Published online Oct 28, 2025. doi: 10.3748/wjg.v31.i40.111375
Revised: July 30, 2025
Accepted: September 16, 2025
Published online: October 28, 2025
Processing time: 121 Days and 14.4 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) and inflammatory bowel disease (IBD) are chronic conditions with complex aetiologies, in which environmental factors and interactions between the gut and liver play a key role. Both conditions are characterised by disturbances in the gut microbiota, which can affect local and systemic inflammatory responses. In particular, inc
Core Tip: Metabolic dysfunction-associated steatotic liver disease (MASLD) in inflammatory bowel disease (IBD) may share a common denominator with the gut microbiota. Through various factors, such as environmental factors, intestinal dysbiosis can occur, disrupting the homeostasis of the intestinal barrier and the entry of, for example, bacterial metabolites that exacerbate inflammation in the body. In the review, we analysed the various pathogenetic mechanisms of MASLD in IBD with an emphasis on the role of the gut microbiota.
- Citation: Sokal-Dembowska A, Ergan K, Jarmakiewicz-Czaja S. Role of gut microbiota in the development of metabolic dysfunction-associated steatotic liver disease in inflammatory bowel disease. World J Gastroenterol 2025; 31(40): 111375
- URL: https://www.wjgnet.com/1007-9327/full/v31/i40/111375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i40.111375
The nomenclature of liver disease has evolved since the 1980s, when cases of non-alcoholic steatohepatitis were first described, excluding alcohol as a causative factor. In 2007, the term ‘non-alcoholic fatty liver disease’ (NAFLD) was introduced, followed by ‘metabolic dysfunction-associated fatty liver disease’ in 2020. NAFLD was permanently withdrawn in 2023 after a multicentre Delphi consensus statement was published. Instead, a new term was adopted, ‘metabolic dysfunction-associated steatotic liver disease’ (MASLD), highlighting its association with metabolic disorders[1].
The defining feature of MASLD is the accumulation of excessive triglycerides in the liver (hepatocytes ≥ 5%), which can lead to inflammation and metabolic dysfunction-associated steatohepatitis (MASH), as well as progressive fibrosis[2,3]. The excess and abnormal metabolism of triacylglycerols and diacylglycerols leads to hepatocyte dysfunction[4]. In addition, patients with MASLD have elevated plasma total cholesterol and low-density lipoprotein cholesterol levels and decreased high-density lipoprotein cholesterol levels. These changes have been linked to an increase in the expression of the sterol regulatory element-binding protein 2 transcription factor, excessive activation of which can lead to cholesterol overload in cells and can exacerbate cellular stress. Lipotoxic ceramides also play a significant role in disease progression. Their elevated levels have been linked to both metabolic syndrome and MASLD. The action of ceramides through activation of protein kinase C ζ and protein phosphatase 2A leads to impaired translocation and dephosphorylation of protein kinase B, which exacerbates hepatic steatosis and insulin resistance. Furthermore, insulin resistance in MASLD contributes to increased very-low-density lipoprotein secretion and lipid dysregulation[5]. The inflammatory response, closely associated with hepatocyte distension and fibrosis, increases the risk of cirrhosis and hepatocellular carcinoma[2,3]. MASLD is estimated to occur in approximately 32.4% of the general population, making it the leading cause of liver disease worldwide[3].
Inflammatory bowel disease (IBD) is characterised by chronic intestinal inflammation in forms such as Crohn’s disease and ulcerative colitis (UC), with systemic effects on liver health. In these diseases, disruption of the intestinal epithelial barrier, particularly loss of function of tight junction proteins and Paneth cell dysfunction, allows pathogenic/inflammatory microbes to enter the portal circulation[6]. This process results in the delivery of lipopolysaccharide (LPS) and secondary bile acid metabolites to the liver. Activation of Toll-like receptors (TLRs) (TLR4, TLR9) in the liver triggers an inflammatory response in both hepatocytes and Kupffer cells, increasing the release of tumour necrosis factor-alpha (TNF-α), interleukin (IL)-6 and IL-1β[7]. These cytokines activate the cellular oxidative stress, endoplasmic reticulum (ER) stress, and hepatic apoptosis pathways, predisposing to steatosis, inflammation, and fibrosis[8].
Current scientific evidence suggests a frequent co-occurrence of MASLD with IBD. Both MASLD and MASH are observed in 8%-59% of patients with IBD. Interestingly, advanced liver fibrosis is common among these patients, and the presence of metabolic syndrome is considered a significant risk factor[9].
IBD is a group of conditions characterised by chronic and recurrent inflammation of the gastrointestinal tract, including UC and Crohn’s disease. The first descriptions of IBD date back to North America and Europe in the late 18th century. At that time, an association was observed between the incidence of these conditions and the economic development of the regions[10,11]. IBD most commonly affects young adults under the age of 20 years. However, it is concerning that it is also increasingly being diagnosed in children, with 5% of cases occurring under the age of 5, and 20% under the age of 10[11].
The molecular mechanisms leading to the development of IBD are not yet fully understood[10]. The disease is assumed to develop as a result of an aberrant or disturbed immune response directed against the gut microbiota in genetically predisposed individuals[11]. IBD patients show changes in the composition of the gut microbiota, including a reduction in the number of bacteria responsible for the production of short-chain fatty acids (SCFAs), such as acetate, propionate and butyrate, and indole derivatives[10]. The interaction between host genetic variants and the gut microbiome may also contribute to the development of IBD. The most common genes studied in the context of IBD are NOD2, CARD9, immunoglobulin-like receptor in myeloid cells, IL-23 receptor and ATG16 L1[12].
In recent years, an increasing number of studies have demonstrated that lifestyle factors, including diet, physical activity, and the use of medications and stimulants have a significant impact on the composition and function of the gut microbiota. These changes may play a key role in the pathogenesis of both IBD and MASLD. The gut microbiota influences lipid metabolism, immune function, and intestinal barrier integrity, and its disruption is considered a potential common pathway that links the two diseases.
Understanding the mechanisms that connect MASLD and IBD is essential to improving our understanding of their coexistence and to developing more effective diagnostic and therapeutic strategies. Therefore, this review places particular emphasis on lifestyle-related factors and their influence on the gut microbiota in individuals affected by MASLD and IBD.
Zhong et al[9] identified 116 shared differentially expressed genes (SDEGs) in MASH and IBD. Analysis of Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathways revealed the significant involvement of SDEGs in defence responses, apoptosis, cell death, and cytokine and chemokine activities, as well as in the phosphoinositide 3-kinase-protein kinase B, TLR, peroxisome proliferator-activated receptor (PPAR), and Ras-proximate-1 signalling pathways. This confirms the common pathogenesis of these conditions and their interplay. Table 1 provides a brief overview of MASLD and IBD[13-16].
| Features | MASLD | IBD |
| Main location[9,14] | Liver | Gastrointestinal tract: UC inflammation of the colonic mucosa (rectum, sigmoid colon, area beyond the sigmoid colon or entire colon up to the caecum); CD inflammation of the terminal ileum and colon |
| Type of disease[9] | Metabolic, chronic | Autoimmune-inflammatory, chronic |
| Risk and progression factors[2,3,9,10,15] | Genetics, epigenetic changes, gut microbiota disorders, cardiometabolic factors, endocrinopathies, obesity, insulin resistance, metabolic syndrome, environmental factors including industrialisation | Genetics, epigenetic changes, gut microbiota disorders, immune response, early life antibiotic therapy, environmental factors, including industrialisation |
| Complications[9,13,14,16] | Liver cirrhosis, hepatocellular carcinoma, circulatory system diseases, type II diabetes, chronic kidney disease | Extraintestinal lesions, including hepatic: Fatty liver, hepatic amyloidosis, sclerosing cholangitis, autoimmune hepatitis, and liver abscess; Colon cancer |
| Link between IBD and MASLD[9] | MASLD and MASH are often co-occurring in IBD patients (twice as high a risk) | |
Risk factors for the development of MASLD in patients with IBD are considered to include diet, changes in the composition of the gut microbiota, disease activity and duration, drug treatment used, and previous gastrointestinal surgery. It is possible that a change in diet after surgery, as well as the advanced course of the disease and accompanying increased inflammation, may influence the development of MASLD. Furthermore, surgical resection of the gastrointestinal tract may lead to metabolic dysfunction that promotes lipid accumulation in the liver[17].
In an observational study by Magrì et al[18], patients with IBD and MASLD showed a higher intake of calories and fat compared to patients without MASLD, regardless of disease activity. In contrast, Glassner et al[19] found that older patients with a longer duration of IBD were more likely to develop MASLD, especially if they had risk factors for metabolic syndrome. Interestingly, patients with concomitant MASLD and IBD had fewer classic metabolic risk factors (such as elevated body weight, hypertension, diabetes, dyslipidemia), which may suggest the existence of other mechanisms leading to inflammation, e.g., related to disease activity, that predispose to the development of MASLD. Similarly, in the study by Abenavoli et al[20], patients with IBD-MASLD were significantly older and had a higher body mass index (BMI), waist circumference, and triglyceride levels. They were also more likely to have cardiometabolic conditions. Patients with IBD-MASLD also showed higher IBD activity and a need for more frequent surgical interventions. Other factors, such as a chronic low-grade condition, alterations in the intestinal microbiota, or genetic predisposition, may play an important role in the pathogenesis of MASLD in patients with IBD[21].
In a study by Rotaru et al[21], patients with IBD-MASLD had more severe hepatic steatosis and elevated levels of systemic inflammation. MASLD was also present in lean subjects, with a prevalence of 34.1% in this group, suggesting the existence of a distinct disease-promoting phenotype in IBD patients. Nevertheless, those with MASLD were characterised by a higher BMI and the presence of visceral obesity. Excess visceral fat has been linked to increased secretion of pro-inflammatory cytokines, development of inflammation, and metabolic disorders. It has been shown that visceral fat reduction may be helpful in reducing inflammatory markers and cardiometabolic disorders[22,23]. This seems important because lipid metabolism disorders occur in patients with IBD-MASLD, presenting as increased triglyceride and reduced high-density lipoprotein levels. Importantly, the results of the study by Rotaru et al[21] did not show differences in typical liver markers such as C-reactive protein (CRP), alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase and alkaline phosphatase, which may limit their diagnostic value in lean individuals with MASLD. The use of the Clínica Universidad de Navarra-Body adiposity estimator index in detecting steatosis compared to traditional indices such as fatty liver index and hepatic steatosis index is also considered to be of higher diagnostic value in lean individuals[24]. According to Zhang et al[22], the presence of lean/non-lean MASLD is associated with an increased risk of death in patients with IBD. However, the risk is higher the greater the number of cardiometabolic risk factors and is markedly higher in lean MASLD patients.
The inflammation accompanying active IBD likely plays a key role in the development of MASLD, regardless of the presence of classical metabolic factors. This suggests that MASLD in patients with IBD requires a distinct preventive and diagnostic approach.
