Published online Oct 21, 2025. doi: 10.3748/wjg.v31.i39.111265
Revised: July 18, 2025
Accepted: September 11, 2025
Published online: October 21, 2025
Processing time: 117 Days and 7.4 Hours
Current diagnostic standards for post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) rely on 24-hour post-ERCP amylase and lipase levels, delaying timely intervention and highlighting the need for earlier predic
To evaluate the utility of 4-hour post-ERCP serum amylase and lipase levels in a large cohort to establish optimal cut-off values and improve early PEP prediction.
This prospective study involved patients with naïve major papillae who under
PEP occurred in 117 patients (6.1%). Diagnostic performance assessment of 4-hour serum amylase and lipase for predicting PEP yielded area under the curves of 0.877 and 0.893, respectively. Optimal cut-off values were 1.2 times the upper nor
Early measurement of 4-hour serum amylase and lipase shows strong predictive capabilities for PEP, with clinically meaningful cut-off values. These biomarkers enable timely interventions, potentially reducing PEP-related adverse events and the overall healthcare burden.
Core Tip: Early prediction of post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP) is crucial for timely intervention and reducing adverse outcomes. This large-scale prospective cohort study evaluated the predictive value of 4-hour post-endoscopic retrograde cholangiopancreatography serum amylase and lipase levels in patients with naïve major papillae. The findings demonstrated excellent diagnostic performance, with optimal cut-off values of 1.2 × and 8 × the upper normal limits for amylase and lipase, respectively. These early biomarkers showed high negative predictive values and were effective in identifying patients at risk of developing moderate to severe PEP. Incorporating 4-hour enzyme measurements into clinical practice may significantly improve risk stratification and enable earlier management of PEP.
- Citation: Jung JH, Lee KJ, Park SW, Park DH, Cha HW, Koh DH, Lee J. Early risk stratification of post-endoscopic retrograde cholangiopancreatography pancreatitis using 4-hour serum amylase and lipase: A prospective study. World J Gastroenterol 2025; 31(39): 111265
- URL: https://www.wjgnet.com/1007-9327/full/v31/i39/111265.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i39.111265
Endoscopic retrograde cholangiopancreatography (ERCP) is a widely used diagnostic and therapeutic procedure for biliary and pancreatic diseases. Despite its effectiveness, post-ERCP pancreatitis (PEP) is one of the most common adverse events (AEs), with incidence rates ranging from 1% to 40%[1,2]. This condition not only leads to increased morbidity but also prolongs hospitalization, adding to the economic burden on healthcare systems. PEP can vary in severity, ranging from mild, self-limiting cases to severe forms that may require intensive care and lead to AEs such as necrosis or organ failure[3]. Early identification of patients at risk of PEP is essential for implementing preventive measures and timely therapeutic interventions, such as aggressive hydration[4-6]. These strategies have been shown to mitigate the severity of PEP when initiated promptly.
The current diagnostic standard for PEP relies on 24-hour post-ERCP amylase and lipase levels combined with the presence of patient symptoms, such as abdominal pain consistent with acute pancreatitis[7]. However, waiting for these results delays clinical decision-making and timely management. Early prediction of PEP could enable prompt interven
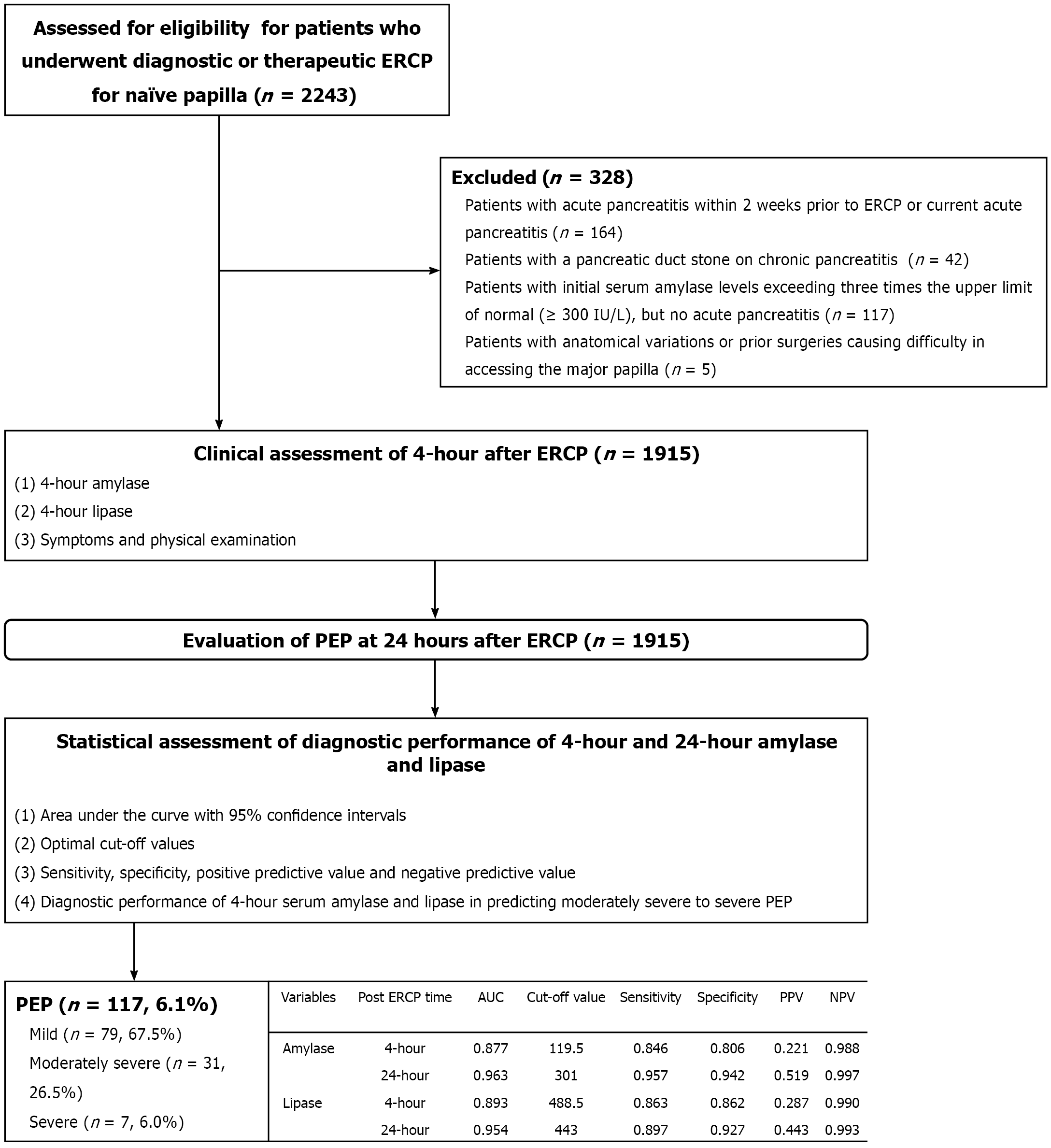
