Published online Nov 14, 2025. doi: 10.3748/wjg.v31.i42.112106
Revised: September 1, 2025
Accepted: October 11, 2025
Published online: November 14, 2025
Processing time: 118 Days and 3.9 Hours
Endoscopic retrograde appendicitis therapy (ERAT) offers an appendix-pre
To assess the efficacy and feasibility of EDAT and compare them with those of ERAT in uncomplicated appen
In this retrospective cohort study, patients diagnosed with uncomplicated appendicitis and treated with ERAT or EDAT between January 2021 and November 2024 were reviewed. The primary outcome was intervention success. Secondary outcomes were guidewire use, stent placement, hospitalization duration, recurrence, and endoscopic direct-view features. Outcomes were compared between groups via appropriate statistical tests.
Of 170 patients, 136 underwent EDAT and 34 ERAT. EDAT showed higher intervention success than ERAT (99.3% vs 82.4%, P < 0.001), with less guidewire assistance and fewer stent placements (both P < 0.001). Hospital stay was shorter with EDAT (P = 0.039). The overall cumulative recurrence rates at 1 year were 10% in EDAT and 24% in ERAT; in the appendicolith subgroup, the recurrence rates were 5% and 14% in EDAT and ERAT, respectively. Findings were consistent in the propensity score-matched (PSM) cohort.
EDAT was demonstrated to be a more effective and feasible approach than ERAT, with a lower overall cumulative recurrence risk and within the appendicolith subgroup. Consistent results after PSM further supported the robust
Core Tip: Endoscopic direct appendicitis therapy (EDAT) shows clear clinical advantages over endoscopic retrograde appendicitis therapy (ERAT) in the management of uncomplicated appendicitis. EDAT achieved significantly higher intervention success and lower recurrence rates, including in patients with appendicoliths, a subgroup at a high risk of antibiotic treatment failure. It also shortens hospital stay and stent replacement, improving procedural efficiency. As the first head-to-head comparison of EDAT and ERAT, this study provided novel evidence supporting EDAT as a more effective, minimally invasive option, with important implications for evolving non-surgical management strategies.
- Citation: Cai J, Lu YB, Lv Y, Zhan XJ, Li T, Yang G, Ma YT, Ren JZ, Li B, Yu H, Liao SH, Guo YT, Qiu QP, Hong XP, Huang LB, Zhang Y, Huang SL. Reduced recurrence rate with a targeted approach in uncomplicated appendicitis treated with endoscopic direct vs retrograde therapy. World J Gastroenterol 2025; 31(42): 112106
- URL: https://www.wjgnet.com/1007-9327/full/v31/i42/112106.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i42.112106
Recent global data underscore the growing burden of appendicitis. According to the Global Burden of Disease study, approximately 17.7 million incident cases were recorded in 2019[1], with an age-standardized incidence rate of 229.9 per 100000, which has been 20% higher since 1990[2]. The updated estimates from 2021 similarly indicated over 17 million new cases worldwide, highlighting the persistent and rising clinical relevance[3]. Although appendectomy has been considered the standard approach for appendicitis[4], advancements in endoscopic technology have allowed a shift toward minimally invasive procedures; consequently, treatments have transitioned from open appendectomy to laparoscopic appendectomy, which is widely accepted with available resources. Yet, emerging evidence has suggested the potential functional roles and important microbiome reservoir of the appendix[5-7], along with the risks associated with negative appendectomy[8,9]; further research has also prompted the consideration of alternative treatments such as organ-preserving approaches and antibiotic therapy[10].
Endoscopic retrograde appendicitis therapy (ERAT), adapted from endoscopic retrograde cholangio-pancreatography (ERCP), has emerged as an appendix-preserving option for uncomplicated appendicitis (UA)[11]. Although comparative studies between ERAT and appendectomy have been reported[12], ERCP has fundamental constraints, including reliance on fluoroscopy for indirect visualization[13] and insufficient evidence on disease-specific features; therefore, this treatment should be further investigated.
In response to these challenges, we introduced the concept of innovative endoscopic direct appendicitis therapy (EDAT), which involves intubating the appendiceal orifice with a flexible appendicoscope through the colonoscope’s therapeutic channel, in which the appendix can be directly and visually observed for diagnosis and subsequently managed. Although limited case series have described similar techniques[14-16], few comparative studies between EDAT and ERAT for UA have been performed. To address this gap, we conducted this retrospective study to assess the efficacy and feasibility of EDAT and compared them with those of ERAT undertaken by individuals with UA.
This retrospective chart review study was approved by the corresponding ethics committees of the participating institutes and conducted in accordance with local regulations and the Declaration of Helsinki[17]. Informed consent was waived because of secondary analyses of deidentified data.
