Published online Nov 7, 2025. doi: 10.3748/wjg.v31.i41.110955
Revised: July 24, 2025
Accepted: September 24, 2025
Published online: November 7, 2025
Processing time: 140 Days and 0.3 Hours
Inflammatory bowel disease (IBD) is a group of chronic, inflammatory disorders that include Crohn’s disease and ulcerative colitis. IBD arises from the interaction of various environmental and genetic factors. Altered gut permeability and mito
To investigate whether GDF15 has a role in IBD and how GDF15 impacts colonic epithelium.
Circulating levels of GDF15 were assessed in plasma samples from IBD patients and healthy controls using an enzyme-linked immunosorbent assay. To study the effects of GDF15 on the colonic mucosa, we employed two different in vitro cul
We found that circulating GDF15 Levels were elevated in IBD patients and cor
In the present study, we describe a novel mechanism in IBD pathophysiology, linking mitochondrial stress to the disruption of the intestinal barrier and increa
Core Tip: Mitochondrial dysfunction in intestinal mucosa is emerging as a novel mechanism in inflammatory bowel disease pathogenesis. In the present study, we show that growth differentiation factor 15 (GDF15), a marker of mitochondrial stress, is elevated in plasma from both ulcerative colitis and Crohn’s disease patients. Furthermore, levels of GDF15 correlate with markers of inflammation and intestinal permeability. In vitro assays using colonic organoids further showed that GDF15 alters the intestinal barrier, reducing the levels of zonula occludens 1 and claudin 1, and subsequently increases intestinal permeability. Targeting GDF15 may offer a promising strategy to prevent disruption of the intestinal barrier, potentially reducing immune overactivation.
-
Citation: Ruiz-Malagón AJ, Herraiz-Vilela M, Serrano-Pino R, García-Ávila P,
Díaz- Suárez L, Carmona-Segovia AD, Becerra-Munoz VM, Jiménez-Navarro M, Arranz-Salas I, López-Villodres JA, Fernández-Castañer A, Gutiérrez-Martínez F, Rodríguez-González FJ, Camargo-Camero R, Alcaín-Martínez G, Rodríguez-Díaz C, García-Fuentes E, Sánchez-Quintero MJ, López-Gómez C. Growth differentiation factor 15 alters intestinal barrier and increases permeability: A new molecular target in inflammatory bowel disease. World J Gastroenterol 2025; 31(41): 110955 - URL: https://www.wjgnet.com/1007-9327/full/v31/i41/110955.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i41.110955
Inflammatory bowel disease (IBD) is a group of chronic, inflammatory disorders that affect the gastrointestinal tract. IBD primarily includes Crohn’s disease (CD) and ulcerative colitis (UC). The development of IBD is thought to result from the interaction of multiple environmental factors in genetically predisposed individuals. Alteration of gut permeability is a key mechanism in IBD pathophysiology, facilitating the contact between epithelial cells and gut microbiota and toxins, which further activate the immune system and trigger proinflammatory pathways[1,2]. Another, yet poorly understood, mechanism in IBD is the disruption of mitochondrial homeostasis[3]. A better understanding of the specific pathogenetic mechanisms involving mitochondrial dysfunction is needed to uncover potential therapeutic targets within this pathway. Our group has previously reported activation of the mitochondrial unfolded protein response (UPRmt) linked to pro-inflammatory signaling in IBD[4]. To further investigate the role of UPRmt in IBD, we aimed to study growth differentiation factor 15 (GDF15), a member of the transforming growth factor (TGF)-β superfamily and a downstream mediator of UPRmt.
GDF15 is considered a biomarker of mitochondrial dysfunction, and its circulating levels are elevated in primary mitochondrial disorders[5,6]. However, GDF15 is also gaining much attention beyond the field of mitochondrial dis
Healthy controls (HC) (volunteers from the colon cancer screening program with non-pathological findings) and patients with a confirmed IBD diagnosis attending for a follow-up colonoscopy were recruited at the Gastroenterology De
| HC (n = 23) | IBD (n = 39) | MI (n = 19) | HC vs IBD | ||
| CD (n = 21) | UC (n = 18) | ||||
| Sex ratio, female/male (%) | 11/12, (47.8/52.2) | 11/10, (52.4/47.6) | 11/7, (61.1/38.9) | 10/9, (52.6/47.4) | NS |
| Age (years) | 45.4 ± 13.6 | 40.48 ± 12.6 | 46.3 ± 15.0 | 78.4 ± 5.8 | NS |
| BMI | 24.0 ± 3.3 | 24.3 ± 5.1 | 24.2 ± 3.5 | 28.8 ± 3.9 | NS |
| Current smoker, yes/no (%) | 4/19, (17.4/82.6) | 8/13, (38.1/61.9) | 3/15, (16.7/83.3) | 0/19, (0.0/100.0) | NS |
| Fecal calprotectin | N/A | 785.9 ± 1128.2 | 663.6 ± 1213.7 | N/A | N/A |
| CRP | 4.97 ± 5.66 | 11.2 ± 17.5 | 8.8 ± 11.3 | 22.1 ± 23.1 | P = 0.006 |
| Glucose | 83.5 ± 10.1 | 85.4 ± 19.9 | 103.7 ± 81.3 | 147.8 ± 43.4 | NS |
| Creatinine | 0.81 ± 0.16 | 0.75 ± 0.12 | 0.76 ± 0.20 | 1.3 ± 0.4 | NS |
| Urea | 26.0 ± 6.3 | 23.3 ± 11.4 | 26.2 ± 7.2 | 52.3 ± 16.2 | NS |
| LDL | 123.7 ± 26.2 | 95.5 ± 32.5 | 102.1 ± 27.9 | 85.1 ± 26.5 | NS |
| Haptoglobin | 130.4 ± 56.6 | 147.4 ± 32.9 | 158.2 ± 97.2 | N/A | NS |
| Crohn’s disease | Ulcerative colitis | |
| n | 21 | 18 |
| Time of disease (years) | 11.3 ± 9.0 | 13.0 ± 8.5 |
| Treatment | ||
| Corticoids | 6 (28.6) | 2 (11.1) |
| 5-aminosalicylic acid | 3 (14.3) | 14 (77.8) |
| Sulfasalazine | 3 (14.3) | 1 (5.6) |
| Azathioprine | 4 (19.0) | 4 (22.2) |
| anti-TNF | 3 (14.3) | 1 (5.6) |
| Disease phenotype | Inflammatory 8 (38.1) | Left-sided colitis 8 (44.4) |
| Stricturing 4 (19.0) | Extensive colitis 9 (50.0) | |
| Fistulizing 7 (33.3) |
Colon biopsy samples were obtained from normal-appearing mucosa from HC and CD patients, and were processed immediately after their reception at the Virgen de la Victoria University Hospital Biobank (Andalusian Public Health System Biobank) either for RNA isolation or for the development of colonic organoids. Blood was collected, and complete biochemistry tests were run. Plasma was isolated immediately and aliquoted for enzyme-linked immunosorbent assay assays. Fecal calprotectin values within ± 1 month were obtained for IBD patients. Blood samples from MI patients were obtained within 24 hours after stabilization, and plasma was isolated and aliquoted immediately.