This study prospectively enrolled patients with naïve major papillae who underwent diagnostic or therapeutic ERCP at Hallym University Dongtan Heart Sacred Hospital between June 2021 and December 2024 (Figure 1). Patients were included if they underwent serum amylase and lipase level measurements before ERCP and 4-hour and 24-hour after ERCP. The exclusion criteria were as follows: (1) Prior diagnosis of chronic pancreatitis; (2) Recent acute pancreatitis within 2 weeks before ERCP or current acute pancreatitis, regardless of recurrence; (3) Initial serum amylase levels exceeding three times the upper normal limit (UNL; ≥ 300 IU/L); (4) Anatomical variations or prior surgeries causing difficulty in accessing the major papilla; and (5) Refusal to comply with the study protocols. Various parameters, including patient demographics, endoscopy-related findings, and clinical outcomes - such as the incidence of PEP - were prospectively collected for analysis. The study adhered to the ethical guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Hallym University Dongtan Sacred Hospital (Approval No. 2020-10-001-001). Informed consent was obtained from all patients prior to the procedure. This trial was registered in the International Clinical Trials Registry Platform (https://cris.nih.go.kr) under registration No. KCT0005950.
The primary endpoint was to determine optimal cut-off values for 4-hour serum amylase and lipase levels for the early prediction of PEP and assess their diagnostic performance. Secondary endpoints included evaluating the diagnostic accuracy of each cut-off value for 4-hour serum amylase and lipase levels for predicting PEP, with a focus on clinical applicability, as well as assessing the diagnostic performance of these biomarkers specifically in patients at high risk for PEP. The total procedure time was defined as the duration from the insertion to the withdrawal of the duodenoscope, while the cannulation time specifically referred to the interval from the proper alignment of the major papilla to the successful deep cannulation of the target duct[13]. All AEs were categorized and graded based on established consensus guidelines[14]. AEs were classified into the following categories: Pancreatitis, bleeding, perforation, post-ERCP cholecystitis, and sedation-related events. The traditional definition of PEP was applied if patients developed new-onset or aggravated abdominal pain consistent with acute pancreatitis and met at least one of the following criteria: Serum lipase or amylase levels elevated to at least three times the UNL or characteristic findings of acute pancreatitis on contrast-enhanced computed tomography approximately 24 hours after ERCP[7]. The normal range for serum amylase was defined as 0-100 IU/L, and that for serum lipase was 13-60 IU/L at our institution. The severity of PEP was classified as mild if hospitalization lasted 2-3 days, moderate if it lasted 4-10 days, and severe if hospitalization extended beyond 10 days or if AEs, such as hemorrhagic pancreatitis, pancreatic necrosis, pancreatic pseudocyst, or the need for percutaneous drainage or surgery, were present[7]. In this study, high-risk patients for PEP were defined by incorpora
All ERCP procedures were performed following a standardized protocol by experienced endoscopists with over 10 years of experience (Lee KJ, Park SW, Koh DH, and Lee J). Cannulation of the target duct was attempted using either a standard catheter or a pull-type sphincterotome, employing a wire-assisted technique. Contrast injection was allowed only after achieving successful selective deep cannulation of the target duct using a guidewire. In cases of difficult cannulation, rescue infundibulotomy was performed as an alternative technique. Notably, primary infundibulotomy with a needle-type knife was carried out directly, without prior attempts at cannulation, in patients with impacted stones at the major papilla or in cases with prominent papillae in which difficult cannulation was expected. Moreover, endoscopic papillary balloon dilation was utilized in patients with large bile duct stones or significant coagulopathy to minimize the risk of post-ERCP bleeding. Pancreatic stent placement was performed only in situations involving intentional or unintentional pancreatic duct cannulation, endoscopic papillectomy, or pancreatic sphincterotomy. Prophylactic rectal nonsteroidal anti-inflammatory drugs were not administered because they were unavailable in South Korea.
