Published online Oct 7, 2025. doi: 10.3748/wjg.v31.i37.110797
Revised: July 4, 2025
Accepted: August 29, 2025
Published online: October 7, 2025
Processing time: 101 Days and 22 Hours
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a recently pro
To assess the prevalence and characteristics of MAFLD among Vietnamese indi
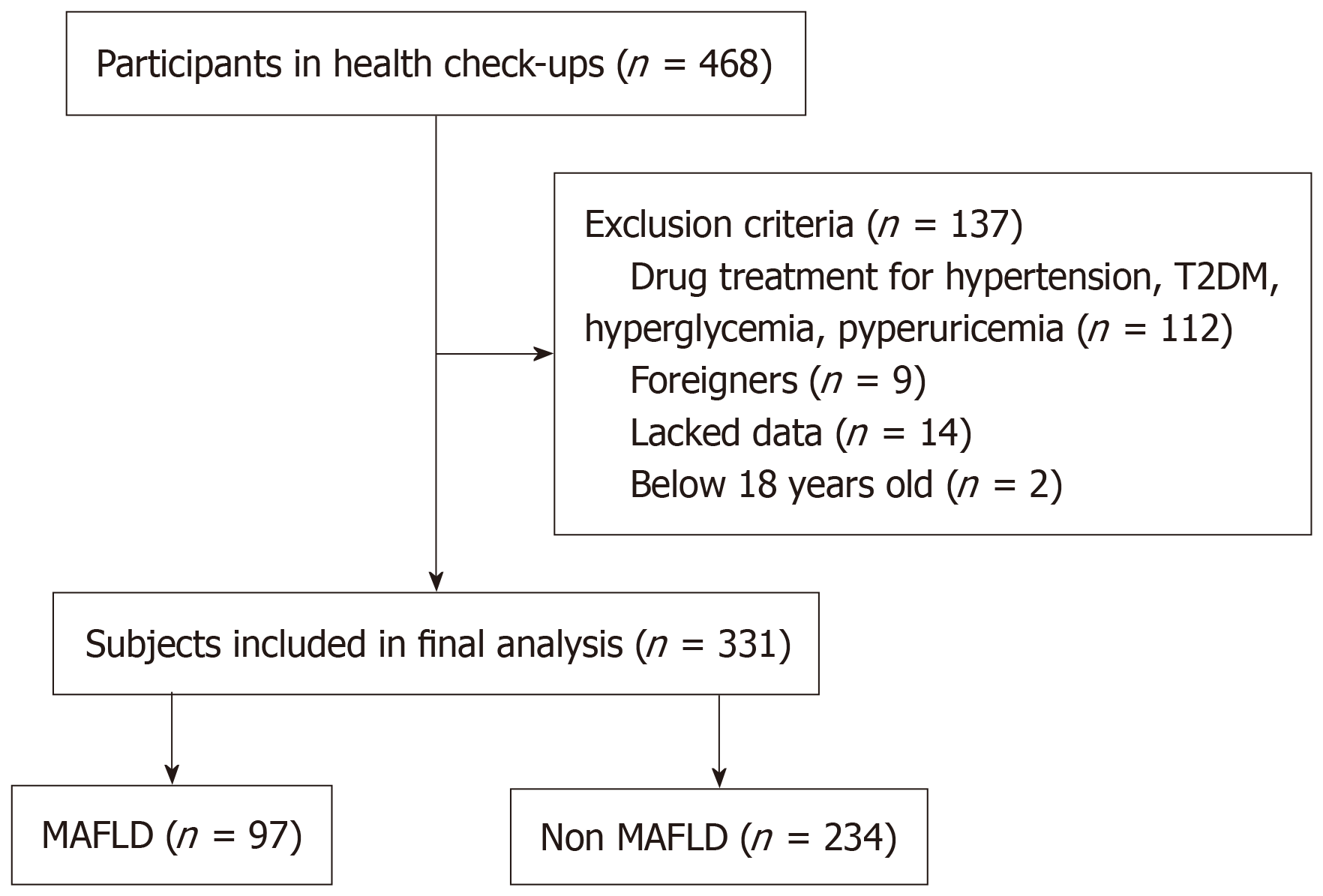
This retrospective study included 331 adults undergoing routine health check-ups at The Health Evaluation and Promotion Center, International University of Hea
MAFLD was identified in 97 of 331 individuals (29.31%). Prevalence was sig
MAFLD is prevalent in Vietnamese adults undergoing health screening. It is strongly associated with sex, age, body mass index, and metabolic disorders, in
Core Tip: This study investigated the prevalence and associated factors of metabolic dy
- Citation: Lu NL, Lam HT, Vo TD. Prevalence of metabolic dysfunction-associated fatty liver disease among health check-up attendees: A retrospective study conducted in Vietnam. World J Gastroenterol 2025; 31(37): 110797
- URL: https://www.wjgnet.com/1007-9327/full/v31/i37/110797.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i37.110797
Non-alcoholic fatty liver disease (NAFLD) has gained attention as a major contributor to chronic liver disease globally, which can lead to cirrhosis and even liver cancer[1]. The global prevalence of NAFLD, which ranges from 13% to 32% in various regions[2], has risen significantly over time, increasing from 18.2% in 1990 to 38.9% in 2020[3]. NAFLD is closely associated with obesity, metabolic disorders, and type 2 diabetes, and can progress to cirrhosis and hepatocellular carcinoma[4,5]. However, the tra
With rapid economic development and lifestyle changes, including sedentary habits and increased carbohydrate intake, there has been a surge in disease prevalence, placing a significant burden on healthcare systems. There are numerous studies investigating the prevalence and characteristics of MAFLD worldwide[11-16]. In China, a study by Li et al[16] in 2020 showed that the prevalence of MAFLD was 31.38%, while a more recent study by Chang et al[12] in 2023 found a higher prevalence of 37.32%. Similarly, studies conducted in South Korea, Iran, and Turkey have documented MAFLD prevalence rates ranging from approximately 26% to over 45%, particularly among individuals with obesity or type 2 diabetes. These findings underscore that MAFLD has become a major public health issue across Asia, closely linked to the rising burden of metabolic disorders and liver-related morbidity[17]. In Vietnam, most studies on fatty liver disease have focused on NAFLD rather than MAFLD. For example, a 2020 study by Tuong et al[18] reported a NAFLD prevalence of 73.3% among patients with type 2 diabetes assessed by FibroScan, while a 2021 study by Thong et al[19] found elevated alanine aminotransferase levels in patients with NAFLD with advanced fibrosis. However, data on MAFLD prevalence and metabolic characteristics in the general Vietnamese population are still lacking, and epidemiological data on this disease in Vietnam remain limited.
