Published online Oct 14, 2025. doi: 10.3748/wjg.v31.i38.111364
Revised: August 2, 2025
Accepted: September 5, 2025
Published online: October 14, 2025
Processing time: 108 Days and 15.4 Hours
Adenoma detection rate (ADR), a key colonoscopy quality metric, varies with patient demographics and procedural factors.
To identify independent predictors of ≥ 25% ADR, develop a risk model, and pro
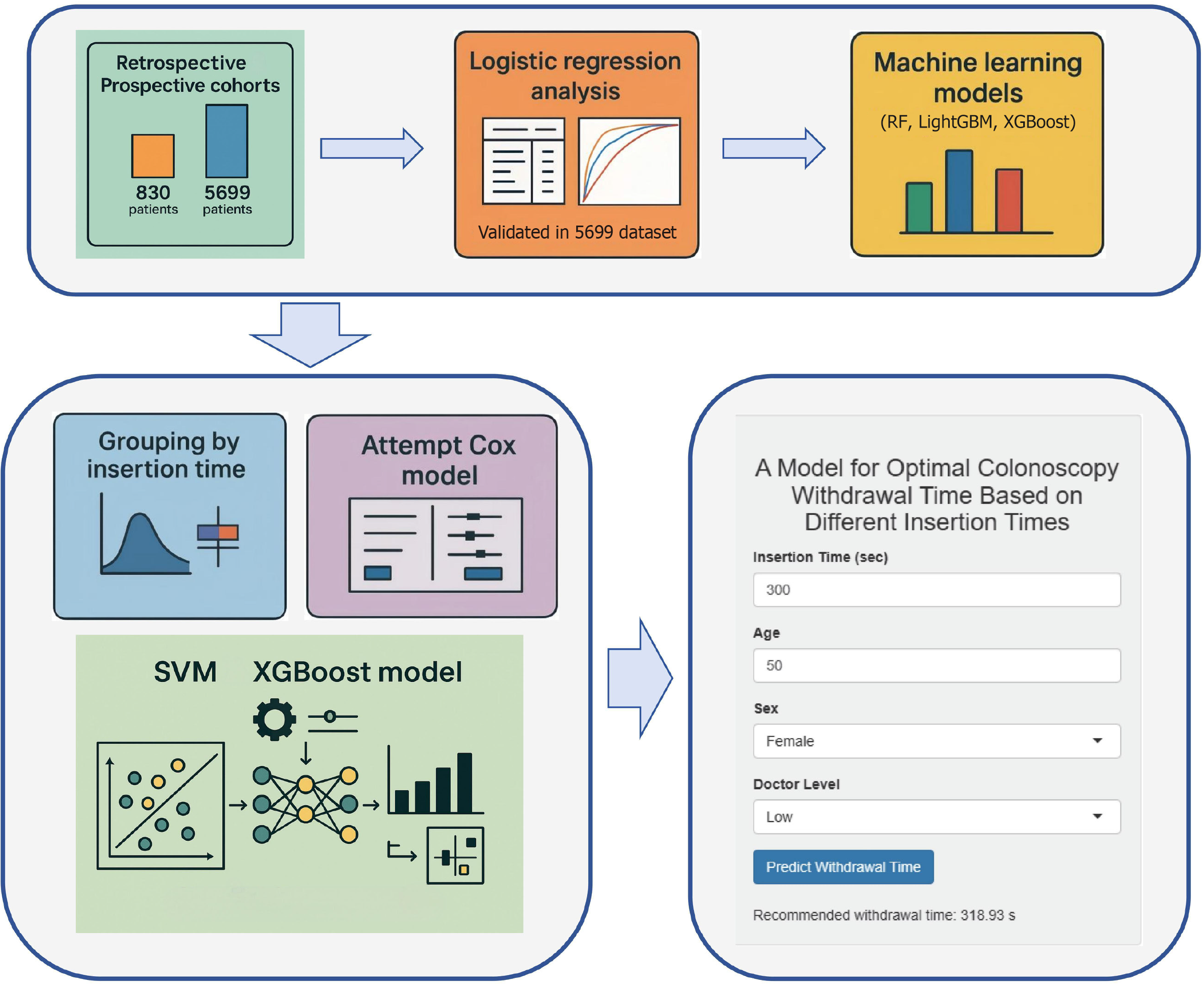
We retrospectively analyzed 830 cases using logistic regression and identified four key factors, validated in a prospective cohort of 5699 patients. Their importance was confirmed using random forest (RF), extreme gradient boosting (XGBoost) and light gradient boosting machine (LightGBM). Attempts to determine target-achieving withdrawal time by grouping cases based on insertion time and Cox re
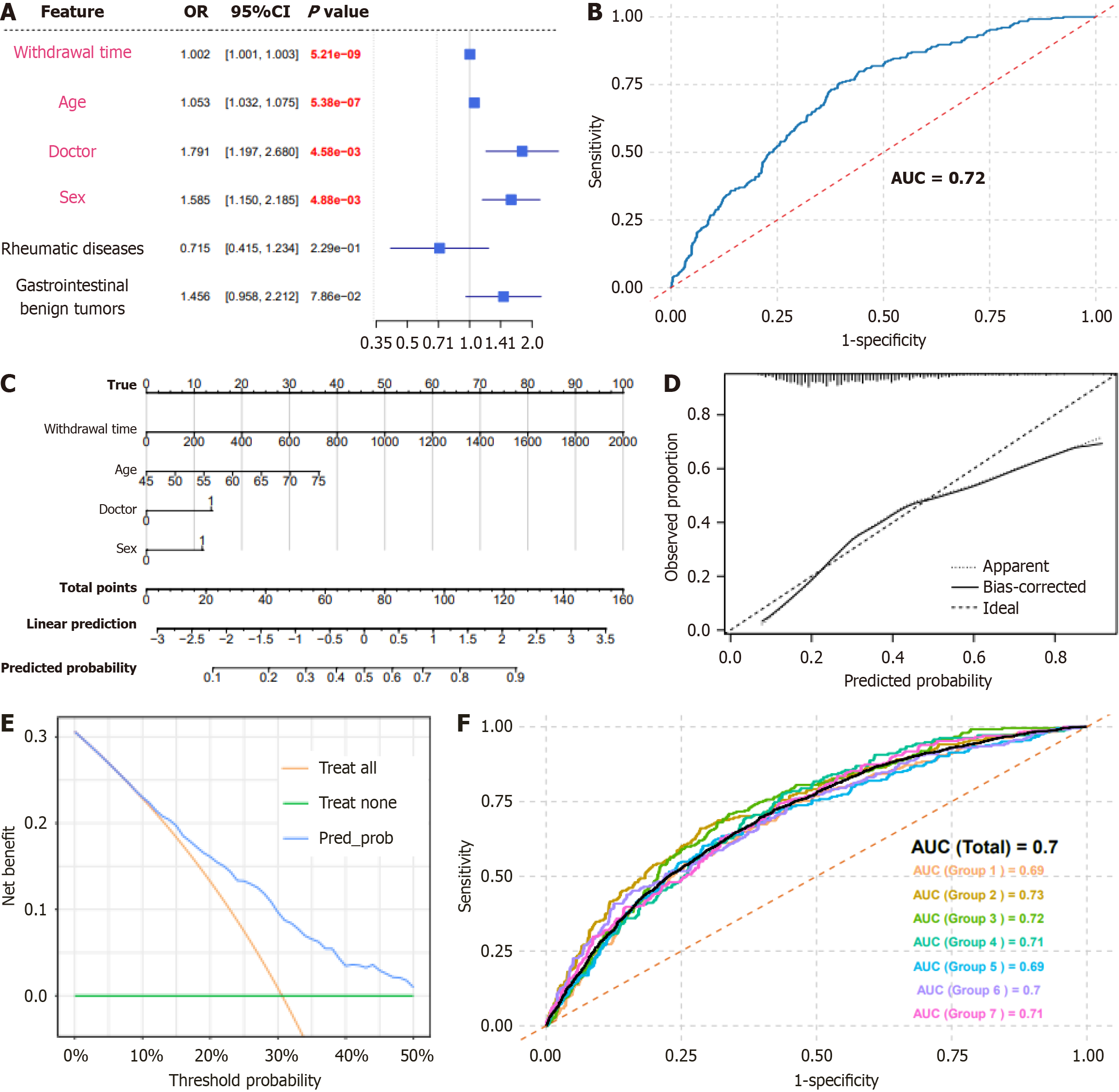
Multivariate logistic regression identified age [odds ratio (OR) = 1.05; 95% confi
A hybrid SVM-XGBoost model using four key endoscopic factors was independently validated and is available as a Shiny app, delivering real-time decision support to streamline endoscopy and enhance clinical outcomes.