Continuous variables are expressed as means with SD, while categorical variables are presented as frequencies with proportions. Comparisons of continuous variables between groups were performed using Student’s t-test or the Mann-Whitney U test for non-normally distributed data. Categorical variables were compared using the χ2 test or Fisher’s exact test, as appropriate. Receiver operating characteristic curves were generated to evaluate the diagnostic performance of 4-hour and 24-hour serum amylase and lipase levels for predicting PEP. The area under the curve (AUC) with 95% confidence intervals (CIs) was calculated to assess the discriminatory ability of each biomarker. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were determined at optimal cut-off values identified using the Youden index (sensitivity + specificity-1). Pairwise comparisons of AUCs for 4-hour and 24-hour biomarkers were performed using the DeLong test to assess statistical significance. Subgroup analyses were conducted to evaluate the diagnostic performance of 4-hour serum amylase and lipase in predicting moderately severe to severe PEP and for assessing high-risk patient groups based on defined clinical characteristics. Statistical analyses were conducted using SPSS version 29.0 (International Business Machines Corporation, NY, United States), or the R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a two-tailed P < 0.05. Sensitivity analyses were performed to validate the robustness of the findings, including assessments stratified by procedural complexity and patient comorbidities.
The study included a total of 1915 patients, with a mean age of 64.1 ± 17.5 years (Table 1). Male patients comprised 55.8% of the cohort, while 44.2% were female. The mean body mass index was 24.1 ± 4.0 kg/m2. Among the cohort, 17.2% reported a history of smoking, and 25.8% reported alcohol consumption, with a mean alcohol intake of 3.1 ± 4.4 g. A history of acute pancreatitis was documented in 2.6% of the patients. Initial laboratory findings showed elevated liver enzyme levels, with mean aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels of 195.7 ± 334.5 IU/L and 185.1 ± 223.2 IU/L, respectively. Gamma-glutamyl transferase (GGT) levels were markedly elevated (423.3 ± 441.7 IU/L), and the mean total bilirubin level was 3.5 ± 5.0 mg/dL. Baseline pancreatic enzyme levels were within normal ranges, with mean amylase and lipase levels of 63.7 ± 41.3 IU/L and 61.1 ± 107.9 IU/L, respectively. Acute cholangitis was present in 42.1% of patients upon admission. Prior to ERCP, 0.9% of patients underwent percutaneous transhepatic biliary drainage, and 2.6% underwent percutaneous transhepatic gallbladder drainage. Regarding procedural findings, the mean total procedure time was 15.5 ± 10.1 minutes, and the mean selective deep cannulation time was 4.7 ± 5.8 minutes. Primary infundibulotomy was performed in 14.4% of patients. Endoscopic interventions included endoscopic papillary balloon dilation in 16.4% of patients, endoscopic sphincterotomy in 89.3%, biliary stent placement in 67.8%, and pancreatic stent placement in 19.8%.