Elevated oxidative stress markers are observed in patients with IBD and MASLD[25,26]. Mierzwa et al[27] observed increased glutathione-S-transferase activity, higher concentrations of reduced glutathione, malondialdehyde (MDA) and nitrite/nitrate in patients with IBD. At the same time, they observed decreased superoxide dismutase-1 and glutathione peroxidase (GPX) activity. In the active form of IBD, oxidative stress is particularly severe, but even in the remission phase, reduced antioxidant levels are observed. Tratenšek et al[28] found elevated levels of antioxidant enzymes and proteins such as catalase, albumin, transferrin, and paraoxonase in patients with IBD during exacerbation. In patients with UC, they also observed an increase in MDA and total antioxidant capacity, and in Crohn’s disease, increased GPX activity and thiol group levels of sulfhydryl group (R-SH).
There is believed to be a three-way relationship between reactive oxygen species (ROS), the microbiome, and the development of MASLD. In the course of MASLD, dysfunction of the adenosine 5’-monophosphate-activated protein kinase (AMPK) and nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant pathways is observed. Mitochondrial dysfunction and ER stress exacerbate cellular stress. Furthermore, the penetration of LPS and harmful gut-derived metabolites such as N-trimethylamine oxide into the liver activates inflammatory cascades. Impaired defence mechanisms, including the AMPK, Nrf2, and PPAR pathways, are unable to effectively counteract damage, leading to liver disease progression and potentially resulting in multi-organ damage[29].
T17 cells play a key role in the inflammatory response in the development and progression of MASLD and IBD. In the normal intestinal environment, Th17 and regulatory T cells (Treg) remain in dynamic balance, and their disruption may be one of the mechanisms driving the pathogenesis of these diseases[30,31]. There are reports that decreased secondary bile acids in IBD patients may lead to an imbalance between Th17 and Treg lymphocytes as a result of impaired signalling via farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 5 (TGR5). A similar mechanism is observed in MASLD, where intestinal dysbiosis leads to a decrease in secondary bile acids and reduced activation of FXR and TGR5, which promotes increased lipogenesis and inflammatory responses[32]. The development of MASH is also thought to be associated with an excessive Th17 response[33].
Metabolic disturbances resulting from a high-fat diet and the development of inflammation in the gut are associated with increased production of pro-inflammatory cytokines such as IL-6, IL-12, TNF-α and IL-1β. This results in low-grade chronic inflammation, a weak intestinal barrier, and an impaired immune response[34]. Polymorphisms of genes encoding proteins involved in maintaining the integrity of the intestinal barrier, such as MYO9B, signalling pathway proteins, e.g., MAGI2, cell adhesion molecules (e.g., E-cadherin), and mucosal layer-forming proteins (e.g., mucin 3A and mucin 19) represent an important component of the complex pathophysiological landscape shared by IBD and MASLD. These changes may affect the expression of tight junction proteins, increasing susceptibility to the development of IBD, chronic inflammation, and MASLD[32]. As is well known, increased intestinal permeability, in addition to an inadequately balanced and highly processed diet, can also be linked to the presence of medical conditions such as obesity and diabetes. This leads to the penetration of bacterial molecules [pathogen-associated molecular patterns (PAMPs), e.g., LPS] into the portal circulation[35]. LPS activates the TLR4 receptor present on Kupffer cells, which in turn causes the activation of the nuclear transcription factor nuclear factor kappa-B (NF-κB) and the inflammasome[32]. Chronic accumulation of lipids in the liver can lead to lipotoxicity, which promotes inflammation and damage to hepatocytes[33]. At the same time, systemic insulin resistance develops[29].
IL-17 secreted by Th-17 cells exacerbates inflammation by inducing ROS and increasing neutrophil infiltration[36]. Increased secretion of IL-17 is observed in the course of MASLD, which promotes increased expression of pro-inflammatory cytokines and chemokines in the hepatic milieu. This is accompanied by increased recruitment of myeloid cells and T cells, increasing inflammation and promoting disease progression. IL-17 may also act directly on the activation of hepatic stellate cells, leading to increased fibrosis[37].
Thielemann et al[33] showed that people with the IL-17A rs2275913 A/A genotype have higher levels of IL-17A cytokines and are more likely to develop liver fibrosis and progress from MASLD to MASH. In addition, it has been observed that progression of MASLD to MASH is characterised by an increase in IL-17A-producing cells in intrahepatic cluster of differentiation 4 + T cells and a higher Th17/Treg ratio in the peripheral blood[38]. Similar mechanisms are observed in IBD. Prananda et al[39] reported elevated IL-17 Levels in IBD patients, particularly the UC group, where IL-17 Levels correlated with disease activity. In addition to IL-17, Lucaciu et al[40] also showed a correlation between IL-23 Levels and the severity of IBD. In contrast, in the study by Menesy et al[41], IL-17 Levels were not related to disease activity. Interestingly, the levels were higher in patients who smoked cigarettes, suggesting concomitant environmental factors on the severity of the inflammatory response.
Dysregulation of the immune and microbial balance within the gut-liver axis together with associated inflammation may represent a common pathogenetic mechanism in the development of IBD and MASLD.
The intestinal microbiota is the totality of microorganisms, consisting mainly of bacteria, but also viruses, fungi, and protozoa. In the large intestine of a healthy adult human, the predominant types are Bacteroidetes and Firmicutes. However, Marchesi et al[42] indicate that at least 10 different types of microorganisms can exert significant effects on the host organism. The correct composition of the intestinal microbiota determines the proper functioning of the intestinal barrier and the maintenance of homeostasis. The commensal intestinal microbiota, as one of the factors, determines the appropriate selective permeability of nutrients necessary for the proper functioning of the body. It also participates in protection against pathogenic microorganisms and in immunity[43]. A normal ‘healthy’ intestinal barrier consists of a layer of mucus (water, glycoproteins mucin), and an outer and inner epithelium, consisting of goblet cells, enterocytes, Paneth cells, microvilli, and enteroendocrine cells, between which there are tight junctions, gap junctions, adherens junctions, and desmosomes[44]. A micro-biotic change in the intestinal tract that reduces the commensal microbiota in favour of the pathogenic one causes intestinal dysbiosis. However, several factors are required for this to occur. Weiss and Hennet[45] point out that these include an inadequate dietary composition, the influence of medications, and the immune system. The result is increased intestinal permeability[46]. It is initially associated with low-grade inflammation, which may later develop into systemic inflammation[47].
In IBD, Halfvarson et al[48] showed significantly higher fluctuations in the composition of the intestinal microbiome compared to the composition in healthy individuals. The authors also point out that the composition of the gut microbiome of IBD patients was periodically like that of the healthy population and then changed to unfavourable. In their work, Imhann et al[49] also indicate that a high genetic risk for IBD may predispose to a change in the composition of the intestinal microbiota (e.g., reduced Roseburia). Genes such as ATG16 L1, NOD2, and CARD9 are associated with a decrease in acetate to butyrate conversion by Roseburia spp.[49]. In addition, CARD9 and NOD2 may be involved in the proper functioning of neutrophils in the body’s defence against pathogenic microorganisms[50]. Another adverse factor in IBD is the interaction of the intestinal microbiota with the intestinal epithelial barrier. Damage to the mechanical intestinal barrier occurs through a decrease in the proper functioning of tight junction proteins, and in addition, the homeostasis of the chemical barrier is also reduced through a decrease in the mucus layer and its colonisation by pathogenic bacteria. Qiu et al[51] state that immune barrier function can be reduced through the secretion of immunosuppressive proteins by a pathogenic microorganism. Kudelka et al[52] point out that the proper glycosylation process of the intestinal epithelium is fundamental to maintaining the integrity of the intestinal barrier, since epithelial glycans are involved in the delivery of nutrients and therefore show a link to the regulation of the intestinal microbiota. In their study, Franzosa et al[53] focused on the metabolic activity of the gut microbiota in IBD patients. They observed a reduction in the metabolic diversity of the gut microbiota in IBD; in addition, some species and their metabolites may have disturbed mechanistic relationships, which may consequently, for example, reduce the body’s response to oxidative stress. Micro-biotic changes in IBD patients are most often characterized by an increase in Proteobacteria and a decrease in Firmicutes and Bacteroidetes. Quaglio et al[54] indicate that patients with IBD show a decrease in the abundance of butyrate producers, e.g., Roseburia spp. and Faecalibacterium prausnitzii, and an increase in pathogenic microorganisms, e.g., Escherichia coli. Other work also indicates an increase in unfavourable bacteria, e.g., Ruminococcus gnavus and Clostridium bolteae, in the active form of the disease[55].
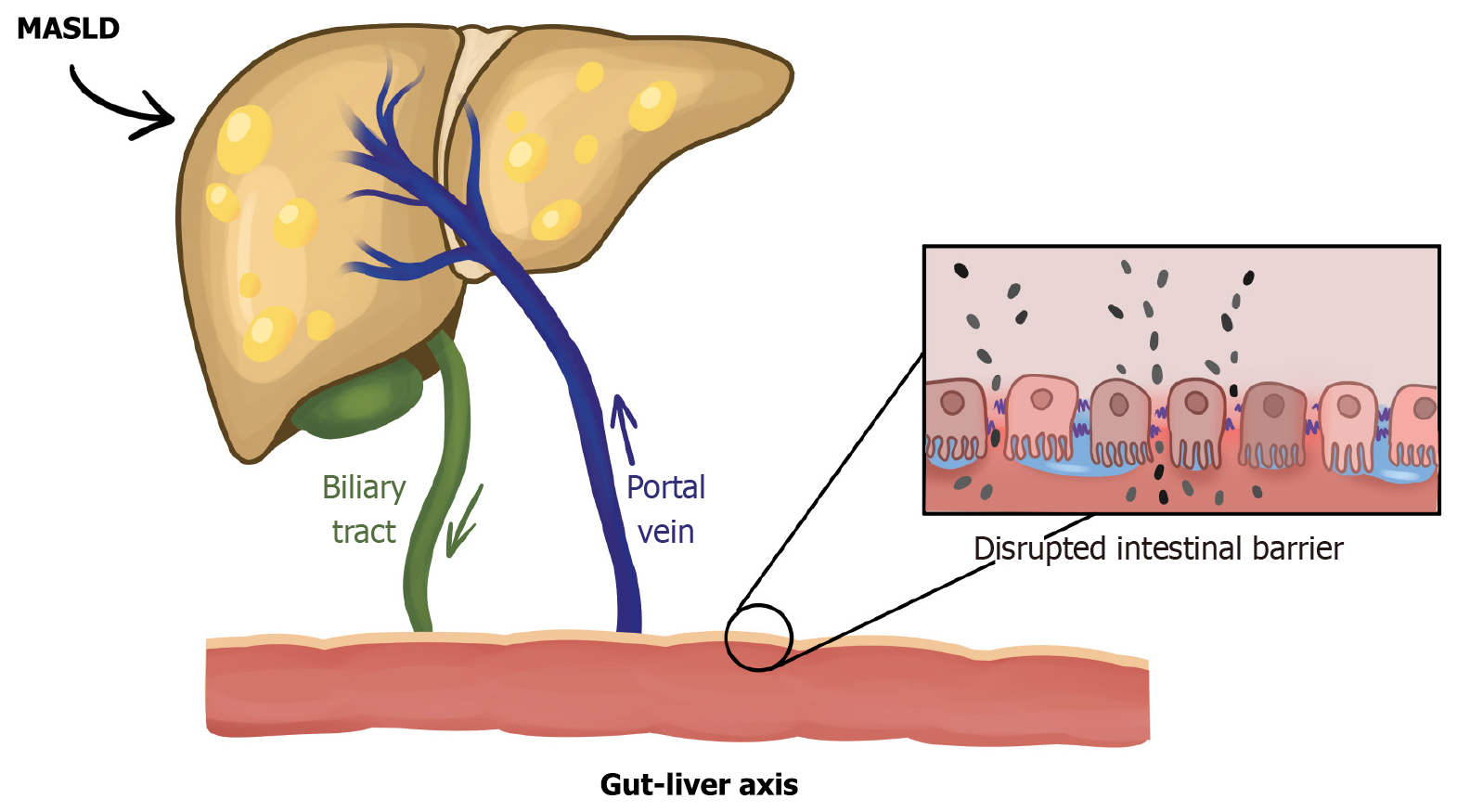
An important role is played by the gut-liver axis (Figure 1), which is functionally and anatomically an interconnected structure that is a two-way ‘communication highway’ between the gut microbiota and the liver. This structure is an important barrier both physically, immunologically and chemically against pathogens[56]. Once pathogens cross the intestinal barrier, they initiate low-grade chronic inflammation. Through lymphoid and vascular barriers, they reach the portal vein, activating Kupffer cells, which flow to the peritumoural area, providing another barrier to intestinal toxins or pathogens[57,58]. Kupffer cells recognise PAMPs and LPS, with increased inflammation, generating the secretion of pro-inflammatory cytokines, e.g., IL-6, TNF-α[59,60]. In the other direction, bile, consisting of bile acids, immunoglobulin A (IgA), bicarbonates, and antimicrobial peptides, can also have an effect on the gut microbiota[61].