Core Tip: This study identifies four key predictors of ≥ 25% adenoma detection rate (ADR), age, sex, endoscopist experience, and withdrawal time and develops a validated hybrid support vector machine extreme gradient boosting model to predict ADR. Using data from 6529 colonoscopies, the model demonstrated stable performance and was implemented in a Shiny app for real-time clinical use. This innovative tool offers personalized guidance to optimize colonoscopy quality and improve clinical outcomes, representing a significant advancement in endoscopic decision support.
- Citation: Xu BX, Xu CZ, Zhang HY, Chen XJ, Wei BN, Yang C. Personalizing withdrawal time by insertion time to achieve target adenoma detection rate in colonoscopy. World J Gastroenterol 2025; 31(38): 111364
- URL: https://www.wjgnet.com/1007-9327/full/v31/i38/111364.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i38.111364
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the second leading cause of cancer related deaths worldwide[1], with incidence rising annually and a trend toward younger onset[2]. CRC develops via the “adenoma-carcinoma” sequence, typically requiring 10-15 years for an adenoma to progress to carcinoma, thus offering a valuable “golden screening window” for early diagnosis and intervention[3]. Early prevention, accurate diagnosis, and effective treatment are essential to reduce CRC mortality, with high-quality colonoscopy being pivotal for this purpose. The adenoma detection rate (ADR) is a vital quality metric for colonoscopy and is influenced by multiple factors, including withdrawal time, bowel-preparation quality, the experience level of the endoscopist, cecal intubation rate, patient characteristics, and intrinsic adenoma features[4]. In asymptomatic, average-risk individuals, the recommended ADR target is ≥ 25% (≥ 30% in men and ≥ 20% in women); each 1% increase in ADR is associated with a 3% reduction in CRC incidence[5]. Therefore, a thorough investigation of colorectal adenoma risk factors and the development of an effective risk prediction model are crucial for enhancing colonoscopy quality and enabling early CRC screening.
Cecal intubation time (CIT), also referred to as “insertion time”, is defined as the interval from the tip of the colono
This study aims to determine, across different insertion-time intervals, the target-achieving withdrawal time required to achieve an ADR of ≥ 25%, thereby providing a robust theoretical foundation and practical guidance for enhancing colonoscopy quality.
This study was conducted in the following two phases at the Digestive Endoscopy Center of Wuxi People’s Hospital Affiliated with Nanjing Medical University.
Phase I (retrospective observational study): In phase I, carried out from January to June 2024, 830 consecutive outpatients undergoing colonoscopy were enrolled. In the retrospective cohort, 1011 patients met the initial inclusion criteria (listed in section “inclusion and exclusion criteria”). Of these, 3 (0.3%) failed cecal intubation, 36 (3.6%) had a history of CRC with concurrent colorectal resection, 1 (0.1%) was diagnosed with familial polyposis, 18 (1.8%) had inflammatory bowel disease, and 123 (12.2%) had incomplete endoscopic data. After these exclusions, 830 patients were included in the final analysis.
Phase II (prospective study): In phase II, conducted from September 2024 to April 2025, 5699 consecutive outpatients undergoing colonoscopy were enrolled. Prospective data collection was completed at submission, while the analysis was still being carried out. In the prospective cohort, 6712 patients met the initial inclusion criteria (section “inclusion and exclusion criteria”). Of these, 5 (0.1%) failed cecal intubation, 234 (3.5%) had a history of CRC with concurrent colorectal resection, 3 (0.04%) were diagnosed with familial polyposis, 89 (1.3%) had inflammatory bowel disease, and 682 (10.2%) had incomplete endoscopic data. After excluding these patients, 5699 were considered in the final analysis.
The study protocol was approved by the Ethics Committee of Wuxi People’s Hospital (No. KY24159) and registered with the Chinese Clinical Trial Registry (No. ChiCTR2400091134).
The inclusion criteria were as follows: Age 45-75 years and adequate bowel preparation, defined as a total Boston bowel preparation scale (BBPS) score ≥ 6. The exclusion criteria were as follows: (1) Diagnosis of CRC or a history of colorectal resection; (2) Familial polyposis syndromes; (3) Inflammatory bowel disease; (4) Inadequate bowel preparation (any bowel segment with BBPS < 2 or total BBPS < 6); (5) Inability to tolerate the procedure or failure to intubate the cecum; and (6) Incomplete clinical or procedural records precluding analysis.
In phase I of the study, we extracted comprehensive patient data comprising basic demographics [age, sex, and body mass index (BMI)], detailed medical history (hypertension, diabetes, hyperlipidemia, nonalcoholic fatty liver disease, coronary artery disease, renal insufficiency, cerebral infarction, hyper- and hypothyroidism, thyroid nodules, chronic obstructive pulmonary disease, rheumatologic and immunologic disorders, psychiatric conditions, benign gastrointes
In the prospective phase, we focused on the variables identified as independently associated with adenoma detection in phase I namely sex, age, experience level of the endoscopist, and withdrawal time alongside insertion time.
Prior to colonoscopy, all patients underwent pre-procedure evaluation, which included a coagulation profile, infectious disease screening, and electrocardiography. They were placed on a low-residue diet one day before the examination and fasted from food and fluids on the day of the procedure. Bowel preparation consisted of dissolving four sachets of polyethylene glycol-electrolyte powder in 3 L of warm water, which was consumed entirely within two hours 4-6 hours before the examination, until clear, watery effluent was achieved. Bowel preparation was assessed based primarily on the BBPS applied during withdrawal following colonic cleansing and suctioning; at this stage, scores were assigned to three colonic segments: The right colon (cecum and ascending colon), the transverse colon (hepatic flexure, transverse colon, and splenic flexure), and the left colon (descending colon, sigmoid colon, and the rectum). Scoring was based on the amount of residual material and the degree of mucosal visibility; each segment was scored from 0 to 3, and the total BBPS score was the sum of the three segmental scores, with higher totals indicating superior bowel preparation quality. A score of 0 denotes that large amounts of solid fecal matter adhere and cannot be cleared, rendering the mucosa invisible; a score of 1 indicates that only a portion of the mucosa is visible, with the remainder obscured by residual feces and/or opaque liquid; a score of 2 signifies that most of the mucosa can be clearly observed, except for small amounts of residual feces and/or opaque liquid covering the lumen; and a score of 3 indicates that the entire segment is completely clean, with no residual feces or opaque liquid and full mucosal visibility. All endoscopists systematically evaluated bowel preparation during colonoscopy using the BBPS.