Therefore, this study estimated the prevalence and characteristics of MAFLD among individuals undergoing health check-ups at a major tertiary referral hospital in southern Vietnam, using the new diagnostic criteria.
A retrospective study used data from the population who participated in the general health check-ups at The Health Evaluation and Promotion Center Cho Ray Hospital, International University of Health and Welfare, Cho Ray Hospital (Hõ Chí Minh, Vietnam) from June to October 2023. The subjects were Vietnamese individuals over 18 years old who had complete data, no history of liver surgery or nephrectomy, no history of ovarian surgery, no liver cirrhosis, liver cancer, and were not currently being treated for conditions such as hypertension, diabetes, dyslipidemia, or hyperuricemia. The recorded information includes medical history, lifestyle habits, anthropometric measurements, clinical examination, abdominal ultrasound results, and the collection of fasting blood samples. The Health Evaluation and Promotion Center Cho Ray Hospital, International University of Health and Welfare Center is a dedicated health screening facility applying standardized Ningen Dock protocols. It mainly serves asymptomatic adults attending voluntary or employer-organized check-ups.
The minimum sample size was calculated using a standard formula for single-proportion studies, assuming a prevalence of 26.1% based on a recent study conducted in Chongqing, China (2021), a 95% confidence level (Z = 1.96), and a precision of ± 5%. Based on this calculation, at least 297 participants were needed. A total of 468 individuals were included in the study. A total of 137 subjects were excluded from the study, including 112 subjects with a history of hypertension, diabetes, dyslipidemia, and hyperuricemia; 9 subjects who were foreigners; 14 subjects who lacked data; and 2 subjects who were age 17. The final sample size of our study was 331 individuals (Figure 1). Ethical approval was granted by the ethics committee of the University of Medicine and Pharmacy at Ho Chi Minh City (No. 836/HDDD-DHYD).
Body mass index (BMI) was determined by height and weight, classified as lean, normal, and overweight/obese according to the criteria in Asian populations. Waist circumference (WC) was calculated at the level of the umbilicus, standing after exhaling normally. Abdominal obesity was defined as WC ≥ 90 cm in men and ≥ 80 cm in women. Blood pressure (BP), including systolic BP and diastolic BP, was measured twice on the upper arm in a sitting position after 5 minutes of rest, by using an electronic automatic sphygmomanometer (Suzuken Kenz BPM AC 05P; Suzuken Co. Ltd., Aichi, Japan). The average BP was obtained after repeated measurements. Hypertension was defined as ≥ 140/90 mmHg according to the European Society of Hypertension 2023[20]. Blood samples were collected from peripheral veins after a minimum of 8 hours of overnight fasting. Routine biochemical blood tests, including tests for liver function, kidney function, serum uric acid level, fasting blood glucose level, blood lipids and glycated hemoglobin (HbA1C), were performed using the Architect Plus Ci4100 Integrated System (Abbott, Chicago, IL, United States) and Adams A1c Automatic Analyzer (Arkray Inc., Kyoto, Japan).
