Published online Oct 14, 2025. doi: 10.3748/wjg.v31.i38.109486
Revised: June 26, 2025
Accepted: September 1, 2025
Published online: October 14, 2025
Processing time: 154 Days and 18.6 Hours
The mortality rate for severe cases of acute pancreatitis (AP), a common gas
To investigate the role of F-box and WD repeat domain containing 11 (FBXW11) in AP models and to assess whether Rut mitigates AP by regulating FBXW11.
AP rat model was established and treated with Rut, followed by biochemical analysis of serum amylase and lipase, hematoxylin and eosin staining of pan
Rut treatment ameliorated AP severity, as evidenced by reduced serum levels of pancreatic enzymes (amylase and lipase) and attenuated histological damage. Rut also decreased inflammatory markers (interleukin-1 beta, interleukin-6, and tumor necrosis factor alpha), tissue oxidative stress (malondialdehyde), and neutrophil infiltration (Ly6G, CD11b, and myeloperoxidase) levels in rats with AP. Moreover, Rut restored pancreatic antioxidant capacity (glutathione and superoxide dismutase). In vitro, Rut pre-incubation enhanced cell viability and suppressed cerulein-induced apoptosis and oxidative stress. Rut increased EZH2 expression while decreasing FBXW11 expression. FBXW11 overexpression eliminated the protective effect of Rut against AP. Further analysis revealed that EZH2 binds to H3 and upregulates H3 methylation levels, thereby inhibiting FBXW11 expression.
Collectively, our findings demonstrate that Rut ameliorates AP by upregulating EZH2, thereby enhancing H3 methylation and suppressing FBXW11 expression.
Core Tip: Rutaecarpine (Rut) is a major bioactive compound with a broad spectrum of pharmacological properties. Our previous study demonstrated that Rut significantly alleviated pancreatic inflammation and necrosis in acute pancreatitis (AP) rat models, primarily through a calcitonin gene-related peptide-dependent manner involving the suppression of inflammatory signaling pathways. However, given the multifactorial pathogenesis of AP and Rut’s inherent polypharmacological properties, the precise molecular mechanisms responsible for its protective effects warrant further investigation. Thus, based on the original research, this study uncovers a previously unrecognized mechanism of Rut (enhancer of zeste homolog 2-dependent F-box and WD repeat domain containing 11 suppression) and provides evidence that traditional Chinese medicine can target epigenetic reprogramming in AP.
- Citation: Jia Y, Shi YX, Gu H, Liu Y, Peng J, Yan L. Rutaecarpine targets F-box and WD repeat domain containing 11 to inhibit inflammatory infiltration and alleviate acute pancreatitis. World J Gastroenterol 2025; 31(38): 109486
- URL: https://www.wjgnet.com/1007-9327/full/v31/i38/109486.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i38.109486
Acute pancreatitis (AP) is a common hospitalization-requiring gastrointestinal disease characterized by a local and systemic inflammatory response and usually manifested by severe upper abdominal pain[1]. The etiology of AP primarily involves three major factors: Gallstone disease, excessive alcohol consumption, and hypertriglyceridaemia, with additional causes including some medications and endoscopic retrograde cholangiopancreatography[2]. Most cases of AP occur in a mild form, resulting in only a few days of hospitalization, while approximately 20% of patients with AP encounter a severe or complicated course of the disease[3]. This severe progression is associated with potentially life-threatening local and/or systemic complications, which dramatically increase mortality rates to 30%-40% compared to the overall AP mortality rate of approximately 1%[4]. The hyperinflammatory response in AP has prompted investigation into immunomodulatory therapies, particularly anti-inflammatory agents. However, clinical trials have yielded largely disappointing results, primarily due to an insufficient understanding of the precise pathophysiological mechanisms underlying AP[5].
The indole alkaloid rutaecarpine (Rut) is a major bioactive compound in Evodia rutaecarpa fruit, which is used in traditional Chinese medicine to relieve pain, inhibit vomiting, and attenuate diarrhea[6]. Contemporary studies have revealed that Rut possesses a broad spectrum of pharmacological properties, such as anti-inflammatory, anti-platelet, vasodilatory, analgesic, anti-tumor, and anti-diabetic[7]. Moreover, Rut has exhibited the potential to alleviate gastroin
F-box and WD repeat domain containing 11 (FBXW11) is an F-box protein that constitutes one subunit of SKP1-cullin-F-box ubiquitin E3 ligase complex and functions as a fundamental regulator in cell-cycle progression and tumorigenesis[12]. Dysregulation of FBXW11 has been implicated in Alzheimer’s disease[13], developmental disorders[14], and various cancers[15-17]. Notably, a recent study identified FBXW11 as a novel inflammatory biomarker with high expression in mice with cerulein-induced AP[18]. However, the function of FBXW11 in AP and the mechanisms regulating its expression remain unknown.
Currently, there are few studies analyzing the therapeutic effect of Rut on AP. Robust efforts are needed to uncover the pathophysiology of AP and promote the translational potential of Rut’s identified therapeutic properties. This study aimed to investigate the role of FBXW11 in animal and cellular models of AP and to assess whether Rut mitigates AP by regulating FBXW11.
Male Sprague-Dawley rats (250 ± 50 g; Experimental Animal Center, Xiangya Hospital, Central South University, China) were housed in a temperature-controlled environment (25 °C; 50% humidity) with a 12-hour circadian rhythm and had free access to standard laboratory food and water. This study was approved by the Experimental Animal Ethics Co
An AP model in rats was established as described previously[11]. Sprague-Dawley rats were fasted for 12 hours, followed by anesthesia with intraperitoneal 3% sodium pentobarbital (40 mg/kg; Sigma-Aldrich, Merck KGaA, MO, United States) and routine disinfection (first disinfect with 2.5% iodine and then wipe twice with 70% alcohol). A midline laparotomy (about 1.5 cm) was performed, followed by induction of AP by retrograde infusion of freshly prepared 5% sodium taurocholate through the biliopancreatic duct. Sham-operated rats were given the same amount of normal saline. Incisions were sutured with continuous silk thread. Rats were euthanized 24 hours after surgery, followed by the collection of arterial blood and pancreatic tissue. Serum was obtained by centrifugation (1500 × g, 5 minutes, 4 °C) and preserved at -20 °C. Before histopathological examination, pancreatic tissue was treated at 4 °C with 4% phosphate-buffered formaldehyde. The rats in the Rut group were injected with 300 μg/kg Rut sublingually 20 minutes before modeling, and the same amount of normal saline was given to the sham and AP model groups. Adeno-associated viral-negative control (AAV-NC) and adeno-associated viral-FBXW11 (AAV-FBXW11) (5 × 1011 viral particles/rat; GeneChem, Shanghai, China) were delivered retrogradely through a catheter into the common bile duct 3 weeks before modeling[19].
Pancreatic tissue was embedded in paraffin and cut into 4 μM serial sections. Hematoxylin and eosin staining was performed to observe pathological changes. Necrotic cells were defined by cytoplasmic swelling, loss of plasma mem
The hospital’s fully automatic biochemical analyzer was used to measure serum amylase and lipase levels. Pancreatic tissue glutathione (GSH) (#S0056; Beyotime, Shanghai, China), malondialdehyde (MDA) (#S0131S; Beyotime, Shanghai, China), and superoxide dismutase (SOD) (#S0101S; Beyotime, Shanghai, China) were detected according to the kits’ instruction. Enzyme-linked immunosorbent assay was performed to measure serum interleukin-1 beta (IL-1β) (#PI303; Beyotime, Shanghai, China), IL-6 (#PI328; Beyotime, Shanghai, China), and tumor necrosis factor alpha (TNF-α) (#PT516; Beyotime, Shanghai, China).
Rat pancreatic AR42J cells (#ZQ0145; Zhong Qiao Xin Zhou, Shanghai, China) were maintained in F-12K medium (#ZQ-599; Zhong Qiao Xin Zhou, Shanghai, China) plus 20% foetal bovine serum (#10099141C; Gibco, NY, United States) at
AR42J cells (logarithmic growth phase) were pre-cultured in a 96-well plate (12000 cells/well) for 24 hours. Then Rut was diluted in culture medium to achieve final concentrations ranging from 10 to 800 μM. Five replicate wells were set up for each concentration. Following a 48-hour incubation at 37 °C, the medium was discarded, and 10 μL/well Cell Counting Kit-8 (CCK-8) solution (Boster, Wuhan, China) was added to further incubate the cells for 1 hour, followed by optical density (OD) at 450 nm (OD450) measurement. The maximum concentration with an OD value that did not significantly reduce compared to the control group was the optimal concentration of Rut. For subsequent experiments, AR42J cells were pre-incubated with Rut for 1 hour before cerulein treatment.