All procedures were performed using an Olympus CV-290 processor and a CF-HQ290I colonoscope, with each examina
ADR was defined as the number of colonoscopies in which at least one adenomatous polyp was detected divided by the total number of colonoscopies, multiplied by 100%. CIT was calculated from the moment the colonoscope tip passed the anal verge to the time it reached the cecum. Withdrawal time was defined as the actual elapsed time from cecal intubation until the colonoscope tip exited the anus; both insertion and withdrawal times were determined from the timestamps of endoscopic photographs. To ensure that the withdrawal time reflected pure mucosal inspection, any extra maneuvers such as chromoendoscopy, biopsy, polypectomy, or cleaning were annotated and excluded by stopping the timer during these interventions and resuming it immediately afterward.
Data analysis was performed in R (version 4.4.1). Continuous variables were expressed as mean ± SD, and categorical variables as counts (percentages). Between-group comparisons were conducted using Student’s t-test or the χ2 test. Univariate logistic regression identified candidate predictors (P < 0.05), which were then entered into a stepwise multivariate logistic regression; odds ratios and 95% confidence intervals were reported, with two-sided P < 0.05 indicating statistical significance. Forest plots and nomograms were generated to visualize the model, and receiver operating characteristic (ROC) curves, calibration curves, and decision curves were plotted to assess discrimination, calibration, and clinical utility. For prospective validation, the second-phase cohort was divided into seven subsets, each approximating the sample size of the retrospective cohort, and ROC curves were used to evaluate model performance. Random forest (RF), extreme gradient boosting (XGBoost) and light gradient boosting machine (LightGBM) algorithms were also applied to confirm factors associated with the ADR.
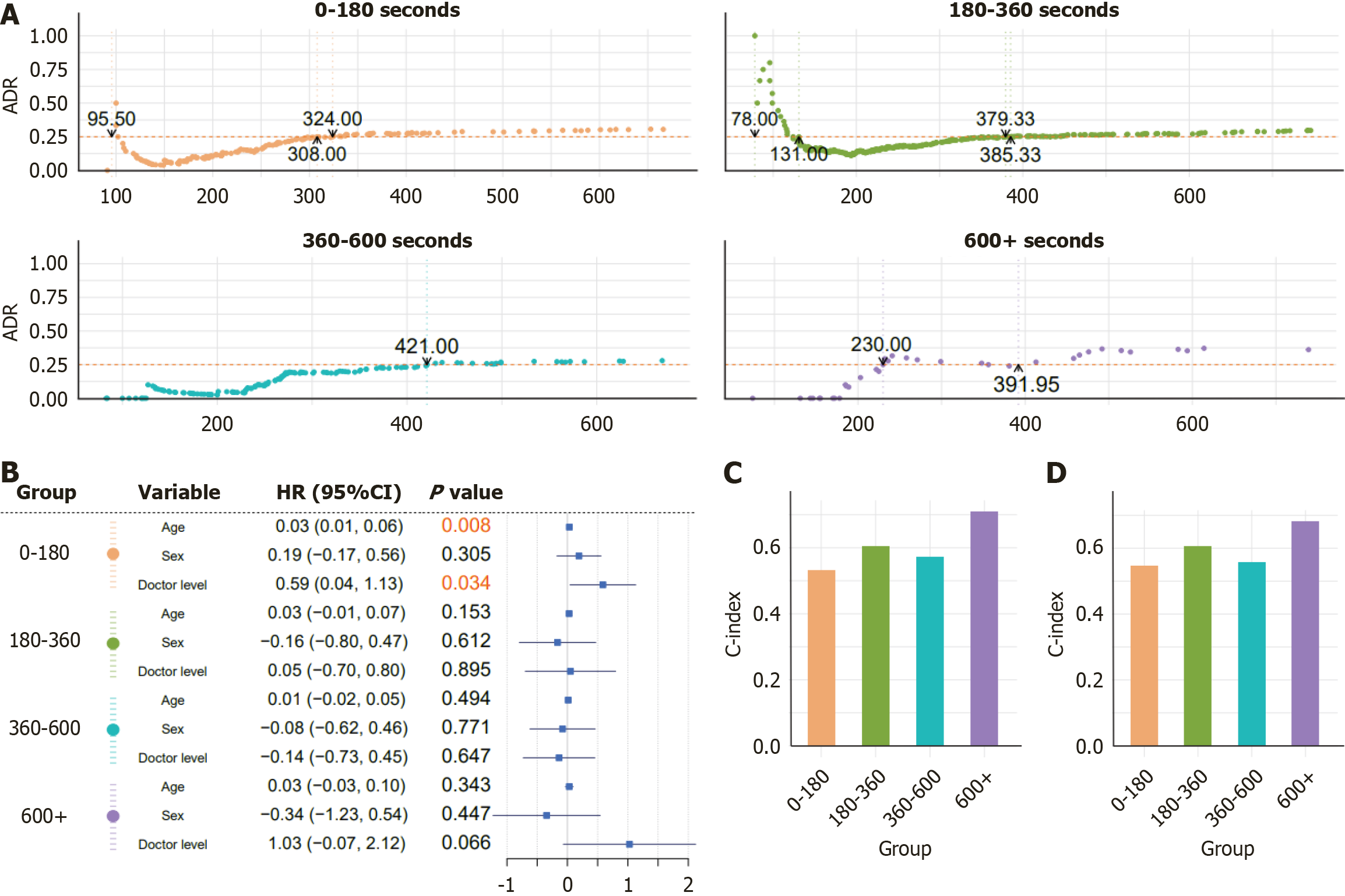
Patient distributions in both cohorts were first summarized. We then assessed the relationship between ADR and withdrawal time by stratifying insertion times into four intervals (< 180 seconds, 180-360 seconds, 360-600 seconds, and > 600 seconds) and estimating, within each stratum, the minimum withdrawal time required to achieve a stable ADR ≥ 25%. Subgroup analyses based on the experience level of the endoscopist, patient age, and sex were also performed to determine the corresponding minimum withdrawal times. Next, a Cox proportional hazards model was constructed and its discrimination was assessed by using the concordance index (C-index), which proved suboptimal. Consequently, after comparing multiple machine learning algorithms, we selected support vector machine (SVM) and XGBoost to develop predictive models of target-achieving colonoscopy withdrawal time across the continuous insertion-time. Model performance and fit were evaluated using residual plots, predicted-versus-observed comparisons, and receiver operating ROC curve analysis.
