and limits the severity of anaphylaxis and stress-induced intestinal permeability. J Allergy Clin Immunol. 2019;143:1865-1877.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Vukman KV, Försönits A, Oszvald Á, Tóth EÁ, Buzás EI. Mast cell secretome: Soluble and vesicular components. Semin Cell Dev Biol. 2017;67:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Guilarte M, Vicario M, Martínez C, de Torres I, Lobo B, Pigrau M, González-Castro A, Rodiño-Janeiro BK, Salvo-Romero E, Fortea M, Pardo-Camacho C, Antolín M, Saperas E, Azpiroz F, Santos J, Alonso-Cotoner C. Peripheral Corticotropin-Releasing Factor Triggers Jejunal Mast Cell Activation and Abdominal Pain in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Am J Gastroenterol. 2020;115:2047-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Keita ÅV, Söderholm JD. Mucosal permeability and mast cells as targets for functional gastrointestinal disorders. Curr Opin Pharmacol. 2018;43:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 22. | Hasler WL, Grabauskas G, Singh P, Owyang C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol Motil. 2022;34:e14339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 23. | Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 317] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 24. | Hannappel E, Xu GJ, Morgan J, Hempstead J, Horecker BL. Thymosin beta 4: a ubiquitous peptide in rat and mouse tissues. Proc Natl Acad Sci U S A. 1982;79:2172-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 121] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Nemolato S, Cabras T, Fanari MU, Cau F, Fraschini M, Manconi B, Messana I, Castagnola M, Faa G. Thymosin beta 4 expression in normal skin, colon mucosa and in tumor infiltrating mast cells. Eur J Histochem. 2010;54:e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Hao M, Zhong K, Bai X, Wu S, Li L, He Y, Wang Z, Sun X, Wang Q, Guo Y, Sun Y, Wu L. Upregulated Tβ4 expression in inflammatory bowel disease impairs the intestinal mucus barrier by inhibiting autophagy in mice. Exp Cell Res. 2024;434:113871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-6773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 318] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (16)] |
| 28. | Stirling ER, Soto-Pantoja DR. In Vitro Cell Impedance Assay to Examine Antigen-Specific T-Cell-Mediated Melanoma Cell Killing to Support Cancer Immunotherapy Drug Discovery. Methods Mol Biol. 2022;2413:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Stark CK, Tarkia M, Kentala R, Malmberg M, Vähäsilta T, Savo M, Hynninen VV, Helenius M, Ruohonen S, Jalkanen J, Taimen P, Alastalo TP, Saraste A, Knuuti J, Savunen T, Koskenvuo J. Systemic Dosing of Thymosin Beta 4 before and after Ischemia Does Not Attenuate Global Myocardial Ischemia-Reperfusion Injury in Pigs. Front Pharmacol. 2016;7:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Ding F, Wu J, Liu C, Bian Q, Qiu W, Ma Q, Li X, Long M, Zou X, Chen J. Effect of Xiaoyaosan on Colon Morphology and Intestinal Permeability in Rats With Chronic Unpredictable Mild Stress. Front Pharmacol. 2020;11:1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 31. | Sun Y, Li H, Liu L, Bai X, Wu L, Shan J, Sun X, Wang Q, Guo Y. A Novel Mast Cell Stabilizer JM25-1 Rehabilitates Impaired Gut Barrier by Targeting the Corticotropin-Releasing Hormone Receptors. Pharmaceuticals (Basel). 2022;16:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 32. | Yu T, Liu B, He Z, Yang M, Song J, Ma C, Ma S, Feng J, Wang X, Li J. Short-term in vitro culture of purity and highly functional rat bone marrow-derived mast cells. In Vitro Cell Dev Biol Anim. 2018;54:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Grabauskas G, Wu X, Gao J, Li JY, Turgeon DK, Owyang C. Prostaglandin E(2), Produced by Mast Cells in Colon Tissues From Patients With Irritable Bowel Syndrome, Contributes to Visceral Hypersensitivity in Mice. Gastroenterology. 2020;158:2195-2207.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 34. | Meurer SK, Neß M, Weiskirchen S, Kim P, Tag CG, Kauffmann M, Huber M, Weiskirchen R. Isolation of Mature (Peritoneum-Derived) Mast Cells and Immature (Bone Marrow-Derived) Mast Cell Precursors from Mice. PLoS One. 2016;11:e0158104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Skaper SD, Facci L. [³H]serotonin release assay using antigen-stimulated rat peritoneal mast cells. Methods Mol Biol. 2012;846:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Malbec O, Roget K, Schiffer C, Iannascoli B, Dumas AR, Arock M, Daëron M. Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J Immunol. 2007;178:6465-6475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Wang Y, Qiao M, Yao X, Feng Z, Hu R, Chen J, Liu L, Liu J, Sun Y, Guo Y. Lidocaine ameliorates intestinal barrier dysfunction in irritable bowel syndrome by modulating corticotropin-releasing hormone receptor 2. Neurogastroenterol Motil. 