Based on the 2009 International Diabetes Federation guidelines[21], metabolic syndrome (MS) was diagnosed by the presence at least three of the following five conditions: Abdominal obesity; hypertriglyceridemia defined as > 150 mg/dL, or being on medication to treat elevated triglycerides (TGs); reduced high-density lipoprotein cholesterol (HDL-c), defined as < 40 mg/dL for men or < 50 mg/dL for women, or being on medication to treat it; BP ≥ 130/85 mmHg or being on medication to treat hypertension; and hyperglycemia defined as fasting blood glucose ≥ 100 mg/dL or being on medication to treat high blood sugar. Dyslipidemia was diagnosed according to the Ministry of Health guidelines for Vietnamese adults as the presence of at least one of the following lipid abnormalities: Total cholesterol level > 200 mg/dL, TG level > 150 mg/dL, low-density lipoprotein cholesterol level > 100 mg/dL, and HDL-c level < 40 mg/dL[22]. Diabetes and pre-diabetes were diagnosed by the criteria of The American Diabetes Association 2020[23]. Hyperuricemia was diagnosed if serum uric acid exceeded 7 mg/dL in males or 6 mg/dL in females[24]. Elevated liver enzymes were defined as enzyme levels of > 33 U/L for men or > 25 U/L for women[25].
MAFLD diagnosis was defined according to the new criteria, based on ultrasonically diagnosed hepatic steatosis and the presence of one of the following three conditions: Overweight or obesity (defined as BMI ≥ 23 kg/m2 in Asians), presence of type 2 diabetes mellitus [fasting plasma glucose (FPG) levels ≥ 126 mg/dL or HbA1c ≥ 6.5%], or evidence of metabolic dysregulation[6]. Metabolic dysregulation was diagnosed by the presence of two or more of the following metabolic risk abnormalities: WC ≥ 90 cm in men and ≥ 80 cm in women, BP ≥ 130/85 mmHg, TG ≥ 150 mg/dL, HDL-c < 40 mg/dL for men and < 50 mg/dL for women, prediabetes (FPG levels 100 mg/dL to 125 mg/dL or HbA1c 5.7% to 6.4%)[6]. According to the Asian Pacific association for the study of the liver recommendation, ultrasound is a useful initial diagnostic tool screening for fatty liver[6]. In our study, experienced sonographers performed abdominal ultrasound. Fatty liver manifests as increased echogenicity of the liver parenchyma compared to the renal cortex, presence of blurring of the walls of the portal veins and hepatic veins, deep attenuation, and blurred diaphragm[26].
All analyses were performed using Stata software, version 17 (StataCorp LLC, College Station, TX, United States). For continuous data, results are expressed as the mean ± SD or as medians with interquartile ranges depending on the distribution. Categorical variables are expressed as proportions. The difference of the continuous variables was analyzed by the t-test or Mann-Whitney test. Groups of categorical variables were compared by the χ2 test or Fisher test. The prevalence of MAFLD in our study was described as a percentage and compared the prevalence differences among different groups by the χ2 test. P < 0.05 was considered statistically significant.
The baseline characteristics of the study participants are summarized in Table 1. A total of 331 individuals undergoing health check-ups were enrolled in our study. The subjects’ mean age was 44.99 ± 10.69 years old. A total of 164 men (49.55%) and 167 women (50.45%) were included in the study. Over half of the study participants were classified as overweight/obese, with a prevalence of 51.96% (172/331). The prevalence of abdominal obesity was 35.05% (116/331). Hypertension, diabetes/pre-diabetes, dyslipidemia, hyperuricemia, elevated liver enzymes, and MS were identified in 7.85% (26/331), 39.27% (130/331), 92.15% (305/331), 41.09% (136/331), 28.70% (95/331), and 21.45% (71/331), respectively.
