Published online Oct 21, 2025. doi: 10.3748/wjg.v31.i39.110115
Revised: July 11, 2025
Accepted: September 15, 2025
Published online: October 21, 2025
Processing time: 145 Days and 7.2 Hours
Cholelithiasis is a prevalent biliary tract disorder primarily characterized by gallbladder or biliary stone formation. Although succinylation has been exten
To investigate the functional role of succinylation in cholelithiasis and determine its underlying molecular mechanisms.
A murine cholelithiasis model was established through high-fat diet feeding, followed by isolation of mouse gallbladder mucosal epithelial cells (GMECs) for in vitro analysis. Gallbladder tissues and serum samples were collected for subsequent analysis. Inflammatory cytokine production was quantified using enzyme-linked immunosorbent assay. Pyroptosis was analyzed by flow cytometry, while succinylation- and pyroptosis-related protein expression was detected via western blot.
Our findings demonstrated that lysine acetyltransferase 2A (KAT2A)-mediated succinylation regulated gallstone formation. KAT2A overexpression inhibited the pyroptosis, inflammatory responses, and promoted the activation of the adenosine monophosphate-activated protein kinase (AMPK)/silent information regulator 1 (SIRT1) sig
KAT2A-mediated succinylation of AMPK inhibited cholelithiasis progression by modulating the AMPK/SIRT1 signaling pathway, offering potential therapeutic strategies for this condition.
Core Tip: Cholelithiasis is a common biliary disorder with complex mechanisms. Lysine succinylation, a protein post-translational modification, remains unstudied in this condition. This study reveals that lysine acetyltransferase 2A-mediated su
- Citation: Wang XX, Ma MZ, Zhu LC, Dai LF, Qin C, Shao S, Xu XW, Gao RX, Zhang ZH. Lysine acetyltransferase 2A-mediated succinylation of adenosine monophosphate-activated protein kinase suppresses gallstone formation by inhibiting inflammation and pyroptosis. World J Gastroenterol 2025; 31(39): 110115
- URL: https://www.wjgnet.com/1007-9327/full/v31/i39/110115.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i39.110115
Cholelithiasis is a prevalent digestive tract disease in clinical practice, characterized by the formation of stones in the gallbladder or biliary tract. Its clinical manifestations are diverse and may lead to complications such as cholecystitis, cholangitis, and pancreatitis[1]. The global prevalence of cholelithiasis is rising, affecting approximately 10% to 20% of adults. Regional disparities exist, with higher incidence rates observed in Europeans and Americans (10% to 15%), Asians (3% to 15%), and less than 5% in Africans[2]. In China, the prevalence has exceeded 10% and continues to increase due to an aging population and evolving dietary patterns[3]. Cholelithiasis disproportionately affects adult women, particularly middle-aged and elderly individuals. Established risk factors include obesity, unhealthy diets, rapid weight loss, pregnancy, and genetic predisposition[4]. Pathogenesis involves disrupted cholesterol homeostasis and abnormal bilirubin metabolism in the biliary tract[5,6]. Despite these insights, the mechanisms underlying cholelithiasis remain complex, primarily influenced by lifestyle, genetic, and physical factors. Postoperative stone recurrence further compli
Post-translational modification (PTM) is a critical regulator of protein function, bridging metabolic processes with physiological and pathological pathways[7]. Lysine succinylation, a widespread PTM in eukaryotes, participates in essential processes such as cell differentiation and metabolism[8]. Lysine acetyltransferase 2A (KAT2A) acts as a succinyltransferase by transferring a succinyl group from succinyl-CoA to lysine residues on histone proteins[9]. This modifi
Adenosine monophosphate-activated protein kinase (AMPK) serves as a central energy sensor and metabolic regulator, governing cell growth, survival, and systemic energy homeostasis[15]. Sirtuin 1 (SIRT1), an NAD+-dependent deacetylase, regulates diverse biological processes, including cell cycle progression, inflammation, autophagy, and aging, while mitigating disease risks[16,17]. SIRT1 modulates AMPK phosphorylation levels[18], and dysregulation of this axis exacerbates inflammation, as seen in obese mice with suppressed AMPK activity[19]. Numerous studies demonstrate that AMPK/SIRT1 signaling suppresses inflammation and pyroptosis in various diseases[20,21]. Notably, activation of this pathway reduces inflammatory damage to gallbladder mucosal epithelial cells, thereby protecting against gallbladder injury and gallstone formation in murine models[22].
This study investigated the role of succinylation in cholelithiasis and its underlying molecular mechanisms. Our findings revealed that KAT2A-mediated succinylation of AMPK inhibited cholelithiasis progression by modulating inflammation and gallstone formation, offering potential therapeutic insights for this condition. This study is the first to investigate the role of lysine succinylation in cholelithiasis pathogenesis, uncovering a novel link between post-translational metabolic regulation and gallstone formation. These findings provide a groundbreaking perspective on choleli
The animal study was approved by the Experimental Animal Welfare Ethics Committee of MDKN Biotechnology Co., Lt (Approval No. MDKN-2024-129). A total of 24 male C57BL/6J mice (20 ± 5 g) were obtained from Aniphe Biolaboratory Inc. (Nanjing, China) and housed in a controlled environment at 24 °C with a 12-hour light/dark cycle, free access to food and water. Mice were randomly assigned to two initial groups: A normal control group (n = 6) and a model group (n = 18). After a 1-week acclimatization period, the normal group was fed a standard diet, while the model group received a high-fat diet (HFD). The commercial HFD composition (containing 40% kcal fat) included 15% butterfat, 1.25% cholesterol, 0.5% sodium cholate, 2% corn oil, 50% sucrose, 20% casein, and essential minerals and vitamins (Dyets Biotech Co., Ltd., Wuxi, China). The model group was further subdivided into three subgroups: Model (n = 6), model + lentivirus (Lv)-NC (n = 6), and model + Lv-KAT2A (n = 6). For four weeks, mice in the Lv-NC and Lv-KAT2A groups were intravenously administered 5 × 107 TU of Lv-NC or Lv-KAT2A in 200 µL phosphate buffer solution (PBS), three times per week. Control mice in the standard diet group received the same volume of normal saline via intravenous injection under identical conditions. After eight weeks of dietary intervention, blood samples were collected via the inferior vena cava and centrifuged at 5000 rpm for 10 minutes to isolate the supernatant, which was stored at -20 °C for subsequent analysis. Mice were weighed and euthanized using 5% isoflurane (Sigma-Aldrich, St. Louis, MO, United States) inhalation. Fresh gallbladder bile was collected and assessed for microscopic and macroscopic gallstone formation according to criteria established by Zhao et al[23]. Gallbladder and liver tissues were then harvested and stored at -80 °C for further experiments.
Primary gallbladder mucosal epithelial cells (GMECs) were isolated and characterized following a previously established protocol[22]. Cells were cultured in the ICell Primary Epithelial Cell Culture System (PriMed-iCell-001; Cellverse Bioscience Technology Co. Ltd., Shanghai, China), supplemented with 2% fetal bovine serum and 1% double antibiotics. Cultures were maintained at 37 °C in a 5% CO2 humidified incubator, with medium replaced every 3 days. To induce an inflammatory injury model in vitro, GMECs were stimulated with lipopolysaccharide (LPS; ST1470; Beyotime Biotech Co., Ltd., Shanghai, China) at a concentration of 200 µg/mL for 24 hours, as described previously[22]. Additionally, GMECs were treated with the AMPK inhibitor dorsomorphin (DOR; purity > 99%; ab120843; 10 μmol/L; Abcam) for 24 hours to assess AMPK signaling involvement.
