Published online Oct 28, 2025. doi: 10.3748/wjg.v31.i40.112033
Revised: August 8, 2025
Accepted: September 16, 2025
Published online: October 28, 2025
Processing time: 103 Days and 17.4 Hours
Colonic diverticular bleeding (CDB) is a leading cause of gastrointestinal blee
To establish a practical benchmark for minimum colonoscopy observation time to ensure reliable SRH detection in patients with CDB.
We retrospectively analyzed patients with acute hematochezia who underwent an initial colonoscopy between January 2017 and December 2024 at a Japanese tertiary hospital. The Observation time was measured from scope insertion to SRH detection (excluding therapeutic time) or withdrawal. The primary outcome, the “5% plateau time”, was defined as the point when the proportion of patients newly identified with SRH in each 5-minute interval consistently dropped below 5%. Computed tomography (CT)-based stratified analyses were performed by endoscopists who conducted ≥ 10% of procedures.
Of the 1039 patients who underwent colonoscopy, 845 (mean age 77 ± 11 years; 64.5% male) were included. Nine board-certified endoscopists performed the procedures. SRH was detected in 286 patients (33.8%), with a median detection time of 19 minutes (interquartile range, 12-28 minutes). The overall 5% plateau time was 40 minutes and varied according to the CT findings: 40, 35, and 30 minutes for no extravasation, right-sided extravasation, and left-sided extravasation, respectively. This time point corresponded to when 80%-90% of SRH cases were detected. De
Although it varied according to the CT findings, the overall 5% plateau time was 40 minutes. This offers a practical benchmark for the minimum observation time without SRH detection.
Core Tip: This study introduces the “5% plateau time” as a novel benchmark for determining the minimum necessary colonoscopy observation time in colonic diverticular bleeding (CDB). By analyzing 845 cases, we found that stigmata of recent hemorrhage (SRH) detection significantly diminished beyond 40 minutes, with stratified times of 40, 35, and 30 mi
- Citation: Ichita C, Goto T, Nishino T, Nakaya S, Shimizu S. Minimum colonoscopy observation time for colonic diverticular bleeding: A new benchmark based on the 5% plateau time. World J Gastroenterol 2025; 31(40): 112033
- URL: https://www.wjgnet.com/1007-9327/full/v31/i40/112033.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i40.112033
Colonic diverticular bleeding (CDB)’s prevalence has increased in the aging population and, by 2017, had surpassed hemorrhagic gastric ulcers as the leading cause of hospitalization for gastrointestinal bleeding in Japan[1,2]. A similar trend has been observed in the United States, where diverticular diseases are a major cause of hospitalization, accounting for over 210000 admissions and more than $2.2 billion in annual healthcare costs[3]. Although many cases resolve spontaneously[4-7], a substantial proportion of patients experience recurrent bleeding[6,8] requiring hospitalization, transfusion, endoscopic therapy, or occasionally surgery[2,9]. As the population ages, CDB is expected to pose a substan
Colonoscopy is the cornerstone of both the diagnosis and treatment of CDB. The detection of stigmata of recent hemorrhage (SRH) during colonoscopy enables definitive endoscopic hemostasis with a reduced risk of rebleeding[6,10]. Several technical and procedural strategies, including early colonoscopy, water-jet scopes, distal attachment caps, and performance by experienced endoscopists, have been explored to improve detection rates[11-14]. Observation time is also considered an important factor; however, few studies have evaluated it, and no clear benchmark exists. Consequently, the observation time remained operator-dependent. Brief observation times may lead to missed SRH and lost treatment opportunities, whereas unnecessarily prolonged procedures, particularly in older patients, may increase the risk of aspiration pneumonia, compound sedation-related burden[15], and be an inefficient use of endoscopy resources.
Currently, no time-based rule for ending observation exists, and a pragmatic benchmark for the minimum observation time is lacking. The absence of such standards contributes to interoperator variability and may lead to both under- and over-observation. Therefore, defining a scientifically grounded lower bound, the minimum time that should be ensured for SRH detection, is essential for improving the diagnostic consistency and procedural efficiency of CDB colonoscopy.
Therefore, in this study, we proposed the “5% plateau time”, defined as the earliest time at which the incremental SRH detection rate falls below 5%, and evaluated it as a scientifically grounded and pragmatic benchmark for the minimum observation time for CDB colonoscopy.
This single-center, retrospective, observational study was conducted at the Shonan Kamakura General Hospital, a 669-bed tertiary care hospital in Japan. The hospital manages over 14000 emergency transportation cases and 42000 emergency department visits annually. More than 5000 colonoscopies are performed each year at the hospital, and all nine board-certified endoscopists have perform at least 1000 colonoscopies in their careers. A 24-hour emergency endoscopy service is available, which includes full coverage during nights and holidays.
This study was approved by the Institutional Review Board of the Future Medical Research Center Ethics Committee (Approval No. TGE1304-024). As this was a non-interventional, observational study using anonymized data, the requirement for informed consent was waived, and an opt-out approach was adopted. This study was conducted in accordance with the principles of the Declaration of Helsinki and is reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
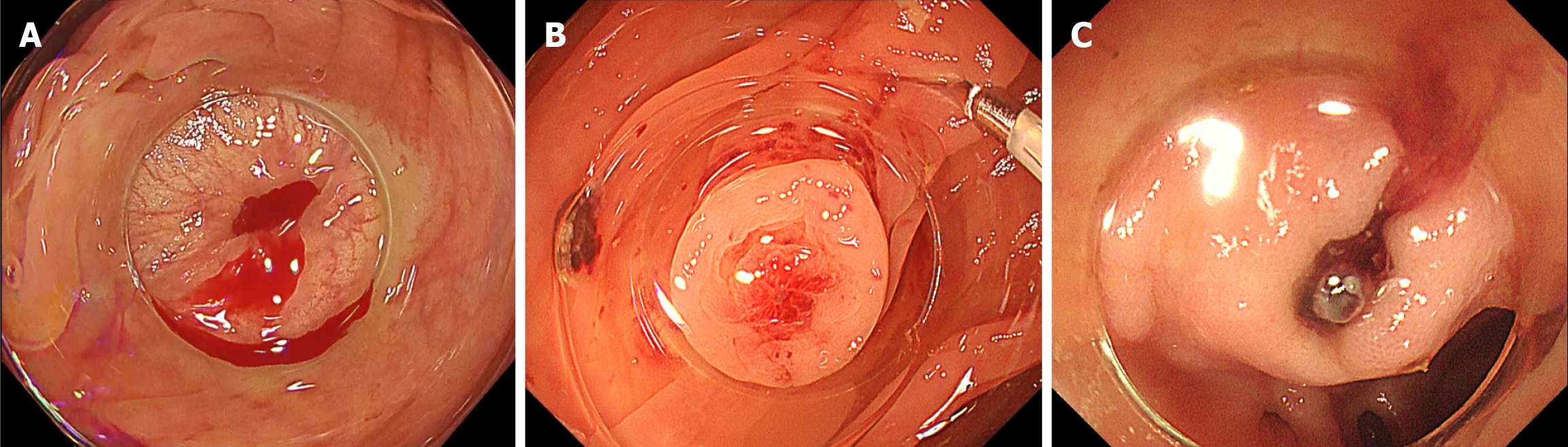
Consecutive patients hospitalized for acute hematochezia who underwent colonoscopy during the same admission were included. CDB was defined as either: (1) Definite CDB, in which clear SRH were identified within a diverticulum[15-17]; or (2) Presumptive CDB, in which no SRH was observed and no alternative bleeding source was identified[16-18]. SRH was defined as active bleeding (Figure 1A), non-bleeding visible vessels (Figure 1B), or adherent clots (Figure 1C) that developed into active bleeding or non-bleeding visible vessels upon removal[17,18]. Patients were excluded if the observation time could not be assessed or if trainees were involved in the colonoscopy, as limited technical proficiency could not only prolong insertion and observation times but also lead to an unjustified reduction in the SRH detection rate.