| Variable | Total (n = 331) | MAFLD (n = 97) | Non-MAFLD (n = 234) | P value |
| Age (years) (mean ± SD) | 44.99 ± 10.69 | 48.02 ± 10.22 | 43.73 ± 10.64 | < 0.001 |
| Age | < 0.001 | |||
| Male | 164 (49.55) | 73 (75.26) | 91 (38.89) | |
| Female | 167 (50.45) | 24 (24.74) | 143 (61.11) | |
| BMI (kg/m2) | 23.1 (21.3-25.3) | 25.4 (23.7-26.8) | 22.4 (20.7-23.8) | < 0.001 |
| Underweight | 8 (2.42) | 0 (0) | 8 (3.42) | |
| Normal | 151 (45.62) | 14 (14.43) | 137 (58.55) | |
| Overweight/obese | 172 (51.96) | 83 (85.57) | 89 (38.03) | |
| WC (cm) | 81 (75-88) | 88 (85-93) | 78.5 (74-84) | < 0.001 |
| Abdominal obesity | 116 (35.05) | 56 (57.73) | 60 (25.64) | |
| SP (mmHg) | 109 (100-120.7) | 117 (109-130) | 105 (98-115) | < 0.001 |
| DP (mmHg) | 72 (66-79) | 78 (72-85) | 69 (64-77) | < 0.001 |
| TC (mg/dL) | 208 (183-233) | 212 (184-247) | 205 (183-230) | 0.112 |
| LDL-c (mg/dL) | 138 (116-166) | 139 (116-165) | 138 (116-166) | 0.851 |
| TG (mg/dL) | 113 (82-182) | 180 (133-258) | 95 (74-138) | < 0.001 |
| HDL-c (mg/dL) | 47 (41-58) | 41 (37-45) | 51 (44-61) | < 0.001 |
| FPG (mg/dL) | 92 (86-99) | 98 (93-105) | 90 (84-96) | < 0.001 |
| HbA1C (%) | 5.5 (5.3-5.7) | 5.7 (5.5-5.9) | 5.4 (5.2-5.6) | < 0.001 |
| AST (U/L) | 19 (16-23) | 22 (18-26) | 19 (16-22) | < 0.001 |
| ALT (U/L) | 20 (15-30) | 26 (20-47) | 18 (13-24) | < 0.001 |
| GGT (U/L) | 30 (18-57) | 55 (31-80) | 25 (16-39) | < 0.001 |
| White blood cell count (G/L) | 6.19 (5.28-7.33) | 6.61 (5.56-7.71) | 6.07 (5.12-7.07) | 0.001 |
| Red blood cell count (T/L) | 4.92 (4.52-5.27) | 5.10 (4.77-5.37) | 4.83 (4.46-5.17) | < 0.001 |
| Platelet (G/L) | 259 (225-304) | 265 (220-302) | 258 (229-304) | 0.838 |
| UA (mg/dL) | 6.2 (5.1-7.3) | 7.2 (6.1-8.5) | 5.7 (4.8-6.8) | < 0.001 |
| Creatinine (mg/dL) | 0.73 (0.62-0.88) | 0.81 (0.69-0.96) | 0.69 (0.61-0.83) | < 0.001 |
| Hypertension | 26 (7.85) | 19 (19.59) | 7 (2.99) | < 0.001 |
| Dyslipidemia | 305 (92.15) | 96 (98.97) | 209 (89.32) | 0.003 |
| Prediabetes/diabetes | 130 (39.27) | 64 (65.98) | 66 (28.21) | < 0.001 |
| Hyperuricemia | 136 (41.09) | 63 (64.95) | 73 (31.19) | < 0.001 |
| Elevated liver enzymes | 95 (28.70) | 45 (46.39) | 50 (21.37) | < 0.001 |
| MS | 71 (21.45) | 53 (54.64) | 18 (7.69) | < 0.001 |