Patients diagnosed with UA or suspected appendicitis based on clinical and laboratory examinations, confirmed by an abdominal computed tomography (CT) and/or ultrasonography (US) findings, and subjected to either ERAT or EDAT from January 2021 to November 2024 were reviewed. Inclusion and exclusion criteria were based on the CODA trial[18], and eligibility age was extended to include adolescents who were offered endoscopic (non-surgical) alternative app
The participants were subjected to bowel preparation with three packs of polyethylene glycol powder. Each pack was mixed with water to obtain 1 L of electrolyte solution that was then consumed within 4 hours before the procedure. The patients were placed in a left horizontal position and appropriately anesthetized.
ERAT[19,20] was performed using a colonoscope (CF-H260AI/CF-HQ290I/CF-H290I, Olympus, Japan; EC-601WM /EC-760R-V/M, Fujifilm, Japan) with a transparent cap to locate the appendix orifice and assess inflammation. A Gerlach flap was gently displaced to expose the opening, and a catheter (PR-233Q, Olympus, Japan) was canulated using a guidewire (M00556581; Boston Scientific, Marlborough, MA, United States) for contrast injection (Ultravist, Bayer Schering Pharma AG, Berlin, Germany). Endoscopic retrograde appendicography was conducted under radiographic surveillance (Supplementary Figure 1). The lumen was irrigated; if present, fecaliths were removed with mesh baskets (CEB01010/CEB00003, Micro-Tech, Nanjing, China). In individuals with a narrowing orifice, a stricture lumen, or considerable pus, a 7-8.5-Fr plastic stent (PBD-234-0708, Olympus, Japan) was placed for drainage. Abdominal radiography was performed 2-4 weeks after the procedure to assess dislodgement or removal. Those with failed canulation were subjected to EDAT, appendectomy, or intravenous antibiotics.
EDAT was performed with a colonoscope (CF-H260AI/CF-HQ290I/CF-H290I, Olympus, Japan; EC-601WM /EC-760R-V/M, Fujifilm, Japan) and a transparent cap to identify the appendiceal orifice. The entrance of the appendix was located, and any signs of inflammation were evaluated. A digital single-operator pancreatociliary scope (EyeMax; Micro-Tech, Nanjing, China) or a cholangioscope (Vedvision, TY-ISS-3, HVDK-ISS-H-200; Vedkang, Jiangsu, China) was then in
The patients were intravenously treated with antibiotics as indicated and given a soft diet on the same day. Standard discharge criteria were clinical improvement and pain control with oral analgesia[21] or absence of pain. Routine follow-up was scheduled at 2 weeks, but an earlier review was set if the patients suffered worsening symptoms. The patients with stent placement were subjected to abdominal ultrasound to assess their stent status and advised to have their colonoscopy removed if spontaneous discharge had not occurred.
The primary outcome variable was intervention success, defined as successful appendiceal cannulation with a catheter in ERAT or intubation with an appendicoscope in EDAT. The secondary outcomes were procedure time (from colonoscopy initiation to treatment completion), appendiceal orifice features, cavity contents, intraluminal mucosa (EDAT only), stent placement rate, hospitalization length, and 1-year cumulative recurrence rate of acute appendicitis.
Descriptive statistics were used to summarize baseline and outcome data. Continuous variables were compared using a t-test for normally distributed data or the Wilcoxon rank sum test for non-normal data. Categorical variables were compared using the χ2 test. Time-to-event outcomes (e.g., hospital discharge) were analyzed with the Kaplan-Meier method and compared using the log-rank test. Results with P < 0.05 were considered statistically significant.
Propensity score matching (PSM) was performed to balance patient characteristics between EDAT and ERAT groups by using the 1:1 nearest-neighbor matching (caliper = 0.5) derived from a logistic regression model that included age and sex. Data were statistically analyzed using Statistical Analysis System software version 9.4 (SAS Institute, Cary, NC, United States).
Of the 170 patients included, 136 underwent EDAT, and 34 had ERAT. Overall, the intervention success of EDAT was higher (99.3% vs 82.4%), and 1-year recurrence was significantly lower (10% vs 24%) than those of ERAT. In the appendi
A total of 185 patients diagnosed with UA and subjected to either ERAT or EDAT were screened for eligibility between January 2021 and November 2024. After the initial introduction of EDAT in 2023, it was broadly applied partly because it required less skillful requirements than ERAT and had no radiation exposure risk; most patients with UA adopted EDAT as a non-surgical alternative from the year during the study period. Consequently, eleven were ineligible because of incomplete interest variables, neoplasm evidence, or suspected complicated appendicitis; therefore, 170 patients (97 males and 73 females) with a median age of 34.0 years were retained. Among them, 34 (20.0%) underwent ERAT, while 136 (80.0%) had EDAT (Supplementary Figure 3). For a better-balanced cohort, a 1:1 propensity score-matched analysis (32 matched pairs by age and sex) was performed to reduce baseline confounders, and the overall findings resembled those of the full cohort. Our primary emphasis remained on the full cohort, with the larger sample size might yield more robust and generalizable outcomes; the PSM results are available in the Supplementary material. Clinical variables, laboratory parameters, and imaging modality confirmation were similar in both groups (Table 1 and Supplementary Table 1).