| Variable | Total patients (n = 1915) |
| Age, years | 64.1 ± 17.5 |
| Sex | |
| Male | 1068 (55.8) |
| Female | 847 (44.2) |
| BMI, kg/m2 | 24.1 ± 4.0 |
| Smoking | 330 (17.2) |
| Alcohol intake | 494 (25.8) |
| Amounts of alcohol (g) | 3.1 ± 4.4 |
| History of acute pancreatitis | 49 (2.6) |
| Initial laboratory findings | |
| WBC, μL | 8536.1 ± 4542.9 |
| Hb, g/dL | 12.6 ± 2.0 |
| Platelet, μL | 225.0 ± 90.2 |
| AST, IU/L | 195.7 ± 334.5 |
| ALT, IU/L | 185.1 ± 223.2 |
| GGT, g/dL | 423.3 ± 441.7 |
| Total bilirubin, mg/dL | 3.5 ± 5.0 |
| Amylase, IU/L | 63.7 ± 41.3 |
| Lipase, IU/L | 61.1 ± 107.9 |
| Acute cholangitis on admission | 807 (42.1) |
| PTBD prior to ERCP | 18 (0.9) |
| PTGBD prior to ERCP | 50 (2.6) |
| Total procedure time, minute | 15.5 ± 10.1 |
| Selective deep cannulation time, minute | 4.7 ± 5.8 |
| Primary infundibulotomy | 275 (14.4) |
| EPBD | 314 (16.4) |
| Endoscopic sphincterotomy | 1710 (89.3) |
| Biliary stent placement | 1298 (67.8) |
| Pancreatic stent placement | 380 (19.8) |
Laboratory findings 4 hours and 24 hours after ERCP showed changes in liver and pancreatic enzyme levels (Table 2). At 4 hours after ERCP, the mean AST and ALT levels were 154 ± 265.5 IU/L and 184.1 ± 214.7 IU/L, respectively. GGT levels were elevated (408.2 ± 398.0 IU/L), and the mean total bilirubin level was 3.4 ± 4.7 mg/dL. Regarding pancreatic enzyme levels at 4 hours, the mean amylase and lipase levels were 131.1 ± 259.3 IU/L and 261.3 ± 894.7 IU/L, respectively. By 24 hours after ERCP, levels of AST, ALT, GGT, and total bilirubin decreased to 114.8 ± 232.2 IU/L, 161.3 ± 188.4 IU/L, 379.8 ± 356.2 IU/L, and 2.9 ± 4.0 mg/dL, respectively. Pancreatic enzyme levels at 24 hours were as follows: 160.4 ± 296.9 IU/L for amylase and 258.7 ± 683.5 IU/L for lipase. PEP occurred in 117 patients, accounting for 6.1% of the total study. Among these, the majority (67.5%) experienced mild PEP, while 26.5% had moderately severe PEP, and 6.0% had severe PEP. Hyperamylasemia was observed in 218 patients (11.4%). Procedure-related AEs were rare, with perforation occurring in 4 patients (0.2%), significant bleeding in 51 patients (2.7%), and post-ERCP cholecystitis in 21 patients (1.1%). The mean length of hospitalization was 8.4 ± 31.6 days. All-cause mortality within 30 days after ERCP was reported for 20 patients (1.9%).
| Variable | Total patients (n = 1915) |
| Laboratory findings at 4 hours after ERCP | |
| AST, IU/L | 154 ± 265.5 |
| ALT, IU/L | 184.1 ± 214.7 |
| GGT, g/dL | 408.2 ± 398.0 |
| Total bilirubin, mg/dL | 3.4 ± 4.7 |
| Amylase, IU/L | 131.1 ± 259.3 |
| Lipase, IU/L | 261.3 ± 894.7 |
| Laboratory findings at 24 hours after ERCP | |
| AST, IU/L | 114.8 ± 232.2 |
| ALT, IU/L | 161.3 ± 188.4 |
| GGT, g/dL | 379.8 ± 356.2 |
| Total bilirubin, mg/dL | 2.9 ± 4.0 |
| Amylase, IU/L | 160.4 ± 296.9 |
| Lipase, IU/L | 258.7 ± 683.5 |