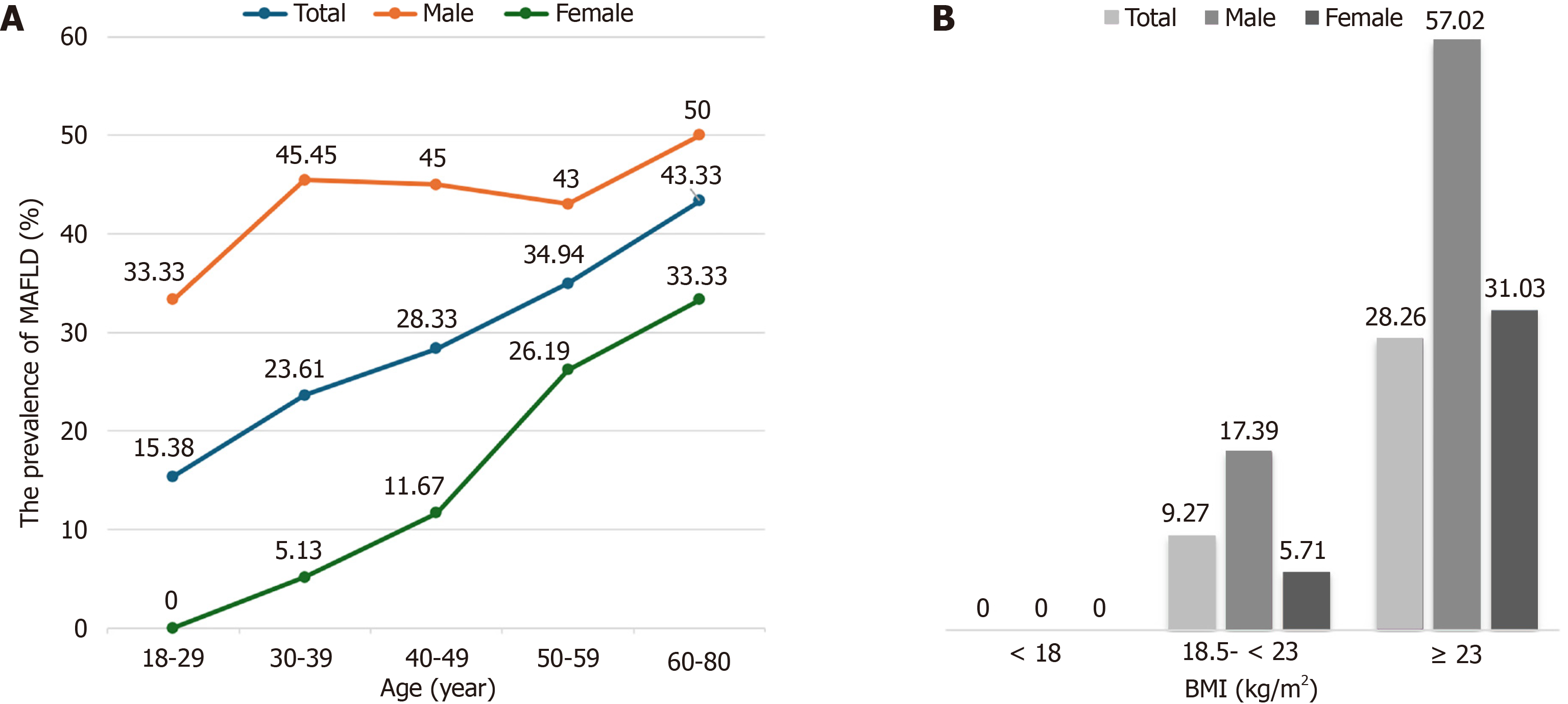
Among the 331 participants enrolled in the study, 97 were diagnosed with MAFLD, representing a prevalence of 29.31% (Table 1). There was a significant difference in the prevalence of MAFLD between sex (P < 0.001), which was higher in men (44.51%) than in women (14.37%). The overall prevalence of MAFLD tended to increase with age; however, this difference was not reported (P = 0.102). Similarly, the prevalence of MAFLD gradually increased with increasing BMI, which was significantly different among different groups (P < 0.001): 0% for the underweight group, 9.27% for the normal group, and 48.26% for the overweight/obese group.
The prevalence of MAFLD also increased with age in males and females (Figure 2). However, a significant age-related increase in MAFLD prevalence was only observed in women (P = 0.008), particularly after the age of 50. For males, the prevalence rose rapidly before 40 years and then decreased; however, it rose slowly and peaked in the 60- to 80-year age range, but there was no difference between different ages in men (P = 0.932). After adjusting for sex, the difference in the MAFLD prevalence between different BMI groups was also found. When stratified by BMI, males exhibited a significantly elevated prevalence of MAFLD compared to their female counterparts (P < 0.05). In both males and females, the prevalence tended to rise with increasing BMI (P < 0.001).
The characteristics of participants with MAFLD are presented in Table 1. Compared to the non-MAFLD group, subjects with MAFLD showed a higher prevalence of males and older age (P < 0.001). The participants with MAFLD also had significantly higher WC, BMI, BP, TG, FPG, HbA1C, liver enzymes, creatinine, serum uric acid, white blood cell count, and red blood cell count but lower HDL-c level (P < 0.001) than the participants without MAFLD. For metabolic disorders, the prevalence of hypertension, dyslipidemia, prediabetes/diabetes, hyperuricemia, elevated liver enzymes, and MS in subjects with MAFLD (19.59%, 98.96%, 65.98%, 64.95%, 46.39%, and 54.64%) was significantly higher than those in subjects without MAFLD (2.99%, 89.32%, 28.20%, 31.19%, 21.37%, and 7.69%) (P < 0.001).