| EDAT (n = 136) | ERAT (n = 34) | P value1 | |
| Clinical characteristics | |||
| Age, years | 33.0 (19.5-46.0) | 35.5 (27.0-49.0) | 0.251 |
| Sex, male | 77 (56.6) | 20 (58.8) | 0.816 |
| Symptoms | |||
| Vomiting/nausea | 26 (19.1) | 11 (32.4) | 0.094 |
| RLQ abdominal pain | 119 (87.5) | 26 (76.5) | 0.104 |
| Fever | 21 (15.4) | 6 (17.6) | 0.753 |
| Laboratory characteristics | |||
| WBC, × 109/L2 | 7.4 (5.9-10.8) | 7.2 (5.1-9.5) | 0.431 |
| Neutrophils proportion > 75% | 41 (31.8) | 10 (29.4) | 0.791 |
| Serum hs-CRP3, mg/L | 5.1 (0.6-28.8) | 9.2 (2.2-21.4) | 0.391 |
| Imaging test | |||
| Computed tomography | 115 (84.6) | 32 (94.1) | 0.145 |
| Ultrasonography | 125 (91.9) | 32 (94.1) | 0.665 |
Intervention success (primary outcome) was achieved in 135/136 (99.3%) patients in the EDAT group with successful intubation and 28/34 (82.4%) patients in the ERAT group with successful cannulation (P < 0.001). In the ERAT group, 6 (14.7%) cases experienced failed cannulation in ERAT (unsuccessful guidewire placement, Supplementary Figure 4), 1 crossed over to EDAT, 2 were transferred for appendectomy, and 3 were treated with intravenous antibiotics. In the EDAT group, 1 case had a severe edematous orifice that could not be identified for intubation and was treated with antibiotics. Two cases of successful intubation with perforation were encountered: One was iatrogenic; the other had an endoscopically identified perforation following fecalith removal (Supplementary Figure 2E), subjected to negative preoperative CT imaging. Both of them crossed over to appendectomy.
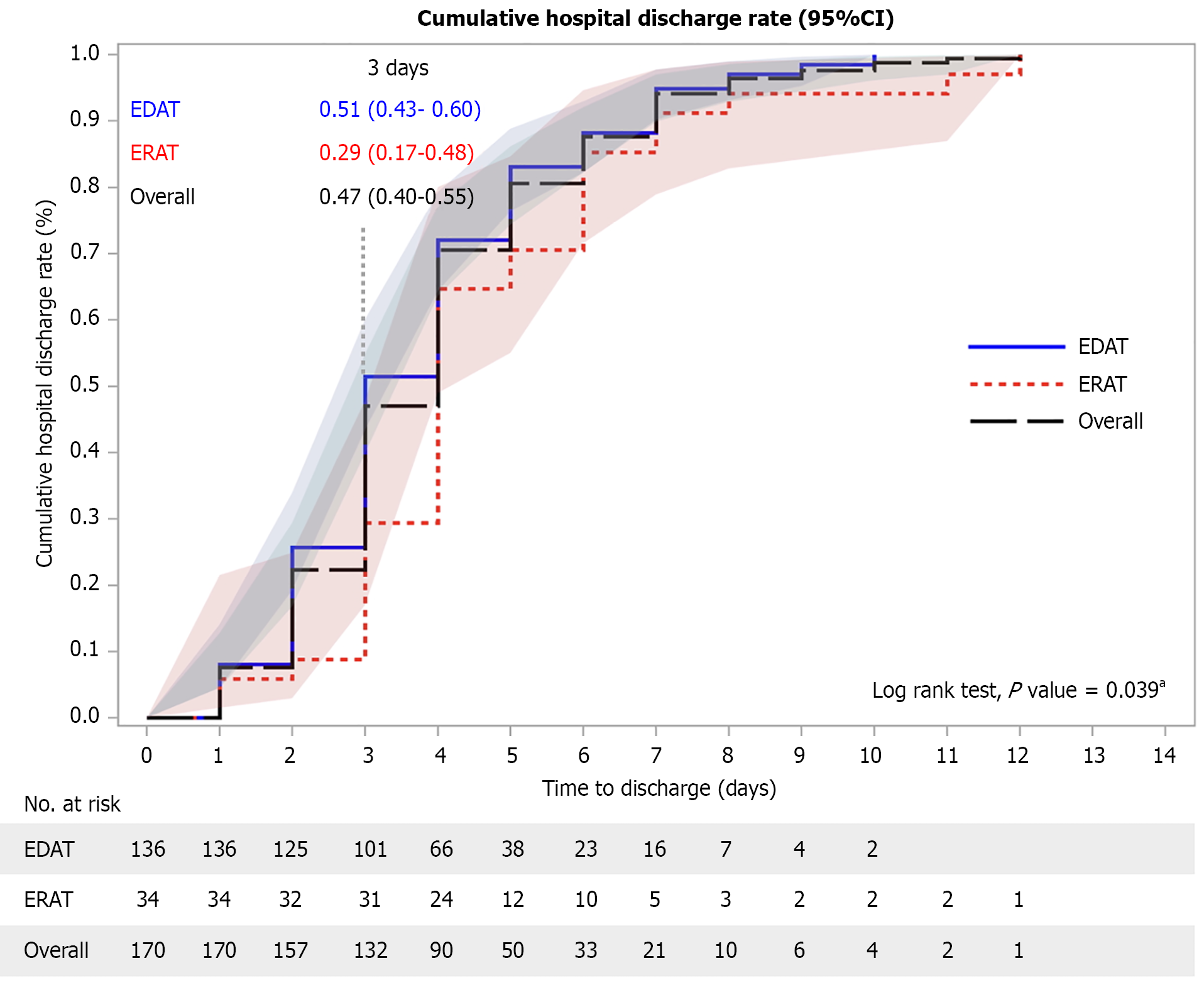
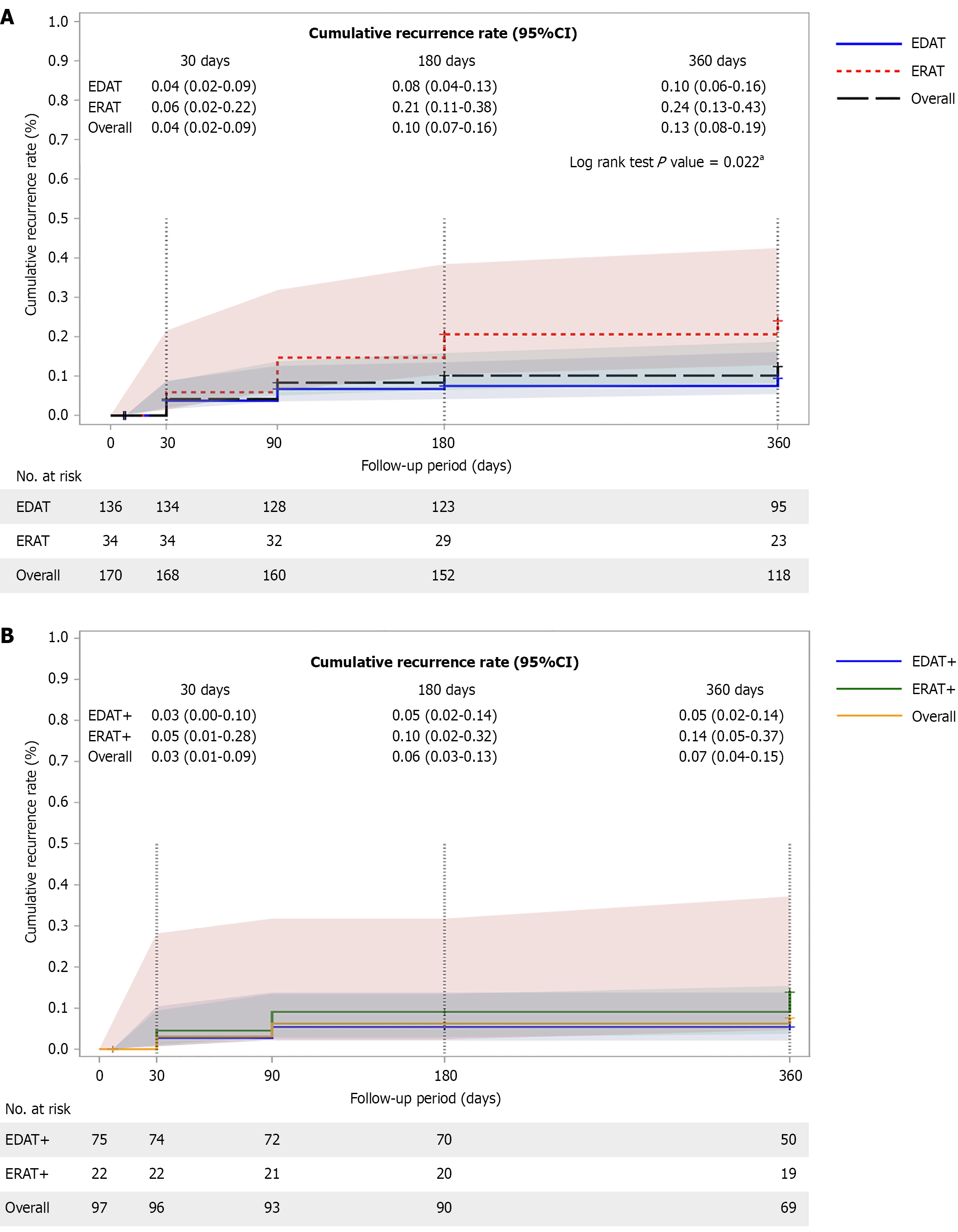
