INTRODUCTION
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic relapsing and remitting inflammatory disorder involving the gastrointestinal (GI) system. Despite the rising incidence rate and recent advancements in therapy, the exact pathophysiology of this disease is unclear[1]. Emerging research has suggested a growing role for the gut microbiome in both GI and extra-luminal disease, linking dysbiosis of the gut microbiome to a wide array of GI diseases including neoplasia, metabolic, autoimmune and inflammatory conditions such as IBD[2,3]. However, despite growing evidence about the gut microbiome, the oral microbiome has only recently been investigated for its potential role in systemic inflammation of immune dysregulation.
The oral microbiome is a diverse and complex network of microorganisms including bacterial, fungal, viral, and archaeal microorganisms. While oral microbial health has previously been studied in the context of oral pathology such as periodontal disease, it has come under attention for its role in metabolic and cardiovascular disease, malignancy, neurologic disorders, and GI disorders[4-6]. In addition to its pathogenic role, recent data has suggested a diagnostic role as well, with specific microbial patterns having been established with increased risk of gastric cancer[7]. Given the recognized connection between the gut microbiome and IBD and the expanding understanding of the systemic inflammatory impact of the oral microbiome, there is a growing interest in its potential role in IBD. This minireview discusses the most recent evidence of the oral microbiome’s role in IBD, including pathogenic developments, diagnostic support, and therapeutic implication in an effort to advance best practices in the management of IBD.
To guide the review, a structured literature search was performed using PubMed, EMBASE, and Scopus databases. Search terms including “inflammatory bowel disease”, “Crohn’s disease”, “ulcerative colitis”, “oral microbiome”, “microbiome”, “periodontal disease”, and “periodontitis”. Articles published in English through 2025 were considered. Inclusion criteria prioritized peer-reviewed original research and review articles, with a focus on studies involving human subjects. However, preclinical studies using murine models were selectively included when they provided mechanistic insight into the oral-gut axis and its relevance to IBD pathogenesis. Reference lists of key articles were also reviewed to identify additional relevant studies.
THE ORAL MICROBIOME AND SYSTEMIC HEALTH
The oral microbiome is composed of different types of microorganisms, including amoebae, archaea, bacteria, flagellates, fungi, and viruses, which colonize the hard and soft tissues of the oral mucosa and teeth. This microbiome is composed of a common or core group of microbiota comparable between all individuals, as well as a more variable microbiome which varies based on lifestyle and genotype. With approximately 400 different taxa and 1500 genomes, each different microbe thrives in different areas of the oral cavity, depending on aerobic and anaerobic capabilities as well as environmental adaptability[8]. Within this, the makeup of the bacterial microbiome includes over 700 different species of bacteria. The predominant species of gram positive bacteria involve the gram positive cocci, including Peptostreptococcus spp. and Streptococcus spp., and the gram positive rods, including Actinomyces spp., Corynebacterium ssp, and Lactobacillus ssp. For the gram negative bacteria, the predominant species of cocci include Moraxella spp. and Neisseria spp., and the predominant gram negative rods include Campylobacter spp., Eikenella spp., Fusobacterium spp., Prevotella spp., and Treponema spp.[8]. The Human Microbiome Project found that the most prevalent group of bacteria in the oral microbiome was Streptococcus (oralis, mitis, peroris) followed by Pasteurellacae (Prevotella, Fusobacterium, Neusseria spp., Corynebacterium, Actinomyces) then Lactobacillales spp. and Lachnospiraceae spp.[9]. Though there has been less research on the other groups of microorganisms, the majority of protozoa are theorized to be Entamoeba gingivalis and the most common fungi is the Candida spp.[8]. This core group of microbiota can differ due to lifestyle, as the oral cavity can be exposed to different diets, exogenous microbes, particulate matter, and even other microbiomes[9].
