Published online Oct 14, 2025. doi: 10.3748/wjg.v31.i38.111363
Revised: July 30, 2025
Accepted: September 1, 2025
Published online: October 14, 2025
Processing time: 108 Days and 17.1 Hours
The high rebleeding rate and severe adverse events have raised concerns re
To compare the efficacy, safety, and procedural characteristics of Clip-CYA vs EUS-CG for treatment of gastric varices (GVs) with spontaneous portosystemic shunts.
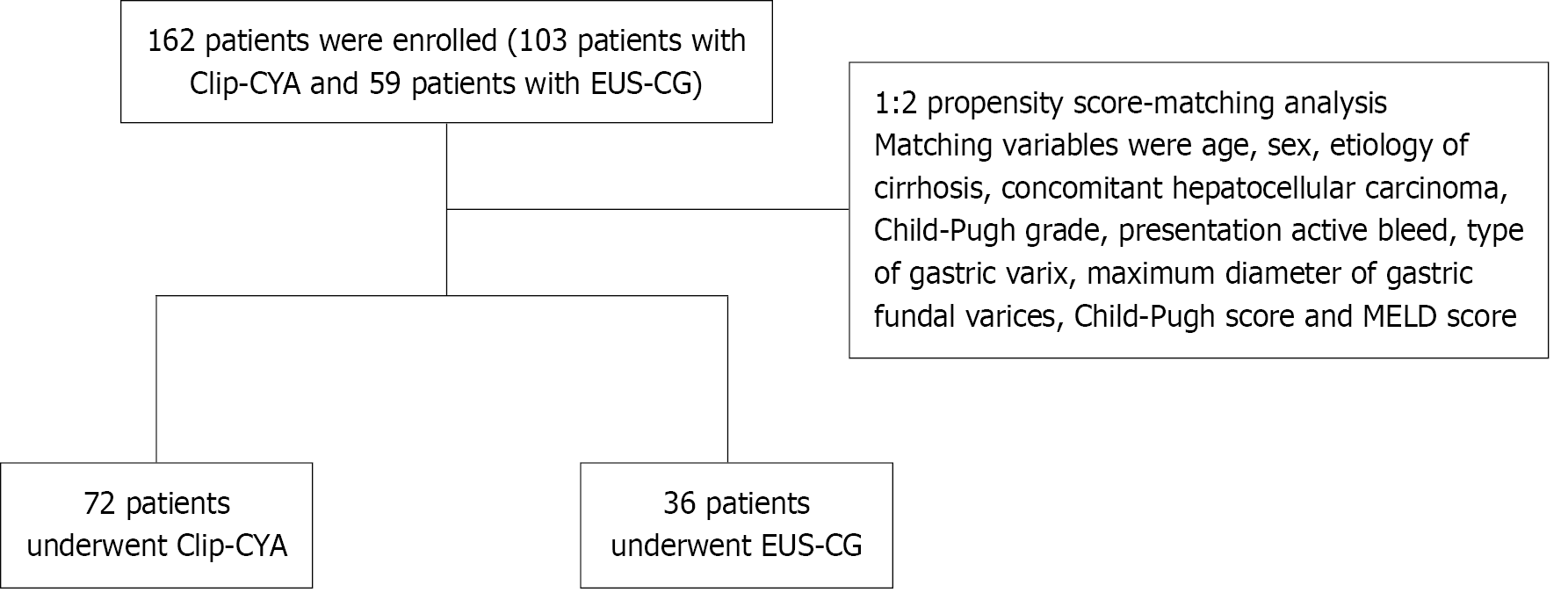
Between April 2019 and August 2023, 162 patients with GVs and concomitant gastrorenal or splenorenal shunts who underwent either Clip-CYA or EUS-CG at our center were included. After 1:2 propensity score matching, 108 patients were included in the final analysis. The evaluated outcomes included the amount of cyanoacrylate, eradication of GVs, cyanoacrylate embolization, all-cause reblee
Of the 108 patients, 72 (male, 83.3%; mean age, 56.2 ± 10.8 years) received Clip-CYA, and 36 (male, 72.2%; mean age, 59.1 ± 10.7 years) received EUS-CG. The amount of cyanoacrylate used, rates of obliteration of GVs and all-cause reblee
Compared with EUS-CG, Clip-CYA of GVs appears to be a safe procedure with shorter operating times and lower endoscopic therapy costs.
Core Tip: This propensity score-matched study compared clip-assisted endoscopic cyanoacrylate injection (Clip-CYA) and endoscopic ultrasound-guided coil and cyanoacrylate injection (EUS-CG) for gastric varices (GVs) with spontaneous portosystemic shunts. Both methods achieved comparable efficacy and safety. However, Clip-CYA required significantly less procedure time and cost, even in patients with large varices (> 3 cm). These findings suggest that Clip-CYA may serve as a more efficient and accessible alternative to EUS-CG in the emergency and real-world treatment of bleeding GVs.