Plasma GDF15 levels and plasma lipopolysaccharide-binding protein (LBP) were determined by enzyme-linked immunosorbent assay (No. EHGDF15X10 for GDF15 and No. EH297RB for LBP, Invitrogen, CA, United States) following the manufacturer’s instructions. One aliquot of plasma from each participating subject was thawed. Each sample was run in duplicate, and the average of the duplicates was calculated. A standard curve was used to determine the concentration of each sample in ng/mL.
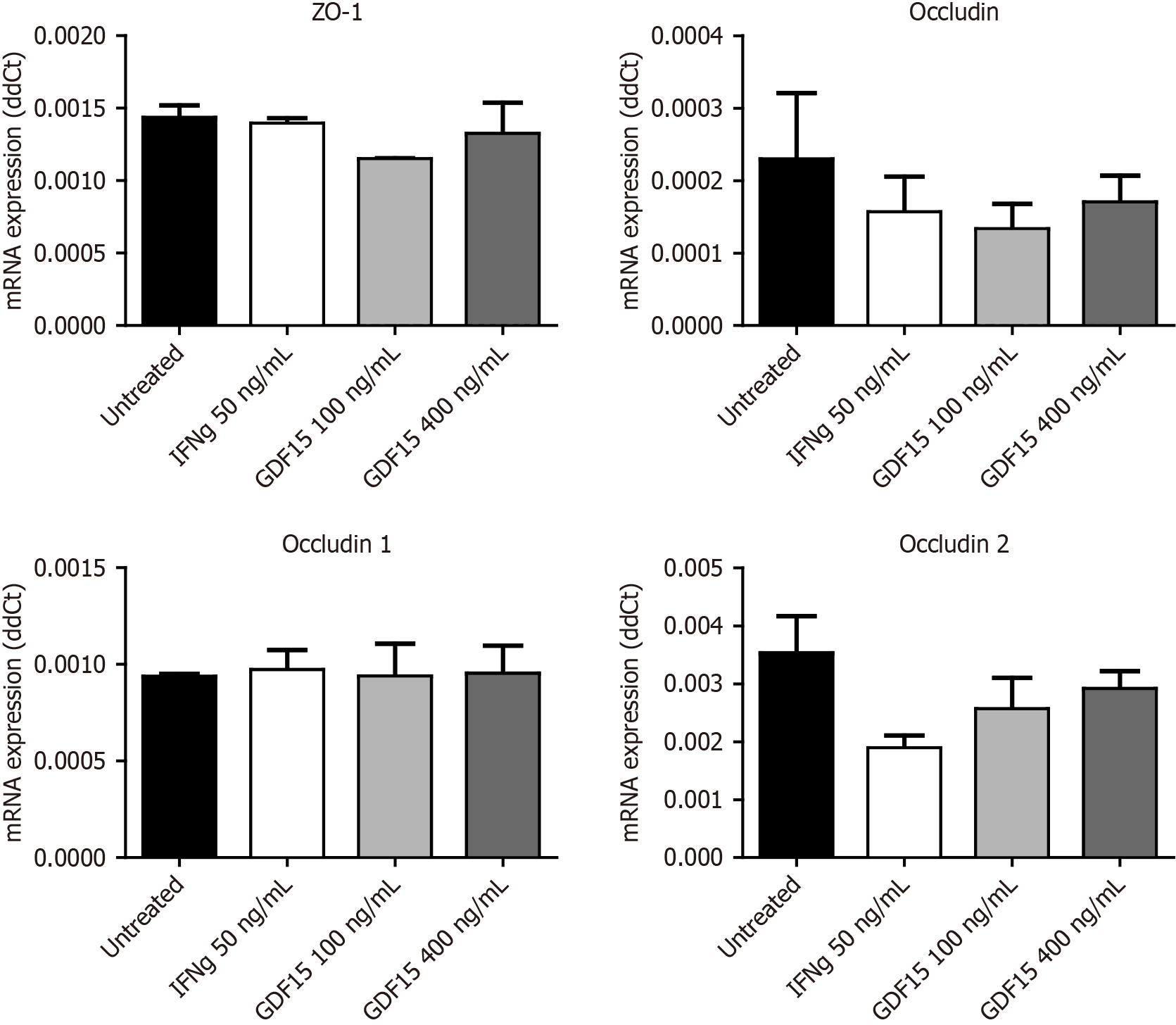
RNA was isolated using QIAzol (QIAGEN Science, Hilden, Germany) following the manufacturer’s procedure and quantified by spectrophotometry (Nanodrop 2000, Thermo Scientific, Waltham, MA, United States). Complementary DNA was synthesized using M-MLV reverse transcriptase (Promega, Madison, WI, United States) from 500 ng of RNA. Gene expression in colonic biopsies was assessed by quantitative polymerase chain reaction in a LightCycler 480 (Roche Diagnostics S.L., Barcelona, Spain) using 1 μL from a 1/5 dilution of the complementary DNA, SensiFAST™ SYBR® Hi-ROX (Bioline, London, United Kingdom). The forward (F) and reverse (R) primers used have been previously described[4]. The ΔCt method was used to analyze the data. S18 was used as a housekeeping gene. Within each assay, each sample was run in triplicate (technical triplicates), and the average of the triplicates was used for statistical analysis. Gene expression in T84 cells was assessed by quantitative polymerase chain reaction in a QuantStudio 12K Flex (384) (Applied Biosystems, Barcelona, Spain) using specific TaqMan™ assays: Zonula occludens (ZO)-1 (No. Hs01551871), occludin (No. Hs05465837), claudin 1 (No. Hs00221623), and claudin 2 (No. Hs00252666). RNase P (Cat. No. 4316844, Applied Biosystems, Barcelona, Spain) was used as a housekeeping gene. Each sample was run in duplicates, and the ΔCt method was used to analyze the data.
