Published online Nov 7, 2025. doi: 10.3748/wjg.v31.i41.110753
Revised: August 12, 2025
Accepted: September 29, 2025
Published online: November 7, 2025
Processing time: 125 Days and 0.8 Hours
Cancer-induced incomplete bowel obstruction presents numerous clinical cha
To compare the efficacy of BGM-UT plus standard palliative care vs standard pa
A retrospective analysis was conducted on 109 patients aged 18-75 years with cancer-induced incomplete bowel obstruction treated at the Foshan Hospital of Traditional Chinese Medicine between October 2023 and September 2024. The participants were categorized into two groups: (1) Regular group receiving stan
Initially, the groups were demographically and clinically comparable. Post-treatment, the BGM-UT group showed significant reductions in opioid intake (P = 0.027), improved gastrointestinal recovery times, and enhanced pain management as reflected by their lower brief pain inventory scores (P < 0.05). Similarly, the improvement in traditional Chinese medicine scores was greater in the BGM-UT group than in the regular group (P < 0.05). Inflammatory markers including interleukin-6, tumor necrosis factor-α, and C-reactive protein decreased significantly in the BGM-UT group, indicating the superior anti-inflammatory effects of this treatment (P < 0.05).
The addition of BGM-UT to standard palliative care enhances pain relief, accelerates gastrointestinal recovery, and effectively reduces inflammatory cytokine levels in patients with cancer-induced incomplete bowel obstruction. This combination therapy offers a promising complementary approach to managing this challenging condition. Further prospective studies are warranted to validate these findings and explore underlying mechanisms.
Core Tip: This study demonstrates that bottle gourd moxibustion combined with umbilical therapy (BGM-UT) significantly enhances standard palliative care for cancer-related incomplete bowel obstruction. In a retrospective analysis of 109 patients, BGM-UT reduced opioid consumption, accelerated gastrointestinal recovery (e.g., faster bowel sound restoration), and improved pain control. Crucially, it also lowered key inflammatory cytokines (interleukin-6, tumor necrosis factor-α, C-reactive protein), suggesting systemic anti-inflammatory effects. These findings highlight BGM-UT as a promising complementary therapy to alleviate symptoms and modulate inflammation in this challenging condition.
- Citation: Wu JW, Li LP, Chen YS. Effects of bottle gourd moxibustion combined with umbilical therapy for cancer-related incomplete bowel obstruction on inflammatory cytokine levels. World J Gastroenterol 2025; 31(41): 110753
- URL: https://www.wjgnet.com/1007-9327/full/v31/i41/110753.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i41.110753
Incomplete bowel obstruction remains a significant clinical challenge in cancer care, often leading to debilitating symp
Inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP), have pivotal roles in the pathophysiology of cancer and its related complications because they mediate systemic inflammation, which exacerbates tissue damage and complicates disease progression[6]. Elevated cytokine levels are associated with poor clinical outcomes and have been implicated in the persistence and intensification of cancer symptoms, in
Traditional Chinese medicine (TCM) offers various therapeutic modalities, such as moxibustion and umbilical therapy, which have been historically utilized to alleviate various gastrointestinal disorders[8]. Moxibustion involves the burning of the mugwort herb on or near the skin to warm and invigorate Qi, a concept equivalent to energy flow in TCM, and thereby promote circulation and reduce stagnation[9]. These practices are believed to harmonize bodily functions and have been increasingly explored in modern integrative healthcare settings for their potential complementary roles along
The specific technique of bottle gourd moxibustion combined with umbilical therapy (BGM-UT) has garnered interest because of its promising clinical outcomes in preliminary studies[11]. Bottle gourd moxibustion is a unique form where moxibustion is administered via a bottle gourd, allowing for concentrated heat application on targeted acupoints[12]. Meanwhile, umbilical therapy involves applying herbal extracts to the umbilical area, which is believed in TCM to be a central locus for affective systemic benefits due to its connectivity with multiple meridians[13].
Despite its traditional uses, rigorous scientific evaluations of BGM-UT have been limited, particularly its influence on inflammatory cytokine levels and consequent clinical benefits to conditions such as cancer-induced incomplete bowel obstruction[14]. Recent investigations have suggested that this treatment can modulate biological responses, offering potential anti-inflammatory and analgesic effects[15]. These effects might be mediated through thermal and herb-induced changes in local circulation and nerve stimulation and subsequent systemic immune modulation[16].
Previous research has highlighted the ability of moxibustion to influence autonomic nervous system activity, poten
Inclusion criteria: (1) Participants aged between 18 and 75 years diagnosed with cancer-induced incomplete bowel obstruction[18]; (2) Monthly use of opioid medications; (3) Clear consciousness with normal verbal communication skills to facilitate cooperation in treatment; (4) Karnofsky performance status (KPS) score below 60, focusing primarily on supportive care; (5) Ability to report pain intensity using the brief pain inventory (BPI) and have a baseline pain level of 4 or higher; and (6) Complete medical records available without any missing data.
Exclusion criteria: (1) Patients who had recently undergone surgery, chemotherapy, and/or radiotherapy; (2) Had increased opioid dosage more than three times within the past 2 weeks; (3) Patients with local skin issues at or near the site of moxibustion; (4) Individuals with any additional complications, poor oral intake, or severe insomnia or depression; and (5) Concurrent use of other TCM external therapies (e.g., acupuncture and herbal compresses) within 4 weeks.