The interplay between the various microbiota allows for the protection of the oral cavity in order to prevent disease development and begin the digestive process. With the beginning of digestion, the oral microbiome is an essential line of defense for pathogens entering the GI system. Bacteria such as Streptococcus sanguinis, Streptococcus salivarius, Streptococcus mitis, Actinomyces naeslundii, and Haemophilus parainfluenzae have demonstrated the ability to decrease adhesion of Porphyromonas gingivalis to the oral cavity, offering direct protection against a pathogenic bacteria linked to periodontal and systemic disease[9]. Additionally, Lactococcus lactis, a bacterium of the oral microbiota, provides systemic benefit due to nisin production. Nisin, a bacteriocin, has been noted to reduce oral tumorigenesis and is currently being studied as a treatment for head and neck squamous carcinoma as it can induce apoptosis and cell cycle arrest, thereby reducing tumor cell proliferation[10]. The oral microbiome works synergistically in creating symbiotic biofilms which balance the pH of the mouth, creating an optimal environment for the growth of the commensal microorganism of the oral microbiome while suppressing pathogenic growth. These biofilms also often contain bacteria which can facilitate the reduction of nitrate to nitrite, with the eventual end product of nitric oxide. Nitric oxide provides multiple benefits systemically, including improving endothelial functioning, the reduction of oxidative stress, and decreasing blood pressure[9]. Furthermore, more locally, the oral microbiome provides protection to the oral cavity while aiding in digestion. Streptococci spp. have developed an amylase binding protein to bind the enzyme, salivary amylase, to further aid in the metabolism of starches[9].
In turn, a dysbiotic disruption in the homeostasis of the oral microbiome cannot only cause adverse effects to the oral cavity, but recent research has demonstrated the effects systemically. Dysbiosis can occur due to the loss or lack of diversity in the microbial population, which often causes the expansion of pathogenic microbes. Additionally, there are multiple influences which can affect this microbiome, such as age, diet, tobacco use, hormonal changes, genetics, underlying medical conditions, medications, hygiene practices, and demographics, which can cause the loss of the balanced synergistic relationships between beneficial microorganisms. This can adversely affect local immune function and promote disease, such as with gingivitis or periodontal disease, increase the carcinogenic metabolites in the oral cavity, as with the loss of Lactococcus creating nisin, and cause vascular suppression, as with the loss of nitrate-reducing bacteria such as Neisseria ssp. Growth of pathogenic bacteria in the oral cavity, such as Porphyromonas gingivalis, has been implicated in atherosclerosis, Alzheimer’s disease, and multiple cancers including head and neck, pancreatic and colorectal cancer[11].
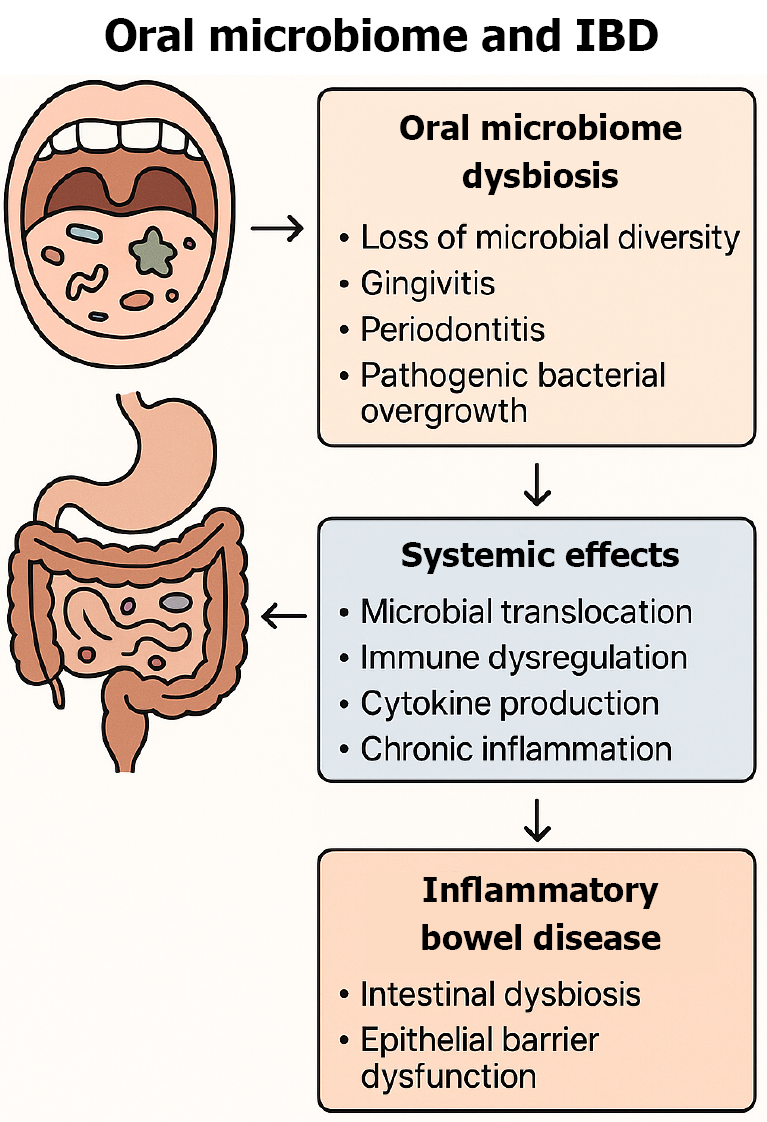
Oral dysbiosis causes an immune cascade through the adaptive immune system due to microbial translocation, immune modulation, and cytokine release. Certain bacteria, including Fusobacterium nucleatum, have evolved virulence factors that allow the microorganism to adhere to the epithelial cells and invade systemically. This invasion can cause the activation of the inflammatory cascade through production of proinflammatory cytokines, such as interleukin (IL)-6, tumor necrosis factor alpha, and IL-1[11]. Pathogenic microbiota, adversely can affect the immune system of the oral cavity will start an inflammatory cascade which releases cytokines and chemokines to attract immune cells to attack the invaders. For example, a recent study noted that the colonization and overgrowth of Klebsiella spp. lead to the recruitment of T helper 1 cells which in turn, caused gut inflammation[12]. The chronic inflammation of the oral cavity can mitigate adverse effects inducing pathways which contribute to the pathogenesis of IBD (Figure 1).