Some of the bile acids are converted by the intestinal microbiota (Eubacterium, Clostridium XIVa and Clostridium XI) into a secondary form[62]. In their study, Yuan et al[63] isolated a potential pathogenic bacterium of MASLD Prevotella copri. Among other things, it affects fatty acid metabolism and immunity. In another paper, Li et al[64] point to an increase in the abundance of Bacteroidetes and a decrease in Prevotella bacteria. Effenberger et al[65] noted a reduction in the abundance of Prevotellaceae, Bacteroidales and Streptococcaceae, among others, in correlation with reduced levels of apelin (one of the adipokines that may correlate with liver damage) in the serum of MASLD patients. An altered Firmicutes/Bacteroidetes ratio can lead to a decrease in the tightness of the intestinal barrier, which consequently promotes the entry of endotoxins into the body[66]. In addition, Proteobacteria may be associated with liver fibrosis[67]. In their article, Yun et al[68] point out that a reduced abundance of Deferribacteriales and Actinomycetales can be observed in patients with MASLD. Zazueta et al[69], however, indicate a lower abundance of Ruminiclostridium-6 and Ruminococcaceae UCG 013 and, in contrast, a higher abundance of Sellimonas. The meta-analysis by Su et al[70] also indicates a decrease in beneficial bacteria (e.g., Coprococcus, Ruminococcaceae) and an increase in pro-inflammatory microorganisms (Escherichia, Fusobac
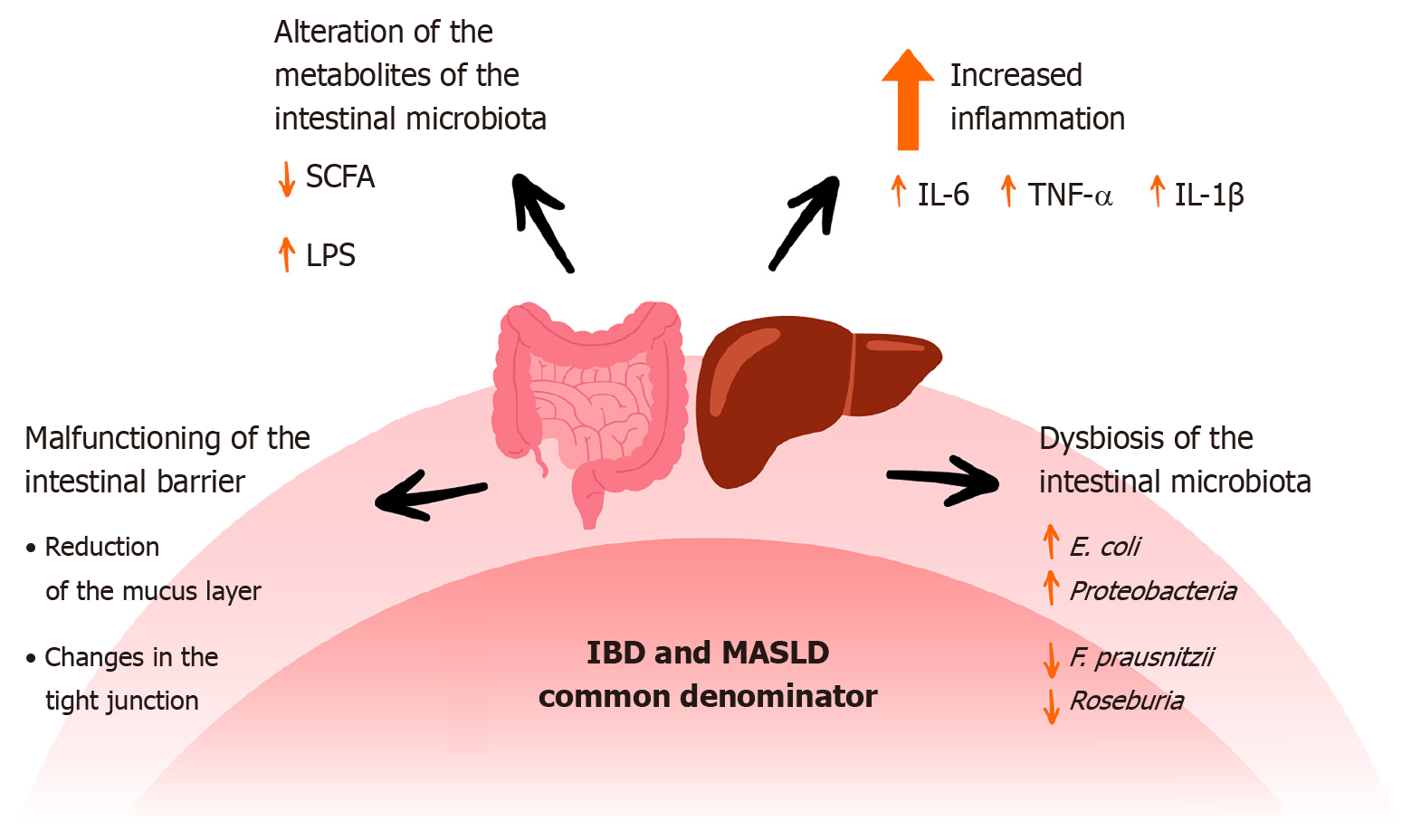
One of the main commonalities in IBD and MASLD is the presence of intestinal dysbiosis, including an increase in Escherichia coli and Proteobacteria, e.g., Klebsiella[71,72]. In another paper, the authors point to a reduction in the number of SCFA producers such as Faecalibacterium prausnitzii, Ruminococcus and Roseburia[73]. By reducing SCFA production, the tightness of the intestinal barrier can be weakened[74]. Intestinal dysbiosis also affects the secretion of mucus and MUC2. Examples of bacteria that negatively affect mucus are Listeria and Salmonella, while those that have a beneficial effect are Clostridium tyrobutyricum and Lactobacillus acidophilus[75]. Another commonality between IBD and MASLD is a reduction in the proper functioning of tight junctions (e.g., a reduction in zonula occludens-1)[76,77]. All abnormalities lead to a ‘leaky gut’ and consequently penetration of endotoxins into the body and increased inflammation (IL-6, TNF-α)[78,79]. Figure 2 shows selected common factors of IBD and MASLD.
Lifestyle, including diet, has an impact on the composition and quantity of the gut microbiota. Complex carbohydrates, specifically dietary fibre metabolised by certain intestinal bacteria, promote the production of SCFAs. The SCFA group includes propionate, acetate, and butyrate. The latter is the main source of energy for intestinal epithelial cells. Martin-Gallausiaux et al[80] indicate that the main producers of butyrate in the gut are the Lachnospiraceae, Ruminococcaceae, and Erysipelotrichaceae. In a paper by García-Montero et al[81], the authors presented the advantages of a Mediterranean diet, based on complex carbohydrates, vegetables, fruits, and poly- and monounsaturated fatty acids. They point out that the composition of the diet influences the normal composition of the intestinal microbiota, thus promoting proper intestinal barrier function and nutrition of the body, and may affect the normal immune response. Perler et al[82] further describe the anti-inflammatory properties of the Mediterranean diet, which also translates into the preservation of intestinal homeostasis. Compounds such as polyphenols and flavonoids can increase the abundance of Bifidobacterium, Enterococcus and Lactobacillus[83]. In addition, care should be taken to ensure a proper supply of minerals (iron, zinc, copper, selenium) because their deficiencies can affect the quantitative change in the composition of the intestinal microbiota. There should also be an adequate supply of vitamins (B group, vitamin C, A, D, E, K)[84]. In contrast, a Western-type diet, rich in total fat, saturated fatty acids, and trans-isomeric unsaturated fatty acids, induces low-grade inflammation. The gut microbiota is qualitatively altered, reducing the number of SCFA producers. A high-fat diet also increases oxidative stress, stimulating stress in the ER, which consequently can disrupt the intestinal barrier homeostasis[85].
Physical activity can affect the gut microbiota both quantitatively and qualitatively. Campaniello et al[86] list a number of potential factors as to why this occurs, including increased SCFA production, decreased intestinal transit time, and increased IgA production[86]. In their paper, Aya et al[87] present that athletes have a higher α-diversity of gut microbiota. However, they also point out that this is probably closely related to their diet. In addition, athletes who practice different sports and at different levels of performance show a different quantitative and qualitative compositions of microorganisms in the gut. In contrast, Wegierska et al[88] indicate that irregular physical exercise, at too-high an intensity, and for prolonged periods without adequate recovery can negatively affect the gut microbiota. Physical exertion not only has an effect on the gut microbiota, but when performed regularly at moderate intensity, it can promote normal intestinal blood flow and maintain normal intestinal barrier function[89]. Exercise can also affect the circulation of bile acids between the intestines and the liver, which can also affect the composition of intestinal microbes[90]. The effects of exercise on the gut microbiota are mainly studied in animal models; in addition, amateur athletes and people performing activities with various diseases are also worth considering in the future[91]. Resistance exercise and aerobic activity effectively change the body composition and reduce body fat. These changes may influence the composition of the intestinal microbiota and the maintenance of normal intestinal barrier homeostasis[92,93].