Kaplan-Meier and log-rank test results showed significant differences in the median in-hospital period (P = 0.039, Figure 1). Table 2 presents the results of additional secondary clinical outcomes. Fewer patients required guidewire-assisted intubation and stent placements (P < 0.001) in the EDAT group than in the ERAT group (P < 0.001). Of the 170 patients, 141 (83%) were followed for more than one year, and survey data on recurrent appendicitis were available for 94% of this subgroup. The cumulative recurrence rates were 4% for EDAT and 6% for ERAT at 30 days, 8% vs 21% at 180 days, and 10% vs 24% at 1 year (Figure 2A). In the appendicolith subgroup, the recurrence rates at 1-year follow-up were 5% in EDAT and 14% in ERAT (Figure 2B). Findings from 1:1 PSM analysis were consistent with those of the overall cohort (Supplementary Tables 1 and 2, Supplementary Figures 5, 6 and 7).
| EDAT (n = 136) | ERAT (n = 34) | P value1 | |
| Outcomes | |||
| Intervention success | 135 (99.3) | 28 (82.4) | < 0.001a |
| Guide-wire assistance2 | 48 (35.3) | 34 (100.0) | < 0.001a |
| Stent deployment | 13 (9.6) | 26 (76.5) | < 0.001a |
| Colonoscopic view of appendiceal orifice | |||
| Edematous/inflammatory | 105 (81.8) | 22 (66.7) | 0.209 |
| Pus exudate | 39 (28.7) | 10 (31.3) | 0.773 |
| Appendiceal content3 | |||
| Appendicolith | 75 (55.6) | 22 (73.3) | 0.074 |
| Pus | 55 (40.7) | 18 (60.0) | 0.055 |
| Lumen stricture | 21 (15.6) | 7 (23.3) | 0.385 |
| Unable to identify | 0 (0) | 4 (11.8) | N/A |
| Appendicoscopic view of appendiceal mucosa4 | |||
| Smooth | 7 (5.3) | N/A | N/A |
| Erosive | 115 (85.2) | N/A | N/A |
The intracavity content with direct-view EDAT or indirect fluoroscopic images of ERAT, including pus and/or intraluminal stricture, fecaliths, were similar (Table 2 and Supplementary Table 2). Specifically, one patient was dia