A retrospective analysis was conducted on 109 patients with cancer-induced incomplete bowel obstruction treated at the Foshan Hospital of Traditional Chinese Medicine between October 2023 and September 2024. The patients were categorized into two groups according to their treatment methods: The regular group consisted of patients receiving standard Western palliative care alone, and the BGM-UT group included those receiving BGM-UT in addition to standard treatment. Data, including demographic information, baseline disease characteristics, analgesic consumption, gastrointes
Standard Western palliative care: The patients in the regular group received standard Western palliative care, which included fasting, gastrointestinal decompression, pain relief, acid suppression, anti-inflammatory treatment, parenteral nutritional support, and timely correction of electrolyte and acid-base imbalances. Routine nursing care involved assis
BGM-UT: In addition to standard palliative care, the patients in the BGM-UT group received BGM-UT. Initially, Taiji abdominal massage was applied to open the acupoints. Navel powder, which was prepared from 10 g of raw rhubarb mixed with 10 g of magnolia bark and ground into a fine powder, was placed at the Shenque acupoint and covered with perforated and heated ginger slices applied to the moxibustion area. A 4 cm-long moxa pillar was inserted into the fixed needle head of the moxibustion device, ignited, and positioned on the base, adjusting the height as needed while main
Prior to the treatments, the KPS scale was used to evaluate the overall health and functional status of the patients by assessing their physical conditions and the intensity of treatment they could tolerate. The scores range from 0 to 100 in increments of 10, with high scores indicating good health status. A score of 100 denotes complete health without disease symptoms or signs, whereas a score of 0 indicates death. Patients with a KPS score above 60 are generally in good health, possessing some functional independence and QoL and capable of enduring intensive treatments to actively cure the disease. By contrast, patients with a KPS score below 60 tend to have poor health. Their treatments focus primarily on supportive and palliative care, emphasizing comfort and QoL improvement. The scale has a Cronbach’s alpha coefficient of 0.87[20].
The BPI was utilized to assess pain intensity and its interference with QoL before treatment and 1 week after treatment to evaluate pain relief. Pain intensity was determined by averaging the scores from four questions regarding the patient’s current pain, the worst and mildest pain over the past 24 hours, and average pain levels. The assessment used a 10-point numerical rating scale, where 0 indicates no pain and 10 represents the most severe pain imaginable. The pain inter
Total TCM clinical symptom score: The TCM syndrome score was applied to compare changes in disease severity between the two groups before treatment and at weeks 1, 2, and 3 after treatment to evaluate efficacy for cancer-induced incomplete bowel obstruction. This scoring followed the “Guidelines for Clinical Research on New Chinese Medicines”, assigning different values to symptoms and signs according to their significance within the syndrome. Key symptoms such as abdominal pain, bloating, nausea, vomiting, flatulence, and defecation patterns were quantified into four levels: None, mild, moderate, and severe scored as 0, 2, 4, and 6, respectively. The total TCM syndrome score was the aggregate of these individual symptom scores (Table 1). These grading criteria were validated through reliability testing and found to have high internal consistency and interrater reliability, with Cronbach’s alpha coefficients ranging from 0.85 to 0.90[22].
| Symptoms | None (0 points) | Mild (1 point) | Moderate (2 points) | Severe (3 points) |
| Flatulence | Normal flatulence | Presence of flatulence | Slight flatulence | Occasional flatulence |
| Defecation | Normal or slightly hard stools, smooth defecation | Hard stools, slightly difficult defecation | No bowel movement for more than 1 day, difficult and dry defecation | No bowel movement for 2 days or more |
| Abdominal pain | Almost none | Mild, occasional, can be relieved spontaneously | Pain localized, tolerable | Severe pain, unbearable, recurrent |
| Abdominal distension | Almost none | Occasionally bloated or postprandial bloating | Significant bloating, lasting longer, tolerable | Obvious bloating or drum-like distension, lasting long |
| Nausea | Almost none | Occasional nausea, short duration | Moderate nausea, lasting longer, still tolerable | Recurrent severe nausea, long duration, intolerable |
| Vomiting | None | Desire to vomit | Intermittent vomiting of saliva or food residue | Frequent vomiting or immediate vomiting after eating, intolerable |
Individual TCM symptom scoring: At 3 weeks posttreatment, the changes in symptom severity for diarrhea frequency, stool characteristics, abdominal pain, and bloating were evaluated using a grading system referenced from the “Guide
Blood samples were collected from the antecubital vein into heparinized tubes from both groups prior to treatment and at weeks 1 and 3 posttreatment (after completion of all three courses). The samples were centrifuged at 3000 rpm for 5 minutes and stored at 4 °C until use. IL-6 and TNF-α serum levels were quantified using the Human Inflammation 20-Plex ProcartaPlex assay kit (EPX200-12185-901, Thermo Fisher Scientific, MA, United States). CRP levels were measured with the CRP Human ProcartaPlex™ Simplex assay kit (EPX01A-10288-901, Thermo Fisher Scientific, MA, United States) to evaluate the impact of the treatments on the levels of the inflammatory factors associated with cancer-induced incom
The European Organization for Research and Treatment of Cancer QoL Questionnaire was used to evaluate QoL before treatment and 3 weeks after treatment (after completion of all three courses) to comprehensively assess the impact of the treatments on the patients’ overall condition. The questionnaire consists of 30 items grouped into five functional scales (physical, role, emotional, cognitive, and social functioning), three symptom scales (fatigue, nausea/vomiting, and pain), and a global health status/QoL scale. For each item, responses were scored on a four-point Likert scale (1 = not at all, 2 = a little, 3 = quite a bit, 4 = very much) or a two-point scale (1 = no, 2 = yes). Raw scores for multi-item scales were linearly transformed to a scale of 0-100. The scores were calculated in accordance with the European Organization for Research and Treatment of Cancer scoring manual, and high scores represent good QoL. The internal consistency of the ques
Data were analyzed using SPSS statistical software (version 29.0; SPSS Inc., IL, United States). Categorical data were expressed as n (%). χ2 tests based on standard formulas were applied to compare categorical variables. Continuous variables were initially assessed for normal distribution using the Shapiro-Wilk test. If the normality assumption was met, the data were reported as mean ± SD. A P value of less than 0.05 was considered statistically significant.
In this study comparing the inflammatory cytokine effects of BGM-UT plus standard palliative care vs standard palliative care alone on cancer-induced incomplete bowel obstruction, the participants’ demographics and baseline characteristics were similar (Table 2). These findings confirm demographic similarity between the two groups, supporting subsequent treatment efficacy comparisons. No significant differences in disease characteristics were found between the groups (Table 3). The causes of obstruction were similar: 63.16% colorectal cancer in regular vs 51.92% in BGM-UT; metastatic tumors accounted for 24.56% and 30.77%, respectively; and other causes accounted 12.28% in regular and 17.31% in BGM-UT (P = 0.486). Obstruction sites were also comparable: Colonic (45.61% regular vs 50.00% BGM-UT), rectal (35.09% vs 32.69%), and other sites (19.30% vs 17.31%; P = 0.898). Regarding obstruction episodes, 53.39% of the regular group and 69.23% of the BGM-UT group had no prior episodes. One episode occurred in 28.07% and 17.31% of the regular and BGM-UT groups, respectively; two episodes in 12.28% and 11.54% of the regular and BGM-UT groups, respectively; and three or more in 5.26% and 1.92% of the regular and BGM-UT groups, respectively (P = 0.364).