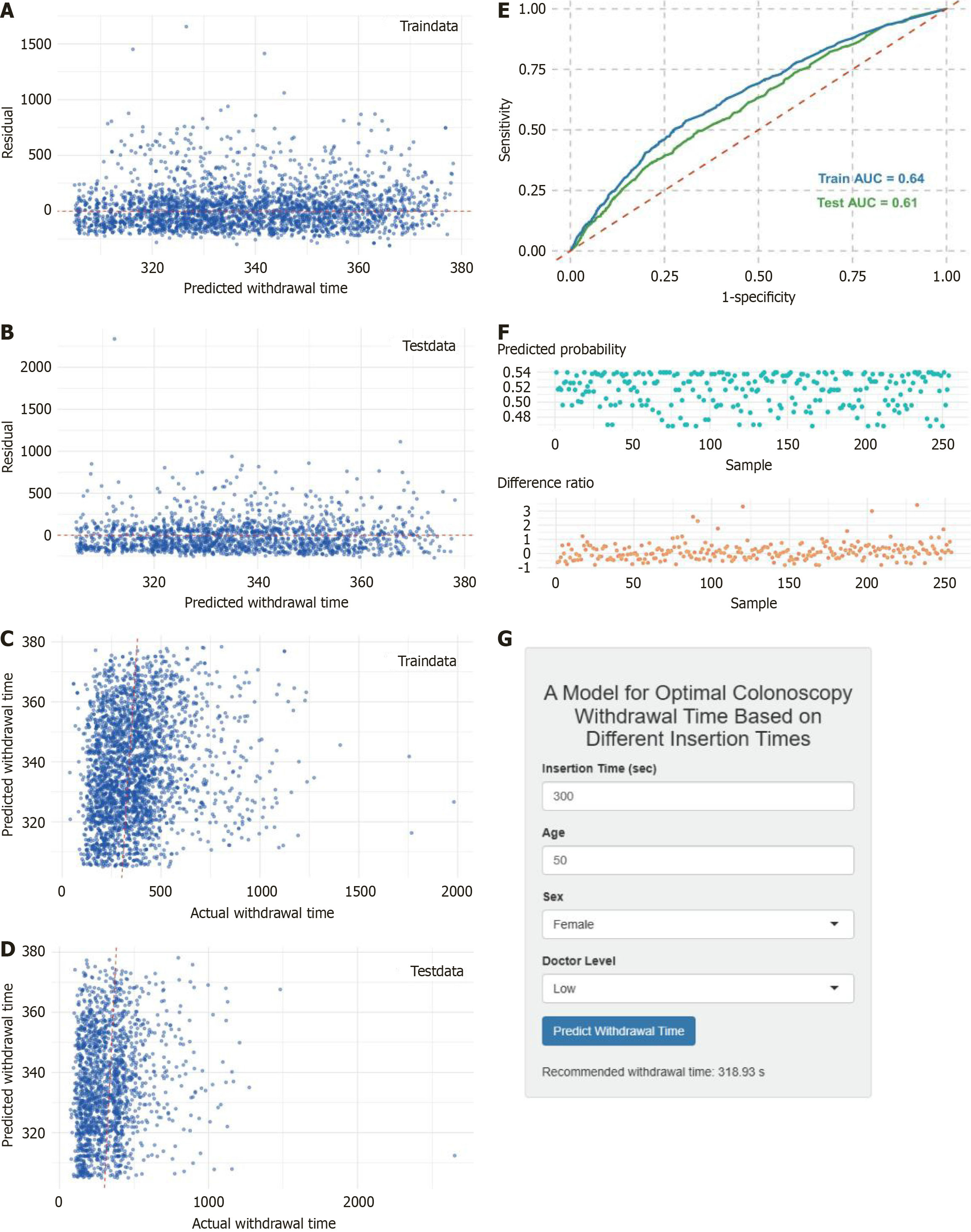
The Shiny application (https://xbxmodel.shinyapps.io/xbx_prediction/) offers a streamlined, single-page interface for real-time, personalized prediction of colonoscopy withdrawal time: Users need to input the cecal insertion time (seconds), patient age, sex (dropdown), and experience level of the endoscopist (“low” or “high”) via numeric fields and selectors, and then click “predict withdrawal time” to display the model-derived minimum withdrawal time required to achieve ≥ 25% ADR. In pre-procedure planning, the anticipated insertion times can be used to establish withdrawal time targets; during live procedures, the countdown timer (not shown) of the app ensures adherence to the calculated withdrawal time. In post-procedure audit and training, the exported predicted vs actual withdrawal time records enable quality-assurance teams to assess compliance, identify training needs, and refine endoscopy protocols.
Of the 830 patients who underwent colonoscopy, 576 were classified as the non-adenoma group and the remaining 254 as the adenoma group based on endoscopic findings. In the non-adenoma group, 262 patients (45.5%) were men, with a mean age of 60.09 ± 8.10 years, and 252 (43.7%) had a BMI of ≥ 24 kg/m². In the adenoma group, 152 patients (59.8%) were men, with a mean age of 63.13 ± 7.29 years, and 111 (43.7%) had a BMI of ≥ 24 kg/m² (Table 1).
| Variables | Total (n = 830) | Non-adenoma (n = 576) | Adenoma (n = 254) | P value |
| Patient characteristics | ||||
| Sex | < 0.001 | |||
| Male | 414 (49.9) | 262 (45.5) | 152 (59.8) | |
| Female | 416 (50.1) | 314 (54.5) | 102 (40.2) | |
| Age | 61.02 ± 7.98 | 60.09 ± 8.10 | 63.13 ± 7.29 | 0.017 |
| BMI | ||||
| < 24 kg/m2 | 467 (56.3) | 324 (56.3) | 143 (56.3) | 0.989 |
| ≥ 24 kg/m2 | 363 (43.7) | 252 (43.7) | 111 (43.7) | |
| Medical history | ||||
| Hypertension | 376 (45.3) | 248 (43.1) | 128 (50.4) | 0.058 |
| Diabetes | 234 (28.2) | 158 (27.4) | 76 (29.9) | 0.462 |
| Hyperlipidemia | 146 (17.6) | 108 (18.8) | 38 (15.0) | 0.186 |
| Hyperlipidemia | 86 (10.4) | 67 (11.6) | 19 (7.5) | 0.071 |
| Coronary heart disease | 121 (14.6) | 92 (16.0) | 29 (11.4) | 0.087 |
| Renal insufficiency | 156 (18.8) | 118 (20.5) | 38 (15.0) | 0.060 |
| Stroke | 121 (14.6) | 92 (16.0) | 29 (11.4) | 0.087 |
| Hyperthyroidism | 11 (1.3) | 5 (0.9) | 6 (2.4) | 0.083 |
| Hypothyroidism | 35 (4.2) | 26 (4.5) | 9 (3.5) | 0.521 |
| Thyroid nodules | 122 (14.7) | 83 (14.4) | 39 (15.4) | 0.723 |
| COPD | 6 (0.7) | 4 (0.7) | 2 (0.8) | 0.884 |
| Rheumatic diseases | 96 (11.6) | 76 (13.2) | 20 (7.9) | 0.033 |
| Mental illness | 39 (4.7) | 28 (4.9) | 11 (4.3) | 0.739 |
| Benign tumors of the gastrointestinal tract | 125 (15.1) | 75 (13.0) | 50 (19.7) | 0.015 |
| Other cancers | 74 (8.9) | 53 (9.2) | 21 (8.3) | 0.664 |
| History of abdominopelvic surgery | 122 (14.7) | 85 (14.8) | 37 (14.6) | 0.943 |
| Clinical symptoms | ||||
| Constipation | 30 (3.6) | 20 (3.5) | 10 (3.9) | 0.741 |
| Bellyache | 230 (27.7) | 166 (28.8) | 64 (25.2) | 0.283 |
| Diarrhea | 119 (14.3) | 84 (14.6) | 35 (13.8) | 0.761 |
| Abdominal distension | 44 (5.3) | 32 (5.6) | 12 (4.7) | 0.622 |
| Blood in the stool | 47 (5.7) | 35 (6.1) | 12 (4.7) | 0.437 |
| Black stool | 17 (2.0) | 14 (2.4) | 3 (1.2) | 0.242 |
| Vomit | 3 (0.4) | 2 (0.3) | 1 (0.4) | 0.918 |
| Personal history | ||||
| Smoking | 93 (11.2) | 57 (9.9) | 36 (14.2) | 0.072 |
| Drinking | 61 (7.3) | 41 (7.1) | 20 (7.9) | 0.701 |
In the adenoma group, 211 (83.1%) colonoscopy examinations were performed by high-experience endoscopists. It was also found that 211 (83.1%) of 254 cases in the adenoma group had good intestinal preparation. In addition, 219 (86.2%) patients underwent the procedure under sedation, 22 (8.7%) had insertion times ≥ 10 minutes, and 108 (42.5%) had withdrawal time ≥ 6 minutes (Table 2).