Only initial colonoscopies performed during hospitalization were included in the analysis. All procedures were performed using adult or pediatric water-jet variable-stiffness colonoscopes (Olympus, Tokyo, Japan) equipped with a distal attachment cap; the choice of scope was at the discretion of the endoscopist. Bowel preparation was also deter
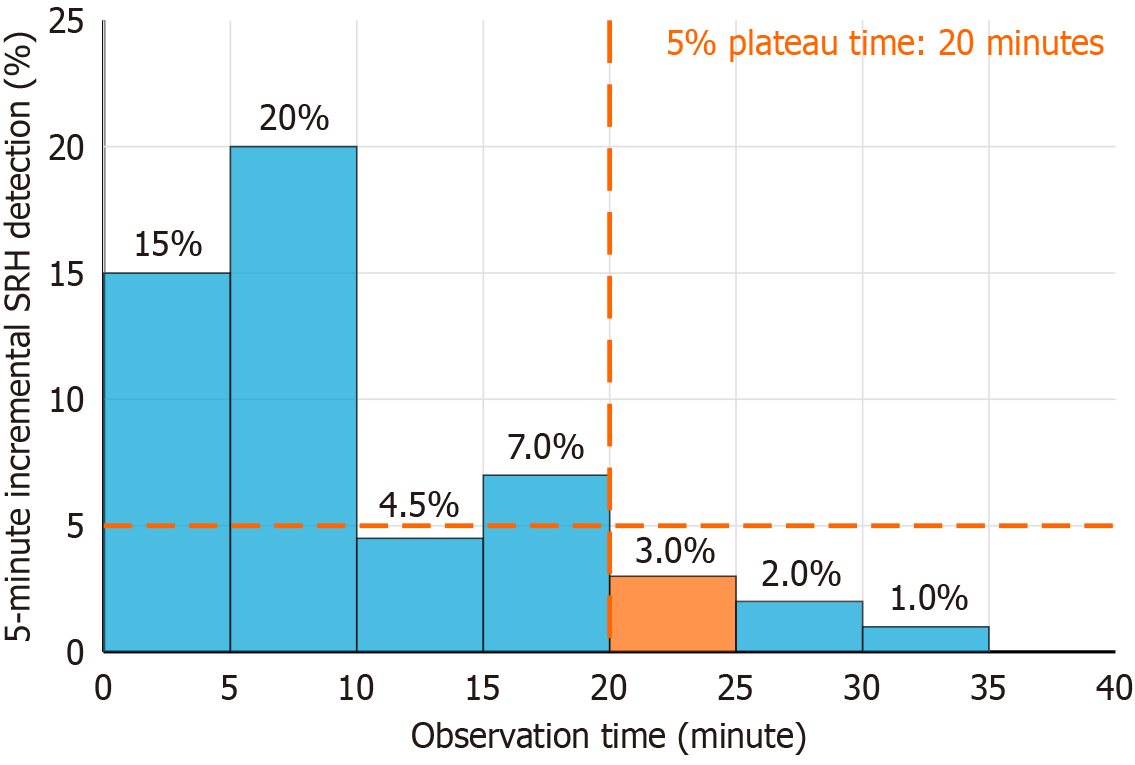
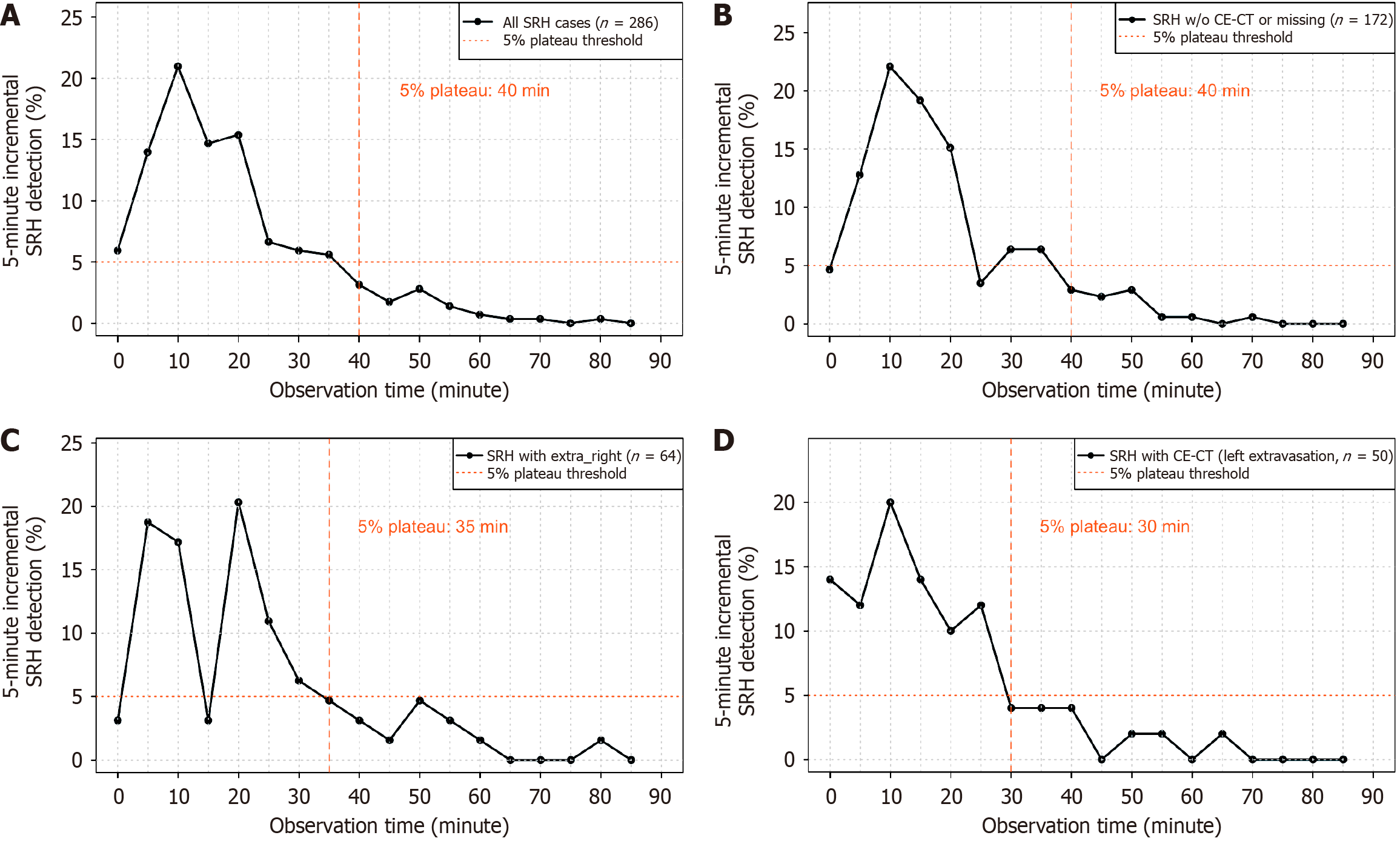
The primary outcome was the “5% plateau time”, defined as the time point at which the incremental detection rate of SRH per 5-minute interval consistently dropped below 5%, indicating a practical lower bound for adequate observation (Figure 2). Secondary outcomes included stratified analyses of the 5% plateau time based on contrast-enhanced CT findings. Patients were classified into three groups: (1) The no extravasation group, defined as those who either underwent contrast-enhanced CT with no extravasation or did not undergo contrast-enhanced CT (including those who received non-contrast CT or no CT); (2) The right-sided extravasation group; and (3) The left-sided extravasation group. Extravasations were defined as right-sided if they occurred between the cecum and the transverse colon, and left-sided if they occurred between the descending colon and sigmoid colon.