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t/χ2 | P value |
| Age (years) | 66.49 ± 9.57 | 67.65 ± 10.14 | 0.612 | 0.542 |
| Male/female | 31 (54.39)/26 (45.61) | 27 (51.92)/25 (48.08) | 0.066 | 0.797 |
| BMI (kg/m2) | 23.78 ± 2.97 | 23.56 ± 3.14 | 0.378 | 0.706 |
| Ethnicity | 0.247 | 0.619 | ||
| Han | 44 (77.19) | 38 (73.08) | ||
| Others | 13 (22.81) | 14 (26.92) | ||
| Smoking history | 0 | 0.983 | ||
| Yes | 33 (57.89) | 30 (57.69) | ||
| No | 24 (42.11) | 22 (42.31) | ||
| Drinking history | 0.952 | 0.329 | ||
| Yes | 20 (35.09) | 23 (44.23) | ||
| No | 37 (64.91) | 29 (55.77) | ||
| Diabetes history | 0.265 | 0.607 | ||
| Yes | 15 (26.32) | 16 (30.77) | ||
| No | 42 (73.68) | 36 (69.23) | ||
| Hypertension history | 0.056 | 0.813 | ||
| Yes | 12 (21.05) | 10 (19.23) | ||
| No | 45 (78.95) | 42 (80.77) | ||
| KPS | 0.096 | 0.756 | ||
| 30-60 | 39 (68.42) | 37 (71.15) | ||
| 0-30 | 18 (31.58) | 15 (28.85) |
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | χ2 | P value |
| Causes of obstruction | 1.443 | 0.486 | ||
| Colorectal cancer | 36 (63.16) | 27 (51.92) | ||
| Metastatic tumor | 14 (24.56) | 16 (30.77) | ||
| Others | 7 (12.28) | 9 (17.31) | ||
| Sites of obstruction | 0.214 | 0.898 | ||
| Colon | 26 (45.61) | 26 (50.00) | ||
| Rectum | 20 (35.09) | 17 (32.69) | ||
| Others | 11 (19.30) | 9 (17.31) | ||
| Numbers of previous obstruction episodes | 3.187 | 0.364 | ||
| 0 | 31 (54.39) | 36 (69.23) | ||
| 1 | 16 (28.07) | 9 (17.31) | ||
| 2 | 7 (12.28) | 6 (11.54) | ||
| ≥ 3 | 3 (5.26) | 1 (1.92) |
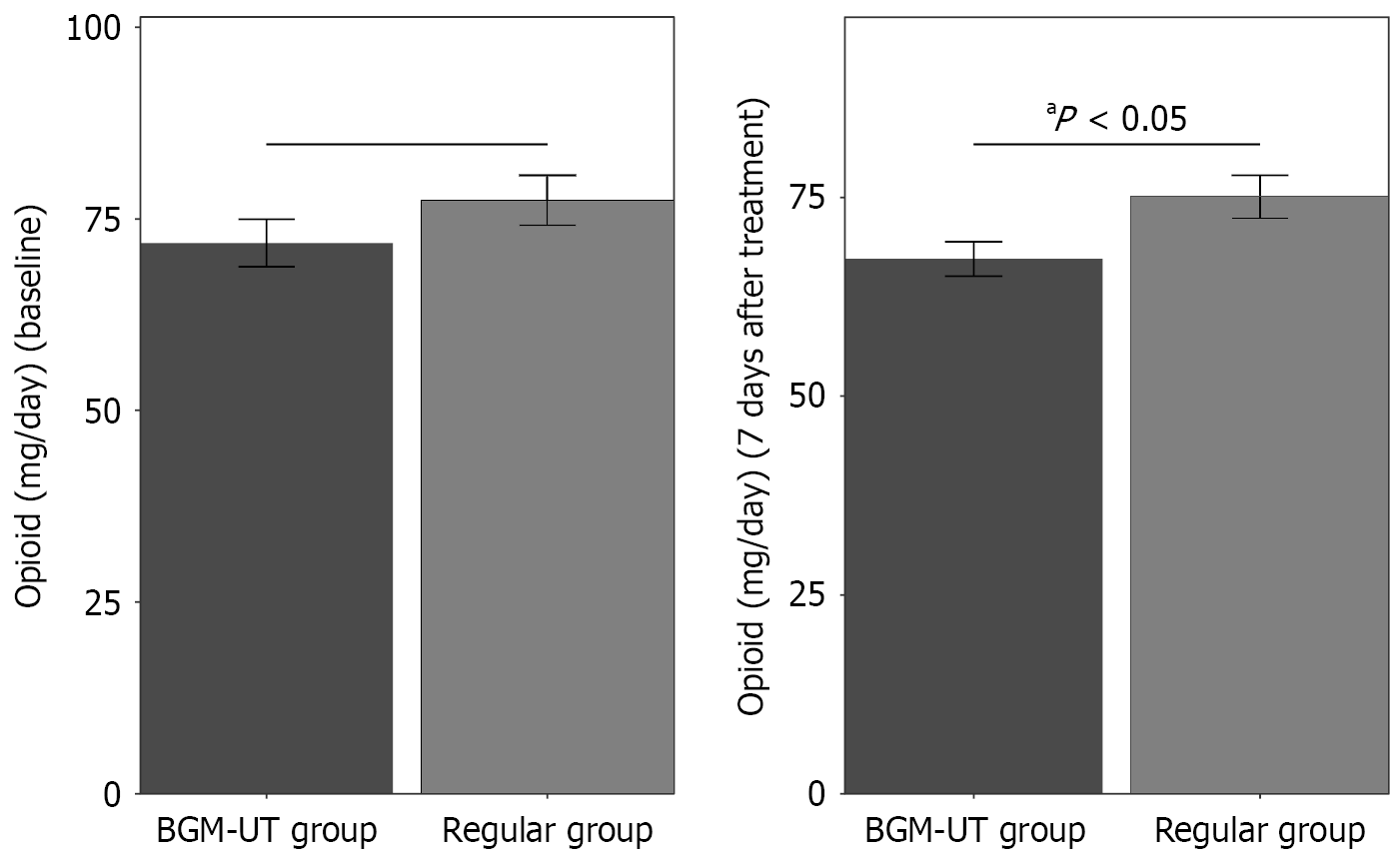
At baseline, opioid intake was similar between the groups (Figure 1): 77.43 ± 24.57 mg/day for the regular group and 71.84 ± 22.29 mg/day for the BGM-UT group (P = 0.217). After 7 days of treatment, the average opioid consumption in the BGM-UT group was 67.29 ± 15.57 mg/day, which represented a significant reduction compared with the 75.08 ± 20.46 mg/day in the regular group (P = 0.027). These findings suggest that BGM-UT may lead to a great reduction in opioid analgesic requirements for patients with cancer-related incomplete bowel obstruction.
