Published online Oct 21, 2025. doi: 10.3748/wjg.v31.i39.111261
Revised: July 25, 2025
Accepted: September 15, 2025
Published online: October 21, 2025
Processing time: 117 Days and 11 Hours
Colonization with multidrug-resistant organisms (MDROs) is frequently observed in critically ill patients with liver cirrhosis admitted to intensive care units (ICUs). However, whether colonization directly leads to infections or adversely impacts clinical outcomes remains unclear. Clarifying this relationship may help deter
To evaluate the clinical relevance of MDRO colonization and infection at ICU admission in patients with cirrhosis.
This retrospective single-center cohort study included 107 ICU admissions of patients with liver cirrhosis at a tertiary care center (2018-2024). Colonization was assessed by rectal and nasal/pharyngeal swabs within 48 hours of ICU admission. Outcomes analyzed included MDRO infection during ICU stay, concordance between colonizing and infecting strains, organ support re
Nearly one-third (29.9%) of patients were colonized with MDROs on admission, more commonly in the acute-on-chronic liver failure phenotype than those with acute decompensation (34.5 vs 10.0%, P = 0.033). Although infections were established in the majority (85%) of cases, of which 17.6% due to MDROs, colonization alone did not independently predict these infections [odds ratio (OR) = 2.18, P = 0.383] nor influenced short-term mortality (OR = 1.14, P = 0.813). However, once MDRO infection occurred, an 82% concordance was observed between colonizing and infecting strains. MDRO infections, unlike colonization, significantly increased the need for organ-support interventions, including mechanical ventilation and vasopressor therapy and prolonged ICU stays. Only severity of organ dysfunction, quantified by the Sequential Organ Failure Assessment score, independently predicted 28-day mortality (OR = 1.38, P = 0.024).
MDRO colonization at ICU admission is frequent among critically ill patients with cirrhosis, particularly those with acute-on-chronic liver failure. While colonization alone does not predict infection or early mortality, its clinical value emerges in guiding empirical antibiotic treatment once infection is suspected. Ultimately, short-term survival appears to be more strongly influenced by the severity of organ failure than by either MDRO colonization or infection.
Core Tip: This retrospective intensive care unit cohort study evaluated the impact of multidrug-resistant colonization in patients with liver cirrhosis. While colonization was common, especially in those with acute-on-chronic liver failure, it was not independently linked to infection or short-term mortality. However, high concordance between colonizing and infecting strains supports its role in guiding empirical antibiotic therapy once infection is suspected. These findings highlight the importance of early screening, local resistance data, and tailored empirical treatment to improve outcomes of critically ill patients with cirrhosis.
- Citation: Kosuta I, Babel J, Domislovic V, Susak F, Peretin L, Varda Brkic D, Marekovic I, Radonic R, Mrzljak A. Clinical impact of multidrug-resistant organisms in liver cirrhosis: A retrospective cohort study in the intensive care setting. World J Gastroenterol 2025; 31(39): 111261
- URL: https://www.wjgnet.com/1007-9327/full/v31/i39/111261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i39.111261
Bacterial infections are a frequent and serious complication in patients with cirrhosis, often triggering clinical deterioration and progression to organ failure[1-3]. These infections remain the predominant trigger of acute decompensation (AD) and acute-on-chronic liver failure (ACLF), particularly among patients admitted to intensive care units (ICU)[1,2,4]. Prompt recognition and antibiotic administration are critical, as even short delays can worsen clinical outcomes and increase mortality risk[5,6]. However, the rising prevalence of multidrug-resistant organisms (MDROs) significantly complicates effective empirical therapy and poses substantial clinical challenges in these critically ill patients[1,4,7-9]. Recent global studies report an increasing burden of MDRO infections in patients with cirrhosis, with an overall prevalence around 34% among hospitalized individuals and similar or slightly higher rates (23%-47%) in ICU settings[1,10]. Over the last two decades, there has been a notable shift toward more healthcare-associated infections, driven by recurrent hospitalizations and antibiotic exposure, along with evolving pathogen profiles characterized by increased detection of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE), particularly in Western countries[1,10,11]. The rising prevalence and changing epidemiology pose substantial challenges to ICU management and liver transplantation, including increased risk of treatment failure, organ dysfunction, transplant delisting, graft loss, and mortality[1,3,10-13].
Colonization with MDROs, particularly at rectal and nasal sites, is prevalent among critically ill patients with cirrhosis, with reported rates between 32% and 47%[14,15]. Colonization strongly predicts subsequent infection, with studies consistently reporting a concordance of 76%-82% between colonizing and infecting strains[14,16,17]. Risk factors include prior antibiotic exposure, overuse of prophylactic antibiotics, and repeated healthcare contact[18-20]. Beyond traditional risk factors, cirrhosis-associated immune dysfunction may could facilitate MDRO colonization and infection. Cirrhosis-associated immune dysfunction involves systemic inflammation alongside impaired innate and adaptive immunity, worsened by gut dysbiosis, increased intestinal permeability, and defective phagocytic function, all promoting bacterial translocation and colonization[6,20-23]. Additionally, patients with ACLF display immune profiles similar to those seen in sepsis, including decreased monocyte human leukocyte antigen-D related expression and impaired tumor necrosis factor-α production, consistent with a state of immune paralysis[24].
Notably, the prevalence and spectrum of colonizing organisms vary substantially across regions[25]. While oxacillinase (OXA)-48-producing Klebsiella pneumoniae (K. pneumoniae) and Acinetobacter baumannii (A. baumanii) predominate in some centers, others report higher rates of vancomycin-resistant enterococci or extended-spectrum beta-lactamases (ESBL)-producing Escherichia coli (E. coli)[6,15,26]. These epidemiological variations emphasize the need for robust local microbiological surveillance to guide empiric therapy and infection control. MDRO infections are associated with poorer out
Given the existing controversy about the prognostic implications of MDRO colonization in critically ill patients with cirrhosis, this study aims to clarify whether colonization at ICU admission independently predicts subsequent MDRO infection or influences short-term survival[15,16,28]. By contributing region-specific data from Southeastern Europe, a region underrepresented in prior research, we intend to address a critical gap regarding local MDRO epidemiology and clinical outcomes. We hypothesized that MDRO colonization upon ICU admission is associated with higher rates of subsequent infection and adverse clinical outcomes. Our primary objective was: To determine whether colonizing MDRO strains identified at ICU admission were concordant with subsequent infecting pathogens. Our secondary objectives were: (1) To describe the local epidemiology of MDRO colonization and infection in critically ill cirrhotic patients within our ICU setting; (2) To compare colonization and infection patterns between patients presenting with AD and ACLF phenotypes; and (3) To evaluate the clinical impact of MDRO colonization and infection, specifically regarding short-term mortality and organ support requirements. Integrating colonization data into clinical decision-making may help refine risk stratification, support appropriate empiric antibiotic selection, and enable earlier intervention in this high-risk population[14,29].
This single-center, retrospective cohort study was conducted at the University Hospital Centre Zagreb, Croatia. The study included critically ill patients with liver cirrhosis admitted to the medical ICU between January 1, 2018 and December 31, 2024.