With the new definition of FLD, many studies worldwide have surveyed the prevalence of MAFLD. Our study is one of the first surveys conducted in Vietnam focusing on the prevalence and characteristics of MAFLD in individuals undergoing physical examination since this new definition was established. The prevalence of NAFLD in Asians ranges from 15%-20%, which is lower than that of Europeans, where the prevalence of NAFLD is about 25%[2]. In our study, the prevalence of MAFLD was 29.31%, which was similar to that in Fan et al’s study (29.2%; 2021)[14], higher than that in Chen et al’s study (26.1%; 2021)[15] and He et al’s study (21.18%; 2024)[11], and lower than that in Li et al’s study (31.38%; 2020)[16] and Chang et al’s study (37.32%; 2023)[12]. A meta-analysis of general populations in the Asia-Pacific region reported that the MAFLD prevalence was approximately 33% (95% confidence interval: 19%-51%) in Southeast Asia. These findings demonstrate that the MAFLD prevalence in Vietnam aligns with regional estimates (about one-third of the adult population) and is comparable to data from Malaysia, Singapore, and Thailand[17]. This suggests a shared regional burden driven by similar socioeconomic and lifestyle transitions. There are many reasons for the difference in the MAFLD’s prevalence, including geographic and racial characteristics, dietary habits, the difference in methods used to diagnose fatty liver [conventional two-dimensional ultrasound; FibroScan (Echosens, Paris, France)], and in study populations. With the development of the economy, changes in living and dietary habits, urbanization, and advances in screening and diagnostic tools, the prevalence of MAFLD has gradually increased. The rising prevalence of MAFLD is not only found in studies conducted in Asia but a United States epidemiological survey[27] also revealed a notable increase in MAFLD prevalence, from 34.4% in 2011 to 38.1% in 2018. In the future, the MAFLD prevalence will also increase further due to the rising rates of obesity, overweight, diabetes, and MS, placing a significant burden on healthcare systems for screening and managing fatty liver disease.
Notably, our study found that there was a significant difference in the prevalence of MAFLD between males and females, which was higher in men (44.51%) than in women (14.37%; P < 0.001). Possible reasons for the different results might be explained by the fact that men tend to have bad habits and a higher risk of metabolic disorders than women. Estrogen plays a role as a protective factor against hepatic steatosis in premenopausal women[28]. Additionally, body fat distribution is significantly different between males and females. Men tend to accumulate more visceral fat creating central obesity, which leads to steatosis in liver, whereas women have fat distributed in their hips and thighs. This result was also found in surveys conducted by Chang et al[12], Chen et al[15], and Li et al[16]. However, a study by Fan et al[14] showed an opposite result. That study reported a higher prevalence of MAFLD in women than in men. A possible reason is the difference in age of the study population. The median age of Fan et al’s study population was 67 years old, which was higher than that in our study and others[14]. Moreover, the results of Fan et al’s study showed a higher percentage of female participants (59.6% vs 40.4%)[14]. Advanced age leads to more postmenopausal individuals, and the decline in estrogen during menopause is a risk factor for MAFLD.
While the prevalence of metabolic disorders appears to increase with age, the trend is not statistically significant (P = 0.102). One possible reason is the difference in participant number in each age group. The small sample size of subjects over 60 years old (n = 30) is a limitation that may compromise the representativeness and generalizability of these findings to this population. Furthermore, the increasing trend of MAFLD prevalence with age was also observed in males and females, with the trend being statistically significant in females (P = 0.008).
The prevalence trend among women differed from that of men. Among males, prevalence increased rapidly until age 40 years, dropped slowly between 40 and 60, and then increased again, peaking in the 60-80 age bracket. This finding can be explained by the fact that men under 40 tend to have higher work pressures and social obligations, leading to unhealthy lifestyle habits such as alcohol consumption, which increases the risk of developing metabolic disorders. For women, the prevalence increased gradually at the younger ages and then accelerated after age 40, a period associated with perimenopause and postmenopause.
We also observed that the prevalence of MAFLD rose with increasing BMI (P < 0.001), in accordance with previous studies[12,14-16], emphasizing the importance of weight management. Thus, higher BMI and age may play important roles in the development and progression of steatosis. In addition, our study also found that people with normal weight had an MAFLD prevalence of 9.27%. Other studies have shown similar results with a prevalence less than 10%[12,14-16,29]. Hepatic steatosis is considered an early predictor and the main cause of metabolic disorders, even in individuals with normal weight.
