Published online Oct 14, 2025. doi: 10.3748/wjg.v31.i38.109528
Revised: June 17, 2025
Accepted: September 1, 2025
Published online: October 14, 2025
Processing time: 152 Days and 18.2 Hours
Colorectal cancer (CRC) frequently metastasizes to the lungs, and image-guided thermal ablation (IGTA) has emerged as a promising treatment for oligometastatic colorectal lung metastases (CRLM). However, high-quality multicenter data remain limited, and the prognostic impact of site-specific extrapulmonary meta
To assess IGTA efficacy in potentially curable oligometastatic CRLM and determine prognostic impacts of extra
This multicenter real-world study analyzed 336 CRLM patients treated with IGTA from 2014 to 2022. Inclusion criteria included pathologically or clinically confirmed oligometastatic CRC, tumor diameter < 50 mm, fewer than 5 metastatic lesions, and ≤ 2 organs involved. Kaplan-Meier and Cox regression methods assessed survival out
The 3-year cumulative local tumor progression rate was 14.0%. Median PFS and OS were 15.6 and 51 months, respectively, with 3- and 5-year OS rates of 59.5% and 41.0%. Poor survival outcomes were associated with a higher tumor burden (larger size and greater number), carcinoembryonic antigen > 20 ng/mL, carbohydrate antigen 19-9 > 37 U/mL, and extrapulmonary metastases. Patients without extrapulmonary metastasis had 1-, 3-, and 5-year PFS rates of 65.4%, 31.0%, and 27.3%, respectively, which were longer than those of CRLM patients with liver metastasis [hazard ratio (HR) = 1.449, P = 0.019] and abdominal cavity metastasis (HR = 1.864, P = 0.010). The 1-, 3-, and 5-year OS rates for patients without extrapulmonary metastasis were 96.4%, 71.0%, and 53.0%, respectively, which were significantly longer than those for patients with bone metastasis (HR = 4.538, P < 0.001), abdominal cavity metastasis (HR = 4.813, P < 0.001), and pelvic cavity metastasis (HR = 3.105, P < 0.001).
Metastatic patterns significantly influence PFS and OS, emphasizing the need for careful patient selection. Notably, patients with liver-only extrapulmonary metastasis demonstrate comparatively favorable outcomes, suggesting a distinct biological behavior and better prognosis within this subgroup.
Core Tip: This multicenter study confirms that thermal ablation provides effective local tumor control and meaningful survival benefit in patients with oligometastatic colorectal lung metastases. Importantly, the pattern of extrapulmonary metastasis significantly influences prognosis. Patients with liver-only metastases show comparatively favorable outcomes, underscoring the importance of detailed metastatic pattern assessment in clinical decision-making. Tailoring treatment strategies based on metastatic distribution can optimize patient selection and improve individualized management in oligometastatic colorectal cancer.
- Citation: Hu XF, Dong XJ, Gu XY, Hu JH, Li XH, Zhao FH, Xia XW, Fan HJ, Xu SF. Extrapulmonary metastases impact survival outcomes of thermal ablation for colorectal lung oligometastases: A multicenter study. World J Gastroenterol 2025; 31(38): 109528
- URL: https://www.wjgnet.com/1007-9327/full/v31/i38/109528.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i38.109528
Colorectal cancer (CRC) ranks as the third most common malignancy and the second leading cause of cancer-related death worldwide, with pulmonary metastases occurring in up to 20% of cases[1-3]. Surgical resection remains the stan
Image-guided thermal ablation (IGTA), including radiofrequency ablation (RFA) and microwave ablation (MWA), has emerged as a valuable treatment option for CRLM in recent years[8,9]. Clinical evidence suggests that IGTA may offer survival outcomes comparable to surgery. A prospective multicenter study involving 70 patients reported a 3-year overall survival (OS) rate of 53% following RFA[8]. Similarly, a study by Cheng et al[10] demonstrated a median OS of 31 months and a 3-year OS rate of 44.4% with MWA.
Despite the promising potential of IGTA in managing oligometastatic CRLM, two key scientific questions remain unresolved. First, current evidence is limited by the lack of large-scale, multicenter studies. Second, the prognostic impact of extrapulmonary metastases particularly their organ-specific or anatomical distribution has not been fully elucidated. To address these gaps, the present study leverages real-world data from multiple tertiary centers to comprehensively evaluate the efficacy and safety of IGTA in oligometastatic CRLM, with a specific focus on the prognostic relevance of extrapulmonary metastatic patterns. Our findings aim to provide high-level evidence to inform clinical decision-making in this complex patient population.
This multicenter real-world study included 336 CRLM patients treated with IGTA between March 2014 and October 2022. The study was approved by the institutional ethics committee (No. IEC-2024-1130), and the requirement for informed consent was waived due to the retrospective nature of the study.
Inclusion criteria: (1) Histopathologically or clinically confirmed CRLM; (2) Received computed tomography (CT)-guided RFA or MWA treatment; (3) Oligometastatic disease, defined as fewer than 5 metastatic lesions with a maximum diameter < 50 mm, involving no more than 2 organs (e.g., liver, lung, peritoneum, with occasional lymph node involvement). In patients with multifocal or bilateral lung lesions, each radiologically identifiable lesion was counted individually, regardless of anatomical location or lobe distribution; (4) Complete clinical and imaging data; (5) Prior R0 resection of the primary tumor; (6) First-time IGTA treatment for CRLM; (7) Eastern Cooperative Oncology Group performance status 0-2; (8) Controllable extrapulmonary metastases, defined as those that can achieve complete or partial response through local or systemic treatment; and (9) Cardio-pulmonary function adequate for ablation.
Exclusion criteria: (1) Age < 18 years or > 85 years; (2) Child-Pugh C liver function; (3) Suboptimal imaging quality for lesion evaluation; (4) Potential pregnancy; (5) Severe lung dysfunction; (6) History of other malignancies within the past 5 years; and (7) Active infection or immunosuppressive state. All participating institutions adhered to a standardized study protocol that clearly defined the inclusion and exclusion criteria, imaging follow-up intervals, and ablation prin
Diagnosis of CRLM was established through histopathological confirmation, resection of pulmonary metastases, or identification of new or enlarging nodules via dynamic contrast-enhanced CT (DCE-CT). Each case was reviewed by a multidisciplinary team comprising experts in thoracic surgery, medical oncology, and interventional radiology. Pre-procedural assessments included comprehensive blood tests, liver and renal function evaluations, coagulation profiles, electrocardiography, pulmonary function testing, and DCE-CT imaging. Patients were informed of the procedure’s benefits, risks, and complications before providing signed procedural consent.
Although minor procedural variations were inevitable in routine clinical practice such as the selection between RFA and MWA or differences in the availability of interventional devices all centers consistently implemented core IGTA techniques in accordance with unified technical standards. The IGTA procedures were conducted by a specialized team of 2-3 interventional radiologists, each with over a decade of experience in oncologic interventions. Patients were positioned (supine, prone, or lateral) according to tumor location, and sedation with analgesia was administered to maintain consciousness while ensuring patient comfort. Continuous vital sign monitoring was performed throughout the procedure. Ablation needle selection (monopolar or multi-electrode radiofrequency: 14-16 Ga; microwave: 17 Ga) was tailored to the tumor’s size and anatomical location. CT imaging was employed to guide needle placement, confirm target positioning, and monitor ablation zones. Following ablation, immediate and 24-hour post-procedure CT scans were performed to evaluate tumor coverage and detect complications. Complications were classified as minor or major in accordance with the Society of Interventional Radiology Clinical Practice Guidelines[11].