Figure 1 Influence of oral microbiome dysbiosis on systemic and intestinal inflammation in inflammatory bowel disease.
IBD: Inflammatory bowel disease.
ORAL MICROBIOME AND ROLE IN IBD
Of the trillions of microorganisms which inhabit the gut and oral-microbiome, the symbiotic relationship between the two is responsible for significant roles in digestion, absorption, immune system development, and metabolic regulation[13]. Factors which influence the development of IBD include genetic predisposition, gut microbiota dysbiosis, and other environmental factors. Several studies have investigated the relationship between the oral microbiome and its role in the pathogenesis of IBD, shedding insight on the importance of understanding its role. The pathogenic involvement and pathways leading to related IBD make sense.
There are several barriers which contribute to the separation of the oral-gut barrier including chemical separations and physical barriers like bile and stomach acid keep the oral and intestinal microbiomes apart[14]. However, the division of the oral-gut axis can be compromised allowing for the translocation of microbes thus causing dysregulated immune activation to the intestinal microbiota. Previous studies have demonstrated the intestinal enrichment of oral-associated bacteria as a potential microbial signature in IBD[15]. This dysregulation of the host–microbiota interactions are integral to the pathogenesis of many oral diseases, specifically for periodontitis. Periodontitis is a chronic and potentially severe inflammatory condition that can lead to several complications including bone loss and systemic diseases. Chronic inflammation during periodontitis may cause the outgrowth of virulent bacteria which continue to drive disease. Further, there are studies investigating the translocation between the oral cavity to distal locations including the intestine which may be associated with increased co-occurrence with IBD as well as throughout the GI tract including in liver diseases and colorectal cancer.
The distinct microbiome of the oral cavity, small intestine, and large intestine consist of different compositions and intestinal dysbiosis consisting of substantial change in the natural microbial environment can lead to pathological changes, which is common in IBD. In the healthy intestine, the oral cavity’s microbes can be divided into the soft buccal, lingual, tongue mucosa, and subgingival surfaces of the teeth which can be classified by various nutrients, oxygen distribution, salivary and pH gradients[15]. In the tongue the microbes include genera Streptococcus, Haemophiuls, Prevotella, Veillonella, and Rothia, which work synergistically to form the structures attached to the host epithelial cells and make up a diverse community which forms multispecies biofilms; additionally, other bacteria found in the mouth include Corynebacterium, Streptococcus, and Actinomyces spp. A study analyzing fecal and salivary microbe datasets has found that about 2% of all intestinal microbiome is composed of oral species in healthy individuals including Fusobacterium spp., Streptococcus spp., Veillonella spp., and Haemophilus spp.[15]. However, the oral microbiota within the intestine have been found to be largely in proximal parts of the GI tract rather than the distal portion due to the acidic conditions of the stomach and the resistance to colonization of the intestine by oral bacteria[15]. In a study conducted in mouse models in which human oral species colonized the intestines of germ-free mice, oral species including Streptococcus spp. and Porphyromonas spp. persisted mainly in the small intestine, suggesting that due to its closer proximity to the oral cavity and lower microbiota stability it may have more overlap in oral-gut microbiota compared to more distal parts[16].