In addition to adequate nutrition and physical activity levels, recovery and maintenance of a normal diurnal rhythm are essential. The composition of the intestinal microbiota itself shows a 10% diurnal variation, mainly in Parabacteroides, Bulleida and Lachnospira[94]. Another study showed diurnal oscillations in Roseburia, Desulfovibrio, Escherichia and Eubacterium[95]. The diurnal rhythm depends on many factors, e.g., genetics, changes in the cycle of light and darkness, and meal schedule[96]. In their work, Sen et al[97] indicate that the vagus nerve, serotonergic system, and immune interactions affect the gut-brain axis, which in turn affects sleep. In the study by Anderson et al[98], the researchers observed that good sleep quality showed a relationship with the proportions of Lentisphaerae and Verrucomicrobia types. Sleep deprivation also affects the activation of the inflammatory response, which, among other things, results in increased permeability of the intestinal barrier. Increased levels of serum zonulin and decreased expression of zonula occludens-1, occludin, Muc-2 and Claudin-1 were demonstrated[99]. Not only do reduced sleep quality and duration affect the gut microbiota, but the gut microbiota has also been shown to affect sleep. Ogawa et al[100] observed in animal models that abnormalities in the gut microbiota can contribute to the duration of the non-rapid eye movement sleep and rapid eye movement sleep phases. The authors indicate that this may be due to an imbalance of neurotransmitters. Wang et al[101] indicate that the gut microbiota may also affect sleep quality via modulation of endocrine conduction, neuronal signalling and bacterial metabolites. Intestinal dysbiosis induced by sleep deprivation can activate the TLR4/NF-κB inflammatory pathway and impair intestinal barrier function[102]. Li et al[103] showed increased abundance of Blautia, Eubacterium hallii and decreased Lachnospira bacteria in patients with insomnia, compared to healthy controls. Finally, the authors showed a correlation between certain bacteria (Blautia, Faecalibacterium) and plasma IL-1β levels in patients with insomnia.
Psychological stress can impair the function of the hypothalamic-pituitary-adrenal (HPA) axis, thereby altering the composition and quantity of the commensal gut microbiota, leading to changes in microbial metabolites[104]. This is a bidirectional relationship, since the gut microbiota changes throughout the day, which also modulates the diurnal concentrations of corticosterone, which responds to stress on the HPA axis[105]. In addition, Tofani et al[106] indicate that micro-biotic changes in the gut can alter the rhythmicity of stress pathways in the hippocampus and amygdala. Altered diurnal rhythms also occur in disorders such as depression and schizophrenia, which may also be linked to intestinal dysbiosis[107,108]. On the other hand, stressful situations can also have an impact on the state of the gut microbiota. In their work, Lai et al[109] describe that exposure to chronic stress can reduce the abundance of Firmicutes. They also present that there is a dysregulation of the stress response with a depleted microbiome. In their review, Cooke et al[110] propose mechanisms of action that could potentially assist in coping with stress, e.g., improving sleep quality, re-educating inflammation, and including a probiotic in a well-balanced diet. Similarly, Zhang et al[111] present that regulating the gut microbiota can help the body cope with stress. Kaur et al[112] indicate that the gut microbiota can be altered in diseases associated with neurological disorders, specifically a reduction in the number of bacteria involved in the metabolism of tryptophan in the gut. In a systematic review, Ma et al[113] observed that the appearance of stress was associated with an increased abundance of Euryarchaeota, along with a decreased abundance of Verrucomicrobia and Proteobacteria. Chronic psychological stress can also negatively affect the homeostasis of the intestinal barrier. It can reduce the expression of tight junction proteins through its association with excessive intestinal permeability[114].
Another factor that modulates intestinal barrier function is smoking. Bai et al[115] observed that in animal models, cigarette smoke can alter the function of the intestinal barrier and ‘dysbiotically’ alter the composition of the intestinal microbiota. Consequently, this can initiate mitogen-activated protein kinase/extracellular regulated protein kinases signalling in the intestinal epithelium, leading to the promotion of colorectal cancer. Similarly, Fan et al[116] point to the occurrence of intestinal dysbiosis after exposure to cigarette smoke. The authors also describe a potential mechanism for the effects of smoking on the gut microbiota through correlations between tryptophan and tyrosine metabolism. Similar conclusions are drawn by Antinozzi et al[117] in their paper. Another factor is alcohol abuse. Leclercq et al[118] noted that transplanting the gut microbiota from humans who abuse alcohol into mice resulted in behavioural changes and higher stress levels in the animals, which may suggest a broad influence of behavioural changes via gut dysbiosis. In their study in animal models, Yang et al[119] also point to changes in the gut microbiota and metabolic pathways after exposure to alcohol. Chronic exposure to ethanol can lead to NLRP3-dependent hippocampal neuroinflammation through disturbance of intestinal barrier homeostasis[120]. In addition to the factors mentioned above that disrupt the proper functioning and composition of the intestinal microbiota, some medications can also have a negative impact. The authors of some studies have noted that the intestinal microbiota is altered with the use of non-steroidal anti-inflammatory drugs, proton pump inhibitors, or antibiotics[121-124].
An anti-healthy lifestyle may be one of several components that predispose to the development of both IBD and MASLD. A high-calorie Western-type diet, high in saturated fatty acids, highly processed foods, characterised by low amounts of vegetables, fruits, and whole-grain cereal products, may be a predictor of the development of some diseases, including MASLD and IBD[125,126]. In addition, cigarette smoking and low levels or a lack of physical activity in correlation with genetic factors may be associated with an increased risk of IBD[127]. A moderate level of regular physical activity is also recommended for diagnosed MASLD, the main goal of which is to lower the BMI[128]. In addition, when diagnosed with MASH, Mucinski et al[129] point to a reduction in fat mass and liver damage following the introduction of exercise and diet. Cigarette smoking, through its effects on the gut microbiota and intestinal barrier, has shown potential effects on the predisposition to the development of both MASLD and IBD[130,131].
All the aforementioned factors can affect the composition and quality of the intestinal microbiota and compromise intestinal barrier homeostasis. As a result, they can increase susceptibility to the development of both IBD and MASLD.
Chronic inflammatory conditions, including IBD, not only cause intestinal inflammation but also profoundly affect eating behaviours[132]. Patients tend to develop restrictive eating habits to reduce gastrointestinal symptoms, both during active and remission periods: Avoiding food, skipping meals, limiting certain food groups, etc. are frequently observed[133]. Avoidant/restrictive food intake disorder (ARFID) is a significant concern among those with IBD[134]. Psychosocial factors such as IBD diagnosis, disease activity, intensity of gastrointestinal symptoms, and gastrointestinal-specific anxiety play a role in the development of ARFID[135].
One of the mechanisms underlying this deterioration in eating behaviours is ‘food literacy’. Low food literacy leads patients to make dietary decisions based on misinformation, which, combined with nutritional deficiencies and psychosocial stress, increases the risk of ARFID and malnutrition. This process progresses in a cyclical model triggered by gastrointestinal symptoms[136].
Eating satisfaction decreases in people with IBD; it has been observed that people avoid eating out during periods of intense symptoms, experience social isolation, and the psychosocial dimension of eating is affected. This situation negatively affects both nutritional diversity and quality of life[137]. Another mechanism that increases the risk of ARFID is food fears caused by gastrointestinal symptoms. In those with more active disease, the development of ARFID is promoted by the expectation of symptoms even though they are not ill. Disease activity stands out as an independent factor in the development of ARFID[135]. Skipping meals and snacking behaviours between meals also play an important role in IBD. Nutritional insufficiency arises from skipping meals, biases against certain foods, and deficits in protein and micronutrients, with 38.6% of patients in remission exhibiting protein consumption below recommended levels[138].
Consumption of ultra-processed food is another factor that increases the risk of both IBD and MASLD. Increased sensitivity to processed foods creates dysbiosis in the gut microbiota, triggering inflammation and gastrointestinal symptoms[139]. Dietary management strategies used in IBD can improve patient nutritional quality, but long-term restrictions can lead to dysbiosis and disturbance of micronutrients[140].
Protein-energy malnutrition due to IBD triggers muscle insulin resistance, which is one of the basic mechanisms of hepatic steatosis[141]. Decreased glucose utilisation in muscle tissue results in increased mobilisation of free fatty acids, favouring the accumulation of triglycerides in the liver. At the same time, conditions such as sarcopenia and muscle proteolysis reduce the buffering function of the liver in lipid metabolism, increasing the ease of developing steatosis[142]. The sarcopenic condition identified in patients with IBD should be regarded as an independent risk factor for MASLD[143].
The anorexia, gastrointestinal symptoms (abdominal pain, diarrhoea, nausea), and inflammation-related appetite suppression seen in the active phases of IBD further restrict energy and nutrient intake. Micronutrient deficiencies, especially vitamin B12, folate, vitamin D, zinc, and iron, are common[143]. These deficiencies have negative effects on hepatic mitochondrial functions, antioxidant defence mechanisms, and cellular redox balance, facilitating lipotoxicity and inflammation in hepatocytes. For example, vitamin D deficiency can increase transforming growth factor-β and inflammatory cytokine expression, which promote both immune dysfunction and liver fibrosis[144].
Malnutrition is not only limited to deficiencies; enteral/parenteral nutrition practices and elimination diets, which are frequently encountered in patients with IBD, can also lead to a disturbance of metabolic balance in the long term[145]. In some individuals, following uniform diets with poor carbohydrate and lipid content can reduce metabolic flexibility, leading to decreased levels of hormones that suppress the lipogenesis pathway[146]. The risk of MASLD is also thought to increase with increased consumption of saturated fat and processed foods[147].
The microbial products (e.g., LPS) that pass to the portal system as a result of intestinal dysbiosis and increased intestinal permeability in IBD increase both systemic inflammation and hepatic inflammatory response[148]. This situation, together with malnutrition, deepens the pathogenesis of MASLD by creating an ‘inflammatory vicious cycle’ in the gut-liver axis. In these individuals, both inflammation and nutritional deficiencies accelerate the pathophysiological process that leads to MASLD[149].
In conclusion, the nutritional deficiencies accompanying IBD are related not only to the gastrointestinal course of the disease but also to the systemic effects on the liver. When factors such as inadequate energy intake, sarcopenia, micronutrient deficiencies, and dysbiosis come together, the risk of developing MASLD in individuals with IBD increases significantly[150]. Therefore, holistic consideration of hepatic evaluation and nutritional support is of great importance in the multidisciplinary management of IBD patients[151].
MASLD represents a complex pathology shaped by the interaction of diet composition, eating behaviours, and the gut microbiota. This interaction explains the influence of modern diets, especially high-calorie, processed and low-fibre diets, on the development of MASLD[152].
Epidemiological analyses using the dietary index of gut microbiota (DI-GM) score, which measures the relationship between diet and gut microbiota, show that high-quality diets significantly reduce the risk of MASLD by increasing the diversity of the gut microbiota; each 1-unit increase in the DI-GM score reduces the risk of MASLD by approximately 10% through hypersensitive-CRP and BMI. Eating behaviours, especially excessive saturated fat, processed meat, and high fructose consumption, create dysbiosis in the intestinal microbiota and increase the rates of inflammatory metabolites, triggering the development of MASLD[153-155]. There are also studies showing the positive effects of diet on gut microbiota. In particular, it has been determined that dietary intake of live microorganisms (e.g., fermented milk products, kefir, probiotic-rich foods) reduces the risk of MASLD and leads to improvements in liver enzymes and inflammation markers[156]. Similarly, high-fibre, plant-based diets similar to the Mediterranean diet support both metabolic parameters and liver health by increasing gut microbiota diversity[157]. Dietary modifications such as intermittent fasting are also among the promising methods to reduce inflammation in MASLD by reshaping gut microbiota profiles[158].