- Citation: Xiong YY, Li DW, Zhou TY, Ma H, Gao JG, Shen Z, Xu CF, Yu CH. Clip-assisted endoscopic cyanoacrylate injection vs endoscopic ultrasound-guided coil and cyanoacrylate injection for gastric varices: A propensity score-matched study. World J Gastroenterol 2025; 31(38): 111363
- URL: https://www.wjgnet.com/1007-9327/full/v31/i38/111363.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i38.111363
Gastroesophageal variceal bleeding is a complication of portal hypertension[1]. Gastric varices (GVs), which are present in approximately 20% of patients with portal hypertension, bleed less frequently but present more catastrophic bleeding and greater mortality than do esophageal varices[2,3]. Endoscopic cyanoacrylate (E-CYA) injection is the first-line treatment for the management of gastric vascular bleeding recommended by the Baveno VII Consensus Workshop[4]. However, a high rebleeding rate (up to 36%) and severe adverse events, such as ectopic embolization related to CYA injection, including pulmonary embolization (PE), splenic infarction, cerebral infarction and myocardial infarction, have been reported[5-9], raising concerns regarding the safety of CYA injection.
Endoscopic ultrasound (EUS)-guided coil and CYA injection (EUS-CG) and clip-assisted endoscopic CYA injection (Clip-CYA) have emerged as newer modalities for obliteration of variceal blood flow during therapy and have been found to be superior to E-CYA[10-14]. The main advantage of EUS-CG is that it enables optimum visualization of the varices as well as their feeder vessels, and GV obliteration can be confirmed in real time with EUS[15]. However, this technique requires a separate ultrasound host and endoscopic instrument, and endoscopists need professional ultrasound training, which may not be available at all centers[16-18]. In contrast to EUS-CG, the Clip-CYA procedure is convenient and easy to master. The inflow or outflow veins of the GVs are first clamped with metal clips to slow down or completely block the gastric variceal blood flow, followed by CYA injection into the GVs, which can reduce the risk of ectopic embolization[11,14]. However, there are limited data on head-to-head comparisons between Clip-CYA and EUS-CG.
In this retrospective cohort study, we compared the two currently used methods of endoscopic therapy for GVs. Compared with EUS-CG, Clip-CYA achieved similar outcomes and had a significantly shorter operating time and lower endoscopic therapy costs.
Patients with cirrhosis who underwent Clip-CYA or EUS-CG between April 2019 and August 2023 at The First Affiliated Hospital, Zhejiang University School of Medicine, were enrolled in this investigation. The inclusion criteria were as follows: (1) Cirrhosis diagnosed on clinical, radiological or laboratory parameters and/or liver biopsy; (2) GVs confirmed by endoscopy, including type 2 gastroesophageal varices (GOV2) and type 1 isolated GVs (IGV1); (3) A gastrorenal or splenorenal shunt confirmed by contrast-enhanced computed tomography (CT); and (4) High-risk GVs, active GVs or a history of bleeding attributable to GVs.
The exclusion criteria were as follows: (1) Age < 18 years or > 80 years; (2) Regional portal hypertension; (3) A history of previous transjugular intrahepatic portosystemic shunt, surgical splenectomy combined with variceal devascularization, retrograde transvenous obliteration or liver transplantation; (4) Significant cardiopulmonary comorbidity; and (5) The presence of hepatocellular carcinoma not meeting the Milan criteria and the presence of other malignancies that significantly affect survival.
This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. 2024IIT803).
The electronic medical records were reviewed for patient demographic details, etiology of the underlying liver disease, laboratory and imaging values, procedural information, and follow-up data. Sarin’s classification was used to categorize the GVs[19]. The Child-Pugh grade, Child-Pugh score and model for end-stage liver disease (MELD) score were used to assess the severity of the disease[20,21]. The maximum diameter of the GVs was measured via EUS or estimated in reference to the diameter of the GIF-Q260J endoscope (Olympus, Tokyo, Japan)[22,23]. We considered patient age, sex, etiology of cirrhosis, concomitant hepatocellular carcinoma, Child-Pugh grade, presentation of active bleeding, type of GVs, maximum diameter of GVs, Child-Pugh score and MELD score as potential confounders for comparison. Thus, propensity score matching (PSM) analysis was adapted to correct for selection bias. Between April 2019 and August 2023, 162 patients who met the criteria were retrospectively identified and enrolled. After 1:2 PSM, 72 patients who underwent Clip-CYA and 36 who underwent EUS-CG were selected for this single-center retrospective cohort study (Figure 1). The primary outcomes were CYA embolization and all-cause rebleeding. The secondary outcomes included the amount of CYA used, the rate of GV eradication, the operating time and the cost of endoscopic therapy.