The empty pcDNA3.1 vector, pcDNA3.1-KAT2A overexpression vector, short hairpin RNA targeting KAT2A (sh-KAT2A), and sh-RNA negative control (sh-NC) were synthesized by HAN Biotech Co., Ltd. (Shanghai, China). Flag-WT, Flag-AMPK-K154E, Flag-AMPK-K154R, and KAT2A-Y645A constructs were designed by Genscript Biotechnology Co., Ltd. (Nanjing, China). GMECs (1 × 106 cells/well) were seeded in six-well plates. Transfection was performed using Lipofectamine 3000 transfection reagent (L3000150; Thermo Fisher Scientific, Waltham, MA, United States) after cells reached 80% confluence. Transfection was carried out for 48 hours. Hepatic total cholesterol (TC), biliary bile cholesterol saturation index (CSI), and total bile acids (TBA) were quantified following a previously established protocol[24].
The concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), TC, total triglycerides (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), tumor necrosis factor (TNF)-α, interleukin (IL)-18, and IL-1β in mouse serum or GMECs were quantified using commercial eenzyme-linked immuno sorbent assay (ELISA) kits (Abcam, Cambridge, MA, United States) according to the manufac
Total RNA from tissues or GMECs was extracted using TRIzol reagent (R0016; Beyotime). The extracted RNA was reverse-transcribed into cDNA using a reverse transcription kit (BL699A; Biosharp Biotech Co. Ltd., Hefei, China). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis was performed using the BeyoFast™ SYBR Green One-Step RT-qPCR Kit (D7268S; Beyotime) under the manufacturer-specified reaction conditions. Gene expression levels were quantified using the 2−ΔΔCT method. The used primers were synthesized by Tsingke Biotech Co. Ltd., (Beijing, China) and listed below: KAT2A, forward, 5′-GCAAGGCCAATGAAACCTGTA-3′ and reverse, 5′-TCCAAGTGGGATACGTGGTCA-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-TGTGGGCATCAATGGATTTGG-3′ and reverse, 5′-ACACCATGTATTCCGGGTCAAT-3′.
Total RNA was extracted from the gallbladder tissues of normal and model group mice using TRIzol reagent (Beyotime) according to the manufacturer’s instructions. RNA quality and concentration were evaluated using a NanoDrop spectrophotometer (Thermo Fisher Scientific) and an Agilent 2100 Bioanalyzer (Agilent Technologies, United States). Only RNA samples with an RNA Integrity Number (RIN) > 7.0 were selected for library construction. RNA-seq libraries were prepared using the NEBNext Ultra II RNA Library Prep Kit (New England Biolabs, United States) following the manufac
The Kyoto Encyclopedia of Genes and Genomes (KEGG; https://www.genome.jp/kegg/) and Gene Ontology (GO; https://www.geneontology.org/) databases were utilized to identify signaling pathways and biological processes enriched in differentially expressed genes (DEGs).
Gallbladder specimens were fixed in 4% neutral-buffered formaldehyde overnight. Following fixation, tissues were embedded in paraffin and sectioned into 4-μm-thick slices. The sections were then stained with Hematoxylin and eosin (H&E). After dewaxing, staining, dehydration, and clearing, the slides were mounted and sealed with a coverslip. Histological examination was performed under a light microscope (Leica, Germany).
Gallbladder tissues and GMECs were lysed using Radio Immunoprecipitation Assay lysis buffer (P0013B; Beyotime) for 10 minutes. The homogenate was further homogenized on ice for 30 minutes and centrifuged at 12000 rpm for 20 minutes at 4 °C to isolate the supernatant. Protein concentration was quantified using the bicinchoninic acid (BCA) assay kit (P0010S; Beyotime). Equal amounts of protein samples were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride membranes (FFP39; Beyotime). The mem
Pyroptosis in GMECs was assessed using a flow cytometry assay. Briefly, a commercial FAM-fluorochrome-labeled inhibitors of caspases (FLICA) in vitro Caspase Detection Kit (ImmunoChemistry Technologies, LLC, United States) was used following the manufacturer’s protocol. Samples were incubated with FLICA and propidium iodide (PI) at 37 °C for 1 hours in the dark. After washing twice with wash buffer, cells were analyzed using a flow cytometer (BD Biosciences, United States).
PI staining was performed to analyze the cell death in GMECs cells using a commercial PI kit (P4170, Sigma). First, the 40 μg/mL of PI working solution was prepared. Cells were resuspended in 500 μL of binding buffer at a concentration of 1 × 106 cells/mL. Next, add 100 μL dye working solution to the cells, gently shake the dye to completely cover the cells, and incubate for 15 minutes at 37 °C. Finally, fluorescence microscopy was used to observe, and the number of PI-positive cells (red fluorescence) was counted using the image analysis software (ImageJ).
A phospho-kinase array was performed to quantify the relative phosphorylation levels of kinase sites. The Human Phospho-Kinase Array Kit was obtained from R&D Systems (Minneapolis, United States). Cells were lysed in lysis buffer at a concentration of 1 × 107 cells/mL and incubated on a rotating platform at 4 °C for 30 minutes. After centrifugation at 14000 × g for 5 minutes, the total protein concentration in the supernatants was quantified. Cell lysates were mixed with biotinylated detection antibodies and incubated overnight with membranes pre-coated with capture and control antibodies. The membranes were then washed, and streptavidin-horseradish peroxidase conjugate and chemiluminescent detection reagents were applied. A chemiluminescent signal was generated at each capture spot, proportional to the amount of phosphorylated protein bound to the corresponding antibody.
Co-immunoprecipitation (Co-IP) was used to detect the binding between liver kinase B1 (LKB1) and AMPK in GMECs. The procedure was conducted according to a previous study[25]. An IP assay combined with western blot was used to assess the succinylation level of AMPK or LKB1 in GMECs. Briefly, GMEC cell lysates were obtained and immunoprecipitated with anti-AMPK or anti-LKB1 antibodies and protein A/G agarose (Novex, Oslo, Norway), followed by western blot analysis for succinylation.
Global Prediction of Generic and Species-specific Succinylation Sites (GPSuc; http://kurata14.bio.kyutech.ac.jp/GPSuc/index.php) was used for site prediction of AMPK. To determine the specific sites at which AMPK was succinylated, Genescript Co. Ltd. was commissioned to generate site-directed mutagenesis of lysine residues (K179, K170, and K204) to arginine (K179R, K170R, and K204R). These mutated plasmids were then transfected into GMECs.
An evaluation of protein stability was conducted to assess the stability of AMPK following overexpression of KAT2A in GMECs. The GMECs were treated with cycloheximide (CHX; #HY-12320; purity: 99.86%; 100 μg/mL; MedChem Express, Monmouth Junction, NJ, United States) at 0, 3, 6, and 9 hours. Subsequently, the protein concentration of AMPK was analyzed via western blot.
Immunofluorescence (IF) assay was performed to determine the distribution of AMPK and LKB1 in GMECs according to a previous study[26]. Antibodies used included anti-AMPK (ab314032; Abcam; 1:200) and anti-LKB1 (PA5-96062; Thermo Fisher; 1:200).
SPSS 21.0 software was used to analyze the data. Data are presented as mean ± SD. Student’s t-test was employed for pairwise comparisons between two groups, while one-way analysis of variance (ANOVA) was utilized for comparisons among multiple groups with Tukey’s post hoc analysis. Statistical analyses were performed using GraphPad Prism software (v8.0.1; GraphPad Software Inc., San Diego, CA, United States). A P value < 0.05 was considered statistically significant.