Colonic organoids were developed from colonic biopsies using the medium IntestiCult™ Organoid Growth Medium for human (catalog number: No. 100-0190, Stemcell Technologies, Saint Égrève, France), following the manufacturer’s protocol. Colonic organoids (passage 3-5) were seeded in a 10 μL dome of 1:1 dilution of DMEM: F12 [15 mmol/L N-2-hydroxyethylpiperazine-N’-2-ethanesulphonic acid, 1% bovine serum albumin (BSA)] and Matrigel on an 18-well μ-slide (Cat. No: 81816, IBIDI GmbH, Gräfelfing, Germany). After 10 minutes of incubation at 37 °C, 100 μL of intesticult organoid growth medium (1 × penicillin/streptomycin) was added. The day after seeding, the medium was replaced with 50 μL of either fresh intesticult medium or fresh intesticult medium containing 100 or 400 ng/mL of GDF15 (catalog number: No. 32-1249, Abeomics, San Diego, CA, United States). The following day, 50 μL of intesticult was added, either alone or containing GDF15 at 100 or 400 ng/mL, or interferon gamma (IFNg) (catalog number: No. 32-6390, Abeomics, San Diego, CA, United States) at 100 ng/mL for a final IFNg concentration of 50 ng/mL. Concentrations of GDF15 and IFNg were based on previous publications[14,15]. For assessment of the intestinal barrier function, 11 μL of 1mg/mL of fluorescein isothiocyanate (FITC)-Dextran 4K (No. FD4-100MG, Sigma-Aldrich, Burlington, MA, United States) was added on the following day, and organoids were visualized on a SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) 24 hours later. Three representative areas from inside and outside of each analyzed organoid were measured (mean gray value), and mean values were calculated. To correct for the background signal, we calculated the ratio between the mean gray value inside and the sum of the mean gray values inside and outside the colon organoid. For each organoid line, at least 10 organoids in three different wells (a total of at least 30 organoids) were analyzed for each condition.
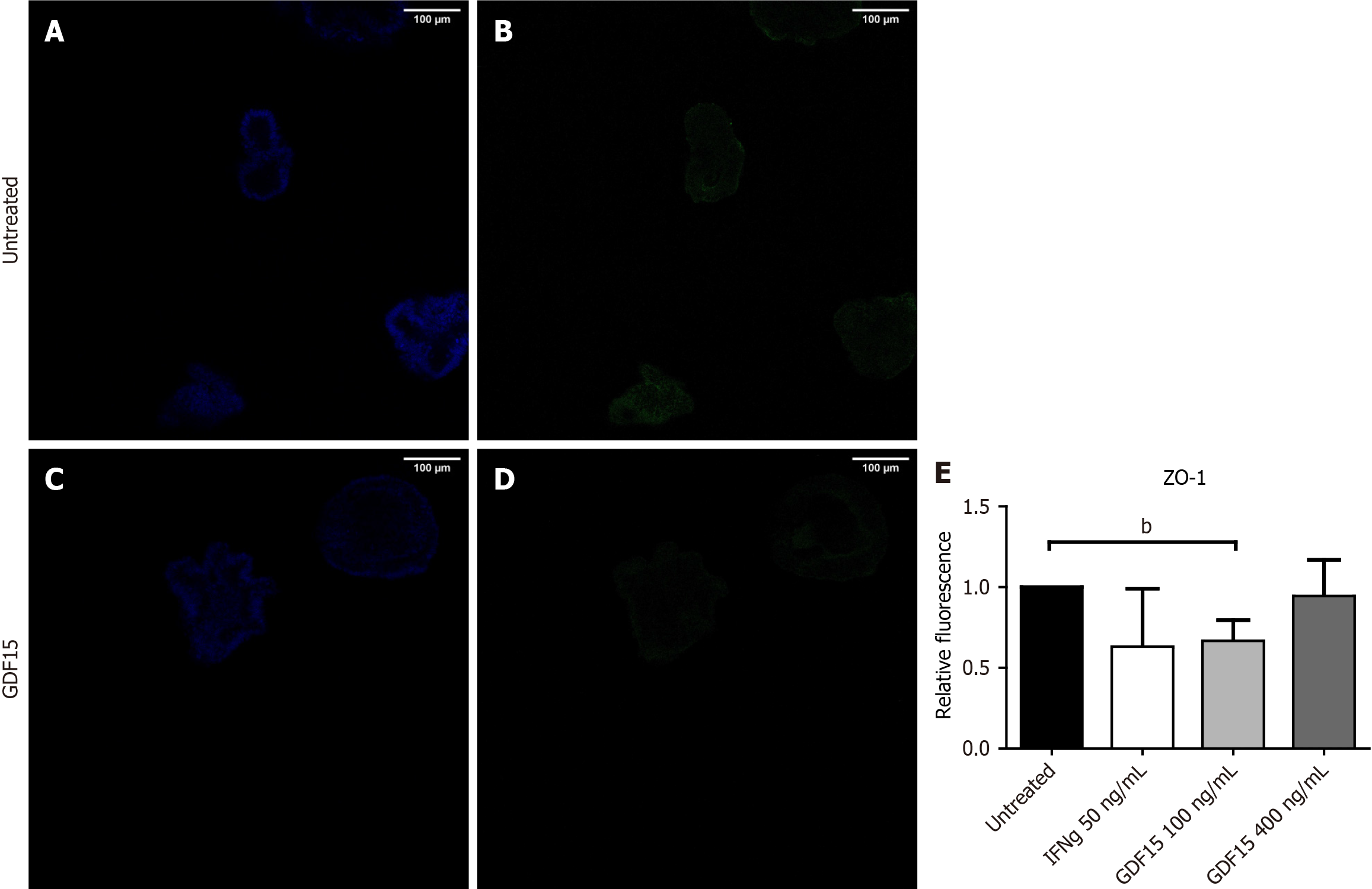
For assessment of tight junction proteins, the media was removed 24 hours after adding IFNg, and organoids were fixed with 10% formalin, permeabilized with Triton X 0.2%, and blocked with phosphate buffered saline (PBS) 1 × BSA 2%. Primary antibody against ZO-1 (No. ZO1-1A12, Invitrogen, CA, United States) was added at a 1:1000 dilution in PBS 1 × 0.1% BSA, and incubated 24 hours at 4 °C. Afterwards, primary antibody was washed and secondary antibody against mouse immunoglobulin G (IgG) AlexaFluor®488 (No. ab150113, Abcam, Cambridge, United Kingdom) was added at a 1:2000 dilution in PBS 1 × and incubated 1 hour at room temperature. Secondary antibodies were washed, and 4’-6-diamidino-2-phenylindole (DAPI) (No. 62247, Thermo Scientific, Waltham, MA, United States) was added. Cells were incubated for 6 minutes, and DAPI was washed to remove excess. Wells were covered with ProLong® diamond antifade mountant (catalog number: No. 39695, Life Technologies, Carlsbad, CA, United States), and organoids were visualized 24 hours later on a SP5 confocal microscope. For each organoid line, at least three organoids in three different wells were analyzed. Fluorescence was measured as integrated density, and values from ZO-1 were normalized to fluorescence from DAPI.