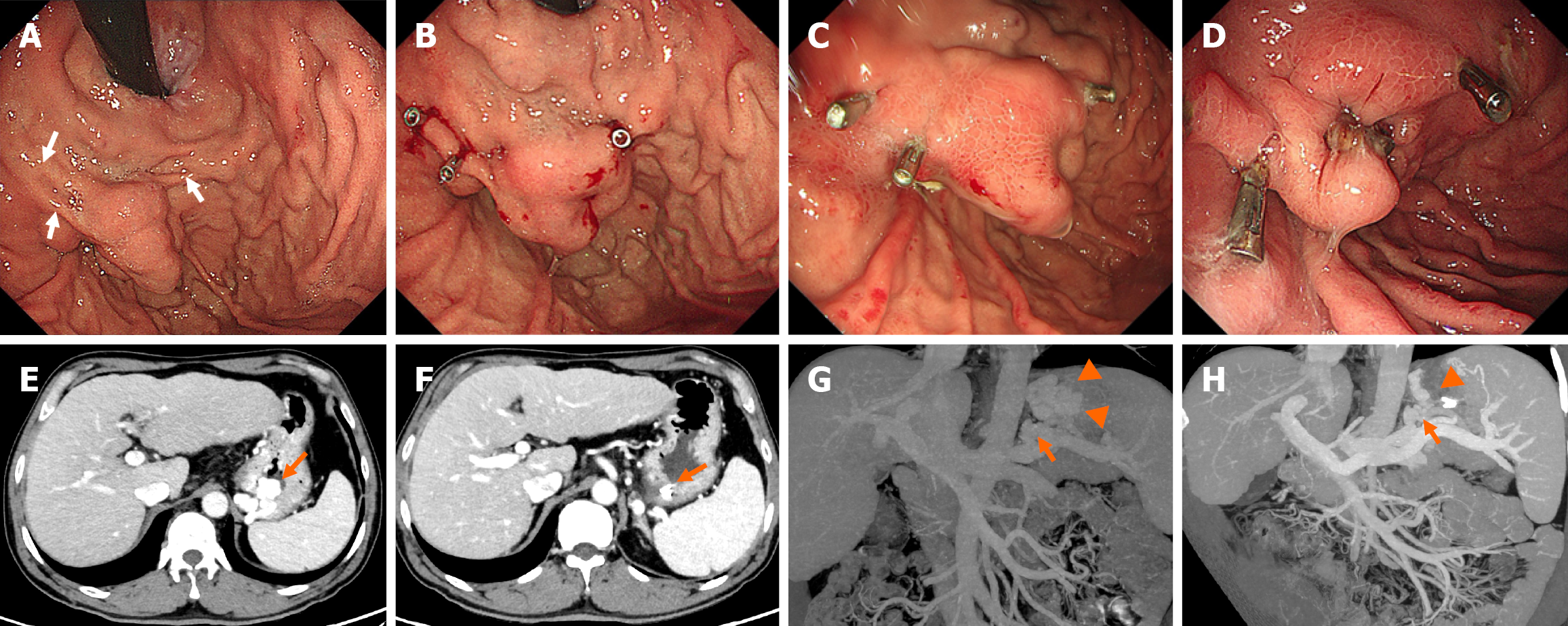
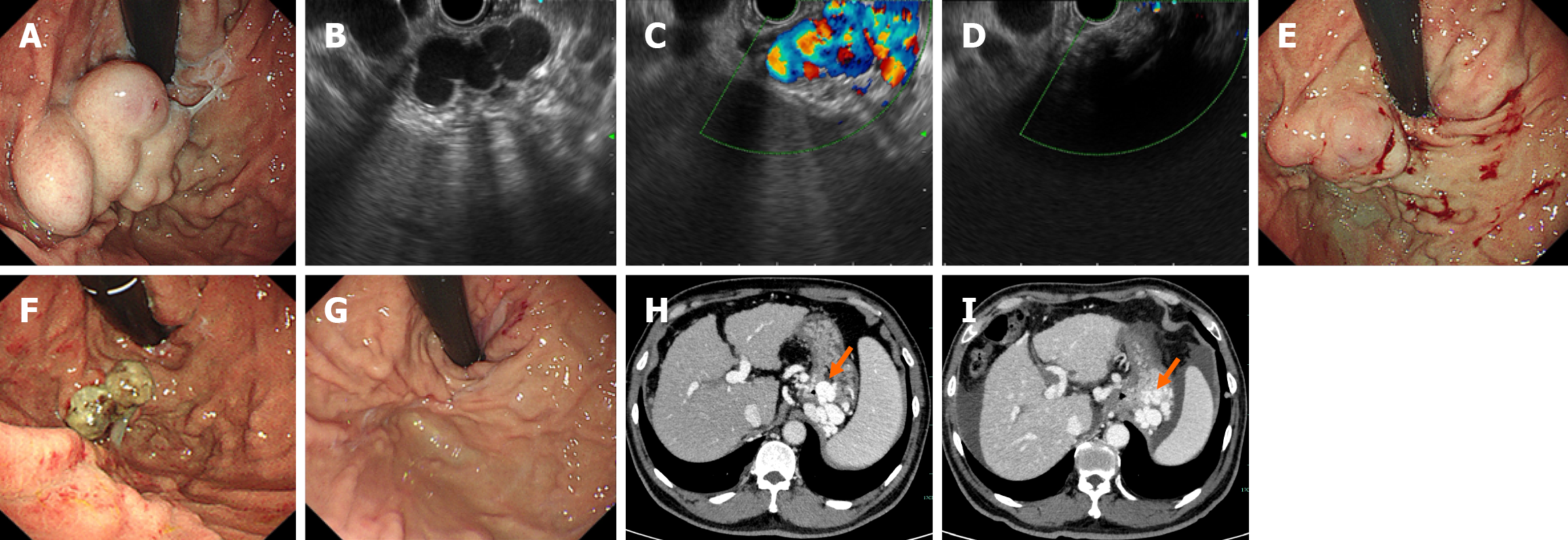
Written informed consent concerning the E-CYA injection procedure was obtained from each patient. All procedures were performed by experienced endoscopists under general anesthesia. The patients underwent standard upper esophagogastroduodenoscopy for evaluation of bleeding and characterization of the GVs. The decision for the type of procedure (EUS-CG or Clip-CYA) was based on the endoscopist’s preference. For the Clip-CYA group, metal clips (HA-I-1, Anrei Scientific, Hangzhou, China or Resolution 360TM Clip, Boston Scientific, Costa Rica) were deployed on the inflow or outflow veins, with the help of contrast-enhanced CT images, to restrict blood flow within the varices. The type of clip chosen depended on the endoscopist’s preference. The clips were placed on the narrow part of the tortuous varices to clamp the whole varices as fully as possible (Figure 2). EUS-CG was performed following the technique described by Samanta et al[12], with transesophageal access using a standard 19-G or 22-G EUS-guided fine-needle aspiration needle (Echotip® Ultra, Endoscopic Ultrasound Needle, Cook Medical). The size of the needle chosen depended on the size of the varix. Once the puncture was performed under EUS guidance, blood was aspirated to confirm the position, after which the needle was flushed with lauromacrogol. Multiple coils (Fibered Platinum Coil, Boston Scientific) were then deployed in the GVs under EUS guidance. A 0.035 coil was used with a 19-G needle, and a 0.021 or 0.018 coil was used with a 22-G needle. The number, length (5 cm-30 cm) and diameter (10 mm-14 mm) of the coils used depended on the size of the varix (Figure 3). After the coil or clip had been placed, a modified sandwich method (lauromacrogol, CYA or lauromacrogol) was used to inject the CYA into the GVs[24]. Endoscopic band ligation was performed on patients with large esophageal varices after Clip-CYA. Antibiotic prophylaxis was routinely administered for 24-72 hours following the injection.