| Variables | Total (n = 830) | Non-adenoma (n = 576) | Adenoma (n = 254) | P value |
| Operating factors | ||||
| Doctor level | 0.016 | |||
| High | 646 (77.8) | 435 (75.5) | 211 (83.1) | |
| Low | 184 (22.2) | 141 (24.5) | 43 (16.9) | |
| BBPS | 0.879 | |||
| Good | 687 (82.8) | 476 (82.6) | 211 (83.1) | |
| Excellent | 143 (17.2) | 100 (17.4) | 43 (16.9) | |
| Anesthesia condition | 0.873 | |||
| No | 112 (13.5) | 77 (13.4) | 35 (13.8) | |
| Yes | 718 (86.5) | 499 (86.6) | 219 (86.2) | |
| Intubation time | 0.809 | |||
| < 10 minutes | 761 (91.7) | 529 (91.8) | 232 (91.3) | |
| ≥ 10 minutes | 69 (8.3) | 47 (8.2) | 22 (8.7) | |
| Withdrawal time | < 0.001 | |||
| < 6 minutes | 592 (71.3) | 446 (77.4) | 146 (57.5) | |
| ≥ 6 minutes | 238 (28.7) | 130 (22.6) | 108 (42.5) |
Univariate logistic regression was performed on the cohort of 830 patients. BMI, history of hypertension, diabetes, hyperlipidemia, coronary artery disease, renal insufficiency, cerebral infarction, hyperthyroidism, hypothyroidism, thyroid nodules, chronic obstructive pulmonary disease, psychiatric disorders, other malignancies, prior abdominal or pelvic surgery, constipation, abdominal pain, diarrhea, altered bowel habits, hematochezia, melena, hematemesis, smoking history, alcohol use, bowel-preparation score, sedation status, and insertion time were found to be not signi
| Variables | Univariable analysis | Multivariable analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Male (%) | 1.79 (1.32-2.41) | < 0.001 | 1.59 (1.15-2.19) | 0.005 |
| Age | 1.05 (1.03-1.07) | < 0.001 | 1.05 (1.03-1.08) | < 0.001 |
| BMI ≥ 24 kg/m2 | 1.00 (0.74-1.34) | 0.989 | ||
| Hypertension | 1.34 (1.00-1.81) | 0.051 | ||
| Diabetes | 1.13 (0.82-1.56) | 0.463 | ||
| Hyperlipidemia | 0.76 (0.51-1.14) | 0.187 | ||
| Hyperlipidemia | 0.61 (0.36-1.05) | 0.073 | ||
| Coronary heart disease | 0.68 (0.43-1.06) | 0.088 | ||
| Renal insufficiency | 0.68 (0.46-1.02) | 0.061 | ||
| Stroke | 0.68 (0.43-1.06) | 0.088 | ||
| Hyperthyroidism | 1.76 (0.84-2.14) | 0.657 | ||
| Hypothyroidism | 0.78 (0.36-1.68) | 0.522 | ||
| Thyroid nodules | 1.08 (0.71-1.63) | 0.723 | ||
| COPD | 1.13 (0.21-6.24) | 0.884 | ||
| Rheumatic diseases | 0.56 (0.34-0.94) | 0.029 | 0.72 (0.42-1.23) | 0.229 |
| Mental illness | 0.89 (0.43-1.81) | 0.739 | ||
| Benign tumors of the gastrointestinal tract | 1.64 (1.11-2.43) | 0.014 | 1.46 (0.96-2.21) | 0.079 |
| Other cancers | 0.89 (0.52-1.51) | 0.664 | ||
| History of abdominopelvic surgery | 0.98 (0.65-1.50) | 0.943 | ||
| Constipation | 1.14 (0.53-2.47) | 0.741 | ||
| Bellyache | 0.83 (0.59-1.16) | 0.283 | ||
| Diarrhea | 0.94 (0.61-1.43) | 0.761 | ||
| Abdominal distension | 0.84 (0.43-1.66) | 0.623 | ||
| Blood in the stool | 0.77 (0.39-1.50) | 0.439 | ||
| Black stool | 0.48 (0.14-1.68) | 0.252 | ||
| Vomit | 1.13 (0.89-3.57) | 0.542 | ||
| Smoking | 1.50 (0.96-2.35) | 0.073 | ||
| Drinking | 1.12 (0.64-1.95) | 0.701 | ||
| Doctor level | 1.59 (1.09-2.32) | 0.016 | 1.79 (1.20-2.68) | 0.005 |
| BBPS | 0.97 (0.66-1.44) | 0.879 | ||
| Intravenous anesthesia | 0.97 (0.63-1.48) | 0.873 | ||
| Intubation time | 1.01 (1.00-1.03) | 0.621 | ||
| Withdrawal time | 1.02 (1.01-1.05) | < 0.001 | 1.02 (1.01-1.05) | < 0.001 |
We visualized multivariate logistic regression results in a forest plot (Figure 1A), discriminative performance, assessed by the area under the ROC curve, was high [area under the curve (AUC) = 0.72; Figure 1B] and a nomogram incorporating sex, age, experience level of the endoscopist, and withdrawal time was generated (Figure 1C). Calibration curves closely followed the ideal reference line, indicating a good model fit (Figure 1D). The decision-curve analysis demonstrated a positive net benefit across threshold probabilities of 10%-50%, suggesting clinical utility (Figure 1E). We split the prospective cohort into seven subsets (approximately n = 800 each) to mirror the retrospective sample size, ensuring stability testing across heterogeneous groups. The overall AUC was 0.700, and the subset AUCs ranged from 0.690 to 0.730 (0.690, 0.730, 0.720, 0.710, 0.690, 0.700, and 0.710 for groups 1-7, respectively; Figure 1F), further confirming robust discrimination and predictive ability of the colorectal adenoma risk-prediction model.