T84 cells (ATCC® CCL-248™) were obtained from the European Collection of Authenticated Cell Cultures. All reagents were from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, United States) unless otherwise specified. T84 cells were cultured in DMEM: F12, 15 mmol/L, N-2-hydroxyethylpiperazine-N’-2-ethanesulphonic acid media supplemented with 10% fetal bovine serum (FBS), 1 mmol/L L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. For gene expression assessment of tight junction markers, T84 cells were seeded on 48-well plates. When cells were semi-confluent (90%), the media were replaced with 0.2 mL of either fresh medium (untreated and IFNg wells) or fresh medium containing 100 or 400 ng/mL of GDF15 (catalog number: No. 32-1249, Abeomics, San Diego, CA, United States). The following day, 0.2 mL of fresh medium was added to untreated wells, 0.2 mL of fresh medium containing 100 ng/mL of IFNg (catalog number: No. 32-6390, Abeomics, San Diego, CA, United States) was addedd to IFNg wells for a final concentration of 50 ng/mL, and 0.2 mL of fresh medium containing 100 or 400 ng/mL of GDF15 was added to GDF15 100 ng/mL and GDF15 400 ng/mL wells, respectively. Cells were collected in QIAzol for RNA isolation. A total of three experiments were run. Within each experiment, conditions were run in triplicate.
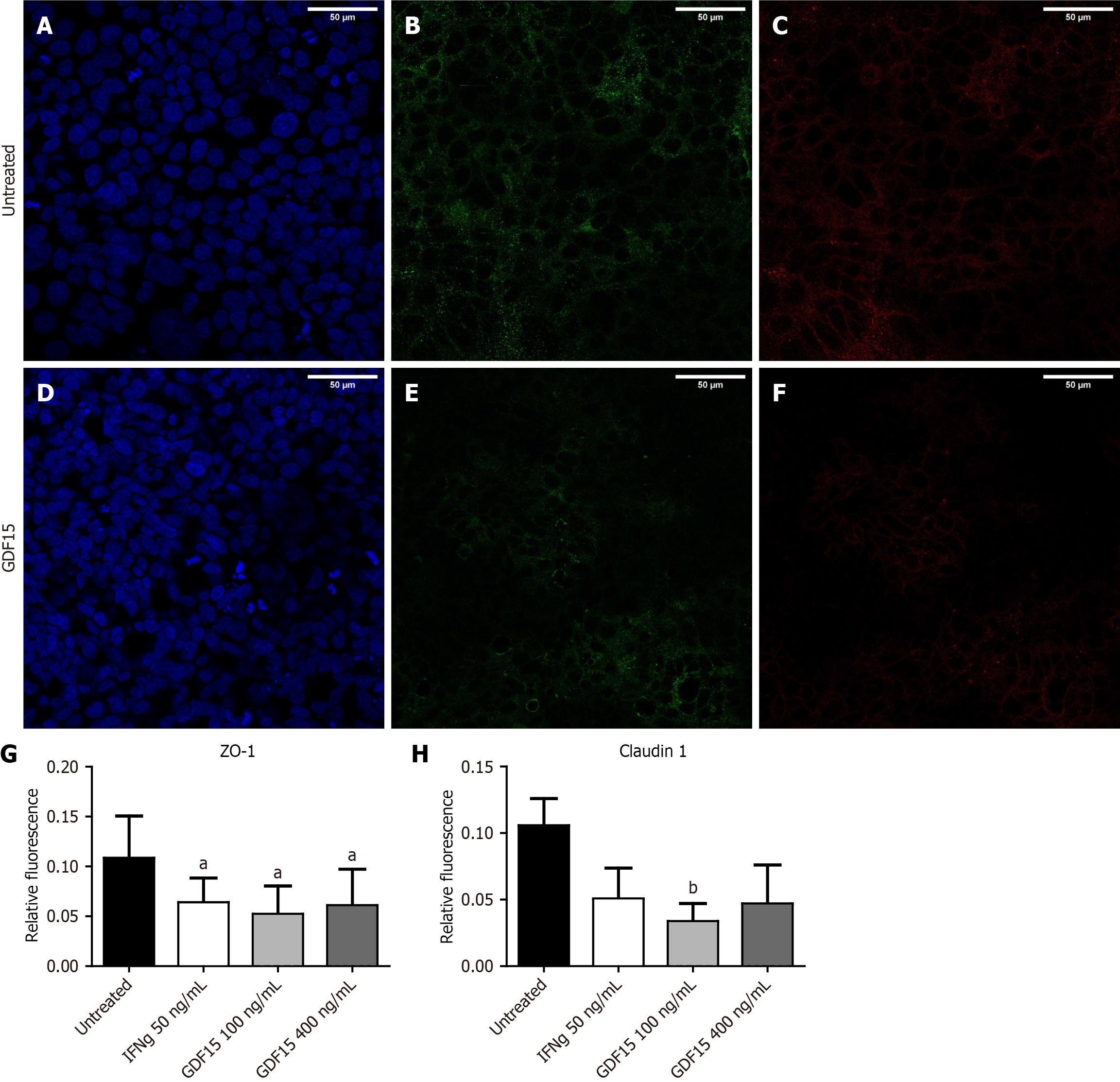
For immunofluorescence analysis of tight junction proteins in the T84 cell line, cells were seeded on an 18-well μ-slide (Cat.No: 81816, IBIDI GmbH, Gräfelfing, Germany). When cells were semi-confluent (90%), the media was replaced following the same protocol as for gene expression assays in T84 cells, but using 0.05 mL instead of 0.2 mL, for a final volume of 0.1 mL in wells. Media was removed 24 hours after adding IFNg, and cells were fixed with 10% formalin, permeabilized with Triton X 0.2%, and blocked with PBS 1 × BSA 2%. Primary antibody against ZO-1 and claudin 1 (No. ab211737, Abcam, Cambridge, United Kingdom) were added at a 1:1000 dilution in PBS 1 × BSA 0.1%, and incubated 24 hours at 4 °C. Afterwards, primary antibody was washed and secondary antibody against mouse IgG AlexaFluor®488 (No. ab150113, Abcam, Cambridge, United Kingdom) and rabbit IgG Alexa Fluor®647 (No. ab150075, Abcam, Cambridge, United Kingdom) were added at a 1:2000 dilution in PBS 1 × and incubated 1 hour at room temperature. Secondary antibodies were washed, and DAPI was added. Cells were incubated for 6 minutes, and DAPI was washed to remove excess. Wells were covered with ProLong® diamond antifade mountant (catalog number: No. 39695, Life Technologies, Carlsbad, CA, United States), and cells were visualized 24 hours later on a SP5 confocal microscope. A Total of three experiments were run, and 5 pictures per condition were analyzed. Fluorescence was measured as integrated density, and values from ZO-1 and claudin 1 were normalized to fluorescence from DAPI.
The Kolmogorov-Smirnov test was used to assess whether data followed a normal distribution. The difference in sex ratio between HC and IBD patients was assessed using the χ2 test. Age differences, body mass index, and biochemical parameters from blood test between HC and IBD patients were assessed using a t-test. The difference in time of disease between CD and UC patients was assessed using a t-test. Data grouped in columns are expressed as either median ± interquartile range or mean ± SD, according to the distribution of the data. Mann-Whitney test (one-tailed) was used to compare plasma levels of GDF15 between different groups, and mRNA levels of intestinal barrier markers between untreated T84 cells and GDF15-treated cells. Paired t-test (one-tailed) was used to compare different conditions within the same organoid/cell line in confocal microscopy assays. Pearson’s or Spearman’s correlation tests were used to assess the relationship between different variables, according to the distribution of the data. For all tests, a P value lower than 0.05 was statistically significant. All authors had access to the study data and had reviewed and approved the final manuscript.