Hospital notes, telephone follow-up, WeChat and/or e-mail follow-up were all used to collect the information. For EUS-CG, technical success was defined as successful deployment of the coil and CYA under EUS guidance with obliteration of blood flow on doppler ultrasound. For Clip-CYA, technical success was defined as the placement of the clips and the injection of CYA for adequate filling of the varices with satisfactory hardening of the varix on the palpation. The eradication of GVs was defined as eradication according to contrast-enhanced CT re-examination after the procedure[11]. The technical success rate, eradication of GVs, CYA embolization, all-cause rebleeding, operating time and endoscopic therapy cost were evaluated. Rebleeding was defined as the occurrence of new-onset hematemesis or fresh blood in the nasogastric aspirate ≥ 6 hours after the procedure, the appearance of melena or hematochezia following normalization of the stool color, or a decrease in the hemoglobin level ≥ 2 g/dL after two consecutive stable hemoglobin measurements taken at least 3 hours apart. Re-intervention was defined as the need for a repeat EUS-CG or Clip-CYA procedure targeting GV during follow-up. Indications for re-intervention included recurrent bleeding from previously treated varices, first-time bleeding in patients who had undergone primary prophylaxis, or incomplete variceal obliteration observed on follow-up endoscopy[10-13]. PE was defined as the presence of a new intraluminal filling defect in a pul
PSM analysis was performed with IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, United States) and R version R 3.5 (R Foundation for Statistical Computing, Vienna, Austria). A 1:2 matched analysis using nearest-neighbor matching with a caliper distance of 0.2 without replacement was performed on the basis of the estimated propensity score of each patient. Continuous variables were analyzed by Student’s t test if normally distributed and by nonparametric tests otherwise. Categorial variables were compared via the c2 test. P < 0.05 was considered to indicate statistical signi
A total of 108 patients were analyzed in the study, of whom 72 (60 males, 83.3%) received Clip-CYA and 36 (26 males, 72.2%) received EUS-CG. Patient characteristics are described in Table 1. There were no significant differences in each possible confounder, including age, sex, etiology of cirrhosis, concomitant hepatocellular carcinoma, Child-Pugh grade, presentation of active bleeding, type of GVs, maximum diameter of GVs, Child-Pugh score or MELD score, between the two groups with PSM. The median follow-up times of the two groups were 29.5 months [interquartile range (IQR): 9.8-53.0 months] and 31.1 months (IQR: 9.8-75.3 months), respectively, and there was no significant difference.
| Variable | Before propensity score matching | After propensity score matching | ||||
| Clip group (n = 103) | EUS group (n = 59) | P value | Clip group (n = 72) | EUS group (n = 36) | P value | |
| Sex | 0.326 | 0.177 | ||||
| Male | 83 (80.6) | 43 (72.9) | 60 (83.3) | 26 (72.2) | ||
| Female | 20 (19.4) | 16 (27.1) | 12 (16.7) | 10 (27.8) | ||
| Age, year | 56.4 ± 11.2 | 57.6 ± 10.0 | 0.494 | 56.2 ± 10.8 | 59.1 ± 10.7 | 0.186 |
| Etiology of cirrhosis | 0.968 | 0.695 | ||||
| Viral hepatitis B | 68 (66.0) | 39 (66.1) | 47 (65.3) | 20 (55.6) | ||
| Viral hepatitis C | 2 (1.9) | 1 (1.7) | 1 (1.4) | 1 (2.8) | ||
| Alcohol consumption | 8 (7.8) | 5 (8.5) | 6 (8.3) | 5 (13.9) | ||
| Autoimmune | 10 (9.7) | 3 (5.1) | 7 (9.7) | 3 (8.3) | ||
| Parasite | 3 (2.9) | 5 (8.5) | 2 (2.8) | 3 (8.3) | ||
| Cryptogenic | 12 (11.7) | 6 (10.2) | 9 (12.5) | 4 (11.1) | ||
| Concomitant HCC | 19 (18.4) | 15 (25.4) | 0.320 | 16 (22.2) | 8 (22.2) | 1.000 |