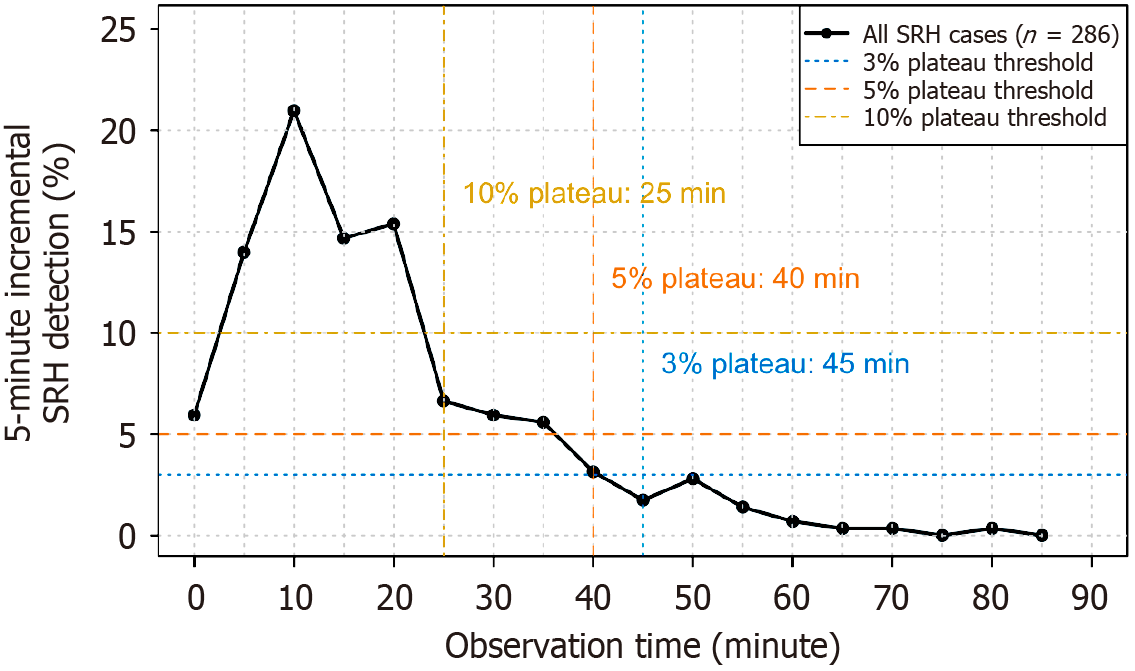
Because the 5% threshold was chosen arbitrarily, we performed sensitivity analyses using 3% and 10% thresholds to assess the robustness and clinical relevance of our findings. For each threshold, we identified the corresponding plateau time and calculated the cumulative SRH detection rate achieved by that time point. This allowed for a comparison of the diagnostic yield and procedural duration across different threshold values.
The patient demographics and clinical characteristics were summarized for the entire cohort. The observation times were described for both the overall population and the subset with SRH identification, and histograms were generated for visualization. Continuous variables are reported as means with standard deviations or medians with interquartile ranges (IQR), and categorical variables as frequencies and percentages.
The cumulative SRH detection rates were plotted over time for definite CDB cases. To assess the plateau in detection, SRH detection rates were calculated in 5-min increments, and both cumulative and interval-based detection trends were visualized to identify the 5% plateau time. Time points corresponding to 50%, 80%, 90%, and 95% of cumulative SRH detections were also determined. Sensitivity analyses with 3% and 10% thresholds were conducted using the same approach to evaluate the robustness and clinical relevance of the 5% threshold.
Additionally, we described the 30-day rebleeding rates according to SRH detection status. Among patients without SRH, the rates were compared between those with observation times above and below the 5% plateau.
Subgroup analyses were conducted based on CT-based localization by individual endoscopists. Categorical variables were analyzed using the Fisher’s exact test for comparison across endoscopists. Parametric continuous variables were evaluated using one-way analysis of variance, and non-parametric continuous variables were compared using the Kruskal-Wallis test. All statistical tests were two-sided, with a P-value < 0.05 considered statistically significant. All analyses were performed using R software (version 4.4.3).