A total of 23 HC, 39 IBD patients, and 19 MI patients were recruited for this study. The demographic characteristics of subjects participating in the study are described in Table 1. We found no statistical differences in sex ratio, age, or body mass index between HC and IBD patients. As expected, MI patients, who were used as positive control for elevated plasma GDF15, were significantly older (P = 0.003) compared to HCs. Results from the blood biochemical test are also shown in Table 1. As expected, IBD patients showed significantly higher levels of C-reactive protein (CRP), as well as albumin. Haptoglobin, another acute-phase protein, was higher in IBD patients, although not reaching statistical significance. Clinical characteristics of IBD patients (time of disease and treatment) are described in Table 2. No significant differences were found in the time of disease between CD and UC patients.
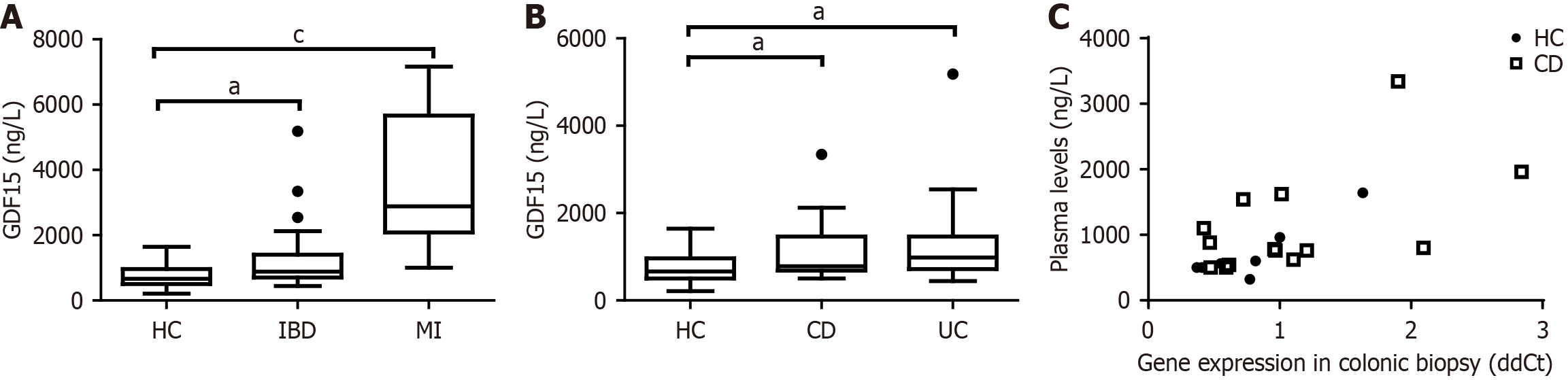
Levels of GDF15 in plasma from IBD patients were significantly increased compared to HC (P = 0.0119) (Figure 1A). When stratified (Figure 1B), both CD and UC patients maintained significantly higher levels (P = 0.0498 and P = 0.0132). We next inquired about the source of plasma levels of GDF15 found in IBD patients. To that end, we assess the correlation between gene expression from colonic biopsies of CD patients with plasma levels from the same subjects (both samples collected on the same day). We found a strong positive correlation between gene expression and plasma levels (Spearman r = 0.6002, P = 0.0020) (Figure 1C), suggesting that the colon may be an important source of circulating GDF15.
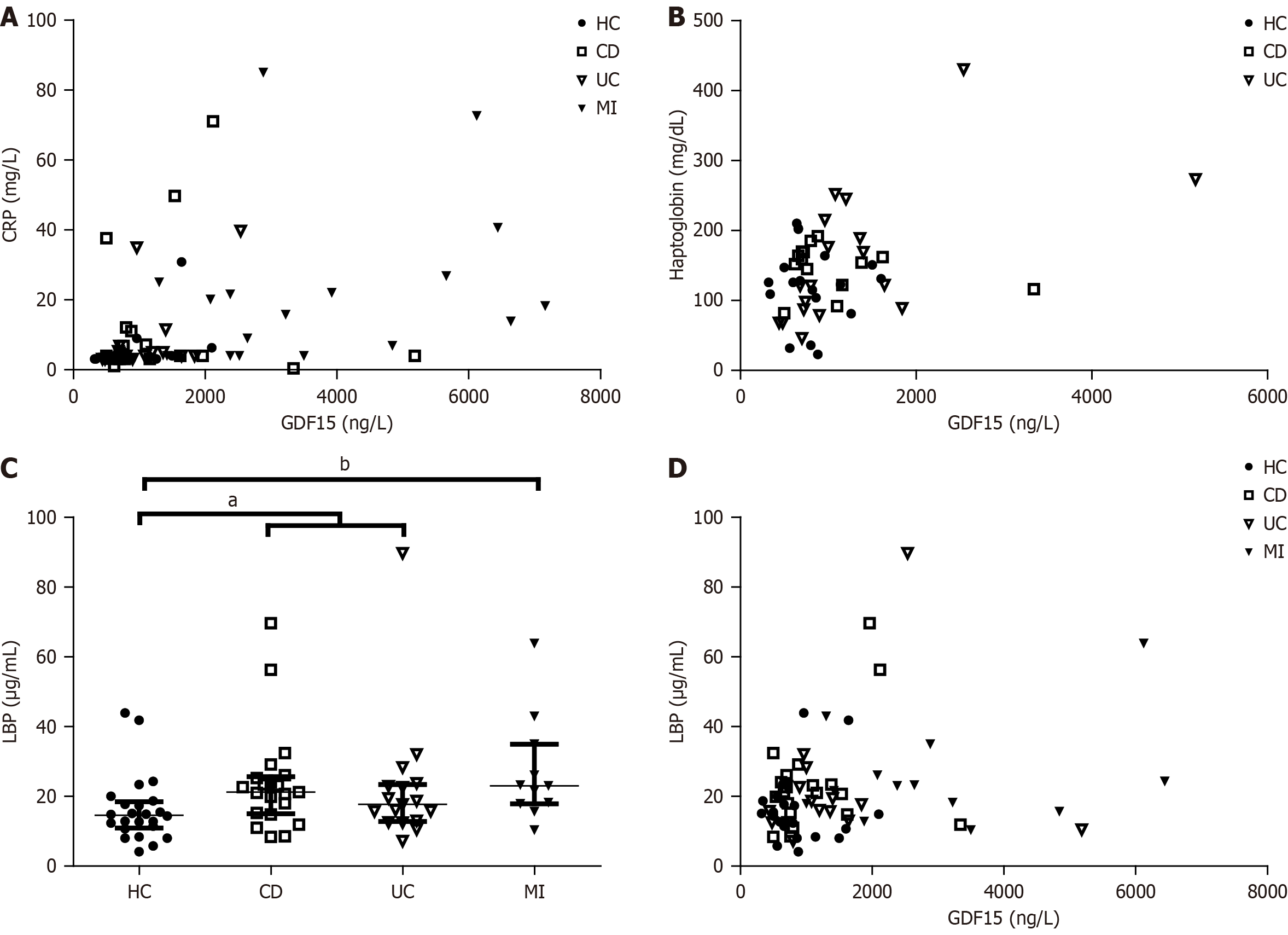
We also assessed the correlation between circulating levels of GDF15 and different markers from the biochemical blood test. We did not find a correlation of plasma levels of GDF15 with calprotectin, a specific marker of intestinal inflammation (data not shown). However, GDF15 showed a positive correlation with CRP (Spearman r = 0.3290, P = 0.0066), a hallmark of inflammation (Figure 2A). These results suggest that GDF15 is not constantly elevated in IBD patients, but rather shows a dynamic behavior, paralleling inflammatory processes. Circulating levels of GDF15 also correlated with plasma levels of haptoglobin (Pearson r = 0.4066, P = 0.0017), another acute-phase protein (Figure 2B). Because haptoglobin is related to intestinal permeability, and leaky gut or dysfunctional intestinal barrier function is frequently observed in IBD patients, we aimed to assess levels of LBP, a validated biomarker of intestinal permeability. As expected, LBP plasma levels were increased in IBD patients compared to HC (P = 0.0127, Figure 2C), and further increased in MI patients (P = 0.0037), who also presented with higher levels of GDF15 in plasma. In fact, we found a correlation between plasma levels of GDF15 and LBP (Spearman r = 0.2032, P = 0.0423, Figure 2D), suggesting a role of GDF15 in intestinal barrier function. We also found a weak correlation between GDF15 plasma levels and glucose (Pearson r = 0.2576, P = 0.0469) and urea (Pearson r = 0.2788, P = 0.0295).