| Child-Pugh grade | 0.104 | 0.202 | ||||
| A | 38 (36.9) | 30 (50.8) | 26 (36.1) | 19 (52.8) | ||
| B | 58 (56.3) | 23 (39.0) | 41 (56.9) | 14 (38.9) | ||
| C | 7 (6.8) | 6 (10.2) | 5 (6.9) | 3 (8.3) | ||
| Presentation active bleed | 73 (70.9) | 38 (64.4) | 0.551 | 52 (72.2) | 24 (66.7) | 0.551 |
| Previous GV bleeding | 61 (59.2) | 45 (76.3) | < 0.001 | 41 (56.9) | 25 (69.4) | 0.209 |
| RCS on GV | 67 (66.3) | 36 (61.0) | 0.500 | 47 (66.2) | 22 (61.1) | 0.603 |
| Concomitant esophageal varices | 88 (85.4) | 51 (86.4) | 0.996 | 60 (83.3) | 29 (80.6) | 0.530 |
| Type of gastric varix | 0.143 | 0.134 | ||||
| GOV2 | 52 (50.5) | 37 (62.7) | 33 (45.8) | 22 (61.1) | ||
| IGV1 | 51 (49.5) | 22 (37.3) | 39 (54.2) | 14 (38.9) | ||
| Maximum diameter of gastric fundal varices, cm | 2.4 ± 1.3 | 2.5 ± 1.2 | 0.481 | 2.5 ± 1.1 | 2.5 ± 1.3 | 0.609 |
| Type of spontaneous portosystemic shunts | 0.082 | 0.319 | ||||
| Gastrorenal shunt | 43 (41.7) | 33 (55.9) | 23 (31.9) | 15 (41.7) | ||
| Splenorenal shunt | 60 (58.3) | 26 (44.1) | 49 (68.1) | 21 (58.3) | ||
| Minimum diameter of shunt varices, cm | 0.5 ± 0.2 | 0.4 ± 0.2 | 0.354 | 0.5 ± 0.2 | 0.4 ± 0.2 | 0.326 |
| Child-Pugh score | 7.3 (1.6) | 6.9 (1.8) | 0.092 | 7.1 (1.3) | 6.8 (1.8) | 0.355 |
| MELD score | 9.6 (3.3) | 8.6 (3.4) | 0.091 | 8.9 (2.3) | 8.2 (4.1) | 0.213 |
| Length of stay, day, median (IQR) | 7 (2-28) | 7 (3-23) | 0.196 | 7 (2-27) | 7.5 (3-23) | 0.889 |
| Follow-up time, month, median (IQR) | 29.4 (9.8-53.0) | 30.3 (9.8-53.3) | 0.041 | 29.5 (9.8-53.0) | 31.1 (9.8-75.3) | 0.093 |
Among the 72 patients (mean age 56.2 ± 10.8 years) undergoing clip-CYA for GVs, the most common underlying etiology was viral hepatitis B infection (65.3%). According to Sarin’s classification, 39 (54.2%) had IGV1, and the remaining 33 (45.8%) had GOV2. There was active bleeding in 52 (72.2%) GV patients. Compared with those in the EUS-CG group (mean age 59.1 ± 10.7 years, P = 0.186), 55.6% (P = 0.695) of the patients in the EUS-CG group had viral hepatitis B infection, 22 (61.1%) had GOV2, 14 (38.9%) had IGV1 (P = 0.134), and 24 (66.7%) had active bleeding in the GVs (P = 0.551).
Clip-CYA injection and EUS-CG injection were successfully performed in all 72 (100%) and 36 (100%) patients, respectively. The median number of clips used was two (1-5), while a single clip was used in 16 (22.2%) patients, two clips were used in 37 (51.4%) patients, and ≥ 3 clips were used in 19 (26.4%) patients. For the EUS-CG group, the median number of coils used was two (1-3), while a single coil was used in 30 (83.3%) patients, two coils were used in two (5.6%) patients, and three coils were used in four (11.1%).
The amount of CYA used was similar between the two groups. Contrast-enhanced CT was performed within 1 month after the procedure, and the rate of obliteration of GVs was high in the EUS-CG group; however, there was no significant difference compared with the Clip-CYA group (P = 0.603). The operating time and endoscopic therapy costs were significantly lower in the clip group than in the EUS group (24.0 ± 9.9 minutes vs 47.1 ± 21.0 minutes, P < 0.001; 7523.4 ± 5719.4 Chinese yuan vs 11153.7 ± 7679.1 Chinese yuan, P = 0.007).
No CYA embolization occurred in the Clip-CYA or EUS-CG group. During the follow-up period, all-cause gastrointestinal rebleeding within 1 year was similar between the two groups (P = 0.623), occurring in 17 (23.6%) patients in the clip group and seven (19.4%) in the EUS group. One patient in the clip group experienced rebleeding of GVs within 5 days after treatment, and the bleeding was controlled by CYA injection (0.5 mL). One patient in each group died due to liver cancer progression.