AR42J cells were seeded in a 96-well plate at 12000 cells/well. A cell model of AP was established when the cell confluence reached 80%. After treatment, the cells were incubated with CCK-8 reagent at 37 °C for 1 hour. OD450 was measured to assess cell viability.
For apoptosis analysis, 1 × 106 cells (80% confluent) were washed twice with prechilled PBS and suspended in 1 × binding buffer. Cell suspensions were then stained with 5 μL Annexin V-fluorescein isothiocyanate and 10 μL propidium iodide (PI; #MA0220; Meilunbio, Dalian, China) away from light for 15 minutes. After incubation, the apoptotic cells were quantified by a flow cytometer (Guava® easyCyte 12; Millipore, MA, United States) equipped with a 488 nm excitation laser. Fluorescence emissions were detected through a 515 nm bandpass filter for fluorescein isothiocyanate and a longpass filter (> 560 nm) for PI. Cell populations were discriminated as follows: Viable cells (Annexin V-/PI-, lower-left quadrant), early apoptotic cells (Annexin V+/PI-, lower-right quadrant), and late apoptotic/necrotic cells (Annexin V+/PI+, upper-right quadrant) in the bivariate dot plot representation.
A reactive oxygen species (ROS) assay kit (#EEA019; Invitrogen, CA, United States) was used to detect ROS in AR42J cells. Cells were incubated at 37 °C for 20 minutes with 10 μM dichloro-dihydro-fluorescein diacetate (diluted 1:1000 in serum-free medium). At the end of the incubation, dichloro-dihydro-fluorescein diacetate that had not entered the cells was removed by washing with serum-free medium. Fluorescence images were obtained through a fluorescence micro
Cellular protein samples were immunoprecipitated with anti-EZH2 (1:1000, #ab307646; Abcam, Cambridge, United Kingdom) or anti-immunoglobulin G antibodies at 4 °C overnight and then rotated with protein G/A beads at 4 °C for 3-5 hours. The mixture was spun at 1000 × g for 5 minutes at 4 °C, and the immune precipitate was washed 3 times with a washing buffer (50 mmol/L Tris-HCl/pH 7.4, 100 mmol/L NaCl, 5 mmol/L CaCl2, 5 mmol/L MgCl2, and 0.1% Nonidet P-40). Immunoprecipitated proteins were eluted in 1 × sodium-dodecyl sulfate polyacrylamide gel electrophoresis loading buffer and subjected to western blot analysis using the following primary antibodies: Anti-histone H3 (tri methyl K4) (1:800, #ab8580; Abcam, Cambridge, United Kingdom), anti-histone H3 (mono methyl K4) (1:800, #ab176877; Abcam, Cambridge, United Kingdom), and anti-histone H3 (1:1000, #ab1791; Abcam, Cambridge, United Kingdom) antibodies.
Total RNA samples were extracted using TRIzol reagent (#DP424; TIANGEN, Hamburg, Germany). A NovoScript RT reagent kit (#E047; Novoprotein, Suzhou, China) was used to synthesize cDNA from 1 μg of total RNA. Quantitative polymerase chain reaction was performed using a SYBR quantitative polymerase chain reaction SuperMix Plus (#E096; Novoprotein, Suzhou, China) and a Bio-Rad CFX384 Touch detection system. All reactions were performed in triplicate. Relative gene expression was calculated using the 2-ΔΔCt method from at least 3 independent runs and was normalized to glyceraldehyde 3-phosphate dehydrogenase. Primer sequences used for amplification are listed in Table 1.
| Gene | Primers | Sequences (5’ to 3’) |
| EZH2 | F | GCTTCCTACATCCCTTTC |
| R | CTGGGTCTGCTACTGTTATT | |
| FBXW11 | F | TTCCAGGTAAGCCATTGCCC |
| R | CACAGGCCCTTCACTGACTC | |
| GAPDH | F | CTTGTGCAGTGCCAGCCTC |
| R | ACCAGCTTCCCATTCTCAGC |
The total protein concentration of cell/tissue lysates was quantified using a bicinchoninic acid kit (#KTD3001; Abbkine, Wuhan, China). After electrophoresis, the separated proteins were transferred onto a polyvinylidene fluoride membrane. After blocking of nonspecific binding sites, the membrane was incubated with anti-EZH2 (1:1500, #ab307646; Abcam, Cambridge, United Kingdom), anti-histone H3 (1:1000, #ab1791; Abcam, Cambridge, United Kingdom), anti-histone H3 (tri methyl K4) (1:800, #ab8580; Abcam, Cambridge, United Kingdom), anti-histone H3 (mono methyl K4) (1:800, #ab176877; Abcam, Cambridge, United Kingdom), anti-FBXW11 (1:1000, #PA5-100524; Invitrogen, CA, United States), anti-glyceraldehyde-3-phosphate dehydrogenase (1:2500, #AF1186; Beyotime, Shanghai, China), and anti-beta tubulin (1:1500, #ab6046; Abcam, Cambridge, United Kingdom) antibodies overnight at 4 °C and then secondary antibodies (1:500, #ab150077; Abcam, Cambridge, United Kingdom) for 1 hour at ambient temperature. Blot images were captured using the BIO-RAD Imaging System (Hercules, CA, United States).
The binding interactions between Rut and EZH2 were analyzed by molecular docking. The EZH2 structure (PDB ID: 5wuk) was prepared by removing water/heteroatoms and adding hydrogens. Rut’s three-dimensional structure (Compound CID: 65752) was energy-minimized. Docking was performed using Auto-Dock Vina centered on EZH2’s active site. The top 10 binding poses were generated and ranked according to their binding affinity (kcal/mol). Protein-ligand interactions were visualized using PyMOL.
Cellular thermal shift assay (CETSA) was employed to evaluate the interaction between Rut and EZH2. Briefly, AR42J cells were seeded into 6-well plates. After adherence, the cells were treated with 200 μM Rut for 48 hours, while the control group received culture medium only. Following incubation, cells were collected, washed with PBS containing protease inhibitors, and resuspended in PBS. The homogeneous suspension was then aliquoted into polymerase chain reaction tubes and heated at specified temperatures (ranging from 37 °C to 67 °C) for 3 minutes. After cooling at room temperature for 3 minutes, three freeze-thaw cycles were performed using liquid nitrogen. The supernatant was collected by centrifugation and mixed with an appropriate volume of loading buffer. Subsequently, the samples were fully denatured by heating and subjected to western blotting to detect EZH2 expression levels.