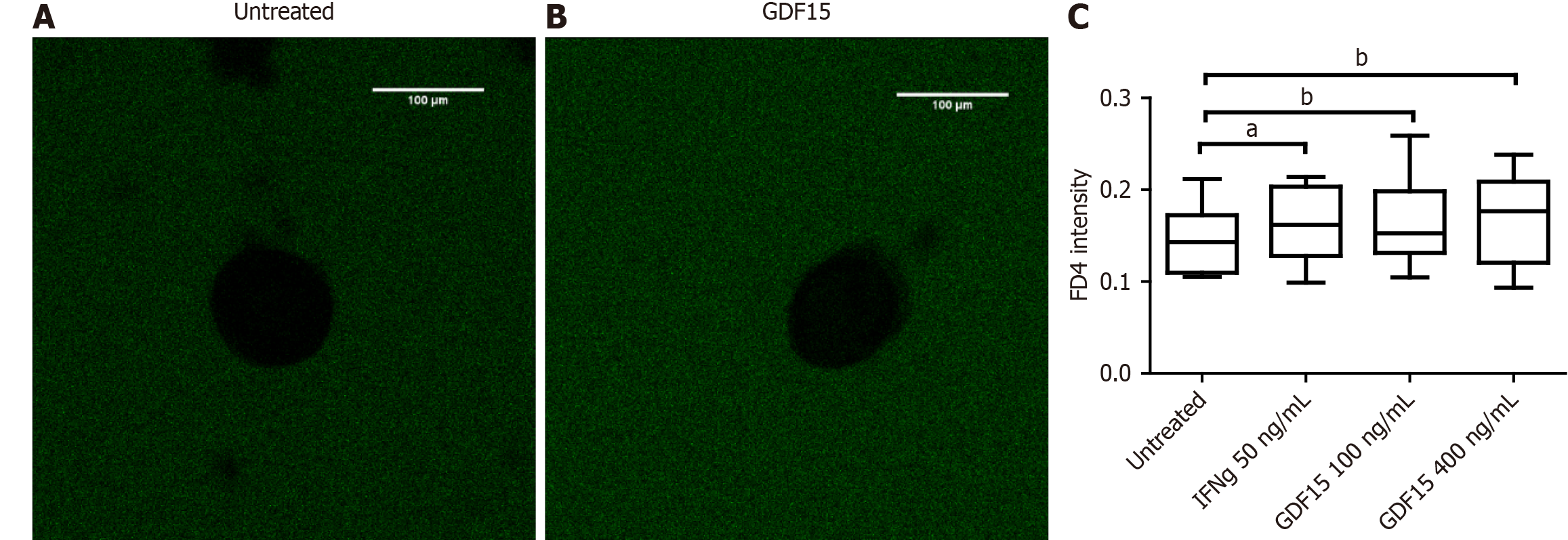
To inquire about the impact of higher levels of GDF15 on the intestinal barrier, we treated colon organoids with GDF15 and performed a permeability assay (Figure 3A and B). Lumen of colon organoids treated with GDF15 showed higher signal from FITC-Dextran 4K compared to untreated colon organoids (Figure 3C), suggesting that GDF15 induces alteration of intestinal barrier (P = 0.0053 for 100 ng/mL dose and P = 0.0096 for 400 ng/mL dose). Colon organoids from both IBD patients and HC showed similar patterns in response to GDF15, with mean increases of dextran 4k-FITC signal in response to the lower dose of 15.7% and 11.1%, respectively. To delve into the mechanism by which GDF15 alters the intestinal barrier, we studied ZO-1, which is a key component of the tight junction. Levels of ZO-1 were assessed in two colon organoid lines from HC and one line from an IBD patient (Figure 4A-D). In all organoid lines assessed, treatment with 100 ng/mL of GDF15 induced a significant down-regulation of ZO-1 (P = 0.0065), while treatment with 400 ng/mL of GDF15 did not induce significant changes (Figure 4E). To confirm the effects of GDF15 on the intestinal barrier, we investigated the levels of ZO-1 and claudin 1 in another model of colonic epithelium, T84 cells. We observed a down-regulation of both ZO-1 and claudin 1 after treatment with GDF15 at either 100 or 400 ng/mL (Figure 5). To further inquire about the mechanisms by which GDF15 induces a down-regulation of tight junction proteins, we assessed the gene expression of different intestinal barrier markers. Opposite to our results on protein levels, we found no differences in the expression of ZO-1 and claudin 1 after treatment with GDF15 (Figure 6). However, we observed a trend towards down-regulation of occludin and claudin 2 in T84 cells treated with either 100 or 400 ng/mL of GDF15.