In subjects with MAFLD, the prevalence of overweight/obese, hypertension, dyslipidemia, prediabetes/diabetes, hyperuricemia, elevated liver enzymes, and MS was all significantly higher than those in subjects without MAFLD (P < 0.001). Chen et al[15] reported that subjects with MAFLD had a higher prevalence of abdominal obesity, dyslipidemia, hypertension, hyperuricemia and MS than those without MAFLD (P < 0.001). The liver plays a pivotal role in lipid and glucose metabolism. Therefore, hepatic steatosis is considered a liver manifestation of systemic metabolic dysfunction[5]. Patients with MAFLD have a high risk of liver dysfunction as well as dyslipidemia, which in turn increases their risk of metabolic disorders, suggesting a strong association between MAFLD and systemic metabolic disorders and indicating that these conditions often coexist and influence each other. Thus, screening for metabolic disorders in individuals with MAFLD is essential to identify and address underlying conditions, improve liver function, and reduce the risk of complications. From a clinical perspective, our study highlights the need for early screening of metabolic risk factors in asymptomatic individuals, especially in routine health check-ups. Identifying MAFLD early provides a valuable window for intervention before the onset of more severe liver or cardiovascular complications. Lifestyle modifications, particularly weight management and metabolic control, should be emphasized as first-line strategies in primary prevention.
A key advantage of our study is that it is the first to survey to the prevalence and characteristics of MAFLD in individuals undergoing health check-ups. Nevertheless, certain limitations should be acknowledged. First, as this was a cross-sectional design, our analysis could not reflect changes over time as well as the natural progression and causal relationships of MAFLD. Second, the participants were individuals who regularly underwent preventive health screenings, potentially reflecting a population that is more engaged in managing their personal health. They are relatively more associated with their health and are likely to be managing their health well on their own. Thus, the findings of this research had some limitations regarding the representativeness and generalizability. Third, excluding those receiving treatment for metabolic disorders could have underestimated MAFLD prevalence and introduced selection bias. Fourth, hepatic steatosis assessment by ultrasound has lower sensitivity compared to magnetic resonance imaging-based methods. Finally, some criteria used to define metabolic dysfunction, such as insulin resistance markers (e.g., Homeostasis Model Assessment of Insulin Resistance) and high-sensitivity C-reactive protein, were not measured in this study, which could have affected diagnostic sensitivity.
In conclusion, our findings showed that the prevalence of MAFLD in the population undergoing health check-ups was 29.31%. There were significant changes in the prevalence of MAFLD between different groups stratified by sex, age, and BMI. MAFLD and systemic metabolic disorders are closely related, they interact with each other, and they may have an influence on each other. Further, more studies are needed to validate factors associated with the development of MAFLD, which may help in the management and prevention of this disease.
The authors would like to express their sincere gratitude to the staff of The Health Evaluation and Promotion Center Cho Ray Hospital, International University of Health and Welfare, Cho Ray Hospital, and the Department of Internal Medicine, University of Medicine and Pharmacy at Ho Chi Minh City, for their valuable support in data collection and scientific guidance.
| 1. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2326] [Article Influence: 155.1] [Reference Citation Analysis (1)] |
| 2. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7944] [Article Influence: 794.4] [Reference Citation Analysis (8)] |
| 3. | Le MH, Yeo YH, Zou B, Barnet S, Henry L, Cheung R, Nguyen MH. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical bayesian approach. Clin Mol Hepatol. 2022;28:841-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 4. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2359] [Article Influence: 87.4] [Reference Citation Analysis (1)] |
| 5. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5228] [Article Influence: 653.5] [Reference Citation Analysis (9)] |
| 6. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 3195] [Article Influence: 532.5] [Reference Citation Analysis (2)] |
| 7. | Kim H, Lee CJ, Ahn SH, Lee KS, Lee BK, Baik SJ, Kim SU, Lee JI. MAFLD Predicts the Risk of Cardiovascular Disease Better than NAFLD in Asymptomatic Subjects with Health Check-Ups. Dig Dis Sci. 2022;67:4919-4928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, Targher G, Byrne CD, Yuan WJ, Zheng MH. MAFLD and risk of CKD. Metabolism. 2021;115:154433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 9. | Lee H, Lee YH, Kim SU, Kim HC. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2021;19:2138-2147.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (7)] |