Subgroup analysis was performed to compare the two modalities for patients whose GVs had a maximum diameter > 3 cm (Table 2). The Child-Pugh and MELD disease severity scores were not significantly different between the two groups when the maximum diameter was > 3 cm. The rates of eradication of GVs, CYA embolization, rebleeding and death were similar. Compared with EUS-CG, Clip-CYA was associated with shorter operating times (24.1 ± 10.1 minutes vs 44.9 ± 21.0 minutes, P < 0.001) and lower endoscopic therapy costs (7147.7 ± 5425.1 Chinese yuan vs 12950.1 ± 6230.0 Chinese yuan, P = 0.005).
| Variable | Clip group (n = 72) | EUS group (n = 36) | P value |
| Technique success | 72 (100) | 36 (100) | |
| Clip number, median (IQR) | 2 (1-5) | ||
| Coil number, median (IQR) | 2 (1-3) | ||
| Amount of cyanoacrylate, mL | 2.0 ± 1.1 | 2.0 ± 0.6 | 0.913 |
| Eradication of gastric fundal varices | 66 (91.7) | 34 (94.4) | 0.603 |
| Operating time, minute | 24.0 ± 9.9 | 47.1 ± 21.0 | < 0.001 |
| Cyanoacrylate embolization | 0 (0) | 0 (0) | |
| Five-day rebleeding | 1 (1.4) | 0 (0) | 0.477 |
| Esophageal varices | 0 (0) | 0 (0) | |
| Gastric varices | 1 (1.4) | 0 (0) | |
| Postinjection ulcers | 0 (0) | 0 (0) | |
| One-year rebleeding | 17 (23.6) | 7 (19.4) | 0.623 |
| Esophageal varices | 4 (5.6) | 2 (5.6) | |
| Gastric varices | 9 (12.5) | 3 (8.3) | |
| Postinjection ulcers | 4 (5.6) | 2 (5.6) | |
| Death | 1 (1.4) | 1 (2.8) | 0.614 |
| Endoscopic therapy costs, Chinese yuan | 7523.4 ± 5719.4 | 11153.7 ± 7679.1 | 0.007 |
Subgroup analysis was performed to compare the two modalities for patients whose GVs had a maximum diameter > 4 cm (Table 3). The Child-Pugh score, the MELD score, the rates of eradication of GVs, CYA embolization, rebleeding and death were similar between the two groups when the maximum diameter of the GVs was > 4 cm. Compared with EUS-CG, Clip-CYA was associated with shorter operating times (27.4 ± 11.1 minutes vs 40.8 ± 10.7 minutes, P = 0.045) and lower endoscopic therapy costs (6764.0 ± 5639.5 Chinese yuan vs 12010.0 ± 6487.4 Chinese yuan, P = 0.042).
| Variable | Maximum diameter of gastric fundal varices ≥ 3 cm | Maximum diameter of gastric fundal varices ≥ 4 cm | ||
| Clip group (n = 30) | EUS group (n = 12) | Clip group (n = 18) | EUS group (n = 7) | |
| Child-Pugh score | 7.2 (1.3) | 6.6 (1.7) | 7.1 (1.2) | 6.1 (1.3) |
| MELD score | 9.1 (2.1) | 7.9 (4.4) | 8.8 (2.4) | 7.0 (3.6) |
| Amount of cyanoacrylate, mL | 2.5 ± 1.3 | 2.2 ± 0.4 | 2.8 ± 1.6 | 2.4 ± 0.6 |
| Eradication of gastric fundal varices | 25 (83.3) | 10 (83.3) | 15 (83.3) | 6 (85.7) |
| Operating time, minute | 24.1 (10.1) | 44.9 (21.0)b | 27.4 (11.1) | 40.8 (10.7)a |
| Cyanoacrylate embolization | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Five-day rebleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| One-year rebleeding | 6 (20.0) | 2 (16.7) | 3 (16.7) | 1 (14.3) |
| Death | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Endoscopic therapy costs, Chinese yuan | 7147.7 ± 5425.1 | 12950.1 ± 6230.0a | 6764.0 ± 5639.5 | 12010.0 ± 6487.4a |
In this study, compared with EUS-CG, Clip-CYA achieved similar outcomes, with a > 90% complete varix obliteration rate at 1 month and 23.6% bleeding after the index procedure at 1 year. Compared with EUS-CG, Clip-CYA had a significantly shorter operating time and lower endoscopic therapy costs.
While GVs constitute a small fraction of portal hypertension cases, their hemorrhage is often catastrophic, with higher mortality than that of esophageal varices. E-CYA injection, which provides excellent immediate control of bleeding, is the conventional treatment for the management of GVs recommended by the Baveno VII consensus Workshop[4], but severe adverse events related to CYA embolization and high rebleeding rates (approximately 36%) offset this advantage[5-9]. EUS-CG management of GVs is emerging as an effective alternative to E-CYA, with encouraging results in recent studies[26]. However, EUS equipment is expensive and has high technical requirements, which makes it difficult to popularize and master[16]. Thus, the most recent European Society of Gastrointestinal Endoscopy guidelines recommend that EUS-CG management of bleeding GVs, combined with the injection of coils and CYA, should be used in centers with expertise and familiarity with this technique[17]. Clip-CYA is another technique with greater safety and a lower rebleeding rate than E-CYA therapy; moreover, this novel approach is not difficult to master and can be performed with ordinary endoscopes[10,11,14,27]. To our knowledge, no reports on a series of patients treated with this approach compared with EUS-CG are available.
In this study, Clip-CYA was successfully performed in all 72 patients. No symptoms related to ectopic embolization were observed, reflecting a similar effect in terms of obliteration and rebleeding with EUS-CG. Obliteration is a more objective assessment of treatment efficacy and is a key factor in preventing rebleeding in GV patients. According to a recent multicenter study[11], GVs managed by Clip-CYA were eradicated in 90.9% of patients, according to contrast-enhanced CT re-examination, and the rebleeding rate was 22.8%, which was similar to that in our study. No symptoms related to ectopic embolization occurred[11,27]. Zhang et al[10] reported that Clip-CYA patients had a significantly lower rebleeding rate than did E-CYA patients. The results showed that Clip-CYA was safe and effective for the treatment of GVs. The precise role of the clip in this technique remains unclear. It has been reported that after clamping with metal clips, the blood flow of inflow or outflow veins of the GVs can be slowed down or completely blocked, followed by CYA injection, which can reduce the risk of ectopic embolization[11,14,27].