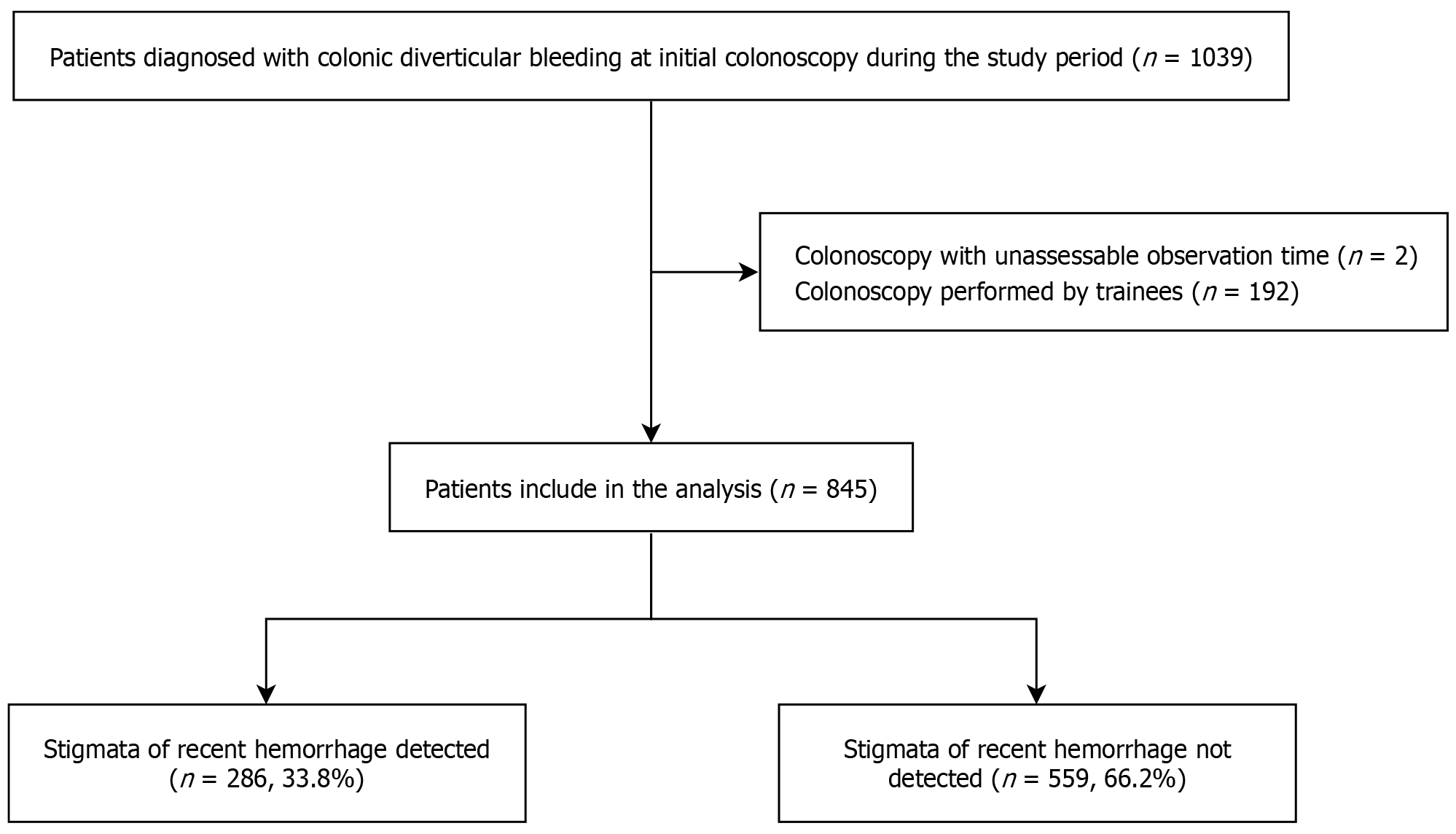
The patient selection process is illustrated in Figure 3. A total of 1039 patients were diagnosed with CDB at initial colonoscopy during the study period. After excluding 194 patients based on the exclusion criteria, 845 patients were included in the final analysis. SRH was detected in 286 patients, yielding a detection rate of 33.8%.
The baseline characteristics of the 845 patients are summarized in Table 1. The mean age of the patients was 77 ± 11 years, and 64.5% were male. The mean body mass index (BMI) of the cohort was 22.9 ± 3.8 kg/m2. Antiplatelet and anticoagulant agents were used in 34.0% and 16.3% of the patients, respectively. A shock index ≥ 1 on admission was observed in 8.0% of the patients. Colonoscopy was performed within 12 hours in 26.6% of patients, and 9.2% of patients underwent enema preparation. Based on contrast-enhanced CT findings, 72.9% had either no extravasation or did not undergo contrast-enhanced CT, 15.3% had right-sided extravasation, and 11.8% had left-sided extravasation. The median observation time for the overall cohort was 28 minutes (IQR, 18-44 minutes). Successful endoscopic hemostasis was achieved in 272 patients (32.0%). Among patients with SRH, the success rate was 95.1% (272/286), whereas endoscopic hemostasis was not attempted in patients without SRH.
| Variable | All cases (n = 845) | SRH detected (n = 286) | SRH not detected (n = 559) |
| Age, mean ± SD (years) | 77 ± 11 | 77 ± 11 | 77 ± 11 |
| Male | 545 (64.5) | 182 (63.6) | 363 (64.9) |
| BMI, mean ± SD (kg/m2) | 22.9 ± 3.8 | 22.8 ± 3.8 | 22.9 ± 3.8 |
| Antiplatelet agent use | 287 (34.0) | 99 (34.6) | 188 (33.6) |
| Anticoagulant agent use | 138 (16.3) | 47 (16.4) | 91 (16.3) |
| Shock index ≥ 1 on admission | 68 (8.0) | 25 (8.7) | 44 (7.9) |
| Syncope episode | 59 (7.0) | 19 (6.6) | 40 (7.2) |
| Colonoscopy within 12 hours | 225 (26.6) | 94 (32.9) | 131 (23.4) |