| PEP | 117 (6.1) |
| Severity of PEP | |
| Mild | 79 (67.5) |
| Moderately severe | 31 (26.5) |
| Severe | 7 (6.0) |
| Hyperamylasemia | 218 (11.4) |
| Procedure-related adverse events | |
| Perforation | 4 (0.2) |
| Significant bleeding | 51 (2.7) |
| Post-ERCP cholecystitis | 21 (1.1) |
| Length of hospitalization, days | 8.4 ± 31.6 |
| All-cause mortality within 30 days | 20 (1.9) |
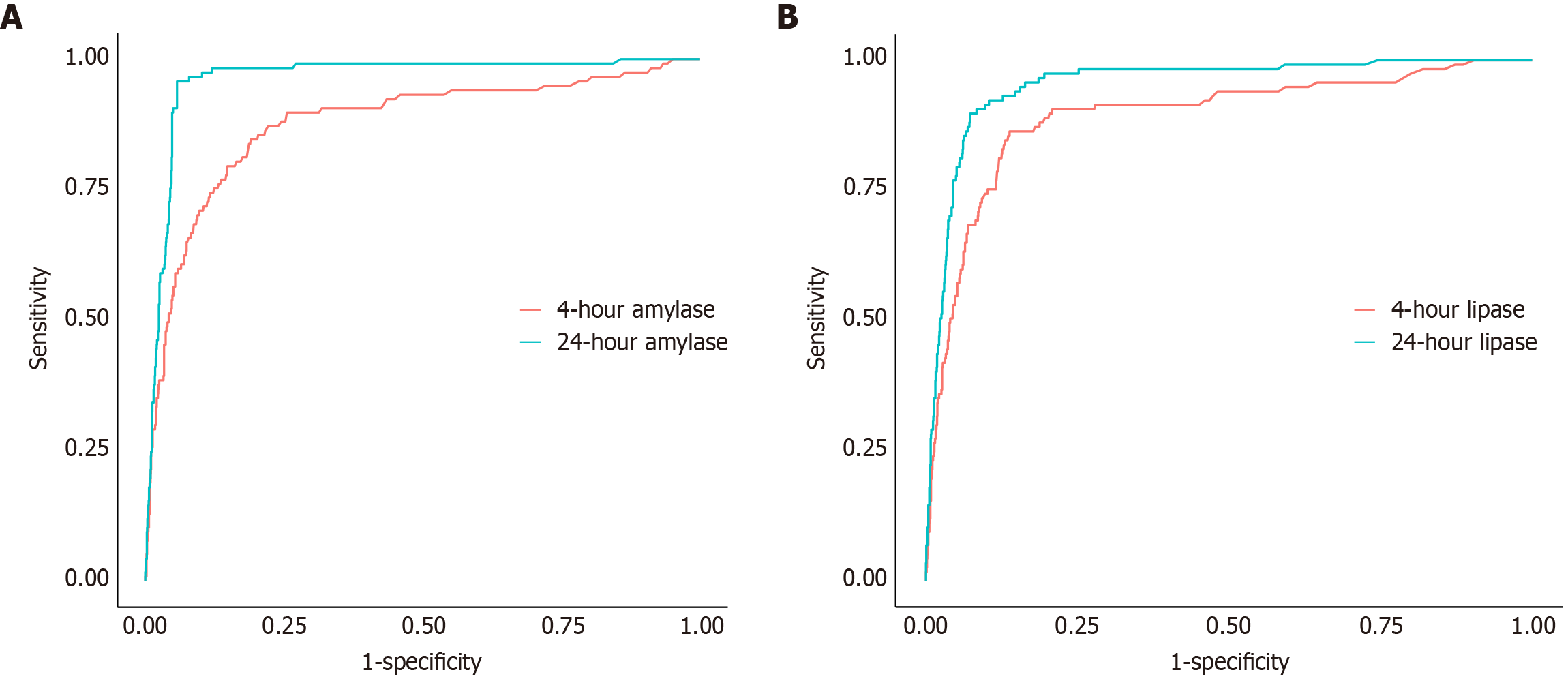
The diagnostic accuracy of serum amylase and lipase levels at 4-hour and 24-hour after ERCP for predicting PEP was evaluated using receiver operating characteristic curves (Table 3). For 4-hour amylase levels, the AUC was 0.877, indicating strong diagnostic performance, with a cut-off value of 119.5 IU/L (Figure 2A). At this threshold, the sensitivity was 0.846, and the specificity was 0.806, with a PPV of 0.221 and an NPV of 0.988. Among the cohort, 18 patients (1.6%) were classified as false negatives, while 342 patients (17.9%) were classified as false positives based on this cut-off value (Supplementary Table 1). In comparison, 24-hour amylase levels demonstrated superior performance, with an AUC of 0.963 and a cut-off value of 301 IU/L, achieving a sensitivity of 0.957, specificity of 0.942, PPV of 0.519, and NPV of 0.997. The difference in AUCs between 4-hour and 24-hour amylase levels was statistically significant (ΔAUC = -0.087; 95%CI: -0.124 to -0.049; P < 0.001). For 4-hour lipase levels, the AUC was 0.893, with a cut-off value of 488.5 IU/L (Figure 2B). This marker showed a sensitivity of 0.863, specificity of 0.862, PPV of 0.287, and NPV of 0.990. At 24-hour, lipase levels exhibited an AUC of 0.954, with a cut-off value of 443 IU/L, yielding a sensitivity of 0.897, specificity of 0.927, PPV of 0.443, and NPV of 0.993. The difference in AUCs for 4-hour and 24-hour lipase levels was also significant (ΔAUC = -0.061; 95%CI: -0.095 to -0.027; P < 0.001).
| Variables | Time point | AUC | Cut-off (IU/L) | Sensitivity | Specificity | PPV | NPV | AUC difference (4 hours-24 hours, 95%CI) | P value |
| Amylase | 4-hour | 0.877 | 119.5 | 0.846 | 0.806 | 0.221 | 0.988 | -0.087 (-0.124 to -0.049) | < 0.001 |
| 24-hour | 0.963 | 301 | 0.957 | 0.942 | 0.519 | 0.997 | |||
| Lipase | 4-hour | 0.893 | 488.5 | 0.863 | 0.862 | 0.287 | 0.990 | -0.061 (-0.095 to -0.027) | < 0.001 |
| 24-hour | 0.954 | 443 | 0.897 | 0.927 | 0.443 | 0.993 |
The cut-off values for 4-hour amylase and lipase levels were further analyzed in terms of their multiples of the UNL (Table 4). For amylase, a cut-off at 1.2 × UNL achieved the highest sensitivity (0.846), while a cut-off at 4.0 × UNL provided the highest specificity (0.971). For lipase, a cut-off at 2.0 × UNL demonstrated the highest sensitivity (0.906), and a cut-off at 8.0 × UNL had the highest specificity (0.927) while maintaining strong diagnostic performance with an NPV of 0.993.