In conclusion, the interaction between dietary behaviours and gut microbiota plays a central role in the development of MASLD. Poor dietary habits increase dysbiosis and inflammation, while improved dietary adjustments allow these processes to be kept at tolerable levels. Therefore, a holistic approach that includes eating quality, microorganism intake and intestinal health should be adopted in MASLD management strategies[159].
IBD profoundly affects the gut microbiota through diet and eating behaviours, with strong implications for disease activity and overall health[160]. The Western-style diet increases dysbiosis by depleting the gut microbiota of nutrients through its high saturated fat, refined sugar, processed foods, and low fibre content. This diet pattern reduces SCFAs levels, such as butyrate and propionate, while promoting the proliferation of pathobiont bacteria[161]. SCFAs maintain epithelial integrity and suppress inflammation by promoting Treg cell differentiation through G protein-coupled receptors (e.g., GPR41/43, GPR109a). The impairment of these mechanisms facilitates increased gut permeability, systemic inflammation, and disease exacerbation[162].
Controlled dietary interventions in IBD patients have shown that diets rich in prebiotics (fibre plant foods), probiotics (fermented dairy products) or omega-3 rapidly provide positive changes in the gut microbiota. This approach resulted in significant decreases in proinflammatory cytokines such as IL-6 and IL-8, leading to an increase in anti-inflammatory SCFA producers such as Roseburia, Faecalibacterium prausnitzii and Bacteroides[161].
Trends in eating behaviours such as restriction, ARFID and food avoidance can lead individuals to long-term low fibre and unbalanced micronutrient intake. A low-fibre diet leads to a reduction in beneficial bacteria such as Bifidobacterium and Lactobacillus and SCFAs that protect the mucus-nerve barrier[162]. Furthermore, low diversity may facilitate pathogenesis, leading to mixed outcomes associated with metabolic syndrome in individuals with IBD[163]. Controlled interventions with diet (e.g., Mediterranean diet) may provide neutral or favourable gut microbiota interactions. However, restrictive diets such as low-FODMAP can lead to a reduction in beneficial species such as Bifidobacterium in the long term; therefore, personalised approaches are essential[164].
In conclusion, eating behaviours in individuals with IBD have important effects on the gut microbiota profile and indirectly on the disease status. When healthy eating behaviours are supported by balanced high fibre, fermented foods and omega-3 diets, an improvement in the gut microbiota is observed, inflammation is reduced, and the epithelial barrier is protected. Therefore, eating behaviour assessments in IBD should be considered as a clinical target that modulates the gut microbiota, not just nutritional status[165].
MASLD and IBD share common risk factors and mechanisms that promote the progression of these diseases. Although the common pathophysiology of these diseases is not yet fully understood, available scientific data make it possible to identify key common points that may explain the development of MASLD in patients with IBD, even in the absence of classical metabolic risk factors.
Dysfunction of the gut-liver axis is considered one of the main pathogenetic mechanisms. Abnormalities in the composition of the gut microbiota and increased intestinal permeability promote translocation of PAMPs, activation of the immune system, and chronic inflammation, which can lead to the development and progression of MASLD in the course of IBD. Therapies targeting the modulation of the gut microbiota can influence bile acid composition and metabolism and restore normal FXR and TGR5 signalling, leading to improved intestinal barrier integrity, normalisation of Treg/Th17 cell ratios, and reduction of inflammation. It is believed that the use of appropriate dietary interventions, supplemented with probiotics and synbiotics, can be an effective strategy to support therapies, through a multidirectional effect on the gut-liver axis and the gut microbiota[166,167].
The use of IL-17 and IL-23 inhibitors could potentially benefit the treatment of MASLD by reducing inflammation and improving metabolic parameters. However, the results of the available studies show inconclusive effects on the liver and, in the case of IL-17 inhibitors, even exacerbations of inflammation in the gut and an increase in fungal infections have been reported[168-171]. Therefore, it is necessary to conduct large, well-designed preclinical and prospective studies to assess the efficacy and safety of these therapies in the course of MASLD/MASH in the group of patients with IBD, but also to search for other therapeutic pathways[168,171].
Both IBD and MASLD are chronic diseases linked by environmental factors. Although the pathogenetic components are not fully understood, they show common mechanisms, e.g., oxidative stress and chronic inflammation. In addition, a Western-type diet, low or no physical activity, chronic psychological stress, and sleep and diurnal rhythm disturbances predispose to intestinal dysbiosis and disruption of intestinal barrier homeostasis. Inflammation in the body can increase through increased ‘leaky gut’ via the gut-liver axis and translocation of bacterial metabolites. Nutritional disorders can exacerbate intestinal dysbiosis, promoting the development of diseases, so it is necessary to incorporate lifestyle factors that promote health in patients, if their condition allows.
We would like to thank Jarmakiewicz A for creating the illustrations.
| 1. | Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr. 2023;32:197-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 450] [Article Influence: 150.0] [Reference Citation Analysis (1)] |
| 2. | Hutchison AL, Tavaglione F, Romeo S, Charlton M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): Beyond insulin resistance. J Hepatol. 2023;79:1524-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 150] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 3. | Ha S, Wong VW, Zhang X, Yu J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut. 2024;74:141-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 73] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 4. | Guo X, Yin X, Liu Z, Wang J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int J Mol Sci. 2022;23:15489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 263] [Reference Citation Analysis (3)] |
| 5. | Syed-Abdul MM. Lipid Metabolism in Metabolic-Associated Steatotic Liver Disease (MASLD). Metabolites. 2023;14:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 6. | Lee VH, Gulati AS. Implications of Paneth cell dysfunction on gastrointestinal health and disease. Curr Opin Gastroenterol. 2022;38:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Shaker ME. The contribution of sterile inflammation to the fatty liver disease and the potential therapies. Biomed Pharmacother. 2022;148:112789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 8. | He Y, Hwang S, Ahmed YA, Feng D, Li N, Ribeiro M, Lafdil F, Kisseleva T, Szabo G, Gao B. Immunopathobiology and therapeutic targets related to cytokines in liver diseases. Cell Mol Immunol. 2021;18:18-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Zhong Z, Xu M, Ge C, Tan J. Exploring shared molecular signatures and regulatory mechanisms in nonalcoholic steatohepatitis and inflammatory bowel disease using integrative bioinformatics analysis. Sci Rep. 2024;14:12085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Dowdell AS, Colgan SP. Metabolic Host-Microbiota Interactions in Autophagy and the Pathogenesis of Inflammatory Bowel Disease (IBD). Pharmaceuticals (Basel). 2021;14:708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Borowitz SM. The epidemiology of inflammatory bowel disease: Clues to pathogenesis? Front Pediatr. 2022;10:1103713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 12. | Zakerska-Banaszak O, Zuraszek-Szymanska J, Eder P, Ladziak K, Slomski R, Skrzypczak-Zielinska M. The Role of Host Genetics and Intestinal Microbiota and Metabolome as a New Insight into IBD Pathogenesis. Int J Mol Sci. 2024;25:9589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 269] [Article Influence: 134.5] [Reference Citation Analysis (1)] |
| 14. | McDowell C, Farooq U, Haseeb M. Inflammatory Bowel Disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. [PubMed] |
| 15. | Törüner M, Ünal NG. Epigenetics of Inflammatory Bowel Diseases. Turk J Gastroenterol. 2023;34:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 16. | Bilson J, Mantovani A, Byrne CD, Targher G. Steatotic liver disease, MASLD and risk of chronic kidney disease. Diabetes Metab. 2024;50:101506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 17. | Maresca R, Mignini I, Varca S, Calvez V, Termite F, Esposto G, Laterza L, Scaldaferri F, Ainora ME, Gasbarrini A, Zocco MA. Inflammatory Bowel Diseases and Non-Alcoholic Fatty Liver Disease: Piecing a Complex Puzzle Together. Int J Mol Sci. 2024;25:3278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Magrì S, Paduano D, Chicco F, Cingolani A, Farris C, Delogu G, Tumbarello F, Lai M, Melis A, Casula L, Fantini MC, Usai P. Nonalcoholic fatty liver disease in patients with inflammatory bowel disease: Beyond the natural history. World J Gastroenterol. 2019;25:5676-5686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Glassner K, Malaty HM, Abraham BP. Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease Among Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Abenavoli L, Scarlata GGM, Borelli M, Suraci E, Marasco R, Imeneo M, Spagnuolo R, Luzza F. Use of Metabolic Scores and Lipid Ratios to Predict Metabolic Dysfunction-Associated Steatotic Liver Disease Onset in Patients with Inflammatory Bowel Diseases. J Clin Med. 2025;14:2973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Rotaru A, Stafie R, Stratina E, Zenovia S, Nastasa R, Minea H, Huiban L, Cuciureanu T, Muzica C, Chiriac S, Girleanu I, Singeap AM, Sfarti C, Stanciu C, Trifan A. Lean MASLD and IBD: Exploring the Intersection of Metabolic Dysfunction and the Gut-Liver Axis. Life (Basel). 2025;15:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Zhang Q, Xu F, Wang Z, Liu S, Zhu S, Zhang S, Wu S. Long-Term Risk of Inflammatory Bowel Disease With MASLD: A Large-Scale Prospective Cohort Study in UK Biobank. J Gastroenterol Hepatol. 2025;40:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Castro-Barquero S, Casas R, Rimm EB, Tresserra-Rimbau A, Romaguera D, Martínez JA, Salas-Salvadó J, Martínez-González MA, Vidal J, Ruiz-Canela M, Konieczna J, Sacanella E, García-Gavilán JF, Fitó M, García-Arellano A, Estruch R. Loss of Visceral Fat is Associated with a Reduction in Inflammatory Status in Patients with Metabolic Syndrome. Mol Nutr Food Res. 2023;67:e2200264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Costa A, Konieczna J, Reynés B, Martín M, Fiol M, Palou A, Romaguera D, Oliver P. CUN-BAE Index as a Screening Tool to Identify Increased Metabolic Risk in Apparently Healthy Normal-Weight Adults and Those with Obesity. J Nutr. 2021;151:2215-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Muro P, Zhang L, Li S, Zhao Z, Jin T, Mao F, Mao Z. The emerging role of oxidative stress in inflammatory bowel disease. Front Endocrinol (Lausanne). 2024;15:1390351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 121] [Reference Citation Analysis (0)] |
| 26. | Peng L, Li L, Liu J, Li Y. New insights into metabolic dysfunction-associated steatotic liver disease and oxidative balance score. Front Nutr. 2023;10:1320238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Mierzwa G, Budzyński J, Kupczyk D, Augustyńska B. Biochemical markers of oxidative stress in patients with inflammatory bowel diseases. Med Res J. 2022;7:234-241. [DOI] [Full Text] |