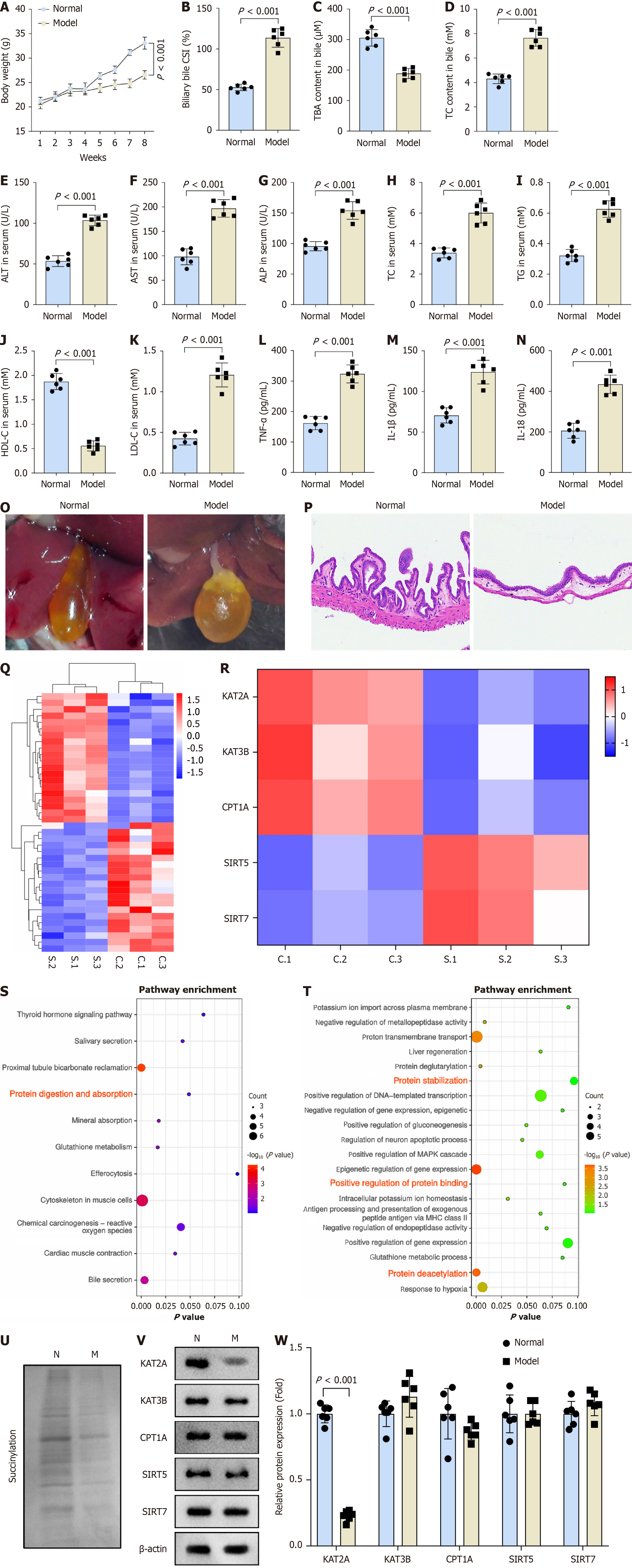
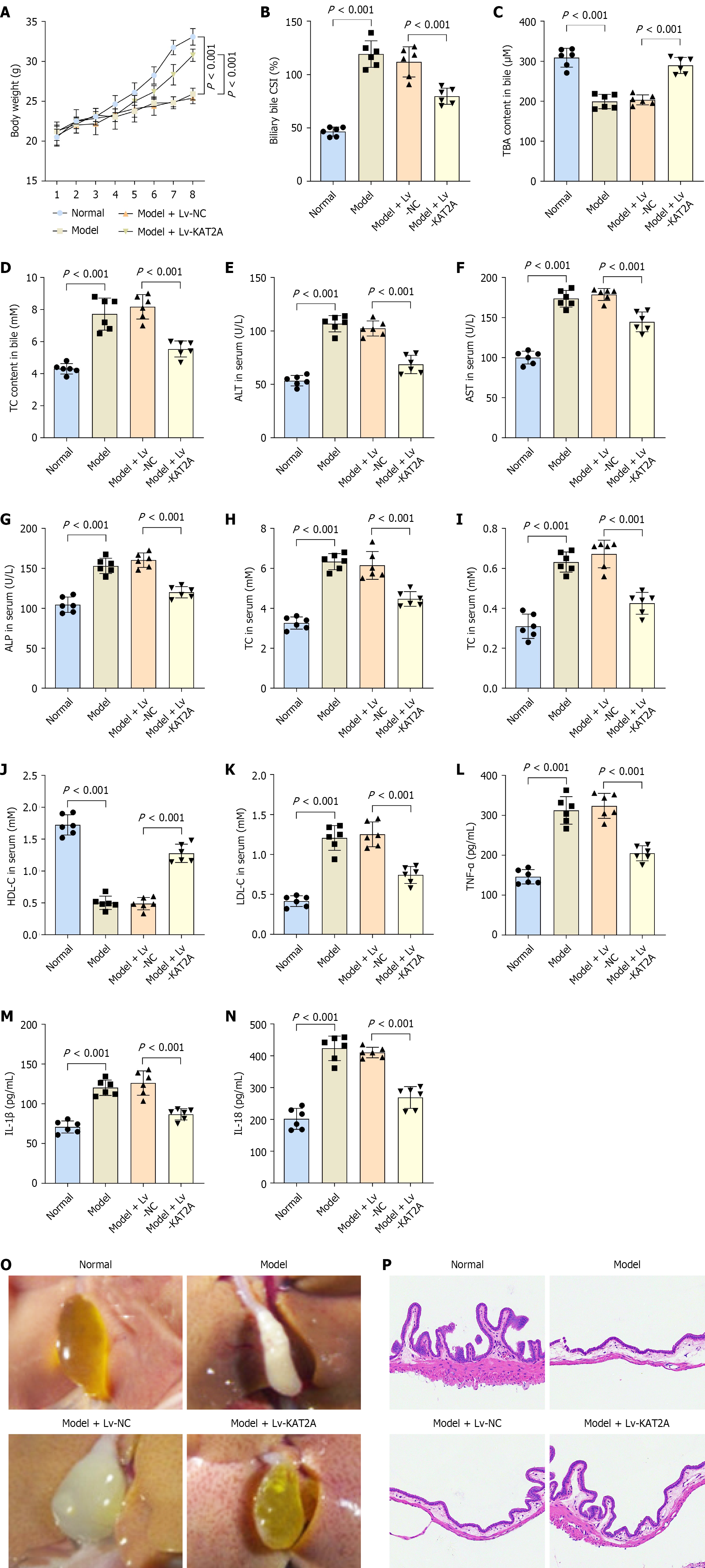
A cholelithiasis mouse model was established in this study. Compared to the normal group, model group mice exhibited decreased body weight (Figure 1A). Furthermore, the model group demonstrated increased biliary bile CSI and TC content, alongside reduced TBA content in bile (Figure 1B-D). ELISA results indicated elevated levels of ALT, AST, ALP, TC, TG, LDH-C, TNF-α, IL-1β, and IL-18 as well as decreased HDL-C level in the model group mice relative to the normal group (Figure 1E-N). Additionally, gallbladder bile from the model group appeared dark yellow with granular stones, and microscopic examination revealed classical cholesterol crystals (Figure 1O). H&E staining confirmed gallbladder inflammation in the model group, characterized by gallbladder wall thickening, inflammatory cell infiltration in the stromal layer, and submucosal vasodilation, compared to the normal group (Figure 1P). RNA-seq analysis was performed to identify DEGs between the normal and model groups (Figure 1Q). Succinylation has been implicated in the pathoge