| Colonoscopy within 24 hours | 520 (61.5) | 198 (69.2) | 322 (57.6) |
| History of colectomy | 50 (5.9) | 12 (4.2) | 38 (6.8) |
| Enema preparation | 78 (9.2) | 34 (11.9) | 44 (7.9) |
| Laboratory data | |||
| White blood cell count, mean ± SD (109/L) | 7.5 ± 2.6 | 7.5 ± 2.6 | 7.5 ± 2.6 |
| Hemoglobin, mean ± SD (g/dL) | 11.2 ± 2.4 | 11.3 ± 2.5 | 11.2 ± 2.4 |
| Hematocrit, mean ± SD (%) | 34 ± 7.0 | 34 ± 7.0 | 34 ± 7.0 |
| CE-CT findings | |||
| No extravasation/no CT performed | 616 (72.9) | 172 (60.1) | 444 (79.4) |
| Right-sided extravasation | 129 (15.3) | 64 (22.4) | 65 (11.6) |
| Left-sided extravasation | 100 (11.8) | 50 (17.5) | 50 (8.9) |
| Observation time (minutes), median (IQR) | 28 (18-44) | 19 (12-28) | 33 (22-48) |
| Successful endoscopic hemostasis | 272 (32.0) | 272 (95.1) | - |
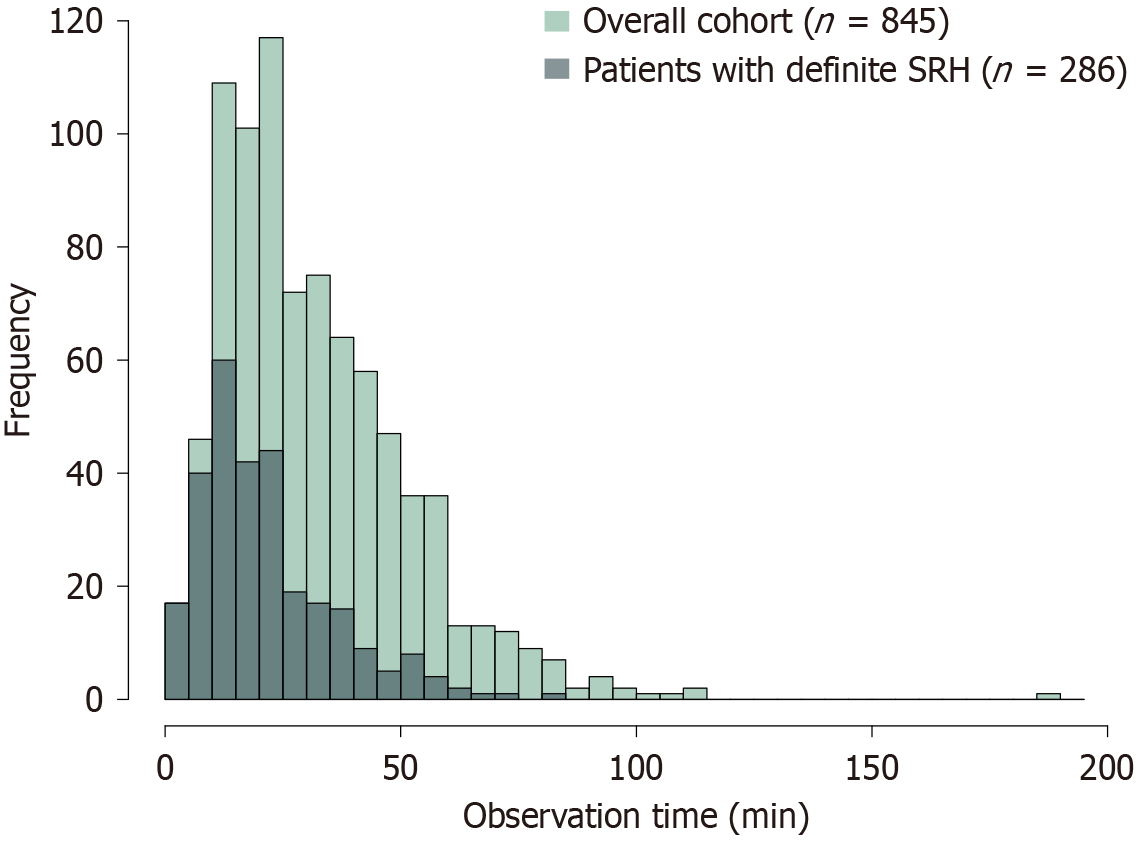
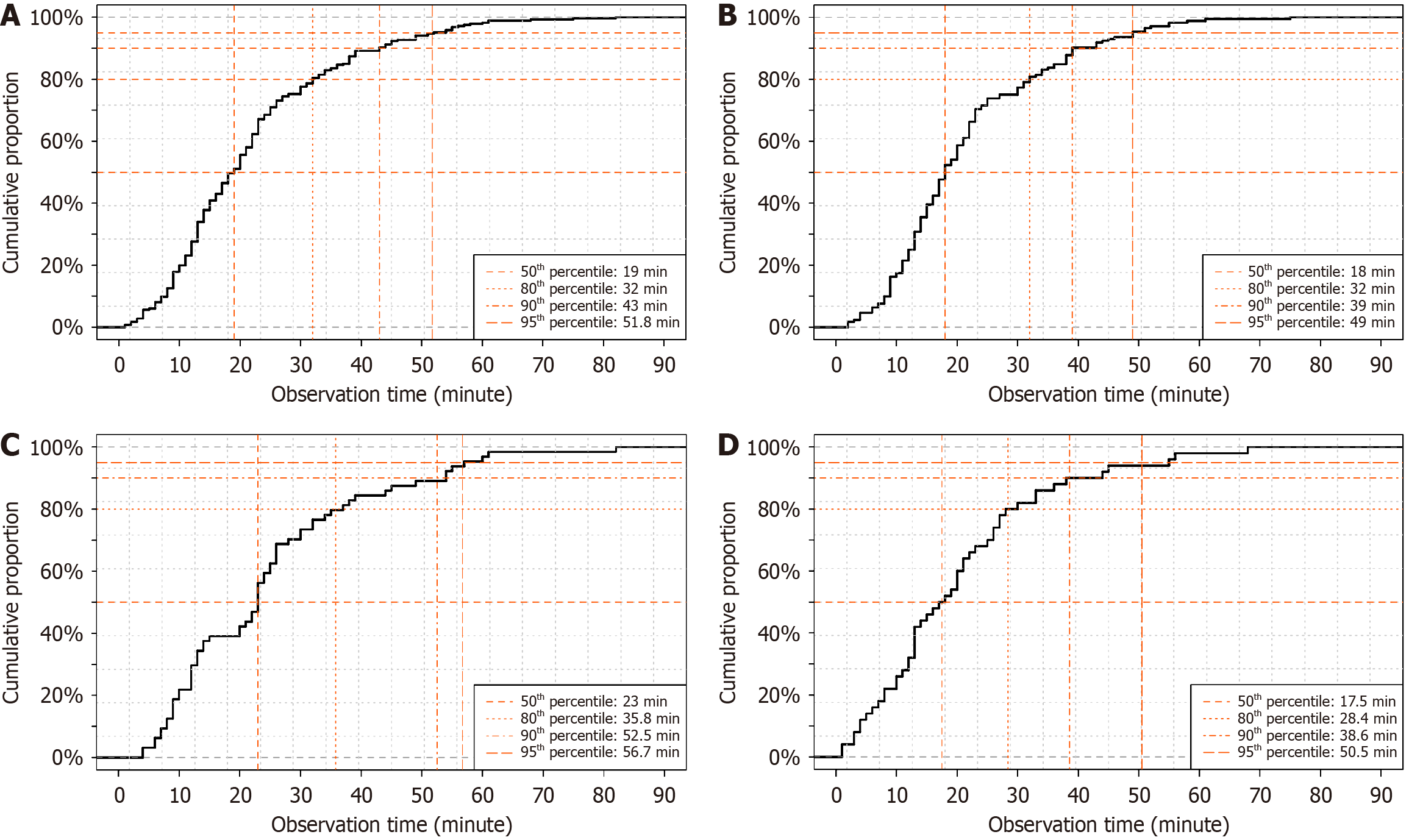
Among the 845 patients included in the final analysis, SRH was detected during the initial colonoscopy in 286 (33.8%). The median observation time until SRH detection was 19 minutes (IQR, 12-28 minutes). A histogram showing the distribution of observation time in the overall cohort and patients with definite SRH is shown in Figure 4. In patients with definite SRH, the frequency of SRH detection increased in the first approximately 15 minutes of observation and subsequently declined. The cumulative detection curve showed that 50%, 80%, 90%, and 95% of SRH cases were detected at 19, 32, 43, and 51.8 minutes, respectively (Figure 5A). The 5% plateau time was at 40 minutes (Figure 6A). These fin
We also evaluated the association between SRH and 30-day rebleeding. Patients with SRH had a significantly lower rebleeding rate than those without SRH (21.3% vs 30.2%, P = 0.007). Among the 559 patients without SRH detection, there was no significant difference in rebleeding rates between those observed for ≥ 5% plateau time and those observed for < 5% plateau time (33.0% vs 28.4%, P = 0.26; Table 2).