Clinicopathological and procedural data were systematically retrieved from institutional electronic medical records and databases. The collected data included patient demographics (age, sex, body mass index), tumor characteristics (primary site, histological differentiation, tumor node metastasis stage), treatment history (surgical resection, chemotherapy, radiotherapy, immunotherapy, targeted therapy), comorbid conditions, and laboratory parameters [e.g., carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125 and CA19-9]. Additional factors such as disease-free interval, synchronous metastases, tumor size, number of lesions, extrapulmonary metastases, and IGTA procedural parameters (e.g., ablation type, power settings, temperature, duration, and puncture depth) were also recorded.
Imaging data were independently reviewed by a panel of 10 radiologists with over 10 years of experience, blinded to the procedural details. CT scans with 2-mm slice thickness were reconstructed using the Picture Archiving and Commu
In accordance with the National Comprehensive Cancer Network guidelines, tumors located within the outer one-third of the hemithorax were categorized as peripheral zone tumors, while those situated in the inner two-thirds of the hemithorax were also classified as peripheral zone lesions[12]. Perivascular and peribronchial lesions were defined based on proximity to vessels/bronchi ≥ 3 mm in diameter[13]. Lesions located within 1 cm of the pleura (mediastinal, parietal, or interlobar) or diaphragm were considered adjacent. Lymph nodes were deemed positive if their short-axis diameter exceeded 1 cm, exhibited extranodal extension > 1 mm, or if malignancy was confirmed pathologically[14]. Patients were grouped based on IGTA timing relative to systemic therapy (FOLFOX, FOLFIRI, CAPOX, and FOLFOXIRI): (1) IGTA alone without systemic treatment; (2) Synchronous therapy (systemic therapy within 1 month of IGTA); (3) Upfront ablation (systemic therapy initiated > 1 month after IGTA); or (4) Delayed ablation (IGTA performed 1-2 months after systemic therapy initiation)[15].
DCE-CT was performed 3-4 weeks after the IGTA procedure to evaluate the ablation zone, with complete ablation being indicative of technical success. Repeat IGTA was conducted for cases with incomplete ablation, and technical success rates were recorded. Local tumor progression (LTP) was defined as recurrence occurring within 1 cm of the ablation zone more than 4 weeks after the procedure. Cases of LTP were managed with repeat IGTA or alternative multimodal therapies, such as radioactive particle implantation, radiotherapy, chemotherapy, or targeted therapy.
Disease surveillance included DCE-CT every 3 months during the first year and every 6 months during the subsequent two years. Follow-up schedules were individualized based on treatment response, baseline characteristics, and patient compliance. For patients unable to attend in-person evaluations, telephone follow-ups were conducted to supplement data collection. The primary endpoints of the study included LTP-free survival (LTPFS), progression-free survival (PFS), and OS. Secondary endpoints encompassed prognostic factor identification, technical success rates, and complications.
Data distribution was evaluated using the Kolmogorov-Smirnov test. Continuous variables conforming to a normal distribution were expressed as mean ± SD and compared using Student’s t-test. Non-normally distributed variables were presented as medians with interquartile ranges (IQRs) and compared using the Mann-Whitney U test. Categorical data were reported as counts and percentages [n (%)] and analyzed using the χ² test or Fisher’s exact test, as appropriate.
To minimize inter-center heterogeneity and ensure data integrity: All baseline characteristics and outcome events were centrally reviewed and validated by an independent data management team using standardized definitions and templates; Sensitivity analyses stratified by treatment center were performed.
Survival analyses were performed using the Kaplan-Meier method, and comparisons were conducted with the log-rank test. Independent prognostic factors were identified through Cox proportional hazards regression models. All candidate prognostic variables including clinical factors and tumor characteristics were first screened by univariable Cox regression (P < 0.05) before multivariable modeling. To guard against multicollinearity, we calculated the variance inflation factor (VIF) for each covariate. Variables with VIF > 5 were examined for redundancy; in cases of high collinearity (for example, between CEA and CA 19-9 Level), we retained the variable with stronger univariable association or greater clinical relevance.
All statistical analyses were performed using SPSS software version 25.0 (IBM Corp.) and Stata version 17.0 (StataCorp). A two-sided P value < 0.05 was considered statistically significant.
The demographic and clinical characteristics of the study population are detailed in Table 1. A total of 336 consecutive patients (62.2% male) with a mean age of 61.1 ± 9.7 years (range: 28-86 years) underwent IGTA for pulmonary metastases. Among these patients, 75 (22.3%) had a history of hypertension, 28 (8.3%) had diabetes mellitus, and 29 (8.6%) presented with other comorbidities. The rectum was identified as the most common primary tumor site (44.9%), with moderate tumor differentiation being the predominant histological grade (43.8%). The majority of patients were classified as T3 stage (55.1%). For those with extrapulmonary metastases, liver metastases were the most prevalent (20.8%). Additionally, 9.2% of patients had undergone prior surgical resection of pulmonary metastases before IGTA, with a median interval of 19 months (IQR: 28.0 months) between surgery and ablation.