We performed an economic efficacy analysis and revealed that, compared with EUS-CG, Clip-CYA had lower endoscopic therapy costs and shorter operating times. This difference between the two treatment modalities was evident in the overall cohort as well as in the subgroup of patients with GVs maximum diameter > 3 cm. Cirrhosis causes a heavy economic burden and is frequently associated with the development of a range of complications, such as gastroesophageal variceal bleeding, ascites, hepatocellular carcinoma and hepatic encephalopathy[28]. Management of variceal bleeding is costly compared with that of other digestive diseases, and the severity of the underlying liver disease has an impact on treatment outcome, leading to higher treatment costs for class C patients than for less-affected patients[29]. However, there is less research regarding the price of different endoscopic treatments for GVs. A small-sample retrospective study (19 patients in the E-CYA group and 17 in the EUS-CG group) revealed that the use of EUS-CG was more cost-effective than the use of E-CYA[30]. Our data revealed that although there was no difference in Child-Pugh classification or intensive care unit occupancy between the two groups, five patients in the Clip-CYA group were admitted to the intensive care unit, and the MELD and Child-Pugh scores were significantly greater than those in the EUS-CG group. A long operation time and failure to effectively stop bleeding may delay the condition, and the increased cost of endoscopic treatment may increase the economic burden on patients. This aspect may have a significant effect on the choice of technique in real-life practice and deserves our attention.
To the best of our knowledge, this study is the first to compare the differences between Clip-CYA and EUS-CG for the management of GVs. Clip-CYA procedures are more convenient and easier to master than EUS-CG procedures are. The rationale for clamping the GVs was to slow down or block the blood flow rate and thereby reduce the risk of CYA embolization. The inflow and outflow veins can be regarded as whole blood flow channels. Regardless of whether the inflow or outflow veins are blocked, the blood flow within the GVs is restricted or slowed, reducing the risk of ectopic embolization during CYA injection. Our results showed that the approach is safe, and no symptoms related to CYA ectopic embolization were observed, which may be attributed to flow-control strategies (clips or coils) that reduce embolic risk[11,12]. Additionally, this approach is effective, and the rates of obliteration and rebleeding of GVs are similar to those of EUS-guided coil and CYA injection. Moreover, we found that Clip-CYA has a lower endoscopic therapy cost and shorter operating time than EUS-CG does.
This study had several limitations. First, the study was retrospective; therefore, the possibility of selection bias cannot be excluded, although patients were screened according to the same inclusion and exclusion criteria. For instance, interventional radiologic procedures such as balloon-occluded retrograde transvenous obliteration/coil-assisted re
Clip-CYA was more convenient and less expensive than EUS-CG was and safe and effective for treating patients with GVs. In the future, larger prospective, randomized controlled clinical trials are warranted to compare this technique with EUS-CG.
We thank the clinical staff at The First Affiliated Hospital, Zhejiang University School of Medicine for their assistance with patient management and data collection.
| 1. | Kaplan DE, Ripoll C, Thiele M, Fortune BE, Simonetto DA, Garcia-Tsao G, Bosch J. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024;79:1180-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 225] [Article Influence: 112.5] [Reference Citation Analysis (1)] |
| 2. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 873] [Article Influence: 25.7] [Reference Citation Analysis (42)] |
| 3. | Kim T, Shijo H, Kokawa H, Tokumitsu H, Kubara K, Ota K, Akiyoshi N, Iida T, Yokoyama M, Okumura M. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 4. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1855] [Article Influence: 463.8] [Reference Citation Analysis (3)] |
| 5. | Qiao W, Ren Y, Bai Y, Liu S, Zhang Q, Zhi F. Cyanoacrylate Injection Versus Band Ligation in the Endoscopic Management of Acute Gastric Variceal Bleeding: Meta-Analysis of Randomized, Controlled Studies Based on the PRISMA Statement. Medicine (Baltimore). 2015;94:e1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Inokuchi M, Kojima K, Kato K, Sugita H, Sugihara K. Laparoscopy versus open distal gastrectomy for advanced gastric cancer: a systematic review and meta-analysis (Qiu J et al. Surg Laparosc Endosc Percutan Tech 2013;23:1-7). Surg Laparosc Endosc Percutan Tech. 2014;24:542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Upadhyay AP, Ananthasivan R, Radhakrishnan S, Zubaidi G. Cortical blindness and acute myocardial infarction following injection of bleeding gastric varices with cyanoacrylate glue. Endoscopy. 2005;37:1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 8. | Kok K, Bond RP, Duncan IC, Fourie PA, Ziady C, van den Bogaerde JB, van der Merwe SW. Distal embolization and local vessel wall ulceration after gastric variceal obliteration with N-butyl-2-cyanoacrylate: a case report and review of the literature. Endoscopy. 