| Status | 30-day rebleed | P value |
| SRH detected | 61/286 (21.3) | 0.007a |
| SRH not detected | 169/559 (30.2) | |
| Observation time ≥ 5% plateau time | 74/224 (33.0) | 0.26 |
| Observation time < 5% plateau time | 95/335 (28.4) |
When stratified by contrast-enhanced CT findings, SRH detection rates were 27.9% (172/616), 49.6% (64/129), and 50.0% (50/100) in the no extravasation, right-sided extravasation, and left-sided extravasation groups, respectively.
In the no extravasation group, the cumulative detection times for 50%, 80%, 90%, and 95% of SRH cases were 18, 32, 39, and 49 minutes, respectively (Figure 5B), and the 5% plateau time was 40 minutes, with a cumulative detection rate of 90.1% at that time point (Figure 6B). In the right-sided extravasation group, the 50%, 80%, 90%, and 95% time points were 23, 35.8, 52.5, and 56.7 minutes, respectively (Figure 5C), with a 5% plateau time of 35 minutes and a corresponding cumulative detection rate of 79.7% (Figure 6C). In the left-sided extravasation group, 50%, 80%, 90%, and 95% of SRH cases were detected by 17.5, 28.4, 38.6, and 50.5 minutes, respectively (Figure 5D), and the 5% plateau time was 30 minutes with a cumulative detection rate of 82.0% (Figure 6D).
A sensitivity analysis using alternative thresholds yielded similar results. The 10%, 5%, and 3% plateau times were 25, 40, and 45 minutes, respectively (Figure 7), corresponding to cumulative SRH detection rates of 71.0%, 89.2%, and 92.3%, respectively.
Among the four endoscopists who performed ≥ 10% of procedures, SRH detection rates ranged from 23.0% to 51.8%, while median observation times ranged from 20 minutes (IQR, 14-33 minutes) to 39 minutes (IQR, 24-51 minutes) (Table 3). Endoscopist C had the longest observation time but the lowest SRH detection rate (26.1%), whereas Endoscopist B achieved the highest detection rate (51.8%) with a relatively long observation time [27 minutes (IQR, 18-45 minutes)]. Patient characteristics, including age, sex, BMI, colonoscopy timing, and enema preparation, were comparable among the four groups. However, the proportion of patients receiving antiplatelet agents was significantly higher in groups A and B (P = 0.01), and contrast-enhanced CT findings differed modestly but significantly between the groups (P = 0.04). The 5% plateau time matched that of the main analysis (40 minutes) for endoscopists A and B, who also had the highest SRH detection rates. Endoscopists C and D showed plateau times of 45 and 35 minutes, respectively, which deviated slightly from the 40-min benchmark but remained generally comparable.
| Endoscopists | A (n = 113) | B (n = 141) | C (n = 88) | D (n = 161) | P value |
| SRH detected | 49 (43.4) | 73 (51.8) | 23 (26.1) | 37 (23.0) | < 0.001a |
| Observation time (minutes), median (IQR) | 20 (14-33) | 27 (18-45) | 39 (24-51) | 23 (16-36) | < 0.001b |
| 5% plateau time (minutes) | 40 | 40 | 45 | 35 | - |
| Age, mean ± SD (years) | 79 ± 10 | 77 ± 11 | 78 ± 10 | 76 ± 13 | 0.42 |
| Male | 73 (64.6) | 89 (63.1) | 45 (51.1) | 107 (66.5) | 0.10 |
| BMI, mean ± SD (kg/m2) | 22.2 ± 3.5 | 22.7 ± 3.5 | 23.1 ± 4.2 | 22.7 ± 3.6 | 0.28 |
| Antiplatelet agent use | 42 (37.2) | 53 (37.6) | 20 (22.7) | 38 (23.6) | 0.01c |
| Anticoagulant agent use | 22 (19.5) | 17 (12.1) | 17 (19.3) | 30 (18.6) | 0.31 |
| Shock index ≥ 1 on admission | 11 (9.7) | 7 (5.0) | 6 (6.8) | 17 (10.6) | 0.29 |
| Colonoscopy within 12 hours | 33 (29.2) | 31 (22.0) | 16 (18.2) | 41 (25.5) | 0.28 |
| History of colectomy | 6 (5.3) | 6 (4.3) | 5 (5.7) | 9 (5.6) | 0.95 |
| Enema preparation | 14 (12.4) | 11 (7.8) | 5 (5.7) | 14 (8.7) | 0.38 |
| CE-CT findings | 0.04d | ||||
| No extravasation/no CT performed | 76 (67.3) | 100 (70.9) | 68 (76.1) | 123 (76.4) | |
| Right-sided extravasation | 26 (23.0) | 17 (12.1) | 9 (10.2) | 25 (15.5) | |
| Left-sided extravasation | 11 (9.7) | 24 (17.0) | 11 (12.5) | 13 (8.1) |
The incremental SRH detection rate did not increase linearly with time; rather, it declined after approximately 15 minutes and gradually plateaued, indicating diminishing returns with prolonged observation. Based on a large single-center dataset of 845 patients, the overall 5% plateau time was 40 minutes. When stratified by contrast-enhanced CT findings, the plateau time was 40, 35, and 30 minutes in the no extravasation, right-sided extravasation, and left-sided extra
The only prior study to quantitatively assess the relationship between observation time and SRH detection in CDB was conducted by Watanabe et al[19], who used receiver operating characteristic curve analysis to identify a 19-minute cutoff and reported that prolonged observation was an independent predictor of SRH detection (odds ratio 10.3, 95% confidence interval: 3.84-27.9, P < 0.001). Although the Watanabe et al’s study[19] highlights observation time as a modifiable factor, it has several limitations. Receiver operating characteristic curve analysis treats SRH detection as a binary outcome and optimizes sensitivity and specificity at a single threshold, which may oversimplify the gradual, time-dependent nature of detection. Clinically, a practical minimum observation time is needed that ensures sufficient diagnostic yield before termination instead of a statistically optimal time point. The 5% plateau time addresses this by defining the point of diminishing returns in the cumulative SRH detection. In addition, the Watanabe et al’s study[19] measured the time from cecal intubation, which may not reflect real-world workflows, in which colonoscopy is often targeted based on CT findings. Moreover, the therapeutic time was included in the observation period, potentially overestimating the actual inspection time. By contrast, our study measured the observation time from scope insertion and excluded the therapeutic duration, providing a more accurate and clinically relevant benchmark. Thus, the 5% plateau time offers a standardized and pragmatic threshold for the minimum inspection time in CDB management.