| Variables | Variables | ||
| Age (years) | 61.1 ± 9.7 | Presence of comorbidities | 132 (39.3) |
| Sex (male) | 209 (62.2) | CEA > 20 ng/mL | 42 (12.5) |
| BMI (kg/m2) | 20.9 ± 3.6 | Extrapulmonary metastasis | |
| Primary tumor location | Liver | 70 (20.8) | |
| Rectum | 151 (44.9) | Bone | 14 (4.2) |
| Sigmoid colon | 68 (20.2) | Abdominal cavity | 21 (6.3) |
| Descending colon | 44 (13.1) | Pelvic cavity | 18 (5.4) |
| Transverse colon | 21 (6.3) | Other conditions | 20 (6.0) |
| Ascending colon | 52 (15.5) | T-stage | |
| Tumor differentiation | T1 | 12 (3.6) | |
| Poor | 24 (7.1) | T2 | 34 (10.1) |
| Poor-to-moderate | 55 (16.4) | T3 | 185 (55.1) |
| Moderate | 147 (43.8) | T4 | 105 (31.3) |
| Moderate-to-well | 70 (20.8) | DFI (< 24 months) | 153 (45.5) |
| Well | 40 (11.9) | ITGA therapy timing | |
| Pneumoresection | 31 (9.2) | Delayed ablation | 111 (33.0) |
| Synchronous metastases | 85 (25.3) | Synchronous ablation | 62 (18.5) |
| CA19-9 > 37 U/mL | 47 (14.0) | Upfront ablation | 65 (19.3) |
| Interval between pulmonary resection and IGTA, median (IQR) | 19.0 (28.0) | Interval from primary resection to IGTA (< 24 months), median (IQR) | 28 (22.0) |
The radiological characteristics of the study cohort are summarized in Table 2. Among the patients, 40.2% presented with a single metastatic lesion. The anatomical distribution of metastases revealed a predominance in the right lung (49.7%), followed by the left lung (39.0%). Notably, 88.4% of metastatic lesions were located within the peripheral lung zones. Across a total of 660 documented metastases, the mean maximum tumor diameter was 17.9 ± 10.8 mm (range: 2.8-
| Variables | Variables | ||
| Tumor size (mm) | 17.9 ± 10.8 | Peridiaphragmatic tumors | 30 (8.9) |
| Tumor size > 3 cm | 66 (19.6) | Near parietal pleural | |
| Tumor number | Near mediastinal pleural | ||
| 1 tumor | 135 (40.2) | Peribronchial tumors | 52 (15.5) |
| 2 tumors | 104 (31.0) | Perivascular tumors | 65 (19.3) |
| 3 tumors | 71 (21.1) | Emphysema | 51 (15.2) |
| 4 tumors | 26 (7.7) | CT imaging features | |
| Lung location | Routine features | 243 (72.3) | |
| Left lung | 131 (39.0) | Air bronchograms | 35 (10.4) |
| Right lung | 167 (49.7) | Cavitation, air bubbles, or bullae | 37 (11.0) |
| Bilateral involvement | 38 (11.3) | ||
| Lung field | Calcifications | 12 (3.6) | |
| Central | 31 (9.2) | Inflammatory changes | 9 (2.7) |
| Peripheral | 297 (88.4) | CT density characteristics | |
| Across multiple field | 8 (2.4) | GGO | 4 (1.2) |
| Lung zone | Solid lesions | 293 (87.2) | |
| Inner zone | 39 (11.6) | Mixed-density lesions | 39 (11.6) |
| Middle zone | 72 (21.4) | Near interlobar fissure | 106 (31.5) |
| Outer zone | 134 (39.9) | Hilar lymph nodes (+) | 46 (13.7) |
| Across multiple zones | 91 (27.1) | Mediastinal lymph nodes (+) | 54 (16.1) |
The IGTA treatment cycle comprised a single session for 314 patients, including 38 individuals with bilateral metastatic disease. MWA was utilized in 193 cases (57.4%), while RFA was performed in 143 cases (42.6%). Additionally, 22 patients with a total of 38 metastases underwent planned fractional ablation sessions, completed within one month. A detailed comparison of therapeutic parameters between MWA and RFA is presented in Table 3. Kaplan-Meier analysis showed no statistically significant difference in LTPFS between the RFA and MWA groups (median not reached, P = 0.675), nor in PFS [15.37 months, 95% confidence interval (CI): 13.038-17.702] vs 15.60 months, 95%CI: 11.870-19.330, P = 0.708], and this was further confirmed by both univariable and multivariable Cox regression analyses (Supplementary Tables 1 and 2). While Kaplan-Meier analysis indicated that the median OS was shorter in the RFA group (42.0 months, 95%CI: 28.968-55.032) compared to the MWA group (53.9 months, 95%CI: 45.091-62.709; P = 0.034), subsequent univariable and multivariable Cox regression analyses demonstrated that ablation modality was not an independent predictor of OS (Supplementary Table 3).