The intervention success rate differed markedly between EDAT and ERAT (99.3% vs 82.4%, P < 0.001). Consistent with a recent randomized clinical trial study[22], the present study revealed that cannulation failure in the ERAT group primarily resulted from factors such as a swollen mucosa and the narrow lumen of the appendix. The appendix base is usually connected to the cecum, but its tip may take various positions, and most appendices show a tortuous curvature between the orifice and lumen. The appendicoscope’s rounded tip facilitates atraumatic passage, while its four-directional adjustability allows direct visualization and careful navigation through these curves and obstructions. As for ERAT, the techniques applied were similar to those of ERCP, which requires radiation exposure and guidewire assistance for appendiceal cannulation. However, the guidewire, characterized by its resilient shaft and remarkably flexible characteristics, poses technical hurdles for precise insertion through the narrowing and convoluted appendiceal passage; as such, its use demands a skillful and experienced operator and a prolonged training period, particularly for clinicians lacking experience in ERCP procedures. In comparison, proficiency in EDAT can be attained through a relatively short-term training, making it more accessible for endoscopists and encouraging its adoption across a broader range of clinical settings. Additionally, the absence of radiation exposure further enhances its clinical appeal for certain individuals, such as pregnant women and children. These advantages may explain the increasing number of EDAT procedures performed in participating centers since its introduction. Furthermore, the presence of a solid fecalith or an edematous orifice can impede guidewire insertion, ultimately leading to unsuccessful ERAT[19]. By contrast, the direct visualization of EDAT leads to an increased rate of successful intervention because it is less constrained by obstructive orifice changes, such as laser lithotripsy, which can facilitate successful intubation.