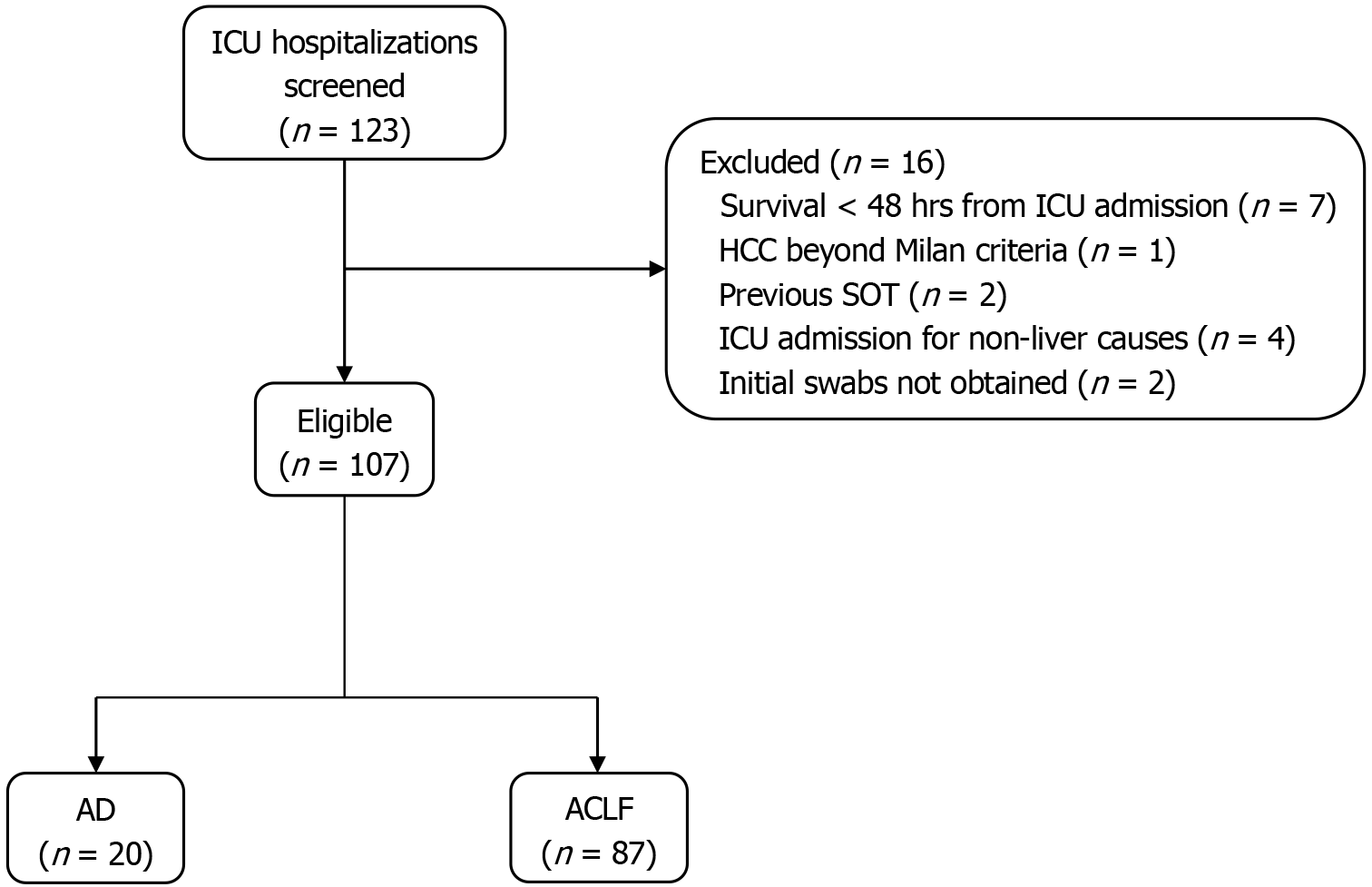
Eligible participants (Figure 1) were adults with established liver cirrhosis who had an ICU stay of at least 48 hours due to liver-related complications. Exclusion criteria included prior solid organ transplantation/cirrhosis of the liver graft, advanced hepatocellular carcinoma (beyond Milan criteria), or ICU admission unrelated to liver disease (e.g., myocardial infarction). For patients with multiple ICU admissions, only admissions separated by more than two months were included as distinct events.
Data were extracted from the electronic medical records and included demographic variables, liver disease aetiology, and comorbidities (diabetes, cardiovascular, respiratory, renal diseases). Pre-admission variables included prior hospitalization, antibiotic exposure, and medication use (e.g., norfloxacin prophylaxis, non-selective beta-blockers, proton pump inhibitors). Clinical and laboratory data at ICU admission included vital signs, need for organ support (mechanical ventilation, vasopressors, renal replacement therapy), and laboratory parameters [white blood cell count (WBC), platelets, bilirubin, creatinine, sodium, albumin, international normalized ratio, liver enzymes, C-reactive protein, procalcitonin]. Disease severity was assessed using Model for End-Stage Liver Disease (MELD), MELD-Na, Sequential Organ Failure Assessment (SOFA), chronic liver failure (CLIF)-organ failure, and CLIF-ACLF/CLIF-AD, as appropriate.
Routine surveillance for MDRO colonization was preformed via nasal/pharyngeal and rectal swabs within 48 hours of ICU admission. In contrast, infections were identified throughout the ICU stay, based on clinical suspicion and confirmed by targeted cultures obtained during ICU hospitalization. Outcomes included ICU and hospital length of stay, organ support requirements, and 28-day transplant-free survival, ICU survival, and overall hospital survival.
Cirrhosis was diagnosed based on histological findings or a combination of clinical indicators, such as variceal bleeding, ascites, or hepatic encephalopathy, along with imaging and laboratory results supportive of the diagnosis. Organ failures and ACLF were defined according to CLIF Consortium criteria[30]. MDRO colonization was defined as a positive scree
The distinction between colonization and infection was based on clinical presentation and microbiological findings. Infection was diagnosed when patients exhibited signs or symptoms consistent with infection (e.g., fever, hypotension, elevated inflammatory markers, leukocytosis) together with positive cultures from typically sterile or clinically relevant sites. Infections were further categorized according to antimicrobial susceptibility into those caused my MDROs and dose caused by susceptible organisms. Colonization was defined by the presence of MDROs in screening cultures (e.g., rectal, nasopharyngeal swabs) without accompanying clinical or laboratory evidence of active infection at the time of sampling. Patients who met criteria for both, i.e., had clinical signs of infection and MDROs identified in both screening and diagnostic cultures, were classified as both colonized and infected.
Infections were defined as follows: (1) Urinary tract infection required the presence of > 10 Leukocytes per high-power field in urinary sediment and positive urine culture, or uncountable leukocytes per field with negative culture; (2) Pneumonia or upper respiratory tract infection required clinical signs of infection with new infiltrates on chest radiography, or clinical signs of infection and a positive sputum or bronchoalveolar lavage culture; (3) Hepatic or intra-abdominal infection was defined by clinical signs of infection with radiological evidence of infected non-solid intra-abdominal or hepatic collections; (4) Surgical wound or soft tissue infection was identified by clinical signs including swelling, erythema, warmth, and tenderness; (5) Bacteremia was defined by the presence of viable bacteria in ≥ set of blood cultures; (6) Cholangitis required cholestasis, right upper quadrant pain and/or jaundice, and radiological evidence of biliary obstruction; (7) Clostridioides infection required compatible clinical symptoms (diarrhoea) and a positive nucleic acid amplification test or stool culture; (8) Spontaneous bacterial peritonitis was defined as bacterial infection of ascitic fluid in the absence of any surgically correctable intra-abdominal infection source, and an ascitic neutrophil count of ≥ 250/mm3; and (9) Unproven bacterial infection was defined by fever ≥ 38 °C and leucocytosis (WBC ≥ 12000/mm3), initiating antibiotic therapy without an identified source.
No targeted decontamination interventions (e.g., selective digestive tract contamination, topical chlorhexidine bathing) were performed based on colonization results. Colonized patients were managed with standard institutional infection-control precautions, including isolation or cohorting and strict adherence to hand hygiene and contact precautions. Empiric antibiotic therapy was guided by clinical suspicion, severity of illness and clinical judgement of treating physicians.
All analyses were conducted using IBM SPSS statistics (version 29.0.0.0). Descriptive statistics were reported as medians (interquartile range) or frequencies (%). Group comparisons used χ2 or Fisher’s exact tests for categorical variables, and t-tests or Mann-Whitney U tests for continuous variables based on Shapiro-Wilk normality testing.