GraphPad Prism 9 was applied to data analysis, with continuous variables expressed as mean ± SD or median (inter
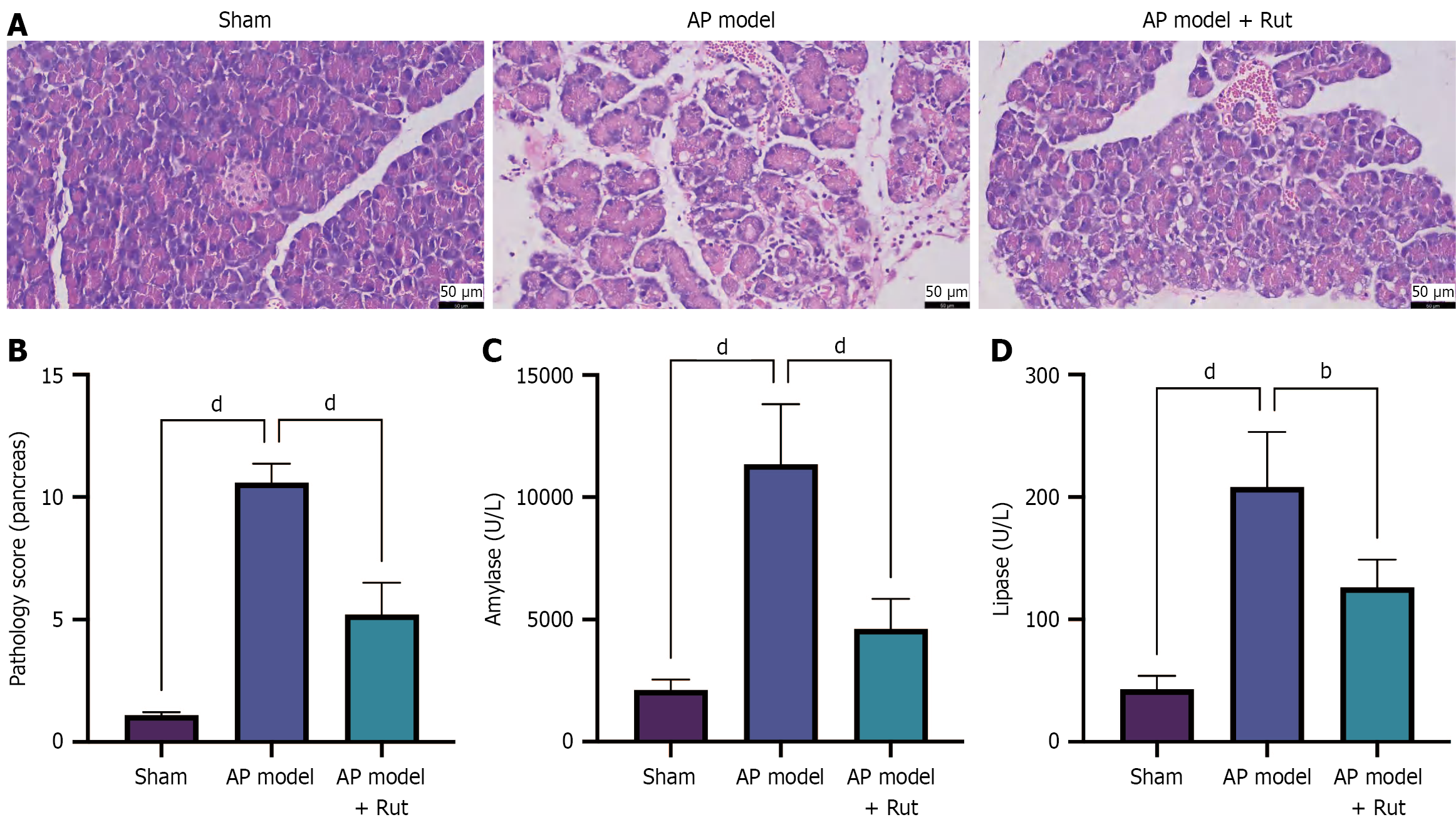
Our previous research showed that Rut could reduce AP-induced pancreatic tissue injury in rats, with the best efficacy at a concentration of 300 μg/kg[11]. However, the exact mechanism of Rut in AP is not fully understood. An AP rat model was established to observe the impact of Rut on pancreatic tissue injury. The pancreatic tissue of the model group displayed pronounced hemorrhage, edema, inflammation, and acinar cell necrosis, as well as obvious lobular structure damage, with a high pathological score (Figure 1A and B, P < 0.05). Moreover, biochemical analyses revealed significantly elevated serum amylase and lipase levels in the model group compared to the sham group (Figure 1C and D, P < 0.05). Notably, Rut treatment (AP model + Rut group) markedly ameliorated these pathological changes, as evidenced by decreased serum amylase, lipase levels, and pathological score relative to the model group (Figure 1A-D, P < 0.05), indicating that Rut treatment can reduce pancreatic tissue injury in rats with AP.
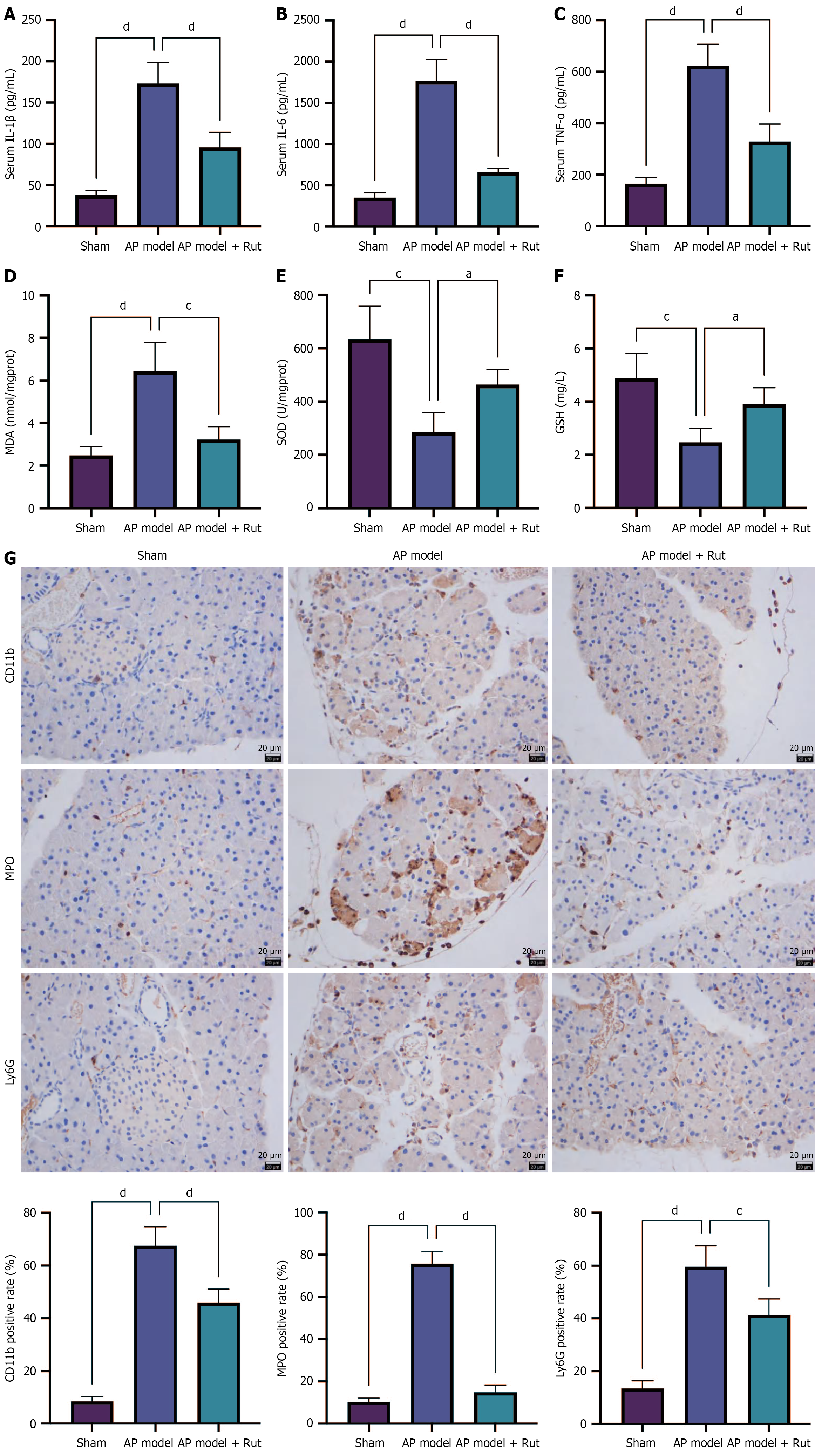
The inflammatory cascade in AP was characterized by significantly elevated serum levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in the model group, which were markedly attenuated by Rut treatment (Figure 2A-C, P < 0.05). Concurrently, we observed significant upregulation of MDA and downregulation of both SOD and GSH activity in the model group, while these indices were reversed following Rut administration (Figure 2D-F, P < 0.05). Immunohistochemistry staining revealed that Rut treatment could reduce the expression of CD11b (immune cell marker), MPO (neutrophil activation marker), and Ly6G (neutrophil-specific marker) in the pancreatic tissue of model rats (Figure 2G). Collectively, these results demonstrate Rut’s dual protective mechanism in AP, simultaneously mitigating systemic inflammation and pancreatic oxidative stress.