In previous comparative studies on non-operative management for UA, both ERAT[23] and EDAT[24] have de
Because appendicoliths are a well-established predictor of antibiotic failure, we specifically examined this subgroup and found that the lower recurrence with endoscopic therapy persisted. In the CODA trial[18,25], the 1-year app
Although acute appendicitis is a common abdominal surgical emergency globally, its accurate diagnosis remains challenging and often leads to negative appendectomy[27]. Nevertheless, advanced diagnostic tools such as CT and US have improved diagnostic accuracy. However, challenges persist because of variations in appendix size and position, as well as individual conditions such as obesity and intestinal gas superposition[28]. The effectiveness of diagnosis via ERAT’s fluoroscopic imaging[20] is also limited. In our study, 10 (29.4%) patients who underwent ERAT could not be diagnosed; among them, 6 had unsuccessful cannulation (Supplementary Figure 4), and 4 had ineffective appendicography. Fluoroscopic appendicography mainly relies on contour changes[20], requires precise contrast injection, and lacks sensitivity for intraluminal issues such as pus or mucosal lining, which may further limit its diagnostic accuracy. Alternatively, an appendicoscope provides a real-time view; thus, the intraluminal content and the lining mucosa can be inspected in detail, thereby supporting the accurate diagnosis of appendicitis. For example, one case of pinworm infestation was successfully identified using EDAT (Supplementary Figure 2H), highlighting a scenario in which ERAT might not detect such an infection. EDAT offers immediate intraluminal inspection, thereby improving the diagnostic accuracy and reducing the incidence of negative appendectomies. Our recent report has demonstrated similar benefits in diverticulitis through the implementation of endoscopic direct diverticulitis therapy[29].
Furthermore, acute appendicitis has been associated with colorectal cancer (CRC)[30-32], but causality remains unclear. Therefore, careful appendiceal assessment should be performed during surveillance colonoscopies. Using an appen
The marked reduction in stent placement with EDAT (9.6% vs 76.5%, P < 0.001) highlights another benefit of direct vision. By reducing uncertainty about intraluminal pathology, EDAT allows more selective stent use and helps avoid complications such as perforation or mucosal injury. Moreover, the significantly shorter hospital stays observed in the EDAT group (P = 0.039) suggested a more efficient resolution of symptoms, likely because of precise intra-appendiceal assessment and thorough treatment of underlying etiologies.
EDAT achieved higher intervention success, lower recurrence, and shorter hospitalization than ERAT did. It also required less technically challenging skills, reduced stent replacements, and avoided radiation exposure compared with ERAT. Therefore, EDAT embodies the principles of precision and individualized medicine. This minimally invasive alternative can be integrated into clinical pathways, particularly for those with appendicoliths at a high risk of antibiotic failure. However, larger multicenter studies with extended follow-up are needed to confirm its role in routine practice.
We acknowledged several limitations. First, the retrospective design may introduce selection bias because patients were not randomly assigned to ERAT or EDAT. Intervention choice could have been influenced by operator experience and patient preference, thereby limiting the generalizability of these findings. Differences in operator background might have also affected our outcomes, and this potential bias should be considered in the interpretation. Moreover, because the procedures were performed in a specialized tertiary setting with experienced endoscopists, the generalizability of EDAT to non-specialized centers may be limited. Validation in broader practice environments is needed. Second, although our cohort of 170 patients provided adequate statistical power (92.5%), the sample size was determined by eligible cases rather than by an a priori calculation. The imbalance between EDAT and ERAT reflected real-world practice, where EDAT is increasingly favored. Although PSM inevitably reduced the effective sample size and modestly decreased the statistical power, this trade-off strengthened the internal validity by minimizing baseline imbalances. Importantly, PSM was applied only as a sensitivity analysis to confirm the robustness of the primary findings, and results were consistent across matched and unmatched cohorts. Third, residual confounding could not be excluded. Although patient selection adhered to the predefined criteria to minimize baseline variability, unmeasured factors such as subtle anatomical variation, immune status, and operator technique were not systematically captured and might have influenced the recurrence risk. Fourth, the absence of follow-up data beyond one year may underrepresent recurrence and late complications. Nevertheless, prior studies indicate an early cluster of recurrences within the first year[24,25], and the mechanistic advantages of EDAT supported the likelihood that its observed lower recurrence than ERAT may persist. However, this finding should be confirmed in future studies.
EDAT achieved higher intervention success and lower recurrence than ERAT did while avoiding radiation exposure and stent-related issues. By enabling direct appendiceal inspection, it offered targeted treatment and streamlined care. These findings supported EDAT as a definitive, minimally invasive alternative treatment for UA. The mechanistic advantages of EDAT suggest that its benefits may be sustained beyond one year but require validation in larger studies with extended follow-up.