| Parameters | RFA (n = 143) | MWA (n = 193) | P value |
| Procedure duration (minute), median (IQR) | 15.0 (11.0) | 7.0 (8.0) | < 0.001 |
| Power output (W), median (IQR) | 180 (110) | 40 (10) | < 0.001 |
| Temperature (°C) | 96.0 ± 8.9 | 98.9 ± 8.3 | 0.071 |
| Number of ablation times, median (IQR) | 2.0 (2.0) | 2.0 (1.5) | 0.079 |
| Puncture depth (cm) | 7.2 ± 2.2 | 7.3 ± 2.0 | 0.784 |
During a median follow-up period of 40.3 months (IQR: 31.3 months), 175 patients (52.1%) died. The estimated cumulative OS rates at 1, 3, and 5 years were 87.5% (SE = 0.018), 59.5% (SE = 0.027), and 41.0% (SE = 0.035), respectively. The median OS was estimated at 51.0 months (95%CI: 44.178-57.822). LTP was documented in 48 patients, with cumulative LTP rates of 6.8%, 12.2%, and 14.0% at 1, 2, and 3 years, respectively. Disease progression following IGTA was observed in 258 patients, of whom 137 (40.8%) experienced progression within 12 months of treatment. The cumulative PFS rates at 1, 2, 3, 4, and 5 years were 59.0% (SE = 0.027), 34.6% (SE = 0.026), 23.9% (SE = 0.024), 21.2% (SE = 0.024), and 15.5% (SE = 0.028), respectively. The median PFS was 15.6 months (95%CI: 13.516-17.684). The results of sensitivity analyses stratified by treatment center revealed no statistically significant differences in OS (P = 0.158), PFS (P = 0.229), or LTPFS (P = 0.462) across participating centers.
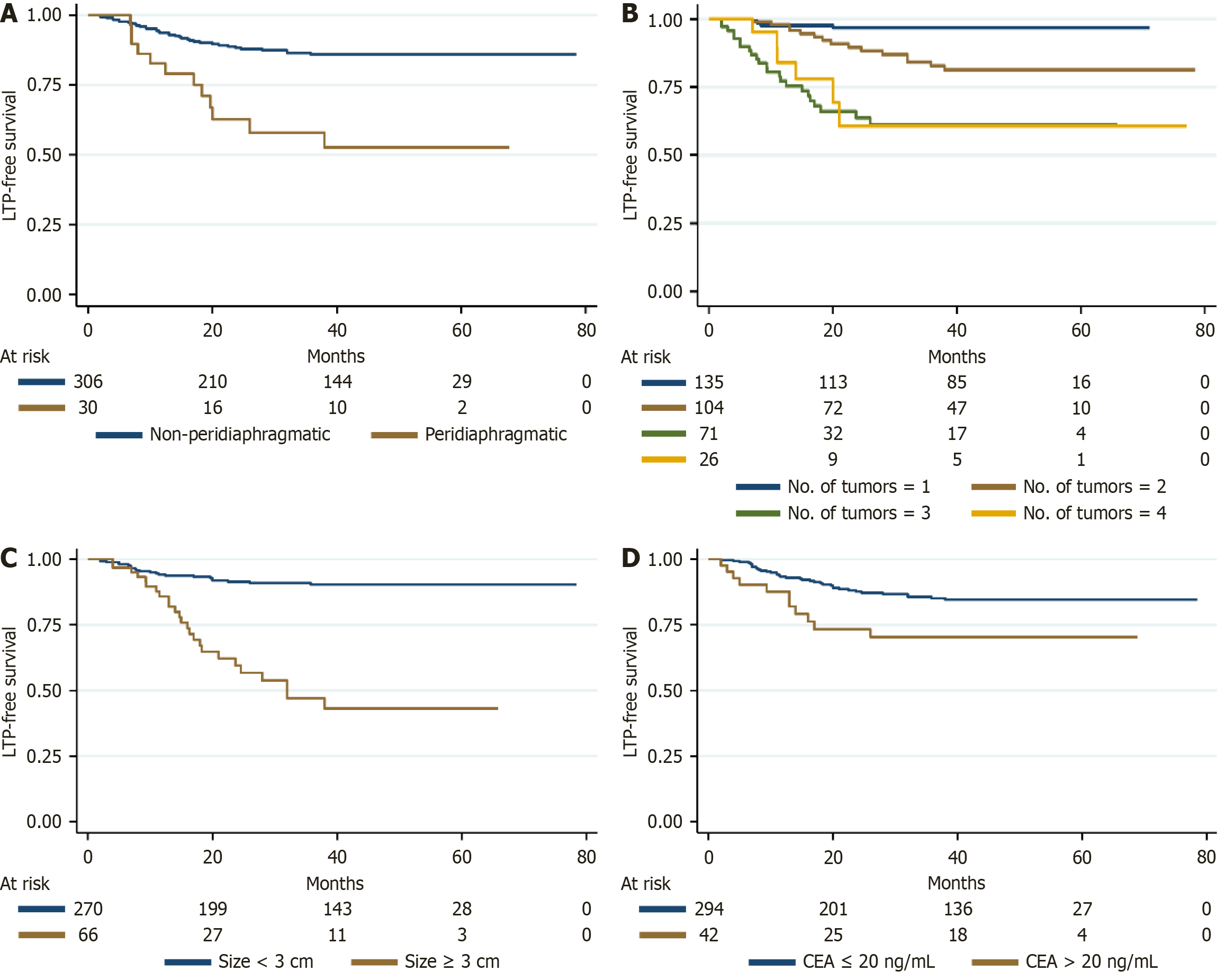
Kaplan-Meier survival analyses, complemented by log-rank tests, demonstrated a significant association between the presence of peridiaphragmatic lesions and reduced LTPFS (P < 0.001) (Figure 1A). This finding was corroborated by both univariable and multivariable Cox proportional hazards regression analyses, with a hazard ratio (HR) of 4.447 (Supple
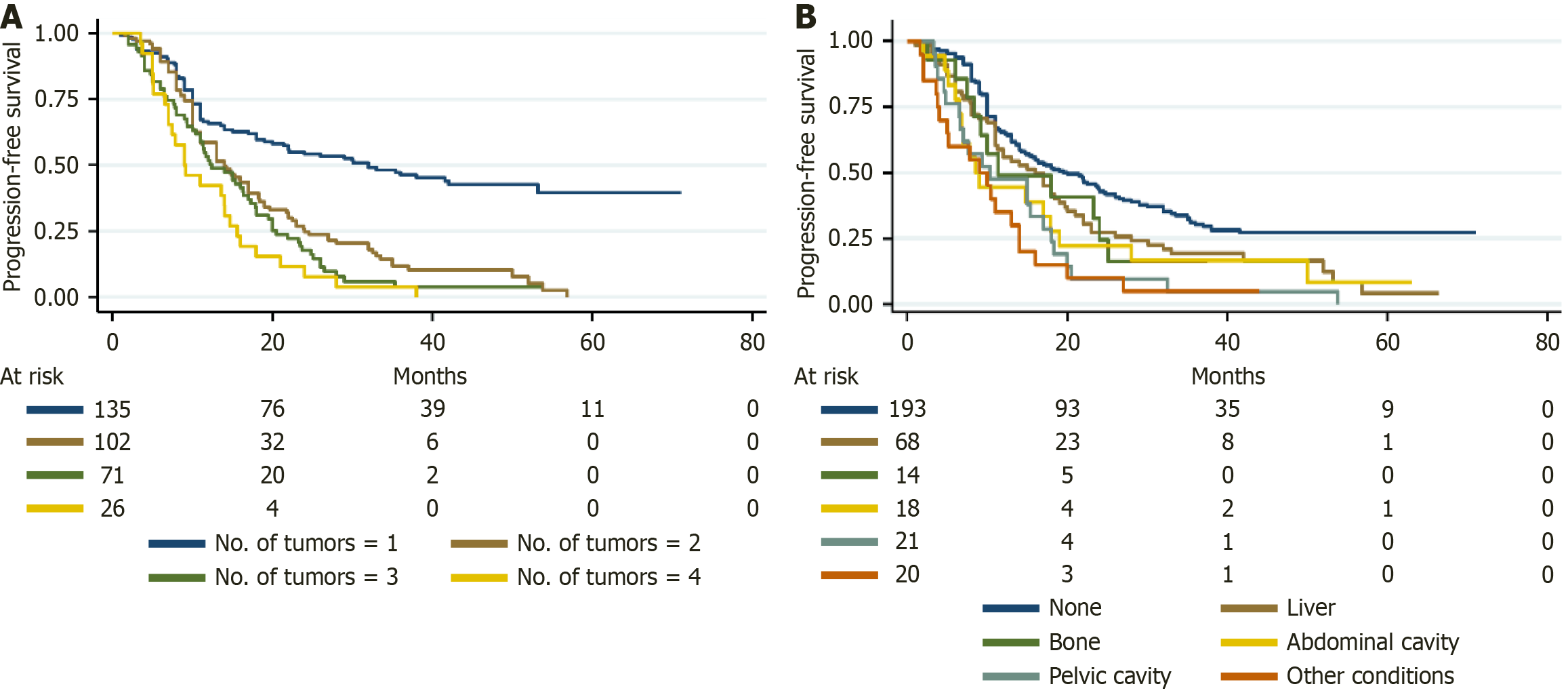
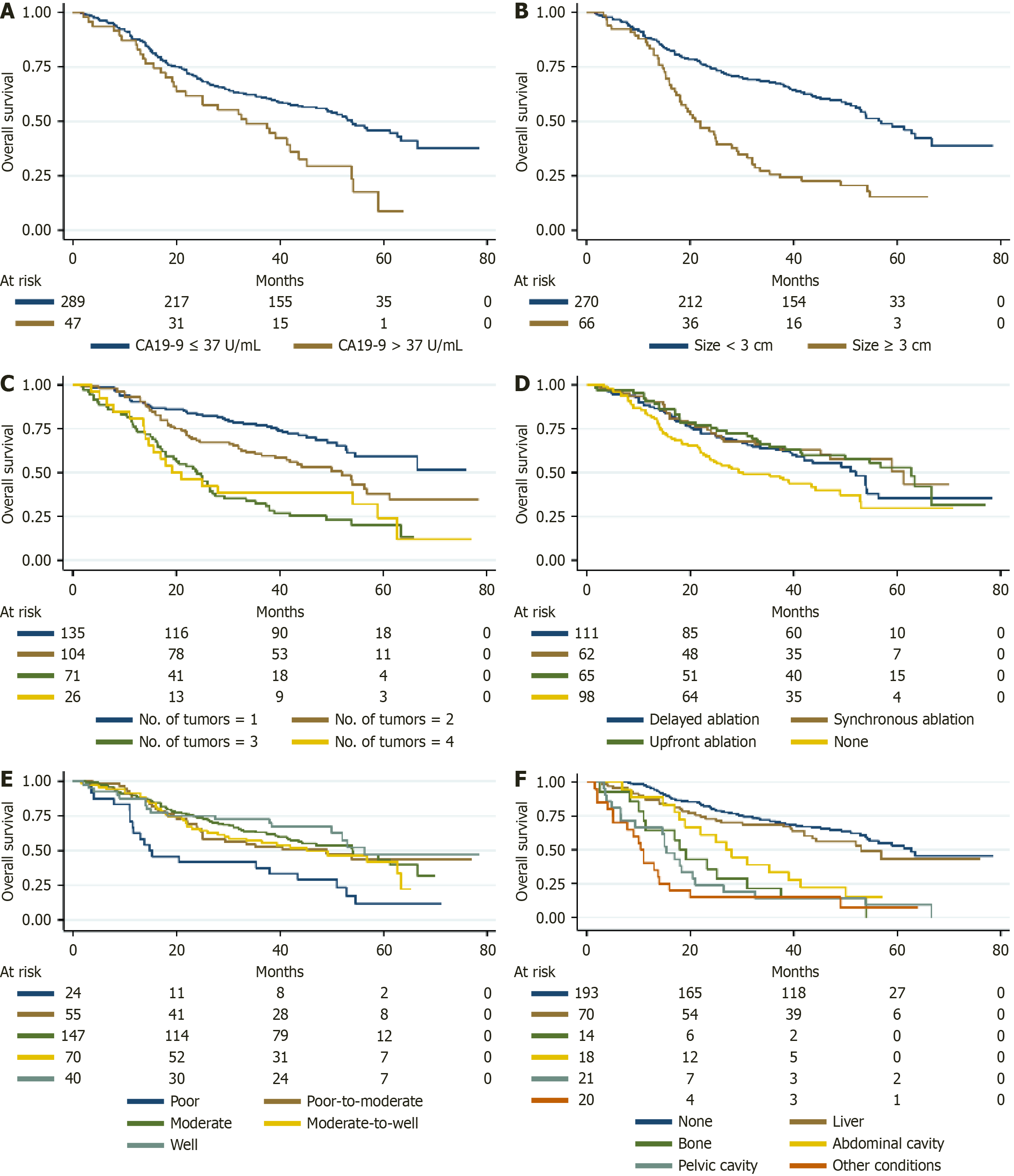
The survival outcomes stratified by the type of extrapulmonary metastasis are summarized in Table 4. Patients without extrapulmonary metastasis had 1-, 3-, and 5-year PFS rates of 65.4%, 31.0%, and 27.3%, respectively, which were longer than those of CRLM patients with liver metastasis (57.1%, 18.7%, 4.0%) (HR = 1.449, P = 0.019) and abdominal cavity metastasis (47.6%, 4.8%, 0%) (HR = 1.864, P = 0.010); however, no statistically significant differences were found in cases with bone metastasis (49.0%, 16.3%, 0%) (HR = 1.256, P = 0.459) and pelvic cavity metastasis (44.4%, 16.7%, 0%) (HR = 1.546, P = 0.106) (Figure 2B). Similarly, the 1-, 3-, and 5-year OS rates for patients without extrapulmonary metastasis were 96.4%, 71.0%, and 53.0%, respectively, which were significantly longer than those for patients with bone metastasis (64.3%, 21.4%, 0%) (HR = 4.538, P < 0.001), abdominal cavity metastasis (66.7%, 14.3%, 0%) (HR = 4.813, P < 0.001), and pelvic cavity metastasis (88.9%, 33.3%, 0%) (HR = 3.105, P < 0.001) (Figure 3F). For CRLM patients with controllable liver metastasis, the 1-, 3-, and 5-year OS rates were 87.1%, 68.6%, and 43.1%, respectively (HR = 1.300, P = 0.222), and liver metastasis was not an independent predictor of OS.