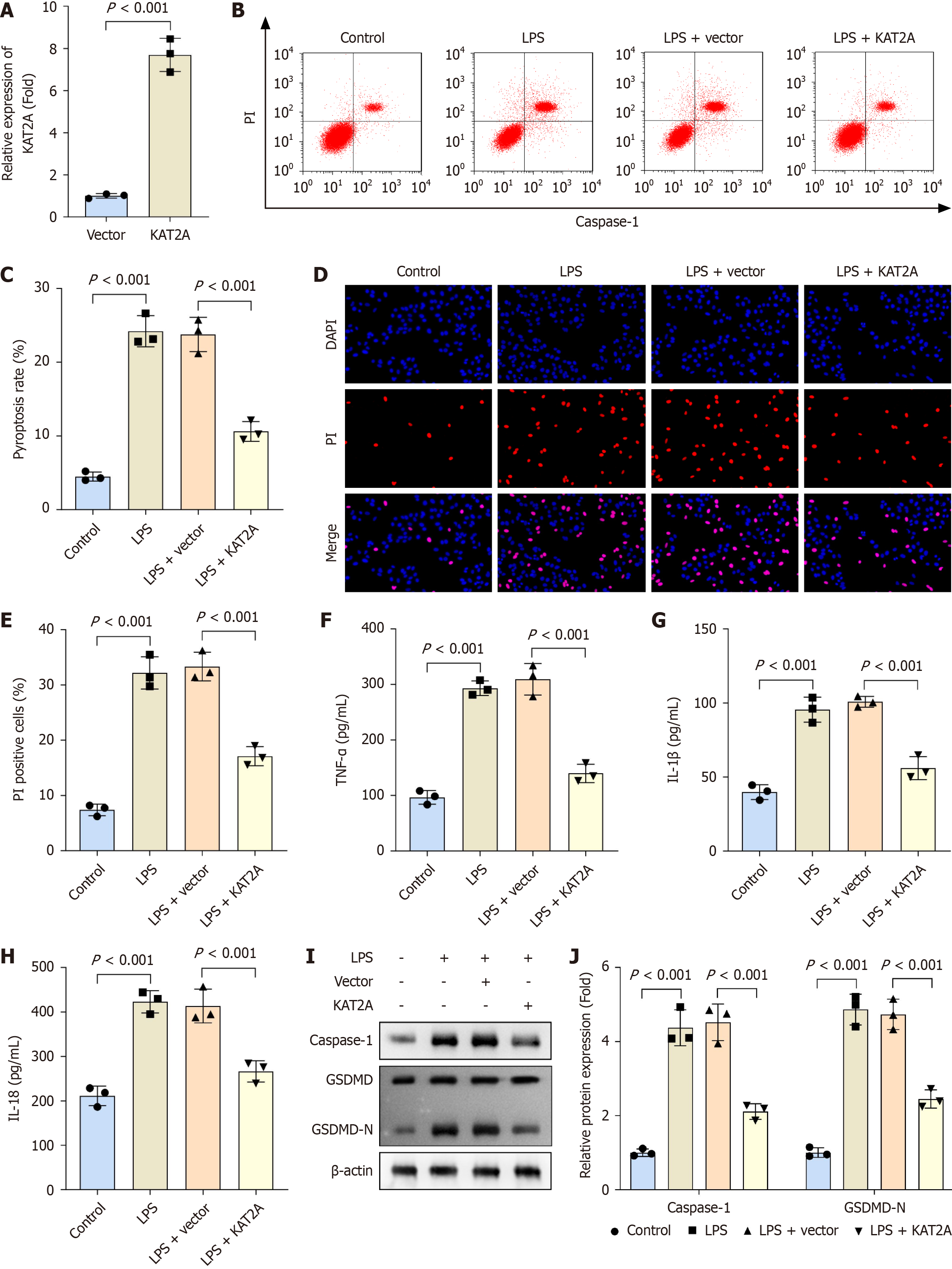
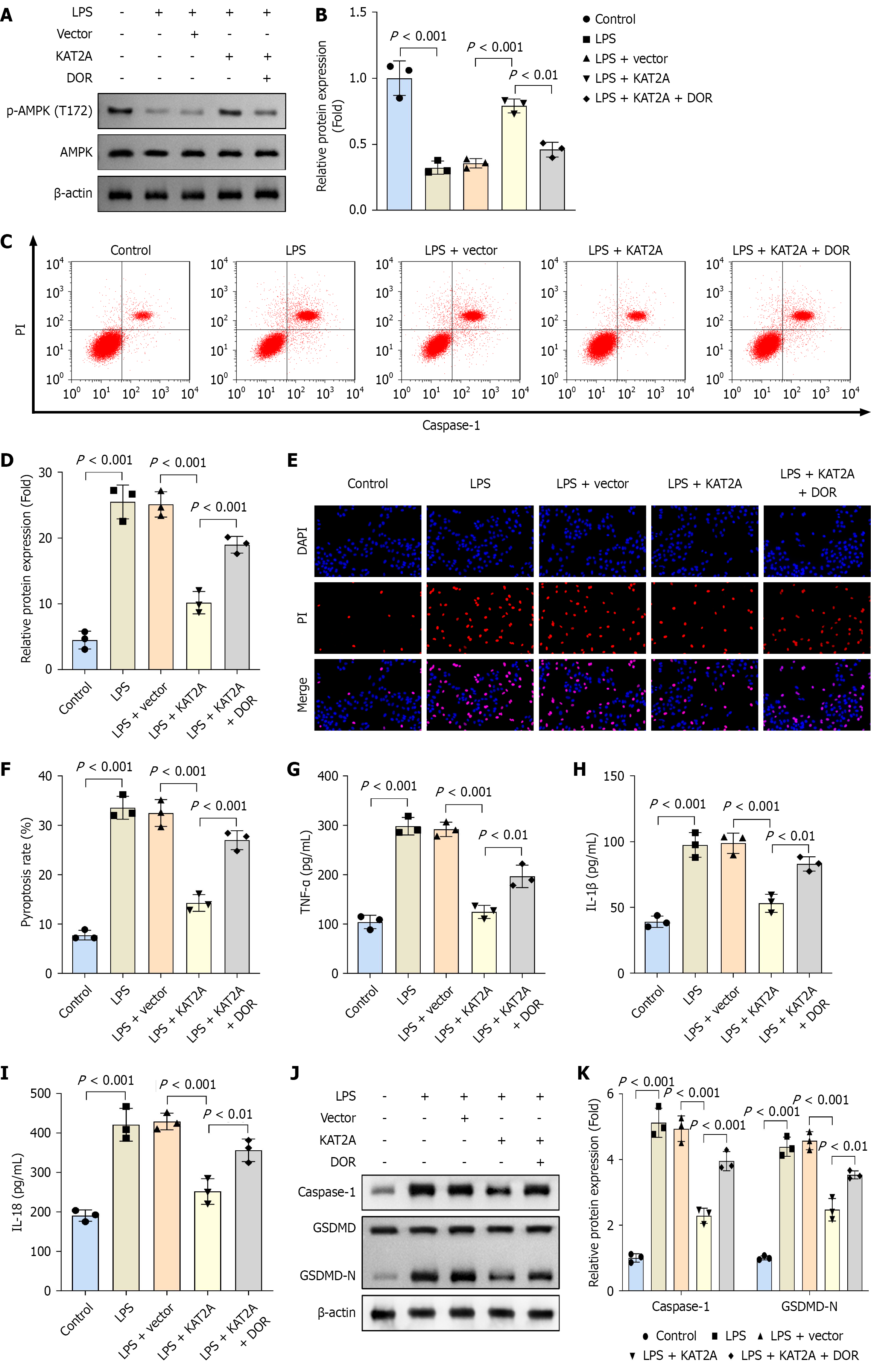
To further investigate the role of KAT2A in cholelithiasis, GMECs were isolated from gallbladder tissues for in vitro experiments. Following transfection of the KAT2A overexpression vector into GMECs, mRNA expression of KAT2A was significantly upregulated (Figure 2A). Additionally, treatment with LPS increased pyroptosis rate, PI positive cells, and inflammatory markers (TNF-α, IL-1β, and IL-18) compared to the control group. Furthermore, KAT2A overexpression reduced pyroptosis rate, PI positive cells, and pro-inflammatory markers (TNF-α, IL-1β, and IL-18) in LPS-treated GMECs (Figure 2B-H). Caspase-1 and GSDMD-N are representative pyroptosis-related proteins. After LPS treatment, protein levels of caspase-1 and GSDMD-N were elevated compared to the control group; however, KAT2A overexpression reversed these increases in the LPS + vector group (Figure 2I and J).