Gastrointestinal function recovery was significantly improved in the BGM-UT group compared with that in the regular group (Table 4). The time to first anal exhaust was shorter in the BGM-UT group (average of 74.35 ± 13.43 hours) than in the regular group (81.85 ± 20.87 hours; P = 0.027). Similarly, the time to first defecation was reduced to 92.71 ± 27.27 hours in the BGM-UT group compared with the 104.7 ± 34.28 hours in the tegular group (P = 0.047). Bowel sound recovery was faster in the BGM-UT group (85.38 ± 12.54 hours) than in the regular group (91.51 ± 13.92 hours; P = 0.018). The BGM-UT group also achieved earlier first solid food intake (123.25 ± 34.87 hours) than the regular group (141.95 ± 56.89 hours; P = 0.039). These results demonstrate that BGM-UT significantly accelerates gastrointestinal recovery in patients with cancer-related incomplete bowel obstruction.
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t | P value |
| The first anal exhaust time (hours) | 81.85 ± 20.87 | 74.35 ± 13.43 | 2.251 | 0.027 |
| The first defecation time (hours) | 104.7 ± 34.28 | 92.71 ± 27.27 | 2.009 | 0.047 |
| The recovery time of bowel sound (hours) | 91.51 ± 13.92 | 85.38 ± 12.54 | 2.408 | 0.018 |
| The first solid food intake time (hours) | 141.95 ± 56.89 | 123.25 ± 34.87 | 2.088 | 0.039 |
Baseline BPI scores showed no significant differences between the groups (Table 5). The total BPI scores were 4.86 ± 1.27 for the regular group and 4.89 ± 1.13 for the BGM-UT group (P = 0.913). The BPI intensity scores were 5.04 ± 1.15 in the regular group and 4.84 ± 1.26 in the BGM-UT group (P = 0.376). Their BPI interference scores were similar, that is, 5.17 ± 1.62 for the regular group and 5.04 ± 1.38 for the BGM-UT group (P = 0.638). These findings indicate similar pain levels between the groups before the intervention. At 1 week posttreatment, the BGM-UT group showed significantly lower BPI scores than the regular group (Table 6). The BPI total scores were 4.62 ± 1.17 in the BGM-UT group vs 5.19 ± 1.45 in the regular group (P = 0.025). Their BPI intensity scores were also reduced, averaging 4.45 ± 1.24 in the BGM-UT group and 5.02 ± 1.43 in the regular group (P = 0.028). Meanwhile, the BPI interference scores were lower for the BGM-UT group at 4.78 ± 1.03 compared with that for the regular group at 5.39 ± 1.72 (P = 0.025). These results suggest that BGM-UT plus standard palliative care is more effective in reducing pain and its impact on daily activities compared with standard care alone.
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t | P value |
| BPI total | 4.86 ± 1.27 | 4.89 ± 1.13 | 0.110 | 0.913 |
| BPI intensity | 5.04 ± 1.15 | 4.84 ± 1.26 | 0.889 | 0.376 |
| BPI interference | 5.17 ± 1.62 | 5.04 ± 1.38 | 0.471 | 0.638 |
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t | P value |
| BPI total | 5.19 ± 1.45 | 4.62 ± 1.17 | 2.267 | 0.025 |
| BPI intensity | 5.02 ± 1.43 | 4.45 ± 1.24 | 2.232 | 0.028 |
| BPI interference | 5.39 ± 1.72 | 4.78 ± 1.03 | 2.276 | 0.025 |
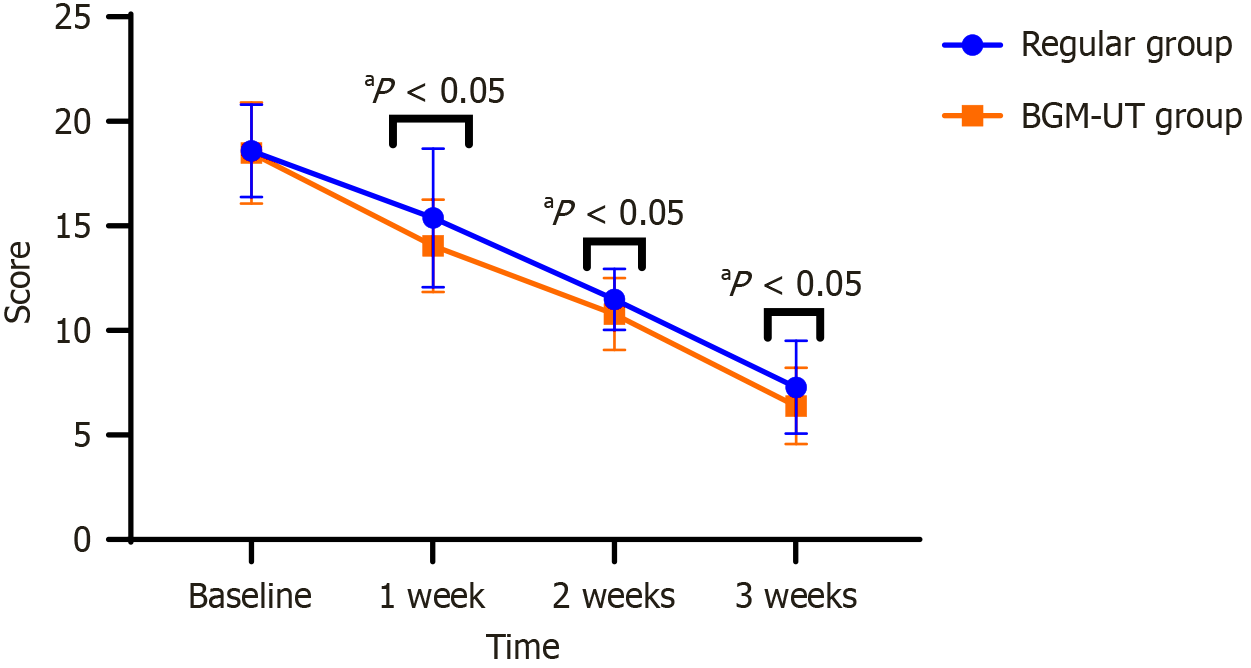
At baseline, the TCM total clinical symptom scores were similar between the regular group (18.59 ± 2.21) and BGM-UT group (18.49 ± 2.41; P = 0.830), indicating no initial differences (Figure 2). After 1 week, both groups improved, but the BGM-UT group had a significantly lower score (14.05 ± 2.21) than the regular group (15.39 ± 3.31; P = 0.014). This trend persisted, with the BGM-UT group scoring 10.79 ± 1.71 at 2 weeks compared with the regular group scoring 11.49 ± 1.45 (P = 0.024). After 3 weeks, the BGM-UT group’s score decreased further to 6.39 ± 1.82 compared with that of the regular group at 7.29 ± 2.21 (P = 0.024). These findings indicate that BGM-UT plus standard palliative care results in more rapid and significant improvement in TCM clinical symptoms than standard care alone.