Among patients with IBD, Campylobacter concisus and Fusobacterium nucleatum were a few of the first oral bacteria enriched in IBD through genomic analysis of stool and by intestinal biopsy sampling[16]. Said et al[17] demonstrated that the dysbiosis of salivary microbiota is associated with inflammatory response in IBD patients. Advances in gene sequencing technology have facilitated expanding data on oral microbial dysbiosis and IBD. In a study conducted in treatment-naive pediatric patients with CD compared to healthy controls, several bacteria including Fusobacterium nucleatum, Haemophilus parainfluenzae, and Veillonella parvula were noted to be present in both the mucosa and stool[18]. Furthermore, a longitudinal analysis showed that severe disease in patients with UC was associated with the presence of oral taxa such as Fusobacterium spp., Veillonella dispar, Veillonella parvula, Haemophilus parainfluenzae, and Campylobacter spp. with Fusobacterium spp. being twice as abundant in intestinal tissue biopsy compared to the other taxa[19]. Fecal samples from patients with IBD have also shown significantly high amounts of Fusobacterium spp. in those with CD, with increased levels correlating with active disease[19].
Among both oral and intestinal sites, the interaction of various factors including the immune system, microbiota, and GI tract integrity and motility are integral to maintain homeostasis. With periodontitis and IBD, the dysregulation in chronic inflammation has been observed to involve overlapping immune pathways such as increased local cytokine production, neutrophil dysfunction, and oxidative stress[20,21]. For example, the junctional epithelium which attaches the mucosa to the tooth is strongly regulated by the gingival immune system which is responsible for neutrophilic response through phagocytosis, degranulation, secretion of antimicrobial peptides[20,21]. Several factors including genetics, microbiota diversity, and environmental factors promote periodontitis. The early stages of this disease are related to inflammatory markers and cytokines including IL-1, IL-6, and tumor necrosis factor alpha causing a cascade of inflammation. Preliminary data has shown that these salivary inflammatory markers are more enriched in those with CD and UC at baseline, which may contribute to the increased severity of oral disease activity in cohorts in this population[21]. Further, studies have shown reduced salivary neutrophil activation and recruitment in patients with periodontal diseases and IBD, aligned with similar defects in neutrophil function within the intestine in patients with CD[22]. In terms of the epithelial barrier which forms the connection between the microbial and host immune interaction within the intestine, this can be divided between innate and adaptive immunity which works against pathogens without hypersensitivity. The epithelial and immune interaction in IBD is largely complex and uncharacterized, but has been known to be associated with feedback between dysbiotic microbiota and host immunity which can be influenced by genetic and environmental factors[23]. Because the host immune response relates to bacteria in both periodontitis and IBD, there are shared inflammatory mechanisms which perpetuate the expansion of oral-intestinal disease in patients with IBD[23]. Studies have found a bidirectional relationship between periodontal disease and IBD. CD was associated with an increased risk for developing periodontitis, and UC was conversely found to be related to UC flares[24]. One mechanism relates to increased concentration of nitrate which is a result of the host inflammatory response[23]. Previous transcriptome analysis of subgingival microbiota in patients with periodontitis has shown greater expression of nitrate reductase genes, which shows the importance of this mechanism in the reproduction of bacteria. Additionally, the T helper 17 inflammatory axis has been shown to be a part of both periodontitis and IBD[23]. The role of T helper 17 inflammatory cells has been shown to drive intestinal inflammation in murine models and its shared role in the pathways of inflammation between the oral cavity and intestine has shown the relationship between the two[23,24].