| None, % (SE) | Liver, % (SE) | Bone, % (SE) | Abdominal cavity, % (SE) | Pelvic cavity, % (SE) | |
| PFS | |||||
| 1 years | 65.4 (0.034) | 57.1 (0.059) | 49.0 (0.136) | 47.6 (0.109) | 44.4 (0.117) |
| 3 years | 31.0 (0.035) | 18.7 (0.048) | 16.3 (0.105) | 4.8 (0.046) | 16.7 (0.088) |
| 5 years | 27.3 (0.035) | 4.0 (0.037) | 0.0 | 0.0 | 0.0 |
| OS | |||||
| 1 years | 96.4 (0.013) | 87.1 (0.040) | 64.3 (0.128) | 66.7 (0.103) | 88.9 (0.074) |
| 3 years | 71.0 (0.033) | 68.6 (0.055) | 21.4 (0.110) | 14.3 (0.076) | 33.3 (0.111) |
| 5 years | 53.0 (0.048) | 43.1 (0.085) | 0.0 | 0.0 | 0.0 |
All AEs related to IGTA are summarized in Table 5. Most AEs were mild and self-limiting (grade A and grade B), with pneumothorax being the most frequent complication, occurring in 50.3% of patients, followed by hemoptysis (25.3%), chest pain (18.5%), and fever (13.7%). Major complications (grade C and grade D) were relatively rare: Pneumothorax included 3.6% grade C and 0.6% grade D cases; Pleural effusion occurred in 16.1% of patients, with 1.5% grade C and no grade D cases; Hemoptysis included 0.9% grade C cases and no grade D cases; Pneumonia was observed with 1.2% grade C and 0.3% grade D cases; Respiratory failure was rare (0.3% grade D). Overall, the rate of major complications was 10.7%. Most patients (88.7%) were discharged within six days after treatment, while 3.3% experienced prolonged hospitalization exceeding 10 days. Management of pneumothorax mainly involved observation or no intervention in 81.1% of cases, with 6.5% requiring needle aspiration and 8.3% needing closed thoracic drainage, with a median drainage duration of three days. Among patients with pleural effusion, five required closed thoracic drainage. Hemorrhagic complications were uncommon, with three cases managed using hemostatic medication and six cases requiring embolization. Across participating centers, management strategies for these complications were consistent and effective, ensuring patient safety. Importantly, no permanent adverse sequelae or treatment-related deaths occurred.
| AEs | Grade | n (%) |
| Pneumothorax | Overall | 169 (50.3) |
| A and B | 155 (46.1) | |
| C | 12 (3.6) | |
| D | 2 (0.6) | |
| Pleural effusion | Overall | 54 (16.1) |
| A and B | 49 (14.6) | |
| C | 5 (1.5) | |
| D | 0 (0) | |
| Subcutaneous hematoma | A and B | 2 (0.6) |
| Hemothorax | A and B | 4 (1.2) |
| Hemoptysis/blood-streaked sputum | Overall | 85 (25.3) |
| A and B | 82 (24.4) | |
| C | 3 (0.9) | |
| D | 0 (0) | |
| Nausea or vomiting | B | 9 (2.7) |
| Fever | Overall | 46 (13.7) |
| A and B | 44 (13.1) | |
| C | 2 (0.6) | |
| D | 0 (0) | |
| Pneumonia | Overall | 5 (1.5) |
| C | 4 (1.2) | |
| D | 1 (0.3) | |
| Respiratory failure | D | 1 (0.3) |
| Chest pain | Overall | 62 (18.5) |
| A and B | 57 (17.0) | |
| C | 5 (1.5) | |
| D | 0 (0) | |
| Abdominal pain | A and B | 6 (1.8) |
| Skin burn | C | 1 (0.3) |
The management of oligometastatic CRC poses a considerable clinical challenge due to the heterogeneity of disease presentation and patient conditions. Unique advantages of IGTA, including reduced invasiveness, preservation of pulmonary function, and repeatability, make it an appealing alternative to pulmonary metastasectomy in selected patients. However, despite its increasing application, the current evidence base remains insufficient to fully establish IGTA’s role in CRLM management. This multicenter study addresses this gap by providing robust survival data and identifying prognostic factors in a real-world setting.