At 3 weeks posttreatment, the BGM-UT group showed significantly better outcomes in TCM individual symptom scores compared with the regular group (Table 7). The BGM-UT group had fewer diarrhea episodes (2.06 ± 0.21) than the regular group (2.31 ± 0.56; P = 0.002). Stool character scores improved in the BGM-UT group (2.16 ± 0.31) vs the regular group (2.34 ± 0.55; P = 0.042). Abdominal pain scores were lower in the BGM-UT group (1.96 ± 0.22) than the regular group (2.21 ± 0.65; P = 0.009). In addition, abdominal distension scores were reduced in the BGM-UT group (2.28 ± 0.28) compared with those in the regular group (2.45 ± 0.31; P = 0.003). These results indicate that BGM-UT plus standard palliative care more effectively alleviates the symptoms of cancer-related incomplete bowel obstruction compared with standard care alone.
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t | P value |
| Number of diarrheas | 2.31 ± 0.56 | 2.06 ± 0.21 | 3.146 | 0.002 |
| Stool character | 2.34 ± 0.55 | 2.16 ± 0.31 | 2.058 | 0.042 |
| Abdominal pain | 2.21 ± 0.65 | 1.96 ± 0.22 | 2.703 | 0.009 |
| Abdominal distension | 2.45 ± 0.31 | 2.28 ± 0.28 | 2.990 | 0.003 |
The baseline levels of inflammatory cytokines were similar between the groups (Table 8). The IL-6 Levels were 38.76 ± 8.41 pg/mL and 39.24 ± 7.31 pg/mL in the regular and BGM-UT groups, respectively (P = 0.749). The TNF-α levels were 59.62 ± 13.75 pg/mL and 57.41 ± 15.43 pg/mL in the regular and BGM-UT groups, respectively (P = 0.430). The CRP levels were 31.65 ± 8.89 mg/L and 32.74 ± 9.12 mg/L in the regular and BGM-UT groups, respectively (P = 0.529). These findings show that the two groups have comparable inflammatory cytokine levels before treatment.
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t | P value |
| IL-6 (pg/mL) | 38.76 ± 8.41 | 39.24 ± 7.31 | 0.321 | 0.749 |
| TNF-α (pg/mL) | 59.62 ± 13.75 | 57.41 ± 15.43 | 0.792 | 0.430 |
| CRP (mg/L) | 31.65 ± 8.89 | 32.74 ± 9.12 | 0.632 | 0.529 |
At 1 week posttreatment, the BGM-UT group showed significant reductions in inflammatory cytokines compared with the regular group (Table 9). The IL-6 Levels decreased to 32.83 ± 7.12 pg/mL in the BGM-UT group and 35.56 ± 7.23 pg/mL in the regular group (P = 0.050). The TNF-α levels were also lower in the BGM-UT group (48.35 ± 10.57 pg/mL) compared with those in the regular group (52.78 ± 12.36 pg/mL) (P = 0.047). Similarly, the CRP levels decreased to 25.86 ± 6.89 mg/L in the BGM-UT group and 28.47 ± 6.58 mg/L in the regular group (P = 0.045). These results suggest that BGM-UT leads to a substantial decrease in inflammatory cytokines, indicating its enhanced anti-inflammatory effects on patients with cancer-related incomplete bowel obstruction.
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t | P value |
| IL-6 (pg/mL) | 35.56 ± 7.23 | 32.83 ± 7.12 | 1.986 | 0.050 |
| TNF-α (pg/mL) | 52.78 ± 12.36 | 48.35 ± 10.57 | 2.005 | 0.047 |
| CRP (mg/L) | 28.47 ± 6.58 | 25.86 ± 6.89 | 2.024 | 0.045 |
At 3 weeks posttreatment after the completion of all three courses, the BGM-UT group continued to show significantly lower levels of inflammatory cytokines compared with the regular group (Table 10). The IL-6 Levels were reduced to 29.45 ± 6.98 pg/mL in the BGM-UT group and 33.12 ± 7.15 pg/mL in the regular group (P = 0.010). The TNF-α levels were 45.23 ± 9.78 pg/mL in the BGM-UT group and 50.12 ± 11.23 pg/mL in the regular group (P = 0.018). The CRP levels decreased to 22.56 ± 6.21 mg/L in the BGM-UT group and 26.14 ± 6.45 mg/L in the regular group (P = 0.004). These findings further support the sustained anti-inflammatory effects of BGM-UT over time.