| Variables | Cut-off value | Sensitivity | Specificity | PPV | NPV |
| Amylase | 1.2 × UNL | 0.846 | 0.806 | 0.221 | 0.988 |
| 2.0 × UNL | 0.684 | 0.911 | 0.333 | 0.978 | |
| 3.0 × UNL | 0.513 | 0.956 | 0.429 | 0.968 | |
| 4.0 × UNL | 0.385 | 0.971 | 0.464 | 0.96 | |
| Lipase | 2.0 × UNL | 0.906 | 0.774 | 0.207 | 0.992 |
| 3.0 × UNL | 0.863 | 0.853 | 0.276 | 0.99 | |
| 4.0 × UNL | 0.752 | 0.886 | 0.3 | 0.982 | |
| 8.0 × UNL | 0.897 | 0.927 | 0.443 | 0.993 |
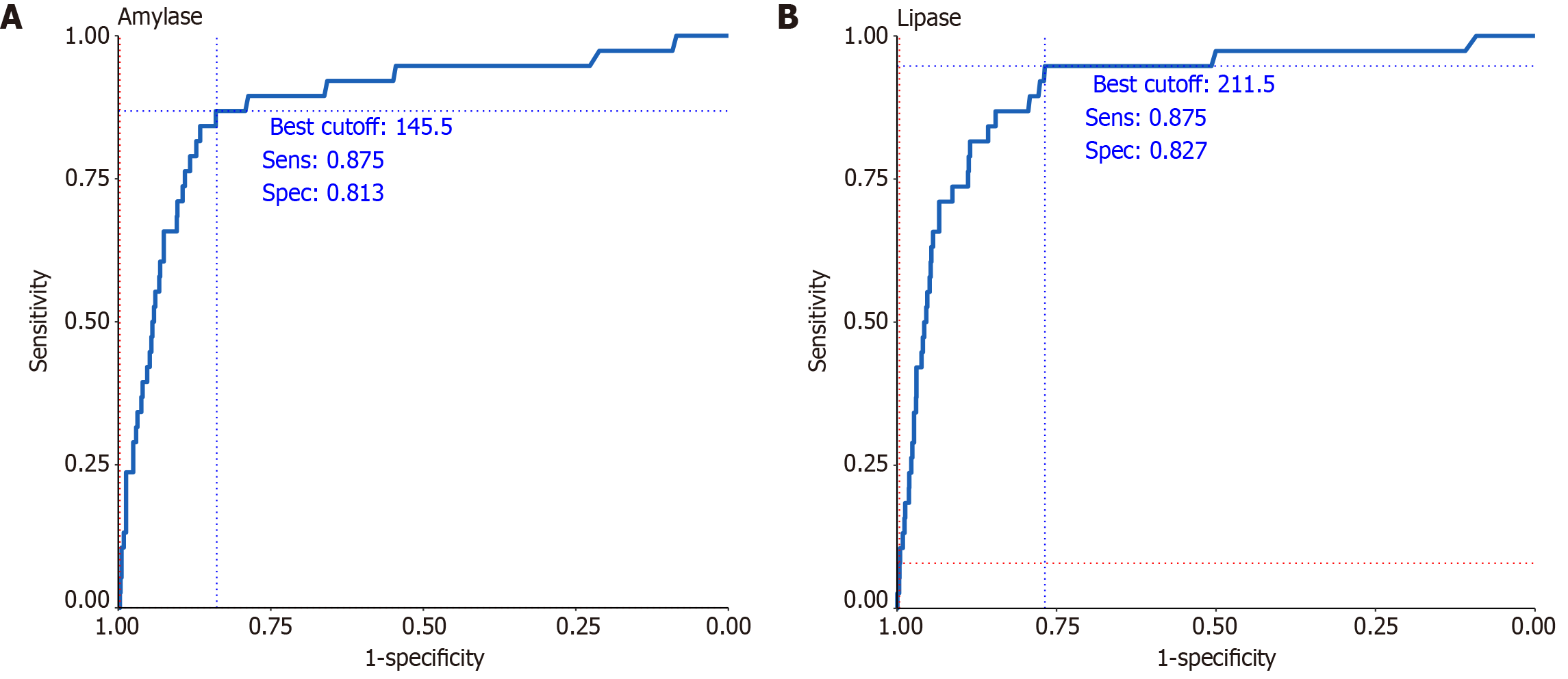
Among patients classified as high-risk based on combined patient and procedural characteristics, 4-hour serum amylase levels demonstrated excellent diagnostic accuracy, with an AUC of 0.881 at an optimal threshold of 145.5 IU/L (Figure 3A). At this cut-off, the sensitivity and specificity were 87.5% and 81.3%, respectively, with a PPV of 9.8% and an NPV of 99.7%. Similarly, 4-hour serum lipase levels yielded an AUC of 0.905 at a cut-off of 211.5 IU/L, with a sensitivity of 87.5%, specificity of 82.7%, PPV of 10.2%, and NPV of 99.7% (Figure 3B).
This study evaluated the diagnostic performance of 4-hour post-ERCP serum amylase and lipase levels as early predictive biomarkers for PEP in a large cohort of patients. Both biomarkers exhibited strong diagnostic capabilities, with amylase and lipase achieving AUCs of 0.877 and 0.893, respectively, for predicting PEP 4-hour after the procedure. Clinically meaningful cut-off values were identified, such as 1.2 times the UNL for 4-hour amylase and 8 times the UNL for 4-hour lipase levels, yielding NPVs of 0.988 and 0.990, respectively. These findings underscore the utility of early biomarker measurements for safely ruling out PEP, enabling timely clinical decision-making, such as initiating massive fluid therapy, facilitating same-day discharge for low-risk patients, and starting an oral diet after ERCP. Additionally, this study is among the first to evaluate the predictive performance of these biomarkers for moderately severe to severe PEP in high-risk patient groups, offering critical insights into their application for personalized risk stratification and proactive management. By facilitating the early identification of patients at risk, the findings of this study could improve clinical outcomes, reduce AEs, and optimize interventions associated with PEP.