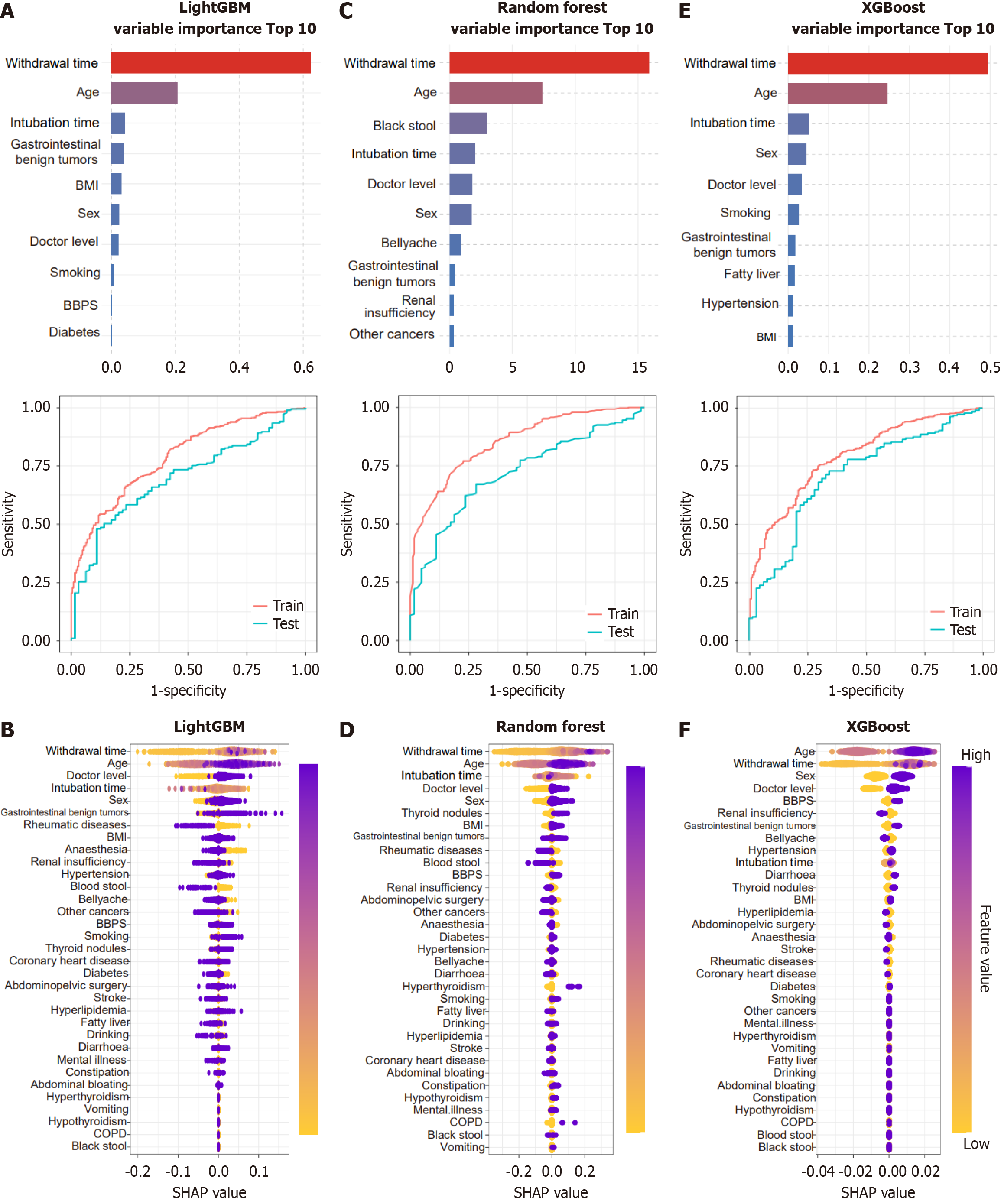
We identified the factors influencing the ADR using RF, XGBoost, and LightGBM algorithms. The following were identified as the top ten predictors in the descending order of importance in the RF model: Withdrawal time, age, smoking history, sex, history of abdominal surgery, BBPS score, alcohol consumption, history of benign gastrointestinal tumors, experience level of the endoscopist, and BMI. In contrast, the following were identified as the most influential features by the XGBoost model: Withdrawal time, age, history of benign gastrointestinal tumors, sex, BBPS score, BMI, insertion time, smoking history, hypertension history, and experience level of the endoscopist. For LightGBM, the leading factors were withdrawal time, age, insertion time, BMI, BBPS score, sex, history of benign gastrointestinal tumors, smoking history, experience level of the endoscopist, and diabetes history. As shown in the ROC curves for the training sets (Figure 2A-C), all three models achieved AUCs close to 1, indicating excellent discrimination. On the test sets, LightGBM demonstrated only a slight decrease in AUC, suggesting robust generalizability. In contrast, XGBoost showed a more pronounced decline, while RF exhibited the largest reduction indicative of overfitting and weaker generalization. These performance patterns were corroborated by SHapley Additive exPlanations (SHAP)-value plots (Figure 2D-F). Collectively, machine learning analyses reinforced that male sex, advanced age, higher endoscopist experience, and longer withdrawal time are independent risk factors for colorectal adenoma.
The distributions of insertion time, withdrawal time, patient sex, and age in the 830- and 5699-patient cohorts are shown in Supplementary Figure 1A-E and Supplementary Figure 2A-E, respectively. Although linear models (Supplementary Figure 1F and G and Supplementary Figure 2F and G) suggest an overall trend, they do not adequately capture the nonlinear patterns of the data. The generalized additive model yielded the best fit, with its smooth curves flexibly accommodating varying trends-particularly in the midrange and effectively characterizing the complex relationship between withdrawal time and ADR. Akaike information criterion comparisons across models confirmed the nonlinear association between withdrawal time and ADR.
Figure 3A and Supplementary Figure 3 show the target-achieving withdrawal times required to achieve a stabilized cumulative ADR of 25%, stratified by insertion-time intervals and further analyzed across subgroups of age, sex, and endoscopist experience for the 830-patient cohort. Supplementary Figure 4 presents the corresponding stratified analyses and target-achieving withdrawal times for the 5699-patient cohort.
A Cox proportional-hazards model was fitted in R using sex, age, and the endoscopist experience level as covariates (Figure 3B). However, the C-index values (0.5-0.7) in both the 830- and 5699-patient cohorts (Figure 3C and D) indicated only a modest predictive accuracy. We therefore developed a hybrid approach combining SVM regression with XGBoost classification, splitting the 5699-patient dataset 7:3 into training and testing sets. The SVM regression model demonstrated good performance in predicting withdrawal time, as shown by residual analyses and prediction-vs-observed plots in both sets (Figure 4A-D), particularly within the central 300-380 seconds range, although it tended to underestimate the extreme withdrawal time values. The XGBoost classifier achieved AUCs of 0.64 and 0.61 on the training and testing sets, res
Recent advancements in endoscopic technology have made colonoscopy increasingly acceptable for the diagnosis and treatment of gastrointestinal diseases. Early colorectal endoscopic screening can reduce CRC mortality by 67%[10], making colonoscopy a cornerstone of CRC screening. The ADR is a critical quality metric for colonoscopy and is closely associated with the incidence of interval CRC[11]; higher ADRs correlate with a lower CRC risk[12]. Therefore, elucidating the factors that influence ADR and implementing targeted management strategies are essential for enhancing colonoscopy quality and improving patient outcomes.
The study flow is presented in Figure 5. In phase I of this study, we constructed a multivariate logistic regression model to predict adenoma detection during colonoscopy, and identified patient age, sex, experience level of the endo
Several domestic and international studies have shown that ADR during colonoscopy is closely associated with patient sex. Men exhibit a significantly higher ADR than women, a trend that also applies to the detection of polyps, serrated lesions, and CRC[13,14], with some studies indicating that the male ADR is up to twice that of women[7]. Our findings similarly confirm the strong relationship between sex and ADR. This disparity likely reflects both physiological and lifestyle factors[15]: Physiologically, estrogen has been demonstrated to mitigate the risk of colorectal carcinogenesis[16], while lifestyle factors such as higher rates of high-fat diets, tobacco and alcohol use, and lower levels of physical activity among men increase the adenoma risk[15]. Moreover, compared with women, men in whom adenomas or serrated lesions are detected at screening colonoscopy face nearly a twofold higher risk of death from CRC[14]. Age is another key determinant of ADR. In our cohort, the mean age of patients with adenomas was 63.13 years; a previous work has shown that adenoma prevalence rises from 1.72% to 3.59% between ages 30 and 50 before increasing sharply after age 50[17]. Consequently, international guidelines recommend CRC screening for average-risk individuals aged 50-75 years[7], whereas domestic recommendations set the screening age from 40 to 74 years[18]. Notably, the incidence and mortality of CRC in those under 50 years are rising rapidly[19]. Although specific epidemiological data on adenoma trends by age are lacking, early-onset adenoma incidence may likewise be increasing, motivating calls to lower the age threshold for screening. Our results provide preliminary support for this proposal, but larger studies are required to confirm these observations. In addition, some variables show different results in regression and machine learning analyses. Although BMI was non-significant in our multivariate regression (P > 0.05), its placement among the top ten predictors in the machine-learning model shows that machine learning can be used to reveal nonlinear and interaction effects; for example, interaction of BMI with insertion time or patient sex influences ADR. This discrepancy suggests latent relationships that merit follow-up using techniques such as partial dependence plots or SHAP interaction analyses.