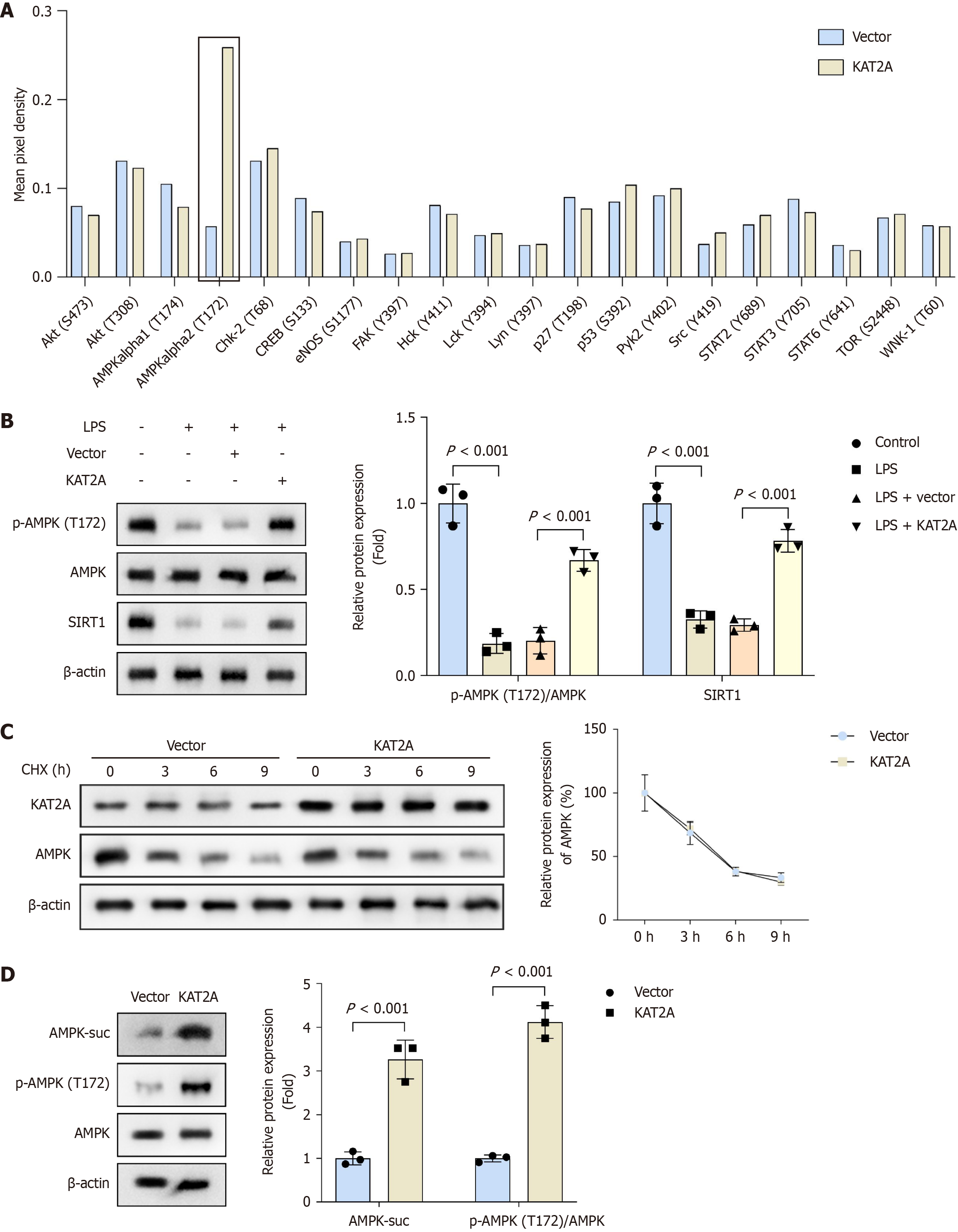
Phospho-kinase array results showed that overexpression of KAT2A did not significantly affect most kinases on the chip, except for the specific upregulation of AMPK alpha2 (T172) phosphorylation (Figure 3A). Consequently, we further analyzed the protein levels of phosphorylated AMPK (p-AMPK), total AMPK, and SIRT1. Our findings revealed that LPS treatment reduced the protein levels of p-AMPK/AMPK and SIRT1 compared to the control group. Additionally, compared to the vector group, KAT2A overexpression increased the protein levels of p-AMPK/AMPK and SIRT1 (Figure 3B). Furthermore, protein stability assay results indicated that KAT2A overexpression did not alter the total AMPK protein level (Figure 3C). Notably, following KAT2A overexpression, the protein levels of succinylated AMPK (AMPK-suc) and p-AMPK/AMPK were upregulated (Figure 3D). These results suggested that KAT2A overexpression activates the AMPK/SIRT1 signaling pathway.