2004;36:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Luo X, Xiang T, Wu J, Wang X, Zhu Y, Xi X, Yan Y, Yang J, García-Pagán JC, Yang L. Endoscopic Cyanoacrylate Injection Versus Balloon-Occluded Retrograde Transvenous Obliteration for Prevention of Gastric Variceal Bleeding: A Randomized Controlled Trial. Hepatology. 2021;74:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Zhang M, Mou H, Wang G, Li P, Kong D, Li S, Feng Q, Sun R, Yan J, Huang G, Shi Y, Tuo B, Zhang C. Clinical outcomes of clip-assisted endoscopic cyanoacrylate injection versus conventional endoscopic cyanoacrylate injection in treating gastric varices with a gastrorenal shunt. Scand J Gastroenterol. 2023;58:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Zhang M, Li P, Mou H, Shi Y, Tuo B, Jin S, Sun R, Wang G, Ma J, Zhang C. Clip-assisted endoscopic cyanoacrylate injection for gastric varices with a gastrorenal shunt: a multicenter study. Endoscopy. 2019;51:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Samanta J, Nabi Z, Facciorusso A, Dhar J, Akbar W, Das A, Birda CL, Mangiavillano B, Auriemma F, Crino SF, Kochhar R, Lakhtakia S, Reddy DN. EUS-guided coil and glue injection versus endoscopic glue injection for gastric varices: International multicentre propensity-matched analysis. Liver Int. 2023;43:1783-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Mohan BP, Chandan S, Khan SR, Kassab LL, Trakroo S, Ponnada S, Asokkumar R, Adler DG. Efficacy and safety of endoscopic ultrasound-guided therapy versus direct endoscopic glue injection therapy for gastric varices: systematic review and meta-analysis. Endoscopy. 2020;52:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Wang G, Peng L, Li P, Mou H, Li X, Li Q, Qi X, Lu K, Yao H, Wang W, Zhao L, Xia Y, Zhang M, Zhu J, Ma J, Li J, Li C, Liu X, Tuo B, Sun X, García-Pagán JC, Zhang C. Adding Clip Before Endoscopic Cyanoacrylate Injection Decreases Ectopic Embolisms in Gastric Varices: A Randomized Controlled Trial. Am J Gastroenterol. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Baliss M, Patel D, Madi MY, Bazarbashi AN. EUS-Guided Vascular Interventions. J Clin Med. 2023;12:2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Patel K. Top tips for EUS-guided embolization of gastric varices (with videos). Gastrointest Endosc. 2024;99:254-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Gralnek IM, Camus Duboc M, Garcia-Pagan JC, Fuccio L, Karstensen JG, Hucl T, Jovanovic I, Awadie H, Hernandez-Gea V, Tantau M, Ebigbo A, Ibrahim M, Vlachogiannakos J, Burgmans MC, Rosasco R, Triantafyllou K. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:1094-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 18. | Laleman W, Vanderschueren E, Mehdi ZS, Wiest R, Cardenas A, Trebicka J. Endoscopic procedures in hepatology: Current trends and new developments. J Hepatol. 2024;80:124-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Sarin SK, Kumar A. Gastric varices: profile, classification, and management. Am J Gastroenterol. 1989;84:1244-1249. [PubMed] |
| 20. | Gantzel RH, Aagaard NK, Vilstrup H, Watson H, Grønbæk H, Jepsen P. Development and validation of the Cirrhotic Ascites Severity model-A patient-reported outcome-based model to predict 1-year mortality. Hepatol Commun. 2022;6:3175-3185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Shaikh S, Ghani H, Memon S, Baloch GH, Jaffery M, Shaikh K. MELD era: is this time to replace the original Child-Pugh score in patients with decompensated cirrhosis of liver. J Coll Physicians Surg Pak. 2010;20:432-435. [PubMed] |
| 22. | Reliability of endoscopy in the assessment of variceal features. The Italian Liver Cirrhosis Project. J Hepatol. 1987;4:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Li ZQ, Linghu EQ, Hu M, Wang XD, Wang HB, Meng JY, Du H. Endoscopic measurement of variceal diameter. World J Gastroenterol. 2015;21:2140-2146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Zeng XQ, Ma LL, Tseng YJ, Chen J, Cui CX, Luo TC, Wang J, Chen SY. Endoscopic cyanoacrylate injection with or without lauromacrogol for gastric varices: A randomized pilot study. J Gastroenterol Hepatol. 2017;32:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 2967] [Article Influence: 593.4] [Reference Citation Analysis (1)] |
| 26. | McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Ryou M. Combination therapy versus monotherapy for EUS-guided management of gastric varices: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:6-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 27. | Yu SY, Wang WH, Xu L. Clip-Assisted Endoscopic Cyanoacrylate Injection: A Novel Technique for Acute Gastroesophageal Variceal Bleeding. J Laparoendosc Adv Surg Tech A. 2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Liu YB, Chen MK. Epidemiology of liver cirrhosis and associated complications: Current knowledge and future directions. World J Gastroenterol. 2022;28:5910-5930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (21)] |
| 29. | Thabut D, Hammer M, Cai Y, Carbonell N. Cost of treatment of oesophageal variceal bleeding in patients with cirrhosis in France: results of a French survey. Eur J Gastroenterol Hepatol. 2007;19:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Robles-Medranda C, Nebel JA, Puga-Tejada M, Oleas R, Baquerizo-Burgos J, Ospina-Arboleda J, Valero M, Pitanga-Lukashok H. Cost-effectiveness of endoscopic ultrasound-guided coils plus cyanoacrylate injection compared to endoscopic cyanoacrylate injection in the management of gastric varices. World J Gastrointest Endosc. 2021;13:13-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/