| 10. | Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, Takahashi H, Anzai K, George J, Torimura T. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40:3018-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 327] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 11. | He Y, Yao N, Tian F, Liu L, Lin X, Chen X, Duan H, Jiang Y, Yu G, Song C, Wang D, Ma Q, Liu L, Wan H, Shen J. Prevalence and risk factors of MAFLD and its metabolic comorbidities in community-based adults in China: A cross-sectional study. Diabetes Metab Syndr. 2024;18:102973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Chang M, Shao Z, Wei W, Shen P, Shen G. Sex-specific prevalence and risk factors of metabolic-associated fatty liver disease among 75,570 individuals in eastern China. Front Endocrinol (Lausanne). 2023;14:1241169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Guan L, Zhang X, Tian H, Jin X, Fan H, Wang N, Sun J, Li D, Li J, Wang X, Zeng Z, Li Y. Prevalence and risk factors of metabolic-associated fatty liver disease during 2014-2018 from three cities of Liaoning Province: an epidemiological survey. BMJ Open. 2022;12:e047588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Fan J, Luo S, Ye Y, Ju J, Zhang Z, Liu L, Yang J, Xia M. Prevalence and risk factors of metabolic associated fatty liver disease in the contemporary South China population. Nutr Metab (Lond). 2021;18:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Chen YL, Li H, Li S, Xu Z, Tian S, Wu J, Liang XY, Li X, Liu ZL, Xiao J, Wei JY, Ma CY, Wu KN, Ran L, Kong LQ. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Li H, Guo M, An Z, Meng J, Jiang J, Song J, Wu W. Prevalence and Risk Factors of Metabolic Associated Fatty Liver Disease in Xinxiang, China. Int J Environ Res Public Health. 2020;17:1818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Eslam M, Fan JG, Yu ML, Wong VW, Cua IH, Liu CJ, Tanwandee T, Gani R, Seto WK, Alam S, Young DY, Hamid S, Zheng MH, Kawaguchi T, Chan WK, Payawal D, Tan SS, Goh GB, Strasser SI, Viet HD, Kao JH, Kim W, Kim SU, Keating SE, Yilmaz Y, Kamani L, Wang CC, Fouad Y, Abbas Z, Treeprasertsuk S, Thanapirom K, Al Mahtab M, Lkhagvaa U, Baatarkhuu O, Choudhury AK, Stedman CAM, Chowdhury A, Dokmeci AK, Wang FS, Lin HC, Huang JF, Howell J, Jia J, Alboraie M, Roberts SK, Yoneda M, Ghazinian H, Mirijanyan A, Nan Y, Lesmana CRA, Adams LA, Shiha G, Kumar M, Örmeci N, Wei L, Lau G, Omata M, Sarin SK, George J. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic dysfunction-associated fatty liver disease. Hepatol Int. 2025;19:261-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 18. | Tuong TTK, Tran DK, Phu PQT, Hong TND, Dinh TC, Chu DT. Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: Evaluation of Hepatic Fibrosis and Steatosis Using Fibroscan. Diagnostics (Basel). 2020;10:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Thong VD, Quynh BTH. Correlation of Serum Transaminase Levels with Liver Fibrosis Assessed by Transient Elastography in Vietnamese Patients with Nonalcoholic Fatty Liver Disease. Int J Gen Med. 2021;14:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Algharably EAE, Azizi M, Benetos A, Borghi C, Hitij JB, Cifkova R, Coca A, Cornelissen V, Cruickshank JK, Cunha PG, Danser AHJ, Pinho RM, Delles C, Dominiczak AF, Dorobantu M, Doumas M, Fernández-Alfonso MS, Halimi JM, Járai Z, Jelaković B, Jordan J, Kuznetsova T, Laurent S, Lovic D, Lurbe E, Mahfoud F, Manolis A, Miglinas M, Narkiewicz K, Niiranen T, Palatini P, Parati G, Pathak A, Persu A, Polonia J, Redon J, Sarafidis P, Schmieder R, Spronck B, Stabouli S, Stergiou G, Taddei S, Thomopoulos C, Tomaszewski M, Van de Borne P, Wanner C, Weber T, Williams B, Zhang ZY, Kjeldsen SE. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41:1874-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 1854] [Article Influence: 618.0] [Reference Citation Analysis (0)] |
| 21. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10963] [Article Influence: 644.9] [Reference Citation Analysis (0)] |
| 22. | Ministry of Health, Vietnam. Guidelines for diagnosis and treatment of Endocrine – Metabolic diseases. [cited 15 May 2025]. Available from: https://kcb.vn/van-ban/huong-dan-chan-doan-va-dieu-tri-benh-noi-tiet-chuyen-hoa.html. |
| 23. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2238] [Article Influence: 373.0] [Reference Citation Analysis (0)] |
| 24. | Chittoor G, Voruganti VS. Hyperuricemia and Gout. In: Caterina RDE, Martinez JA, Kohlmeier M. Principles of Nutrigenetics and Nutrigenomics. MA, United States: Academic Press, 2020: 389-394. [DOI] [Full Text] |
| 25. | Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112:18-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 789] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 26. | Idilman IS, Ozdeniz I, Karcaaltincaba M. Hepatic Steatosis: Etiology, Patterns, and Quantification. Semin Ultrasound CT MR. 2016;37:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Wong RJ, Cheung R. Trends in the Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in the United States, 2011-2018. Clin Gastroenterol Hepatol. 2022;20:e610-e613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402-409. [RCA] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Yuan Q, Wang H, Gao P, Chen W, Lv M, Bai S, Wu J. Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease among 73,566 Individuals in Beijing, China. Int J Environ Res Public Health. 2022;19:2096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/