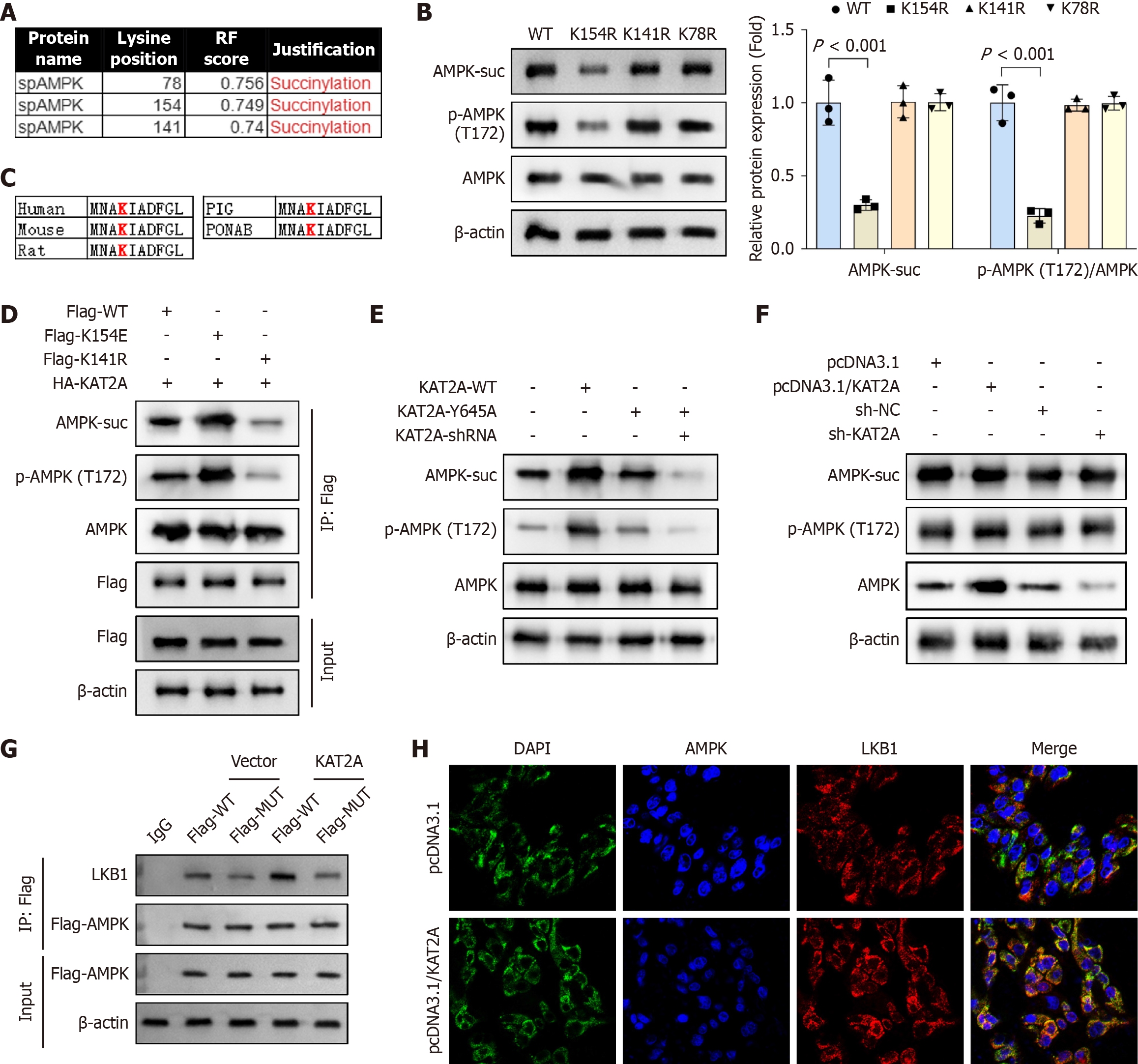
Three lysine residues (K78, K154, and K141) were predicted by GPSuc to be potential succinylation sites (Figure 4A). To determine the specific site(s) at which AMPK is succinylated, plasmids encoding the K78R, K154R, and K141R mutations were transfected into GMECs. Western blot analysis revealed that transfection with K154R significantly reduced the protein levels of AMPK-suc and p-AMPK/AMPK compared to K141R and K78R transfections, indicating that K154 is the critical succinylation site (Figure 4B). Notably, K154 is highly conserved across multiple species, including human, mouse, rat, pig, and Pongo abelii (Figure 4C). To further investigate the functional role of K154 succinylation, mutant constructs encoding AMPK-K154R (a non-succinylatable variant) and AMPK-K154E (a mimic of AMPK-suc) were generated. The K154R mutation blocks succinylation, while the K154E mutation enhances it. Results showed that FLAG-tagged K154E significantly upregulated AMPK-suc and p-AMPK/AMPK levels compared to FLAG-tagged WT AMPK, whereas FLAG-tagged K154R downregulated these levels (Figure 4D). To validate the role of KAT2A in AMPK succinylation, a KAT2A-Y645A mutant plasmid (which disrupts KAT2A’s succinylase activity) was constructed. Compared to the KAT2A-WT group, the KAT2A-Y645A group exhibited reduced AMPK-suc and p-AMPK/AMPK levels in GMECs (Figure 4E). As LKB1 encodes a serine/threonine kinase that directly phosphorylates and activates AMPK, functioning as a key metabolic sensor[29], we hypothesized that KAT2A might regulate AMPK phosphorylation via LKB1. Western blot analysis demonstrated that neither overexpression nor silencing of KAT2A altered the expression levels of LKB1-suc or total LKB1 in GMECs (Figure 4F). However, Co-IP experiments revealed that AMPK mutation suppressed the interaction between LKB1 and AMPK; moreover, when KAT2A was overexpressed along with the AMPK site mutation, the binding was also attenuated (Figure 4G). Furthermore, IF imaging confirmed that KAT2A overexpression promoted LKB1-AMPK binding in GMECs (Figure 4H). These findings demonstrated that KAT2A promotes AMPK succinylation, thereby strengthening the LKB1-AMPK interaction and ultimately enhancing AMPK phospho
To inhibit AMPK expression, GMECs were treated with DOR. Western blot results showed that after DOR treatment, the relative protein level of p-AMPK (T172)/AMPK was decreased (Figure 5A and B). In addition, DOR group exhibited increased pyroptosis rates, PI positive cells, pro-inflammatory markers (TNF-α, IL-1β, and IL-18), and elevated protein levels of caspase-1 and GSDMD-N compared to the LPS + KAT2A group (Figure 5C-K).
In the in vivo study, overexpression of KAT2A resulted in increased body weight compared to the Lv-NC group (Figure 6A). Furthermore, the KAT2A overexpression group exhibited decreased biliary bile CSI and TC content, alongside increased TBA content in bile compared to the Lv-NC group (Figure 6B-D). Additionally, ELISA results revealed reduced levels of ALT, AST, ALP, TC, TG, LDL-C, TNF-α, IL-1β, and IL-18 and increased HDL-C level in the Lv-KAT2A group mice compared to the Lv-NC group (Figure 6E-N). Moreover, gallstone formation was alleviated in the Lv-KAT2A group compared to the Lv-NC group (Figure 6O). H&E staining demonstrated reduced gallbladder inflammation in the Lv-KAT2A group, characterized by lessened wall thickening, inflammatory cell infiltration, and submucosal vasodilation, compared to the Lv-NC group (Figure 6P).