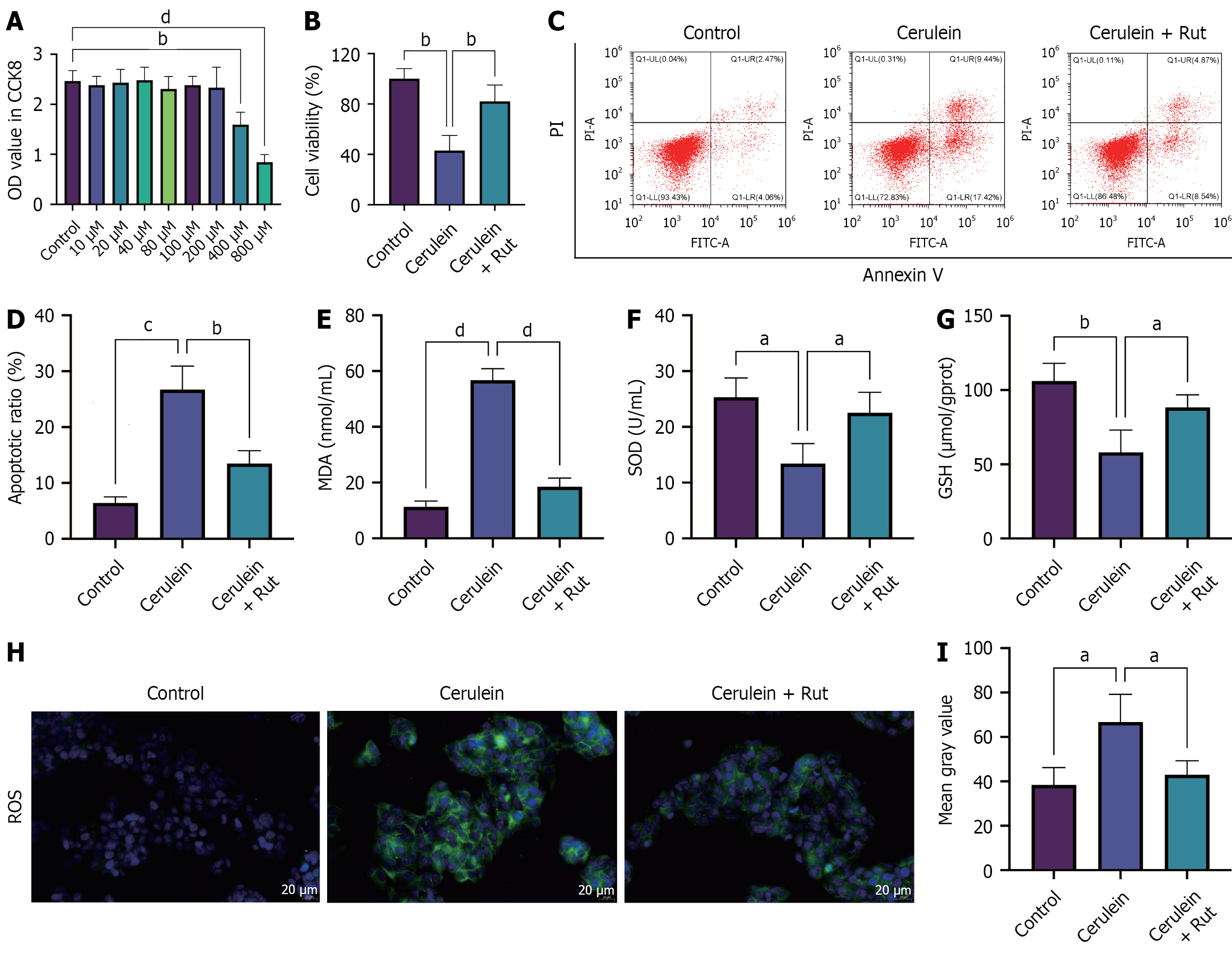
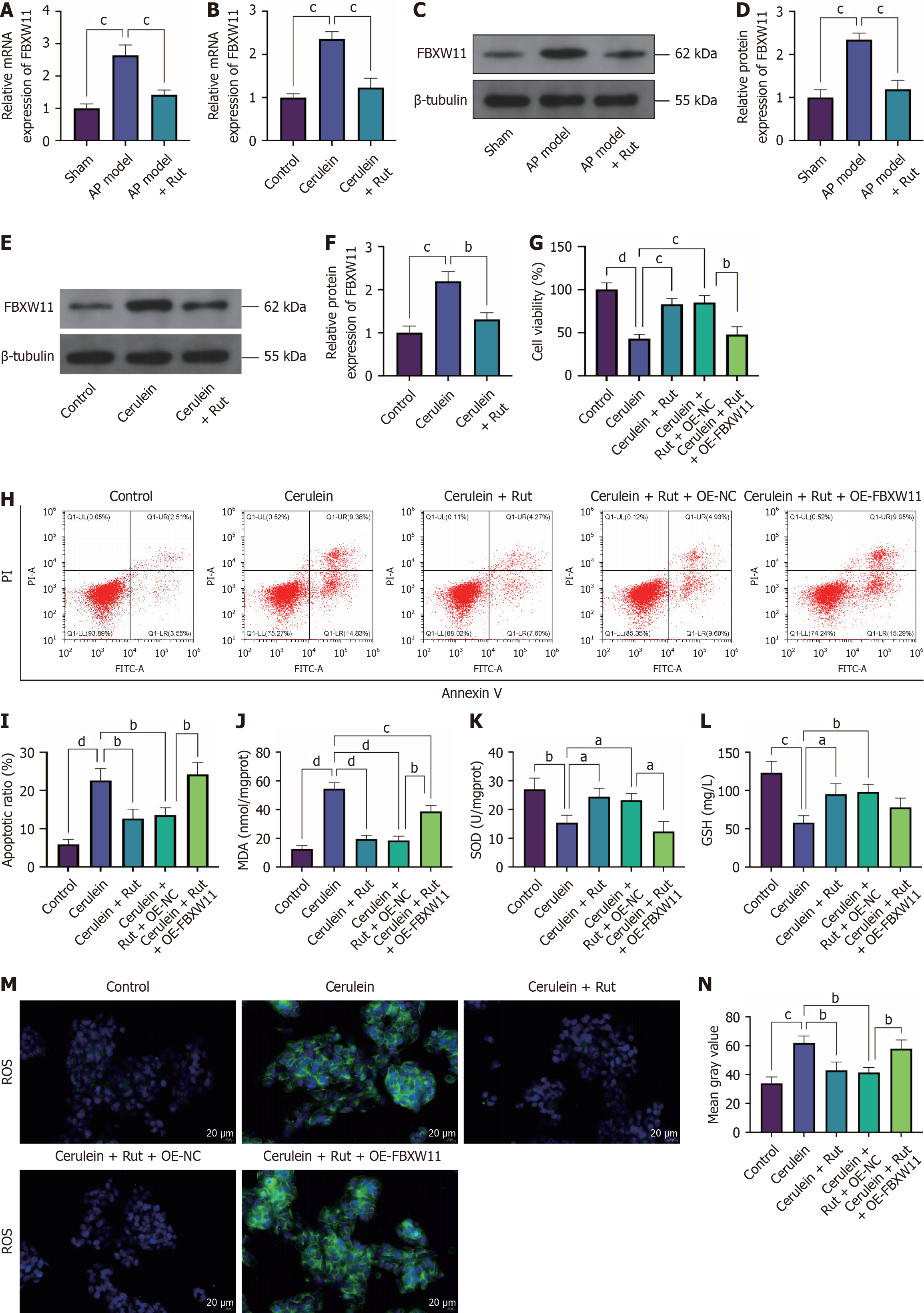
To analyze the effects of Rut on AP cells, AR42J cells were treated with varying concentrations of Rut to determine the safe concentration. The maximum drug concentration (200 μM) at which the OD value showed no statistically significant difference compared to the control group was determined to be the optimal concentration (Figure 3A, P < 0.05). To assess the protective effects of Rut on AP cells, AR42J cells were pre-incubated with Rut before cerulein stimulation, and then relevant cell phenotypes were detected. Cerulein treatment reduced cell viability, increased apoptosis, decreased GSH and SOD levels, and elevated the level of MDA and positive rate of ROS. Rut pretreatment partially mitigated these changes (Figure 3B-I, P < 0.05). These data suggest that Rut exerted a protective effect against cerulein-induced injury in AR42J cells.