Two multivariable logistic regression models evaluated whether MDRO colonization predicted: (1) Subsequent infection; and (2) 28-day transplant-free mortality. Both models adjusted for MELD-Na, SOFA, CLIF-ACLF scores, age and sex. MELD was excluded due to collinearity with MELD-Na. Covariates were selected a priori based on their known clinical relevance and prognostic significance in critically ill patients with cirrhosis. MELD-Na, SOFA, and CLIF-ACLF scores reflect liver disease severity and organ dysfunction, while age and sex are recognized demographic predictors[31,32]. Prior to model fitting, multicollinearity was formally assessed using variance inflation factor. All predictor variables demonstrated acceptable variance inflation factor values (< 2), indicating no significant multicollinearity. Model ade
Kaplan-Meier survival analyses assessed 28-day survival from ICU admission to death or censoring (discharge or day 28). Unadjusted survival curves were stratified by colonization and infection status between AD and ACLF subgroups, and compared using the log-rank test. A predefined missing data strategy was applied. Variables with more than 50% missing data were excluded. For variables with less than 10% missing data, complete case analysis was used. Cases missing survival or phenotype status were excluded from subgroup analyses. A two-tailed alpha of 0.05 was used, and P < 0.05 was considered statistically significant. No correction for multiple testing was applied due to the exploratory nature of the study.
The study was approved by the Institutional Review Board of the University Hospital Centre Zagreb (Approval No: 02/013 AG). All study procedures were conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice, and the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies. Given the retrospective design and anonymized data collection, the requirement for individual informed consent was waived.
A total of 107 ICU admissions from 105 patients with liver cirrhosis were included in the final cohort. The median age was 60 years (interquartile range: 53-67), and 69.2% were male. Most patients were admitted with ACLF (81.3%), while the remainder presented with AD (18.7%). The most common etiology of cirrhosis was alcohol-associated liver disease (82.2%), followed by chronic viral hepatitis (10.3%) and metabolic dysfunction associated steatotic liver disease (5.6%). Multiple etiologies could be assigned per patient where applicable. Baseline characteristics of the cohort are shown in Table 1.
| Characteristics | Total (n = 107) | Without MDRO colonization (n = 75) | With MDRO colonization (n = 32) | P value1 | AD (n = 20) | ACLF (n = 87) | P value1 |
| Clinical and laboratory data | |||||||
| Age (years), median | 60.0 (53.0, 67.0) | 59 (53.0, 66.0) | 61.0 (52.5, 69.0) | 0.343 | 56.0 (50.3, 63.0) | 61.0 (53.0, 68.0) | 0.118 |
| Gender | 0.123 | 0.475 | |||||
| Male | 74 (69.2) | 48 (64.0) | 26 (81.2) | 12 (60.0) | 62 (71.3) | ||
| Female | 33 (30.8) | 27 (36.0) | 6 (18.8) | 8 (40.0) | 25 (28.7) | ||
| Hospital admission within last 3 months | 40 (37.4) | 25 (33.3) | 15 (46.9) | 0.268 | 7 (35.0) | 33 (37.9) | 1 |
| ICU admission within last 3 months | 12 (11.2) | 7 (9.3) | 5 (15.6) | 0.542 | 4 (20.0) | 8 (9.2) | 0.231 |
| Systemic antibiotics within last 3 months | 38 (35.5) | 23 (30.7) | 15 (46.9) | 0.167 | 5 (25.0) | 33 (37.9) | 0.406 |
| Long-term norfloxacin prophylaxis | 3 (2.8) | 3 (4.0) | 0 (0) | 0.553 | 1 (5.0) | 2 (2.3) | 0.466 |
| NSBB | 41 (38.3) | 27 (36.0) | 14 (43.8) | 0.591 | 9 (45.0) | 32 (36.8) | 0.67 |
| IPP prior to hospital | 58 (54.2) | 39 (52.0) | 19 (59.4) | 0.659 | 12 (60.0) | 46 (52.9) | 0.774 |
| IPP prior to ICU | 76 (71.0) | 50 (66.7) | 26 (81.2) | 0.197 | 15 (75.0) | 61 (70.1) | 0.872 |
| TIPSS | 8 (7.5) | 5 (6.7) | 3 (9.4) | 0.694 | 1 (5.0) | 7 (8.0) | 1 |
| WBC (× 109/L) | 12.2 (7.8, 16.8) | 12.4 (7.2, 17.8) | 11.6 (9.2, 14.3) | 0.498 | 9.8 (5.1, 12.1) | 12.7 (8.3, 17.2) | 0.034 |
| Platelets (× 109/L) | 88.0 (57.5, 122.0) | 100.0 (64.0, 132.0) | 76.5 (45.0, 100.0) | 0.004 | 101.0 (76.3, 138.0) | 84.0 (55.5, 118.5) | 0.166 |
| Serum creatinine (μmol/L) | 150.0 (84.0, 278.5) | 150.0 (88.0, 305.5) | 140.5 (79.8, 220.0) | 0.586 | 67.5 (59.5, 100.5) | 185.0 (101.0, 317.0) | 0.094 |
| Serum sodium (mmol/L) | 134.0 (130.0, 138.0) | 134.0 (130.5, 138.0) | 135.0 (129.0, 138.0) | 0.751 | 136.5 (129.8, 142.8) | 134.0 (130.0, 137.5) | 0.246 |
| Serum albumin (g/L) | 26.0 (22.7, 29.5) | 26.0 (22.4, 29.3) | 26.0 (23.3, 30.0) | 0.935 | 27.5 (22.0, 31.3) | 26.0 (23.0, 29.0) | 0.584 |
| Serum bilirubin (μmol/L) | 102.0 (45.0, 219.0) | 100.0 (47.6, 207.0) | 139.5 (42.5, 213.0) | 0.586 | 69.5 (37.5, 94.3) | 114.0 (49.0, 280.5) | 0.094 |
| INR | 1.7 (1.4, 2.2) | 1.6 (1.3, 2.2) | 1.77 (1.6, 2.4) | 0.156 | 1.4 (1.3, 1.5) | 1.8 (1.5, 2.4) | 0 |
| CRP (mg/L) | 36.1 (17.0, 73.5) | 32.4 (15.4, 75.1) | 36.6 (21.3, 68.9) | 0.617 | 37.5 (13.0, 55.1) | 35.0 (17.4, 80.5) | 0.29 |
| PCT (ng/mL) | 1.1 (0.5, 2.8) | 1.1 (0.5, 2.8) | 1.2 (0.6, 2.2) | 0.824 | 0.5 (0.1, 1.1) | 1.3 (0.6, 2.9) | 0.003 |