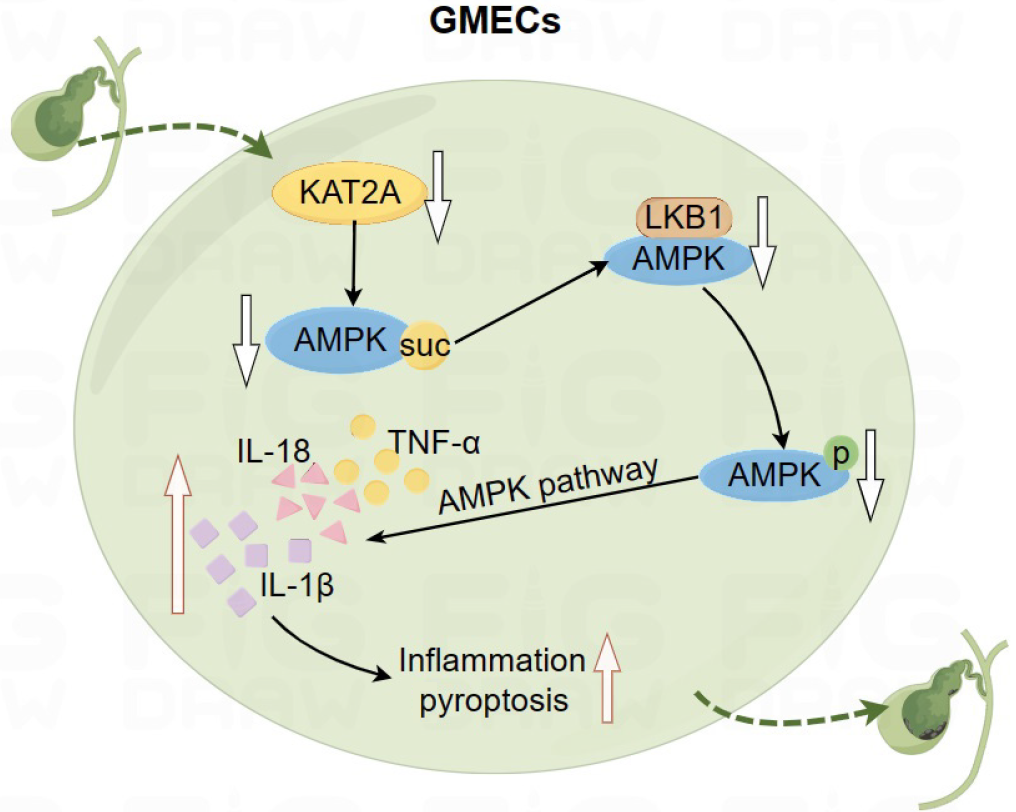
Down-regulation of KAT2A reduces AMPK succinylation. Reduced AMPK succinylation inhibits the interaction between AMPK and LKB1, thereby suppressing AMPK phosphorylation. Inhibition of AMPK phosphorylation promotes pyroptosis and inflammation in GMECs, ultimately leading to gallstone formation (Figure 7).
Cholelithhiasis is primarily categorized into two types: Gallbladder stones and bile duct stones. Stones in the gallbladder or bile duct stimulate the mucosa, causing inflammatory reactions and potentially leading to secondary infections. These conditions significantly impact patients’ quality of life and health, often resulting in severe abdominal pain[22]. Consequently, patients with cholelithiasis frequently exhibit elevated inflammatory markers. In our study, we established a cholelithiasis mouse model by feeding mice a HFD and observed that the model group exhibited increased inflammation and gallstone formation in gallbladder tissues. Similar findings have been reported in studies showing elevated inflammation and apoptosis in patients with cholelithiasis, acute cholecystitis, or gallbladder adenomyomatosis[30-32]. Furthermore, chronic, unresolved inflammation is a primary carcinogenic mechanism in gallbladder cancer[30]. There
Succinylation has been implicated in various digestive tract tumors and non-neoplastic diseases. For instance, Zhang
The AMPK/SIRT1 signaling pathway is widely involved in the progression of gastrointestinal diseases, such as colon neoplasia[39]. In this study, we observed that KAT2A overexpression enhanced AMPK/SIRT1 signaling activation in GMECs. Furthermore, KAT2A overexpression increased the succinylation level of AMPK at the K170 site, suggesting that KAT2A-mediated AMPK succinylation may inhibit cholelithiasis progression through modulation of the AMPK/SIRT1 pathway. Subsequent functional rescue experiments revealed that DOR treatment elevated pyroptosis rates, inflammatory responses, and the protein levels of caspase-1 and GSDMD-N, which corroborated these findings. Similarly, a previous study demonstrates that activation of the AMPK/SIRT1 signaling pathway inhibits gallbladder damage and gallstone formation[22]. Additionally, SIRT1 regulates the phosphorylation level of AMPK[18]. The role of KAT2A in AMPK/SIRT1 signaling has not been discovered before. However, other succinyltransferases have been found to regulate the activation of the AMPK signaling pathway. Zhang et al[40] reveal that SIRT5 knockout protects against transverse aortic constriction-induced cardiac remodeling by modulating cardiac energy metabolism and enhancing AMPK signaling.
The current study has several limitations: (1) It lacks validation using samples from patients with cholelithiasis, which restricts the direct translation of our findings to the clinical setting of human cholelithiasis; (2) This study mainly focuses on KAT2A-mediated succinylation. It is not yet clear whether there are other PTMs in cholelithiasis; and (3) Although we observed that KAT2A overexpression influences the AMPK-LKB1 interaction, we did not investigate the precise conformational mechanisms by which succinylation drives this change. We aim to address these limitations in our future research.
This study first demonstrates that KAT2A-mediated succinylation of AMPK inhibits cholelithiasis progression by modulating the AMPK/SIRT1 signaling pathway, which might offer novel insights into the molecular mechanisms underlying cholelithiasis.
| 1. | Wilkins T, Agabin E, Varghese J, Talukder A. Gallbladder Dysfunction: Cholecystitis, Choledocholithiasis, Cholangitis, and Biliary Dyskinesia. Prim Care. 2017;44:575-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | E S, Srikanth MS, Shreyas A, Desai S, Mehdi S, Gangadharappa HV, Suman, Krishna KL. Recent advances, novel targets and treatments for cholelithiasis; a narrative review. Eur J Pharmacol. 2021;908:174376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Dan WY, Yang YS, Peng LH, Sun G, Wang ZK. Gastrointestinal microbiome and cholelithiasis: Current status and perspectives. World J Gastroenterol. 2023;29:1589-1601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 4. | Pak M, Lindseth G. Risk Factors for Cholelithiasis. Gastroenterol Nurs. 2016;39:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Dutt MK, Murphy GM, Thompson RP. Unconjugated bilirubin in human bile: the nucleating factor in cholesterol cholelithiasis? J Clin Pathol. 2003;56:596-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Hyogo H, Tazuma S, Cohen DE. Cholesterol gallstones. Curr Opin Gastroenterol. 2002;18:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Han ZJ, Feng YH, Gu BH, Li YM, Chen H. The post-translational modification, SUMOylation, and cancer (Review). Int J Oncol. 2018;52:1081-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 8. | Zhao G, Zhen J, Liu X, Guo J, Li D, Xie J, Xie L. Protein post-translational modification by lysine succinylation: Biochemistry, biological implications, and therapeutic opportunities. Genes Dis. 2023;10:1242-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Guo YR, Liu K, Yin Z, Liu R, Xia Y, Tan L, Yang P, Lee JH, Li XJ, Hawke D, Zheng Y, Qian X, Lyu J, He J, Xing D, Tao YJ, Lu Z. KAT2A coupled with the α-KGDH complex acts as a histone H3 succinyltransferase. Nature. 2017;552:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 417] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 10. | Ye L, Yu Z, He L, Yuan J, Zhang X, Li L, Huang X, Ma Y, Zhang L. KAT2A-mediated succinylation modification of notch1 promotes the proliferation and differentiation of dental pulp stem cells by activating notch pathway. BMC Oral Health. 2024;24:407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Zeng K, Yin H. KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1. Open Life Sci. 2024;19:20220785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Zhang C, Huang Z. KAT2A Promotes the Succinylation of PKM2 to Inhibit its Activity and Accelerate Glycolysis of Gastric Cancer. Mol Biotechnol. 2024;66:1446-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Tong Y, Guo D, Yan D, Ma C, Shao F, Wang Y, Luo S, Lin L, Tao J, Jiang Y, Lu Z, Xing D. KAT2A succinyltransferase activity-mediated 14-3-3ζ upregulation promotes β-catenin stabilization-dependent glycolysis and proliferation of pancreatic carcinoma cells. Cancer Lett. 2020;469:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Zhu Y, Lu F. Astragaloside IV inhibits cell viability and glycolysis of hepatocellular carcinoma by regulating KAT2A-mediated succinylation of PGAM1. BMC Cancer. 2024;24:682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 15. | Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 3041] [Article Influence: 337.9] [Reference Citation Analysis (0)] |