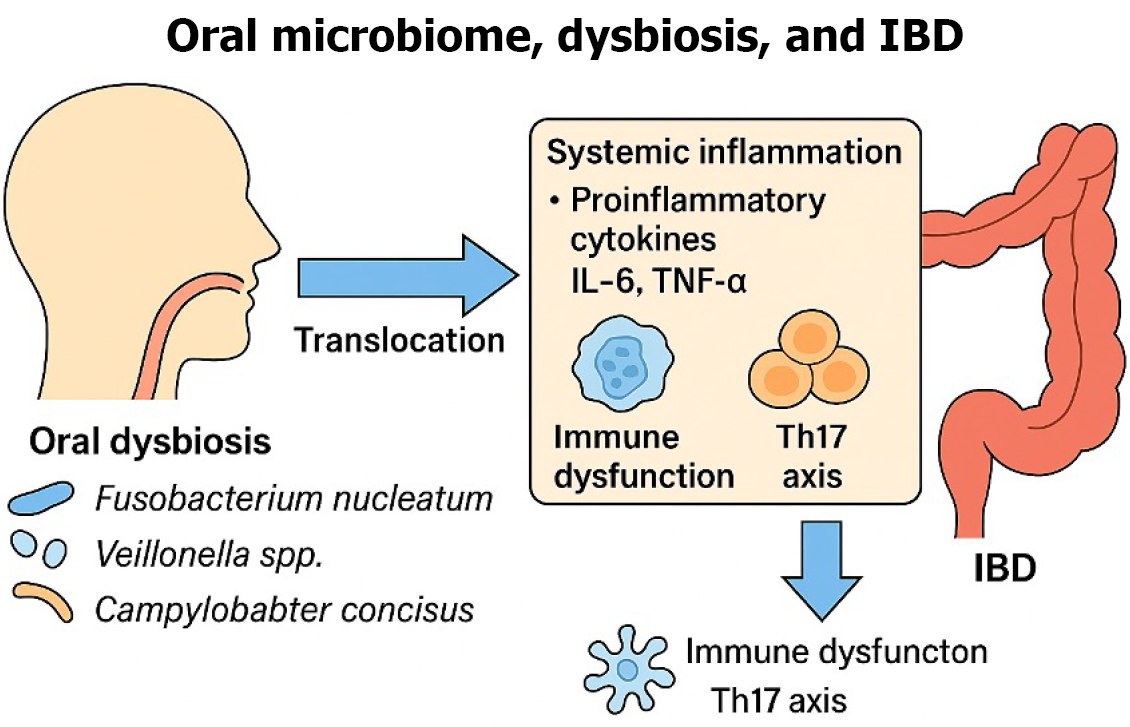
In addition to the previously discussed immunologic pathways, emerging evidence highlights several metabolic mechanisms by which oral pathogens exacerbate intestinal inflammation in IBD. For example, oral bacteria such as Fusobacterium nucleatum and Porphyromonas gingivalis produce metabolic byproducts like hydrogen sulfate, which can compromise intestinal barrier integrity, increasing permeability and ease of bacterial translocation, resulting in further inflammation[25]. Oral microbial dysbiosis also influences gut metabolism of short-chain fatty acids, such as butyrate, a critical component in maintaining barrier health and immune regulation. Decreased short-chain fatty acid has been directly linked to increased inflammatory cytokine production and altered immune cell function within the gut[25]. Additionally, oral bacteria have been shown to metabolize dietary components into inflammatory mediators such as arachidonic acid derivates, further contributing to a pro-inflammatory state in IBD[25]. Collectively, these metabolic pathways underscore the intricate link between microbial metabolism and intestinal inflammation, offering new insights into targeted therapeutic strategies aimed at modulating the oral microbiome to impact intestinal disease severity (Figure 2).
Figure 2 Proposed mechanism of oral microbiome dysbiosis influencing inflammatory bowel disease.
IBD: Inflammatory bowel disease; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor alpha; Th17: T helper 17.
THERAPEUTIC IMPLICATIONS AND EMERGING TREATMENTS
The mainstay of medical treatment in IBD is based on reducing inflammatory activity of the gut. Pharmacologic options typically consist of corticosteroids, aminosalicylates, immunomodulators, biologics, and Janus kinase inhibitors. Research suggests that such pharmacotherapies may alter the makeup of the gut microbiome and this may, in part, play a role in mediating their therapeutic benefits[26-28]. Treatments intended to specifically target or alter the gut and/or oral microbiome, however, have yet to be fully adopted in clinical practice.