In addition to patient‐related determinants, we evaluated the impact of endoscopist expertise and procedural factors on adenoma detection. Our findings demonstrate that senior endoscopists achieve significantly higher ADRs. Rather than relying solely on case volume or years of practice, we devised a comprehensive, center-specific competency scale through multiple expert consultations to score and stratify operators into “senior” and “non-senior” groups, thereby capturing a more holistic measure of endoscopic proficiency. This approach aligns with domestic data showing marked ADR differences by operator seniority 24.33% in the 5-10 years cohort vs 11.57% in those with < 5 years of experience[20]. According to Telford et al[21] too, stronger endoscopic skills correlate with higher detection rates for adenomas, non-polypoid serrated lesions, and proximal serrated polyps. A meta-analysis of eight studies confirmed that targeted educational interventions can significantly improve ADR[22]. Another review of 12 trials found that feedback and performance review are associated with ADR gains[23]. Given our single-center design and limited number of participating endoscopists, more multicenter studies are warranted to further elucidate how operator factors influence adenoma detection.
Withdrawal time, a pivotal quality-control parameter in colonoscopy, has garnered widespread attention. A 2006 observational study published in the New England Journal of Medicine demonstrated that endoscopists whose mean withdrawal time exceeded 6 minutes achieved significantly higher ADRs[24], prompting the adoption of a 6-minute minimum withdrawal standard in multiple national guidelines and consensus statements. Subsequent research found that extending withdrawal time from 6 to 9 minutes increased the ADR from 27.1% to 36.6%, particularly in the proximal colon and among less experienced endoscopists[25]. Similarly, Desai et al[26] reported that, after excluding time devoted to polypectomy and mucosal cleansing, each additional minute of withdrawal corresponded to a 6% increase in ADR. Our team further proposed segment-specific optimization of withdrawal time: Shortening withdrawal in the ascending colon, where lesions are less subtle, while reallocating time toward segments such as the sigmoid colon and rectum, where adenomas are more readily detected[27]. However, withdrawal time is not indefinitely beneficial ADR gains plateau beyond 10 minutes[28], and under conditions of poor bowel preparation, prolonging withdrawal does not significantly increase the polyp yield[29]. Moreover, longer withdrawal may prolong the total procedure time, potentially necessitate deeper sedation, and may cause patient discomfort; these trade-offs should be carefully weighed by endoscopy teams. As one of the most controllable and modifiable factors in colonoscopy, withdrawal time even with a universally recommended minimum of 6 minutes remains insufficiently tailored to endoscopist experience and individual patient factors. Hence, developing customized withdrawal strategies for specific clinical scenarios is essential to ensure consistently high ADRs.
Building on this foundation, the present study innovatively incorporated insertion time as a key covariate in our predictive model. von Renteln et al[30] demonstrated that longer CITs corresponded with lower detection rates of both advanced adenomas and non-advanced adenomas, suggesting that insertion difficulty may compromise subsequent mucosal inspection. Prolonged CIT during colonoscopy serves as a validated surrogate for procedural complexity reflecting anatomic challenges such as colonic redundancy, loop formation, and adhesions that require additional maneuvers and sedation and is associated with increased difficulty in adenoma detection[30]. In a multicenter cohort, Choi et al[31] similarly identified insertion time as a surrogate for procedural complexity, reflecting the attentional and cognitive load of the endoscopist and thereby indirectly affecting adenoma detection. The cognitive burden of sustained procedural demands and endoscopist fatigue impairs attentional resources during withdrawal, leading to decreased vigilance and ADR in later or more challenging cases. Fritz et al[32] further showed that when withdrawal time is maintained above average levels, the negative impact of prolonged insertion can be offset by extended withdrawal, underscoring the need to model the interaction between insertion and withdrawal durations. Our preliminary literature review[8] indicated that insertion time is largely determined by patient characteristics, endoscopist factors, and technical nuances effectively serving as a composite marker of these influences. By stratifying withdrawal time according to insertion time, the allocation of inspection effort can be made proportionate to procedural difficulty: Cases flagged by extended insertion time receive longer withdrawal time, thereby compensating for heightened complexity and attenuated attention, and mitigating the risk of missed lesions inherent to uniform withdrawal time protocols.
However, this study has several limitations. First, the determinants of ADR are multifaceted and complex; because colonoscopies were performed by multiple endoscopists and the sample size was relatively small, we did not observe significant associations for other potential predictors, such as BMI, diabetes, and bowel-preparation score, highlighting the need for larger confirmatory studies. Second, the generalizability and feasibility of our model across different institutions and patient populations remain untested and should be evaluated in prospective, multicenter cohorts. Furthermore, as the model is derived from existing data, its performance in clinical practice may be influenced by subjective factors such as operator adherence, procedural techniques, and real-time teamwork. In addition, operator adherence to app-generated guidance is yet to be evaluated in routine workflows and may vary by institutional culture and individual practice. Besides, our machine learning model was trained on all colonoscopy cases, both adenoma-positive and adenoma-negative, to predict whether an individual procedure would achieve an ADR ≥ 25%. Detailed adenoma attributes (e.g., size, location, and morphology) are only recorded when a lesion is detected and thus are not available for the negative cases, and their inclusion would introduce post-hoc information and limit real-time appli
This study discusses a clinical application framework in which, during screening colonoscopy, endoscopists use patient age, sex, operator experience, and actual insertion time together with the predictive model developed here to estimate the target-achieving withdrawal duration. We propose a minimum withdrawal time framework personalized by insertion time to achieve ≥ 25% ADR. While the modest discrimination (AUC = 0.64) of the model requires refinement, its integration into a Shiny app enables real-time clinical testing. This framework provides real-time decision support: Smooth, low-complexity insertion can maintain a conventional withdrawal time (e.g., 6-9 minutes), whereas high-difficulty procedures characterized by prolonged insertion should receive an appropriately extended withdrawal (e.g., ≥ 10 minutes) to ensure a thorough mucosal inspection, which will maximize ADR and reduce the CRC risk. However, the model, with a ROC AUC of 0.640, has modest discrimination and predictive power. This is an exploratory study, which requires further validation and refinement.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12706] [Article Influence: 6353.0] [Reference Citation Analysis (6)] |
| 2. | Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 1335] [Article Influence: 445.0] [Reference Citation Analysis (13)] |
| 3. | Helsingen LM, Kalager M. Colorectal Cancer Screening - Approach, Evidence, and Future Directions. NEJM Evid. 2022;1:EVIDra2100035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (3)] |