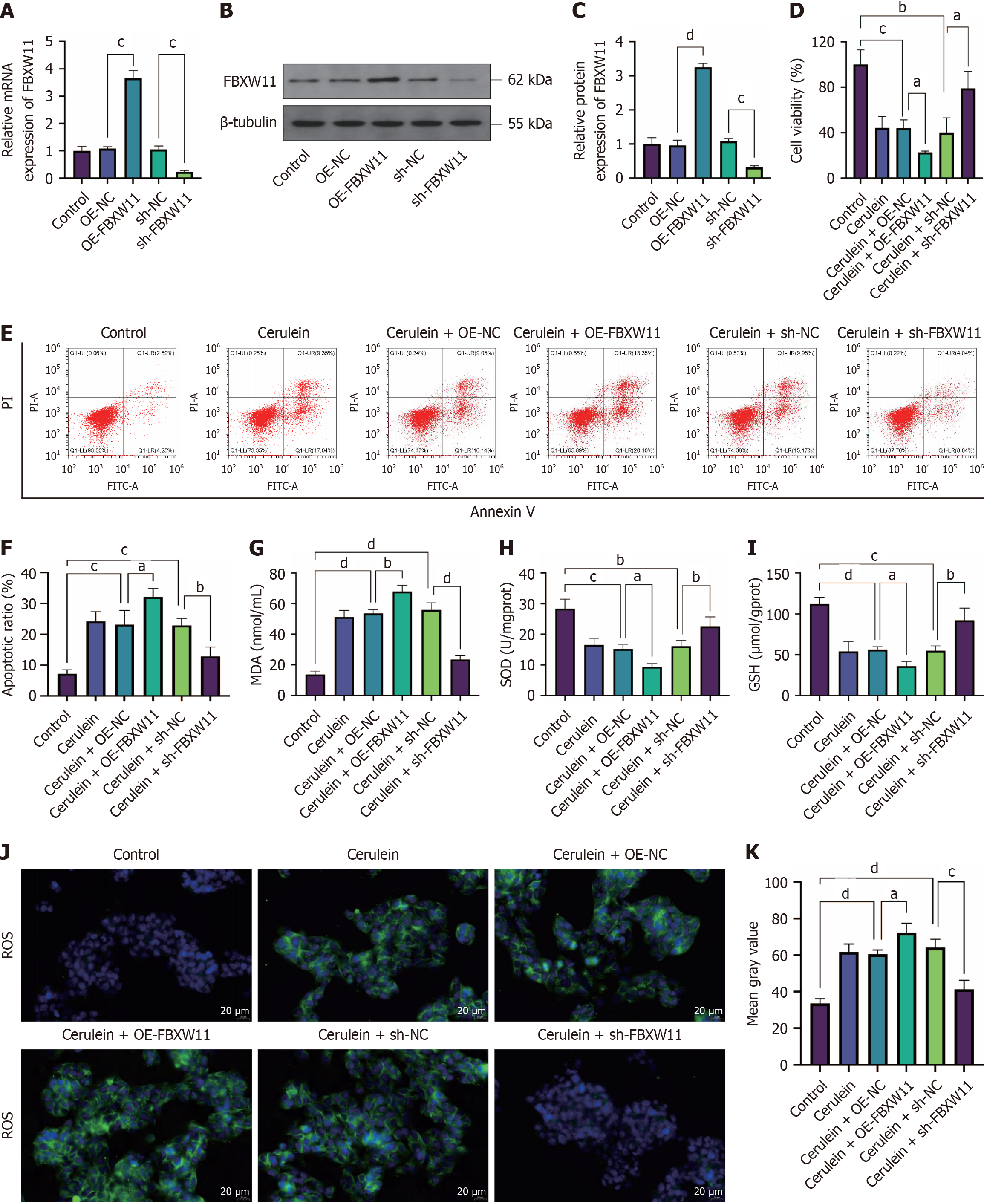
FBXW11 is highly expressed in mice with cerulein-induced AP[18], but its function in AP has not been studied. To elucidate the impact of differential expression of FBXW11 on AP, we established a cell model of AP in AR42J cells transfected with FBXW11-related vectors. First, we checked the effects of the overexpression vector OE-FBXW11 and knockdown vector sh-FBXW11 and obtained satisfactory results (Figure 4A-C, P < 0.05). Further detection of cell phenotypes showed that FBXW11 knockdown restored viability, inhibited apoptosis, elevated GSH and SOD levels, and reduced the level of MDA and positive rate of ROS in cerulein-treated AR42J cells. In contrast, FBXW11 overexpression aggravated cerulein-induced cell damage (Figure 4D-K, P < 0.05). These findings indicate that FBXW11 knockdown confers protection against cerulein-induced oxidative stress and damage in AR42J cells.
To find whether Rut regulates FBXW11 expression in AP, we measured FBXW11 expression in both animal and cell models of AP. Rut treatment significantly downregulated FBXW11 expression (Figure 5A-F, P < 0.05). To determine whether the protective effect of Rut on AP cells is mediated through FBXW11 regulation, we pre-incubated OE-FBXW11-transfected AR42J cells with Rut before AP induction. The cerulein + Rut + OE-FBXW11 group exhibited decreases in viability, GSH and SOD levels and increases in the apoptosis rate, MDA level, and ROS positive rate in comparison with the cerulein + Rut + OE-NC group (Figure 5G-N), indicating that overexpressing FBXW11 can eliminate the protective effect of Rut on AP cells.
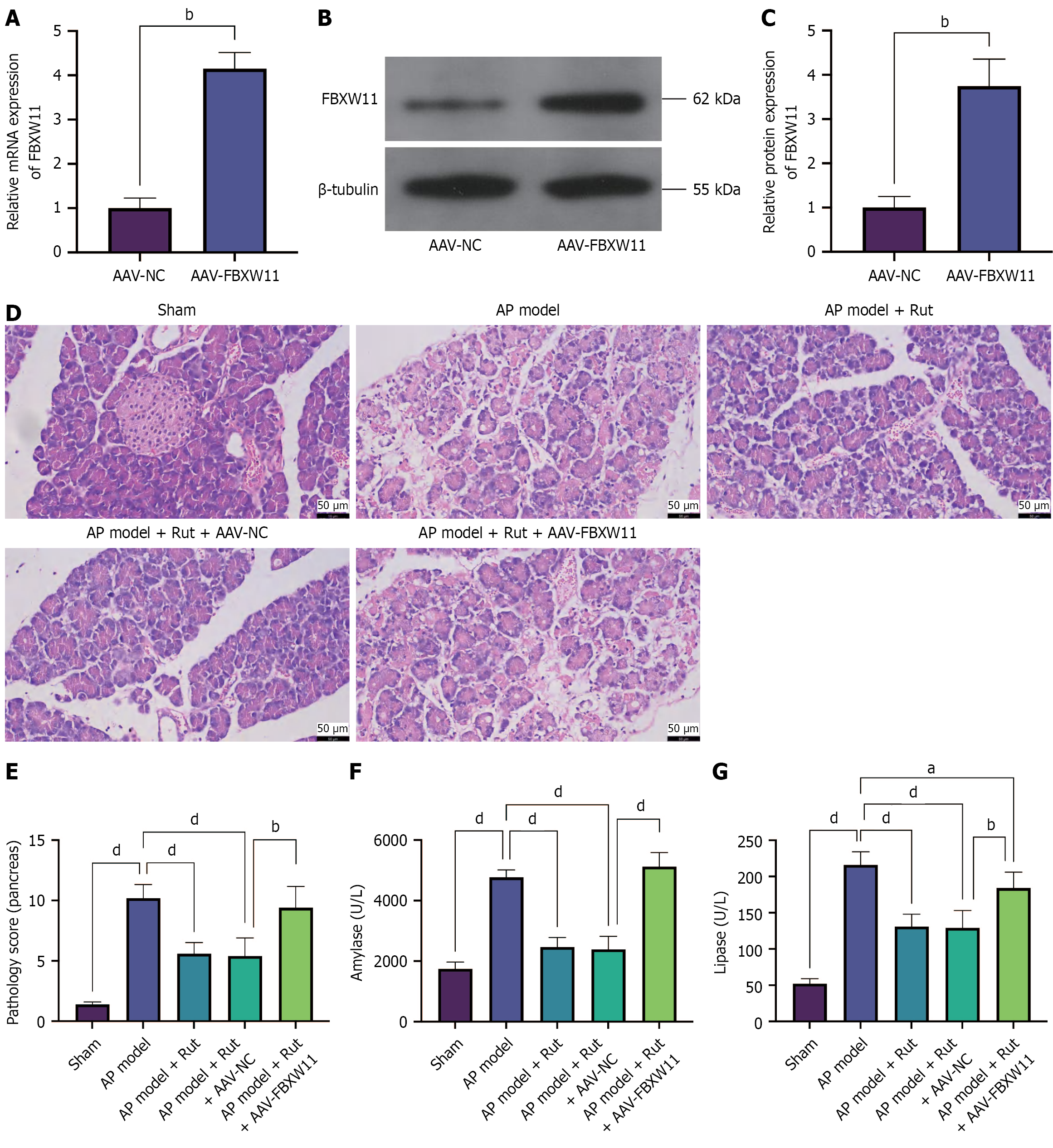
To investigate the impact of overexpressed FBXW11 on Rut’s regulation of AP in vivo, we infected rats with AAV-FBXW11 and established an AP model. Successful upregulation of FBXW11 expression following AAV-FBXW11 infection was confirmed (Figure 6A-C, P < 0.05). Compared with the AP model + Rut + AAV-NC group, the AP model + Rut + AAV-FBXW11 group showed higher serum amylase and lipase levels, along with higher pancreatic histopathological scores (Figure 6D-G, P < 0.05). These results indicate that FBXW11 overexpression attenuates the protective effects of Rut on pancreatic tissue in AP rats.