GDF15 is a mitokine expressed by various tissues in response to stress, particularly mitochondrial stress[16]. The role of GDF15 in human health remains controversial, with studies suggesting both protective and detrimental effects. For example, GDF15 prevents hepatic steatosis by inhibiting oxidative stress, mitochondrial dysfunction, and inflammasome activation[17], and also prevents obesity and insulin resistance by increasing lipolysis in adipocytes and hepatocytes[18]. In contrast, GDF15 has been shown to promote the proliferation of cervical cancer cells[19] and to upregulate mucin expression in alveolar cells exposed to cigarette smoke, contributing to the pathogenesis of chronic obstructive pul
In the present study, we observed elevated plasma levels of GDF15 in patients with IBD, consistent with our previous findings showing activation of the UPRmt in colon tissues from IBD patients[4]. This finding supports mitochondrial stress in the colon as a pathophysiological mechanism in IBD. Additionally, we confirmed previous reports of elevated circulating GDF15 levels in IBD patients[13]. Importantly, we observed significantly higher levels in both CD and UC patients. We next observed a correlation between GDF15 and CRP, a hallmark of inflammation, and haptoglobin. Haptoglobin is well known for its role in binding hemoglobin from lysed erythrocytes, preventing oxidative damage[21]. However, it has also been reported to bind lipopolysaccharide, thus reducing immune overactivation[22]. Recent studies have identified associations between haptoglobin and plasma levels of lipopolysaccharide, as well as other markers of increased intestinal permeability, such as LBP[23-25]. To confirm an association between GDF15 and increased intestinal permeability, we evaluated its correlation with plasma levels of LBP, a validated biomarker of increased intestinal permeability. We found a positive correlation between GDF15 and LBP, which led us to investigate the effects of GDF15 on the intestinal barrier in vitro. Using colonic organoids, we demonstrated for the first time that GDF15 increases in
This effect of GDF15 on the gastrointestinal tract has not been previously described and raises new questions about its mode of action. The only currently known receptor for GDF15, glial-derived neurotrophic factor receptor alpha-like, is expressed exclusively in the brain[26]. It has been hypothesized that GDF15 might interact with the Transforming growth factor (TGF)-β receptors TGFBR1 and TGFBR2[27]. However, studies suggesting such interactions should be interpreted with caution, as commercially purified GDF15 has been found to be contaminated with TGF-β[14]. The source of this contamination is likely the constitutive overexpression of TGF-β in mammalian cell cultures used for the overexpression of GDF15. To avoid contamination in our study, we used GDF15 purified from Escherichia coli, ensuring that the observed effects on intestinal tight junctions were induced by GDF15. Further studies are needed to identify the specific receptor mediating the effects of GDF15 on intestinal tight junctions.
The source of GDF15 Likely varies across different conditions, with the placenta exhibiting the highest expression levels[16]. In patients with cardiovascular diseases, it might seem logical to assume that the heart is the primary source of elevated plasma GDF15. However, the literature indicates that, while GDF15 gene expression in the heart correlates with circulating levels, extra-cardiac expression (particularly in the liver) contributes more significantly to plasma GDF15[28]. The gastrointestinal tract may also serve as a major source of circulating GDF15 under certain conditions. For example, the gastrointestinal tract has been implicated in the increase in plasma GDF15 levels induced by metformin treatment[29]. In our study, we found a positive correlation between plasma GDF15 levels and GDF15 gene expression in colonic biopsies, suggesting that the colon may be a major source of circulating GDF15. Comparative studies of GDF15 gene expression across different tissues in a mouse model of colitis are necessary to better clarify the source of circulating GDF15. Nonetheless, the parallelism between circulating GDF15 levels and gene expression in the colon supports a potential impact of GDF15 on the gastrointestinal tract.
Intestinal barrier dysfunction has been consistently reported in IBD as a key factor contributing to immune system dysregulation[1]. In our study, we report a novel mechanism by which GDF15 affects the intestinal tract, altering the intestinal barrier and increasing permeability. Increased intestinal permeability facilitates contact between epithelial cells, gut microbiota, and toxins, potentially leading to immune system overactivation. Furthermore, the correlation between circulating GDF15 levels and CRP may reflect immune overactivation resulting from increased intestinal permeability. This finding is relevant not only for IBD patients, but also for patients with cardiovascular diseases. Elevated plasma GDF15 levels have been found in several cardiovascular conditions[10]. At the same time, there is growing interest in studying the impact of intestinal microbiota on cardiovascular health as an important factor in the development of cardiovascular disorders[30-32]. In fact, our cohort of patients with MI, which served as a positive control for elevated plasma GDF15 levels, also showed elevated plasma LBP levels, highlighting the effect of GDF15 on the intestinal barrier in disorders other than IBD.
Our study confirms increased circulating levels of GDF15 in IBD patients and reveals a novel molecular mechanism by which this mitokine disrupts the intestinal barrier. Targeting GDF15 may offer a promising strategy to prevent disruption of the intestinal barrier, potentially reducing immune overactivation.
Experiments on confocal imaging were performed in the ICTS “NANBIOSIS”, Unit U28 at IBIMA Plataforma-BIONAND.
| 1. | Michielan A, D'Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015;2015:628157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 518] [Article Influence: 47.1] [Reference Citation Analysis (1)] |
| 2. | Ahmad R, Sorrell MF, Batra SK, Dhawan P, Singh AB. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol. 2017;10:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 3. | Sánchez-Quintero MJ, Rodríguez-Díaz C, Rodríguez-González FJ, Fernández-Castañer A, García-Fuentes E, López-Gómez C. Role of Mitochondria in Inflammatory Bowel Diseases: A Systematic Review. Int J Mol Sci. 2023;24:17124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Martín-Reyes F, Bernal M, Rodríguez-Díaz C, Rodríguez-de Los Reyes D, Ho-Plagaro A, Rodríguez-Pacheco F, Camacho-Martel L, Camargo-Camero R, Rodríguez-González FJ, Alcain-Martínez G, Martín-Masot R, Navas-López VM, Villanueva-Paz M, Lucena MI, García-Fuentes E, López-Gómez C. Mitochondrial Stress Links Environmental Triggers with Pro-Inflammatory Signaling in Crohn’s Disease. Antioxidants (Basel). 2023;12:2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Li Y, Li S, Qiu Y, Zhou M, Chen M, Hu Y, Hong S, Jiang L, Guo Y. Circulating FGF21 and GDF15 as Biomarkers for Screening, Diagnosis, and Severity Assessment of Primary Mitochondrial Disorders in Children. Front Pediatr. 2022;10:851534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Suomalainen A. Blood biomarkers of mitochondrial disease-One for all or all for one? Handb Clin Neurol. 