The variation in plateau times across contrast-enhanced CT findings is likely attributable to differences in the anatomical route and the time required to reach the bleeding site during colonoscope insertion. In left-sided colonic lesions, early access to the antegrade insertion from the rectum allows shorter observation times. This aligns with the 30-minute plateau time observed in the left-sided extravasation group. By contrast, right-sided lesions, which are anatomically distant, may require longer insertion and detection times. Additionally, SRH detection patterns in the right-sided group exhibited a bimodal distribution, possibly reflecting two distinct scenarios: Cases with ongoing active bleeding and those in which bleeding temporarily ceased, necessitating meticulous inspection. In the no extravasation group, comprehensive observation of the entire colon was often required, which resulted in the longest plateau time.
This study is the first to propose a quantitative indicator that addresses both the quality and duration of colonoscopic observations in CDB, providing a clinically interpretable threshold for adequate SRH detection. The derived 5% plateau time roughly corresponded to the time point at which 80%-90% of SRH cases were detected, supporting its validity as a clinically meaningful benchmark. Rather than aiming to maximize detection indefinitely, this indicator offers a practical guide for determining when additional observation time is unlikely to provide substantial additional benefits, thus helping clinicians decide when to conclude an examination. This is particularly important in CDB, a condition that primarily affects older adults who are more vulnerable to complications associated with prolonged sedation-assisted procedures, including aspiration pneumonia[15]. Although we did not conduct a formal cost-effectiveness analysis, the 5% plateau time may offer utility in resource-limited settings by supporting a more standardized and efficient use of endoscopic resources. Future prospective studies incorporating health economic evaluations are needed to clarify the cost-benefit profile. Despite advances in hemostatic techniques such as the shift from indirect to direct clipping and the adoption of endoscopic band ligation, which have reduced rebleeding rates from approximately 40% to below 20%[20-23], SRH detection during initial colonoscopy remains suboptimal at approximately 30%[16]. To further improve outcomes, future development should focus on enhancing SRH detectability, including the integration of artificial intelligence-assisted diagnostic systems and advanced imaging technologies.
First, this was a retrospective, single-center study, and institutional factors may have influenced the findings. However, rather than imposing a strict observation protocol, this study allowed procedural discretion to reflect the variation observed in actual practice and identify the minimum observation time applicable to diverse operator behaviors. Notably, we observed considerable interendoscopist differences in both SRH detection rates and observation times, reflecting the inherent heterogeneity of clinical practice. Despite this variation, the 5% plateau time remained relatively consistent (35-45 minutes) among endoscopists, suggesting that the proposed threshold may serve as a practical lower bound for ade
We introduced a novel concept, the “5% plateau time”, defined as the earliest time at which the incremental SRH detection rate falls below 5%. The overall plateau time was 40 minutes, with subgroup-specific values of 40, 35, and 30 minutes for the no, right-sided, and left-sided extravasation groups, respectively. The 5% plateau time provides a scientifically grounded and practical minimum observation time for colonoscopy in patients with CDB, offering a benchmark to standardize practice, reduce the risk of underdetection, and support consistent and efficient management.
We thank the team members of the Gastroenterology Medicine Center and Emergency Department at Shonan Kamakura General Hospital for their dedication to daily clinical practice.
| 1. | Ichita C, Goto T, Sasaki A, Shimizu S. National trends in hospitalizations for gastrointestinal bleeding in Japan. J Clin Biochem Nutr. 2024;75:60-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Ichita C, Kishino T, Aoki T, Machida T, Murakami T, Sato Y, Nagata N. Updated evidence on epidemiology, diagnosis, and treatment for colonic diverticular bleeding. DEN Open. 2026;6:e70122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, Lund JL, Moon AM, Pate V, Barnes EL, Schlusser CL, Baron TH, Shaheen NJ, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2022;162:621-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 584] [Article Influence: 146.0] [Reference Citation Analysis (1)] |
| 4. | Tanaka Y, Motomura Y, Akahoshi K, Iwao R, Komori K, Nakama N, Osoegawa T, Itaba S, Kubokawa M, Hisano T, Ihara E, Nakamura K, Takayanagi R. Predictive factors for colonic diverticular rebleeding: a retrospective analysis of the clinical and colonoscopic features of 111 patients. Gut Liver. 2012;6:334-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Niikura R, Nagata N, Yamada A, Akiyama J, Shimbo T, Uemura N. Recurrence of colonic diverticular bleeding and associated risk factors. Colorectal Dis. 2012;14:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Gobinet-Suguro M, Nagata N, Kobayashi K, Yamauchi A, Yamada A, Omori J, Ikeya T, Aoyama T, Tominaga N, Sato Y, Kishino T, Ishii N, Sawada T, Murata M, Takao A, Mizukami K, Kinjo K, Fujimori S, Uotani T, Fujita M, Sato H, Suzuki S, Narasaka T, Hayasaka J, Funabiki T, Kinjo Y, Mizuki A, Kiyotoki S, Mikami T, Gushima R, Fujii H, Fuyuno Y, Gunji N, Toya Y, Narimatsu K, Manabe N, Nagaike K, Kinjo T, Sumida Y, Funakoshi S, Kawagishi K, Matsuhashi T, Komaki Y, Miki K, Watanabe K, Uemura N, Itawa E, Sugimoto M, Fukuzawa M, Kawai T, Kaise M, Itoi T. Treatment strategies for reducing early and late recurrence of colonic diverticular bleeding based on stigmata of recent hemorrhage: a large multicenter study. Gastrointest Endosc. 2022;95:1210-1222.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Doi H, Sasajima K, Takahashi M, Sato T, Ootsu I, Chinzei R. Effectiveness of Conservative Treatment without Early Colonoscopy in Patients with Colonic Diverticular Hemorrhage. Can J Gastroenterol Hepatol. 2020;2020:3283940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Ichita C, Nakajima M, Ohbe H, Kaszynski RH, Sasaki A, Miyamoto Y, Kondo Y, Sasabuchi Y, Fushimi K, Matsui H, Yasunaga H. Effectiveness of early colonoscopy in patients with colonic diverticular hemorrhage: Nationwide inpatient analysis in Japan. Dig Endosc. 2023;35:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | Obeidat M, Teutsch B, Rancz A, Tari E, Márta K, Veres DS, Hosszúfalusi N, Mihály E, Hegyi P, Erőss B. One in four patients with gastrointestinal bleeding develops shock or hemodynamic instability: A systematic review and meta-analysis. World J Gastroenterol. 2023;29:4466-4480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (6)] |