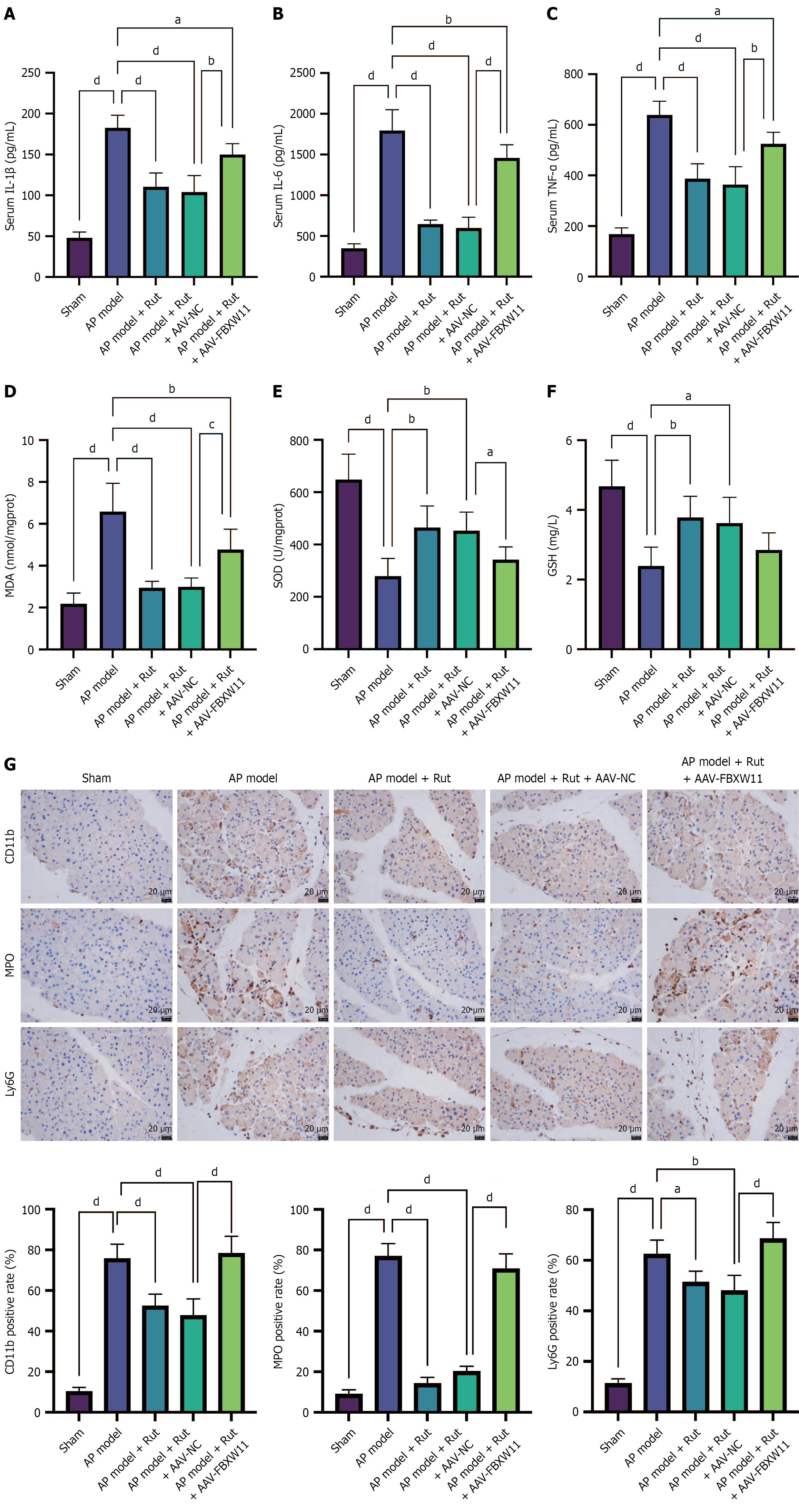
Further detection of oxidative stress and inflammatory markers revealed that the AP model + Rut + AAV-FBXW11 group had higher IL-1β, IL-6, TNF-α and MDA levels, along with lower SOD level than the AP model + Rut + AAV-NC group (Figure 7A-F). In addition, elevated expression of Ly6G, CD11b, and MPO was observed in the AP model + Rut + AAV-FBXW11 group (Figure 7G). The above results show that Rut can repress FBXW11 expression to reduce AP-caused damage in animal and cell models.
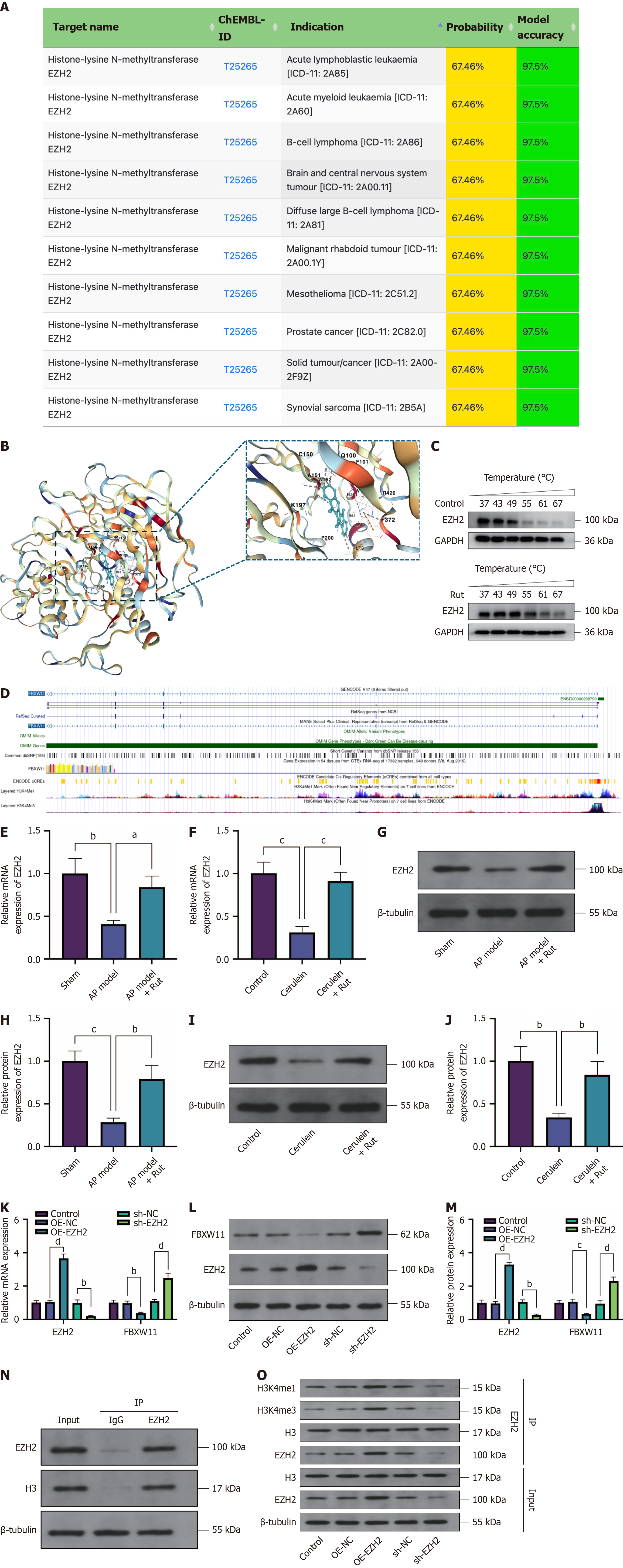
From the above, Rut modulates AP through FBXW11, but the underlying molecular mechanism remains unclear. SuperPred (https://prediction.charite.de/) was used to predict downstream targets of Rut, with results showing that Rut targeted the histone methyltransferase EZH2 (Figure 8A). Subsequently, molecular docking analysis was performed to evaluate the binding affinity between Rut and EZH2. The results revealed that Rut formed a stable binding with EZH2 primarily through hydrophobic and electrostatic interactions, along with several key hydrogen bonds (Figure 8B), with a docking score of -9.7 kcal/mol. CETSA results showed that EZH2 expression in the control group was largely degraded when the temperature increased to 55 °C, whereas in AR42J cells treated with Rut, EZH2 maintained relatively high expression levels even at temperatures as high as 67 °C (Figure 8C). These data suggest that Rut may effectively bind to EZH2, supporting its potential as a therapeutic target. Previous studies have reported that EZH2 can promote acinar cell regeneration in acute/chronic pancreatitis[21]. EZH2 methylates histone H3, leading to transcriptional repression of affected target genes. According to the University of California Santa Cruz database (https://genome.ucsc.edu/), H3 methylation peaks are present in the promoter region of FBXW11 (Figure 8D), suggesting that the FBXW11 promoter is affected by EZH2-mediated H3 methylation. Based on this, we speculate that Rut regulates FBXW11 expression in AP by targeting EZH2. Consistent with this hypothesis, Rut treatment significantly increased EZH2 mRNA and protein levels in both animal and cell models of AP (Figure 8E-J, P < 0.05).
EZH2-related vectors were further transfected into AR42J cells to detect the influence of EZH2 on FBXW11. OE-EZH2 transfection reduced FBXW11 expression, whereas sh-EZH2 transfection promoted FBXW11 expression (Figure 8K-M, P < 0.05). The identification of the protein co-immunoprecipitated with EZH2 in AR42J cells showed that EZH2 bound to the H3 protein (Figure 8N). Moreover, EZH2 overexpression enhanced H3 methylation levels, whereas EZH2 knockdown decreased them (Figure 8O, P < 0.05). In conclusion, Rut inhibits FBXW11 expression by targeting EZH2 to promote H3 methylation, thereby alleviating AP.