| 4. | Huang ES, Huang Q, Kenkare P, Mudiganti S, Martinez MC, Liang SY. Prevalence of "one and done" phenomenon in adenoma detection within a large community-based healthcare system. Gastrointest Endosc. 2025;101:1038-1050.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG 2nd, Park WG, Rizk MK, Sawhney MS, Shaheen NJ, Wani S, Weinberg DS. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 6. | Chung GE, Lim SH, Yang SY, Song JH, Kang HY, Kang SJ, Kim YS, Yim JY, Park MJ. Factors that determine prolonged cecal intubation time during colonoscopy: impact of visceral adipose tissue. Scand J Gastroenterol. 2014;49:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 538] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 8. | Chen XJ, Zhu XL, Ji L, Yang C, Zhan Q. [Research status of influencing factors of endoscopic insertion time and its relationship with adenoma detection]. Zhonghua Xiaohua Neijing Zazhi. 2023;40:165-168. [DOI] [Full Text] |
| 9. | Gao M, Lv J, Yu C, Guo Y, Bian Z, Yang R, Du H, Yang L, Chen Y, Li Z, Zhang X, Chen J, Qi L, Chen Z, Huang T, Li L; China Kadoorie Biobank (CKB) Collaborative Group. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: A cohort study. PLoS Med. 2020;17:e1003351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 10. | Doubeni CA, Corley DA, Quinn VP, Jensen CD, Zauber AG, Goodman M, Johnson JR, Mehta SJ, Becerra TA, Zhao WK, Schottinger J, Doria-Rose VP, Levin TR, Weiss NS, Fletcher RH. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018;67:291-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 302] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 11. | Cross AJ, Robbins EC, Saunders BP, Duffy SW, Wooldrage K. Higher Adenoma Detection Rates at Screening Associated With Lower Long-Term Colorectal Cancer Incidence and Mortality. Clin Gastroenterol Hepatol. 2022;20:e148-e167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Ishtiaq R, Zulfiqar L, Gangwani MK, Aziz M. Adenoma detection rate vs. adenoma per colonoscopy as quality indicators for colon cancer screening. Transl Gastroenterol Hepatol. 2023;8:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 13. | Valian H, Hassan Emami M, Heidari A, Amjadi E, Fahim A, Lalezarian A, Ali Ehsan Dehkordi S, Maghool F. Trend of the polyp and adenoma detection rate by sex and age in asymptomatic average-risk and high-risk individuals undergoing screening colonoscopy, 2012-2019. Prev Med Rep. 2023;36:102468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Waldmann E, Jiricka L, Zessner-Spitzenberg J, Majcher B, Rockenbauer LM, Penz D, Hinterberger A, Trauner M, Ferlitsch M. Differences between men and women with respect to colorectal cancer mortality despite screening colonoscopy. Gastrointest Endosc. 2024;99:998-1005.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Choi Y, Kim N. Sex Difference of Colon Adenoma Pathway and Colorectal Carcinogenesis. World J Mens Health. 2024;42:256-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Stevanato Filho PR, Aguiar Júnior S, Begnami MD, Ferreira FO, Nakagawa WT, Spencer RMSB, Bezerra TS, Boggiss PE, Lopes A. Estrogen Receptor β as a Prognostic Marker of Tumor Progression in Colorectal Cancer with Familial Adenomatous Polyposis and Sporadic Polyps. Pathol Oncol Res. 2018;24:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Pendergrass CJ, Edelstein DL, Hylind LM, Phillips BT, Iacobuzio-Donahue C, Romans K, Griffin CA, Cruz-Correa M, Tersmette AC, Offerhaus GJ, Giardiello FM. Occurrence of colorectal adenomas in younger adults: an epidemiologic necropsy study. Clin Gastroenterol Hepatol. 2008;6:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Expert Group on Early Diagnosis and Treatment of Cancer; Chinese Society of Oncology; Chinese Medical Association. [Expert consensus on the early diagnosis and treatment of colorectal cancer in China (2023 edition)]. Zhonghua Yi Xue Za Zhi. 2023;103:3896-3908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Liu H, Xu Z, Song C, Lu Y, Li T, Zheng Z, Li M, Ye H, Wang K, Shi J, Wang P. Burden of gastrointestinal cancers among people younger than 50 years in China, 1990 to 2019. Public Health. 2024;234:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Qian C, Xu Z. [Analysis of characteristics and detection factors of colorectal adenoma]. Linchuang Heli Yongyao Zazhi. 2019;12:32-42. [DOI] [Full Text] |
| 21. | Telford J, Gondara L, Pi S, Gentile L, Enns R. Higher adenoma detection, sessile serrated lesion detection and proximal sessile serrated lesion detection are associated with physician specialty and performance on Direct Observation of Procedural Skills. BMJ Open Gastroenterol. 2021;8:e000677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Causada-Calo NS, Gonzalez-Moreno EI, Bishay K, Shorr R, Dube C, Heitman SJ, Hilsden RJ, Rostom A, Walsh C, Anderson JT, Keswani RN, Scaffidi MA, Grover SC, Forbes N. Educational interventions are associated with improvements in colonoscopy quality indicators: a systematic review and meta-analysis. Endosc Int Open. 2020;8:E1321-E1331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Bishay K, Causada-Calo N, Scaffidi MA, Walsh CM, Anderson JT, Rostom A, Dube C, Keswani RN, Heitman SJ, Hilsden RJ, Shorr R, Grover SC, Forbes N. Associations between endoscopist feedback and improvements in colonoscopy quality indicators: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92:1030-1040.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 974] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 25. | Zhao S, Yang X, Wang S, Meng Q, Wang R, Bo L, Chang X, Pan P, Xia T, Yang F, Yao J, Zheng J, Sheng J, Zhao X, Tang S, Wang Y, Wang Y, Gong A, Chen W, Shen J, Zhu X, Wang S, Yan C, Yang Y, Zhu Y, Ma RJ, Wang R, Ma Y, Li Z, Bai Y. Impact of 9-Minute Withdrawal Time on the Adenoma Detection Rate: A Multicenter Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2022;20:e168-e181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 26. | Desai M, Rex DK, Bohm ME, Davitkov P, DeWitt JM, Fischer M, Faulx G, Heath R, Imler TD, James-Stevenson TN, Kahi CJ, Kessler WR, Kohli DR, McHenry L, Rai T, Rogers NA, Sagi SV, Sathyamurthy A, Vennalaganti P, Sundaram S, Patel H, Higbee A, Kennedy K, Lahr R, Stojadinovikj G, Campbell C, Dasari C, Parasa S, Faulx A, Sharma P. Impact of withdrawal time on adenoma detection rate: results from a prospective multicenter trial. Gastrointest Endosc. 2023;97:537-543.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Chen X, Xu B, Wei B, Ji L, Yang C, Zhan Q. Relationship Between Adenoma Detection Rate and Respective Withdrawal Time in Different Colon Segments: A Retrospective, Single-Center Study. JGH Open. 2025;9:e70095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Gellad ZF, Weiss DG, Ahnen DJ, Lieberman DA, Jackson GL, Provenzale D. Colonoscopy withdrawal time and risk of neoplasia at 5 years: results from VA Cooperative Studies Program 380. Am J Gastroenterol. 2010;105:1746-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Widjaja D, Bhandari M, Loveday-Laghi V, Glandt M, Balar B. Withdrawal time in excellent or very poor bowel preparation qualities. World J Gastrointest Endosc. 2014;6:186-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | von Renteln D, Robertson DJ, Bensen S, Pohl H. Prolonged cecal insertion time is associated with decreased adenoma detection. Gastrointest Endosc. 2017;85:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Choi JM, Lim SH, Han YM, Lee J, Jin EH, Seo JY, Kim J. Association Between Longer Cecal Intubation Time and Detection and Miss Rate of Colorectal Neoplasms. J Clin Med. 2024;13:7080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Fritz CDL, Smith ZL, Elsner J, Hollander T, Early D, Kushnir V. Prolonged Cecal Insertion Time Is Not Associated with Decreased Adenoma Detection When a Longer Withdrawal Time Is Achieved. Dig Dis Sci. 2018;63:3120-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/