The early prediction of PEP using 4-hour serum amylase and lipase levels holds significant clinical implications. Endoscopic instrumentation during ERCP - particularly in cases involving pancreatic duct manipulation, high-pressure injection, or difficult cannulation - can precipitate early ductal injury and acinar disruption[9,16]. This results in enzyme leakage into the systemic circulation within 2 hours to 4 hours, explaining the diagnostic utility of early post-ERCP enzyme levels. This early injury promotes intraductal hypertension and zymogen activation, initiating the inflammatory cascade that characterizes PEP. Timely identification of at-risk patients facilitates the early initiation of appropriate fluid therapy, a critical measure for mitigating severe PEP[4-6]. Additionally, same-day discharge for low-risk patients and earlier resumption of oral intake could streamline patient management and reduce hospitalization durations[9,10,12]. The cut-off values established in this study (1.2 × UNL for amylase and 8 × UNL for lipase) align with findings from previous studies that have reported the utility of similar thresholds for predicting PEP[9-12,16]. For instance, a study by Testoni et al[9] evaluated 409 patients who underwent ERCP, measuring serum amylase levels 2 hours, 4 hours, 8 hours, and 24 hours after the procedure. The study suggested that a cut-off value of five times the UNL for 4-hour serum amylase levels is a reliable predictor of PEP, demonstrating strong diagnostic performance. Sutton et al[11] conducted a study involving 886 ERCP procedures, reporting a PEP incidence of 4.4%. The study recommended that patients who underwent pancreatography be admitted if their 4-hour serum amylase levels exceeded 2.5 times the upper limit of the reference range, as this threshold reliably predicted the development of PEP. Moreover, a Korean study involving a cohort of 516 patients highlighted the utility of early amylase measurements, with 16 patients (3.1%) diagnosed with PEP[12]. The study found that an amylase level greater than 1.5 × the UNL was useful for PEP exclusion, demonstrating a sensitivity of 93.8%, while a threshold of 4 × UNL was recommended for guiding preventive therapy, achieving a specificity of 93.2%. The present study comprehensively addresses the shortcoming of these previous studies by including a larger sample size and analyzing high-risk subgroups.
A notable consideration in the early prediction of PEP is the occurrence of asymptomatic hyperamylasemia. While hyperamylasemia is often observed after ERCP, it does not necessarily indicate PEP unless accompanied by abdominal pain consistent with pancreatitis. In this study, hyperamylasemia was observed in 11.4%, yet only 6.1% of patients were diagnosed with PEP. This emphasizes the importance of correlating amylase and lipase levels with clinical symptoms, as isolated hyperamylasemia without symptoms does not warrant intervention or prolonged hospitalization. Furthermore, the routine use of imaging, such as computed tomography, for confirming PEP is generally unnecessary unless warranted by diagnostic uncertainty or severe symptoms. Using the 4-hour amylase cut-off value of 119.5 IU/L, this study revealed a low false negative rate of only 1.6%, indicating strong reliability in ruling out PEP. However, the false positive rate was relatively high, at 17.9%, corresponding to 342 patients who may have been classified as at risk despite not developing PEP. Although this was a prospective study, early hydration was not protocolized or implemented according to bio
In clinical practice, concern regarding PEP is particularly high in high-risk patients following ERCP, emphasizing the need for reliable early predictive tools. This study highlights the strong predictive performance of 4-hour serum amylase and lipase levels for identifying moderately severe to severe PEP in high-risk patients. In this subgroup, 4-hour serum amylase levels demonstrated an AUC of 0.881, with a sensitivity of 0.875 and a specificity of 0.813 at a cut-off value of 145.5 IU/L. Similarly, 4-hour serum lipase levels showed an AUC of 0.905, with a sensitivity of 0.875 and a specificity of 0.827 at a cut-off value of 211.5 IU/L. When the cut-off values were adjusted to 1.5 × the UNL for amylase and 3.5 × the UNL for lipase, the predictive performance for moderately severe and severe pancreatitis improved significantly, enhancing the clinical utility of these biomarkers. These findings provide a solid basis for early risk stratification and tailored management strategies in high-risk populations. Importantly, the application of these cut-off values in high-risk patients enables clinicians to initiate aggressive hydration and monitor patients more closely, potentially reducing the severity of PEP and improving outcomes.
This study has several strengths, including its large sample size of 1915 patients, which enhances the generalizability of the findings. The prospective design and comprehensive data collection further strengthen the reliability of the results. Additionally, efforts were made to minimize bias by excluding patients with current pancreatitis or those with baseline serum amylase levels exceeding three times the UNL (≥ 300 IU/L), ensuring a more homogenous study population. These exclusion criteria enabled an accurate evaluation of the diagnostic performance of 4-hour biomarkers in predicting PEP, unaffected by confounding factors, such as pre-existing pancreatic inflammation.