| 10. | Aoki T, Sadashima E, Kobayashi K, Yamauchi A, Yamada A, Omori J, Ikeya T, Aoyama T, Tominaga N, Sato Y, Kishino T, Ishii N, Sawada T, Murata M, Takao A, Mizukami K, Kinjo K, Fujimori S, Uotani T, Fujita M, Sato H, Hayakawa Y, Fujishiro M, Kaise M, Nagata N; CODE BLUE-J Study collaborators. High risk stigmata and treatment strategy for acute lower gastrointestinal bleeding: a nationwide study in Japan. Endoscopy. 2024;56:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Green BT, Rockey DC, Portwood G, Tarnasky PR, Guarisco S, Branch MS, Leung J, Jowell P. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100:2395-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Nagata N, Niikura R, Sakurai T, Shimbo T, Aoki T, Moriyasu S, Sekine K, Okubo H, Imbe K, Watanabe K, Yokoi C, Yanase M, Akiyama J, Uemura N. Safety and Effectiveness of Early Colonoscopy in Management of Acute Lower Gastrointestinal Bleeding on the Basis of Propensity Score Matching Analysis. Clin Gastroenterol Hepatol. 2016;14:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Shiratori Y, Ishii N, Aoki T, Kobayashi K, Yamauchi A, Yamada A, Omori J, Aoyama T, Tominaga N, Sato Y, Kishino T, Sawada T, Murata M, Takao A, Mizukami K, Kinjo K, Fujimori S, Uotani T, Fujita M, Sato H, Suzuki S, Narasaka T, Hayasaka J, Funabiki T, Kinjo Y, Mizuki A, Kiyotoki S, Mikami T, Gushima R, Fujii H, Fuyuno Y, Gunji N, Toya Y, Narimatsu K, Manabe N, Nagaike K, Kinjo T, Sumida Y, Funakoshi S, Kobayashi K, Matsuhashi T, Komaki Y, Miki K, Watanabe K, Yamamoto K, Yoshimoto T, Takasu A, Ikeya T, Omata F, Fukuda K, Kaise M, Nagata N. Timing of colonoscopy in acute lower GI bleeding: a multicenter retrospective cohort study. Gastrointest Endosc. 2023;97:89-99.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Niikura R, Nagata N, Aoki T, Shimbo T, Tanaka S, Sekine K, Kishida Y, Watanabe K, Sakurai T, Yokoi C, Yanase M, Akiyama J, Mizokami M, Uemura N. Predictors for identification of stigmata of recent hemorrhage on colonic diverticula in lower gastrointestinal bleeding. J Clin Gastroenterol. 2015;49:e24-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Bielawska B, Hookey LC, Sutradhar R, Whitehead M, Xu J, Paszat LF, Rabeneck L, Tinmouth J. Anesthesia Assistance in Outpatient Colonoscopy and Risk of Aspiration Pneumonia, Bowel Perforation, and Splenic Injury. Gastroenterology. 2018;154:77-85.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Nagata N, Kobayashi K, Yamauchi A, Yamada A, Omori J, Ikeya T, Aoyama T, Tominaga N, Sato Y, Kishino T, Ishii N, Sawada T, Murata M, Takao A, Mizukami K, Kinjo K, Fujimori S, Uotani T, Fujita M, Sato H, Suzuki S, Narasaka T, Hayasaka J, Funabiki T, Kinjo Y, Mizuki A, Kiyotoki S, Mikami T, Gushima R, Fujii H, Fuyuno Y, Gunji N, Toya Y, Narimatsu K, Manabe N, Nagaike K, Kinjo T, Sumida Y, Funakoshi S, Kawagishi K, Matsuhashi T, Komaki Y, Miki K, Watanabe K, Fukuzawa M, Itoi T, Uemura N, Kawai T, Kaise M. Identifying Bleeding Etiologies by Endoscopy Affected Outcomes in 10,342 Cases With Hematochezia: CODE BLUE-J Study. Am J Gastroenterol. 2021;116:2222-2234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Jensen DM, Ohning GV, Kovacs TO, Jutabha R, Ghassemi K, Dulai GS, Machicado GA. Natural history of definitive diverticular hemorrhage based on stigmata of recent hemorrhage and colonoscopic Doppler blood flow monitoring for risk stratification and definitive hemostasis. Gastrointest Endosc. 2016;83:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Nagata N, Ishii N, Manabe N, Tomizawa K, Urita Y, Funabiki T, Fujimori S, Kaise M. Guidelines for Colonic Diverticular Bleeding and Colonic Diverticulitis: Japan Gastroenterological Association. Digestion. 2019;99 Suppl 1:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 19. | Watanabe S, Sato A, Kobayashi K, Miyakawa A, Uchida H, Machida T, Kobashi K, Yauchi T. Colonoscopic observation time as a predictor of stigmata of recent hemorrhage identification in colonic diverticular hemorrhage. Scand J Gastroenterol. 2023;58:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Kishino T, Nagata N, Kobayashi K, Yamauchi A, Yamada A, Omori J, Ikeya T, Aoyama T, Tominaga N, Sato Y, Ishii N, Sawada T, Murata M, Takao A, Mizukami K, Kinjo K, Fujimori S, Uotani T, Fujita M, Sato H, Suzuki S, Narasaka T, Hayasaka J, Funabiki T, Kinjo Y, Mizuki A, Kiyotoki S, Mikami T, Gushima R, Fujii H, Fuyuno Y, Gunji N, Toya Y, Narimatsu K, Manabe N, Nagaike K, Kinjo T, Sumida Y, Funakoshi S, Kawagishi K, Matsuhashi T, Komaki Y, Miki K, Watanabe K, Kaise M. Endoscopic direct clipping versus indirect clipping for colonic diverticular bleeding: A large multicenter cohort study. United European Gastroenterol J. 2022;10:93-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Nagata N, Ishii N, Kaise M, Shimbo T, Sakurai T, Akiyama J, Uemura N. Long-term recurrent bleeding risk after endoscopic therapy for definitive colonic diverticular bleeding: band ligation versus clipping. Gastrointest Endosc. 2018;88:841-853.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Kobayashi K, Nagata N, Furumoto Y, Yamauchi A, Yamada A, Omori J, Ikeya T, Aoyama T, Tominaga N, Sato Y, Kishino T, Ishii N, Sawada T, Murata M, Takao A, Mizukami K, Kinjo K, Fujimori S, Uotani T, Fujita M, Sato H, Suzuki S, Narasaka T, Hayasaka J, Kaise M; CODE BLUE-J study collaborators. Effectiveness and adverse events of endoscopic clipping versus band ligation for colonic diverticular hemorrhage: a large-scale multicenter cohort study. Endoscopy. 2022;54:735-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Kishino T, Kitamura Y, Okuda T, Okamoto N, Sawa T, Yamakawa M, Kanemasa K. Useful treatment selection strategy for endoscopic hemostasis in colonic diverticular bleeding according to endoscopic findings (with video). Endosc Int Open. 2025;13:a24711016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/