We gratefully acknowledge Dr. Sandy Hui-Shan Hsieh for valuable editorial assistance and biostatistician Betty Cheng for support with statistical analysis.
| 1. | Guan L, Liu Z, Pan G, Zhang B, Wu Y, Gan T, Ouyang G. The global, regional, and national burden of appendicitis in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. BMC Gastroenterol. 2023;23:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 2. | Yang Y, Guo C, Gu Z, Hua J, Zhang J, Qian S, Shi J. The Global Burden of Appendicitis in 204 Countries and Territories from 1990 to 2019. Clin Epidemiol. 2022;14:1487-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | GBD 2021 Appendicitis Collaborator Group. Trends and levels of the global, regional, and national burden of appendicitis between 1990 and 2021: findings from the Global Burden of Disease Study 2021. Lancet Gastroenterol Hepatol. 2024;9:825-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Sceats LA, Trickey AW, Morris AM, Kin C, Staudenmayer KL. Nonoperative Management of Uncomplicated Appendicitis Among Privately Insured Patients. JAMA Surg. 2019;154:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Wu T, Yang Y, Wu Y, Lu L, Dong S. Complications after appendectomy in patients with treated appendicitis: results from a retrospective study. Ann Palliat Med. 2021;10:12546-12553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Song MY, Ullah S, Yang HY, Ahmed MR, Saleh AA, Liu BR. Long-term effects of appendectomy in humans: is it the optimal management of appendicitis? Expert Rev Gastroenterol Hepatol. 2021;15:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Masahata K, Umemoto E, Kayama H, Kotani M, Nakamura S, Kurakawa T, Kikuta J, Gotoh K, Motooka D, Sato S, Higuchi T, Baba Y, Kurosaki T, Kinoshita M, Shimada Y, Kimura T, Okumura R, Takeda A, Tajima M, Yoshie O, Fukuzawa M, Kiyono H, Fagarasan S, Iida T, Ishii M, Takeda K. Generation of colonic IgA-secreting cells in the caecal patch. Nat Commun. 2014;5:3704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Ambe PC. Negative Appendectomy. It is Really Preventable? J Invest Surg. 2019;32:474-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Jeon BG. Predictive factors and outcomes of negative appendectomy. Am J Surg. 2017;213:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Salminen P, Paajanen H, Rautio T, Nordström P, Aarnio M, Rantanen T, Tuominen R, Hurme S, Virtanen J, Mecklin JP, Sand J, Jartti A, Rinta-Kiikka I, Grönroos JM. Antibiotic Therapy vs Appendectomy for Treatment of Uncomplicated Acute Appendicitis: The APPAC Randomized Clinical Trial. JAMA. 2015;313:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 566] [Article Influence: 51.5] [Reference Citation Analysis (1)] |
| 11. | Liu BR, Song JT, Han FY, Li H, Yin JB. Endoscopic retrograde appendicitis therapy: a pilot minimally invasive technique (with videos). Gastrointest Endosc. 2012;76:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Sarraf P, Prabhu A, Love J, Agrawal R, Ghoulam E, Villa E. Endoscopic retrograde appendicitis therapy in adults with uncomplicated acute appendicitis: a systematic review and meta-analysis. iGIE. 2023;2:522-528.e3. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Kang J, Zhang W, Zeng L, Lin Y, Wu J, Zhang N, Xie X, Zhang Y, Liu X, Wang B, Yang R, Jiang X. The modified endoscopic retrograde appendicitis therapy versus antibiotic therapy alone for acute uncomplicated appendicitis in children. Surg Endosc. 2021;35:6291-6299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Kong LJ, Liu D, Zhang JY, Ullah S, Zhao L, Li D, Yang H, Liu BR. Digital single-operator cholangioscope for endoscopic retrograde appendicitis therapy. Endoscopy. 2022;54:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Kong LJ, Zhang JY, Ullah S, Liu BR. SpyGlass-Guided Laser Lithotripsy for the Treatment of Giant Appendiceal Fecalith: First Human Case Report. Am J Gastroenterol. 2021;116:1981-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Wang F, Zhou XR, Zhang Y, Wang Y, Du ZQ, Liu WH. Digital single-operator cholangioscopy-guided appendiceal intubation for endoscopic retrograde appendicitis therapy in a pregnant woman (with video). Gastrointest Endosc. 2023;98:1034-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16597] [Cited by in RCA: 20295] [Article Influence: 1561.2] [Reference Citation Analysis (9)] |