The therapeutic value of pulmonary metastasectomy in CRLM remains controversial, as outcomes across different studies vary substantially[16]. A recent clinical trial evaluating adjuvant chemotherapy with the mFOLFOX6 regimen after pulmonary metastasectomy in patients with colorectal adenocarcinoma (with ≤ 4 resected lung lesions and no prior chemotherapy) reported a 5-year OS rate of 85.2% and a 5-year disease-free survival rate of 60.2%[17]. Conversely, a previous retrospective study reported a median OS of 34.73 months with an estimated 5-year survival rate of only 24.39%[18]. Another study found that after curative (non-palliative) surgical resection of 1-5 metastatic lung lesions from CRC, the mean OS was 71 ± 35 months, with a median of 25 months[19]. In comparison, our study demonstrated 3- and 5-year cumulative OS rates of 59.5% and 41.0%, respectively, with a median OS of 51.0 months (95%CI: 44.178-57.822). These findings support the role of IGTA as a viable and minimally invasive alternative to surgery, particularly for patients who are ineligible for resection or refuse surgery. However, surgical resection remains the standard of care for selected patients with resectable disease.
In parallel, stereotactic body radiotherapy (SBRT) has emerged as a noninvasive option for liver and lung oligometastases. Petrelli et al[20] showed that in 656 patients with approximately 3 cm CRC liver metastases, SBRT achieved 2-year local control and PFS rates of 59% and 56%, respectively[20]. A separate series of oligometastatic lung tumors reported 1-, 3-, and 5-year local recurrence-free survival of 80.6%, 68.6%, and 68.6%, with high-dose SBRT [biologically effective dose (BED)10 = 132 Gy] outperforming lower doses (BED10 ≤ 105.6 Gy)[21]. Across 1033 oligometastatic patients (including CRC primaries), median PFS was 12.9 months and OS 44.2 months, with 1-, 3-, and 5-year OS of 84.1%, 56.7%, and 35.2%[22]. In a registry of 772 SBRT-treated lung metastases, the CRC subgroup achieved a median OS of 30 months and 3-year OS of 35.8%[23].
Complementing surgery and SBRT, IGTA offers a repeatable, percutaneous approach with low morbidity. In our mul
Together, surgical resection, SBRT, and IGTA form a continuum of minimally invasive strategies. IGTA, in particular, bridges the gap between surgery and radiotherapy, offering repeatable local control for patients who are ineligible for or refuse resection, while synergizing effectively with systemic agents.
Multivariate analyses identified key prognostic factors for OS, PFS, and LTPFS. Increased tumor burden, reflected by larger size and higher number of metastases, was independently associated with poorer outcomes. Larger tumors pose challenges to complete ablation due to heat-sink effects, while higher tumor burden indicates more advanced disease. Larger metastatic lesions frequently harbor hypoxic, necrotic regions that disrupt uniform heat delivery during ablation and activate hypoxia inducible factor-1α-mediated angiogenesis and glycolytic adaptation, thereby promoting resistance to both thermal and cytotoxic therapies[28]. Additionally, a higher tumor burden increases intratumoral heterogeneity, raising the probability of pre-existing resistant clones that survive local interventions and drive recurrence[29]. Peridiaphragmatic lesions strongly predicted reduced LTPFS due to technical challenges in achieving complete ablation, con
Mediastinal lymph node involvement was associated with significantly poorer outcomes for both PFS (HR = 1.716) and OS (HR = 2.336) in univariate analysis (Supplementary Tables 2 and 3). However, it was not included in the final multivariate Cox model. This may be due to potential collinearity with other factors such as extrapulmonary metastases or overall tumor burden, which could have diminished its independent prognostic value after adjustment. Another contributing factor might be the limited sample size of patients with mediastinal node positivity, potentially affecting its statistical robustness. These observations indicate a need for further studies to clarify the prognostic significance and biological role of mediastinal lymph node metastasis in patients with pulmonary spread from CRC.
The presence of extrapulmonary metastases emerged as a critical prognostic factor, significantly reducing both PFS and OS. Survival outcomes stratified by metastatic site reveal distinct prognoses. As shown in Table 4, patients with no extrapulmonary metastases had the most favorable outcomes, with a 3-year PFS of 31.0% and a 5-year OS of 53.0%. Conversely, patients with liver or abdominal cavity metastases had worse outcomes, with 3-year PFS rates of 18.7% and 4.8%, respectively, and 5-year OS rates of 43.1% and 0.0%. Bone metastases were associated with the poorest prognosis, with no 5-year survivors. These findings highlight the need for tailored therapeutic approaches based on metastatic patterns[33]. Bone metastases occur in only 10%-15% of CRC patients and carry a five-year survival rate of < 5%, largely due to skeletal-related events such as trabecular and cortical weakening, bone pain, and increased fracture risk that dramatically worsen prognosis and quality of life[34,35]. These lesions typically emerge later than hepatic or pulmonary metastases and often present alongside multi-site dissemination, with their severe morbidity underscoring the need for earlier detection and timely intervention[34]. In contrast, patients with concomitant liver-only metastases achieved favorable long-term outcomes (5-year OS: 43.1%), likely reflecting both more advanced local treatment options such as thermal ablation, transcatheter arterial chemoembolization, radioembolization, stereotactic radiotherapy, hepatic artery infusion chemotherapy, and surgical resection and distinct tumor–microenvironment interactions in hepatic lesions. Biologically, metastatic tropism to different organs reflects underlying tumor phenotypes and microenvironments. Abdominal and pelvic dissemination often signifies either an invasive cellular program or widespread micro-metastatic seeding that evades focal interventions, driven by differences in organ affinity, epithelial-mesenchymal transition, angiogenesis, inflammatory signaling, and supportive stromal niches that collectively promote tumor progression[36]. By contrast, liver metastases remain highly susceptible to repeat locoregional therapies thanks to the organ’s rich perfusion, accessible anatomy, and well-established efficacy of targeted hepatic modalities. These observations underscore the heterogeneity of extrapulmonary CRLM. When metastases are confined and technically controllable particularly in the liver IGTA can achieve durable local control and synergize with systemic agents. Accordingly, we advocate a stratified treatment algorithm: Patients with limited, liver-only extrapulmonary disease should be considered for IGTA combined with systemic therapy, whereas those with abdominal, pelvic or skeletal involvement warrant prioritization of intensified systemic approaches.
Despite these challenges, IGTA may still provide meaningful palliation and local control in selected patients with extrapulmonary disease, particularly when combined with systemic therapy[37]. The integration of IGTA with systemic regimens such as FOLFOX, FOLFIRI, or immunotherapy may enhance long-term outcomes[37,38]. Prospective studies are needed to refine patient selection criteria and determine the optimal use of IGTA in this context.