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t | P value |
| IL-6 (pg/mL) | 33.02 ± 7.15 | 29.45 ± 6.98 | 2.632 | 0.010 |
| TNF-α (pg/mL) | 50.12 ± 11.23 | 45.23 ± 9.78 | 2.412 | 0.018 |
| CRP (mg/L) | 26.14 ± 6.45 | 22.56 ± 6.21 | 2.944 | 0.004 |
The baseline levels of QoL scores were similar between the groups (Table 11). The physical functioning scores were 52.13 ± 12.36 in the regular group and 51.85 ± 11.92 in the BGM-UT group (P = 0.906). The role functioning scores were 49.54 ± 13.27 in the regular group and 48.92 ± 12.85 in the BGM-UT group (P = 0.805). The emotional functioning scores were 50.12 ± 11.53 in the regular group and 49.82 ± 11.22 in the BGM-UT group (P = 0.888). The cognitive functioning scores were 51.38 ± 10.72 in the regular group and 50.93 ± 10.44 in the BGM-UT group (P = 0.825). The social functioning scores were 48.76 ± 12.16 in the regular group and 48.44 ± 11.82 in the BGM-UT group (P = 0.892). These findings show that the two groups have comparable baseline QoL scores before treatment.
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t | P value |
| Physical functioning | 52.13 ± 12.36 | 51.85 ± 11.92 | 0.119 | 0.906 |
| Role functioning | 49.54 ± 13.27 | 48.92 ± 12.85 | 0.248 | 0.805 |
| Emotional functioning | 50.12 ± 11.53 | 49.82 ± 11.22 | 0.141 | 0.888 |
| Cognitive functioning | 51.38 ± 10.72 | 50.93 ± 10.44 | 0.222 | 0.825 |
| Social functioning | 48.76 ± 12.16 | 48.44 ± 11.82 | 0.136 | 0.892 |
At 3 weeks posttreatment, the BGM-UT group showed significantly higher improvements in QoL scores compared with the regular group (Table 12). The physical functioning scores improved to 70.51 ± 12.59 in the BGM-UT group and 64.09 ± 12.25 in the regular group (P = 0.008). The role functioning scores increased to 71.32 ± 12.77 in the BGM-UT group and 64.66 ± 12.39 in the regular group (P = 0.007). The emotional functioning scores were enhanced to 63.25 ± 11.58 in the BGM-UT group and 57.64 ± 11.32 in the regular group (P = 0.012). The cognitive functioning scores rose to 66.15 ± 11.42 in the BGM-UT group and 59.63 ± 10.73 in the regular group (P = 0.003). The social functioning scores increased to 65.08 ± 11.94 in the BGM-UT group and 58.49 ± 11.48 in the regular group (P = 0.004). These results indicate that BGM-UT has a positive impact on multiple aspects of the patients’ QoL within 3 weeks, highlighting its potential efficacy over conventional methods in enhancing patient well-being.
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | t | P value |
| Physical functioning | 64.09 ± 12.25 | 70.51 ± 12.59 | 2.698 | 0.008 |
| Role functioning | 64.66 ± 12.39 | 71.32 ± 12.77 | 2.760 | 0.007 |
| Emotional functioning | 57.64 ± 11.32 | 63.25 ± 11.58 | 2.559 | 0.012 |
| Cognitive functioning | 59.63 ± 10.73 | 66.15 ± 11.42 | 3.076 | 0.003 |
| Social functioning | 58.49 ± 11.48 | 65.08 ± 11.94 | 2.936 | 0.004 |
Safety profiles were comparable between the groups (all P > 0.05). In the BGM-UT group, two patients (3.85%) developed transient skin erythema at moxibustion sites (resolved within 24 hours with topical cooling), and one patient (1.92%) reported mild pruritus requiring antihistamines. No burns occurred. In the regular group, routine monitoring also did not reveal any significant adverse events. Standard palliative care procedures were well tolerated by all the participants (Table 13). These findings indicate that both treatments are safe and highly tolerable for patients.
| Parameters | Regular group (n = 57) | BGM-UT group (n = 52) | χ2 | P value |
| Skin erythema | 0 (0.00) | 2 (3.85) | 0.608 | 0.435 |
| Allergic reaction | 0 (0.00) | 1 (1.92) | 0.142 | 0.877 |
| Skin burns | 0 (0.00) | 0 (0.00) | 0.000 | 1.000 |
Significant negative correlations were observed between the reduction in inflammatory cytokine levels and gastrointes
| Gastrointestinal indicator | IL-6 reduction | TNF-α reduction | CRP reduction | |||
| r | P value | r | P value | r | P value | |
| First anal exhaust time | -0.31 | 0.006 | -0.41 | < 0.001 | -0.49 | < 0.001 |
| First defecation time | -0.42 | < 0.001 | -0.35 | 0.002 | -0.52 | < 0.001 |
| Bowel sound recovery | -0.29 | 0.011 | -0.47 | < 0.001 | -0.45 | < 0.001 |
| First solid food intake | -0.38 | 0.001 | -0.37 | 0.001 | -0.51 | < 0.001 |
This study presents evidence supporting the potential benefits of BGM-UT in managing cancer-induced incomplete bowel obstruction. Our findings indicate that BGM-UT enhances gastrointestinal function recovery, reduces the need for opioid analgesics, and significantly reduces inflammatory cytokine levels such as IL-6, TNF-α, and CRP. The mechanisms underlying these outcomes appear to be multifaceted and rooted in TCM perspectives and modern pharmacological understandings[24]. Moxibustion is traditionally viewed as a therapeutic modality that enhances the flow of Qi and blood, thereby restoring balance and health[25]. The use of acupoints such as Guanyuan, Qihai, Shenque, and Tianshu aligns with the principle of unblocking Qi stagnation and harmonizing the interior[26]. The warming nature of moxibus
From a modern biomedical perspective, the anti-inflammatory effects of BGM-UT could be explained by the observed modulation of systemic inflammatory markers[29]. IL-6 and TNF-α are central to the inflammatory cascade associated with cancer-induced complications, promoting a pro-inflammatory environment that can exacerbate symptoms and impede functional recovery[30]. The reduction in these cytokines posttreatment suggests that BGM-UT might exert systemic anti-inflammatory effects[30]. The thermal stimulus provided by moxibustion affects local tissues and the neu
Opioid analgesics, while effective for pain management, pose significant challenges because of their potential for addiction and adverse effects, including constipation and bowel obstruction exacerbation[32]. Owing to the effective analgesia provided by BGM-UT, the patients showed reduced opioid requirements, which are suggestive of an improve
The parallel reductions in opioid use, pain scores, and cytokines suggest that BGM-UT disrupts the vicious cycle between inflammation and neuropathic pain. In bowel obstruction, IL-6 sensitizes the transient receptor potential vanilloid 1 channels on visceral afferents, and TNF-α promotes synaptic glutamate release in dorsal horn neurons. BGM-UT likely reduces peripheral sensitization by lowering the levels of these cytokines, which explains the decreased BPI scores despite the lower opioid doses[34].