| 28. | Tratenšek A, Locatelli I, Grabnar I, Drobne D, Vovk T. Oxidative stress-related biomarkers as promising indicators of inflammatory bowel disease activity: A systematic review and meta-analysis. Redox Biol. 2024;77:103380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 29. | Das A, De AJ, Mohanty T, Aich P. Role of oxidative stress, gut microbiota and derived metabolites in the etiology and progression of nonalcoholic fatty liver disease. Redox Expe Med. 2023;2023. [DOI] [Full Text] |
| 30. | Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, Han X, Peng Y, Chen X, Shen L, Qiu D, Li Z, Ma X. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011;166:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 31. | Amerikanou C, Papada E, Gioxari A, Smyrnioudis I, Kleftaki SA, Valsamidou E, Bruns V, Banerjee R, Trivella MG, Milic N, Medić-Stojanoska M, Gastaldelli A, Kannt A; MAST4HEALTH, Dedoussis GV, Kaliora AC. Mastiha has efficacy in immune-mediated inflammatory diseases through a microRNA-155 Th17 dependent action. Pharmacol Res. 2021;171:105753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Abenavoli L, Giubilei L, Procopio AC, Spagnuolo R, Luzza F, Boccuto L, Scarpellini E. Gut Microbiota in Non-Alcoholic Fatty Liver Disease Patients with Inflammatory Bowel Diseases: A Complex Interplay. Nutrients. 2022;14:5323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 33. | Thielemann N, Siliceo SL, Rau M, Schöninger A, Reus N, Aldejohann AM, Shehata A, Behr IS, Nieuwenhuizen NE, Herz M, Hermanns HM, Mirhakkak M, Löffler J, Dandekar T, Hünniger-Ast K, Martin R, Panagiotou G, Geier A, Kurzai O. Mycobiome Dysbiosis and Genetic Predisposition for Elevated IL-17A Drive Fibrosis in MASLD. 2024 Preprint. [DOI] [Full Text] |
| 34. | Chen L, Ruan G, Cheng Y, Yi A, Chen D, Wei Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front Immunol. 2022;13:1055914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 35. | Riedel S, Pheiffer C, Johnson R, Louw J, Muller CJF. Intestinal Barrier Function and Immune Homeostasis Are Missing Links in Obesity and Type 2 Diabetes Development. Front Endocrinol (Lausanne). 2021;12:833544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Wu Q, Yang Y, Lin S, Geller DA, Yan Y. The microenvironment in the development of MASLD-MASH-HCC and associated therapeutic in MASH-HCC. Front Immunol. 2025;16:1569915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 37. | Abdelnabi MN, Hassan GS, Shoukry NH. Role of the type 3 cytokines IL-17 and IL-22 in modulating metabolic dysfunction-associated steatotic liver disease. Front Immunol. 2024;15:1437046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 38. | Rau M, Schilling AK, Meertens J, Hering I, Weiss J, Jurowich C, Kudlich T, Hermanns HM, Bantel H, Beyersdorf N, Geier A. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J Immunol. 2016;196:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 39. | Prananda MF, Abdullah M, Koesnoe S, Sinto R. S1166 Serum Interleukin 17 Levels in Ulcerative Colitis and Crohn’s Disease: Inflammatory Bowel Disease Patients in Indonesia. Am J Gastroenterol. 2024;119:S828-S828. [DOI] [Full Text] |
| 40. | Lucaciu LA, Ilieș M, Vesa ȘC, Seicean R, Din S, Iuga CA, Seicean A. Serum Interleukin (IL)-23 and IL-17 Profile in Inflammatory Bowel Disease (IBD) Patients Could Differentiate between Severe and Non-Severe Disease. J Pers Med. 2021;11:1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Menesy A, Hammad M, Aref S, Abozeid FAM. Level of interleukin 17 in inflammatory bowel disease and its relation with disease activity. BMC Gastroenterol. 2024;24:135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 42. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1639] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 43. | Lacerda JF, Lagos AC, Carolino E, Silva-Herdade AS, Silva M, Sousa Guerreiro C. Functional Food Components, Intestinal Permeability and Inflammatory Markers in Patients with Inflammatory Bowel Disease. Nutrients. 2021;13:642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2024;19:275-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 612] [Article Influence: 306.0] [Reference Citation Analysis (0)] |
| 45. | Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74:2959-2977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 497] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 46. | Christovich A, Luo XM. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front Immunol. 2022;13:946248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 47. | Arifuzzaman M, Collins N, Guo CJ, Artis D. Nutritional regulation of microbiota-derived metabolites: Implications for immunity and inflammation. Immunity. 2024;57:14-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 48. | Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D'Amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE, Dunklebarger MF, Knight R, Jansson JK. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2:17004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 862] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 49. | Imhann F, Vich Vila A, Bonder MJ, Fu J, Gevers D, Visschedijk MC, Spekhorst LM, Alberts R, Franke L, van Dullemen HM, Ter Steege RWF, Huttenhower C, Dijkstra G, Xavier RJ, Festen EAM, Wijmenga C, Zhernakova A, Weersma RK. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 588] [Article Influence: 73.5] [Reference Citation Analysis (1)] |
| 50. | Danne C, Skerniskyte J, Marteyn B, Sokol H. Neutrophils: from IBD to the gut microbiota. Nat Rev Gastroenterol Hepatol. 2024;21:184-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 126] [Reference Citation Analysis (1)] |
| 51. | Qiu P, Ishimoto T, Fu L, Zhang J, Zhang Z, Liu Y. The Gut Microbiota in Inflammatory Bowel Disease. Front Cell Infect Microbiol. 2022;12:733992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 366] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 52. | Kudelka MR, Stowell SR, Cummings RD, Neish AS. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat Rev Gastroenterol Hepatol. 2020;17:597-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 53. | Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, Vatanen T, Hall AB, Mallick H, McIver LJ, Sauk JS, Wilson RG, Stevens BW, Scott JM, Pierce K, Deik AA, Bullock K, Imhann F, Porter JA, Zhernakova A, Fu J, Weersma RK, Wijmenga C, Clish CB, Vlamakis H, Huttenhower C, Xavier RJ. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4:293-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 888] [Cited by in RCA: 1354] [Article Influence: 193.4] [Reference Citation Analysis (0)] |
| 54. | Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022;28:4053-4060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 219] [Cited by in RCA: 286] [Article Influence: 71.5] [Reference Citation Analysis (116)] |
| 55. | Haneishi Y, Furuya Y, Hasegawa M, Picarelli A, Rossi M, Miyamoto J. Inflammatory Bowel Diseases and Gut Microbiota. Int J Mol Sci. 2023;24:3817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 137] [Reference Citation Analysis (0)] |
| 56. | Pabst O, Hornef MW, Schaap FG, Cerovic V, Clavel T, Bruns T. Gut-liver axis: barriers and functional circuits. Nat Rev Gastroenterol Hepatol. 2023;20:447-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 236] [Reference Citation Analysis (0)] |
| 57. | Vallianou NG, Kounatidis D, Psallida S, Vythoulkas-Biotis N, Adamou A, Zachariadou T, Kargioti S, Karampela I, Dalamaga M. NAFLD/MASLD and the Gut-Liver Axis: From Pathogenesis to Treatment Options. Metabolites. 2024;14:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 58. | Long Q, Luo F, Li B, Li Z, Guo Z, Chen Z, Wu W, Hu M. Gut microbiota and metabolic biomarkers in metabolic dysfunction-associated steatotic liver disease. Hepatol Commun. 2024;8:e0310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 45] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 59. | Chen J, Deng X, Liu Y, Tan Q, Huang G, Che Q, Guo J, Su Z. Kupffer Cells in Non-alcoholic Fatty Liver Disease: Friend or Foe? Int J Biol Sci. 2020;16:2367-2378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 60. | Li W, Yang Y, Yang L, Chang N, Li L. Monocyte-derived Kupffer cells dominate in the Kupffer cell pool during liver injury. Cell Rep. 2023;42:113164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 61. | Mohanty I, Mannochio-Russo H, Schweer JV, El Abiead Y, Bittremieux W, Xing S, Schmid R, Zuffa S, Vasquez F, Muti VB, Zemlin J, Tovar-Herrera OE, Moraïs S, Desai D, Amin S, Koo I, Turck CW, Mizrahi I, Kris-Etherton PM, Petersen KS, Fleming JA, Huan T, Patterson AD, Siegel D, Hagey LR, Wang M, Aron AT, Dorrestein PC. The underappreciated diversity of bile acid modifications. Cell. 2024;187:1801-1818.e20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 136] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 62. | Cai J, Rimal B, Jiang C, Chiang JYL, Patterson AD. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. 2022;237:108238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 296] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 63. | Yuan H, Wu X, Wang X, Zhou JY, Park S. Microbial Dysbiosis Linked to Metabolic Dysfunction-Associated Fatty Liver Disease in Asians: Prevotella copri Promotes Lipopolysaccharide Biosynthesis and Network Instability in the Prevotella Enterotype. Int J Mol Sci. 2024;25:2183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 64. | Li Y, Yang P, Ye J, Xu Q, Wu J, Wang Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024;23:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 141] [Reference Citation Analysis (0)] |
| 65. | Effenberger M, Grander C, Hausmann B, Enrich B, Pjevac P, Zoller H, Tilg H. Apelin and the gut microbiome: Potential interaction in human MASLD. Dig Liver Dis. 2024;56:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Hamamah S, Iatcu OC, Covasa M. Dietary Influences on Gut Microbiota and Their Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Nutrients. 2024;17:143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 67. | Jasirwan COM, Muradi A, Hasan I, Simadibrata M, Rinaldi I. Correlation of gut Firmicutes/Bacteroidetes ratio with fibrosis and steatosis stratified by body mass index in patients with non-alcoholic fatty liver disease. Biosci Microbiota Food Health. 2021;40:50-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 68. | Yun Y, Kim HN, Lee EJ, Ryu S, Chang Y, Shin H, Kim HL, Kim TH, Yoo K, Kim HY. Fecal and blood microbiota profiles and presence of nonalcoholic fatty liver disease in obese versus lean subjects. PLoS One. 2019;14:e0213692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 69. | Zazueta A, Valenzuela-Pérez L, Ortiz-López N, Pinto-León A, Torres V, Guiñez D, Aliaga N, Merino P, Sandoval A, Covarrubias N, Pérez de Arce E, Cattaneo M, Urzúa A, Roblero JP, Poniachik J, Gotteland M, Magne F, Beltrán CJ. Alteration of Gut Microbiota Composition in the Progression of Liver Damage in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Int J Mol Sci. 2024;25:4387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 70. | Su X, Chen S, Liu J, Feng Y, Han E, Hao X, Liao M, Cai J, Zhang S, Niu J, He S, Huang S, Lo K, Zeng F. Composition of gut microbiota and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Obes Rev. 2024;25:e13646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 71. | Verdugo-Meza A, Ye J, Dadlani H, Ghosh S, Gibson DL. Connecting the Dots Between Inflammatory Bowel Disease and Metabolic Syndrome: A Focus on Gut-Derived Metabolites. Nutrients. 2020;12:1434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 72. | Scarlata GGM, Abenavoli L. Gut microbiota: the pathogenetic bridge between inflammatory bowel disease and metabolic-associated steatotic liver disease. Expert Rev Gastroenterol Hepatol. 2025;19:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 73. | Saha P, Hartmann P. Impact of Gut Microbiome on Gut Permeability in Liver and Gut Diseases. Microorganisms. 2025;13:1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 74. | Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, van Tol R, Vaughan EE, Verbeke K. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11:411-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 717] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 75. | Liu Y, Yu Z, Zhu L, Ma S, Luo Y, Liang H, Liu Q, Chen J, Guli S, Chen X. Orchestration of MUC2 - The key regulatory target of gut barrier and homeostasis: A review. Int J Biol Macromol. 2023;236:123862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 76. | Otani T, Furuse M. Tight Junction Structure and Function Revisited: (Trends in Cell Biology 30, 805-817, 2020). Trends Cell Biol. 2020;30:1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 77. | Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB, Abraham C, Turner JR. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology. 2021;161:1924-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 352] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 78. | Bansal SK, Bansal MB. Pathogenesis of MASLD and MASH - role of insulin resistance and lipotoxicity. Aliment Pharmacol Ther. 2024;59 Suppl 1:S10-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 79. | Souza RF, Caetano MAF, Magalhães HIR, Castelucci P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J Gastroenterol. 2023;29:2733-2746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (4)] |