| AST (U/L) | 97.0 (49.0, 214.0) | 110.0 (49.0, 262.0) | 79.5 (50.0, 133.8) | 0.187 | 76.0 (42.8, 130.8) | 106.0 (52.0, 228.0) | 0.303 |
| ALT (U/L) | 48.0 (21.0, 117.5) | 60.0 (22.0, 141.0) | 29.5 (18.3, 79.5) | 0.039 | 39.5 (22.0, 101.5) | 49.0 (20.50, 117.50) | 0.851 |
| GGT (U/L) | 67.0 (34.0, 162.5) | 80.0 (39.0, 225.0) | 47.5 (24.0, 87.8) | 0.011 | 141.5 (51.8, 564.5) | 60.0 (30.5, 119.5) | 0.01 |
| ALP (U/L) | 108.0 (87.0, 154.5) | 109.0 (86.5, 179.5) | 106.0 (90.8, 129.0) | 0.492 | 100.0 (82.5, 187.3) | 109.0 (87.0, 144.5) | 0.876 |
| Cirrhosis etiology | |||||||
| ALD | 88 (82.2) | 64 (85.3) | 24 (75.0) | 0.315 | 16 (80.0) | 72 (82.8) | 0.752 |
| Chronic viral hepatitis | 11 (10.3) | 9 (12.0) | 2 (6.2) | 0.5 | 1 (5.0) | 10 (11.5) | 0.685 |
| MASLD | 6 (5.6) | 3 (4.0) | 3 (9.4) | 0.361 | 2 (10.0) | 4 (4.6) | 0.312 |
| PSC/PBC | 1 (0.9) | 1 (1.3) | 0 (0) | 1 | 1 (5.0) | 0 (0) | 0.187 |
| AIH | 1 (0.9) | 1 (1.3) | 0 (0) | 1 | 1 (5.0) | 0 (0) | 0.187 |
| Cryptogenic | 8 (7.5) | 5 (6.7) | 3 (9.4) | 0.694 | 0 (0) | 8 (9.2) | 0.347 |
| HCC | 3 (2.8) | 1 (1.3) | 2 (6.2) | 0.212 | 1 (5.0) | 2 (2.3) | 0.466 |
| Comorbidities | |||||||
| Diabetes mellitus | 25 (23.4) | 13 (17.3) | 12 (37.5) | 0.045 | 5 (25.0) | 20 (23.0) | 1 |
| Cardiovascular diseases | 50 (46.7) | 33 (44.0) | 17 (53.1) | 0.513 | 7 (35.0) | 43 (49.4) | 0.359 |
| Respiratory diseases | 15 (14.0) | 9 (12.0) | 6 (18.8) | 0.537 | 2 (10.0) | 13 (14.9) | 0.732 |
| Chronic kidney diseases | 13 (12.1) | 8 (10.7) | 5 (15.6) | 0.692 | 2 (10.0) | 11 (12.6) | 1 |
| Chronic hemodialysis | 1 (0.9) | 1 (1.3) | 0 (0) | 1 | 0 (0) | 1 (1.1) | 1 |
| Disease severity at admission | |||||||
| MELD score | 12.0 (10.0, 13.0) | 11.0 (10.0, 13.0) | 12.0 (10.8, 13.3) | 0.365 | 10.0 (8.0, 11.0) | 12.0 (10.5, 13.0) | 0 |
| MELD-Na score | 27.4 (21.3, 33.5) | 27.0 (21.4, 34.0) | 29.08 (20.9, 32.6) | 0.979 | 19.7 (16.0, 23.3) | 29.3 (24.2, 35.2) | 0 |
| SOFA score | 8.0 (6.0, 11.0) | 8.0 (6.0, 10.0) | 8.5 (7.0, 11.0) | 0.247 | 5.5 (4.0, 7.0) | 9.0 (7.0, 11.0) | 0 |
| CLIF ACLF score | 61.0 (53.8, 66.0) | 61.0 (56.0, 65.8) | 58.0 (53.3, 65.8) | 0.558 | 71.0 (71.0, 71.0) | 61.0 (53.5, 65.5) | 0.168 |
| Type of ICU admission | |||||||
| Organ failure | 48 (44.9) | 36 (48.0) | 12 (37.5) | 0.431 | 9 (45.0) | 39 (44.8) | 1 |
| Bleeding | 11 (10.3) | 9 (12.0) | 2 (6.2) | 0.5 | 5 (25.0) | 6 (6.9) | 0.046 |
| Infection | 35 (32.7) | 18 (24.0) | 17 (53.1) | 0.007 | 2 (10.0) | 33 (37.9) | 0.017 |
| Other | 13 (12.1) | 12 (16.0) | 1 (3.1) | 0.103 | 4 (20.0) | 9 (10.3) | 0.259 |
| ACLF at ICU admission | 87 (81.3) | 57 (76.0) | 30 (93.8) | 0.033 | 0 (0.0) | 87 (100.0) | 0 |
| Organ support at admission | 63 (58.9) | 44 (58.7) | 19 (59.4) | 1 | 7 (35.0) | 56 (64.4) | 0.031 |
| Mechanical ventilation | 45 (42.1) | 30 (40.0) | 15 (46.9) | 0.656 | 7 (35.0) | 38 (43.7) | 0.647 |
| Vasopressors | 45 (42.1) | 32 (42.7) | 13 (40.6) | 1 | 0 (0.0) | 45 (51.7) | 0 |
| Outcomes | |||||||
| Mechanical ventilation | 57 (53.3) | 38 (50.7) | 19 (59.4) | 0.539 | 9 (45) | 48 (55.2) | 0.566 |
| Acute RRT | 36 (33.6) | 23 (30.7) | 13 (40.6) | 0.438 | 1 (5) | 35 (40.2) | 0.003 |
| Vasopressors | 52 (48.6) | 34 (45.3) | 18 (56.2) | 0.41 | 1 (5) | 51 (58.6) | 0 |
| 28-day transplant free survival | 49 (45.8) | 37 (49.3) | 12 (37.5) | 0.361 | 15 (75) | 34 (39.1) | 0.008 |
| Overall ICU survival | 55 (51.4) | 41 (54.7) | 14 (43.8) | 0.41 | 17 (85) | 38 (43.7) | 0.001 |
| Overall hospital survival | 45 (42.1) | 35 (46.7) | 10 (31.2) | 0.206 | 15 (75) | 30 (34.5) | 0.002 |
| ICU LOS | 8.0 (4.0, 16.0) | 8.0 (4.0, 14.0) | 10.0 (3.0, 18.3) | 0.513 | 5.0 (3.0, 10.5) | 9.0 (5.0, 17.0) | 0.084 |
| Hospital LOS | 22.0 (12.0, 36.0) | 21.0 (12.0, 31.0) | 27.0 (17.8, 47.0) | 0.083 | 20.5 (11.8, 37.0) | 24.0 (12.0, 36.0) | 0.946 |
MDRO colonization at ICU admission was identified in 32 patients (29.9%). Compared to non-colonized patients, those with colonization had significantly lower platelet counts (76.5 × 109/L vs 100.0 × 109/L, P = 0.004), lower enzyme levels (alanine aminotransferase, P = 0.039; gamma-glutamyl transpeptidase, P = 0.011), and a higher prevalence of diabetes mellitus (37.5% vs 17.3%, P = 0.045). Infection was more frequently the reason for ICU admission in colonized patients (53.1% vs 24.0%, P = 0.007), and ACLF was more common in this group (93.8% vs 76.0%, P = 0.033). No significant differences were observed in MELD-Na, SOFA, or CLIF-ACLF scores, nor in most other comorbidities. When comparing patients with AD and ACLF, those with ACLF had significantly higher inflammatory markers (WBC: 12.7 × 109/L vs 9.8 × 109/L, P = 0.034; procalcitonin: 1.2 ng/mL vs 0.5 ng/mL, P = 0.003), as well as greater disease severity reflected by higher MELD-Na (29.2 vs 19.7, P < 0.001) and SOFA scores (9.0 vs 5.5, P < 0.001). Short-term outcomes were significantly worse in ACLF patients, including lower transplant- free survival (39.1% vs 75%, P = 0.008), ICU survival (43.7% vs 85.0%, P = 0.001), and overall hospital survival (34.5% vs 75.0%, P = 0.002).