However, several limitations should be acknowledged. First, as this was a single-center study conducted at a tertiary institution in Korea, the findings may not fully capture interinstitutional or international variations in ERCP techniques, patient characteristics, or clinical practices. Notably, the absence of prophylactic rectal nonsteroidal anti-inflammatory drugs - due to their unavailability in South Korea - may limit the applicability of our results to regions in which such interventions are standard practice. Furthermore, excluding patients with chronic pancreatitis or anatomical alterations of the ampulla or duodenum may reduce the generalizability of the findings to broader and more heterogeneous clinical settings. Finally, this study did not evaluate the clinical impact of early interventions guided by 4-hour biomarker levels. As such, the utility of these markers in directing real-time clinical decisions - such as early fluid resuscitation or discharge planning - remains hypothetical and should be evaluated in future prospective interventional trials.
This study demonstrates that 4-hour post-ERCP serum amylase and lipase levels are reliable early predictors of PEP, offering clinically meaningful cut-off values for timely intervention. These biomarkers enable the identification of patients at risk, allowing for personalized management strategies that can reduce AEs, improve clinical outcomes, and optimize healthcare resource utilization. While our findings support the high predictive value of 4-hour enzyme levels for ruling out PEP, their use in guiding clinical decisions such as early discharge or preventive hydration requires prospective validation through interventional trials.
| 1. | Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, Huang Q, Zhang X, He LP, Sun WS, Zhao Q, Shi RH, Tian ZB, Li YQ, Li W, Zhi FC. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009;104:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 341] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 2. | Pekgöz M. Post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review for prevention and treatment. World J Gastroenterol. 2019;25:4019-4042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, Yadlapati RH, Gardner TB. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. 2024;119:419-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 205] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 4. | Park CH, Paik WH, Park ET, Shim CS, Lee TY, Kang C, Noh MH, Yi SY, Lee JK, Hyun JJ, Lee JK. Aggressive intravenous hydration with lactated Ringer's solution for prevention of post-ERCP pancreatitis: a prospective randomized multicenter clinical trial. Endoscopy. 2018;50:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Cho E, Kim SH, Park CH, Yoon JH, Lee SO, Kim TH, Chon HK. Tailored Hydration With Lactated Ringer's Solution for Postendoscopic Retrograde Cholangiopancreatography Pancreatitis Prevention: A Randomized Controlled Trial. Am J Gastroenterol. 2024;119:2426-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Wu M, Jiang S, Lu X, Zhong Y, Song Y, Fan Z, Kang X. Aggressive hydration with lactated ringer solution in prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e25598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2086] [Article Influence: 59.6] [Reference Citation Analysis (2)] |
| 8. | Park CH, Park SW, Lee KJ, Park DH, Cha H, Choi A, Koh DH, Lee J, Cho E. Prospective validation and revision of predictive models for post-ERCP pancreatitis: focus on procedure-related factors and a novel risk stratification approach. Surg Endosc. 2025;39:1207-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Testoni PA, Bagnolo F, Caporuscio S, Lella F. Serum amylase measured four hours after endoscopic sphincterotomy is a reliable predictor of postprocedure pancreatitis. Am J Gastroenterol. 1999;94:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Thomas PR, Sengupta S. Prediction of pancreatitis following endoscopic retrograde cholangiopancreatography by the 4-h post procedure amylase level. J Gastroenterol Hepatol. 2001;16:923-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Sutton VR, Hong MK, Thomas PR. Using the 4-hour Post-ERCP amylase level to predict post-ERCP pancreatitis. JOP. 2011;12:372-376. [PubMed] |
| 12. | Lee YK, Yang MJ, Kim SS, Noh CK, Cho HJ, Lim SG, Hwang JC, Yoo BM, Kim JH. Prediction of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis Using 4-Hour Post-Endoscopic Retrograde Cholangiopancreatography Serum Amylase and Lipase Levels. J Korean Med Sci. 2017;32:1814-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Park CH, Jung JH, Hyun B, Kan HJ, Lee J, Kae SH, Jang HJ, Koh DH, Choi MH, Chung MJ, Bang S, Park SW. Safety and efficacy of early feeding based on clinical assessment at 4 hours after ERCP: a prospective randomized controlled trial. Gastrointest Endosc. 2018;87:1040-1049.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 2017] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 15. | Matsubayashi H, Fukutomi A, Kanemoto H, Maeda A, Matsunaga K, Uesaka K, Otake Y, Hasuike N, Yamaguchi Y, Ikehara H, Takizawa K, Yamazaki K, Ono H. Risk of pancreatitis after endoscopic retrograde cholangiopancreatography and endoscopic biliary drainage. HPB (Oxford). 2009;11:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Testoni PA, Caporuscio S, Bagnolo F, Lella F. Twenty-four-hour serum amylase predicting pancreatic reaction after endoscopic sphincterotomy. Endoscopy. 1999;31:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/