| 18. | CODA Collaborative, Flum DR, Davidson GH, Monsell SE, Shapiro NI, Odom SR, Sanchez SE, Drake FT, Fischkoff K, Johnson J, Patton JH, Evans H, Cuschieri J, Sabbatini AK, Faine BA, Skeete DA, Liang MK, Sohn V, McGrane K, Kutcher ME, Chung B, Carter DW, Ayoung-Chee P, Chiang W, Rushing A, Steinberg S, Foster CS, Schaetzel SM, Price TP, Mandell KA, Ferrigno L, Salzberg M, DeUgarte DA, Kaji AH, Moran GJ, Saltzman D, Alam HB, Park PK, Kao LS, Thompson CM, Self WH, Yu JT, Wiebusch A, Winchell RJ, Clark S, Krishnadasan A, Fannon E, Lavallee DC, Comstock BA, Bizzell B, Heagerty PJ, Kessler LG, Talan DA. A Randomized Trial Comparing Antibiotics with Appendectomy for Appendicitis. N Engl J Med. 2020;383:1907-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 377] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 19. | Liu BR, Ma X, Feng J, Yang Z, Qu B, Feng ZT, Ma SR, Yin JB, Sun R, Guo LL, Liu WG. Endoscopic retrograde appendicitis therapy (ERAT) : a multicenter retrospective study in China. Surg Endosc. 2015;29:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Li Y, Mi C, Li W, She J. Diagnosis of Acute Appendicitis by Endoscopic Retrograde Appendicitis Therapy (ERAT): Combination of Colonoscopy and Endoscopic Retrograde Appendicography. Dig Dis Sci. 2016;61:3285-3291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Gumaa D, Shrestha S, Jenner D. 620 Daycase Appendicectomy and Same Day Discharge After Laparoscopic Appendicectomy. Br J Surg. 2021;108:znab259.519. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Shen Z, Sun P, Jiang M, Zhen Z, Liu J, Ye M, Huang W. Endoscopic retrograde appendicitis therapy versus laparoscopic appendectomy versus open appendectomy for acute appendicitis: a pilot study. BMC Gastroenterol. 2022;22:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Li D, Yang B, Liao J, Li Y, Liu D, Zhao L, Meng X, Hu H, Kong L, Podda M, Ullah S, Liu B. Endoscopic retrograde appendicitis therapy or antibiotics for uncomplicated appendicitis. Br J Surg. 2023;110:635-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Zhan K, Bai Y, Liu T, Su X, Yang Q, Liu Y, Zhou X, Zhang Y, Tang J, Jiang Z, Yang X, Liu W. Visual Endoscopic Retrograde Appendicitis Therapy Vs Antibiotic Therapy for Treatment of Uncomplicated Acute Appendicitis. Am J Gastroenterol. 2025;120:1036-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | CODA Collaborative, Davidson GH, Flum DR, Monsell SE, Kao LS, Voldal EC, Heagerty PJ, Fannon E, Lavallee DC, Bizzell B, Lawrence SO, Comstock BA, Krishnadasan A, Winchell RJ, Self WH, Thompson CM, Farjah F, Park PK, Alam HB, Saltzman D, Moran GJ, Kaji AH, DeUgarte DA, Salzberg M, Ferrigno L, Mandell KA, Price TP, Siparsky N, Glaser J, Ayoung-Chee P, Chiang W, Victory J, Chung B, Carter DW, Kutcher ME, Jones A, Holihan J, Liang MK, Faine BA, Cuschieri J, Evans HL, Johnson J, Patton JH, Coleman N, Fischkoff K, Drake FT, Sanchez SE, Parsons C, Odom SR, Kessler LG, Talan DA. Antibiotics versus Appendectomy for Acute Appendicitis - Longer-Term Outcomes. N Engl J Med. 2021;385:2395-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 26. | Tao L, Wang H, Guo Q, Guo S, Guo X, Liu S. Appendicoscopy using single-operator cholangioscope in the management of acute obstructive appendicitis: a novel alternative (with video). Gastrointest Endosc. 2024;100:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Henriksen SR, Christophersen C, Rosenberg J, Fonnes S. Varying negative appendectomy rates after laparoscopic appendectomy: a systematic review and meta-analysis. Langenbecks Arch Surg. 2023;408:205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Giljaca V, Nadarevic T, Poropat G, Nadarevic VS, Stimac D. Diagnostic Accuracy of Abdominal Ultrasound for Diagnosis of Acute Appendicitis: Systematic Review and Meta-analysis. World J Surg. 2017;41:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Cai J, Huang S, Lu Y, Liao S, Yang G, Li B, Ren J. First report of the use of a digital single-operator cholangioscope for endoscopic direct diverticulitis therapy. Endoscopy. 2024;56:E466-E467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Viennet M, Tapia S, Cottenet J, Bernard A, Ortega-Deballon P, Quantin C. Increased risk of colon cancer after acute appendicitis: a nationwide, population-based study. EClinicalMedicine. 2023;63:102196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 31. | Hajibandeh S, Hajibandeh S, Morgan R, Maw A. The incidence of right-sided colon cancer in patients aged over 40 years with acute appendicitis: A systematic review and meta-analysis. Int J Surg. 2020;79:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Liu Z, Ma X, Zhu C, Fang JY. Risk of colorectal cancer after appendectomy: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2023;38:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/