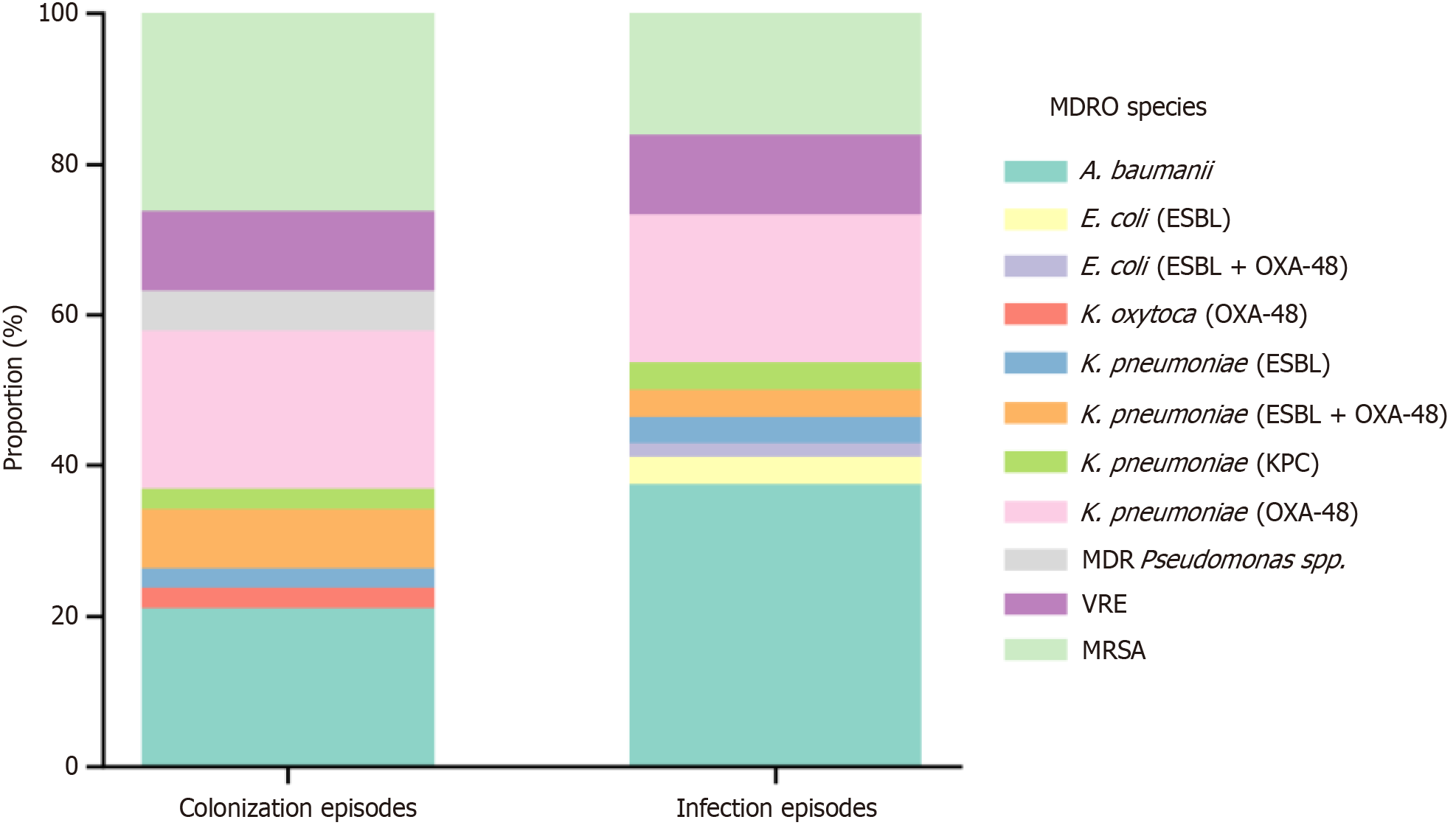
In the 107 ICU admissions, 38 distinct MDRO colonizing strains were identified in 32 patients (29.9%), indicating some had harbored multiple organisms. The most common were MRSA (26.3%), A. baumanii (21.1%), and OXA-48-producing K. pneumoniae (21.1%), while VRE was found in 10.5% of cases (Table 2; Figure 2). Infection occurred in 91 patients (85.0%), with a total of 56 distinct MDRO strains identified in 16 MDRO-positive infection episodes, suggesting some infections involved multiple sites and/or multiple resistant organisms. The most frequently infecting pathogens were A. baumanii (37.5% of MDRO infections), K. pneumoniae with various resistance profiles (approximately 28.6%), and MRSA (16.1%). Some organisms such as ESBL-producing E. coli and certain Klebsiella spp. were detected exclusively during infection and not colonization, highlighting gaps in routine surveillance (Table 2). Overall, the distribution of specific MDROs did not significantly differ between colonization and infection (P > 0.1 for all comparisons). However, the variety of resistance mechanisms observed across isolates highlights the clinical complexity of managing infections in these populations.
| MDRO species | Total (n) | Colonized | Infected | P value1 |
| MDR A. baumanii | 29 | 8 (21.1) | 21 (37.5) | 0.142 |
| Escherichia coli, ESBL | 2 | 0 (0.0) | 2 (3.6) | 0.513 |
| Escherichia coli, ESBL and OXA-48 | 1 | 0 (0.0) | 1 (1.8) | 1 |
| Klebsiella oxytoca, OXA-48 | 1 | 1 (2.6) | 0 (0.0) | 0.404 |
| Klebsiella pneumoniae, ESBL | 3 | 1 (2.6) | 2 (3.6) | 1 |
| Klebsiella pneumoniae, ESBL and OXA-48 | 5 | 3 (7.9) | 2 (3.6) | 0.391 |
| Klebsiella pneumoniae, KPC | 3 | 1 (2.6) | 2 (3.6) | 1 |
| Klebsiella pneumoniae, OXA-48 | 19 | 8 (21.1) | 11 (19.6) | 0.925 |
| MDR Pseudomonas spp. | 2 | 2 (5.3) | 0 (0.0) | 0.161 |
| VRE | 10 | 4 (10.5) | 6 (10.7) | 1 |
| MRSA | 19 | 10 (26.3) | 9 (16.1) | 0.341 |
Patterns of MDRO colonization and infection varied by clinical phenotype. Colonization with A. baumanii and OXA-48-producing K. pneumoniae was more frequently observed in patients with ACLF than AD (87.5% vs 12.5%, P = 0.508), although subgroup comparisons did not reach statistical significance. Notably, nearly all isolates of ESBL and OXA-48 K. pneumoniae, VRE, and MRSA were identified in ACLF patients (Supplementary Tables 1 and 2). The spectrum of infection types also differed between phenotypes. Pneumonia was the most common infection in both groups (72.4% in ACLF vs 60.0% in AD), followed by urinary tract infections and bacteriaemia. Skin and soft tissue infections and intra-abdominal infections were rare and observed exclusively in ACLF patients. However, none of these phenotype specific differences reached statistical significance (Supplementary Table 3).
Clinical outcomes differed by MDRO infection status but were less influenced by colonization alone. In colonized patients, there were no statistically significant differences in major outcomes compared to non-colonized peers; rates of mechanical ventilation (59.4% vs 50.7%, P = 0.539), renal replacement therapy (40.6% vs 30.7%, P = 0.438), vasopressor use (56.2% vs 45.3%, P = 0.410), and 28-day transplant free survival (37.5% vs 49.3%, P = 0.361) were similar between groups. ICU and hospital survival were also not statistically different (ICU: 43.8% vs 54.7%, P = 0.410; hospital 32.2% vs 46.7%, P = 0.206) neither were median ICU or hospital lengths of stay (Table 3). In contrast, MDRO infection was associated with significantly worse outcomes. Patients with documented MDRO infections had higher rates of mechanical ventilation (59.3% vs 18.8%, P = 0.005) and vasopressor use (53.8% vs 18.8%, P = 0.013) compared to non-infected patients. ICU length of stay was longer (median 9.0 days vs 5.0 days, P = 0.003), as was hospital length of stay (24.0 days vs 14.0 days, P = 0.030). Hospital survival was significantly lower in infected patients (37.4% vs 68.8%, P = 0.038), while ICU and 28-day transplant-free survival was also lower, but did not reach statistical significance (Table 3).