The safety profile of IGTA was reaffirmed in this study, with a low rate of major complications (10.7%) and no treatment-related mortality. Pneumothorax was the most common adverse event (50.3%), but it was effectively managed in the majority of cases with conservative or minimally invasive interventions. These findings underscore the feasibility of IGTA in routine clinical practice, particularly in outpatient or short-stay hospital settings. Compared to pulmonary metastasectomy, IGTA offers significant advantages in terms of lower perioperative morbidity, shorter recovery times, and broader applicability in patients with comorbidities or poor performance status.
Both RFA and MWA demonstrated efficacy in this cohort, with MWA being slightly more prevalent (57.4%). MWA offers advantages such as faster heating, larger ablation zones, and reduced sensitivity to tissue impedance, making it particularly suited for larger tumors or challenging perivascular anatomical locations[39]. However, RFA remains a viable alternative for smaller lesions or patients with limited disease burden. Although Kaplan-Meier analysis suggested a longer OS in the MWA group, this difference was no longer significant after adjusting for potential confounders in multivariate Cox regression. This indicates that the apparent survival advantage of MWA was likely attributable to underlying patient or tumor characteristics, rather than the ablation technique itself. Therefore, both RFA and MWA can be considered effective modalities for local control in appropriately selected patients. Direct comparisons of these modalities in randomized controlled trials are warranted to clarify their relative benefits and guide clinical decision-making.
While IGTA demonstrates significant promise, challenges remain. LTP remains a concern, particularly for larger lesions or those in anatomically challenging locations, as reflected by a cumulative 3-year LTP rate of 14.0%. Advances in ablation technology, including multi-electrode systems[40], optimized energy delivery, artificial pleural or peritoneal effusion[41], and real-time imaging guidance, hold the potential to enhance ablation completeness and efficacy.
The role of IGTA in patients with extrapulmonary metastases requires further exploration. Liver, bone, and abdominal cavity metastases significantly worsen prognosis, but IGTA may still play a role in palliation and disease control within a multimodal framework. The timing and sequencing of IGTA relative to systemic therapy also warrant investigation. While this study did not stratify patients based on treatment sequencing, emerging evidence suggests that upfront IGTA followed by systemic therapy may enhance tumor control in select cases[15].
This study has several limitations. The results of this study may be influenced by selection bias and unmeasured confounding factors. Although the multicenter design enhances generalizability, variability in procedural techniques and follow-up protocols may have influenced the results. Furthermore, the study cohort was limited to patients with < 5 Lesions and tumor size < 5 cm, which may not reflect the broader CRLM population. Future prospective, randomized controlled trials are needed to validate these findings and establish standardized treatment guidelines.
IGTA is a safe, minimally invasive option that offers significant survival benefits in CRLM, particularly when combined with systemic therapies. Our findings support a stratified approach: Patients with limited liver-only metastases may achieve durable control and should be considered for IGTA plus systemic treatment, while those with extensive abdo
The authors would like to thank colleagues, institutions, and funding agencies for their valuable contributions and support throughout the study.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12727] [Article Influence: 6363.5] [Reference Citation Analysis (8)] |
| 2. | Tan KK, Lopes Gde L Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. J Gastrointest Surg. 2009;13:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Parnaby CN, Bailey W, Balasingam A, Beckert L, Eglinton T, Fife J, Frizelle FA, Jeffery M, Watson AJ. Pulmonary staging in colorectal cancer: a review. Colorectal Dis. 2012;14:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Davini F, Ricciardi S, Zirafa CC, Romano G, Alì G, Fontanini G, Melfi FMA. Lung metastasectomy after colorectal cancer: prognostic impact of resection margin on long term survival, a retrospective cohort study. Int J Colorectal Dis. 2020;35:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Yu WS, Bae MK, Choi JK, Hong YK, Park IK. Pulmonary Metastasectomy in Colorectal Cancer: A Population-Based Retrospective Cohort Study Using the Korean National Health Insurance Database. Cancer Res Treat. 2021;53:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Stefanou AJ. Surgical and Interventional Management of Lung Metastasis: Surgical Assessment, Resection, Ablation, Percutaneous Interventions. Clin Colon Rectal Surg. 2024;37:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Li J, Yuan Y, Yang F, Wang Y, Zhu X, Wang Z, Zheng S, Wan D, He J, Wang J, Ba Y, Bai C, Bai L, Bai W, Bi F, Cai K, Cai M, Cai S, Chen G, Chen K, Chen L, Chen P, Chi P, Dai G, Deng Y, Ding K, Fan Q, Fang W, Fang X, Feng F, Fu C, Fu Q, Gu Y, He Y, Jia B, Jiang K, Lai M, Lan P, Li E, Li D, Li J, Li L, Li M, Li S, Li Y, Li Y, Li Z, Liang X, Liang Z, Lin F, Lin G, Liu H, Liu J, Liu T, Liu Y, Pan H, Pan Z, Pei H, Qiu M, Qu X, Ren L, Shen Z, Sheng W, Song C, Song L, Sun J, Sun L, Sun Y, Tang Y, Tao M, Wang C, Wang H, Wang J, Wang S, Wang X, Wang X, Wang Z, Wu A, Wu N, Xia L, Xiao Y, Xing B, Xiong B, Xu J, Xu J, Xu N, Xu R, Xu Z, Yang Y, Yao H, Ye Y, Yu Y, Yu Y, Yue J, Zhang J, Zhang J, Zhang S, Zhang W, Zhang Y, Zhang Z, Zhang Z, Zhao L, Zhao R, Zhou F, Zhou J, Jin J, Gu J, Shen L. Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). J Hematol Oncol. 2019;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Hasegawa T, Takaki H, Kodama H, Yamanaka T, Nakatsuka A, Sato Y, Takao M, Katayama Y, Fukai I, Kato T, Tokui T, Tempaku H, Adachi K, Matsushima Y, Inaba Y, Yamakado K. Three-year Survival Rate after Radiofrequency Ablation for Surgically Resectable Colorectal Lung Metastases: A Prospective Multicenter Study. Radiology. 2020;294:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | de Baère T, Aupérin A, Deschamps F, Chevallier P, Gaubert Y, Boige V, Fonck M, Escudier B, Palussiére J. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015;26:987-991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 283] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 10. | Cheng G, Shi L, Qiang W, Wu J, Ji M, Lu Q, Li X, Xu B, Jiang J, Wu C. The safety and efficacy of microwave ablation for the treatment of CRC pulmonary metastases. Int J Hyperthermia. 2018;34:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199-S202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1366] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 12. | Deng HY, Zhou J, Wang RL, Jiang R, Zhu DX, Tang XJ, Zhou Q. Lobe-Specific Lymph Node Dissection for Clinical Early-Stage (cIA) Peripheral Non-small Cell Lung Cancer Patients: What and How? Ann Surg Oncol. 2020;27:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Hiraki T, Sakurai J, Tsuda T, Gobara H, Sano Y, Mukai T, Hase S, Iguchi T, Fujiwara H, Date H, Kanazawa S. Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: evaluation based on a preliminary review of 342 tumors. Cancer. 2006;107:2873-2880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Kann BH, Likitlersuang J, Bontempi D, Ye Z, Aneja S, Bakst R, Kelly HR, Juliano AF, Payabvash S, Guenette JP, Uppaluri R, Margalit DN, Schoenfeld JD, Tishler RB, Haddad R, Aerts HJWL, Garcia JJ, Flamand Y, Subramaniam RM, Burtness BA, Ferris RL. Screening for extranodal extension in HPV-associated oropharyngeal carcinoma: evaluation of a CT-based deep learning algorithm in patient data from a multicentre, randomised de-escalation trial. Lancet Digit Health. 2023;5:e360-e369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Li J, Liu G, Xie X, Zhang D, Zheng R, Yang H, Zhong H, Dai G, Yu J, Liang P. Outcomes Following Different Thermal Ablation Strategies in Patients with Unresectable Colorectal Liver Metastases. Radiology. 2023;308:e223135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Treasure T. Questioning the benefit of lung metastasectomy in colorectal cancer. BMJ. 2020;368:m264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Machida N, Okumura T, Boku N, Kishimoto J, Nishina T, Suyama K, Ohde Y, Shinozaki K, Baba H, Tokunaga S, Kawakami H, Tsuda T, Kotaka M, Okuda H, Yasui H, Yamazaki K, Hironaka S, Muro K, Hyodo I. A phase 2 study of adjuvant chemotherapy with 5-fluorouracil/leucovorin and oxaliplatin after lung metastasectomy for colorectal cancer (WJOG5810G). Cancer. 2025;131:e35807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Younes RN, Abrao F, Gross J. Pulmonary metastasectomy for colorectal cancer: long-term survival and prognostic factors. Int J Surg. 2013;11:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Olmez OF, Cubukcu E, Bayram AS, Akcali U, Evrensel T, Gebitekin C. Clinical outcomes of lung metastasectomy in patients with colorectal cancer. World J Gastroenterol. 2012;18:662-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Petrelli F, Comito T, Barni S, Pancera G, Scorsetti M, Ghidini A; SBRT for CRC liver metastases. Stereotactic body radiotherapy for colorectal cancer liver metastases: A systematic review. Radiother Oncol. 2018;129:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Wang X, Zamdborg L, Ye H, Grills IS, Yan D. A matched-pair analysis of stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer versus early stage non-small cell lung cancer. BMC Cancer. 2018;18:962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Poon I, Erler D, Dagan R, Redmond KJ, Foote M, Badellino S, Biswas T, Louie AV, Lee Y, Atenafu EG, Ricardi U, Sahgal A. Evaluation of Definitive Stereotactic Body Radiotherapy and Outcomes in Adults With Extracranial Oligometastasis. JAMA Netw Open. 2020;3:e2026312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Ricco A, Davis J, Rate W, Yang J, Perry D, Pablo J, D'Ambrosio D, Sharma S, Sundararaman S, Kolker J, Creach KM, Lanciano R. Lung metastases treated with stereotactic body radiotherapy: the RSSearch® patient Registry's experience. Radiat Oncol. 2017;12:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Ferguson J, Alzahrani N, Zhao J, Glenn D, Power M, Liauw W, Morris DL. Long term results of RFA to lung metastases from colorectal cancer in 157 patients. Eur J Surg Oncol. 2015;41:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Matsui Y, Hiraki T, Gobara H, Iguchi T, Fujiwara H, Nagasaka T, Toyooka S, Kanazawa S. Long-term survival following percutaneous radiofrequency ablation of colorectal lung metastases. J Vasc Interv Radiol. 2015;26:303-10;quiz 311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Li J, Pang C, Liu G, Xie X, Zhang DZ, Li K, Li Z, He G, Xu E, Zhong H, Yang H, Lu M, Lou K, Xie X, Lan S, Li Q, Dai G, Yu J, Liang P. Thermal ablation with and without adjuvant systemic therapy: a nationwide multicenter observational cohort study of solitary colorectal liver metastases. Int J Surg. 2024;110:4240-4248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2540] [Article Influence: 254.0] [Reference Citation Analysis (41)] |
| 28. | Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2389] [Cited by in RCA: 2662] [Article Influence: 190.1] [Reference Citation Analysis (0)] |
| 29. | Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 2548] [Article Influence: 283.1] [Reference Citation Analysis (3)] |
| 30. | Tual A, Revel MP, Canniff E, Garin A, Chassagnon G. Risk of pleural and diaphragmatic complications following percutaneous radiofrequency ablation of basal lung nodules. Diagn Interv Imaging. 2022;103:324-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Iguchi T, Hiraki T, Gobara H, Fujiwara H, Sakurai J, Matsui Y, Mitsuhashi T, Toyooka S, Kanazawa S. Radiofrequency ablation of pulmonary tumors near the diaphragm. Diagn Interv Imaging. 2017;98:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2893] [Cited by in RCA: 3120] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 33. | Meyer Y, Olthof PB, Grünhagen DJ, de Hingh I, de Wilt JHW, Verhoef C, Elferink MAG. Treatment of metachronous colorectal cancer metastases in the Netherlands: A population-based study. Eur J Surg Oncol. 2022;48:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 34. | Gigante I, Tutino V, De Nunzio V, Notarnicola M. Colorectal Cancer and Bone Tissue: Fantastic Relations and Where to Find Them. Cancers (Basel). 2020;12:2029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Khattak MA, Martin HL, Beeke C, Price T, Carruthers S, Kim S, Padbury R, Karapetis CS. Survival differences in patients with metastatic colorectal cancer and with single site metastatic disease at initial presentation: results from South Australian clinical registry for advanced colorectal cancer. Clin Colorectal Cancer. 2012;11:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Pretzsch E, Bösch F, Neumann J, Ganschow P, Bazhin A, Guba M, Werner J, Angele M. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J Oncol. 2019;2019:7407190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 37. | Timmerman RD, Bizekis CS, Pass HI, Fong Y, Dupuy DE, Dawson LA, Lu D. Local surgical, ablative, and radiation treatment of metastases. CA Cancer J Clin. 2009;59:145-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | de Baere T, Tselikas L, Yevich S, Boige V, Deschamps F, Ducreux M, Goere D, Nguyen F, Malka D. The role of image-guided therapy in the management of colorectal cancer metastatic disease. Eur J Cancer. 2017;75:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Gaia S, Ciruolo M, Ribaldone DG, Rolle E, Migliore E, Mosso E, Vola S, Risso A, Fagoonee S, Saracco GM, Carucci P. Higher Efficiency of Percutaneous Microwave (MWA) Than Radiofrequency Ablation (RFA) in Achieving Complete Response in Cirrhotic Patients with Early Hepatocellular Carcinoma. Curr Oncol. 2021;28:1034-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Laimer G, Bauer M, Scharll Y, Schullian P, Bale R. Multi-Probe RFA vs. Single-Probe MWA in an Ex Vivo Bovine Liver Model: Comparison of Volume and Shape of Coagulation Zones. Biology (Basel). 2023;12:1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Zhang D, Liang P, Yu X, Cheng Z, Han Z, Yu J, Liu F. The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperthermia. 2013;29:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/