| 80. | Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 956] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 81. | García-Montero C, Fraile-Martínez O, Gómez-Lahoz AM, Pekarek L, Castellanos AJ, Noguerales-Fraguas F, Coca S, Guijarro LG, García-Honduvilla N, Asúnsolo A, Sanchez-Trujillo L, Lahera G, Bujan J, Monserrat J, Álvarez-Mon M, Álvarez-Mon MA, Ortega MA. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients. 2021;13:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 82. | Perler BK, Friedman ES, Wu GD. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu Rev Physiol. 2023;85:449-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 202] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 83. | Beam A, Clinger E, Hao L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients. 2021;13:2795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 363] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 84. | Fan L, Xia Y, Wang Y, Han D, Liu Y, Li J, Fu J, Wang L, Gan Z, Liu B, Fu J, Zhu C, Wu Z, Zhao J, Han H, Wu H, He Y, Tang Y, Zhang Q, Wang Y, Zhang F, Zong X, Yin J, Zhou X, Yang X, Wang J, Yin Y, Ren W. Gut microbiota bridges dietary nutrients and host immunity. Sci China Life Sci. 2023;66:2466-2514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 85. | Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Mądry E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells. 2021;10:3164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 495] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 86. | Campaniello D, Corbo MR, Sinigaglia M, Speranza B, Racioppo A, Altieri C, Bevilacqua A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients. 2022;14:2456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 87. | Aya V, Flórez A, Perez L, Ramírez JD. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS One. 2021;16:e0247039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 88. | Wegierska AE, Charitos IA, Topi S, Potenza MA, Montagnani M, Santacroce L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022;52:2355-2369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 89. | Hughes RL, Holscher HD. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv Nutr. 2021;12:2190-2215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 90. | de Sire A, de Sire R, Petito V, Masi L, Cisari C, Gasbarrini A, Scaldaferri F, Invernizzi M. Gut-Joint Axis: The Role of Physical Exercise on Gut Microbiota Modulation in Older People with Osteoarthritis. Nutrients. 2020;12:574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 91. | Pedersini P, Turroni S, Villafañe JH. Gut microbiota and physical activity: Is there an evidence-based link? Sci Total Environ. 2020;727:138648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 92. | Bonomini-Gnutzmann R, Plaza-Díaz J, Jorquera-Aguilera C, Rodríguez-Rodríguez A, Rodríguez-Rodríguez F. Effect of Intensity and Duration of Exercise on Gut Microbiota in Humans: A Systematic Review. Int J Environ Res Public Health. 2022;19:9518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 93. | Solitano V, Moschetta A. Metabolic Dysfunction-Associated Steatotic Liver Disease in Inflammatory Bowel Disease: A Proposed Stepwise Approach. Clin Gastroenterol Hepatol. 2024;22:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 1011] [Article Influence: 84.3] [Reference Citation Analysis (1)] |
| 95. | Kaczmarek JL, Musaad SM, Holscher HD. Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am J Clin Nutr. 2017;106:1220-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 96. | Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53:101340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 341] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 97. | Sen P, Molinero-Perez A, O'Riordan KJ, McCafferty CP, O'Halloran KD, Cryan JF. Microbiota and sleep: awakening the gut feeling. Trends Mol Med. 2021;27:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 98. | Anderson JR, Carroll I, Azcarate-Peril MA, Rochette AD, Heinberg LJ, Peat C, Steffen K, Manderino LM, Mitchell J, Gunstad J. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 2017;38:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 99. | Han M, Yuan S, Zhang J. The interplay between sleep and gut microbiota. Brain Res Bull. 2022;180:131-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 100. | Ogawa Y, Miyoshi C, Obana N, Yajima K, Hotta-Hirashima N, Ikkyu A, Kanno S, Soga T, Fukuda S, Yanagisawa M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci Rep. 2020;10:19554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 101. | Wang Z, Wang Z, Lu T, Chen W, Yan W, Yuan K, Shi L, Liu X, Zhou X, Shi J, Vitiello MV, Han Y, Lu L. The microbiota-gut-brain axis in sleep disorders. Sleep Med Rev. 2022;65:101691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 187] [Reference Citation Analysis (0)] |
| 102. | Sun J, Fang D, Wang Z, Liu Y. Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications. Int J Mol Sci. 2023;24:9603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 103. | Li Y, Zhang B, Zhou Y, Wang D, Liu X, Li L, Wang T, Zhang Y, Jiang M, Tang H, Amsel LV, Fan F, Hoven CW. Gut Microbiota Changes and Their Relationship with Inflammation in Patients with Acute and Chronic Insomnia. Nat Sci Sleep. 2020;12:895-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 104. | Hyder N, Raza ML. Stress and the gut microbiota-brain axis. Prog Brain Res. 2025;291:175-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 105. | Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, Naudon L, Rabot S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 424] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 106. | Tofani GSS, Leigh SJ, Gheorghe CE, Bastiaanssen TFS, Wilmes L, Sen P, Clarke G, Cryan JF. Gut microbiota regulates stress responsivity via the circadian system. Cell Metab. 2025;37:138-153.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
| 107. | Walker WH 2nd, Walton JC, DeVries AC, Nelson RJ. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020;10:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 580] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 108. | Sun Q, Ho CT, Zhang X, Liu Y, Zhang R, Wu Z. Strategies for circadian rhythm disturbances and related psychiatric disorders: a new cue based on plant polysaccharides and intestinal microbiota. Food Funct. 2022;13:1048-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Lai TT, Liou CW, Tsai YH, Lin YY, Wu WL. Butterflies in the gut: the interplay between intestinal microbiota and stress. J Biomed Sci. 2023;30:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 110. | Cooke MB, Catchlove S, Tooley KL. Examining the Influence of the Human Gut Microbiota on Cognition and Stress: A Systematic Review of the Literature. Nutrients. 2022;14:4623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 111. | Zhang H, Wang Z, Wang G, Song X, Qian Y, Liao Z, Sui L, Ai L, Xia Y. Understanding the Connection between Gut Homeostasis and Psychological Stress. J Nutr. 2023;153:924-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 112. | Kaur H, Bose C, Mande SS. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Front Neurosci. 2019;13:1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 113. | Ma L, Yan Y, Webb RJ, Li Y, Mehrabani S, Xin B, Sun X, Wang Y, Mazidi M. Psychological Stress and Gut Microbiota Composition: A Systematic Review of Human Studies. Neuropsychobiology. 2023;82:247-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 114. | Ilchmann-Diounou H, Menard S. Psychological Stress, Intestinal Barrier Dysfunctions, and Autoimmune Disorders: An Overview. Front Immunol. 2020;11:1823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 115. | Bai X, Wei H, Liu W, Coker OO, Gou H, Liu C, Zhao L, Li C, Zhou Y, Wang G, Kang W, Ng EK, Yu J. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut. 2022;71:2439-2450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 116. | Fan J, Zhou Y, Meng R, Tang J, Zhu J, Aldrich MC, Cox NJ, Zhu Y, Li Y, Zhou D. Cross-talks between gut microbiota and tobacco smoking: a two-sample Mendelian randomization study. BMC Med. 2023;21:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 117. | Antinozzi M, Giffi M, Sini N, Gallè F, Valeriani F, De Vito C, Liguori G, Romano Spica V, Cattaruzza MS. Cigarette Smoking and Human Gut Microbiota in Healthy Adults: A Systematic Review. Biomedicines. 2022;10:510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 118. | Leclercq S, Le Roy T, Furgiuele S, Coste V, Bindels LB, Leyrolle Q, Neyrinck AM, Quoilin C, Amadieu C, Petit G, Dricot L, Tagliatti V, Cani PD, Verbeke K, Colet JM, Stärkel P, de Timary P, Delzenne NM. Gut Microbiota-Induced Changes in β-Hydroxybutyrate Metabolism Are Linked to Altered Sociability and Depression in Alcohol Use Disorder. Cell Rep. 2020;33:108238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 119. | Yang J, Wang H, Lin X, Liu J, Feng Y, Bai Y, Liang H, Hu T, Wu Z, Lai J, Liu J, Zou Y, Wei S, Yan P. Gut microbiota dysbiosis induced by alcohol exposure in pubertal and adult mice. mSystems. 2024;9:e0136624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 120. | Yao H, Zhang D, Yu H, Yuan H, Shen H, Lan X, Liu H, Chen X, Meng F, Wu X, Zhang G, Wang X. Gut microbiota regulates chronic ethanol exposure-induced depressive-like behavior through hippocampal NLRP3-mediated neuroinflammation. Mol Psychiatry. 2023;28:919-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 121. | Mäkivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr. 2010;103:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 122. | Tiihonen K, Tynkkynen S, Ouwehand A, Ahlroos T, Rautonen N. The effect of ageing with and without non-steroidal anti-inflammatory drugs on gastrointestinal microbiology and immunology. Br J Nutr. 2008;100:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 123. | Kiecka A, Szczepanik M. Proton pump inhibitor-induced gut dysbiosis and immunomodulation: current knowledge and potential restoration by probiotics. Pharmacol Rep. 2023;75:791-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 124. | Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as Major Disruptors of Gut Microbiota. Front Cell Infect Microbiol. 2020;10:572912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 553] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 125. | Singh N, Bernstein CN. Environmental risk factors for inflammatory bowel disease. United European Gastroenterol J. 2022;10:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 126. | Liu J, Li C, Yang Y, Li J, Sun X, Zhang Y, Liu R, Chen F, Li X. Special correlation between diet and MASLD: positive or negative? Cell Biosci. 2025;15:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 127. | Sun Y, Yuan S, Chen X, Sun J, Kalla R, Yu L, Wang L, Zhou X, Kong X, Hesketh T, Ho GT, Ding K, Dunlop M, Larsson SC, Satsangi J, Chen J, Wang X, Li X, Theodoratou E, Giovannucci EL. The Contribution of Genetic Risk and Lifestyle Factors in the Development of Adult-Onset Inflammatory Bowel Disease: A Prospective Cohort Study. Am J Gastroenterol. 2023;118:511-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 128. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 955] [Article Influence: 477.5] [Reference Citation Analysis (1)] |