| Outcome | Colonized patients (n = 32) | Non-colonized (n = 75) | P value1 | Infected patients (n = 91) | Non-infected (n = 16) | P value1 |
| Mechanical ventilation | 19 (59.4) | 38 (50.7) | 0.539 | 54 (59.3) | 3 (18.8) | 0.005a |
| Renal replacement therapy | 13 (40.6) | 23 (30.7) | 0.438 | 31 (34.1) | 5 (31.2) | 1 |
| Vasopressor use | 18 (56.2) | 34 (45.3) | 0.41 | 49 (53.8) | 3 (18.8) | 0.013b |
| ICU LOS | 10.0 (15.2) | 8.0 (10.0) | 0.513 | 9.0 (12.0) | 5.0 (2.2) | 0.003c |
| 28-day transplant free survival | 12 (37.5) | 37 (49.3) | 0.361 | 38 (41.8) | 11 (68.8) | 0.084 |
| ICU survival | 14 (43.8) | 41 (54.7) | 0.41 | 44 (48.4) | 11 (68.8) | 0.217 |
| Hospital survival | 10 (31.2) | 35 (46.7) | 0.206 | 34 (37.4) | 11 (68.8) | 0.038d |
| Hospital LOS (days) | 27.0 (29.2) | 21.0 (19.0) | 0.083 | 24.0 (26.5) | 14.0 (15.0) | 0.03e |
Two multivariable logistic regression models were constructed to evaluate whether MDRO colonization at ICU admission predicted adverse clinical outcomes, specifically subsequent MDRO infection and 28-day transplant-free mortality. In the first model (n = 87), colonization was not independently associated with infection [odds ratio (OR) = 2.18; 95% confidence internal (CI): 0.47-10.10; P = 0.383]. None of the included covariates, MELD-Na score, SOFA score, CLIF-ACLF score, age or sex, were statistically significant predictors of infection. CLIF-ACLF score approached significance (P = 0.066), and male sex appeared to have a protective association (OR = 0.17), but this did not reach statistical significance (Table 4). In the second model evaluating predictors of 28-day mortality, colonization again showed no significant association with outcome (OR = 1.14; 95%CI: 0.37-3.50; P = 0.813). However, SOFA score emerged as a significant predictor, with higher scores associated with increased risk of death (OR = 1.38; 95%CI: 1.04-1083; P = 0.024). CLIF-ACLF score also neared significance (P = 0.062). MELD-Na, age, and sex were not significant in this model (Table 5).
| Predictor | OR | 95%CI | P value1 |
| MDRO colonization | 2.18 | 0.47-10.10 | 0.383 |
| MELD-Na score | 0.94 | 0.82-1.08 | 0.321 |
| SOFA score | 1.03 | 0.68-1.54 | 0.394 |
| CLIF-ACLF score | 1.11 | 0.99-1.23 | 0.903 |
| Age | 0.94 | 0.86-1.02 | 0.066 |
| Sex (male vs female) | 0.17 | 0.02-1.61 | 0.152 |
Outcomes were further examined according to decompensation phenotype. MDRO colonization was significantly more common among patients with ACLF compared to those with AD (34.5% vs 10.0%, P = 0.033). Although MDRO infection was prevalent in both groups (86.2% in ACLF vs 80.0% in AD) the difference was not statistically significant (P = 0.494). In patients who developed infection, strain concordance with colonization was observed in all AD cases (2/2) and in 81.8% of ACLF cases (18/22; P = 1.000). Clinical outcomes were worse in the ACLF subgroup. Patients with ACLF had significantly lower 28-day transplant-free survival (39.1% vs 75.0%, P = 0.002) compared to those with AD. Although ICU length of stay was longer in the ACLF patients (median 9.0 days vs 5.0 days), this did not reach statistical significance (P = 0.084). Hospital length of stay was similar between two groups (P = 0.946; Table 6).
| Outcome | AD (n = 20) | ACLF (n = 87) | P value1 |
| MDRO colonized | 2 (10.0) | 30 (34.5) | 0.033a |
| Total infections | 16 (80.0) | 75 (86.2) | 0.494 |
| Strain-concordant infection | 2 (100.0) | 18 (81.8) | 1.000 |
| ICU length of stay (days) | 5.0 (7.5) | 9.0 (12.0) | 0.084 |
| 28-day transplant-free survival | 15 (75.0) | 34 (39.1) | 0.008b |
| ICU survival | 17 (85.0) | 38 (43.7) | 0.001c |
| Hospital survival | 15 (75.0) | 30 (34.5) | 0.002d |
| Hospital LOS (days) | 20.5 (25.2) | 24.0 (24.0) | 0.946 |
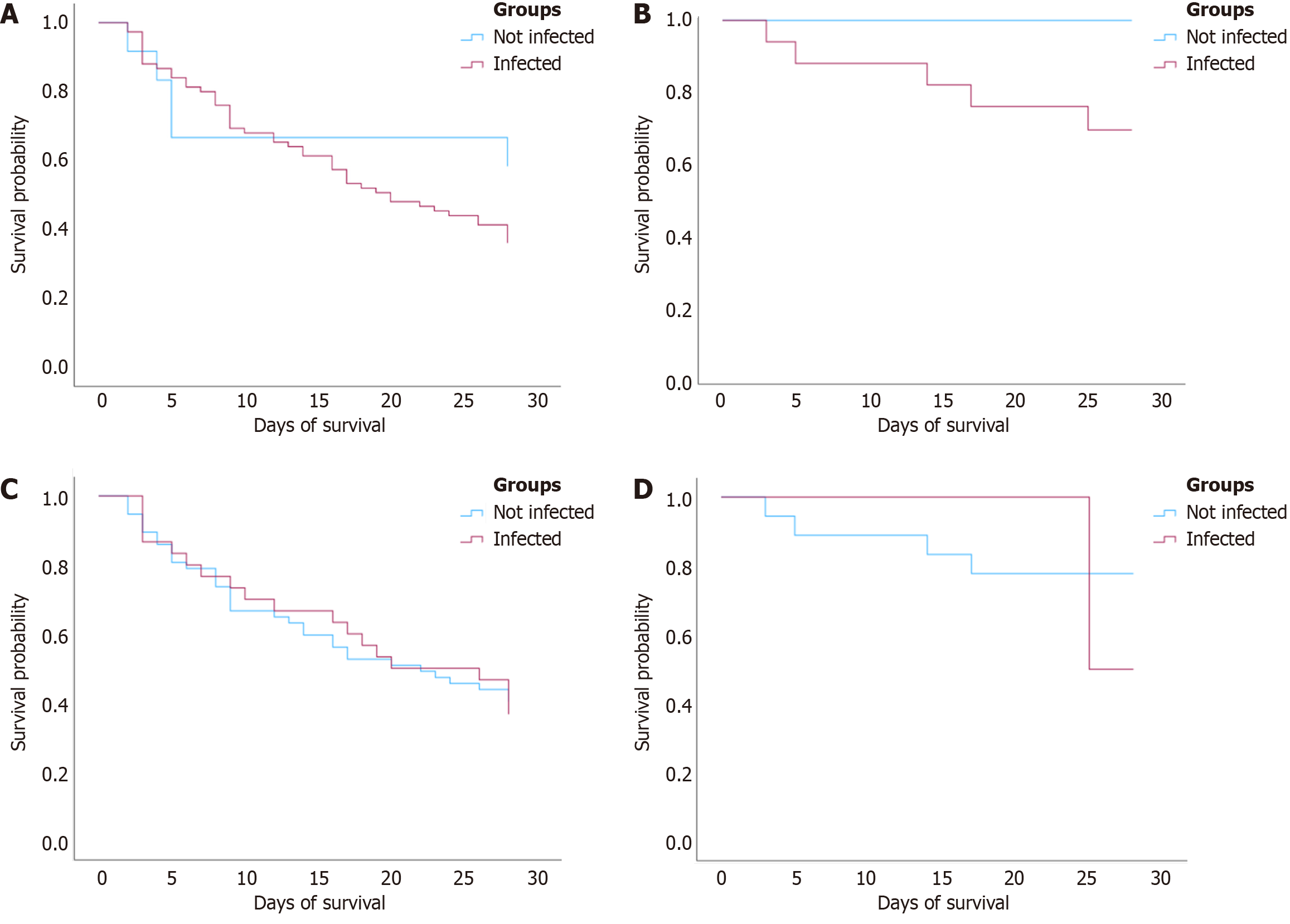
Kaplan-Meier analysis revealed no statistically significant differences in 28-day survival based on colonization or infection status in either AD or ACLF groups. Kaplan-Meier was conducted to assess 28-day transplant free survival stratified by MDRO colonization and infection status within both AD and ACLF subgroups. In patients with ACLF, MDRO infection was associated with a lower survival probability over the 28-day period compared to non-infected peers. However, the difference did not reach statistical significance (P = 0.257). A similar pattern was observed in AD patients, where those with MDRO infection had worse survival, however, again not statistically significant (P = 0.225). MDRO colonization status did not significantly impact 28-day survival in either clinical group. In ACLF patients, survival curves for colonized and non-colonized individuals were nearly superimposable (P = 0.956). Among AD patients, no survival difference by colonization was observed (P = 0.542; Figure 3).