| 16. | Yang Y, Liu Y, Wang Y, Chao Y, Zhang J, Jia Y, Tie J, Hu D. Regulation of SIRT1 and Its Roles in Inflammation. Front Immunol. 2022;13:831168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 359] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 17. | Lu C, Zhao H, Liu Y, Yang Z, Yao H, Liu T, Gou T, Wang L, Zhang J, Tian Y, Yang Y, Zhang H. Novel Role of the SIRT1 in Endocrine and Metabolic Diseases. Int J Biol Sci. 2023;19:484-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 18. | Liou CJ, Lee YK, Ting NC, Chen YL, Shen SC, Wu SJ, Huang WC. Protective Effects of Licochalcone A Ameliorates Obesity and Non-Alcoholic Fatty Liver Disease Via Promotion of the Sirt-1/AMPK Pathway in Mice Fed a High-Fat Diet. Cells. 2019;8:447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 19. | Ren FF, Xie ZY, Jiang YN, Guan X, Chen QY, Lai TF, Li L. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol Sin. 2022;43:1721-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Yang M, Lv H, Liu Q, Zhang L, Zhang R, Huang X, Wang X, Han B, Hou S, Liu D, Wang G, Hou J, Yu B. Colchicine Alleviates Cholesterol Crystal-Induced Endothelial Cell Pyroptosis through Activating AMPK/SIRT1 Pathway. Oxid Med Cell Longev. 2020;2020:9173530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Li F, Chen Y, Li Y, Huang M, Zhao W. Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur J Pharmacol. 2020;886:173449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 22. | Wang G, Zhang H, Zhou Z, Jin W, Zhang X, Ma Z, Wang X. AQP3-mediated activation of the AMPK/SIRT1 signaling pathway curtails gallstone formation in mice by inhibiting inflammatory injury of gallbladder mucosal epithelial cells. Mol Med. 2023;29:116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Zhao F, Ma S, Zhou Y, Wei B, Hao Z, Cui X, Xing L, Liu G, Jin L, Ma T, Shi L. miRNA-223 Suppresses Mouse Gallstone Formation by Targeting Key Transporters in Hepatobiliary Cholesterol Secretion Pathway. Int J Biol Sci. 2021;17:4459-4473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Yang B, Cao P, Bao G, Wu M, Chen W, Wu S, Luo D, Bi P. Inhibiting miRNA-146a suppresses mouse gallstone formation by regulating LXR/megalin/cubilin-media cholesterol absorption. Heliyon. 2024;10:e36679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Wu S, Han Y, Shi H, Yuan J, Cui W. LncRNA SH3PXD2A-AS1 facilitates cisplatin resistance in non-small cell lung cancer by regulating FOXM1 succinylation. BMC Cancer. 2024;24:848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Wu Y, Chen W, Miao H, Xu T. SIRT7 promotes the proliferation and migration of anaplastic thyroid cancer cells by regulating the desuccinylation of KIF23. BMC Cancer. 2024;24:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 27. | Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, Xu Y, Zhou L, Chu Y, Zhang C, Qin X, Yang P, Yu H. SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1β Production and to Prevent DSS-Induced Colitis in Mice. Cell Rep. 2017;19:2331-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 28. | Qin YP, Yu HB, Yuan SY, Yang Z, Ren F, Wang Q, Li F, Ren JH, Cheng ST, Zhou YJ, He X, Zhou HZ, Zhang Y, Tan M, Yang ML, Zhang DP, Wen X, Dong ML, Zhang H, Liu J, Li ZH, Chen Y, Huang AL, Chen WX, Chen J. KAT2A Promotes Hepatitis B Virus Transcription and Replication Through Epigenetic Regulation of cccDNA Minichromosome. Front Microbiol. 2021;12:795388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 29. | Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1563] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 30. | Espinoza JA, Bizama C, García P, Ferreccio C, Javle M, Miquel JF, Koshiol J, Roa JC. The inflammatory inception of gallbladder cancer. Biochim Biophys Acta. 2016;1865:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Hong K, Yang Q, Liu G, Qiu H, Yu B. Cyclophilin D Regulates Oxidative Stress and Apoptosis via Mitochondrial Permeability Transition Pore in Acute Acalculous Cholecystitis. Curr Mol Med. 2023;23:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Lee HJ, Chung WS, Kim JY, An JH, Park S. Chronic inflammation-related radiological findings of gallbladder adenomyomatosis. Jpn J Radiol. 2020;38:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Yang F, Bettadapura SN, Smeltzer MS, Zhu H, Wang S. Pyroptosis and pyroptosis-inducing cancer drugs. Acta Pharmacol Sin. 2022;43:2462-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 34. | Burdette BE, Esparza AN, Zhu H, Wang S. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11:2768-2782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 497] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 35. | Cao Y, Wang Z, Yan Y, Ji L, He J, Xuan B, Shen C, Ma Y, Jiang S, Ma D, Tong T, Zhang X, Gao Z, Zhu X, Fang JY, Chen H, Hong J. Enterotoxigenic Bacteroidesfragilis Promotes Intestinal Inflammation and Malignancy by Inhibiting Exosome-Packaged miR-149-3p. Gastroenterology. 2021;161:1552-1566.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 36. | Chen R, Hu J, Zhang Y, Liu Y, Zhu J, Pan Z, Yang H, Wang Q, Chen Y, Tang S, Min B. Total glucosides of paeony ameliorates chemotherapy-induced neuropathic pain by suppressing microglia pyroptosis through the inhibition of KAT2A-mediated p38 pathway activation and succinylation. Sci Rep. 2024;14:31875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 37. | Ouyang C, Mu J, Lu Q, Li J, Zhu H, Wang Q, Zou MH, Xie Z. Autophagic degradation of KAT2A/GCN5 promotes directional migration of vascular smooth muscle cells by reducing TUBA/α-tubulin acetylation. Autophagy. 2020;16:1753-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Dong Z, He W, Lin G, Chen X, Cao S, Guan T, Sun Y, Zhang Y, Qi M, Guo B, Zhou Z, Zhuo R, Wu R, Liu M, Liu Y. Histone acetyltransferase KAT2A modulates neural stem cell differentiation and proliferation by inducing degradation of the transcription factor PAX6. J Biol Chem. 2023;299:103020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 39. | Talero E, Alcaide A, Ávila-Román J, García-Mauriño S, Vendramini-Costa D, Motilva V. Expression patterns of sirtuin 1-AMPK-autophagy pathway in chronic colitis and inflammation-associated colon neoplasia in IL-10-deficient mice. Int Immunopharmacol. 2016;35:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Zhang M, Wu J, Sun R, Tao X, Wang X, Kang Q, Wang H, Zhang L, Liu P, Zhang J, Xia Y, Zhao Y, Yang Y, Xiong Y, Guan KL, Zou Y, Ye D. SIRT5 deficiency suppresses mitochondrial ATP production and promotes AMPK activation in response to energy stress. PLoS One. 2019;14:e0211796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/