Probiotics, live microbiota introduced to impart therapeutic benefits, are an area of interest in IBD management. In systematic reviews and metanalyses of available clinical trials, probiotics were found to have potential benefit in clinical remission of UC but with a low degree of certainty given study heterogeneity[29,30]. The role of probiotics in CD, however, is unclear as studies are mixed in demonstrating clinical benefit[31]. Fecal microbiota transplantation (FMT), aimed to restore the beneficial intestinal microbiome in recipients, is best known in treatment of recurrent Clostridioides difficile colitis; however, FMT for IBD is also a growing area of interest. Studies suggest FMT to be promising in achieving clinical remission in both UC and CD[32,33]. Large scale clinical trials in both probiotics and FMT are necessary to better understand their role in IBD treatment and if they may be beneficial as adjuncts to existing pharmaceutical options.
Periodontal disease is another area of interest in the study of IBD, with consideration that changes in the microbiome of either the oral cavity or GI tract may have downstream implications on the microbiome and health of the other[34]. Patients with IBD have been found to have significant differences in oral microbiome and a greater degree of dysbiosis when compared to those without IBD, demonstrating the potential utility of the oral microbiome in evaluation of IBD and disease severity[35,36]. Recent literature suggests that interventions targeting oral dysbiosis such as oral probiotics, antimicrobial rinses, regular dental hygiene, and periodontal therapy may offer potential novel therapeutic pathways[37,38]. One study demonstrated that the addition of oral probiotics in the form of fermented lingonberry juice supported oral Lactobacillus populations and improved oral dysbiosis[36]. Given the established crosstalk between the oral and gut microbiome, this study offers the potential for modulation of IBD course via the oral and gut microbiomes. Periodontal health and the oral microbiome is a promising frontier in IBD research, as it may help contribute to the diagnosis of IBD, aid in evaluating for severity of disease, and may further lead to areas of therapeutic intervention.
CONCLUSION
The oral microbiome has emerged as a critical yet underrecognized contributor to the complex pathogenesis of IBD. While history examined in the context of periodontal disease, growing evidence now supports its systemic impact on immune modulation, microbial translocation, and chronic inflammation. Several noteworthy reviews have recently explored the oral-gut microbiome relationship in IBD, each from distinct perspectives. Read et al[23] provided a broad overview of the oral-gut axis, introducing key concepts regarding microbial translocation and generalized gut inflammation but without specific therapeutic implications. Elmaghrawy et al[36] offered valuable insights into clinical oral manifestations in IBD patients, particularly pediatric cohorts, focusing largely on microbial biomarkers rather than mechanistic or therapeutic approaches. Xiang et al[25] emphasized detailed metabolic interactions drive by oral bacteria, uniquely highlighting specific microbial metabolites implicated in intestinal inflammation. Han et al[39] closely examined oral microbiota-driven pathogenic mechanisms, clearly delineating the immune and metabolic pathways involved in oral-intestinal crosstalk.
This minireview builds upon these important foundational works by distinctly highlighting the overlapping immunologic mechanisms, shared microbial signatures, and functional crosstalk between the oral and gut microbiomes in patients with IBD. Additionally, there is an increased focus on the bidirectional associations between the oral and gut microbiome as well as potential diagnostic and therapeutic applications. Despite these developing insights, the oral microbiome remains an underutilized domain in the diagnosis and treatment of IBD. Exploring the role of dysregulation in the oral microbiome may provide further insight into the pathogenesis of IBD as well as the potential for future treatments. Future research is needed to elucidate casual pathways, define microbial signatures as adjuncts to inflammatory markers in assessing disease activity, and assess the efficacy of oral microbiome-targeted interventions such as probiotics, enhanced emphasis on regular dental hygiene and checkups, as well as appropriate referrals for periodontal therapy as adjunctive strategies for IBD management. A key impediment, however, is that medical care providers who recognizably are not dentists need to start the discussion asking about dental hygiene. The scientific study as well as the integration of oral health into IBD management will likely more clearly identify the pathogenic relationships as well as better identify treatment strategies which allow a more personalized approach for treatment, and ultimately improvement in clinical outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade B
Novelty: Grade A, Grade A, Grade B
Creativity or Innovation: Grade A, Grade B, Grade B
Scientific Significance: Grade A, Grade A, Grade A
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Leonan-Silva B, DDS, Professor, Researcher, Brazil; Wang YH, PhD, Assistant Professor, Post Doctoral Researcher, China S-Editor: Wu S L-Editor: A P-Editor: Wang WB