2023;35:e14677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Zhang R, Chen YN, Zhang J, Liu J. Elevated serum levels of diamine oxidase, D-lactate and lipopolysaccharides are associated with metabolic-associated fatty liver disease. Eur J Gastroenterol Hepatol. 2023;35:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 39. | Kang X, Jia M, Zhao L, Zhang S. Bu-Zhong-Yi-Qi Granule Enhances Colonic Tight Junction Integrity via TLR4/NF-κB/MLCK Signaling Pathway in Ulcerative Colitis Rats. Evid Based Complement Alternat Med. 2021;2021:6657141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Zhang K, Chen L, Zhu C, Zhang M, Liang C. Current Knowledge of Th22 Cell and IL-22 Functions in Infectious Diseases. Pathogens. 2023;12:176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 41. | Talbot J, Hahn P, Kroehling L, Nguyen H, Li D, Littman DR. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature. 2020;579:575-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 42. | Leeanansaksiri W, DeSimone SK, Huff T, Hannappel E, Huff TF. Thymosin beta 4 and its N-terminal tetrapeptide, AcSDKP, inhibit proliferation, and induce dysplastic, non-apoptotic nuclei and degranulation of mast cells. Chem Biodivers. 2004;1:1091-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Wyczółkowska J, Walczak-Drzewiecka A, Wagner W, Dastych J. Thymosin beta4 and thymosin beta4-derived peptides induce mast cell exocytosis. Peptides. 2007;28:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tόth J, Holvoet L, Farré R, Van Oudenhove L, Boeckxstaens G, Verbeke K, Tack J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 45. | Saito-Nakaya K, Hasegawa R, Nagura Y, Ito H, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterol Motil. 2008;20:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Hanning N, Edwinson AL, Ceuleers H, Peters SA, De Man JG, Hassett LC, De Winter BY, Grover M. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol. 2021;14:1756284821993586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 47. | Edogawa S, Edwinson AL, Peters SA, Chikkamenahalli LL, Sundt W, Graves S, Gurunathan SV, Breen-Lyles M, Johnson S, Dyer R, Graham R, Chen J, Kashyap P, Farrugia G, Grover M. Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS. Gut. 2020;69:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Labus JS, Hubbard CS, Bueller J, Ebrat B, Tillisch K, Chen M, Stains J, Dukes GE, Kelleher DL, Naliboff BD, Fanselow M, Mayer EA. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology. 2013;145:1253-1261.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (9)] |
| 49. | Kanamori A, Tanaka F, Ominami M, Nadatani Y, Fukunaga S, Otani K, Hosomi S, Kamata N, Nagami Y, Taira K, Fujiwara Y. Psychological Stress Exacerbates Inflammation of the Ileum via the Corticotropin-Releasing Hormone-Mast Cell Axis in a Mouse Model of Eosinophilic Enteritis. Int J Mol Sci. 2022;23:8538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 50. | Saito K, Kasai T, Nagura Y, Ito H, Kanazawa M, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks brain-gut activation induced by colonic distention in rats. Gastroenterology. 2005;129:1533-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 347] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, Kelleher DL, Tillisch K, Naliboff BD, Mayer EA. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491-12500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 53. | Chen C, Grigoriadis DE. NBI 30775 (R121919), an orally active antagonist of the corticotropin‐releasing factor (CRF) type‐1 receptor for the treatment of anxiety and depression. Drug Dev Res. 2005;65:216-226. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Künzel HE, Zobel AW, Nickel T, Ackl N, Uhr M, Sonntag A, Ising M, Holsboer F. Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res. 2003;37:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Keir M, Yi Y, Lu T, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med. 2020;217:e20192195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 56. | Khan SA, Kojour MAM, Han YS. Recent trends in insect gut immunity. Front Immunol. 2023;14:1272143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 57. | Wang X, Li L, Yuan G, Zhu L, Pei C, Hou L, Li C, Jiang X, Kong X. Interleukin (IL)-22 in common carp (Cyprinus carpio L.): Immune modulation, antibacterial defense, and activation of the JAK-STAT signaling pathway. Fish Shellfish Immunol. 2022;131:796-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 58. | Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 59. | Maciag G, Hansen SL, Krizic K, Kellermann L, Inventor Zøylner MJ, Ulyanchenko S, Maimets M, Baattrup AM, Riis LB, Khodosevich K, Sato T, Bressan RB, Nielsen OH, Jensen KB. JAK/STAT signaling promotes the emergence of unique cell states in ulcerative colitis. Stem Cell Reports. 2024;19:1172-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/