As evidenced by the earlier first anal exhaust, defecation, bowel sound recovery, and solid food intake times, gas
Our correlation analysis provides mechanistic insights into the clinical benefits of BGM-UT. The significant inverse relationships between IL-6, TNF-α, and CRP reductions and gastrointestinal recovery times suggest that systemic inflammation directly impairs gut motility and mucosal barrier function. TNF-α disrupts intestinal smooth muscle contractility and increase mucosal permeability, and IL-6 promotes enteric neuronal dysfunction. The strong correlation between CRP reduction and all functional outcomes may reflect CRP’s role as a sensitive integrator of global inflammatory burden. These findings align with previous studies showing that cytokine-mediated inflammation prolongs postoperative ileus and impairs epithelial restitution. Thus, BGM-UT likely accelerates functional recovery partly through the downregulation of these key inflammatory mediators[37]. Our study shows notable changes in TCM symptom scores, which evaluate multifactorial clinical presentations, including pain, bloating, and nausea. These improvements hint at the holistic mechanism of BGM-UT, i.e., addressing obstructions physically and systemically by influencing the balance and function of body systems.
At the cellular level, anti-inflammatory responses might be further explained by the activation of heat-shock proteins induced by thermal stimulation[38]. Heat-shock proteins can modulate immune responses, promoting the degradation of potentially inflammatory molecules and stabilizing cellular environments[38]. By maintaining cellular integrity and reducing inappropriate immune responses, such proteins could lower inflammation and improve recovery in bowel obstruction[38].
The significant decrease in blood inflammatory markers in the BGM-UT group aligns well with the therapeutic reduction in inflammation and pain. CRP, a sensitive marker of systemic inflammation, increases in response to cytokines such as IL-6[39]. Its significant reduction further corroborates the systemic anti-inflammatory effects of BGM-UT. This reduction could be particularly influential in cancer, where systemic inflammation is a staple of disease progression and symptomatology. Reducing these inflammatory burdens can potentially alleviate a range of cancer-related symptoms, thus enhancing patient QoL[39].
Despite these promising results, several limitations of this study must be acknowledged. Although no statistical difference in baseline conditions was found between the two groups, we did not clarify whether the grouping was based on patients’ treatment preferences. Patients with mild symptoms might have been willing to receive BGM-UT, leading to potential selection bias. Future studies should consider randomization or stratification methods to minimize this bias and ensure comparability between groups. All the samples were collected from a single center, which limits the generalizability of our findings. A multicenter study design would provide a broad and diverse patient population, enhancing the external validity of the results. In addition, subgroup analyses based on tumor types (e.g., colorectal cancer and ovarian cancer) were not conducted, which narrows the applicability of the results. Future research should include subgroup analyses to explore differential responses across various cancer types. Owing to its retrospective design, our study inherently carries limitations related to data collection and completeness. Prospective studies with well-defined protocols and rigorous follow-up procedures would strengthen the evidence base for BGM-UT’s efficacy and safety.
Given the pilot nature of our exploration, future studies on the pathways and mediators involved in BGM-UT’s effects are necessary. Research could extend into molecular investigations, assessing how moxibustion’s heat nature induces specific immune modulations or triggers particular neurotransmitter releases that might broadly explain its clinical effects. Moreover, analysis of individual patient variability regarding responses to BGM-UT might identify subpopulations that would benefit the most from this modality, offering a tailored approach to cancer care that is essential considering cancer’s heterogeneity.
The study’s implications are promising, suggesting a viable complementary therapy within integrative oncology care frameworks. As cancer treatments evolve, patient-centered care with emphasis on QoL becomes integral. Therapies such as BGM-UT, with minimal side effects and multiple symptomatic relief avenues, hold immense potential in achieving these care goals. However, integrating such traditional therapies into standard care necessitates validation through extensive clinical trials and mechanistic explorations to ensure that safety and efficacy standards are universally upheld.
BGM-UT represents a beneficial intervention strategy for enhancing recovery and diminishing systemic inflammation in patients with cancer-related incomplete bowel obstructions. The results of this study call for enhanced interdisciplinary approaches to healthcare, combining insights from traditional and modern medicine to comprehensively improve patient outcomes.