2023;194:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Conte M, Sabbatinelli J, Chiariello A, Martucci M, Santoro A, Monti D, Arcaro M, Galimberti D, Scarpini E, Bonfigli AR, Giuliani A, Olivieri F, Franceschi C, Salvioli S. Disease-specific plasma levels of mitokines FGF21, GDF15, and Humanin in type II diabetes and Alzheimer’s disease in comparison with healthy aging. Geroscience. 2021;43:985-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Siddiqui JA, Pothuraju R, Khan P, Sharma G, Muniyan S, Seshacharyulu P, Jain M, Nasser MW, Batra SK. Pathophysiological role of growth differentiation factor 15 (GDF15) in obesity, cancer, and cachexia. Cytokine Growth Factor Rev. 2022;64:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 9. | Wang D, Day EA, Townsend LK, Djordjevic D, Jørgensen SB, Steinberg GR. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021;17:592-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 10. | di Candia AM, de Avila DX, Moreira GR, Villacorta H, Maisel AS. Growth differentiation factor-15, a novel systemic biomarker of oxidative stress, inflammation, and cellular aging: Potential role in cardiovascular diseases. Am Heart J Plus. 2021;9:100046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Wan Y, Fu J. GDF15 as a key disease target and biomarker: linking chronic lung diseases and ageing. Mol Cell Biochem. 2024;479:453-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Yamamoto H, Takeshima F, Haraguchi M, Akazawa Y, Matsushima K, Kitayama M, Ogihara K, Tabuchi M, Hashiguchi K, Yamaguchi N, Miyaaki H, Kondo H, Nakao K. High serum concentrations of growth differentiation factor-15 and their association with Crohn’s disease and a low skeletal muscle index. Sci Rep. 2022;12:6591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 13. | Kučerka O, Blahutová M, Kosek V, Mináriková P, Horáček JM, Urbánek P, Malý M. Exploring the Role of GDF-15 in Inflammatory Bowel Disease: A Case-Controlled Study Comparing Crohn’s Disease and Ulcerative Colitis with Non-Inflammatory Controls. Metabolites. 2024;14:185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Olsen OE, Skjærvik A, Størdal BF, Sundan A, Holien T. TGF-β contamination of purified recombinant GDF15. PLoS One. 2017;12:e0187349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Sayoc-Becerra A, Krishnan M, Fan S, Jimenez J, Hernandez R, Gibson K, Preciado R, Butt G, McCole DF. The JAK-Inhibitor Tofacitinib Rescues Human Intestinal Epithelial Cells and Colonoids from Cytokine-Induced Barrier Dysfunction. Inflamm Bowel Dis. 2020;26:407-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Johann K, Kleinert M, Klaus S. The Role of GDF15 as a Myomitokine. Cells. 2021;10:2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Chen C, Chen J, Sang T, Peng H, Lin X, Zhao Q, Chen S, Eling T, Wang X. Overexpression of NAG-1/GDF15 prevents hepatic steatosis through inhibiting oxidative stress-mediated dsDNA release and AIM2 inflammasome activation. Redox Biol. 2022;52:102322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 18. | Chung HK, Ryu D, Kim KS, Chang JY, Kim YK, Yi HS, Kang SG, Choi MJ, Lee SE, Jung SB, Ryu MJ, Kim SJ, Kweon GR, Kim H, Hwang JH, Lee CH, Lee SJ, Wall CE, Downes M, Evans RM, Auwerx J, Shong M. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol. 2017;216:149-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 19. | Li S, Ma YM, Zheng PS, Zhang P. GDF15 promotes the proliferation of cervical cancer cells by phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp Clin Cancer Res. 2018;37:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 20. | Wu Q, Jiang D, Chu HW. Cigarette smoke induces growth differentiation factor 15 production in human lung epithelial cells: implication in mucin over-expression. Innate Immun. 2012;18:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | MacKellar M, Vigerust DJ. Role of Haptoglobin in Health and Disease: A Focus on Diabetes. Clin Diabetes. 2016;34:148-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Zein L, Grossmann J, Swoboda H, Borgel C, Wilke B, Awe S, Nist A, Stiewe T, Stehling O, Freibert SA, Adhikary T, Chung HR. Haptoglobin buffers lipopolysaccharides to delay activation of NFκB. Front Immunol. 2024;15:1401527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Zhao Y, Yu S, Zhao H, Li L, Li Y, Liu M, Jiang L. Integrated multi-omics analysis reveals the positive leverage of citrus flavonoids on hindgut microbiota and host homeostasis by modulating sphingolipid metabolism in mid-lactation dairy cows consuming a high-starch diet. Microbiome. 2023;11:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 24. | Silva BC, Godoi LA, Supapong C, Bitsie B, Valadares Filho SC, Schoonmaker JP. Effect of a molasses-based liquid supplement on gastrointestinal tract barrier function, inflammation, and performance of newly received feedlot cattle before and after a transport stress. J Anim Sci. 2023;101:skac295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Goetz BM, Abeyta MA, Rodriguez-Jimenez S, Opgenorth J, McGill JL, Fensterseifer SR, Arias RP, Lange AM, Galbraith EA, Baumgard LH. Effects of a multistrain Bacillus-based direct-fed microbial on gastrointestinal permeability and biomarkers of inflammation during and following feed restriction in mid-lactation Holstein cows. J Dairy Sci. 2024;107:6192-6210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjær SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Nørgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, Jørgensen SB. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017;23:1158-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 514] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 27. | Conte M, Giuliani C, Chiariello A, Iannuzzi V, Franceschi C, Salvioli S. GDF15, an emerging key player in human aging. Ageing Res Rev. 2022;75:101569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 28. | Du W, Piek A, Schouten EM, van de Kolk CWA, Mueller C, Mebazaa A, Voors AA, de Boer RA, Silljé HHW. Plasma levels of heart failure biomarkers are primarily a reflection of extracardiac production. Theranostics. 2018;8:4155-4169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Kincaid JWR, Rimmington D, Tadross JA, Cimino I, Zvetkova I, Kaser A, Richards P, Patel S, O’Rahilly S, Coll AP. The gastrointestinal tract is a major source of the acute metformin-stimulated rise in GDF15. Sci Rep. 2024;14:1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Sánchez-Quintero MJ, Delgado J, Martín Chaves L, Medina-Vera D, Murri M, Becerra-Muñoz VM, Estévez M, Crespo-Leiro MG, Paz López G, González-Jiménez A, A G Ranea J, Queipo-Ortuño MI, Plaza-Andrades I, Rodríguez-Capitán J, Pavón-Morón FJ, Jiménez-Navarro MF. Multi-Omics Approach Reveals Prebiotic and Potential Antioxidant Effects of Essential Oils from the Mediterranean Diet on Cardiometabolic Disorder Using Humanized Gnotobiotic Mice. Antioxidants (Basel). 2023;12:1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Sánchez-Quintero MJ, Delgado J, Medina-Vera D, Becerra-Muñoz VM, Queipo-Ortuño MI, Estévez M, Plaza-Andrades I, Rodríguez-Capitán J, Sánchez PL, Crespo-Leiro MG, Jiménez-Navarro MF, Pavón-Morón FJ. Beneficial Effects of Essential Oils from the Mediterranean Diet on Gut Microbiota and Their Metabolites in Ischemic Heart Disease and Type-2 Diabetes Mellitus. Nutrients. 2022;14:4650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Rivera K, Gonzalez L, Bravo L, Manjarres L, Andia ME. The Gut-Heart Axis: Molecular Perspectives and Implications for Myocardial Infarction. Int J Mol Sci. 2024;25:12465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/