This study explored the clinical relevance of MDRO colonization at ICU admission in patients with cirrhosis, with a particular focus on its relationship to subsequent infection, patient outcomes, and local epidemiological patterns. While colonization at ICU admission was frequent and often concordant with the organisms’ causing infection, it was not independently associated with MDRO infection or short-term mortality. These findings diverge from previous reports and suggest that, in a population with advanced liver disease and high baseline infection rates, the prognostic signi
We observed a high concordance (approximately 82%) between colonizing and infecting strains, aligning with previous studies that emphasize the predictive value of colonization in anticipating subsequent infection strains[14,17]. This observation supports the consideration of colonization data when selecting empiric antibiotic regimens, particularly when infection is clinically suspected and prior MDRO colonization has been documented. However, the absence of certain pathogens, such as ESBL-producing E. coli, in screening samples indicates that reliance on colonization data alone could lead to incomplete empirical coverage. Thus, clinicians should consider colonization status alongside local microbiological data and clinical suspicion rather than as an isolated guide.
MDRO colonization at ICU admission was observed in nearly 30% of our cohort, consistent with rates reported in critically ill patients with cirrhosis[16]. However, the predominant colonizing strains in our population, MRSA, A. baumanii, and OXA-48-producing K. pneumoniae, differ from those reported in Western European centers, where ESBL-producing E. coli and VRE are more frequently observed[6,15,26]. The spectrum of MDROs varies widely across regions, and local surveillance data are essential for guiding empirical therapy and infection control[6,11]. Regional data from Eastern Europe suggest a particularly high prevalence of carbapenem-resistant organisms in ICU-admitted patients with cirrhosis, which is reflected in our cohort by the predominance of A. baumanii and OXA-48 producing K. pneumoniae[33].
Infection was identified in 85% of patients, with 17.6% of these involving MDROs. This is comparable to the prevalence reported in other ICU cohorts of patients with cirrhosis[16]. However, the spectrum of infecting organisms also showed some divergence. In our cohort, Gram-negative non-fermenters such as A. baumanii were particularly prominent, an observation consistent with Eastern and Southern European epidemiology[1,34]. The overall severity of illness in our cohort, reflected in high CLIF-ACLF, MELD-Na, and SOFA scores, confirms a critically ill population with substantial baseline risk[6]. MDRO colonization was notably more frequent in patients with ACLF, potentially reflecting the immunological dysfunction inherent to this phenotype[20,21]. This association persisted even when global severity scores were comparable, supporting the notion that ACLF-related immune derangements may predispose patients to microbial colonization independently of liver function. However, despite the high prevalence of colonization and MDRO infection, we did not observe a statistically significant association between colonization at ICU admission and MDRO infection nor short-term mortality in either group. These findings contrast with previous studies that report a clearer link between colonization and infection[14,16,17]. Several factors may account for this discrepancy, including the fact that over 80% of patients in our cohort were already infected at ICU admission limiting opportunities to detect new infections and potentially reducing the colonization’s predictive value. Additionally, the predominance of patients with advanced liver disease and high baseline severity may have diminished the independent prognostic importance of colonization.
Clinically, these findings suggest phenotype-specific considerations: In ACLF patients, characterized by higher MDRO colonization rates, empirical therapy might need broader initial coverage informed by local MDRO profiles. Repeated colonization screening during prolonged hospital stays or pre-ICU admission could enhance detection and tailor empirical treatment, although prospective validation of such strategies is needed. Regarding infection control, although standard isolation and contact precaution protocols were employed for MDRO-colonized patients, our study did not specifically investigate transmission events, and no targeted decontamination strategies (e.g., selective digestive decontamination or chlorhexidine bathing) were implemented. Enhanced surveillance using molecular typing could potentially clarify transmission dynamics and further refine infection control measures.
This study has several limitations. The retrospective, single-center design limits generalizability, and its observational nature precludes causal inference. In particular, our findings reflect a Southeastern European resistance profile, limiting direct applicability to settings with different epidemiological patterns. Additionally, the lack of molecular strain typing prevented definitive confirmation of colonizing and infecting strain concordance. Another limitation was the inability to clearly differentiate colonization timing relative to infection onset, particularly since many patients were infected at ICU admission. Thus, the exact predictive window and causal relationships remain uncertain. Nonetheless, this study offers several notable strengths. It focuses on a relatively homogeneous, well-characterized population of critically ill patients with cirrhosis, minimizing heterogeneity in baseline liver disease and clinical trajectories. Microbiological data were obtained from systematic screening and diagnostic cultures, enabling a detailed comparison between colonizing and infecting strains. Additionally, outcomes were stratified by both colonization and decompensation phenotype (AD vs ACLF), offering insights into host-pathogen interactions across different immunological states. Compared to earlier studies, our data contribute region-specific evidence from a high-risk ICU-setting with a distinct MDRO profile, under
In critically ill patients with cirrhosis, especially those with ACLF, colonization with MDROs at ICU admission is common but not independently associated with short-term mortality or subsequent infection, highlighting the complexity of infection risk in this population. The high concordance between colonizing and infecting strains supports the clinical value of colonization data for guiding empirical therapy when infection is clinically suspected. MDRO infections were associated with significantly higher resource utilization, including increased need for mechanical ventilation, vasopressors, and renal replacement therapy, emphasizing their clinical impact despite the limited predictive value of colonization status alone. Our findings highlight that short-term mortality is more strongly driven by the extent of organ dysfunction than by MDRO colonization status, suggesting that physiological status remains the dominant factor in early outcome prediction.
Given that infections are the leading ICU admission trigger, incorporating earlier MDRO screening, phenotype-tailored empirical therapy, and enhanced surveillance might improve clinical management. Local epidemiological insight, targeted surveillance, and region-adapted empirical strategies are essential to support timely, effective treatment. However, caution is warranted to avoid overinterpreting colonization as a standalone determinant for empirical coverage, given its limited predictive performance demonstrated herein. Prospective studies that incorporate antimicrobial susceptibility profiling and track infection dynamics over time are warranted to refine screening strategies and improve microbiological precision in this at-risk population.