| 1. | Ahmed M, Elkahly M, Gorski T, Mahmoud A, Essien F. Meckel's Diverticulum Strangulation. Cureus. 2021;13:e14817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (2)] |
| 2. | Ahmed M, Saeed R, Allawi A, Zajicek J. Meckel's Diverticulum With Perforation. Cureus. 2024;16:e67026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Aliabadi T, Saberi EA, Motameni Tabatabaei A, Tahmasebi E. Antibiotic use in endodontic treatment during pregnancy: A narrative review. Eur J Transl Myol. 2022;32:10813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Bauschert L, Sermet K, Fréalle E, Khodr J, Magro L, Yakoub-Agha I, Alfandari S, Beauvais D. A case of subacute bowel obstruction revealing slowly-evolutive gastro-intestinal mucormycosis following allogeneic hematopoietic cell transplantation. J Mycol Med. 2022;32:101312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Blask AR, Fagen KE, Rubio EI, Badillo AT, Bulas DI. Prenatal diagnosis of intestinal nonrotation using magnetic resonance imaging: Is it possible? Pediatr Radiol. 2021;51:1332-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Carroll W, Green J, Gilchrist FJ. Interventions for preventing distal intestinal obstruction syndrome (DIOS) in cystic fibrosis. Cochrane Database Syst Rev. 2021;12:CD012619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Chen P, Hu Q, Wu J, Sun Y. A Rare Cause of Small Bowel Obstruction: A Case Report. Front Surg. 2022;9:855904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Chiang HC, Chen CY, Chuang CH, Hsu HL. Incomplete small bowel obstruction in a patient with ankylosing spondylitis. Radiol Case Rep. 2021;16:2505-2508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | De Dyn S, Demirci I, Prescher A, Kopp A, Klosterhalfen B, Janßen H. Mechanical ileus of the small bowel due to an inflamed Meckel's diverticulum with an enterolith - a case report with literature review. Acta Chir Belg. 2023;123:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Doll A, Grimm L. Congenital Incomplete Rotation of the Colon With Adhesive Obstruction in an Adult. Am Surg. 2023;89:2803-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Elzeneini WMA, Cusick E. A large single-center experience in management of pediatric intussusception. Pediatr Int. 2023;65:e15495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Farooq R, Sahibole AS, Misiriyyah N, Ahmed H, Margossian H. Small Bowel Obstruction as a Complication of Uterine Fibroids: A Case Report. Cureus. 2023;15:e36902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Figueroa-Giralt M, Torrealba A, Gonzalez T, Almeida P, Braghetto I, Csendes A. Risk factors for reoperation, morbidity, and mortality in patients with small bowel obstruction submitted to surgical treatment. Arq Bras Cir Dig. 2022;35:e1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Gilchrist FJ, Green J, Carroll W. Interventions for treating distal intestinal obstruction syndrome (DIOS) in cystic fibrosis. Cochrane Database Syst Rev. 2021;12:CD012798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Guo JF, Zhao YT, Du QY, Ren Y, Wang Y, Wang ZX, Jin W. The Network Pharmacology Study of Dahuang Fuzi Decoction for Treating Incomplete Intestinal Obstruction. Biomed Res Int. 2022;2022:2775434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Hong SM, Jung SH, Baek DH. Diagnostic Yields and Clinical Impacts of Capsule Endoscopy. Diagnostics (Basel). 2021;11:1842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Jabra SB, Chaouch MA, Moussa A, Jallali M, Toumi O, Noomen F. Incomplete common mesentery with Ladd's band and Meckel's diverticulum: A rare cause of small bowel obstruction. Int J Surg Case Rep. 2023;106:108159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Madariaga A, Lau J, Ghoshal A, Dzierżanowski T, Larkin P, Sobocki J, Dickman A, Furness K, Fazelzad R, Crawford GB, Lheureux S. MASCC multidisciplinary evidence-based recommendations for the management of malignant bowel obstruction in advanced cancer. Support Care Cancer. 2022;30:4711-4728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Yao F, Zhang Y, Kuang X, Zhou Q, Huang L, Peng J, Du S. Effectiveness and Safety of Moxibustion on Constipation: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2020;2020:8645727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 21. | Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 967] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 22. | Chien TJ, Song YL, Lin CP, Hsu CH. The correlation of traditional chinese medicine deficiency syndromes, cancer related fatigue, and quality of life in breast cancer patients. J Tradit Complement Med. 2012;2:204-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11936] [Article Influence: 361.7] [Reference Citation Analysis (0)] |
| 24. | Jazayeri-Moghadass BS, Sutherland R, Patel LD, Cebotaru V. Small Bowel Obstruction with a Transition Point in a Patient on Peritoneal Dialysis. Case Rep Nephrol Dial. 2022;12:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kaneishi K, Imai K, Nishimura K, Sakurai N, Kohara H, Ishiki H, Kanai Y, Oyamada S, Yamaguchi T, Morita T, Iwase S. Olanzapine versus Metoclopramide for Treatment of Nausea and Vomiting in Advanced Cancer Patients with Incomplete Malignant Bowel Obstruction. J Palliat Med. 2020;23:880-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Li E, Feng N, Zeng Q, Sanchez-Tacuba L, Kawagishi T, Branham G, Hou G, Wang Z, Greenberg HB, Ding S. Rhesus rotavirus NSP1 mediates extra-intestinal infection and is a contributing factor for biliary obstruction. PLoS Pathog. 2024;20:e1012609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Liang JT, Liao YT, Chen TC, Huang J, Hung JS. Changing patterns and surgical outcomes of small bowel obstruction in the era of minimally invasive surgery for colorectal cancer. Int J Surg. 2024;110:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Lim JH, Lee WY, Yun SH, Kim HC, Cho YB, Huh JW, Park YA, Shin JK. Comparison of Oncologic Outcomes Between Incomplete Obstructive Colon Cancer and Non-Obstructive Colon Cancer by Tumor Location. Front Oncol. 2022;12:914299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Zhao Z, Li J, Wen J, He Y, Sun Z. Effect of Moxibustion on Inflammatory Cytokines for Low Back Pain: A Systematic Review, Meta-Analysis and Meta-Regression. Ther Clin Risk Manag. 2023;19:811-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 30. | Mejri A, Trigui E. Phytobezoar: A train can hide another. Int J Surg Case Rep. 2021;81:105814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Liao F, Zhang C, Bian Z, Xie D, Kang M, Li X, Wan Y, Chen R, Yi M. Characterizing heat-sensitization responses in suspended moxibustion with high-density EEG. Pain Med. 2014;15:1272-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Merrill C, Wilson SR. Ultrasound of the bowel with a focus on IBD: the new best practice. Abdom Radiol (NY). 2025;50:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Pan S, Wang S, Li J, Yuan H, Xue X, Liu Y, Yue Z. Moxibustion for Primary Dysmenorrhea: An Adjuvant Therapy for Pain Relief. Evid Based Complement Alternat Med. 2022;2022:6864195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Kadhim S, McDonald J, Lambert DG. Opioids, gliosis and central immunomodulation. J Anesth. 2018;32:756-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Saalabian K, Friedmacher F, Theilen TM, Keese D, Rolle U, Gfroerer S. Prenatal Detection of Congenital Duodenal Obstruction-Impact on Postnatal Care. Children (Basel). 2022;9:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Schudrowitz N, Shahan CP, Moss T, Scarborough JE. Bowel Preparation Before Nonelective Sigmoidectomy for Sigmoid Volvulus: Highly Beneficial but Vastly Underused. J Am Coll Surg. 2023;236:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Chen MK, Liu SZ, Zhang L. Immunoinflammation and functional gastrointestinal disorders. Saudi J Gastroenterol. 2012;18:225-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Hoter A, Naim HY. The Functions and Therapeutic Potential of Heat Shock Proteins in Inflammatory Bowel Disease-An Update. Int J Mol Sci. 2019;20:5331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Sugarbaker PH, Chang D. Incomplete cytoreduction with peritoneal metastases from appendiceal mucinous neoplasms. J Surg Oncol. 2022;126:1462-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/