AP is a sudden-onset inflammatory condition of the pancreas, with a mortality rate of 30%-40% for severe cases. Rut, a natural compound with known anti-inflammatory properties, has previously been shown in our study to exert anti-inflammatory and pancreas-protective effects in rat models of AP, partly through modulation of inflammation-related pathways. This study not only uncovers a previously unrecognized mechanism of Rut (EZH2-dependent FBXW11 suppression), but also provides evidence that traditional Chinese medicine can target epigenetic reprogramming in AP. This expands the therapeutic paradigm beyond conventional anti-inflammatory approaches. FBXW11 is a newly identified inflammatory biomarker associated with AP. This study found that Rut targeted the histone methyltransferase EZH2 to increase H3 methylation in FBXW11, thereby repressing FBXW11 expression and reducing AP-induced pan
The anti-inflammatory activity of Rut has been primarily attributed to its modulation of the nuclear factor-kappa B (NF-κB), phosphatidylinositol 3-kinase/protein kinase B, and mitogen-activated protein kinase (MAPK) pathways. Some studies found that Rut reduced the production of nitric oxide and the expression of inducible nitric oxide synthase, cyclooxygenase-2, and IL-1β in lipopolysaccharide and lipoteichoic acid-stimulated RAW macrophages by blocking NF-κB and MAPK pathways[22,23]. Consistent with these findings, Rut also attenuated inflammation and cartilage degradation in osteoarthritis by inhibiting phosphatidylinositol 3-kinase/protein kinase B/NF-κB and MAPK pathways, possibly via activation of integrin αVβ3[24]. Moreover, Rut exerts antioxidative properties by activating nuclear factor erythroid-2-related factor 2 in various disease conditions, including acute liver injury[25], colitis[26], traumatic brain injury[27], and migraine[28]. In the context of AP, our previous work found that Rut mitigated cerulein-induced inflammation in mice and AR42J cells by upregulating calcitonin gene-related peptide, and then suppressing MAPK and NF-κB signaling[10,11]. Based on this, the present study further probed into the therapeutic effect of Rut on AP. Given that no single model can fully replicate human AP, we adopted a combinatorial strategy. The cerulein-induced cellular model was used to elucidate mechanisms, while the sodium taurocholate-induced animal model was employed to validate therapeutic efficacy in severe AP. This approach allowed us to verify the therapeutic effects of Rut at both cellular and animal levels. We found that Rut protected the pancreas from sodium taurocholate-induced oxidative stress and inflammation in rats. Furthermore, Rut enhanced viability and reduced apoptosis and oxidative stress in cerulein-treated AR42J cells. These findings prompted us to explore the potential involvement of FBXW11, a key inflammation-related regulator, in mediating the protective effects of Rut.
FBXW11 is a subunit of SKP1-cullin-F-box ubiquitin E3 ligase complexes that target proteins for proteasome-mediated degradation. FBXW11 mediates the proteasomal degradation of IL-17 receptor A and Act1 phosphorylated in IL-17 signaling pathways, which is thought to inhibit IL-17-dependent inflammatory responses, but FBXW11 can also ubiquitinate phosphorylated IκBα to promote nuclear translocation of NF-κB p50/p65 and transcription of downstream genes[29,30]. Additionally, FBXW11 Leads to abnormal activation of MAPK/NF-κB signaling in amyloid-β-stimulated microglia by promoting apoptosis signal-regulating kinase 1 ubiquitination, contributing to neuroinflammation in Alzheimer’s disease models[13]. In a model of intervertebral disc degeneration, nanoparticles delivering the miR-150-5p inhibitor attenuated puncture-induced intervertebral disc degeneration in mice by targeting FBXW11 and suppressing transforming growth factor-beta-activated kinase 1 ubiquitination, thereby downregulating NF-κB signaling activity[31]. Moreover, FBXW11 is further implicated in the suppression of NF-κB signaling by the human immunodeficiency virus type 1 viral protein U[32], and is found to be upregulated in patients with systemic lupus erythematosus[33], highlighting its role in autoimmunity. Recent evidence indicates that FBXW11 expression is significantly elevated in mice with cerulein-induced AP, where it correlates with increased macrophage infiltration and NF-κB activation[18]. Therefore, we further analyzed the function of FBXW11 in AP. The experimental data showed that FBXW11 undermined viability, promoted apoptosis, and exacerbated oxidative stress in AP cells. Importantly, FBXW11 overexpression eliminated the protective effect of Rut on the rat and cell models of AP, indicating that Rut alleviated AP through inhibition of FBXW11.
To investigate the mechanism by which Rut regulates FBXW11 expression, SuperPred was used to predict downstream targets of Rut. Among the predicted targets, EZH2 was identified and found to exhibit strong binding affinity with Rut based on molecular docking and CETSA analysis. Notably, EZH2 expression was upregulated in Rut-treated AP models. EZH2 is an enzymatic catalytic subunit of polycomb repressive complex 2 that can repress gene expression by methylating histone H3[34]. Li et al[35] have found that EZH2 can inhibit the expression of angiotensin-converting enzyme 2 in mammals by mediating histone H3 methylation, thereby contributing to the treatment of coronavirus disease 2019. Similarly, in a study on atrial fibrosis, EZH2 has been shown to suppress the transcription of CDKN2a (p16, p19) and Timp4 genes by establishing canonical histone H3 (H3K27me3) modifications at their promoter regions[36]. Therefore, Rut may repress FBXW11 expression via EZH2-mediated histone modification. Moreover, previous studies have reported that EZH2 promotes pancreatic tissue renewal in cerulein-induced pancreatitis models by silencing nuclear factor of activated T cells cytoplasmic 1[21]. Our results verified that EZH2 catalyzes H3 methylation in FBXW11 and inhibits FBXW11 expression.
While this study provides insights into the role of EZH2-mediated FBXW11 suppression in Rut’s therapeutic effects on AP, certain limitations should be acknowledged. First, the regulatory relationship between EZH2 and FBXW11 was primarily validated through bioinformatic predictions (University of California Santa Cruz database) and changes in FBXW11 expression upon EZH2 modulation. Direct evidence (e.g., chromatin immunoprecipitation assay) demonstrating EZH2 binding to the FBXW11 promoter would further strengthen our conclusions. Second, despite Rut’s promising anti-inflammatory and pancreas-protective effects, its clinical translation faces challenges, including low oral bioavailability, rapid metabolism into hydroxylated derivatives, and moderate bioactivity. To mitigate these limitations, we administered Rut intravenously in this study. Future research should explore structural modifications or nanodelivery systems to enhance Rut’s pharmacokinetic properties and therapeutic efficacy[7,37].
In summary, Rut alleviates AP by upregulating EZH2 to silence FBXW11. This study elucidates a mechanism of action of Rut on AP, which may contribute to its future clinical application in AP treatment. Additionally, the findings of FBXW11 as a functional contributor to AP pathogenesis provide new insights into the molecular mechanisms driving pancreatic inflammation and injury.