| 129. | Mucinski JM, Salvador AF, Moore MP, Fordham TM, Anderson JM, Shryack G, Cunningham RP, Lastra G, Gaballah AH, Diaz-Arias A, Ibdah JA, Rector RS, Parks EJ. Histological improvements following energy restriction and exercise: The role of insulin resistance in resolution of MASH. J Hepatol. 2024;81:781-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 130. | Mignini I, Galasso L, Piccirilli G, Calvez V, Termite F, Esposto G, Borriello R, Miele L, Ainora ME, Gasbarrini A, Zocco MA. Interplay of Oxidative Stress, Gut Microbiota, and Nicotine in Metabolic-Associated Steatotic Liver Disease (MASLD). Antioxidants (Basel). 2024;13:1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 131. | Sigvardsson I, Ludvigsson J, Andersson B, Størdal K, Mårild K. Tobacco Smoke Exposure in Early Childhood and Later Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study. J Crohns Colitis. 2024;18:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 132. | Moran GW, Thapaliya G. The Gut-Brain Axis and Its Role in Controlling Eating Behavior in Intestinal Inflammation. Nutrients. 2021;13:981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 133. | Day AS, Yao CK, Costello SP, Andrews JM, Bryant RV. Food avoidance, restrictive eating behaviour and association with quality of life in adults with inflammatory bowel disease: A systematic scoping review. Appetite. 2021;167:105650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 134. | Yelencich E, Truong E, Widaman AM, Pignotti G, Yang L, Jeon Y, Weber AT, Shah R, Smith J, Sauk JS, Limketkai BN. Avoidant Restrictive Food Intake Disorder Prevalent Among Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2022;20:1282-1289.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 135. | Nicholas JK, van Tilburg MAL, Pilato I, Erwin S, Rivera-Cancel AM, Ives L, Marcus MD, Zucker NL. The diagnosis of avoidant restrictive food intake disorder in the presence of gastrointestinal disorders: Opportunities to define shared mechanisms of symptom expression. Int J Eat Disord. 2021;54:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 136. | Yin T, Tu W, Li Y, Yang M, Huang L, Zhang S, Xu G. Risk of avoidant/restrictive food intake disorder in patients with inflammatory bowel disease: predictive value of disease phenotype, disease activity and food literacy. J Eat Disord. 2023;11:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 137. | Zhu W, Zhang Y, Wang LD, Li J, Hou S. Factors influencing food-related quality of life in patients with inflammatory bowel disease: A systematic review. J Eval Clin Pract. 2025;31:e14133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Peters V, Tigchelaar-Feenstra EF, Imhann F, Dekens JAM, Swertz MA, Franke LH, Wijmenga C, Weersma RK, Alizadeh BZ, Dijkstra G, Campmans-Kuijpers MJE. Habitual dietary intake of IBD patients differs from population controls: a case-control study. Eur J Nutr. 2021;60:345-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 139. | Kim KA, Im HW, Choi S, Shon WJ, Kim JS, Kim BG, Im JP, Kim SH, Kim JW, Kang HW, Kim KW, Kim ES, Koh SJ. P1316 Increased ultra-processed food consumption is associated with inflammation-promoting shift of gut microbiota in Inflammatory Bowel Disease. J Crohns Colitis. 2025;19:i2371-i2373. [DOI] [Full Text] |
| 140. | Chen L, Srinivasan A, Vasudevan A. Examining dietary interventions in Crohn's disease. World J Gastroenterol. 2024;30:3868-3874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 141. | Gibiino G, Sartini A, Gitto S, Binda C, Sbrancia M, Coluccio C, Sambri V, Fabbri C. The Other Side of Malnutrition in Inflammatory Bowel Disease (IBD): Non-Alcoholic Fatty Liver Disease. Nutrients. 2021;13:2772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 142. | Li AA, Kim D, Ahmed A. Association of Sarcopenia and NAFLD: An Overview. Clin Liver Dis (Hoboken). 2020;16:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 143. | Nishikawa H, Nakamura S, Miyazaki T, Kakimoto K, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Inflammatory Bowel Disease and Sarcopenia: Its Mechanism and Clinical Importance. J Clin Med. 2021;10:4214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 144. | Triantos C, Aggeletopoulou I, Thomopoulos K, Mouzaki A. Vitamin D-Liver Disease Association: Biological Basis and Mechanisms of Action. Hepatology. 2021;74:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 145. | Nelson AD, Elkins JR, Stocchi L, Farraye FA, Hashash JG. Use and Misuse of Parenteral Nutrition in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2022;28:1592-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 146. | Palmer BF, Clegg DJ. Metabolic Flexibility and Its Impact on Health Outcomes. Mayo Clin Proc. 2022;97:761-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 147. | Mellemkjær A, Kjær MB, Haldrup D, Grønbæk H, Thomsen KL. Management of cardiovascular risk in patients with metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med. 2024;122:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 148. | Fu Y, Lyu J, Wang S. The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Front Immunol. 2023;14:1277102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 149. | Schwärzler J, Grabherr F, Grander C, Adolph TE, Tilg H. The pathophysiology of MASLD: an immunometabolic perspective. Expert Rev Clin Immunol. 2024;20:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 150. | Nardone OM, de Sire R, Petito V, Testa A, Villani G, Scaldaferri F, Castiglione F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front Immunol. 2021;12:694217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 151. | Bischoff SC, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Forbes A, Montoro M, Burgos Peláez R. [ESPEN guideline: Clinical nutrition in inflammatory bowel disease]. Nutr Hosp. 2022;39:678-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 152. | Jiménez-González C, Alonso-Peña M, Argos Vélez P, Crespo J, Iruzubieta P. Unraveling MASLD: The Role of Gut Microbiota, Dietary Modulation, and AI-Driven Lifestyle Interventions. Nutrients. 2025;17:1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 153. | Pu Y, Tan Z, Wu Y, Zeng S, He H, Zhang J, Dong W. Association of gut microbiota dietary index with metabolic dysfunction-associated steatotic liver disease: the mediating roles of inflammation and body mass index. Front Nutr. 2025;12:1573636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 154. | Hussain M, Umair Ijaz M, Ahmad MI, Khan IA, Bukhary SUF, Khan W, Hussain S, Hashmi MS, Li C. Gut inflammation exacerbates hepatic injury in C57BL/6J mice via gut-vascular barrier dysfunction with high-fat-incorporated meat protein diets. Food Funct. 2020;11:9168-9176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 155. | Rahimi-Sakak F, Maroofi M, Emamat H, Hekmatdoost A. Red and Processed Meat Intake in Relation to Non-Alcoholic Fatty Liver Disease Risk: Results from a Case-Control Study. Clin Nutr Res. 2022;11:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 156. | Abd El-Salam MH, El-Shibiny S, Assem FM, El-Sayyad GS, Hasanien YA, Elfadil D, Soliman TN. Impact of Fermented Milk On Gut Microbiota And Human Health: A Comprehensive Review. Curr Microbiol. 2025;82:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 157. | Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, Topf M, Gonzalez CG, Van Treuren W, Han S, Robinson JL, Elias JE, Sonnenburg ED, Gardner CD, Sonnenburg JL. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184:4137-4153.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 834] [Article Influence: 166.8] [Reference Citation Analysis (0)] |
| 158. | Gallage S, Ali A, Barragan Avila JE, Seymen N, Ramadori P, Joerke V, Zizmare L, Aicher D, Gopalsamy IK, Fong W, Kosla J, Focaccia E, Li X, Yousuf S, Sijmonsma T, Rahbari M, Kommoss KS, Billeter A, Prokosch S, Rothermel U, Mueller F, Hetzer J, Heide D, Schinkel B, Machauer T, Pichler B, Malek NP, Longerich T, Roth S, Rose AJ, Schwenck J, Trautwein C, Karimi MM, Heikenwalder M. A 5:2 intermittent fasting regimen ameliorates NASH and fibrosis and blunts HCC development via hepatic PPARα and PCK1. Cell Metab. 2024;36:1371-1393.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 60] [Reference Citation Analysis (1)] |
| 159. | Popov J, Despot T, Avelar Rodriguez D, Khan I, Mech E, Khan M, Bojadzija M, Pai N. Implications of Microbiota and Immune System in Development and Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients. 2024;16:1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 160. | Sugihara K, Kamada N. Diet-Microbiota Interactions in Inflammatory Bowel Disease. Nutrients. 2021;13:1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 161. | Olendzki B, Bucci V, Cawley C, Maserati R, McManus M, Olednzki E, Madziar C, Chiang D, Ward DV, Pellish R, Foley C, Bhattarai S, McCormick BA, Maldonado-Contreras A. Dietary manipulation of the gut microbiome in inflammatory bowel disease patients: Pilot study. Gut Microbes. 2022;14:2046244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 162. | Lee JE, Kim KS, Koh H, Lee DW, Kang NJ. Diet-Induced Host-Microbe Interactions: Personalized Diet Strategies for Improving Inflammatory Bowel Disease. Curr Dev Nutr. 2022;6:nzac110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 163. | Wark G, Kaakoush NO, Samocha-Bonet D, Ghaly S, Danta M. Dietary Determinants of Metabolic and Gut Microbial Health in Patients with Inflammatory Bowel Disease. Nutrients. 2024;16:3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 164. | Staudacher HM, Rossi M, Kaminski T, Dimidi E, Ralph FSE, Wilson B, Martin LD, Louis P, Lomer MCE, Irving PM, Whelan K. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol Motil. 2022;34:e14241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 165. | Peters JE, Basnayake C, Hebbard GS, Salzberg MR, Kamm MA. Prevalence of disordered eating in adults with gastrointestinal disorders: A systematic review. Neurogastroenterol Motil. 2022;34:e14278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 166. | Baars A, Oosting A, Knol J, Garssen J, van Bergenhenegouwen J. The Gut Microbiota as a Therapeutic Target in IBD and Metabolic Disease: A Role for the Bile Acid Receptors FXR and TGR5. Microorganisms. 2015;3:641-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 167. | Yan JB, Luo MM, Chen ZY, He BH. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J Immunol Res. 2020;2020:8813558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 168. | González Fernández J, Prieto-Torres L, Ara Martín M, Martínez-Domínguez SJ. MASLD and liver fibrosis in patients with psoriasis receiving IL-17 or IL-23 inhibitors: a systematic review. Therap Adv Gastroenterol. 2025;18:17562848251335824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 169. | Fauny M, Moulin D, D'Amico F, Netter P, Petitpain N, Arnone D, Jouzeau JY, Loeuille D, Peyrin-Biroulet L. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann Rheum Dis. 2020;79:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 170. | Jiraskova Zakostelska Z, Reiss Z, Tlaskalova-Hogenova H, Rob F. Paradoxical Reactions to Anti-TNFα and Anti-IL-17 Treatment in Psoriasis Patients: Are Skin and/or Gut Microbiota Involved? Dermatol Ther (Heidelb). 2023;13:911-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 171. | Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:374-388.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/