The authors wish to sincerely thank the medical and nursing staff of the Intensive Care Unit and the Department of Clinical Microbiology at University Hospital Centre Zagreb for their invaluable support in data collection and dedication to patient care.
| 1. | Terra C, de Mattos ÂZ, Chagas MS, Torres A, Wiltgen D, Souza BM, Perez RM. Impact of multidrug resistance on the management of bacterial infections in cirrhosis. World J Clin Cases. 2023;11:534-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 2. | Wong F, Piano S, Singh V, Bartoletti M, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bruns T, Yoon EL, Girala M, Pyrsopoulos NT, Kim TH, Yim SY, Juanola A, Gadano A, Angeli P; International Club of Ascites Global Study Group. Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure. J Hepatol. 2021;74:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 3. | Ferrarese A, Senzolo M, Cattelan AM, Sasset L, Battistella S, Zanetto A, Germani G, Russo FP, Gambato M, Pelizzaro F, Vio S, Bassi D, Cillo U, Burra P. Bacterial Infections in End-Stage Liver Disease: Implications for Liver Transplantation. Transplantology. 2024;5:129-139. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Martin Mateos R, Albillos A. Sepsis in Patients With Cirrhosis Awaiting Liver Transplantation: New Trends and Management. Liver Transpl. 2019;25:1700-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Chen G, To U. Inpatient management of bacterial infections in patients with cirrhosis: A clinical review. Clin Liver Dis (Hoboken). 2024;23:e0214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, Garcia E, Acevedo J, Fuhrmann V, Durand F, Sánchez C, Papp M, Caraceni P, Vargas V, Bañares R, Piano S, Janicko M, Albillos A, Alessandria C, Soriano G, Welzel TM, Laleman W, Gerbes A, De Gottardi A, Merli M, Coenraad M, Saliba F, Pavesi M, Jalan R, Ginès P, Angeli P, Arroyo V; European Foundation for the Study of Chronic Liver Failure (EF-Clif). Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 269] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 7. | Fernández J, Piano S, Bartoletti M, Wey EQ. Management of bacterial and fungal infections in cirrhosis: The MDRO challenge. J Hepatol. 2021;75 Suppl 1:S101-S117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (5)] |
| 8. | Piano S, Bunchorntavakul C, Marciano S, Rajender Reddy K. Infections in cirrhosis. Lancet Gastroenterol Hepatol. 2024;9:745-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 9. | Ferguson Toll J, Solà E, Perez MA, Piano S, Cheng A, Subramanian AK, Kim WR. Infections in decompensated cirrhosis: Pathophysiology, management, and research agenda. Hepatol Commun. 2024;8:e0539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Onorato L, Monari C, Capuano S, Grimaldi P, Coppola N. Prevalence and Therapeutic Management of Infections by Multi-Drug-Resistant Organisms (MDROs) in Patients with Liver Cirrhosis: A Narrative Review. Antibiotics (Basel). 2022;11:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Trad N, Mohamed G, Bizid S, Abdallah HB, Bouali R, Abdelli MN. Clinical impact of multidrug-resistant bacterial infections in patients with cirrhosis. Future Sci OA. 2024;10:FSO945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Ferrarese A, Senzolo M, Burra P. Multidrug-Resistant Bacterial Infections before and after Liver Transplantation. OBM Transplant. 2020;4:110. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Incicco S, Tonon M, Zeni N, Gambino C, Gagliardi R, Calvino V, Barone A, Zilio G, Feltracco P, Burra P, Cillo U, Angeli P, Piano S. Impact of bacterial infections prior to liver transplantation on post-transplant outcomes in patients with cirrhosis. JHEP Rep. 2023;5:100808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 14. | Prado V, Hernández-Tejero M, Mücke MM, Marco F, Gu W, Amoros A, Toapanta D, Reverter E, Peña-Ramirez C, Altenpeter L, Bassegoda O, Mezzano G, Aziz F, Juanola A, Rodríguez-Tajes S, Chamorro V, López D, Reyes M, Hogardt M, Kempf VAJ, Ferstl PG, Zeuzem S, Martínez JA, Vila J, Arroyo V, Trebicka J, Fernandez J. Rectal colonization by resistant bacteria increases the risk of infection by the colonizing strain in critically ill patients with cirrhosis. J Hepatol. 2022;76:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | Verma N, Divakar Reddy PV, Vig S, Angrup A, Biswal M, Valsan A, Garg P, Kaur P, Rathi S, De A, Premkumar M, Taneja S, Ray P, Duseja A, Singh V. Burden, risk factors, and outcomes of multidrug-resistant bacterial colonisation at multiple sites in patients with cirrhosis. JHEP Rep. 2023;5:100788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 16. | Kim M, Cardoso FS, Pawlowski A, Wunderink R, Ladner DP, Abraldes JG, Karvellas CJ. The impact of multidrug-resistant microorganisms on critically ill patients with cirrhosis in the intensive care unit: a cohort study. Hepatol Commun. 2023;7:e0038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Pouriki S, Alexopoulos T, Vasilieva L, Vrioni G, Alexopoulou A. Rectal colonization by resistant bacteria is associated with infection by the colonizing strain and high mortality in decompensated cirrhosis. J Hepatol. 2022;77:1207-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Crocombe D, O'Brien A. Antimicrobial prophylaxis in decompensated cirrhosis: friend or foe? Hepatol Commun. 2023;7:e0228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Kutmutia R, Tittanegro T, China L, Forrest E, Kallis Y, Ryder SD, Wright G, Freemantle N, O'Brien A. Evaluating the Role of Antibiotics in Patients Admitted to Hospital With Decompensated Cirrhosis: Lessons From the ATTIRE Trial. Am J Gastroenterol. 2023;118:105-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 20. | Liakina V. Antibiotic resistance in patients with liver cirrhosis: Prevalence and current approach to tackle. World J Clin Cases. 2023;11:7530-7542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (2)] |
| 21. | Hasa E, Hartmann P, Schnabl B. Liver cirrhosis and immune dysfunction. Int Immunol. 2022;34:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | McGettigan B, Hernandez-Tejero M, Malhi H, Shah V. Immune Dysfunction and Infection Risk in Advanced Liver Disease. Gastroenterology. 2025;168:1085-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Tranah TH, Kronsten VT, Shawcross DL. Implications and Management of Cirrhosis-Associated Immune Dysfunction Before and After Liver Transplantation. Liver Transpl. 2022;28:700-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM, Matern S, Lammert F. Patients with acute on chronic liver failure display "sepsis-like" immune paralysis. J Hepatol. 2005;42:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 405] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 25. | Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P; International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. 2019;156:1368-1380.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 360] [Article Influence: 51.4] [Reference Citation Analysis (4)] |
| 26. | Kremer WM, Gairing SJ, Kaps L, Ismail E, Kalampoka V, Hilscher M, Michel M, Siegel E, Schattenberg JM, Galle PR, Sprinzl MF, Wörns MA, Nagel M, Labenz C. Characteristics of bacterial infections and prevalence of multidrug-resistant bacteria in hospitalized patients with liver cirrhosis in Germany. Ann Hepatol. 2022;27:100719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Revelli L, Dirchwolf M. Editorial: Terlipressin and MDRO colonisation in cirrhosis-Is there a causal link? Aliment Pharmacol Ther. 2024;59:1284-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Roy A, Premkumar M. Progress in Hepatology-rectal Colonization by Resistant Bacteria Increases the Risk of Infection by the Colonizing Strain in Critically Ill Patients with Cirrhosis. J Clin Exp Hepatol. 2022;12:1574-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Santoro-Lopes G, de Gouvêa EF, Monteiro RC, Branco RC, Rocco JR, Halpern M, Ferreira AL, de Araújo EG, Basto ST, Silveira VG, Ribeiro-Filho J. Colonization with methicillin-resistant Staphylococcus aureus after liver transplantation. Liver Transpl. 2005;11:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 779] [Article Influence: 64.9] [Reference Citation Analysis (2)] |
| 31. | Zhang C, Zhang X, Sun Z, Liu X, Shen B. MetaSepsisBase: a biomarker database for systems biological analysis and personalized diagnosis of heterogeneous human sepsis. Intensive Care Med. 2023;49:1015-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2215] [Article Influence: 110.8] [Reference Citation Analysis (3)] |
| 33. | Lingiah VA, Pyrsopoulos NT. Bacterial Infections in Cirrhotic Patients in a Tertiary Care Hospital. J Clin Transl Hepatol. 2021;9:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Fischer P, Pandrea S, Dan Grigorescu M, Stefanescu H, Tefas C, Hadade A, Procopet B, Ionescu D. The threat of carbapenem resistance in Eastern Europe in patients with decompensated cirrhosis admitted to intensive care unit. Dig Liver Dis. 2022;54:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/