| 1. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 687] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 2. | Szatmary P, Grammatikopoulos T, Cai W, Huang W, Mukherjee R, Halloran C, Beyer G, Sutton R. Acute Pancreatitis: Diagnosis and Treatment. Drugs. 2022;82:1251-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 337] [Reference Citation Analysis (1)] |
| 3. | Gliem N, Ammer-Herrmenau C, Ellenrieder V, Neesse A. Management of Severe Acute Pancreatitis: An Update. Digestion. 2021;102:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, Coward S, Forbes N, Heitman SJ, Shaheen AA, Swain M, Buie M, Underwood FE, Kaplan GG. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology. 2022;162:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 494] [Article Influence: 123.5] [Reference Citation Analysis (1)] |
| 5. | van den Berg FF, Boermeester MA. Update on the management of acute pancreatitis. Curr Opin Crit Care. 2023;29:145-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Chen L, Hu Y, Ye Z, Li L, Qian H, Wu M, Qin K, Li N, Wen X, Pan T, Ye Q. Major Indole Alkaloids in Evodia Rutaecarpa: The Latest Insights and Review of Their Impact on Gastrointestinal Diseases. Biomed Pharmacother. 2023;167:115495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 7. | Li D, Huang Z, Xu X, Li Y. Promising derivatives of rutaecarpine with diverse pharmacological activities. Front Chem. 2023;11:1199799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Ren S, Wei Y, Niu M, Li R, Wang R, Wei S, Wen J, Wang D, Yang T, Chen X, Wu S, Tong Y, Jing M, Li H, Wang M, Zhao Y. Mechanism of rutaecarpine on ethanol-induced acute gastric ulcer using integrated metabolomics and network pharmacology. Biomed Pharmacother. 2021;138:111490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | He Y, Liu HH, Zhou XL, He TT, Zhang AZ, Wang X, Wei SZ, Li HT, Chen LS, Chang L, Zhao YL, Jing MY. Rutaecarpine Ameliorates Murine N-Methyl-N'-Nitro-N-Nitrosoguanidine-Induced Chronic Atrophic Gastritis by Sonic Hedgehog Pathway. Molecules. 2023;28:6294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Huang H, Wang M, Guo Z, Wu D, Wang H, Jia Y, Liu H, Ding J, Peng J. Rutaecarpine alleviates acute pancreatitis in mice and AR42J cells by suppressing the MAPK and NF-κB signaling pathways via calcitonin gene-related peptide. Phytother Res. 2021;35:6472-6485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 11. | Yan L, Li QF, Rong YT, Chen YH, Huang ZH, Wang ZZ, Peng J. The protective effects of rutaecarpine on acute pancreatitis. Oncol Lett. 2018;15:3121-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Shi L, Du D, Peng Y, Liu J, Long J. The functional analysis of Cullin 7 E3 ubiquitin ligases in cancer. Oncogenesis. 2020;9:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Sun J, Qin X, Zhang X, Wang Q, Zhang W, Wang M. FBXW11 deletion alleviates Alzheimer's disease by reducing neuroinflammation and amyloid-β plaque formation via repression of ASK1 signaling. Biochem Biophys Res Commun. 2021;548:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Holt RJ, Young RM, Crespo B, Ceroni F, Curry CJ, Bellacchio E, Bax DA, Ciolfi A, Simon M, Fagerberg CR, van Binsbergen E, De Luca A, Memo L, Dobyns WB, Mohammed AA, Clokie SJH, Zazo Seco C, Jiang YH, Sørensen KP, Andersen H, Sullivan J, Powis Z, Chassevent A, Smith-Hicks C, Petrovski S, Antoniadi T, Shashi V, Gelb BD, Wilson SW, Gerrelli D, Tartaglia M, Chassaing N, Calvas P, Ragge NK. De Novo Missense Variants in FBXW11 Cause Diverse Developmental Phenotypes Including Brain, Eye, and Digit Anomalies. Am J Hum Genet. 2019;105:640-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Chen C, Zhou H, Zhang X, Liu Z, Ma X. Association of FBXW11 levels with tumor development and prognosis in chondrosarcoma. Cancer Biomark. 2022;35:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Yao J, Yang J, Yang Z, Wang XP, Yang T, Ji B, Zhang ZY. FBXW11 contributes to stem-cell-like features and liver metastasis through regulating HIC1-mediated SIRT1 transcription in colorectal cancer. Cell Death Dis. 2021;12:930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Zhang Q, Yin X, Zhang Y. MicroRNA-221 Promotes Cell Proliferation and Inhibits Apoptosis in Osteosarcoma Cells by Directly Targeting FBXW11 and Regulating Wnt Signaling. Arch Med Res. 2021;52:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Tan P, Cai S, Huang Z, Li M, Liu S, Chen J, Fu W, Zhao L. E3 ubiquitin ligase FBXW11 as a novel inflammatory biomarker is associated with immune infiltration and NF-κB pathway activation in pancreatitis and pancreatic cancer. Cell Signal. 2024;116:111033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Quirin KA, Kwon JJ, Alioufi A, Factora T, Temm CJ, Jacobsen M, Sandusky GE, Shontz K, Chicoine LG, Clark KR, Mendell JT, Korc M, Kota J. Safety and Efficacy of AAV Retrograde Pancreatic Ductal Gene Delivery in Normal and Pancreatic Cancer Mice. Mol Ther Methods Clin Dev. 2018;8:8-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 694] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | Chen NM, Neesse A, Dyck ML, Steuber B, Koenig AO, Lubeseder-Martellato C, Winter T, Forster T, Bohnenberger H, Kitz J, Reuter-Jessen K, Griesmann H, Gaedcke J, Grade M, Zhang JS, Tsai WC, Siveke J, Schildhaus HU, Ströbel P, Johnsen SA, Ellenrieder V, Hessmann E. Context-Dependent Epigenetic Regulation of Nuclear Factor of Activated T Cells 1 in Pancreatic Plasticity. Gastroenterology. 2017;152:1507-1520.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Jayakumar T, Lin KC, Chang CC, Hsia CW, Manubolu M, Huang WC, Sheu JR, Hsia CH. Targeting MAPK/NF-κB Pathways in Anti-Inflammatory Potential of Rutaecarpine: Impact on Src/FAK-Mediated Macrophage Migration. Int J Mol Sci. 2021;23:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Jayakumar T, Yang CM, Yen TL, Hsu CY, Sheu JR, Hsia CW, Manubolu M, Huang WC, Hsieh CY, Hsia CH. Anti-Inflammatory Mechanism of An Alkaloid Rutaecarpine in LTA-Stimulated RAW 264.7 Cells: Pivotal Role on NF-κB and ERK/p38 Signaling Molecules. Int J Mol Sci. 2022;23:5889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Wan J, Li M, Yuan X, Yu X, Chen A, Shao M, Kang H, Cheng P. Rutaecarpine ameliorates osteoarthritis by inhibiting PI3K/AKT/NFκB and MAPK signalling transduction through integrin αVβ3. Int J Mol Med. 2023;52:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 25. | Choi JH, Jin SW, Lee GH, Han EH, Hwang YP, Jeong HG. Rutaecarpine Protects against Acetaminophen-Induced Acute Liver Injury in Mice by Activating Antioxidant Enzymes. Antioxidants (Basel). 2021;10:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Zhang Y, Yan T, Sun D, Xie C, Wang T, Liu X, Wang J, Wang Q, Luo Y, Wang P, Yagai T, Krausz KW, Yang X, Gonzalez FJ. Rutaecarpine inhibits KEAP1-NRF2 interaction to activate NRF2 and ameliorate dextran sulfate sodium-induced colitis. Free Radic Biol Med. 2020;148:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 27. | Xu M, Li L, Liu H, Lu W, Ling X, Gong M. Rutaecarpine Attenuates Oxidative Stress-Induced Traumatic Brain Injury and Reduces Secondary Injury via the PGK1/KEAP1/NRF2 Signaling Pathway. Front Pharmacol. 2022;13:807125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Xu M, Shi Z, He Z, Ling X, Wang W, Liu H, Gong M. Rutaecarpine alleviates migraine in nitroglycerin-induced mice by regulating PTEN/PGK1 signaling pathway to activate NRF2 antioxidant system. Biomed Pharmacother. 2023;166:115300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 29. | Jin B, Moududee SA, Ge D, Zhou P, Wang AR, Liu YZ, You Z. SCF(FBXW11) Complex Targets Interleukin-17 Receptor A for Ubiquitin-Proteasome-Mediated Degradation. Biomedicines. 2024;12:755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Shi P, Zhu S, Lin Y, Liu Y, Liu Y, Chen Z, Shi Y, Qian Y. Persistent stimulation with interleukin-17 desensitizes cells through SCFβ-TrCP-mediated degradation of Act1. Sci Signal. 2011;4:ra73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Jiang H, Qin H, Yang Q, Huang L, Liang X, Wang C, Moro A, Xu S, Wei Q. Effective delivery of miR-150-5p with nucleus pulposus cell-specific nanoparticles attenuates intervertebral disc degeneration. J Nanobiotechnology. 2024;22:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 32. | Pickering S, Sumner J, Kerridge C, Perera M, Neil S. Differential dysregulation of β-TrCP1 and -2 by HIV-1 Vpu leads to inhibition of canonical and non-canonical NF-κB pathways in infected cells. mBio. 2023;14:e0329322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Yang F, Zhai Z, Luo X, Luo G, Zhuang L, Zhang Y, Li Y, Sun E, He Y. Bioinformatics identification of key candidate genes and pathways associated with systemic lupus erythematosus. Clin Rheumatol. 2020;39:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 674] [Article Influence: 112.3] [Reference Citation Analysis (7)] |
| 35. | Li Y, Li H, Zhou L. EZH2-mediated H3K27me3 inhibits ACE2 expression. Biochem Biophys Res Commun. 2020;526:947-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Li Y, Fang G, Cao W, Yuan J, Song S, Peng H, Wang Y, Wang Q. Ezh2 Inhibits Replicative Senescence of Atrial Fibroblasts Through Promotion of H3K27me3 in the Promoter Regions of CDKN2a and Timp4 Genes. J Inflamm Res. 2022;15:4693-4708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 37. | Liang Z, Lei F, Deng J, Zhang H, Wang Y, Li J, Shi T, Yang X, Wang Z. Design, synthesis and bioactivity evaluation of novel evodiamine derivatives with excellent potency against gastric cancer. Eur J Med Chem. 2022;228:113960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/